- 1Department of Biochemistry, Kwara State University, Ilorin, Nigeria
- 2Food and Nutrition Sciences Laboratory, International Institute of Tropical Agriculture, Ibadan, Nigeria
- 3Food and Nutrition Sciences Laboratory, International Institute of Tropical Agriculture, Southern Africa, Research and Administration Hub (SARAH), Lusaka, Zambia
- 4Food Quality Laboratory, International Institute of Tropical Agriculture, Dar es Salaam, Tanzania
- 5Cassava Breeding Unit, International Institute of Tropical Agriculture, Ibadan, Nigeria
Biofortification of cassava roots has improved its health benefits by raising the quantity and bioavailability of bioactive compounds, particularly carotenoids. This study analyzed the bioactive constituents (carotenoids, tannins, total phenolics, and flavonoids), antioxidant, starch-digesting enzymes (α-amylase and α-glucosidase) inhibitory and pasting properties of flours of 18 elite yellow-fleshed cassava genotypes grown at the International Institute of Tropical Agriculture, Ibadan, using standard laboratory methods. Generally, the concentrations of the bioactive constituents (9-cis-β-carotene, 13-cis-β-carotene, all trans-β-carotene, total β-carotene, total carotenoids, tannins, total phenolics, and total flavonoids) of the different cassava genotypes varied. The antioxidant activity varied significantly among the different cassava genotypes, with IITA-TMS-IBA183001 having the highest reducing power (2.59 GAE mg/g) and most potent DPPH· scavenging ability (SC50: 14.56 mg/mL). However, the starch-hydrolysing enzymes (alpha-amylase and alpha-glucosidase) inhibitory and the pasting properties of the different genotypes were generally comparable. Total carotenoids content significantly correlated with the DPPH· SC50 (r = −0.495), while total phenolics content correlated with ABTS·+(r = 0.839) and DPPH· SC50 (r = −0.870). Also, tannins content significantly correlated with ABTS·+ (r = 0.553), while total flavonoids content was significantly correlated with α-amylase IC50 (r = −0.532). These findings suggest the potential of flours of the 18 elite yellow-fleshed cassava genotypes to serve as important dietary sources of antioxidants and starch-hydrolysing enzyme inhibitors, which may be beneficial in oxidative stress and postprandial hyperglycaemic conditions.
Introduction
Cassava (Manihot esculenta Crantz) roots are the primary calorie sources for ~700 million people living in the world's tropical and sub-tropical regions (Ferraro et al., 2016). Cassava has some agronomic advantages over some other staple crops. It withstands drought stress and soil infertility and thrives in suboptimal conditions. After maturation, it can be stored underground for several months, without losing its nutrient quality (Ukwuru and Egbonu, 2013). Hence, it is regarded as a viable staple crop for achieving the United Nations Sustainable Development Goal (SDG) of food and nutritional security (Kanter et al., 2016). Traditionally, cassava roots are rich in starch, but lack fat, protein, and micronutrients (Aragón et al., 2018). However, biofortification of cassava roots has improved its micronutrients and bioactive compounds levels, especially provitamin A carotenoids (pVAC). Interestingly, the pVAC level in yellow-fleshed cassava roots has been increased considerably to about 25 μg/g FW, a concentration that surpasses the initial breeding target of 15 μg/g FW (Ceballos et al., 2017).
Presently, pVAC cassava varieties serve as a source of energy. They are also important dietary sources for the alleviation of vitamin A deficiency, especially in developing nations (Aidoo et al., 2022). Furthermore, the carotenoids in yellow-fleshed cassava roots are well-known to possess antioxidant activities. They also protect against some chronic non-communicable diseases including cancer (Agarwal and Rao, 2000) and cardiovascular diseases (Gammone et al., 2017). According to Seifried et al. (2007), the antioxidant activity of carotenoids stands out as the main mechanism underlying their health benefits. Also, Sugiura et al. (2015) reported that consuming carotenoids-rich diets could protect against developing type 2 diabetes (T2D). Aside from the carotenoids in yellow-fleshed cassava, cassava flour also contains phenolic compounds (Irondi et al., 2019; Adefegha et al., 2021), flavonoid glycosides, alkaloids (Osipitan et al., 2015; Pinto-Zevallos et al., 2016), and fiber (Nwose et al., 2017); all of which are prominent for their health benefits. Most of the existing reports on these bioactive constituents, aside carotenoids, were on white-fleshed cassava root and its traditional products, leaving the bioactive components and bioactivities of yellow-fleshed cassava genotypes under-reported. However, this trend is changing as recent research has focused on the bioactive constituents and bioactivity of different traditional products, from biofortified (yellow-fleshed) cassava. For example, our recent study demonstrated the bioactive constituents, antioxidant, and antihyperglycaemic activities of different traditional products (fufu, gari, and lafun) from pVAC biofortified cassava (Kareem et al., 2022). Similarly, Ogbonna et al. (2018) reported the blood glucose response in humans due to the consumption of gari produced from different varieties of pVAC biofortified cassava. In a related study, Oluba et al. (2017) investigated the glycaemic index (GI) of gari eba produced from pVAC cassava in healthy adult humans. These authors (Oluba et al., 2017; Ogbonna et al., 2018) concluded that the gari eba made from pVAC biofortified cassava had a significantly lower GI than that of gari eba produced from white-fleshed (non-provitamin A biofortified) cassava roots.
With this available evidence, it is pertinent to appreciate the possible mechanism underpinning the lower GI of pVAC biofortified cassava compared to non-pVAC biofortified cassava. In this context, inhibition of enzymes (α-amylase and α-glycosidase) that digest dietary starch by the bioactive constituents in foods is a crucial mechanism to the diminution of postprandial blood glucose response has been attributed (Butterworth et al., 2022; Irondi et al., 2022a). Alpha-amylase and alpha-glucosidase are responsible for catalyzing dietary carbohydrates digestion into simple sugars, and for this reason, their activities affect postprandial blood glucose levels (Irondi et al., 2019). Furthermore, cassava roots are processed into different products, such as high-quality cassava flour and cassava starch, that have applications in the food and non-food industries. The end-use that these products are suitable for is partly determined by their pasting properties. Pasting properties, including peak, breakdown, trough, final, set back viscosities, peak time, and pasting temperature, represent the behavior of starch when subjected under shear forces to a definite cycle of heating and cooling (Irondi et al., 2019). Hence, to provide insight into the possible mechanism behind the lower GI of pVAC biofortified cassava and its potential industrial uses, this study aimed to determine the bioactive compounds' levels, antioxidant, starch-hydrolysing enzymes inhibitory, and pasting properties of flours of 18 elite yellow-fleshed cassava genotypes.
Materials and methods
Samples collection and preparation
Freshly harvested root samples of 18 elite yellow-fleshed cassava genotypes (IITA-TMS-IBA070337, 070593, 182924, 182926, 182959, 182961, 182962, 182963, 182984, 182986, 182993 183001, 183007, 183008, 183023, 183028, 183037, and 183044) were collected from the research farm of the International Institute of Tropical Agriculture's (IITA) Cassava Breeding Unit in Ibadan, Nigeria. The roots of the various genotypes were washed thoroughly with tap water, peeled, and cut into chunks using a stainless-steel kitchen knife. A fresh sample of each genotype was portioned into two; one portion was packed in light-resistant sample bags and immediately analyzed for carotenoids (β-carotene profile) using HPLC. The second portion was oven-dried at 60°C for a period of 72 h, after which it was ground into flour using a Laboratory Hammer Mill (Perten 3102). Subsequently, the cassava flour samples were dispensed in sample containers, and kept in a 4°C refrigerator for the other analyses (tannins, total phenolics, total flavonoids, antioxidants, starch-hydrolysing enzymes, and pasting properties).
Quantification of carotenoids by a reverse-phase HPLC
The carotenoid contents (total carotenoid and β-carotene profile) of the yellow-fleshed cassava genotypes were quantified using Maziya-Dixon et al. (2016) method. The total carotenoid content was determined using the spectrophotometric method, while the β-carotene profile [all-trans-β-carotene (βC), 9-cis-β-carotene (9-cis-βC), and 13-cis-β-carotene (13-cis-βC)] was quantified using a reverse-phase HPLC system (Waters Corporation, Milford, MA, USA), comprising a binary HPLC pump (Waters 626), C30 YMC carotenoid column (4.6 × 250 mm, 3 μM), photodiode array detector (Waters 2996), auto-sampler (Waters 717), and Waters Empower 1 software. The carotenoids'chromatograms were acquired at 450 nm, and the identification and quantification of the individual β-carotenes were achieved by comparing their absorption spectrum and elution time with those of their corresponding pure external standards. To guard against the degradation of the carotenoids induced by light, the analysis was conducted under subdued light, with the extracts collected into a vial wrapped with an aluminum foil.
Preparation of cassava flour extract
The cassava flour extract was prepared by soaking 0.5 g of the cassava flour sample in 25 mL of methanol in an air-tight centrifuge tube of 50 mL capacity. After shaking the mixture for 1 h with a mechanical shaker, it was left for 12 h. After that, the mixture was centrifuged at 3,000 g for 10 min. Theresulting supernatant (subsequently referred to as flour extract) was collected and kept at −4°C until further analysis (Irondi et al., 2022a).
Assay for tannins content
The procedure described by Joslyn (1970) was adopted to assay for the tannins content of the flour. A 0.5 g portion of the cassava flour was soaked for 15 min in 5 mL of acidified methanol (1% HCl in methanol), after which the mixture was vortexed and centrifuged for a period of 10 min at 3,000 g. Afterwards, 0.1 mL of the supernatant, distilled water (7.5 mL), Folin-Dennis reagent (0.5 mL), and Na2CO3 solution (1 mL) were sequentially added and mixed. The volume of the reaction mixture was diluted to 10 mL by adding distilled water (0.9 mL). At 760 nm, the absorbance measurement was conducted following 30 min of incubation at room temperature. The tannin content of the flours was calculated using a tannic acid calibration curve and presented as tannic acid equivalent (TAE) in mg/g.
Assay for total phenolics content
The protocol described by Elemosho et al. (2021) was adopted to quantify the total phenolic content of the cassava flours extract. This was achieved by dispensing 300 μL of each extract into a test tube, to which Folin–Ciocalteu reagent (1.5 mL, 10 times dilution with distilled water) and Na2CO3 solution (1.2 mL, 7.5% w/v) were sequentially added. The reaction mixture was vortexed and allowed to incubate at room temperature for 30 min before the absorbance was recorded at 765 nm in comparison to a control sample. For the blank preparation, distilled water (300 μL) was dispensed instead of the flours extract, with all other reagents added. The total phenolic level was computed using a standard curve of gallic acid and expressed in gallic acid equivalents (GAE) in mg per g.
Assay for total flavonoids content
The protocol outlined by Meda et al. (2005) was followed to assay for the total flavonoids content of the cassava flours extract. To accomplish this,0.5 mL of the extract, methanol (1.5 mL), 10% aluminum chloride (0.1 mL), 1M potassium acetate (0.1 mL), and distilled water (2.8 mL) were mixed in a test tube. Afterwards, the reaction mixture was vortexed and incubated for a period of 30 min (at room temperature), before the absorbance reading was taken at 514 nm. The total flavonoids content in the extract was computed using a quercetin standard curve and presented as quercetin equivalent (QE) in mg per g.
Assay for 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) radical cation (ABTS·+) scavenging capacity
The method described by Re et al. (1999) was adopted to assay for the ABTS·+ scavenging capacity of the cassava flours extract. First, a working reagent of ABTS·+ was prepared by mixing aqueous solutions of ABTS·+ (7 millimole/L) and K2S2O8 (2.45 millimole/L) in equal volumes. Then, for a total of 16 h, the mixture was incubated at room temperature in the dark. After that, the reagent's absorbance reading at 734 nm was adjusted to 0.70 ± 0.02 using ethanol (95%). Next, an aliquot of 2.0 mL of the ABTS·+ working reagent and the fours extract (0.2 mL) were dispensed in a test tube, vortexed, and subjected to 15 min of incubation at room temperature in a dark condition. The absorbance reading was then taken at 734 nm, and the extract's ABTS·+ scavenging capacity was computed from a Trolox calibration curve and presented as Trolox Equivalent Antioxidant Capacity (TEAC) in millimole/g.
Assay for 2,2-diphenyl-2-picrylhydrazyl radical (DPPH·) scavenging capacity
The procedure described by Cervato et al. (2000) was followed to assay for the DPPH radical scavenging capacity of the cassava flours extract. For this purpose, a mixture of 1.0 mL of different extract concentrations and 60 micromolar DPPH radical solution (3.0 mL) was subjected to incubation at room temperature in a dark condition for 30 min. Subsequently, the absorbance reading was taken at 517 nm in a spectrophotometer, and the DPPH radical scavenging capacity of the extract was computed and presented as SC50 (extract's concentration that scavenged DPPH· by 50%).
Assay fo ferric reducing power
The method described by Oyaizu (1986) was used to assay for the ferric reducing power of the cassava flour extract. This was achieved by incubating at 50°C for 20 min a mixture consisting of 2.5 mL each of the extract, 200 mM sodium phosphate buffer (pH 6.6), and 1% potassium ferricyanide. This was followed by the addition of 2.5 mL of 10% trichloroacetic acid to the mixture, which was then subjected to centrifugation for a period of 10 min at 650 × g. The supernatant was portioned into aliquots of 2.5 mL in different test tubes and distilled water (2.5 mL) and ferric chloride solution (1 mL, 0.1%) were sequentially added to each test tube. The reaction mixture was vortexed and the absorbance was subsequently read at 700 nm. The extract's ferric reducing power was expressed as gallic acid equivalent in mg per g.
Assay for alpha-amylase inhibitory activity
An assay for the cassava flour extract's ability to inhibit α-amylase activity was performed using the procedure outlined by Kwon et al. (2008). For this purpose, α-amylase (EC 3.2.1.1) from porcine pancreas and its substrate (soluble starch) were used. A mixture of different dilutions of flour extract totaling 500 μL and 500 μL of α-amylase (0.5 mg/mL, prepared in 0.02 mole/L sodium phosphate buffer, pH 6.9, containing 0.006 M NaCl) was subjected to incubation for 10 min at 37°C. Afterwards, starch solution (500 μL, 1% in 0.02 mole/L sodium phosphate buffer) was added to the mixture, which was incubated for a period of 15 min at 37°C. After that, the reaction was stopped by adding 1.0 mL of the DNSA color reagent (1% 3,5-dinitrosalicylic acid and 12% sodium potassium tartrate in 0.4 mole/L NaOH). The reaction mixture was then diluted with 10 mL of distilled water, after incubating in a boiling water bath for a period of 5 min and cooled to a room temperature. The absorbance was read at 540 nm. The percentage of -amylase inhibition was determined using the following equation:
Where AR is the mean absorbance reading of the reference; AS is the mean absorbance reading of the sample.
Assay for α-glucosidase inhibitory activity
An assay for the ability of the cassava flour extract to inhibit α-glucosidase activity was performed as per the procedure outlined by Kim et al. (2005), in which α-glucosidase (EC 3.2.1.20) from Bacillus stearothermophillus and it substrate, para-nitrophenylglucopyranoside (PNPG) were used. In this assay, α-glucosidase (5 units) was incubated with the flour extract (20 g/mL) for a period of 15 min. Following that, 3 millimole/L PNPG in 20 millimole/L phosphate buffer (pH 6.9) was added to initiate the hydrolytic reaction for 20 min, during incubation at 37°C. The hydrolytic reaction was terminated by dispensing 2 mL of 0.1 M Na2CO3 into the mixture. The absorbance reading of the yellow p-nitrophenol formed during PNPG hydrolysis was taken at 400 nm. The inhibition of alpha-glucosidase was calculated thus:
Where AR is the mean absorbance reading of the reference; AS is the mean absorbance reading of the sample.
Analysis of pasting properties
The cassava flour samples' pasting characteristics (peak, breakdown, trough, final, set back viscosities, peak time, and pasting temperature) were analyzed using a Perten Scientific instrument's Rapid Visco Analyzer (RVA) (RVA-4500, Springfield, IL) (Irondi et al., 2022b). The RVA was attached to a Personal computer (PC) running Thermocline software. The pasting properties of the flour suspension (3.45 g of flour in 25 mL of distilled water) were read on the PC through the Thermocline software. The assay was performed at a 160 rpm constant stirring rate as per the following protocol: a 5 min holding period at 25°C; a 5°C/min heating rate between 25 and 95°C; a 5 min holding period at 95°C; a 5°C/min cooling rate to 25°C; and a 5 min holding period at 25°C. Viscosity was expressed in Rapid Visco Analyzer units (RVU); peak time was in minutes, while pasting temperature was expressed in °C.
Statistical analysis of data
Data from replicate experiments (mean ± standard deviation) were analyzed using one-way ANOVA and Duncan multiple range test (DMRT) at a 95% confidence level, using the 17th version of SPSS statistical software. The correlations between the bioactive constituents, antioxidant activity, and starch-hydrolysing enzymes inhibitory capacity of the samples were established by the Pearson correlation analysis.
Results
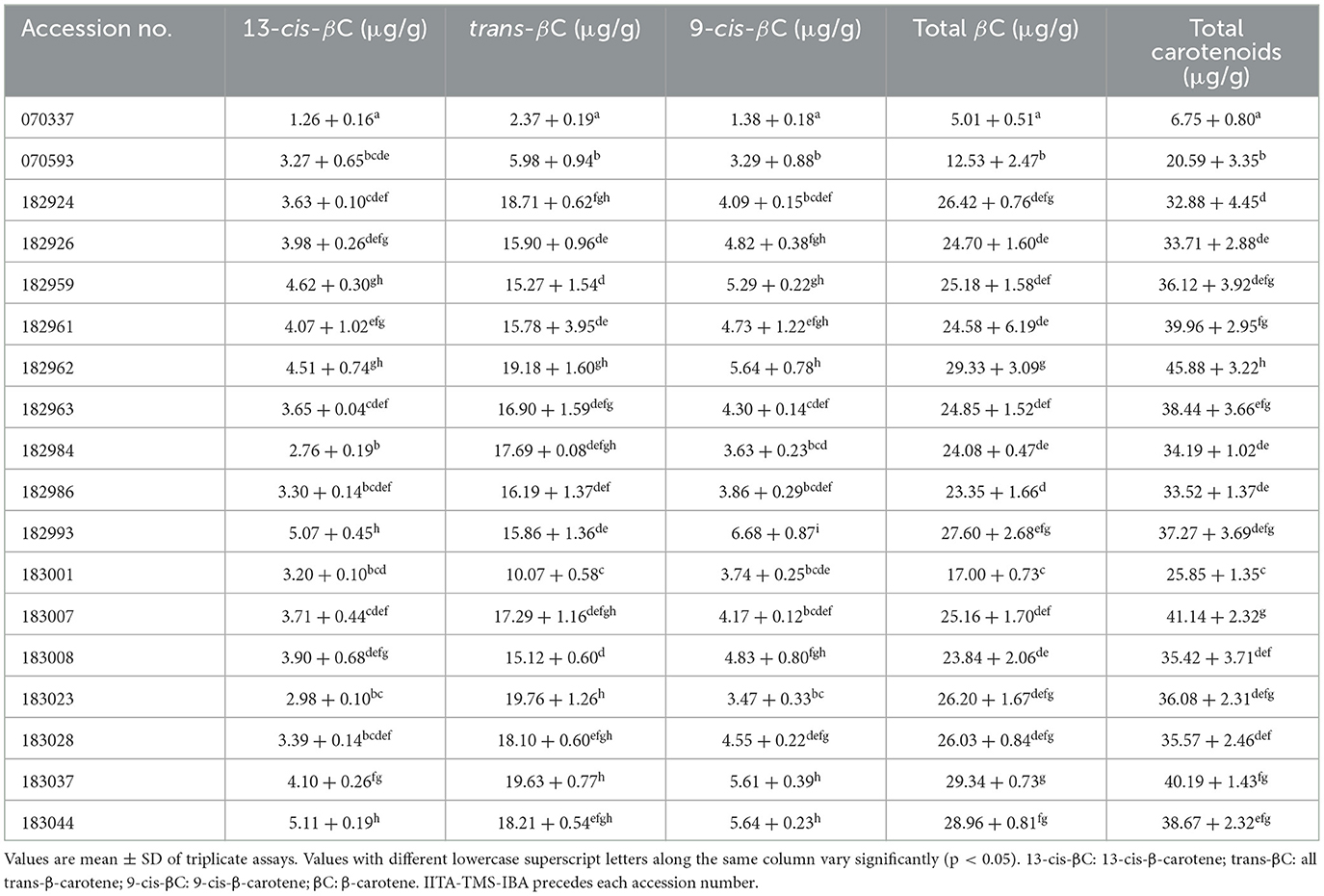
Carotenoids profile of 18 elite yellow-fleshed cassava genotypes
The carotenoid profile (on dry weight base) of the 18 elite yellow-fleshed cassava genotypes is presented in Table 1. The level of 13-cis-β-carotene ranged from 1.26 μg/g in IITA-TMS-IBA070337 to 5.11 μg/g in IITA-TMS-IBA183044; trans-β-carotene ranged from 2.37 to 19.76 μg/g in IITA-TMS-IBA070337 and IITA-TMS-IBA183023; 9-cis-β-carotene ranged from 1.38 μg/g in IITA-TMS-IBA070337 to 6.68 μg/g in IITA-TMS-IBA182993. The total β-carotene content ranged from 5.01 μg/g in IITA-TMS-IBA070337 to 29.34 μg/g in IITA-TMS-IBA183037, while the total carotenoids ranged from 6.75 μg/g in IITA-TMS-IBA070337 to 45.88 μg/g in IITA-TMS-IBA182962.
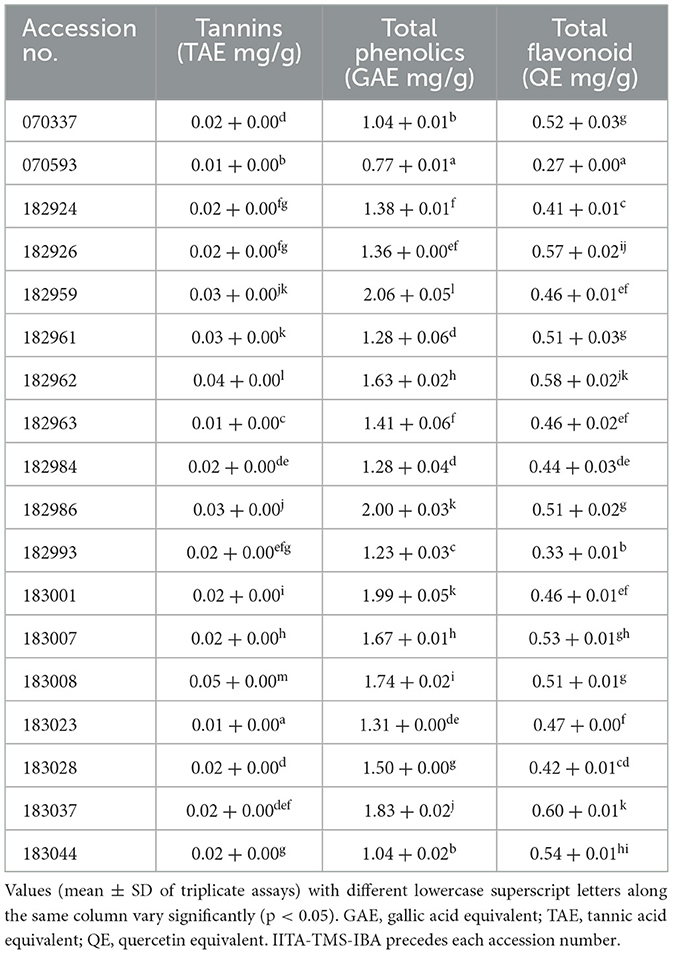
Polyphenolic constituents of 18 elite yellow-fleshed cassava genotypes
The polyphenolic constituents of the 18 elite yellow-fleshed cassava genotypes are presented in Table 2. Tannins content ranged from 0.01 TAE mg/g in IITA-TMS-IBA183023 to 0.05 TAE mg/g in IITA-TMS-IBA183008; total phenolics content ranged from 0.77 GAE mg/g in IITA-TMS-IBA070593 to 2.06 GAE mg/g in IITA-TMS-IBA182959, and total flavonoids ranged from 0.27 QE mg/g in IITA-TMS-IBA070593 to 0.60 QE mg/g in IITA-TMS-IBA183037. As presented in the Table, the level of total phenolic in IITA-TMS-IBA182959 was significantly higher (p < 0.05) than those of the other genotypes.
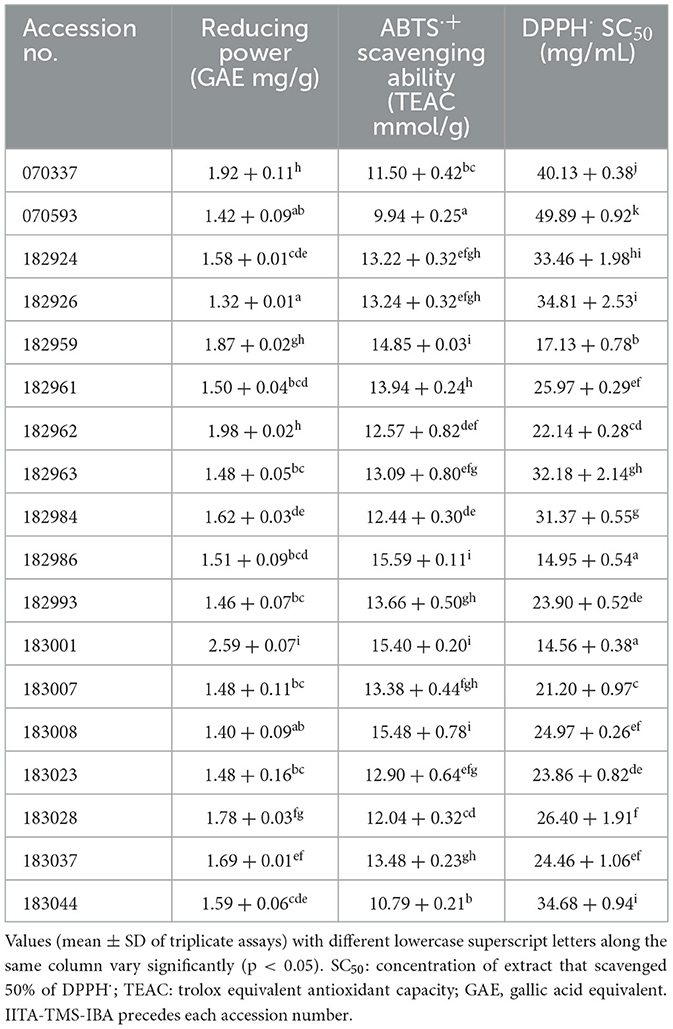
Antioxidant activity of 18 elite yellow-fleshed cassava genotypes
The antioxidant activity of the 18 elite yellow-fleshed cassava flours is presented in Table 3. The reducing power (Fe3+ to Fe2+) of the flours ranged from 1.32 GAE mg/g in IITA-TMS-IBA070337 to 2.59 GAE mg/g in IITA-TMS-IBA183001. The ABTS·+ scavenging capacity ranged from 9.94 TEAC mmol/g in IITA-TMS-IBA070593 to 15.59 TEAC mmol/g in IITA-TMS-IBA182986, and the DPPH· SC50 ranged from 14.56 mg/mL (strongest) in IITA-TMS-IBA183001 to 49.89 mg/mL (weakest) in IITA-TMS-IBA070593. There were significant variations (p < 0.05) in the antioxidant activity of the flours of the 18 cassava genotypes studied.
Starch-hydrolysing enzymes inhibitory activity of 18 elite yellow-fleshed cassava genotypes
The inhibitory activity of the samples against the starch-hydrolysing enzymes (alpha-amylase and alpha-glucosidase) is presented in Table 4 as IC50 values (extract's concentration that produced 50% inhibition of the enzyme's catalytic activity). The IC50 values against alpha-amylase and alpha-glucosidase ranged from 21.75 to 33.18 mg/mL in IITA-TMS-IBA070593 and IITA-TMS-IBA182963, respectively, and 23.06 mg/mL in IITA-TMS-IBA182961 to 40.64 mg/mL in IITA-TMS-IBA182986. The starch-hydrolysing enzymes inhibitory activity of the 18 genotypes were significantly different (p < 0.05).
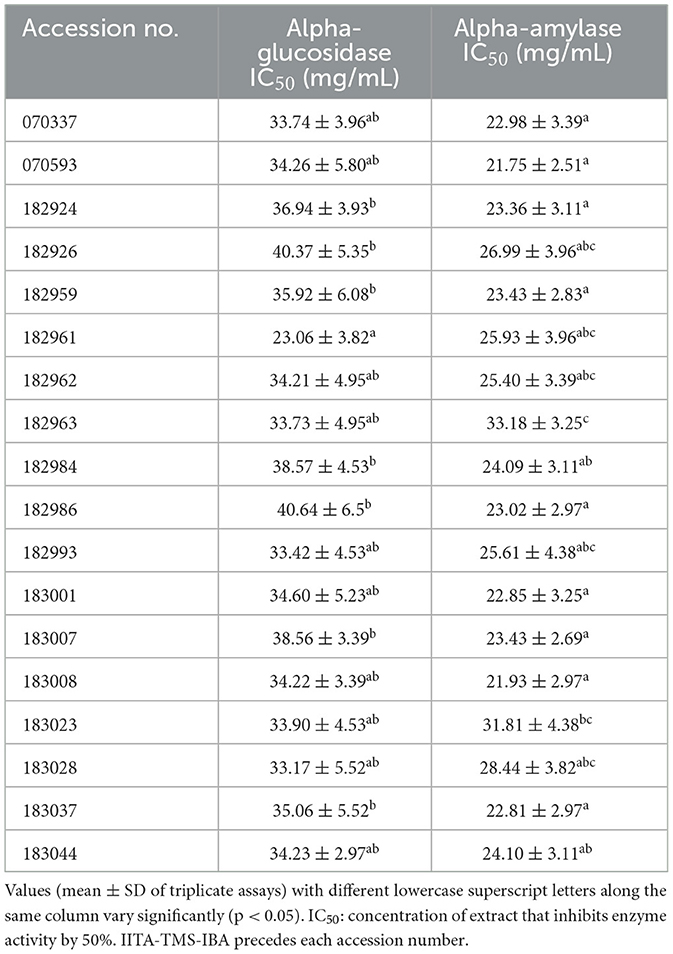
Table 4. Starch-hydrolysing enzymes inhibitory activity of 18 elite yellow-fleshed cassava genotypes.
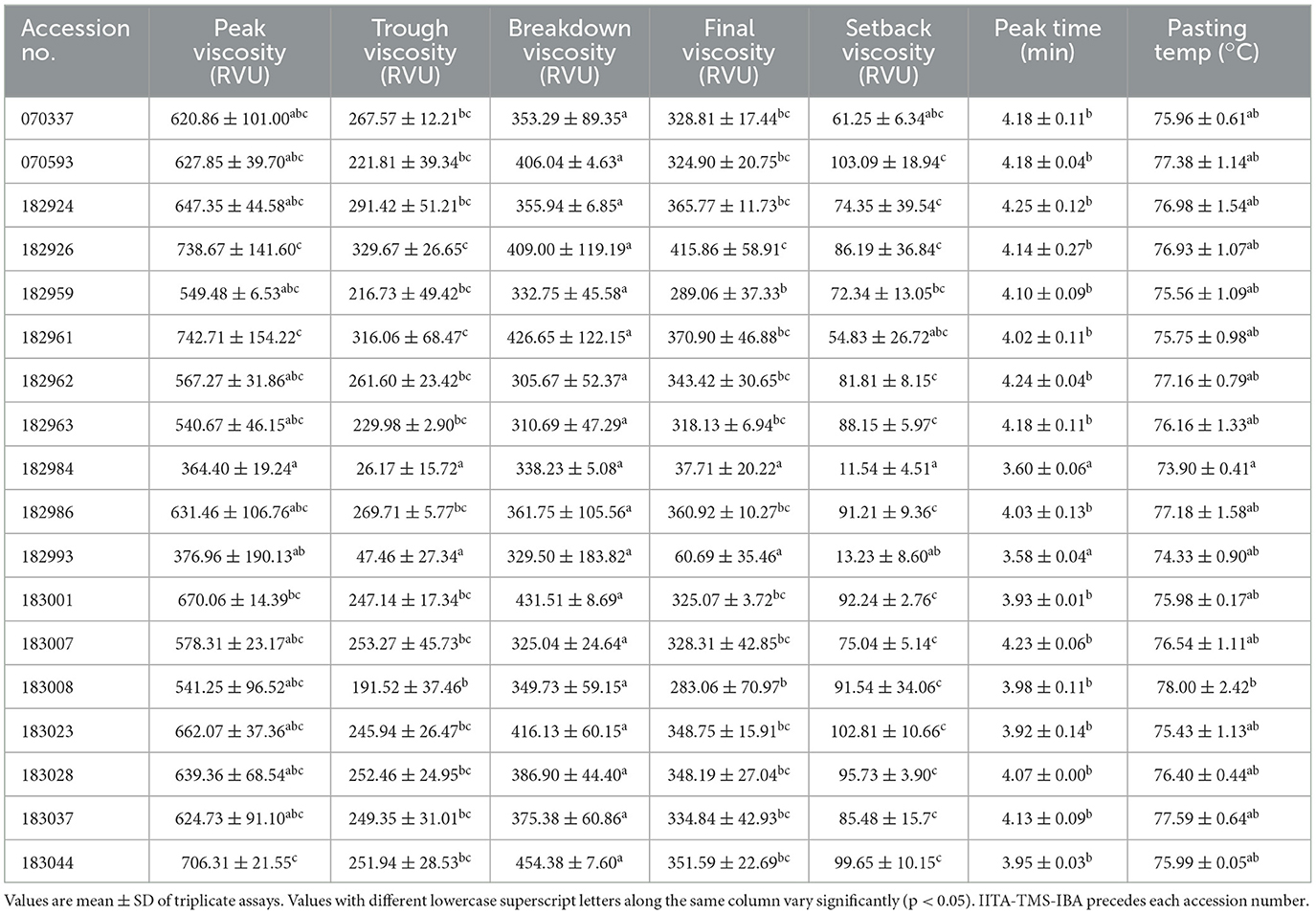
Pasting properties of 18 elite yellow-fleshed cassava genotypes
Table 5 presents the pasting properties of the 18-elite yellow-fleshed cassava genotypes. The peak, trough, and breakdown viscosities (in RVU) ranged from 93.83 to 972.60 in IITA-TMS-IBA182993 and IITA-TMS-IBA182961, 15.25–373.0 in IITA-TMS-IBA182984 and IITA-TMS-IBA182959, and 63.17 to 599.6 in IITA-TMS-IBA18299 and IITA-TMS-IBA182926, respectively. The final and setback viscosities (in RVU) ranged from 23.17 to 488.3 in IITA-TMS-IBA182984 and IITA-TMS-IBA182926, and 3.920 to 128.1 in IITA-TMS-IBA182993 and IITA-TMS-IBA182926, respectively. The peak time ranged from 3.53 to 4.40 min in IITA-TMS-IBA182993 and IITA-TMS-IBA182924, while the pasting temperature ranged from 73.50 to 80.80°C in IITA-TMS-IBA182984 and IITA-TMS-IBA183008, respectively. Generally, the pasting properties of the different genotypes were comparable (p > 0.05).
Correlations among the bioactive components, antioxidant, and starch-hydrolysing enzymes inhibitory activities of flours of 18 elite yellow-fleshed cassava genotypes
Table 6 shows a significant and positive correlation (p ≤ 0.0001, r = 0.951) between the total β-carotene and total carotenoid levels of the 18 elite yellow-fleshed cassava genotypes. The total carotenoid content negatively correlated significantly with the DPPH· SC50 (p ≤ 0.05, r = −0.495). Total phenolic content correlated significantly with ABTS·+ (p < 0.0001, r = 0.839) and DPPH· SC50 (p < 0.0001, r = −0.870). Tannins content was significantly correlated with ABTS·+ (p < 0.05, r = 0.553). Further, total flavonoids content was significantly correlated with alpha-amylase IC50 (p ≤ 0.05, r = −0.532), while each of the total carotenoids (p ≤ 0.001, r = 0.710) and total β-carotene (p ≤ 0.001, r = 0.724) had a significant positive correlation with α-glucosidase IC50.
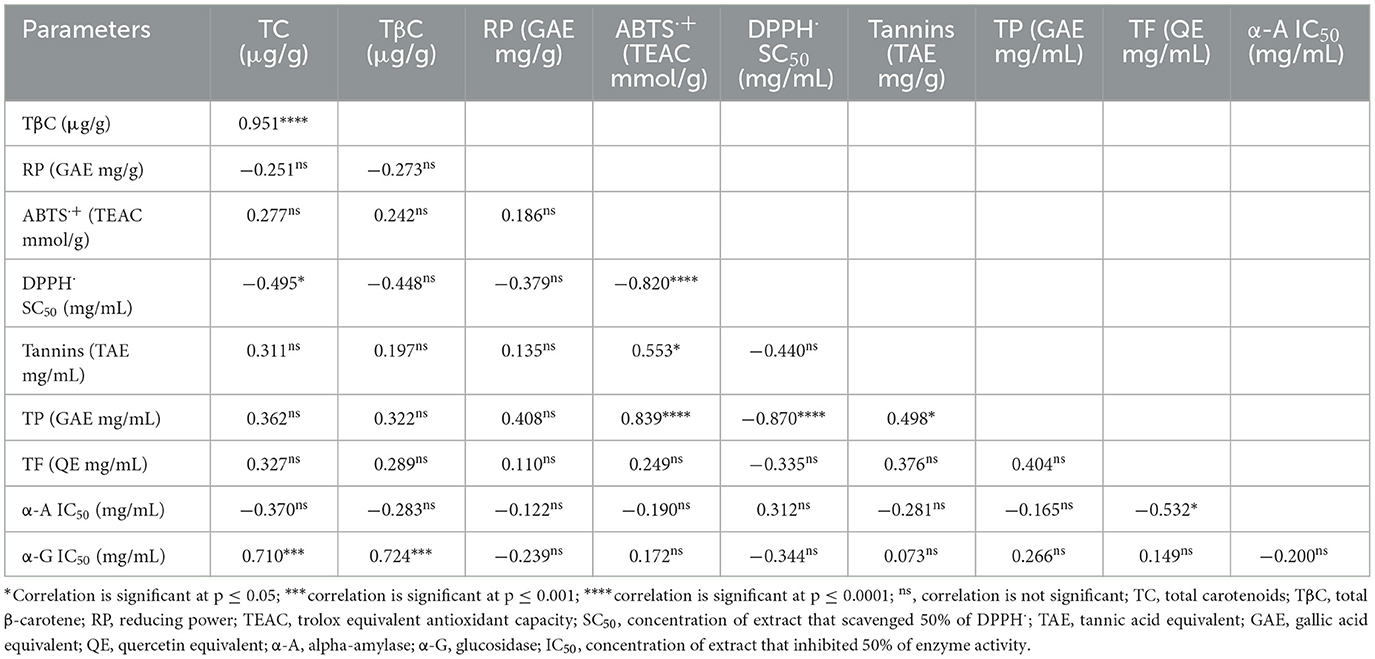
Table 6. Correlations among the bioactive constituents, antioxidant and starch-hydrolysing enzymes inhibitory activities of flours of 18 elite yellow-fleshed cassava genotypes.
Discussion
Carotenoids profile of 18 elite yellow-fleshed cassava genotypes
Among the 18 elite yellow-fleshed cassava genotypes, IITA-TMS-IBA182962 had the highest (p < 0.05) level of total carotenoids. Carotenoids, which are the pigments in yellow-fleshed cassava, were reported to have some desirable health-promoting activities such as antioxidant activities, immune function enhancement, mammary tumor growth suppression, prevention of blindness dueto age-related macular degeneration, and cardiovascular diseases (Chew and Park, 2004; Ahmed et al., 2005; Elemosho et al., 2021; Kareem et al., 2022). The range of β-carotene level, the predominant carotenoid in yellow-fleshed cassava, quantified in this study is higher than the range (1.12–6.53 μg/g) reported by Odoemelam et al. (2020) for yellow root cassava. Similarly, when compared to the range of total carotenoids level in fresh roots of sweet yellow cassava (2.64–14.15 μg/g) (Carvalho et al., 2012) and bitter yellow cassava (3.65–18.92 μg/g) cultivars (Oliveira et al., 2010), the total carotenoids level (6.75 to 45.88 μg/g) in the eighteen elite yellow-fleshed cassava genotypes is higher. These differences could be attributed abiotic and biotic factors, period of sample collection, and method of extraction variations. Other factors that may have contributed to the variations include differences in the sample variety, composition of farm soil, and climatic conditions, all of which have been documented to influence the concentrations and biological activities of plant secondary metabolites (Mpofu et al., 2006; Braga et al., 2014; Dusuki et al., 2020). Generally, we observed a variation in the β-carotene level in the 18 elite yellow-fleshed cassava genotypes. The observed variation may be attributed to differences in their genetic makeup (Ayetigbo et al., 2018). Beta-carotene is notable for its provitamin A activity. Although an adequate intake or the recommended dietary allowance for carotenoids is not yet established (Andarwulan et al., 2021), with a β-carotene range of 5.01 to 29.34 μg/g, the 18 elite yellow-fleshed cassava genotypes may serve as a rich dietary source of vitamin A in human nutrition. Moreover, with the emergence of COVID-19, β-carotene was also reported to offer protection against the disease (Anand et al., 2022).
Polyphenolic constituents of 18 elite yellow-fleshed cassava genotypes
Tannins and flavonoids, as phenolic compounds, possess antioxidant activities due to their redox properties. Thus, they act as singlet oxygen quenchers, reducing agents, and hydrogen donors (Chang et al., 2001). In addition to these mechanisms, Andarwulan et al. (2021) noted that flavonoids could indirectly scavenge free radicals by inducing key antioxidant enzymes that protect against oxidative stress and electrophilic toxicants, such as S-transferase glutathione and NAD(P)H quinone oxidoreductase. The tannins, total phenolics, and flavonoids levels of the 18 elite yellow-fleshed cassava genotypes examined in this research differ from the values reported by Adefegha et al. (2021) in roasted and sun-dried cassava flours. For example, whereas we detected a total phenolics level in the range of 0.77–2.00 mg/g in the yellow-fleshed cassava genotypes, Adefegha et al. reported 51.35 and 64.82 mg/g as the total phenolics level in roasted and sun-dried cassava flours, respectively. As explained earlier, the differences may be related to variations in various factors, including the sample variety, farm soil compositions, climatic conditions, and abiotic and biotic factors, which were reported to affect the levels and bioactivities of phytochemicals (Braga et al., 2014; Dusuki et al., 2020). Apart from their antioxidant activities, studies have shown that polyphenolics possess starch-digesting enzymes inhibitory activity, which they exhibit through non-specific binding to the enzymes, thereby denaturing them (Villiger et al., 2015; Elemosho et al., 2021). Additionally, phenolic compounds were reported to possess anti-diabetic, anti-hypertensive and anti-obesity properties (Irondi et al., 2018).
Antioxidant activity of flours of 18 elite yellow-fleshed cassava genotypes
The ability of the cassava flours to scavenge DPPH· (denoted by SC50) represents their free radical scavenging capacity. DPPH (a stable free radical) is converted to α,α-diphenyl-β-picryl hydrazine when reacted with antioxidants. As reported in previous studies, a lower DPPH· SC50 of a plant extract/bioactive compound represents a stronger free radical scavenging capacity (Irondi et al., 2018; Alamu et al., 2021). Thus, among the 18 elite yellow-fleshed cassava genotypes, IITA-TMS-IBA18298, with the least DPPH· SC50, had the strongest DPPH· scavenging capacity.
ABTS cation radical is more reactive than the DPPH radical. The reactions involving ABTS cation radicals require an electron transfer process as opposed to the DPPH reactions, which involve the transfer of a H atom (Srikanth et al., 2010). It is well-known that in the body, a preponderance of oxidants (free radicals and reactive oxygen species) over the antioxidant level leads to oxidative stress, a denominator in the pathogenesis of many diseases (Irondi et al., 2018, 2019). Taken together, the DPPH and ABTS free radicals scavenging capacities of the cassava genotypes suggest that they can serve as dietary sources for retarding free radicals generation, and mopping them up from the body, thereby mitigating oxidative stress (Alamu et al., 2021). Similarly, the reducing power (Fe3+ to Fe2+) of the cassava genotypes indicates their potential to alleviate Fe2+-mediated hydroxyl radical (OH·) formation from hydrogen peroxide (H2O2) (Oboh et al., 2008) and lipids oxidation (Hsu et al., 2003), also guarding against oxidative stress.
The antioxidant activity values of flours of the 18 elite yellow-fleshed cassava genotypes in this study differ from those previously reported in cassava flour. For instance, Irondi et al. (2019) reported a DPPH SC50 of 0.48 ± 0.06 mg/mL and an ABTS cation radical scavenging capacity of 120.55 ± 1.7 μmol TEAC/g in high-quality cassava flour made from the roots of white-fleshed cassava genotypes. Similarly, Adefegha et al. (2021) reported an ABTS cation radical scavenging capacity of 58.46 ± 0.33 and 99.92 ± 0.65 mmol TEAC/g for roasted and sun-dried cassava flours, respectively. As earlier stated, these variations may be due to genotypic, climatic conditions, and other abiotic and biotic factors differences, which affect phytochemicals' levels and, consequently, their bioactivities, including antioxidant activity (Braga et al., 2014; Dusuki et al., 2020).
Starch-hydrolysing enzymes inhibitory activity of flour extracts of 18 elite yellow-fleshed cassava genotypes
The capacity of plant/food extracts to inhibit starch-hydrolysing enzymes (alpha-amylase and alpha-glucosidase) has been used as an index of their potential for managing T2D (Irondi et al., 2021). Thus, the ability of the 18 elite yellow-fleshed cassava genotypes to inhibit alpha-amylase and alpha-glucosidase suggests that they may be of benefit for controlling postprandial hyperglycaemia characterizing T2D. Alpha-amylase and α-glucosidase play important catalytic roles in hydrolysing dietary carbohydrates. Alpha-amylase is responsible in hydrolysing the α-1,4 bonds of starch to form oligosaccharides such as malt-ose and dextrins, and disaccharides. On the other hand, α-glucosidase found in the small intestine brush bor-der, completes the digestion of starch by hydrolysing the glycosidic bonds of the oligosaccha-rides and disaccharides, producing absorbable monosaccha-rides, including glucose and fructose (Tucci et al., 2010). Hence, inhibiting these two enzymes is a crucial mechanism of action of many anti-diabetic agents (Kim et al., 2005), including drugs, natural products, and functional foods.
The starch-hydrolysing enzymes inhibitory activity of the 18 elite yellow-fleshed cassava genotypes observed in this study could be as a resultthe phenolic compounds and carotenoids present in them. Previous studies revealed the ability of these two classes of phytochemicals to inhibit starch-digesting enzymes, thereby exhibiting anti-hyperglycaemic activity (Sugiura et al., 2015; Irondi et al., 2019; Elemosho et al., 2021).
Pasting properties of flours of 18 elite yellow-fleshed cassava genotypes
According to Berski et al. (2011), pasting properties are important parameters used to determine the possible application of starch in the food industry. They represent the behavior of starch (changes in the physical and chemical structures) upon subjection under shear forces to a definite cycle of heating and cooling (Irondi et al., 2019). Peak viscosity, according to Alamu et al. (2017), denotes starch granules' ability to freely swell before their physical breakdown, which can be used to predict the final product quality of flour samples.
IITA-TMS-IBA182926 had the highest final viscosity among all the genotypes. The final viscosity is a measure of the cooked starch's stability and the paste's resistance to shear force during stirring (Maziya-Dixon et al., 2005). Thus, IITA-TMS-IBA182926 may be a good choice among all the genotypes for food and non-food products requiring high-viscosity starch. The stability of paste and its capacity to retrograde are assessed using setback viscosity (Liang and King, 2003). When the cooked paste cools to 50°C, the amylose and amylopectin components of the starch retrograde or reassociate, defining the viscosity of the cooked paste. The minimum temperature required to cook a starchy sample, according to Offia-Olua (2014), is the pasting temperature. Among all the genotypes, IITA-TMS-IBA182984 had the least pasting temperature, suggesting that the energy cost of developing products with it may be comparatively cheaper than those of the other genotypes.
Correlations among the bioactive components, antioxidant and starch-hydrolysing enzymes inhibitory activities of flours of 18 elite yellow-fleshed cassava genotypes
The significant positive correlation between each of the total phenolics, tannin contents and the ABTS·+ scavenging capacity indicates that polyphenolics were majorly responsible for the ABTS·+ scavenging activity of the cassava genotypes. In contrast, a negative correlation between a bioactive compound and DPPH· SC50 represents a stronger scavenging capacity since a lower DPPH· SC50 denotes a more potent free radical scavenging activity (Alamu et al., 2021; Elemosho et al., 2021). Thus, the negative correlations between each of total phenolics, total carotenoids, and DPPH· SC50 suggest that the phenolics and carotenoids in the 18 elite yellow-fleshed cassava flours might have contributed synergistically to the DPPH radical scavenging capacity of the flours.
Among the bioactive constituents in the cassava flours, only total flavonoids had a significant negative correlation with α-amylase IC50 (p < 0.05; r,−0.532). As with the DPPH SC50, a lower IC50 reflects a stronger enzyme inhibitory capacity. Hence, flavonoids (a class of polyphenolics) contributed majorly to the α-amylase inhibitory activity of the cassava flours. This is consistent with an earlier report by Elemosho et al. (2021), who also suggested that phenolic compounds significantly contribute to the starch-hydrolysing enzymes inhibitory capacity of yellow-orange maize hybrids.
Conclusions
Flours of the 18 elite yellow-fleshed cassava genotypes evaluated in this study were rich in carotenoids. They also contained polyphenolic compounds and exhibited antioxidant and starch-hydrolysing enzymes (alpha-amylase and alpha-glucosidase) inhibitory activities. Generally, the carotenoids and polyphenolics compositions of the different genotypes varied, but their starch-hydrolysing enzymes inhibitory and pasting properties were comparable. Correlation analysis revealed that total carotenoids and phenolics contents were associated with the antioxidant activity of cassava flour. In contrast, the total flavonoid was associated with their α-amylase inhibitory activity. Therefore, flours of the 18 elite yellow-fleshed cassava genotypes may serve as important dietary sources of antioxidants and starch-hydrolysing enzymes inhibitor, which may offer some benefits in oxidative stress and postprandial hyperglycaemic conditions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
EAl and EI: conceptualization. EI, EAl, and BK: methodology. BK: investigation, software, and formal analysis. AA, BM-D, EP, EAl, and EAj: resources, review, and editing. BK and EI: writing—original draft preparation. EI, EAl, and EAj: supervision. EAl and AA: funding acquisition. EAl, AA, EP, and BM-D: project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This study and the APC were funded by the CGIAR Research Program on Roots, Tubers, and Bananas (RTB) and the Bill & Melinda Gates Foundation (BMGF)–OPP1019962.
Acknowledgments
The authors acknowledged the support received from the International Institute of Tropical Agriculture (IITA), Ibadan, Department of Biochemistry, Kwara State University, staff and interns of the Food and Nutrition Sciences Laboratory (especially Mr. Michael Adesokan), and Cassava Breeding Unit (especially Mr. Peter Illuebey and Mrs. Bukky Ogungbesan and), IITA, Ibadan, Nigeria.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adefegha, S. A., Okeke, B. M., Oyeleye, S. I., and Oboh, G. (2021). Effects of processing on starch composition, glycemic indices, phenolic profile, and possible anti-diabetic properties of cassava (Manihot esculenta) flours. J. Food Process. Preserv. 45, e15586. doi: 10.1111/jfpp.15586
Agarwal, L. S., and Rao, A. V. (2000). Carotenoids and chronic diseases. Drug Metabol. Drug Interact. 17, 189–210. doi: 10.1515/DMDI.2000.17.1-4.189
Ahmed, S. S., Lott, M. N., and Marcus, D. M. (2005). The macular xanthophylls. Surv. Ophthalmol. 50, 183–193. doi: 10.1016/j.survophthal.2004.12.009
Aidoo, R., Oduro, I. N., Agbenorhevi, J. K., Ellis, W. O., and Pepra-Ameyaw, N. B. (2022). Physicochemical and pasting properties of flour and starch from two new cassava accessions. Int. J. Food Propert. 25, 561–569. doi: 10.1080/10942912.2022.2052087
Alamu, E. O., Maziya-Dixon, B., and Dixon, A. G. (2017). Evaluation of proximate composition and pasting properties of high quality cassava flour (HQCF) from cassava genotypes (Manihot esculenta Crantz) of β-carotene-enriched roots. LWT. 86, 501–506. doi: 10.1016/j.lwt.2017.08.040
Alamu, E. O., Maziya-Dixon, B., Menkir, A., Irondi, E. A., and Olaofe, O. (2021). Bioactive composition and free radical scavenging activity of fresh orange maize hybrids: impacts of genotype, maturity stages, and processing methods. Front. Nutr. 8, 640563. doi: 10.3389/fnut.2021.640563
Anand, R., Mohan, L., and Bharadvaja, N. (2022). Disease prevention and treatment using β-carotene: the ultimate provitamin A. Rev. Bras. Farmacogn. 32, 491–501. doi: 10.1007/s43450-022-00262-w
Andarwulan, N., Cahyarani, P. N., and Saraswati, Srednicka-Tober, D. (2021). Antioxidants such as flavonoids and carotenoids in the diet of Bogor, Indonesia residents. Antioxidants. 10, 587. doi: 10.3390/antiox10040587
Aragón, I. J., Ceballos, H., Dufour, D., and Ferruzzi, M. G. (2018). Pro-vitamin A carotenoids stability and bioaccessibility from elite selection of biofortified cassava roots (Manihot esculanta, Crantz) processed to traditional flours and porridges. Food Funct. 9, 4822–4835. doi: 10.1039/C8FO01276H
Ayetigbo, O., Latif, S., Abass, A., and Müller, J. (2018). Comparing characteristics of root, flour and starch of biofortified yellow-flesh and white-flesh cassava variants, and sustainability considerations: a review. Sustainability. 10, 3089. doi: 10.3390/su10093089
Berski, W., Ptaszek, A., Ptaszek, P., Ziobro, R., Kowalski, G., Grzesik, M., et al. (2011). Pasting and rheological properties of oat starch and its derivatives. Carbohydr. Polym. 83, 665–671. doi: 10.1016/j.carbpol.2010.08.036
Braga, T. V., Dores, R. G., Ramos, C. S., Evange, F. C. G., Tinoco, L. M., and Varotti, F. (2014). Antioxidant, antibacterial and antitumor activity of ethanolic extract of the Psidium guajava leaves. Am. J. Plant Sci. 5, 3492–3500. doi: 10.4236/ajps.2014.523365
Butterworth, P. J., Bajka, B. H., Edwards, C. H., Warren, F. J., and Ellis, P. R. (2022). Enzyme kinetic approach for mechanistic insight and predictions of in vivo starch digestibility and the glycaemic index of foods. Trends Food Sci. Technol. 120, 254–264. doi: 10.1016/j.tifs.2021.11.015
Carvalho, L. J., Oliveira, A. G., Godoy, R. O., Pacheco, S., Nutti, M., de Carvalho, J. V., et al. (2012). Retention of total carotenoid and b-carotene in yellow sweet cassava (Manihot esculenta Crantz) after domestic cooking. Food Nutr. Res. 56, 15788. doi: 10.3402/fnr.v56i0.15788
Ceballos, H., Davrieux, F., Talsma, E. F., Belalcázar, J., Chavarriaga, P., and Andersson, M. S. (2017). “Carotenoids in cassava roots”, in Carotenoids, G. Nikolic, ed. (London: IntechOpen) doi: 10.5772/intechopen.68279
Cervato, G., Carabelli, M., Gervasio, S., Cittera, A., Cazzola, R., and Cestaro, B. (2000). Antioxidant properties of oregano (Origanum vulgare) leaf extracts. J. Food Biochem. 24, 453–465. doi: 10.1111/j.1745-4514.2000.tb00715.x
Chang, S. T., Wu, J. H., Wang, S. Y., Kang, P. L., Yang, N. S., and Shyur, L. F. (2001). Antioxidant activity of extracts from Acacia confusa bark and heartwood. J. Agric. Food Chem. 49, 3420–3424. doi: 10.1021/jf0100907
Chew, B. P., and Park, J. S. (2004). Carotenoid action on the immune response. J. Nutr. 134, 257. doi: 10.1093/jn/134.1.257S
Dusuki, N. J. S., Abu Bakar, M. F., Abu Bakar, F. I., Ismail, N. A., and Azman, M. I. (2020). Proximate composition and antioxidant potential of selected tubers peel. Food Res. 4, 121–126. doi: 10.26656/fr.2017.4(1).178
Elemosho, A. O., Irondi, E. A., Alamu, E. O., Ajani, E. O., Menkir, A., and Maziya-Dixon, B. (2021). Antioxidant and starch-hydrolysing enzymes inhibitory properties of striga-resistant yellow-orange maize hybrids. Molecules. 26, 6874. doi: 10.3390/molecules26226874
Ferraro, V., Piccirillo, C., Tomlins, K., and Pintado, M. E. (2016). Cassava (Manihot esculenta Crantz) and yam (Dioscorea spp.) crops and their derived foodstuffs: safety, security and nutritional value. Crit. Rev. Food Sci. Nutr. 56, 2714–2727. doi: 10.1080/10408398.2014.922045
Gammone, M. A., Pluchinotta, F. R., Bergante, S., Tettamanti, G., and D'Orazio, N. (2017). Prevention of cardiovascular diseases with carotenoids. Front. Biosc. 9, 165–171. doi: 10.2741/s480
Hsu, C. C., Chen, C. L., Chen, J. J., and Sung, J. M. (2003). Accelerated aging-enhanced lipid peroxidation in bitter gourd seeds and effects of priming and hot water soaking treatments. Sci. Hortic. 98, 201–212. doi: 10.1016/S0304-4238(03)00002-5
Irondi, E. A., Adewuyi, A. E., and Aroyehun, T. M. (2022a). Effect of endogenous lipids and proteins on the antioxidant, in vitro starch digestibility, and pasting properties of sorghum flour. Front. Neurol. 8, 809330. doi: 10.3389/fnut.2021.809330
Irondi, E. A., Agboola, S. O., and Boligon, A. A. (2018). Inhibitory effects of tropical almond leaf extract on xanthine oxidase, pancreatic lipase, and angiotensin I-converting enzyme, in vitro. J. Food Biochem. 42, e12481 doi: 10.1111/jfbc.12481
Irondi, E. A., Ajani, E. O., Aliyu, O. M., Olatoye, K. K., Abdulameed, H. T., and Ogbebor, O. F. (2021). Bioactive components, enzymes inhibitory and antioxidant activities of biofortified yellow maize (Zea mays L.) and cowpea (Vigna unguiculata L. Walp) composite biscuits. Ann. Univ. 45, 86–101. doi: 10.35219/foodtechnology.2021.1.06
Irondi, E. A., Awoyale, W., Oboh, G., and Boligon, A. A. (2019). Phenolics composition, antioxidant and pasting properties of high-quality cassava flour substituted with Brachystegia eurycoma seed flour. Ann. Univ. 43, 9–23. doi: 10.35219/foodtechnology.2019.1.01
Irondi, E. A., Imam, Y. T., and Ajani, E. O. (2022b). Physicochemical, antioxidant and starch-digesting enzymes inhibitory properties of pearl millet and sweet detar gluten-free flour blends, and sensory qualities of their breads. Food Sci. Technol. 2, 974588. doi: 10.3389/frfst.2022.974588
Joslyn, M. A. (1970). “Tannins and related phenolics,” in Methods in Food Analysis. New York, NY: Academic Press. 701–725.
Kanter, D. R., Schwoob, M. H., Baethgen, W. E., Bervejillo, J. E., Carriquiry, M., Dobermann, A., et al. (2016). Translating the sustainable development goals into action: a participatory backcasting approach for developing national agricultural transformation pathways. Global Food Security. 10, 71–79. doi: 10.1016/j.gfs.2016.08.002
Kareem, B., Irondi, E. A., Alamu, E. O., Ajani, E. O., Abass, A., Adesokan, M., et al. (2022). Influence of traditional processing on the antioxidant, starch-digesting enzymes inhibitory activities and glycaemic index of yellow-fleshed cassava genotypes. Front. Nutr. 9, 894843. doi: 10.3389/fnut.2022.894843
Kim, Y. M., Jeong, Y. K., Wang, M. H., Lee, W. Y., and Rhee, H. I. (2005). Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycaemia. Nutrition. 21, 756–761. doi: 10.1016/j.nut.2004.10.014
Kwon, Y. I., Apostolidis, E., and Shetty, K. (2008). Inhibitory potential of wine and tea against α-amylase and α-glucosidase for management of hyperglycaemia linked to type 2 diabetes. J. Food Biochem. 32, 15–31. doi: 10.1111/j.1745-4514.2007.00165.x
Liang, X., and King, J. M. (2003). Pasting and crystalline property differences of commercial and isolated rice starch with added amino acids. J. Food Sci. 68, 832–838. doi: 10.1111/j.1365-2621.2003.tb08251.x
Maziya-Dixon, B., Adebowale, A. A., Onabanjo, O. O., and Dixon, A. G. O. (2005). Effect of variety and drying methods on physico-chemical properties of high quality cassava flour from yellow cassava roots. Afr. Crop Sci. 7, 635–641.
Maziya-Dixon, B., Alamu, E. O., and Dixon, A. G. O. (2016). Variation in the evaluation of cis-and trans- < beta>-carotene in yellow-fleshed cassava (Manihot esculenta Cranz) varieties as a function of the storage root portion and sampling method. LWT. 70, 296–3001. doi: 10.1016/j.lwt.2016.03.002
Meda, A., Lamien, C. E., Romito, M., Millogo, J., and Nacoulma, O. G. (2005). Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 91, 571–577. doi: 10.1016/j.foodchem.2004.10.006
Mpofu, A., Sapirstein, H. D., and Beta, T. (2006). Genotype and envinronmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem. 54, 1265–70. doi: 10.1021/jf052683d
Nwose, E. U., Onodu, B. C., Anyasodor, A. E., Sedowo, M. O., Okuzor, J. N., and Culas, R. J. (2017). Ethnopharmacological values of cassava and its potential for diabetes and dyslipidemia management: Knowledge survey and critical review of report. J. Intercultural Ethnopharmacol. 6, 260–266. doi: 10.5455/jice.20170606094119
Oboh, G., Raddatz, H., and Henle, T. (2008). Antioxidant properties of polar and non-polar extracts of some tropical green leafy vegetables. J. Sci. Food Agric. 88, 2486–2492. doi: 10.1002/jsfa.3367
Odoemelam, C. S., Percival, B., Ahmad, Z., Chang, M. W., Scholey, D., Burton, E., et al. (2020). Characterization of yellow root cassava and food products: investigation of cyanide and β-carotene concentrations. BMC Res. Notes. 13, 1–7. doi: 10.1186/s13104-020-05175-2
Offia-Olua, B. I. (2014). Chemical, functional and pasting properties of wheat (Triticumspp)-walnut (Juglans regia) flour. Food Sci. Nutr. 5, 16. doi: 10.4236/fns.2014.516172
Ogbonna, O. C., Fadeiye, E. O., Ikem, R. T., Oladipo, K. O., Soyoye, D. O., Olulana, T. M., et al. (2018). Blood glucose response on consumption of cassava varieties (Garri) in healthy Nigerian subjects. J. Nutr. Health. 2, 22–27. doi: 10.35841/nutrition-human-health.2.1.22-27
Oliveira, R. A., De Carvalho, M. L., Nutti, R. M., and De Carvalho, L. J. (2010). Assessment and degradation study of total carotenoid and-carotene in bitter yellow cassava (Manihot esculenta Crantz) varieties. Afr. J. Food Sci. 4, 148–155.
Oluba, O. M., Oredokun-Lache, A. B., and Odutuga, A. A. (2017). Effect of vitamin A biofortification on the nutritional composition of cassava flour (gari) and evaluation of its glycemic index in healthy adults. J. Food Biochem. 42, e12450. doi: 10.1111/jfbc.12450
Osipitan, A. A., Sangowusi, V. T., Lawal, O. I., and Popoola, K. O. (2015). Correlation of chemical compositions of cassava varieties to their resistance to Prostephanus truncatus horn (Coleoptera: Bostrichidae). J. Insect Sci. 15, 173. doi: 10.1093/jisesa/ieu173
Oyaizu, M. (1986). Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. J. Acad. Nutr. Diet. 44, 307–315. doi: 10.5264/eiyogakuzashi.44.307
Pinto-Zevallos, D. M., Pareja, M., and Ambrogi, B. G. (2016). Current knowledge and future research perspectives on cassava (Manihot esculenta Crantz) chemical defenses: an agroecological view. Phytochemistry. 130, 10–21. doi: 10.1016/j.phytochem.2016.05.013
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 26, 1231–1237. doi: 10.1016/S0891-5849(98)00315-3
Seifried, H. E., Anderson, D. E., Fisher, E. I., and Milner, J. A. (2007). A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 18, 567–579. doi: 10.1016/j.jnutbio.2006.10.007
Srikanth, G., Babu, S. M., Kavitha, C. H. N., Rao, M. B., Vijaykumar, N., and Pradeep, C. H. (2010). Studies on in-vitro antioxidant activities of Carica papaya aqueous leaf extract. Res. J. Pharmaceutical Biol. Chemical Sci. 1, 59–65.
Sugiura, M., Nakamura, M., Ogawa, K., Ikoma, Y., and Yano, M. (2015). High-serum carotenoids associated with lower risk for developing type 2 diabetes among Japanese subjects: Mikkabi cohort study. BMJ Open Diabetes Res. Care. 3, e000147. doi: 10.1136/bmjdrc-2015-000147
Tucci, S. A., Boyland, E. J., and Halford, J. C. (2010). The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: a review of current and emerging therapeutic agents. Diabetes Metab. Syndr. Obes. 3, 125. doi: 10.2147/DMSO.S7005
Ukwuru, M. U., and Egbonu, S. E. (2013). Recent development in cassava-based products research. Academia J. Food Res. 1, 001–013. doi: 10.15413/ajfr.2012.0105
Keywords: antioxidant activity, bioactive composition, pasting properties, starch-digesting enzymes, yellow-fleshed cassava
Citation: Kareem B, Irondi EA, Alamu EO, Ajani EO, Abass A, Parkes E and Maziya-Dixon B (2023) Antioxidant, starch-digesting enzymes inhibitory, and pasting properties of elite yellow-fleshed cassava genotypes. Front. Sustain. Food Syst. 7:1129807. doi: 10.3389/fsufs.2023.1129807
Received: 22 December 2022; Accepted: 27 February 2023;
Published: 16 March 2023.
Edited by:
Paras Sharma, National Institute of Nutrition, IndiaReviewed by:
Kolawole Banwo, University of Ibadan, NigeriaAditya Parmar, University of Greenwich, United Kingdom
Copyright © 2023 Kareem, Irondi, Alamu, Ajani, Abass, Parkes and Maziya-Dixon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emmanuel Oladeji Alamu, b2FsYW11QGNnaWFyLm9yZw==
 Babajide Kareem
Babajide Kareem Emmanuel Anyachukwu Irondi
Emmanuel Anyachukwu Irondi Emmanuel Oladeji Alamu
Emmanuel Oladeji Alamu Emmanuel Oladipo Ajani
Emmanuel Oladipo Ajani Adebayo Abass
Adebayo Abass Elizabeth Parkes
Elizabeth Parkes Busie Maziya-Dixon2
Busie Maziya-Dixon2