
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 30 March 2023
Sec. Agro-Food Safety
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.1127445
This article is part of the Research TopicFood Safety in Low and Middle Income CountriesView all 25 articles
 Shingo Asakura1,2*
Shingo Asakura1,2* Borin Khieu3
Borin Khieu3 Sokerya Seng3
Sokerya Seng3 Samkol Pok3
Samkol Pok3 Chhay Ty3
Chhay Ty3 Chiv Phiny3
Chiv Phiny3 Teng Srey4
Teng Srey4 Stuart D. Blacksell5,6
Stuart D. Blacksell5,6 Jeffrey Gilbert1,7
Jeffrey Gilbert1,7 Delia Grace1,8
Delia Grace1,8 Silvia Alonso1*
Silvia Alonso1*Background: Most of human diarrheal pathogens are zoonotic, and transmission of the pathogens can occur by contaminated food, water, environment and direct contact with animals especially for livestock keepers. Yet little is known of the relative importance of different risk factors especially in under-studied countries. The objectives of this study were to identify risk factors for diarrhea in livestock keepers in Cambodia and detect diarrhea-causing pathogenic bacteria in both humans and livestock within a One Health approach. Of special interest were the links between diarrhea and food consumption and livestock-keeping.
Materials and methods: We used an existing dataset from a questionnaire survey conducted in 400 livestock farms in Prey Veng and Kampot Prefectures between February and March 2013 as well as laboratory results on bacterial isolation from fecal and swab samples from livestock and poultry, and human stool samples. Laboratory results were available for up to three animals of each species kept by a household, and for up to three human samples from households reporting at least one case of human diarrhea in the previous 2 weeks. Presence of Escherichia coli, Shigella spp. and Salmonella spp. was investigated in both animal and human samples, in addition to Aeromonas spp., Vibrio spp. and Plesiomonas spp. in animal samples and Campylobacter spp. in human samples. Univariable and multivariable risk factor analyses were performed by generalized linear mixed model.
Results: Household-level diarrhea incidence rate was 9.0% (36/400). The most statistically significant factor associated with diarrhea in multivariable analysis was water treatment for drinking and cooking (OR = 0.33, 95%CI: 0.16–0.69, p = 0.003), followed by number of days consuming egg within 2 weeks (OR = 1.16, 95%CI: 1.04–1.29, p = 0.008), number of children under 5 years old (OR = 1.99, 95%CI: 1.14–3.49, p = 0.016) and keeping poultry (OR = 0.36, 95%CI: 0.14–0.92, p = 0.033). Animal samples for bacterial culture test were collected at 279 cattle, 165 pig and 327 poultry farms, and bacteria were detected from 6 farms with the isolation of Escherichia coli O157 (non H7) from 1 cattle and 1 pig sample, Aeromonas caviae from 1 pig sample and Salmonella spp. from 3 chicken samples. In human samples, 17 out of 67 individual samples were positive for the culture test, detecting Escherichia coli O157 (non H7) from 7 samples and Shigella spp. from 10 samples. None of the households where target bacteria were detected from animal samples had human samples collected due to lack of diarrhea episodes in the household.
Conclusions: It has often been hypothesized that keeping livestock may increase the incidence of diarrhea through multiple pathways. Contrary to this, we found livestock-keeping was not associated with increased risk, but food-related behavior and children under 5 years of age were strongly associated with increased risk. We discuss mediating and confounding factors and make recommendations for reducing the burden of diarrheal disease in Cambodia and more widely in low- and middle-income countries.
Diarrhea is one of the most common symptoms of foodborne illness (Kosek et al., 2003). It is responsible for about 3.6% of the total disability-adjusted life year (DALY) global burden of disease, and causes ~1.45 million deaths annually worldwide (Lozano et al., 2012; Murray et al., 2012). Most deaths are among children under the age of 5 years, with diarrhea being the second largest cause of mortality in this age group globally. Children's death by diarrhea is often associated with underlying malnutrition, which makes them more vulnerable to diarrhea and many infectious diseases (Pelletier et al., 1993; Schroeder and Brown, 1994).
In 2005 in Cambodia, diarrhea was responsible for 17% of deaths among children under the age of 5 (Borapich and Warsh, 2010). Over the past decades, Cambodia has made significant improvements and has been able to reduce by 80% the diarrhea-related DALYs from 1990 to 2010. This decreasing trend still continues, according to data available until 2019 (Institute for Health Metrics Evaluation, 2022). Despite these improvements, diarrhea is still a significant problem responsible for 6% of all deaths in children under 5 years of age in 2015 in Cambodia (Cambodia Health Data, 2015), and its impact is considerable especially in rural areas due to the poor resources of medical facilities. Diarrhea is a preventable but widespread condition in the country, and understanding its drivers and causes remains essential for its control.
Diarrheal diseases are caused by ingestion of bacterial, viral or parasitic pathogens mainly through contaminated food or water. Environmental contamination from human feces is also considered important in the epidemiology of human diarrhea (Laborde et al., 1993). In addition, two-thirds of emerging and re-emerging diseases are considered to be zoonotic and contact between animals and humans increases the risk of the transmission of diseases (Jones et al., 2008; Christou, 2011; Coker et al., 2011). In this regard, animals can also be causes of human diarrheal cases through contact with humans and environmental contamination from animal feces. For example, enteric pathogens including Escherichia coli O157:H7, Campylobacter spp., Giardia spp., Salmonella spp. and Cryptosporidium spp. are found in animals and are known for their zoonotic transmission (Feachem et al., 1983; Crawford and Vermund, 1988).
Livestock and poultry raising in close distance to the human living environment is common in many parts of the world, especially in low- and middle-income countries (LMICs), where animal husbandry is closely linked with human lifestyle and is a primary source of income and nutritious foods such as milk and meat (Sansoucy et al., 1995). Household-livestock keeping increases the opportunity of direct contact of humans with animals and the risk of fecal contamination within the household living environment, indicating potentially high transmission risk of zoonoses. However, solid evidence on the links between livestock keeping and contact with animals and its role in diarrhea is still limited (Coker et al., 2011).
The objective of this study was to identify risk factors for diarrhea among livestock-keeping households in Cambodia and to investigate the presence of foodborne pathogens concurrently in animals and humans living in close contact. The results will shed light on the intrahousehold transmission of zoonotic pathogens and household and farming practices that may be associated with diarrhea in humans.
We used an existing dataset from a cross-sectional survey conducted among 400 livestock farms in four Districts in Prey Veng and Kampot Provinces between February and March 2013, as part of the Ecosystem approaches to the better management of zoonotic emerging infectious diseases in the Southeast Asia region (EcoZD) project (https://www.ilri.org/ecozd). The study that generated the data worked in two provinces that had contrasting agro-ecologies: Prey Veng Province is located in lower Mekong flood plain, which is a main agricultural production area in Cambodia; Kampot Province is located in the coastal area where rising sea levels and increased salinization are having a great impact in farming and fishery industries and in the availability of safe drinking water (Figure 1). Within each province, 2 districts were selected for the study to capture areas with contrasting diarrhea incidence. To do that, the study used 2010 bloody diarrhea incidence estimates to identify the districts with the highest and lowest bloody diarrhea incidence within each province; Preah Sdach (high: 3,492/100,000 people) and Prey Veng (low: 291/100,000 people) Districts were selected from Prey Veng Province, and Kampot (high: 1,386/100,000 people) and Angkor Chey (low: 974/100,000 people) Districts from Kampot Province (National Institute of Statistics, Directorate General for Health, and ICF Macro, 2011). Using the sampling frame of all the villages in each district, two villages were randomly selected from each district, and 50 livestock-keeping households were randomly selected from each village based on a sampling frame built with the help of the community. Thus, 100 households from each district were included in the study for a total of 400 households. Data were obtained via a structured questionnaire designed to collect information on diarrhea episodes in the household in the 2 weeks prior to the visit, as well as household and farming factors potentially associated with diarrhea, including household characteristics, animals kept, food consumption practices and water sources. An episode of diarrhea was defined as having soft stools at least three times within 24 h in the 2 weeks prior to the visit, and a household with at least one household member having experienced at least one episode of diarrhea was considered a diarrhea-positive household. The questionnaire was designed in English and translated into Khmer, the national language of Cambodia.
Data were also obtained from laboratory results on bacterial isolation from fecal and swab samples collected from livestock and poultry, as well as human stool samples from consenting households where at least one person had diarrhea in the previous 2 weeks. One to three individual animals of each species kept by the household were sampled; when more than one animal was sampled of a given species, the samples were pooled into a single tube. All animal species kept in the household were tried being sampled. In consenting diarrhea-positive households (i.e., where at least one person reported having experienced diarrhea in the 2 weeks prior to the visit), human samples were collected from one and up to three household members, with particular interest in those who prepared food, children and those having contact with animals. All samples were collected and placed in Cary-Blair transport medium and kept in a cool box until sent to the National Institute of Public Health (NIPH), in Phnom Penh, Cambodia. The samples were stored at −20°C at NIPH.
Samples were tested following published protocols for isolation and identification of the target pathogens (Ernest Jawetz et al., 1989; Henry and Todd, 1991; Centers for Disease Control and Prevention, 1999; Forbes et al., 2002; Lynne and Henry, 2007). Human samples were tested at NIPH for presence of Escherichia coli O157:H7, Salmonella spp., Shigella spp. and Campylobacter spp. Briefly, to detect Escherichia coli, Salmonella and Shigella, the samples were directly inoculated in MacConkey Agar (MAC) and Salmonella Shigella Agar (SSA) and then incubated at 37°C for 18–24 h. Moreover, to increase the sensitivity of the culture test, enrichment of bacteria included in the samples was done using Selenite broth with incubation of 37°C for 18–24 h. After the incubation, the Selenite broth was subjected to SSA and then the SSA was incubated. Screening and identification were performed based on the morphology and biochemistry characteristics using Triple Sugar Iron (TSI) tube agar, Sulfide Indol Motility (SIM) tube agar, Simmons citrate agar, Analytical Profile Index 20E kit (API20E, bioMérieux) and oxidase test. Escherichia coli isolates were further tested for serotype O157:H7 by agglutination test. To detect Campylobacter spp., the sample was inoculated in Campylobacter agar with Blood and Blaser formula supplemented and incubated in microaerophilic condition at 42°C for 48 h. Colony identification was done using Gram stain, Oxidase and Catalase tests.
Animal samples were sent to Mahidol-Oxford Tropical Medicine Research Unit, Mahidol University, Bangkok, Thailand and tested for the presence of Escherichia coli O157:H7, Salmonella spp., Shigella spp., Plesiomonas spp., Vibrio spp. and Aeromonas spp. The samples from animals were cultured using MAC, SSA and Thiosulfate Citrate Bile-Salts Sucrose agar (TCBS) with the incubation of 37°C for 18–24 h. In addition, for enrichment of bacteria, the samples were also placed into Alkaline Peptone Water (APW) tubes and the tubes were incubated at 37°C for 6–8 h. Then the APW were inoculated to TCBS and the TCBS were incubated as described above. Colony identification was performed using Kligler Iron Agar, Manitol Mobility agar, Urea Indole broth, API20E, Oxidase and Catalase tests.
Data from the questionnaires were obtained electronically in Microsoft Excel files and data cleaning was performed. Statistical analyses were performed using statistic software R version 3.5.0 (R Core Team, 2018). For comparisons of the occurrence of diarrhea in the households between provinces and between districts, Pearson's Chi-squared test with Yates' continuity correction was used. Univariable risk factor analysis was performed by generalized linear mixed model (GLMM) with binomial errors using lme4 package (Bates et al., 2011) to identify household-level diarrhea risk factors. Only variables for which a plausible biological link to diarrhea were included as explanatory variables in the analysis. Univariable models included the occurrence of diarrhea in households as response variable and prefectures and districts as random effects.
Multivariable analyses were performed using GLMM. The models included variables having p values < 0.1 in the univariable analyses as explanatory variables and diarrhea occurrence as response variable. The association between the selected explanatory variables was checked using GLMM with cut-off p < 0.05. Backward stepwise simplification was conducted using the likelihood ratio test.
Table 1 shows the characteristics of the households participating in the study. Households had an average of 4–5 members in all districts. Rice farming was a major activity in most of the households across the two provinces. Livestock farming (animal husbandry) was practiced by the majority of households in Kampot Province, but was rarely practiced by households in Prey Veng Province. Crop farming was also most common in Kampot Province than in Prey Veng Province. The majority of the households kept cattle and chicken, and pigs and ducks were kept by a moderate number of households. Livestock farms were smallholders, keeping on average 2–3 heads of large livestock, 2–6 pigs and from 15 to 30 chickens.
The number of households reporting at least one member experiencing diarrhea over 2 weeks prior to the visit was 36, for a household-level diarrhea prevalence of 9.0% (95% CI: 6.5–12.3). The diarrhea prevalence in Prey Veng Province was 16.0% (95%CI: 9.7–25.0) and 6.0% (95%CI: 2.5–13.1) in Preah Sdach and Prey Veng Districts, respectively. In Kampot Province, the prevalence was 5.0% (95%CI: 1.9–11.8) and 9.0% (95%CI: 4.5–16.8) in Kampot and Angkor Chey Districts, respectively. In the comparison of the district prevalence within the provinces, a statistically significant difference was observed between Preah Sdach and Prey Veng Districts in Prey Veng Province (p = 0.04), while the difference between Kampot and Angkor Chey Districts in Kampot Province was not statistically significant (p = 0.41). In addition, the difference of the prevalence between Prey Veng and Kampot Provinces was not statistically significant (p = 0.22).
A total of 67 human stool samples were collected from 25 diarrhea-positive households. Pathogenic bacteria were detected in 17 human samples (25.4%); Escherichia coli O157 (non O157:H7) was found in 7 samples and Shigella spp in 10 samples (Table 2).
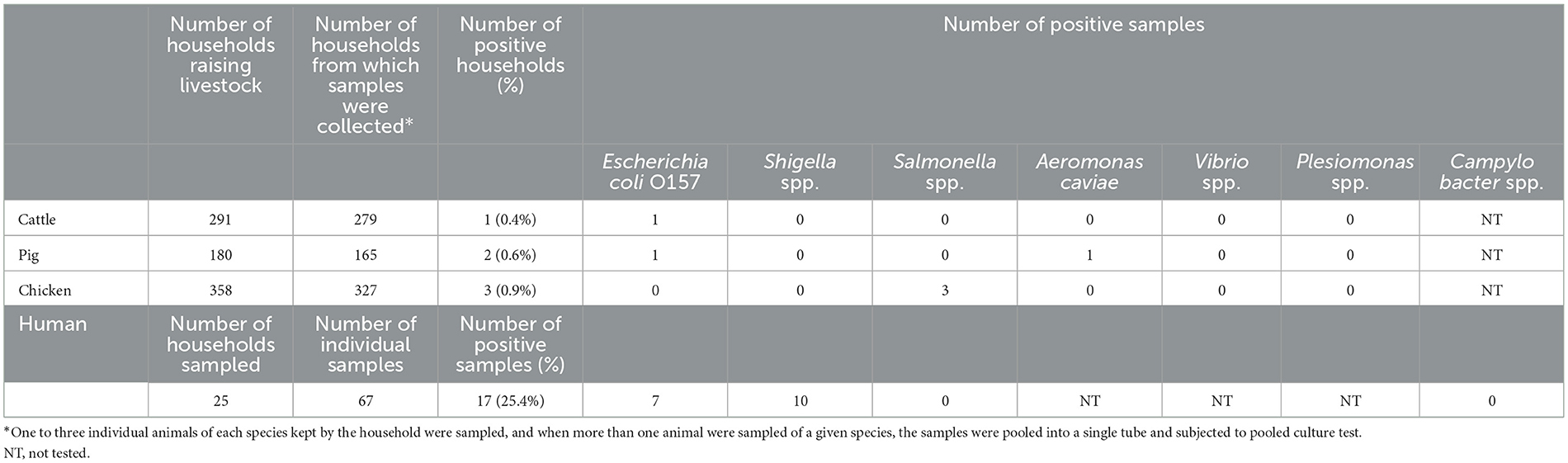
Table 2. Result of bacteria culture test of livestock fecal and swab samples and human rectal or stool swab samples.
Animal samples were collected from 95.9% (279/291), 91.7% (165/180) and 91.3% (327/358) of households keeping cattle, pigs and poultry, respectively. Escherichia coli O157 (non O157:H7) was isolated from 1 cattle and 1 pig sample, Aeromonas caviae from 1 pig sample and Salmonella spp. was isolated from 3 chicken samples. All the 6 culture-positive samples belonged to different households. None of the households where animal samples were found to be carrying the target bacteria had human samples collected due to lack of diarrhea episodes in the household in the 2 weeks prior to the visit. The same pathogen was not detected simultaneously in different animal species and human samples belonging to the same household. Among households reporting diarrhea in the previous 2 weeks, only one had an animal positive for any of the target bacteria; in particular one poultry sample carrying Salmonella spp.
Tables 3, 4 show the results of univariable and multivariable analysis for diarrhea. At univariable analysis, water treatment for drinking and cooking appeared as a potential preventive factor, and family size, number of children under 5 years old, not having a toilet facility, and number of days consuming eggs within the previous 2 weeks appeared as risk factors for diarrhea. In multivariable analysis, the final model included four factors (Table 4). In line with the results of univariable analysis, water treatment for drinking and cooking appeared as a protective factor for diarrhea in the household, while frequency of egg consumption and children under 5 years old appeared as risk factors. In addition, keeping poultry showed a negative association with diarrhea.
Diarrhea is a symptom of infections caused by bacterial, viral and parasitic organisms. Common causative agents of diarrhea are zoonotic, and it has often been hypothesized that keeping livestock, which is closely associated with human lifestyle, especially in LMICs, may increase the incidence of diarrhea through multiple pathways such as direct contact with them and environmental and water contamination by the animal feces with infectious organisms. Our study did not find evidence supporting this hypothesis, at least for the pathogens that our studied targeted. We did not find higher diarrhea prevalence in the districts where households were most likely to keep livestock and, accordingly, livestock keeping did not appear as a risk factor in our analysis. Moreover, poultry keeping was revealed as a protective factor for diarrhea in our study. In a review paper exploring the association between human diarrhea and domestic animal husbandry, of the 23 studies included in the systematic review, 21 indicated having found a positive association between livestock keeping and human diarrhea (Zambrano et al., 2014). The other two studies included in the systematic review reported negative associations between animal husbandry and diarrhea, as is the case of our study (Huttly et al., 1987; Kimani et al., 2012; Zambrano et al., 2014). Moreover, we did not find that livestock and humans were sharing any of the target pathogens in our study, which further supports the idea that, in the context we studied, human diarrhea may more likely be driven by non-animal related factors. Zoonotic transmission is influenced by many factors, including the nature and closeness of interaction between animals and humans. It is likely that livestock keeping in our study areas, while practiced at home, does not involve co-habitation and instead involves separation of living areas for animals and humans. This certainly would reduce pathogen transmission, and explain the lack of pathogen sharing between animals and humans. These findings show the importance of contextualizing the investigation of livestock keeping and the meaning it has in veterinary public health. Considering diarrhea remains a challenge worldwide and the growing interest in livestock raising as an economic activity in emerging nations, more context-specific research is needed in this field. The One Health approach (Zinsstag, 2012; Cleaveland et al., 2017) should continue to be used to look at animal-human interactions in the context of diarrhea.
While no association was found between animal husbandry and human diarrhea, we found several significant factors associated with diarrhea occurrence. Water treatment was the most significant and protective factor identified in this study. Many previous studies have reported the protective association of safe water against diarrhea, suggesting access to safe drinking water is one of the key preventive measures (Quick et al., 2002; Arnold and Colford Jr, 2007; Daud et al., 2017). In addition, a randomized controlled intervention trial of drinking water filters was performed in a rural village in Cambodia, which is a similar setting to that of the current study, showing a preventive effect of diarrheal disease (Brown et al., 2008). Thus, great effort should be put into safe water security to mitigate human diarrheal illness. As food-related factors, consuming eggs and washing root vegetables showed an association with diarrhea, although the latter was not included in the final model in multivariable analysis. Salmonella spp. is a well-known diarrhea causative agent, and one of the most important pathogens associated with egg consumption (Mc and Eisele, 1951; Hennessy et al., 2004; Schroeder et al., 2005). Although the number of days consuming eggs within 2 weeks was a risk factor statistically significant in our analysis, the effect size showed an odds ratio −1.14, showing a relatively minor effect. In the current study, while washing other vegetables had no association, washing root vegetables showed a slight protective association (p = 0.065) with diarrhea in univariable analysis. Our result may suggest that root vegetables have high risk of contamination by enteropathogenic species in soil because they grow underground, and washing may more effectively remove the pathogens of diarrheal diseases from root vegetables. Nevertheless, several studies have reported the limitations of washing fruits and vegetables for preventing food-borne diseases, which is compatible with our general finding that washing vegetables practices did not appear to be associated with diarrhea in the household (Burnett and Beuchat, 2001; Sivapalasingam et al., 2004; Lynch et al., 2009).
Although not included in the final model in multivariable analysis, washing hands with soap before eating and after animal handling were moderate preventive factors (p = 0.082 and 0.087, respectively) and no access to toilet facility was a significant risk factor in univariable analyses. These factors were hypothesized by us to be important due to the previously reported evidence (Koopman, 1978; Black et al., 1981; Pickering et al., 1986; Luby et al., 2004; Asfaha et al., 2018; Ejemot-Nwadiaro et al., 2021). According to World Health Organization, along with access to safe drinking water and food hygiene, as mentioned above, use of improved sanitation including hand washing with soap and toilet facilities are the key preventive measures (World Health Organization, 2014).
Regarding the children under 5 years old as a risk factor for diarrhea, in line with our result, several studies have reported the incidence of diarrheal diseases are greatest among children in this age group (Fischer Walker et al., 2012; Farthing et al., 2013). Since diarrhea in children causes growth faltering, malnutrition, and impaired cognitive development as well as fatal cases in resource-limited countries, great attention should be paid to them (Pelletier et al., 1993).
In order to mitigate diarrheal disease, health and food safety education plays an important role as well as the factors mentioned above (World Health Organization, 2014). In addition, not only household-based interventions but also community-based interventions to reinforce the knowledge and practices toward diarrhea and other diseases have been recognized as effective disease mitigation strategies in developing countries (Sheth and Obrah, 2004; Haroun et al., 2010; Mashoto et al., 2014; Abdel-Aziz et al., 2015).
In addition to risk factor analysis, this study also investigated the concurrent presence of bacterial organisms in both domestic animal and human samples in a One Health framework. While most of other studies which focused on association between animal husbandry and human diarrhea set single pathogen as a target and only human samples were utilized for culture test, the strength of the current study is that it investigated several pathogenic bacteria simultaneously (O'Brien et al., 2001; Belongia et al., 2003; Alyousefi et al., 2011; Leung et al., 2013; Zambrano et al., 2014). Only six animal samples were positive to any of our target bacteria. Among the six positives, one chicken-positive household with Salmonella spp. was human diarrhea positive at household level in questionnaire survey, but no human samples were available for that case. As one of the limitations of this study, not all animals kept at each household were sampled, and the samples were pooled into a single tube for every animal species within each household for culture test. While this makes laboratory analysis more efficient, it does reduce the sensitivity of the test, and it may have led to false negative results. For bacteria culture of human samples targeting Escherichia coli, Salmonella spp., Shigella spp. and Campylobacter spp., 67 individual samples were collected from diarrhea positive households and culture tests were positive for 17 (25.4%) of them, detecting Escherichia coli O157: non-H7 and Shigella spp. Although the individuals sampled in a household had not necessarily experienced diarrhea in the 2 weeks prior to the visit while at least one household member had done diarrhea in the period, other pathogens such as norovirus, rotavirus, Cryptosporidium and Giardia, which were not investigated in this study, may have caused diarrhea. Although there are limited data on diarrhea etiology from developing countries, a study conducted in Cambodia reported that among the stool samples collected from 600 children with acute diarrhea (cases) and 578 children without diarrhea (controls), the most frequently isolated pathogens in these cases were enteroaggregative Escherichia coli (20%) and rotavirus (26%) (Meng et al., 2011). In addition, from the perspective of the season, while bacterial diarrheal diseases often occur during rainy season, the sampling was performed in dry season in the current study, which may be behind the low overall diarrhea incidence observed (Bonkoungou et al., 2013; Kraay et al., 2020). Season could have also explained the low isolate rates of some of the target bacteria, which may be less prevalent in dry season (Picard and Goullet, 1987; Shigematsu et al., 2000; Barkocy-Gallagher et al., 2003; Perencevich et al., 2008; Akil et al., 2014; Bhattacharya et al., 2014; Lee et al., 2017). The available data does not allow to further investigate if this is indeed the case. Further research is desirable to understand the diarrhea situation more generally, in particular in the context of its zoonotic transmission.
General preventive measures against diarrhea, such as good food hygiene, water hygiene, handwashing with soap and good sanitary facilities, are effective methods to contain diarrhea in the studied livestock-keeping households in Cambodia. These are universally well known and effective methods to reduce gastro-intestinal disease. Livestock keeping, while it may be related to other zoonoses not targeted in our study, does not seem to be a factor that may be behind diarrhea in the households in our study areas. Livestock keeping remains an important source of livelihoods and food in many communities, and remains a potential risk activity in terms of a number of zoonotic infections. Further studies should expand, using the One Health approach, our understanding of what health risks for humans livestock keeping may pose to the communities in our study area.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by International Livestock Research Institute. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. The animal study was reviewed and approved by International Livestock Research Institute. Written informed consent was obtained from the owners for the participation of their animals in this study.
SAs performed data cleaning and analysis and wrote the majority of the manuscript. BK, SS, SP, CT, CP, and TS contributed to field surveys, data collection, and entry. SB contributed to bacterial culture test. JG and DG designed and coordinated the research. SAl designed and coordinated the research, advised analysis, and finalized the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the International Development Research Centre (IDRC) and the International Livestock Research Institute (ILRI) through the EcoZD project. The authors thank all funders who supported this research through their contributions to the CGIAR Trust Fund: https://www.cgiar.org/funders/. SB was funded by the Wellcome Trust of the United Kingdom. This research was funded, in whole or in part, by the Wellcome Trust (220211).
We acknowledge all the participants involved in this study and all those who helped in the data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Aziz, S. B., Mowafy, M. A., and Galal, Y. S. (2015). Assessing the impact of a community-based health and nutrition education on the management of diarrhea in an Urban District, Cairo, Egypt. Glob. J. Health Sci. 8, 46–55. doi: 10.5539/gjhs.v8n2p46
Akil, L., Ahmad, H. A., and Reddy, R. S. (2014). Effects of climate change on Salmonella infections. Foodborne Pathog. Dis. 11, 974–980. doi: 10.1089/fpd.2014.1802
Alyousefi, N. A., Mahdy, M. A., Mahmud, R., and Lim, Y. A. (2011). Factors associated with high prevalence of intestinal protozoan infections among patients in Sana'a City, Yemen. PLoS ONE 6, e22044. doi: 10.1371/journal.pone.0022044
Arnold, B. F., and Colford Jr, J. M. (2007). Treating water with chlorine at point-of-use to improve water quality and reduce child diarrhea in developing countries: a systematic review and meta-analysis. Am. J. Trop. Med. Hyg. 76, 354–364.
Asfaha, K. F., Tesfamichael, F. A., Fisseha, G. K., Misgina, K. H., Weldu, M. G., Welehaweria, N. B., et al. (2018). Determinants of childhood diarrhea in Medebay Zana District, Northwest Tigray, Ethiopia: a community based unmatched case-control study. BMC Pediatr. 18, 120. doi: 10.1186/s12887-018-1098-7
Barkocy-Gallagher, G. A., Arthur, T. M., Rivera-Betancourt, M., Nou, X., Shackelford, S. D., Wheeler, T. L., et al. (2003). Seasonal prevalence of Shiga toxin-producing Escherichia coli, including O157:H7 and non-O157 serotypes, and Salmonella in commercial beef processing plants. J. Food Prot. 66, 1978–1986. doi: 10.4315/0362-028x-66.11.1978
Bates, D., Maechler, M., and Bolker, B. (2011). Package lme4. R. Available online at: http://cran.r-project.org/web/packages/lme4/lme4.pdf (accessed October 2, 2022).
Belongia, E. A., Chyou, P. H., Greenlee, R. T., Perez-Perez, G., Bibb, W. F., and DeVries, E. O. (2003). Diarrhea incidence and farm-related risk factors for Escherichia coli O157:H7 and Campylobacter jejuni antibodies among rural children. J. Infect. Dis. 187, 1460–1468. doi: 10.1086/374622
Bhattacharya, D., Bhattacharya, H., Thamizhmani, R., Sayi, D. S., Reesu, R., Anwesh, M., et al. (2014). Shigellosis in Bay of Bengal Islands, India: clinical and seasonal patterns, surveillance of antibiotic susceptibility patterns, and molecular characterization of multidrug-resistant Shigella strains isolated during a 6-year period from 2006 to 2011. Eur. J. Clin. Microbiol. Infect. Dis. 33, 157–170. doi: 10.1007/s10096-013-1937-2
Black, R. E., Dykes, A. C., Anderson, K. E., Wells, J. G., Sinclair, S. P., Gary Jr, G. W., et al. (1981). Handwashing to prevent diarrhea in day-care centers. Am. J. Epidemiol. 113, 445–451. doi: 10.1093/oxfordjournals.aje.a113112
Bonkoungou, I. J., Haukka, K., Österblad, M., Hakanen, A. J., Traoré, A. S., Barro, N., et al. (2013). Bacterial and viral etiology of childhood diarrhea in Ouagadougou, Burkina Faso. BMC Pediatr. 13, 36. doi: 10.1186/1471-2431-13-36
Borapich, D., and Warsh, M. (2010). Improving child health in Cambodia: social marketing of diarrhea treatment kit, results of a pilot project. Cases Public Health Commun Market. 4, 4–22.
Brown, J., Sobsey, M. D., and Loomis, D. (2008). Local drinking water filters reduce diarrheal disease in Cambodia: a randomized, controlled trial of the ceramic water purifier. Am. J. Trop. Med. Hyg. 79, 394–400.
Burnett, S. L., and Beuchat, L. R. (2001). Human pathogens associated with raw produce and unpasteurized juices, and difficulties in decontamination. J. Ind. Microbiol. Biotechnol. 27, 104–110. doi: 10.1038/sj.jim.7000199
Cambodia Health Data (2015). 2015 Profile. Countdown to 2030 (2015). Available online at: http://countdown2030.org/country-profiles/cambodia (accessed October 26, 2022
Centers for Disease Control and Prevention (1999). Laboratory Methods for the Diagnosis of Epidemic Dysentery and Cholera. Atlanta, Georgia: CDC.
Christou, L. (2011). The global burden of bacterial and viral zoonotic infections. Clin. Microbiol. Infect. 17, 326–330. doi: 10.1111/j.1469-0691.2010.03441.x
Cleaveland, S., Sharp, J., Abela-Ridder, B., Allan, K. J., Buza, J., Crump, J. A., et al. (2017). One Health contributions towards more effective and equitable approaches to health in low- and middle-income countries. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 372(1725). doi: 10.1098/rstb.2016.0168
Coker, R., Rushton, J., Mounier-Jack, S., Karimuribo, E., Lutumba, P., Kambarage, D., et al. (2011). Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infect. Dis. 11, 326–331. doi: 10.1016/s1473-3099(10)70312-1
Crawford, F. G., and Vermund, S. H. (1988). Human cryptosporidiosis. Crit. Rev. Microbiol. 16, 113–159. doi: 10.3109/10408418809104469
Daud, M. K., Nafees, M., Ali, S., Rizwan, M., Bajwa, R. A., Shakoor, M. B., et al. (2017). Drinking Water Quality Status and Contamination in Pakistan. Biomed Res. Int. 2017, 7908183. doi: 10.1155/2017/7908183
Ejemot-Nwadiaro, R. I., Ehiri, J. E., Arikpo, D., Meremikwu, M. M., and Critchley, J. A. (2021). Hand-washing promotion for preventing diarrhoea. Cochrane Database Syst. Rev. 12, CD004265. doi: 10.1002/14651858.CD004265.pub4
Ernest Jawetz, J. L. M., Edward, A., Adelberg, G. F., Brooks, J. S, and Butel, S. (1989). Medical Microbiology. Norwalk, CI: Appleton and Lange.
Farthing, M., Salam, M. A., Lindberg, G., Dite, P., Khalif, I., Salazar-Lindo, E., et al. (2013). Acute diarrhea in adults and children: a global perspective. J. Clin. Gastroenterol. 47, 12–20. doi: 10.1097/MCG.0b013e31826df662
Feachem, R. G., Bradley, D. J., Garelick, H., and Mara, D. D. (1983). Sanitation and Disease. Health Aspects of Excreta and Wastewater Management. The World Bank.
Fischer Walker, C. L., Perin, J., Aryee, M. J., Boschi-Pinto, C., and Black, R. E. (2012). Diarrhea incidence in low- and middle-income countries in 1990 and 2010: a systematic review. BMC Public Health 12, 220. doi: 10.1186/1471-2458-12-220
Forbes, B. A., Sahm, D. F., and Weissfeld, A. S. (2002). Bailey and Scott's Diagnostic Microbiology, 11th ed. St. Louis: Mosby.
Haroun, H. M., Mahfouz, M. S., El Mukhtar, M., and Salah, A. (2010). Assessment of the effect of health education on mothers in Al Maki area, Gezira state, to improve homecare for children under five with diarrhea. J. Family Community Med. 17, 141–146. doi: 10.4103/1319-1683.74332
Hennessy, T. W., Cheng, L. H., Kassenborg, H., Ahuja, S. D., Mohle-Boetani, J., Marcus, R., et al. (2004). Egg consumption is the principal risk factor for sporadic Salmonella serotype Heidelberg infections: a case-control study in FoodNet sites. Clin Infect Dis 38 Suppl 3, S237–243. doi: 10.1086/381593
Henry, B. J., and Todd, C. J. (1991). Clinical Diagnosis and Management by Laboratory Methods 18th ed. Saint Louis: W B Saunders Co Ltd.
Huttly, S. R., Blum, D., Kirkwood, B. R., Emeh, R. N., and Feachem, R. G. (1987). The epidemiology of acute diarrhoea in a rural community in Imo State, Nigeria. Trans. R. Soc. Trop. Med. Hyg. 81, 865–870. doi: 10.1016/0035-9203(87)90055-1
Institute for Health Metrics and Evaluation (2022). Global Burden of Disease Profile. Available online at: https://www.healthdata.org/gbd (accessed September 20, 2022).
Jones, K. E., Patel, N. G., Levy, M. A., Storeygard, A., Balk, D., Gittleman, J. L., et al. (2008). Global trends in emerging infectious diseases. Nature 451, 990–993. doi: 10.1038/nature06536
Kimani, V. N., Mitoko, G., McDermott, B., Grace, D., Ambia, J., Kiragu, M. W., et al. (2012). Social and gender determinants of risk of cryptosporidiosis, an emerging zoonosis, in Dagoretti, Nairobi, Kenya. Trop. Anim. Health Prod. 44 Suppl 1, S17–23. doi: 10.1007/s11250-012-0203-4
Koopman, J. S. (1978). Diarrhea and school toilet hygiene in Cali, Colombia. Am. J. Epidemiol. 107, 412–420. doi: 10.1093/oxfordjournals.aje.a112559
Kosek, M., Bern, C., and Guerrant, R. L. (2003). The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. World Health Organ. 81, 197–204.
Kraay, A. N. M., Man, O., Levy, M. C., Levy, K., Ionides, E., and Eisenberg, J. N. S. (2020). Understanding the impact of rainfall on diarrhea: testing the concentration-dilution hypothesis using a systematic review and meta-analysis. Environ. Health Perspect. 128, 126001. doi: 10.1289/ehp6181
Laborde, D. J., Weigle, K. A., Weber, D. J., and Kotch, J. B. (1993). Effect of fecal contamination on diarrheal illness rates in day-care centers. Am. J. Epidemiol. 138, 243–255. doi: 10.1093/oxfordjournals.aje.a116853
Lee, H. S., Ha Hoang, T. T., Pham-Duc, P., Lee, M., Grace, D., Phung, D. C., et al. (2017). Seasonal and geographical distribution of bacillary dysentery (shigellosis) and associated climate risk factors in Kon Tam Province in Vietnam from 1999 to 2013. Infect. Dis. Poverty 6, 113. doi: 10.1186/s40249-017-0325-z
Leung, D. T., Das, S. K., Malek, M. A., Ahmed, D., Khanam, F., Qadri, F., et al. (2013). Non-typhoidal Salmonella gastroenteritis at a diarrheal hospital in Dhaka, Bangladesh, 1996–2011. Am. J. Trop. Med. Hyg. 88, 661–669. doi: 10.4269/ajtmh.12-0672
Lozano, R., Naghavi, M., Foreman, K., Lim, S., Shibuya, K., Aboyans, V., et al. (2012). Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128. doi: 10.1016/s0140-6736(12)61728-0
Luby, S. P., Agboatwalla, M., Painter, J., Altaf, A., Billhimer, W. L., and Hoekstra, R. M. (2004). Effect of intensive handwashing promotion on childhood diarrhea in high-risk communities in Pakistan: a randomized controlled trial. JAMA 291, 2547–2554. doi: 10.1001/jama.291.21.2547
Lynch, M. F., Tauxe, R. V., and Hedberg, C. W. (2009). The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol. Infect. 137, 307–315. doi: 10.1017/s0950268808001969
Lynne, S. G., and Henry, D. I. (2007). Clinical Microbiology Procedures Handbook, 2nd ed. Washington, DC: ASM Press.
Mashoto, K. O., Malebo, H. M., Msisiri, E., and Peter, E. (2014). Prevalence, one week incidence and knowledge on causes of diarrhea: household survey of under-fives and adults in Mkuranga district, Tanzania. BMC Public Health 14, 985. doi: 10.1186/1471-2458-14-985
Mc, C. N., and Eisele, C. W. (1951). Experimental human salmonellosis. III. Pathogenicity of strains of Salmonella newport, Salmonella derby, and Salmonella bareilly obtained from spray-dried whole egg. J. Infect. Dis. 89, 209–213. doi: 10.1093/infdis/89.3.209
Meng, C. Y., Smith, B. L., Bodhidatta, L., Richard, S. A., Vansith, K., Thy, B., et al. (2011). Etiology of diarrhea in young children and patterns of antibiotic resistance in Cambodia. Pediatr. Infect. Dis. J. 30, 331–335. doi: 10.1097/INF.0b013e3181fb6f82
Murray, C. J., Vos, T., Lozano, R., Naghavi, M., Flaxman, A. D., Michaud, C., et al. (2012). Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2197–2223. doi: 10.1016/s0140-6736(12)61689-4
National Institute of Statistics, Directorate General for Health, and ICF Macro. (2011). Cambodia Demographic and Health Survey 2010. Phnom Penh, Cambodia; Calverton, MD. Available online at: https://dhsprogram.com/pubs/pdf/Fr249/Fr249.pdf (accessed December 15, 2011).
O'Brien, S. J., Adak, G. K., and Gilham, C. (2001). Contact with farming environment as a major risk factor for Shiga toxin (Vero cytotoxin)-producing Escherichia coli O157 infection in humans. Emerging Infect. Dis. 7, 1049–1051. doi: 10.3201/eid0706.010626
Pelletier, D. L., Frongillo Jr, E. A., and Habicht, J. P. (1993). Epidemiologic evidence for a potentiating effect of malnutrition on child mortality. Am. J. Public Health 83, 1130–1133. doi: 10.2105/ajph.83.8.1130
Perencevich, E. N., McGregor, J. C., Shardell, M., Furuno, J. P., Harris, A. D., Morris Jr, J. G., et al. (2008). Summer peaks in the incidences of gram-negative bacterial infection among hospitalized patients. Infect. Control Hosp. Epidemiol. 29, 1124–1131. doi: 10.1086/592698
Picard, B., and Goullet, P. (1987). Seasonal prevalence of nosocomial Aeromonas hydrophila infection related to aeromonas in hospital water. J. Hosp. Infect. 10, 152–155. doi: 10.1016/0195-6701(87)90141-1
Pickering, L. K., Bartlett, A. V., and Woodward, W. E. (1986). Acute infectious diarrhea among children in day care: epidemiology and control. Rev. Infect. Dis. 8, 539–547. doi: 10.1093/clinids/8.4.539
Quick, R. E., Kimura, A., Thevos, A., Tembo, M., Shamputa, I., Hutwagner, L., et al. (2002). Diarrhea prevention through household-level water disinfection and safe storage in Zambia. Am. J. Trop. Med. Hyg. 66, 584–589. doi: 10.4269/ajtmh.2002.66.584
R Core Team (2018). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org
Sansoucy, R., Jabbar, M., Ehui, S., Fitzhugh, H., Wilson, R., Ehui, S., et al. (1995). “The contribution of livestock to food security and sustainable development,” in Proceedings of the Joint FAO/ILRI Roundtable on Livestock Development Strategies for Low Income Countries, ILRI, Addis Ababa, Ethiopia, 27 February−2 March 1995. Nairobi: Food and Agriculture Organization/International Livestock Research Institute.
Schroeder, C. M., Naugle, A. L., Schlosser, W. D., Hogue, A. T., Angulo, F. J., Rose, J. S., et al. (2005). Estimate of illnesses from Salmonella enteritidis in eggs, United States, 2000. Emerg. Infect. Dis. 11, 113–115. doi: 10.3201/eid1101.040401
Schroeder, D. G., and Brown, K. H. (1994). Nutritional status as a predictor of child survival: summarizing the association and quantifying its global impact. Bull. World Health Organ. 72, 569–579
Sheth, M., and Obrah, M. (2004). Diarrhea prevention through food safety education. Indian J. Pediatr. 71, 879–882. doi: 10.1007/bf02830824
Shigematsu, M., Kaufmann, M. E., Charlett, A., Niho, Y., and Pitt, T. L. (2000). An epidemiological study of Plesiomonas shigelloides diarrhoea among Japanese travellers. Epidemiol. Infect. 125, 523–530. doi: 10.1017/s0950268800004817
Sivapalasingam, S., Friedman, C. R., Cohen, L., and Tauxe, R. V. (2004). Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 67, 2342–2353. doi: 10.4315/0362-028x-67.10.2342
World Health Organization (2014). Preventing Diarrhoea Through Better Water, Sanitation and Hygiene: Exposures and Impacts in Low- and Middle-Income Countries. Geneva, Switzerland: World Health Organization.
Zambrano, L. D., Levy, K., Menezes, N. P., and Freeman, M. C. (2014). Human diarrhea infections associated with domestic animal husbandry: a systematic review and meta-analysis. Trans. R. Soc. Trop. Med. Hyg. 108, 313–325. doi: 10.1093/trstmh/tru056
Keywords: diarrhea, animal husbandry, risk factor, One Health, Cambodia
Citation: Asakura S, Khieu B, Seng S, Pok S, Ty C, Phiny C, Srey T, Blacksell SD, Gilbert J, Grace D and Alonso S (2023) Diarrhea illness in livestock keeping households in Cambodia: An analysis using a One Health framework. Front. Sustain. Food Syst. 7:1127445. doi: 10.3389/fsufs.2023.1127445
Received: 19 December 2022; Accepted: 16 March 2023;
Published: 30 March 2023.
Edited by:
John Franklin Leslie, Kansas State University, United StatesReviewed by:
Monica Ponder, Virginia Tech, United StatesCopyright © 2023 Asakura, Khieu, Seng, Pok, Ty, Phiny, Srey, Blacksell, Gilbert, Grace and Alonso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shingo Asakura, czIxNDQxMDAxQGcucmFrdW5vLmFjLmpw; Silvia Alonso, Uy5BbG9uc29AY2dpYXIub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.