- 1Department of Microbiology and Biotechnology, University School of Science, Gujarat University, Navrangpura, Ahmedabad, India
- 2Department of Microbiology, Gujarat Arts and Science College, Ellisbridge, Ahmedabad, India
- 3Department of Plant Pathology, Tamil Nadu Agricultural University, Coimbatore, India
- 4Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, Saudi Arabia
- 5Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia
- 6Department of Geology and Pedology, Mendel University in Brno, Brno, Czechia
Co-cultures of bacteria are more metabolically flexible and more tolerant to changes in the environment than single cultures. In order to test for plant growth promotion in a medicinal herb Anethum graveolens L, potent phosphate-solubilizing rhizobacteria were selected, characterized and assessed for their compatibility with each other. Molecular identification of isolates was made by 16s rRNA sequence, and they were identified as Pseudomonas aeruginosaNJC4 (OP289324), Serratia marcescens NJC21 (OP289323) and Bacillus spp. Dual species consortia, namely, Bacillus spp. + Serratia marcescens NJC21 (T1), and Pseudomonas aeruginosa NJC4 + Serratia marcescens NJC21 (T2), were tested for their ability to produce multiple plant beneficial activities such as phosphate solubilization, and ammonia and indole acetic acid production. The best isolate and consortium were evaluated for plant growth promotion activity. A plant treated with consortia T-2 seemed most effective in seed emergence at 84.66%, which was four times superior to the control. Growth and yield characters, along with all different rhizobacterial treatments, were examined by principal component analysis (PCA), where PC1 can explain 51.37% of the total variance and PC2 can explain 26.75%. PC1 was associated with wet biomass, chlorophyll b, and total chlorophyll content, which reflect the strong influence of consortia T-1. At the same time, PC2 was found to be related to dry biomass and chlorophyll a content. This study lends credence to the theory that microbial consortiums consisting of more than one efficient strains may be more effective than single cultures in boosting the increase of agricultural output in a sustainable way.
Introduction
Plants have formed the cornerstone of complex traditional medicine systems for hundreds of years and continue to deliver revolutionary therapies to humanity (Jabborova et al., 2021b). India's varied topography and climate have made it a veritable treasure trove for medicinal plants (Sharma et al., 2015). Every component of medicinal plants, including the roots, stems, leaves, and seeds, has therapeutic potential and can treat various infections (Shahzad et al., 2015). The secondary metabolites or marker compounds that medicinal plants secrete are also important because they have medicinal properties, pesticidal properties and can be used to treat and prevent human illnesses (Shahzad et al., 2015; Arora et al., 2022). In addition to helping treat diseases, medicinal plants can make cosmetics and other healthcare products that meet consumer demands (Rustamova et al., 2022). As a result, medicinal plants are grown all over the world to meet domestic industry demands as well as for therapeutic purposes. Nearly 60 to 80% of the world's population currently uses traditional herbal medicines for some aspect of primary healthcare (Sharma et al., 2015). Because of their diverse therapeutic potentials, the presence of various phytochemicals and a spike in medication resistance in neoteric times has resulted in an increased demand for potent medicinal resources (Bhattacharjee et al., 2020). As a result, the global market for medicinal plant cultivation is steadily growing. Hence, the emphasis is shifting to cultivating various therapeutically useful medicinal plants.
Among all the cultivation techniques, microbial-based biofertilizers are the low-cost natural resource and fundamental base that increase crop yield and impact sustainable agriculture (Basu et al., 2021; Khan et al., 2021). Regarding sustainable agriculture, plant growth-promoting rhizobacteria (PGPR) are widely regarded as key players in positively affecting plant growth (Lynch, 1990; Shah et al., 1998; Sayyed et al., 2019; Ali et al., 2022). For enhanced activity and more prolonged survival of PGPR, creating a plant-growth-promoting consortium (PGPC) has been proposed as a viable strategy (Jabborova et al., 2021a). Each strain in the inocula consortium competes with the others for the rhizospheric establishment and provides a complementary function in promoting plant growth (Shenoy and Kalagudi, 2003). The individual strains in multi-strain consortia can work together to increase plant growth and nutrient availability through processes like siderophore production for iron chelation, mineral solubilization (phosphate), nitrogen fixation, phytohormone synthesis (auxin), and the production of oxidizing and reducing enzymes (Singh and Singh, 2017; Patel et al., 2018; Jabborova et al., 2020b; Kalam et al., 2020). Mostly, compatible microbes favor mutual physical and biochemical activities which enhance one another's performance, resulting in enhanced positive aspects. Many studies have shown that consortia improve nutrient uptake and plant growth across various plant species in stressed and non-stressed environments (Buddhi and Min-Ho, 2013; Sagar et al., 2022). Co-inoculation of PGPR has become common in recent decades as it has proven more effective than single-strain inoculation at promoting plant growth in a unified fashion (Guetsky et al., 2002; Najafi et al., 2021). Therefore, using consortiums is the most environmentally friendly and practical method for keeping crop yields high and pollution low.
Acknowledging the value of medicinal plants and rhizobacteria, the present study was planned and executed to evaluate the effects of multifunctional rhizobacterial two species consortia on one of the traditional medicine, Anethum graveolens L. known as “shatapushpa” in Ayurveda and also recognized as Dill or Sowa. Anethum graveolens L. makes more than 56 ayurvedic medicines, such as Dasmoolarishtam, Dhanwanthararishtam, Mrithasanjeevani, Saraswatharishtam, Gugguluthiktaquatham, Maharasnadikashayam, Dhanwantharamquatham, and more (Jana and Shekhawat, 2010). Different parts of the plant and the plant's essential oil have antifungal, antibacterial, antioxidant, and anti-inflammatory properties. This plant is also a flavoring agent but is mainly used to stimulate milk flow in lactating mothers (Jana and Shekhawat, 2010). To utilize these medicinal characteristics of the plant to the fullest, this study focuses on the growth promotion of the dill plant i.e., Anethum graveolens L. by the isolates Pseudomonas aeruginosa NJC4 (OP289324), Serratia marcescens NJC21 (OP289323) and Bacillus spp. and two consortia developed from it to check the growth promotion effect of isolates as well as consortia and their difference on plant growth promotion.
Materials and methods
Sample collection and isolation of rhizobacteria
The rhizospheric soil sample was collected from the rhizosphere of Anethum graveolens L., from the Amrapur village, situated near Gandhinagar, Gujarat. The pH (determined using a pH meter) and electrical conductivity (determined using a conductometer) were 7.48 and 160 μsiemens/cm, respectively, at the time of collection. The plant was carefully pulled up to collect rhizospheric soil, and the soil adhering to the plant roots was collected for isolation. The samples were placed in plastic bags and stored at 4°C in the laboratory, and further analysis was done within 24 h. These soil samples were used to isolate the potent phosphate solubilizers using the serial dilution (10−1 to 10−3) technique on Pikovskaya agar media, where 0.1 mL of each dilution was inoculated on Pikovskaya media by spread plate method. All the plates were incubated at 28°C ± 3°C for 24 to 48 h. Colonies surrounded by clear halo zone were considered phosphate solubilizers. These phosphate solubilizers were further purified by re-streaking on a plate. Colonies with different morphology on nutrient agar plates were selected for further studies and preserved on nutrient agar slant at 4°C.
Compatibility test
The compatibility of the isolates NJC 1, NJC 4 and NJC 21 with one another was examined. In this test, a single isolate was streaked on a nutrient agar plate in a straight line, while test cultures were streaked perpendicularly across the initial culture and incubated at 28°C for 24 to 48 h (Manasa et al., 2021). The absence of microbial growth or the presence of an inhibitory zone demonstrated the antagonistic nature of the bacterial suspension against each other.
Interaction of microbes (Growth profile assessment)
To study the interaction between microbes under liquid culture conditions, 100 mL nutrient broth was prepared to inoculate the active cultures of each isolate at 1% inoculum levels. The growth curves of isolates were determined. The growth profiles of two species consortia were governed by inoculating active culture of each isolates in equal amount having 1 × 104 CFU/mL in given combination at their early exponential phase. For every combination, equal volumes (0.5 mL)of the early exponential phase of each culture were added aseptically. Samples were withdrawn at every 30 min intervals and absorbance was noted at 535 nm. The formula calculated the mean growth rate constant: K = 3.322 (log Nt- log N0)/Dt, where Nt and N0 denote the final cell number and initial cell number, respectively, and Dt express the difference in culture time (Jha and Saraf, 2012).
The combination of isolates prepared for rhizobacterial consortia treatment is as follows
T-1: Bacillus spp.-NJC1 + Serratia marcescens- NJC 21
T-2: Pseudomonas aeruginosa- NJC 4 + Serratia marcescens- NJC 21.
Identification and biochemical characterization of isolates
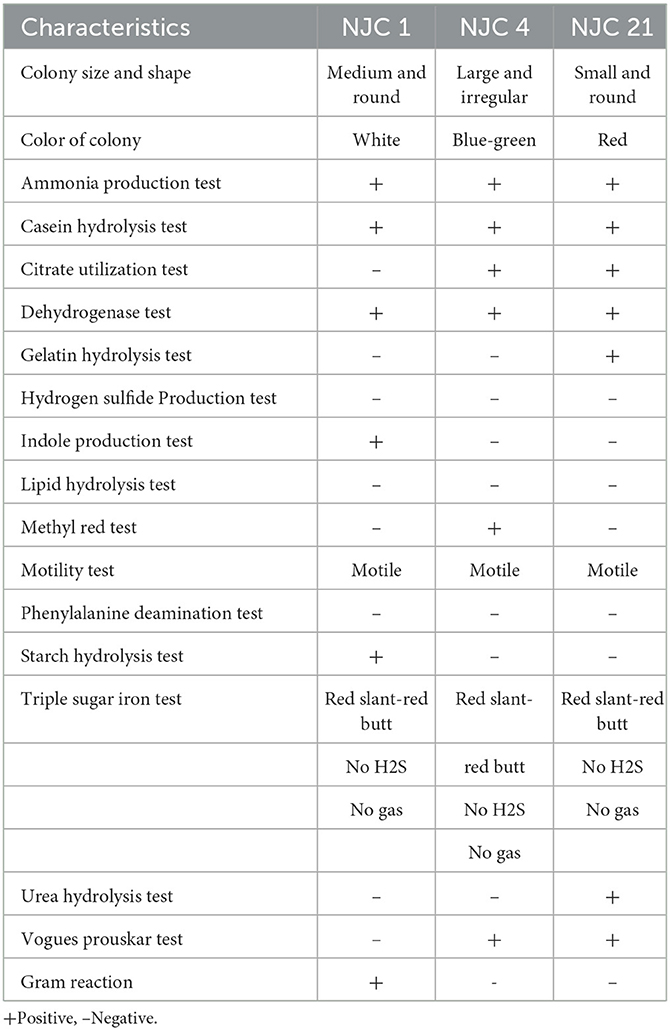
Isolates were examined for different morphological features like colony color, size and shape after 24 h of growth on nutrient agar plates. Each isolates were assayed for Gram reaction and biochemical assessments likethe MRVP test (Methyl Red, Voges-Proskauer), starch hydrolysis, nitrate reduction test, casein hydrolysis, urease test and gelatin liquefaction were also performed.
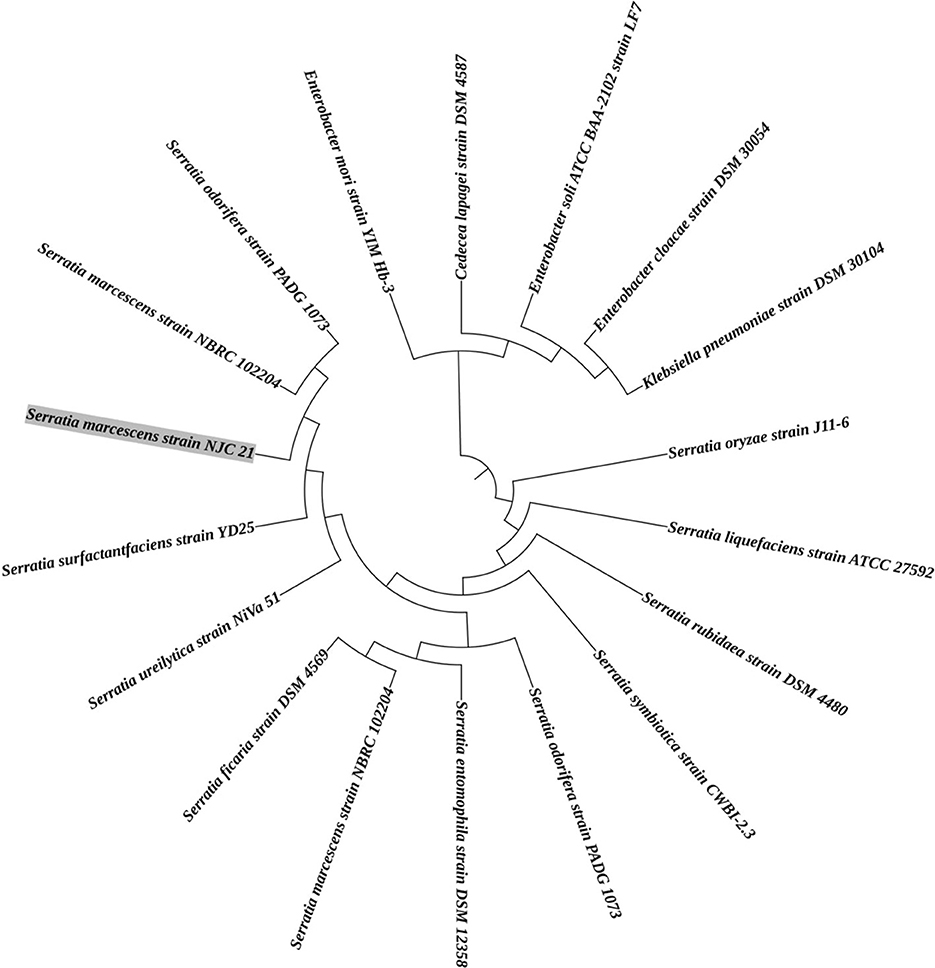
Molecular identification of isolates was made by the Sanger sequencing method. Amplification of 16s rRNA was first carried out using 27F (-AGAGTTTGATCCTGGCTCAG-) and 1492R (-TACGGTTACCTTGTTACGACTT-) primer. The NCBI-BLAST server was used to determine gene sequence homology. The BLAST output identified the query sequences by their percentage coverage and their identity to the query sequences. Using MEGA 10.0 software, the acquired gene sequence was aligned by ClustalW, and a neighbor-joining (NJ) tree with a bootstrap value of 1,000 was created. The gene sequence was uploaded to Gene Bank, and an accession number was obtained for each isolate.
Characterization of plant growth-promoting traits
Phosphate solubilization
A qualitative phosphate solubilization assay used Pikovoskya media, which contained tri-calcium phosphate [Ca3(PO4)2]. Phosphate solubilization was performed, both qualitatively and quantitatively, on the consortia and the individual isolates. Bacterial cultures were spotted on the medium and incubated at 27°C ± 2°C for 6 to 7 days. A clear halo zone around the spot culture indicated the cultures' phosphate solubilization ability, and the solubilization index was calculated after 7 days (Pandey et al., 2008). Qualitative phosphate solubilization was done only for the isolates.
The quantitative phosphate solubilization capacity of each isolate and developed consortia tested were using insoluble tri-calcium phosphate [Ca3(PO4)2] as the sole P source in Pikovskya liquid media (Khumairah et al., 2022). Pikovskya liquid media was inoculated with single strain and mixed strains at 27°C ± 2.0°C for up to 7 days. During incubation, the 1 mL of sample was withdrawn at the 3rd, 5th, and 7th day. Estimation of the soluble phosphate in the supernatant was determined by the stannous chloride (SnCl2.2H2O) method (Gaur, 1990). The pH drop induced by organic acid generation from sugar consumed by the test isolate was determined. The amount of phosphate solubilized was calculated from the standard graph.
Catalase production assay
Catalase test was performed according to Clarke and Cowan (1952) method. For the qualitative analysis, bacterial colonies of isolates on nutrient agar exposed to 6% H2O2 and the effervescences of O2 released from the colony exhibit a positive result.
HCN production assay
Isolates were tested for hydrogen cyanide (HCN) production using the Picrate assay (Castric, 1975). In this assay, isolate were inoculated glycine-supplemented (0.44 g%) nutrient agar plates used and the Whatman filter paper no. 1 which was soaked in 2% sodium carbonate dissolved in 0.5% picric acid solution was placed between the Petri plate's base. Plates were wrapped with parafilm and incubated for 4 days at 27°C ± 2.0°C. The transformation of yellow filter paper to orange-brown during incubation suggests the release of HCN by a bacterial isolate (Nithyapriya et al., 2021).
IAA production assay
IAA production assay was analyzed by Salkowsky's method. Briefly, erlenmeyer flasks containing 25 mL of nutrient broth amended with the precursor of IAA, i.e., tryptophan 50 μg/mL were inoculated with freshly prepared single bacterial culture and developed consortia. Uninoculated sterile media served as control. All the cultures were incubated at 28°C for 24 h on a rotary shaker. Each broth was centrifuged at 10,000 rpm for 15 min. The 2 mL supernatant was taken from each broth, and 4 mL Salkowsky reagent was added and incubated for 30 min in the dark at room temperature. The IAA production was confirmed by the development of pink color and absorbance was taken at 530 nm (Chaiharn and Lumyong, 2011). The estimation of IAA production by isolates and consortia was calculated from the standard graph.
Ammonia production assay
The ammonia production assay method suggested by Cappucino and Sherman (1992) was done for the single isolated bacteria and the developed consortium. Freshly grown cultures were inoculated to peptone broth and incubated at 28°C ± 2°C for 48 h. The development of brown to yellow color after adding Nessler's reagent indicated the presence of ammonia.
Pot experiment
The effects of all designed consortia and single isolates on a medicinal plant like Anethum graveolens L. were studied in a pot experiment. All seeds were soaked for 2–3 min in 0.0.2% sodium hypochlorite and washed five times with distilled water that had been sterilized. As an adhesive, 1% carboxymethyl cellulose was used to cover the seeds. The seeds were then treated for 30 min with both single cultures of bacteria and the consortia of bacteria that had been made. Each consortium was put into a 250-mL flask with 100 mL of nutrient broth and kept there for 48 h at 28°C ± 2°C. To get a uniform cell density of 108-109 CFU/mL, an optical density of 0.5 was observed at 535 nm. The pots were filled with sterile soil and watered regularly. In each pot, 10 treated seeds were embedded, and the pot with untreated seeds was used as a control. For each treatment, three pots were kept, and one replicate consisted of the mean of three randomly chosen plants. These experiments were conducted three times. Up to 21 days of experiments, daily data were collected to evaluate the rate of seed emergence from soil. After 21 days, measurements of the plant's height, root length, shoot length, number of shoots per branch, etc., and the emergence percentage, and seedling vigor index were calculated using the following formula (Abdul-Baki and Anderson, 1973).
Estimation of chlorophyll contents from Leaf tissue
The method described by HISCOX and ISRAELSTAM (Hiscox and Israelstam, 1979) was used, but it was changed to fit the needs. With the help of a pestle and mortar, 100 mg of leaf tissue was broken up. They added 15 mL of acetone and let it sit in a water bath at 60°C for 3 h. The OD was taken at 645 nm to estimate chlorophyll a and at 663 nm to estimate chlorophyll b. Use the following formula to figure out chlorophyll a, b, and the total amount of chlorophyll.
Microelement analysis in soil and plants
At the beginning and end of the experiment, three soil samples were taken from each experiment. DTPA (diethylenetriaminepentaacetic acid) extraction methods were used to find concentrations of microelements like Zn, Fe, Mn, and Cu in the soils (Lindsay and Norwell, 1969). In brief, 0.005 M DTPA extracting solution was prepared and 10 grams of air-dried soil were placed in a 250 mL conical flask with 20 ml of the extracting solution. Immediately after shaking the covered flask for 2 h, Whatman no. 42 filter paper was used to filter the suspension. With the use of atomic absorption spectrophotometry and appropriate standards, micronutrients present in the filtrates were determined.
9 mL of freshly prepared acid mixture of 65 % nitric acid and 37 % HCl was added to 0.5 grams of root and shoot parts from control and treated plants. For 5–6 h (or until the sample had completely dissolved), the mixture was gently boiled over a water bath. The inner side of the beakers were rinse with 2 mL of deionized water during digestion procedures to avoid loss of samples. The samples were filtered with Whatman 42 filter paper at the end of digestion and make up to 50 mL using deionized water. Thus, prepared samples were analyzed by Atomic absorption spectroscopy (Uddin et al., 2016).
Statistical analysis
Each bacterial treatments and biometric observations for the Anethum graveolens L, were set up using three replications of each treatment, repeated three times. A statistical analysis was conducted to determine the bioformulation treatment's effect on seedling growth. This ANOVA was performed on triplicate values to discover significant differences between treated and untreated seeds for each yield contributing parameter. Tukey's test compared the mean values of triplicates at the P ≤ 0.05 significance level. Principal component analysis was used to compare the means of the treatments and the parameters that contributed to the yield.
Results
Screening of bacterial isolates
The soil from which the bacteria were isolated had a pH of 7.34 and electrical conductivity of 160 μS/cm. Approximately 30 bacteria were isolated from the soil sample based on their colony characteristics. Five potent phosphate solubilizing isolates were selected for the in vitro plant growth-promoting traits. Among these, the highest phosphate solubilization was demonstrated by isolate NJC 4 followed by NJC 1.
Compatibility and growth profile study
For the formation of consortia, five isolates were tested for their compatibility. All PGPR strains were grown perpendicularly on nutrient agar with different combinations.
The formation of consortia was based on the compatibility of strains. The combination that showed compatibility is as follows:
1) NJC 1+NJC 21
2) NJC 4+ NJC 21
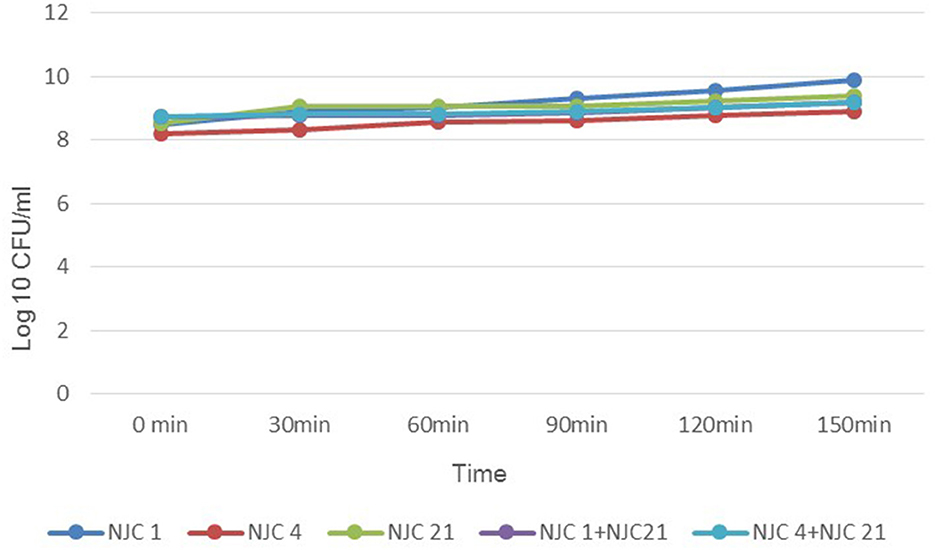
Co-inoculation was done by mixing an equal volume of bacterial culture to obtain 1 × 108 CFU/mL of total population density. All isolates were fast-growing, and for the single cultures, K-values of NJC 1, NJC 4, and NJC 21 were 1.86 ± 0.01, 0.92 ± 0.01, and 1.15 ± 0.09/h, respectively. In mixed species culture, the mean growth rate constant of NJC 1+NJC 21 and NJC 4+NJC 21 were 0.61 ± 0.03 and 0.63 ± 0.01/h, respectively. Growth profile study evidenced that each isolate of formed consortia favors the growth of others, reflecting the commensalism relationship among them (Figure 1).
Biochemical and molecular characterization of isolates
Biochemical characterization of all the potent phosphate solubilizing isolates revealed the presence of dehydrogenase activity and motility. All the isolates were lipase, hydrogen sulfide negative. Strain NJC 1 showed amylase and protease activities (Table 1). As per Bergey's manual of determinative bacteriology, it can be concluded that the NJC 1 bacterial strain belonged to the Bacillus genera as a positive, catalase-positive, and endospore-forming organism.
All bacterial isolates identified at the molecular level were subjected to 16S rRNA sequencing. The obtained sequences were uploaded to the NCBI Gene Bank database using BLAST (basic local alignment tool). Based on the sequence data, a phylogenetic tree of the sequence was constructed using MEGA 10.0. Similarity tests revealed that NJC4 was identified as Pseudomonas aeruginosa, and NJC21 was identified as Serratia marcescens. Pseudomonas aeruginosa NJC4 (Figure 2) and Serratia marcescens NJC21 (Figure 3) have the accession numbers OP289324 and OP289323, respectively, for the sequences of these strains.
PGP traits for isolates and consortia
Phosphate solubilization
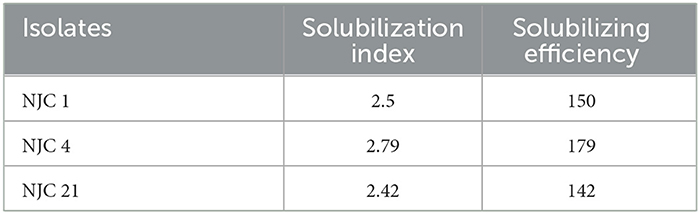
After 3 days of incubation on Pikovskyaya's medium, all bacterial isolates with a clear halo zone around the colony confirmed that all selected isolates were phosphate solubilizers. Significant zones were observed in all isolates. Maximum solubilization indexes 2.79 and 2.5 were observed in NJC 4 and NJC 1, respectively. The highest solubilization efficiency was seen in isolate NJC 4 (Table 2).
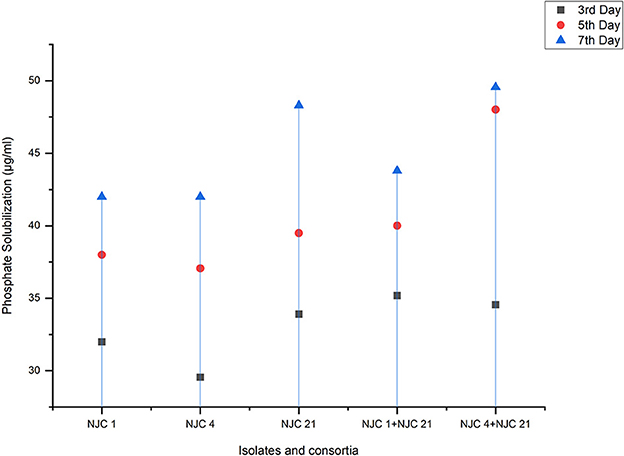
Figure 4 shows the highest quantitative estimation of phosphate solubilization seen in the co-inoculated consortia NJC 4+NJC 21 after 7 days of incubation, i.e., 49.6 μg/mL. In our in vitro analysis of quantitative phosphate solubilization, it was found that the highest solubilization among isolates was found in NJC 21, i.e., 47.4 μg/mL. The difference between the average phosphate solubilization of isolate and consortia is 26.54%. But the difference between most potential isolates and consortia wasn't significant. All liquid cultures were found to decrease their pH, which indicated the production of acid, which can be regarded as phosphate solubilization (Whitelaw, 1999).
IAA production and ammonia production
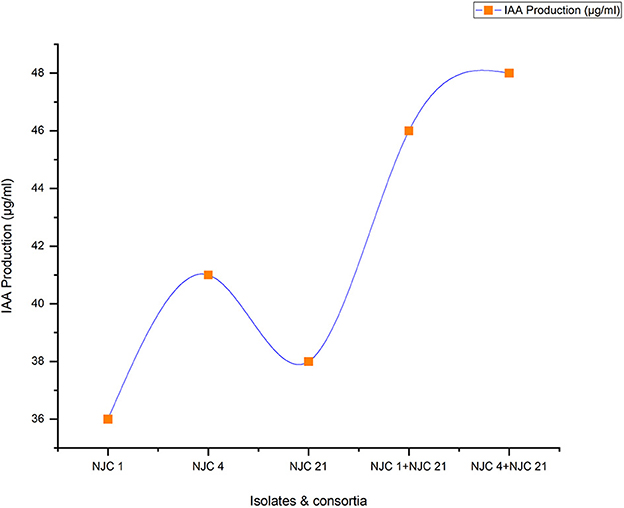
The production of IAA (Indole Acetic Acid) varied from 36 to 48 μg/mL (Figure 5). Selected single strains and developed two-species cultures triggered IAA's production and favored ammonia production. The highest amount of IAA production was seen in the developed two-species culture NJC 4+NJC 21, which suggested that co-inoculated plant growth-promoting strains had a greater capacity to produce IAA than single strains. In the present study, the lowest IAA producer among consortia i.e., NJC 1+NJC 21 generates 12% higher IAA than the highest generator among isolates i.e., NJC 21, while the other i.e., NJC 4+NJC 21 produced 17% higher IAA than the same while on average, consortia generated 65% higher IAA than the isolates.
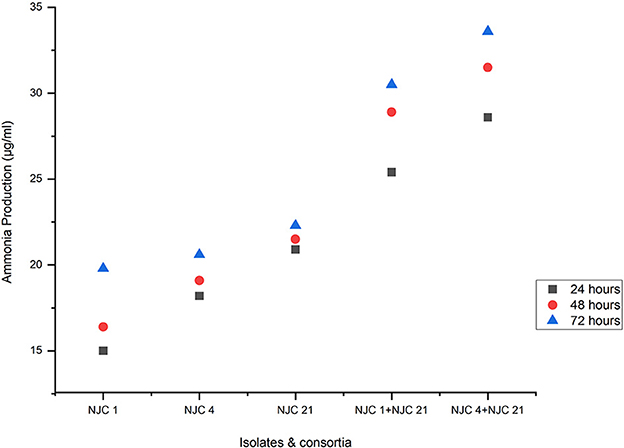
Ammonia production by isolates and consortia was determined from peptone water at 24, 48, and 72 h. The highest amount of ammonia produced by isolates was 22.3 μg/mL produced by NJC 21 after 72 h of incubation, while consortia NJC 4+NJC 21 produced 33.6 μg/mL ammonia after the same incubation period, which is 50% higher than the maximum amount of ammonia produced by the most potential isolates (Figure 6). Even the other consortia generated 37% more ammonia than the isolates.
HCN production and catalase test
At 27 ± 2°C, NJC 1 and NJC 4 were discovered to be generating HCN. A filter paper strip impregnated with 0.5% picric acid and 2.0% sodium carbonate turned orange after 72 h, indicating the production of HCN, while NJC 21 does not produce HCN.
Catalase production was seen in all the isolates. When a 6% H2O2 solution was flooded over nutritional agar colonies, a substantial effervescence of O2 was seen, indicating a favorable qualitative result for catalase production.
Assessment of growth-promoting attributes Anethem graveolens L.
Pot experiments were carried out to observe the effect of a combined rhizobacterial strain and the effect of a single rhizobacterial culture on Anethem graveolens. The Anethem graveolens plant was chosen for its annual life span and easy pot maintenance. After 3 weeks, all the pots were harvested and single and multiple rhizobacteria treated seeds showed magnification in various physiological parameters like root length, shoot length, shoot branches, etc. Both treatments (single and consortia) showed improved chlorophyll content than the control. This difference has become even more visible in the case of total chlorophyll content, where consortia shows an significant difference at p < 0.05, which is almost 3 fold increase in the chlorophyll content than the control and the isolates (Table 3).
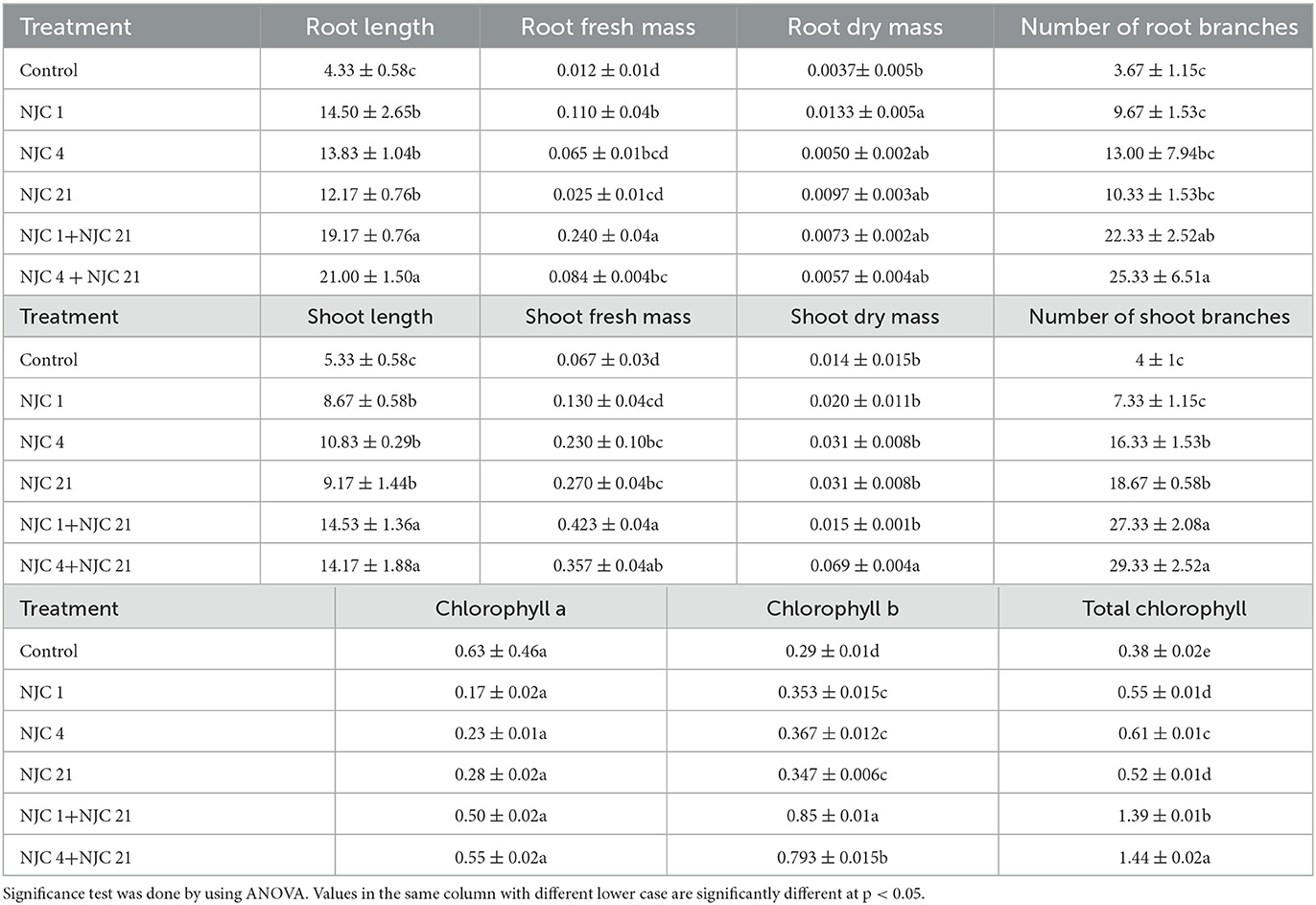
Table 3. Yield contributing parameters treated with two species consortia and single isolates in comparison with control.
This investigation showed that combined PGPR strains were proven to be better growth boosters than mono-species culture and control. Stastically noteworthy (p < 0.05) most intensive root growth stimulation was seen in treatment NJC 1+NJC 21 which were nearly 6 times greater than the control. While treatment NJC 4+NJC 21 seems most effective in seed emergence, 84.66% was four times superior compared to control and showed the maximum shoot elongation. Shoot branches were higher in T-2-treated plants and performed well in wet shoot weight. It was noticed that the degree of seed emergence in isolates ranges from 37.77 to 73.77% when control has only 20%, with the while in consortia, it ranges from 80% to nearly 86% (Figure 7).
The seedling vigor index, which includes all of the characteristics of the seed that affect its potential level of activity and performance, is one of the most crucial quantitative factors. Among the five isolated bacterial strain treatments to the seeds, the application of NJC 4, a PGPR strain, shows a maximum level of seedling vigor index than any other monospecies culture application (Figure 8). However, our observation of the present study exhibits seedling vigor index more pronounced when the PGPR strains were mixed as two species culture and applied to the seeds. The combination of NJC 1+NJC 21 gave maximum growth improvement as it's seedling vigorindex manifold higher than control and individual treatments.
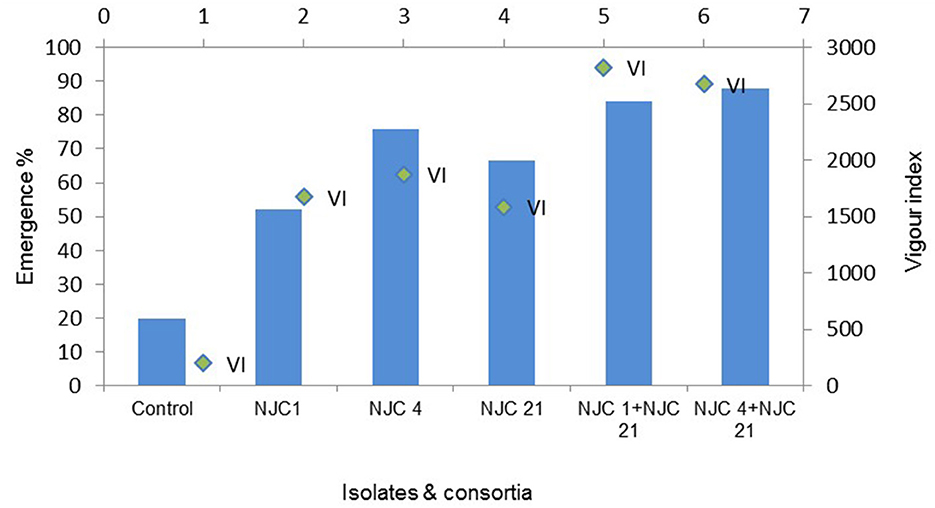
Figure 8. Emergence percentage and vigor index of Anethum graveolens treated with isolates and consortia.
Micronutrient uptake in plant
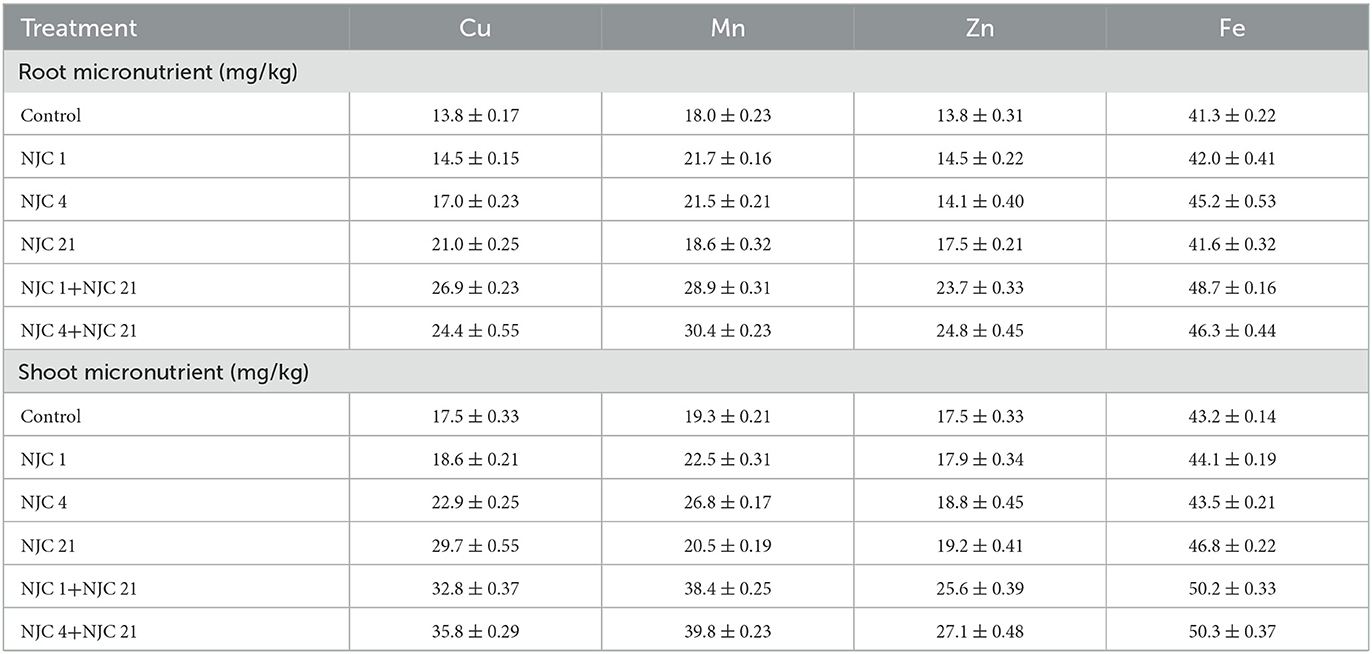
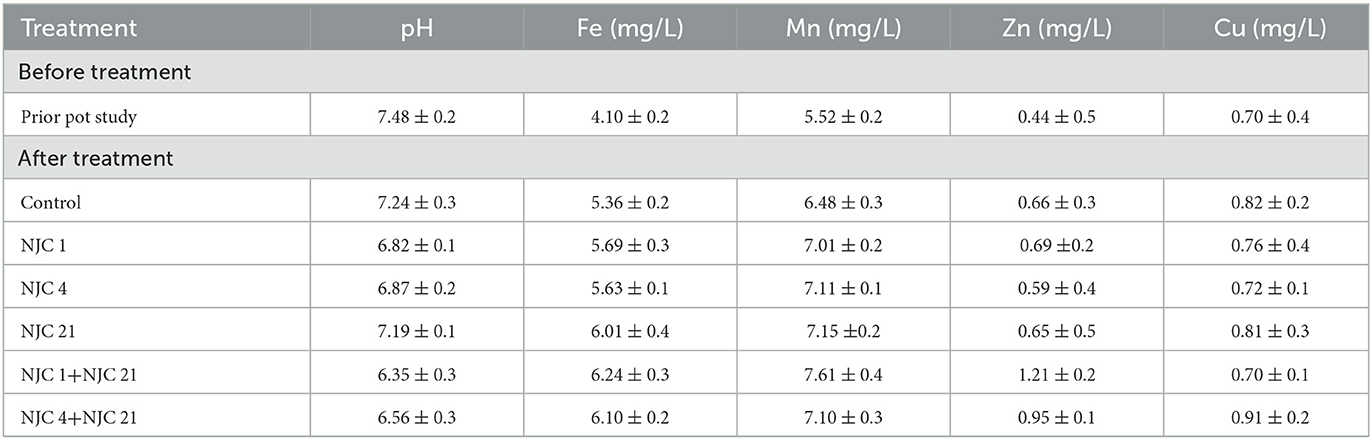
A clear correlation was proved between bacterial treatment and plant metal content when micronutrient analysis of the plants was performed (Table 4). Isolates tend to give better results than the control. In contrast, consortia performed even better than the isolates. The highest increment was seen in the copper content of NJC 1+NJC 21 treated plants, and the shoot of NJC 4+NJC 21 resulted in a two-fold increase in the copper content than the control. For isolates, the increment ranges from 5 to 69%. The results were also significant for manganese and zinc. Isolates show an increase of 3 to 38%; consortia show a 69% increase in root while concentration doubled in shoot manganese content. For zinc, consortia could produce 79% more concentration of zinc in root micronutrients. The slightest difference was observed in iron content. Isolate-treated plants showed a maximum increase of 9%, while it is 18% for consortia. Hence, the copper content is most influenced by the bacteria, while manganese and zinc are moderately influenced in comparison, and the treatment of bacteria least influences iron content.
Soil analysis
Soil analysis shows an essential insight into the effect of the PGPR on the plant. Soil treated with isolates showed more metal concentration than the control, while consortia-treated soil generally showed higher micronutrient concentration than soil treated with the isolates. A significant decrease in the soil pH was observed in PGPR-treated soil. On the contrary, we found an increase in the micronutrient concentration after the treatment. An increase in micronutrient content in the range from up to 17% to up to 56% was observed in isolate-treated soil than the control (Table 5).
In all the soils treated with consortia, there is a significant difference in the micronutrient content to the control. In consortia-treated soil, the bioavailability of zinc increases drastically as NJC 1+NJC 21 treated soil gives a 175% gain in zinc content. In contrast, micronutrient content increased by 30 to 175% of consortia-treated soil. Still, the copper content in NJC 1+NJC 21 has remained an exception as the copper content remains the same as the control.
Assessments of results by PCA
Growth and yield characteristics were examined through Principal component analysis (PCA). According to the PCA, the PC1 and PC2 cumulatively explained 78.12% of the total variance. PC1 can explain a significant 51.37% of the total variance, while 26.75% can be explained by PC2 (Figure 9).
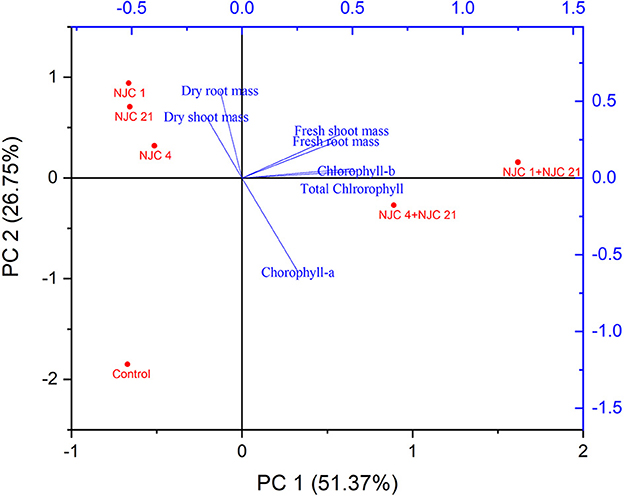
Figure 9. Principal component analysis (PCA) of growth and yield characteristics of Anethum graveolens L. where NJC 1 -Bacillus sp., NJC 21 –Serratia marcescens, NJC 4 - Pseudomonas aeruginosa.
Wet weight of the root and shoot, total chlorophyll content, and chlorophyll b content were all related to PC1. The dry weight of the root and shoot and an increase in chlorophyll a were both correlated with PC2.
Wet biomass, total chlorophyll content, and chlorophyll b content were positively correlated to treatment with PC1. In contrast, Chlorophyll b content associated with treatment with PC2 and dry biomass linked to single culture inoculation.
Discussion
In the present study, organisms were grown as two species cultures based on their compatibility. The growth profile data shows the compatible behavior among the co-inoculation. Here, we reported that our isolates belonged to distinct genera though they could establish interaction like commensalism or synergism, which designates their mutual ecological niche. Each organism was able to enhance the growth of the tested plant at the physiological as well as biochemical levels. This encourages us to evaluate the effects of our two species of consortia on the Anethum graveolens L.
Due to the elevated availability of nutrients from the root system, the rhizosphere serves as a healthier niche for organisms. Consortia, which associate with different organisms, are more effective at promoting plant growth than isolates. It is suggested that using co- culture of PGPRs in the preparation of biofertilizers might be desirable to using just one bacterium to achieve the synergistic effect of nutrient mobilization, improved efficacy, stability, and consistency when applied to various fields (Stockwell et al., 2011). According to some researches, inoculation with consortia promotes plant development more meritoriously than individual inoculations because each strain complements the others' advantageous features (Panwar et al., 2014; Singh et al., 2014). In the present study, two rhizobacterial consortia were selected based on their PGP traits, which can benefit Anethum graveolans. One contains the consortia of NJC 1+ NJC 21, and the other includes NJC 4+NJC 21.
Consortia have been checked for various PGP traits like phosphate solubilization, IAA production, ammonia production, HCN production, urease activity, and catalase test and exhibited growth promotion in Anethum graveolans L.
Phosphate solubilization is a determiner feature of plant growth promotion as microorganism aid plants in utilizing the insoluble phosphate by making it obtainable to the host plant (Jabborova et al., 2020a; Bhat et al., 2022). Rhizobacteria that solubilize phosphate are necessary for phosphate accumulation and phosphate transformation in plant roots. The majority of phosphate is absorbed during vegetative growth and is found in seeds and fruits as re-trans phosphorus (Allahdadi et al., 2010). According to several investigation, different soil bacteria typically produce low molecular weight organic acids, which aid in the solubilization of inorganic phosphorus (Zaidi et al., 2009; Hamid et al., 2021). It is believed that the primary mechanism for phosphate solubilization is a drop in pH in the vicinity of organic acid-producing microorganisms (Sperber, 1958). Abd El-Azeem et al. (2007) discovered that isolates that solubilize tricalcium phosphate (TCP) reduce the pH of the medium by producing organic acids. Isolates from the current study also demonstrated medium to acidic pH manifestation. This reduction in pH of various organic acids is made possible by the use of sugars present in media (Abd El-Azeem et al., 2007). Phosphate solubilizers produce a clear zone of P solubilization (i.e., Tricalcium Phosphate – TCP) on Pikovaskya's agar and consortia have a better solubilization proficiency than isolates, which is a clear evidence of their improved ability to solubilize phosphate when compared to isolates.
IAA production is another significant attribute of PGPR because this phytohormone aid in the absorption of nutrient by allowing the plant to develop a thoroughly formulated root system (Tsavkelova et al., 2007; Gowtham et al., 2022). Raut in 2017 reported that consortia generate more IAA than their isolated counterparts, which was also true for the present study (Raut et al., 2017).
Ammonia production and HCN production are significant features of PGPR as well. Ammonia indirectly influences plant growth by accumulating nitrogen to help promote plant growth (Geetha et al., 2014). The interaction of HCN produced by the rhizospheric bacteria appears to be a significant factor in controlling soil-borne diseases. It contributes to the effective inhibitor for the growth of plant pathogens infecting the root (Geetha et al., 2014). In vitro experiment of this study shows higher production of ammonia in consortia than those of isolates, while each isolate gave positive results for HCN production.
PGPR consortia treatment considerably affected the soil metal content. The application of bacteria changed the soil nutrient elements significantly. A perceptible increase in the metal content after the bacterial treatment indicates the mineralization ability of the inoculated PGPR. Apart from that, the pH of the soil also decreases after the treatment of the PGPR, which shows organic acid production, which in turn may increase the mineralization capacity of the PGPR (Gaind and Gaur, 1989; Orhan et al., 2006). The important observation about the change in the metal content is that even the NJC 4 treated soil has less zinc content than the control, NJC 4 containing consortia (i.e., NJC 1+NJC 4) found to have the highest amount of zinc content, and that is far more than each isolate treated soil had. The same happened with the copper content. Soil treated with NJC 1 has a copper concentration less than the control, but soil treated with consortia containing NJC 1 (i.e., NJC 1+NJC 21) was found to have the highest amount of copper. Though in the case of copper, the difference is not as significant as that of zinc content, this can indicatethe synergistic effect of component isolates in the consortia. Consortia can rise the bioavailability of micronutrients in the soil via mineralization.
The positive impact of individual and combined effects on the growth, seed germination, root length, shoot length, root branch, shoot branch, fresh mass and dry mass of root, and fresh mass and dry mass of shoot reflected the biopotential of consortia. The results indicate that a higher proportion of seed emergence could be obtained using consortia as PGPR. Other than that, plants inoculated with consortia exhibit a greater degree of development for nearly every characteristic considered here as an indicator, i.e., root length, shoot length, etc.
Micronutrients are essential to plant growth because they contribute to organic structures. With organic ligands, every micronutrient forms stable complexes. They operate as biological catalysts when complexes to proteins (metalloenzymes) (Ipek et al., 2017; Jabborova et al., 2021b). They are also essential as enzyme components or activators. They also serve as electron transporters and Osmo-regulators. Micronutrients play vital roles in metabolic control, reproduction, and resistance to abiotic and biotic stressors. In our study, inoculation of proficient rhizobacteria heightened the plant's vegetative growth and influenced the micronutrient concentration in Anethum graveolens L. The enhanced growth of Anethum graveolens L. can be elucidated by the improved nutrient concentration obtained in the current study. The inoculation of Serratia marcescens and Bacillus spp. together had the greatest impact on the concentration of micronutrients, increasing it by 5 to 69% compared to the uninoculated control under pot conditions. Under pot conditions, all treatments showed higher increases in micronutrient concentrations than the control. However, consortia-treated plants showed a superior increase in micronutrients and were more productive in sustainable agriculture.
All the isolates and designed consortia were found to enhance plants' yield contributing parameters and the micronutrient uptake of plants consistently compared to the control.
Conclusion
The formation of consortia can give a slight edge on the performance of PGPR. As the results suggested, consortia performed better for every PGP trait than the control and the isolates. Applying isolated PGPR strain as two species consortia to the seeds can promote the seedling attributes, which benefits early seedling establishment and, subsequently, efficient crop development. The preliminary results present here to fulfill the prognosis of the consortia having copious amounts of adaptability as PGP characteristics which can aid in getting more crops with better nutrient availability and capacity to open novel aspects of the future research scopes. This also opens the possibility of employment of consortia for the growth promotion of medicinal plants to meet the global requirements. This study provides a powerful insight that the consortia can be a tool to utilize the maximum growth-promoting potential of rhizobacteria. Not only for medicinal plants but the utilization of consortia for agriculture can be a further step toward sustainable agriculture as it has the potential to eliminate the application of chemical fertilizers as well as harmful pesticides. If PGPR is a gateway to sustainable agriculture, consortia can be a path for it.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
NJ and CJ designed the research. NJ performed the experiments and interpreted the data and wrote the manuscript. SAlh, SAlf, and RD performed analysis and writing–review and editing. CJ and MS revised the manuscript. SAlh and SAlf fund acquisition. All authors contributed to the article and approved the submitted version.
Acknowledgments
This project was supported by Researchers Supporting Project Number (RSP2023R5), King Saud University, Riyadh, Saudi Arabia and the Scheme of Developing High-Quality Research (SHODH) Program of the Government of Gujarat, India.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1126621/full#supplementary-material
References
Abd El-Azeem, S., Mehana, T., and Shabayek, A. (2007). Some plant growth promoting traits of rhizobacteria isolated from Suez Canal region, Egypt. African Crop Sci. Conf. Proceed. 8, 1517–1525.
Abdul-Baki, A. A., and Anderson, J. D. (1973). Vigor determination in soybean seed by multiple criteria 1. Crop Sci. 13, 630–633. doi: 10.2135/cropsci1973.0011183X001300060013x
Ali, S. A. M., Sayyed, R. Z., Mir, M. I., Hameeda, B., Khan, Y., Alkhanani, M. F., et al. (2022). Production, characterization and gene expression of surfactin of bacillus velezensis ms20 and evaluation of its induced systemic resistance and antibiofilm activity. Front. Microbiol. 13:1219. doi: 10.3389/fmicb.2022.879739
Allahdadi, I., Fallah, R., Akbari, G. A., and Mohaddesi, A. (2010). Effect of phosphate solubilizing bacteria and phosphate fertilizers on rice growth parameters. Iran. J. Soil Res. 23, 229–238. doi: 10.22092/IJSR.2010.126954
Arora, H., Sharma, A., Poczai, P., Sharma, S., Haron, F. F., Gafur, A., et al. (2022). Plant-derived protectants in combating soil-Borne fungal infections in tomato and Chilli. J. Fungi 8, 213. doi: 10.3390/jof8020213
Basu, A., Prasad, P., Das, S. N., Kalam, S., Sayyed, R., Reddy, M., et al. (2021). Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: recent developments, constraints, and prospects. Sustainability 13, 1140. doi: 10.3390/su13031140
Bhat, B. A., Tariq, L., Nissar, S., Islam, S. T., Islam, S. U., Mangral, Z., et al. (2022). The role of plant-associated rhizobacteria in plant growth, biocontrol and abiotic stress management. J. Appl. Microbiol. 133, 2717–2741. doi: 10.1111/jam.15796
Bhattacharjee, T., Sen, S., Chakraborty, R., Maurya, P. K., and Chattopadhyay, A. (2020). Cultivation of Medicinal Plants: Special Reference to Important Medicinal Plants of India in Herbal Medicine in India. Cham: Springer, 101–115.
Buddhi, C. W., and Min-Ho, Y. (2013). In vitro solubilization of inorganic phosphates by phosphate solubilizing microorganisms. Afr. J. Microbiol. Res. 7, 3534–3541. doi: 10.5897/AJMR2013.5861
Cappucino, J. C., and Sherman, N. (1992). Micorbiology: A Laboratory Manual, 3rd Edn. New York, NY: Benjamin/Cumming Pub Co.
Castric, P. A. (1975). Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can. J. Microbiol. 21, 613–618. doi: 10.1139/m75-088
Chaiharn, M., and Lumyong, S. (2011). Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Curr. Microbiol. 62, 173–181. doi: 10.1007/s00284-010-9674-6
Clarke, P. H., and Cowan, S. (1952). Biochemical methods for bacteriology. Microbiology 6, 187–197. doi: 10.1099/00221287-6-1-2-187
Gaind, S., and Gaur, A. (1989). Effect of pH on phosphate solubilization by microbes. Curr. Sci. 58, 1208–1211.
Gaur, A. (1990). Physiological Functions of Phosphate Solubilizing Micro-Organisms. Phosphate Solubilizing Micro-Organisms as Biofertilizers. New Delhi: Omega Scientific Publishers, 16–72. doi: 10.1016/j.bcab.2020.101685
Geetha, K., Venkatesham, E., Hindumathi, A., and Bhadraiah, B. (2014). Isolation, screening and characterization of plant growth promoting bacteria and their effect on Vigna Radita (L.) R. Int. J. Curr. Microbiol. Appl. Sci 3, 799–899.
Gowtham, H. G., Singh, S. B., Shilpa, N., Aiyaz, M., Nataraj, K., Udayashankar, A. C., et al. (2022). Insight into recent progress and perspectives in improvement of antioxidant machinery upon PGPR augmentation in plants under drought stress: a review. Antioxidants 11, 1763. doi: 10.3390/antiox11091763
Guetsky, R., Shtienberg, D., Elad, Y., Fischer, E., and Dinoor, A. (2002). Improving biological control by combining biocontrol agents each with several mechanisms of disease suppression. Phytopathology 92, 976–985. doi: 10.1094/PHYTO.2002.92.9.976
Hamid, B., Zaman, M., Farooq, S., Fatima, S., Sayyed, R., Baba, Z. A., et al. (2021). Bacterial plant biostimulants: a sustainable way towards improving growth, productivity, and health of crops. Sustainability 13, 2856. doi: 10.3390/su13052856
Hiscox, J., and Israelstam, G. (1979). A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 57, 1332–1334. doi: 10.1139/b79-163
Ipek, M., Aras, S., Arıkan, Ş., Eşitken, A., Pırlak, L., Dönmez, M. F., et al. (2017). Root plant growth promoting rhizobacteria inoculations increase ferric chelate reductase (FC-R) activity and Fe nutrition in pear under calcareous soil conditions. Sci. Hortic. 219, 144–151.
Jabborova, D., Annapurna, K., Fayzullaeva, M., Sulaymonov, K., Kadirova, D., Jabbarov, Z., et al. (2020a). Isolation and characterization of endophytic bacteria from ginger (Zingiber officinale Rosc.). Ann. Phytomed. 9, 116–121. doi: 10.21276/ap.2020.9.1.14
Jabborova, D., Kannepalli, A., Davranov, K., Narimanov, A., Enakiev, Y., Syed, A., et al. (2021a). Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep. 11, 1–9. doi: 10.1038/s41598-021-01337-9
Jabborova, D., Sayyed, R., Azimov, A., Jabbarov, Z., Matchanov, A., Enakiev, Y., et al. (2021b). Impact of mineral fertilizers on mineral nutrients in the ginger rhizome and on soil enzymes activities and soil properties. Saudi J. Biol. Sci. 28, 5268–5274. doi: 10.1016/j.sjbs.2021.05.037
Jabborova, D., Wirth, S., Kannepalli, A., Narimanov, A., Desouky, S., Davranov, K., et al. (2020b). Co-inoculation of rhizobacteria and biochar application improves growth and nutrientsin soybean and enriches soil nutrients and enzymes. Agronomy 10, 1142. doi: 10.3390/agronomy10081142
Jana, S., and Shekhawat, G. (2010). Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacognosy Rev. 4, 179. doi: 10.4103/0973-7847.70915
Jha, C. K., and Saraf, M. (2012). Evaluation of multispecies plant-growth-promoting consortia for the growth promotion of Jatropha curcas L. J. Plant Growth Reg. 31, 588–598. doi: 10.1007/s00344-012-9269-5
Kalam, S., Basu, A., Ahmad, I., Sayyed, R., El-Enshasy, H. A., Dailin, D. J., et al. (2020). Recent understanding of soil acidobacteria and their ecological significance: a critical review. Front. Microbiol. 11, 580024. doi: 10.3389/fmicb.2020.580024
Khan, N., Ali, S., Shahid, M. A., Mustafa, A., Sayyed, R., Curá, J. A., et al. (2021). Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: a review. Cells 10, 1551. doi: 10.3390/cells10061551
Khumairah, F. H., Setiawati, M. R., Fitriatin, B. N., Simarmata, T., Alfarraj, S., Ansari, M. J., et al. (2022). Halotolerant plant growth promoting rhizobacteria isolated from saline soil improve nitrogen fixation and alleviate salt stress in rice plants. Front. Microbiol. 1607. doi: 10.3389/fmicb.2022.905210
Lindsay, W., and Norwell, W. (1969). Development of a DTPA micronutrient soil test. Sci. Am. Proc. 35, 600–602.
Lynch, J. (1990). Beneficial interactions between micro-organisms and roots. Biotechnol. Adv. 8, 335–346. doi: 10.1016/0734-9750(90)91069-S
Manasa, M., Ravinder, P., Gopalakrishnan, S., Srinivas, V., Sayyed, R., El Enshasy, H. A., et al. (2021). Co-inoculation of Bacillus spp. for growth promotion and iron fortification in sorghum. Sustainability 13, 12091. doi: 10.3390/su132112091
Najafi, S., Nazari Nasi, H., Tuncturk, R., Tuncturk, M., Sayyed, R. Z., Amirnia, R., et al. (2021). Biofertilizer application enhances drought stress tolerance and alters the antioxidant enzymes in medicinal pumpkin (Cucurbita pepo convar. pepo var. Styriaca). Horticulturae 7, 588. doi: 10.3390/horticulturae7120588
Nithyapriya, S., Lalitha, S., Sayyed, R., Reddy, M., Dailin, D. J., El Enshasy, H. A., et al. (2021). Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in sesame. Sustainability 13, 5394. doi: 10.3390/su13105394
Orhan, E., Esitken, A., Ercisli, S., Turan, M., and Sahin, F. (2006). Effects of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient contents in organically growing raspberry. Sci. Hortic. 111, 38–43. doi: 10.1016/j.scienta.2006.09.002
Pandey, A., Das, N., Kumar, B., Rinu, K., and Trivedi, P. (2008). Phosphate solubilization by Penicillium spp. isolated from soil samples of Indian Himalayan region. World J. Microbiol. Biotechnol. 24, 97–102. doi: 10.1007/s11274-007-9444-1
Panwar, M., Tewari, R., and Nayyar, H. (2014). Microbial consortium of plant growth-promoting rhizobacteria improves the performance of plants growing in stressed soils: an overview. Phosphate Solub. Micro. 257–285. doi: 10.1007/978-3-319-08216-5_11
Patel, P., Shaikh, S., and Sayyed, R. (2018). Modified chrome azurol S method for detection and estimation of siderophores having affinity for metal ions other than iron. Environ. Sust. 1, 81–87. doi: 10.1007/s42398-018-0005-3
Raut, V., Shaikh, I., Naphade, B., Prashar, K., and Adhapure, N. (2017). Plant growth promotion using microbial IAA producers in conjunction with azolla: a novel approach. Chem. Biol. Technol. Agric. 4, 1–11. doi: 10.1186/s40538-016-0083-3
Rustamova, N. I., Litao, N., Bozorov, K. H., Sayyed, R. I., Aisa, H. A., and Yili, A. B. (2022). Plant-associated endophytic fungi: a source of structurally diverse and bioactive natural products. Plant Cell Biotechnol. Mol. Biol. 23, 1–19. Available online at: https://www.ikppress.org/index.php/PCBMB/article/view/7454
Sagar, A., Yadav, S., Sayyed, R., Sharma, S., and Ramteke, P. (2022). Bacillus subtilis: A Multifarious Plant Growth Promoter, Biocontrol Agent, and Bioalleviator of Abiotic Stress in Bacilli in Agrobiotechnology. Cham: Springer, 561–580.
Sayyed, R., Seifi, S., Patel, P., Shaikh, S., Jadhav, H., Enshasy, H. E., et al. (2019). Siderophore production in groundnut rhizosphere isolate, Achromobacter sp. RZS2 influenced by physicochemical factors and metal ions. Environ. Sust. 2, 117–124. doi: 10.1007/s42398-019-00070-4
Shah, S., Li, J., Moffatt, B. A., and Glick, B. R. (1998). Isolation and characterization of ACC deaminase genes from two different plant growth-promoting rhizobacteria. Can. J. Microbiol. 44, 833–843. doi: 10.1139/w98-074
Shahzad, S. M., Arif, M. S., Ashraf, M., Abid, M., Ghazanfar, M. U., Riaz, M., et al. (2015). Alleviation of Abiotic Stress in Medicinal Plants by PGPR in Plant-growth-promoting rhizobacteria (PGPR) and Medicinal Plants. Cham: Springer 135–166.
Sharma, S., Singh, V., Kumar, V., Devi, S., Shukla, K. P., Tiwari, A., et al. (2015). Plant Growth-Promoting Rhizobacteria (PGPR): Emergence and Future Facets in Medicinal Plants in Plant-Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants. Cham: Springer, 109–131.
Shenoy, V., and Kalagudi, G. (2003). “Meta-bug and near-isogenic strain consortia concepts for plant growth promoting rhizobacteria,” in: 6th International PGPR Workshop, India, Section VII—Mechanism of Biocontrol, Vol. 108 (Heidelberg: Springer-Verlag).
Singh, A., Jain, A., Sarma, B. K., Upadhyay, R. S., and Singh, H. B. (2014). Rhizosphere competent microbial consortium mediates rapid changes in phenolic profiles in chickpea during Sclerotium rolfsii infection. Microbiol. Res. 169, 353–360. doi: 10.1016/j.micres.2013.09.014
Singh, J., and Singh, A. V. (2017). Microbial Strategies for Enhanced Phytoremediation of Heavy Metal-Contaminated Soils in Environmental pollutants and Their Bioremediation Approaches. London: CRC Press, 257–272.
Sperber, J. I. (1958). Solution of apatite by soil microorganisms producing organic acids. Austr. J. Agric. Res. 9, 782–787. doi: 10.1071/AR9580782
Stockwell, V., Johnson, K., Sugar, D., and Loper, J. (2011). Mechanistically compatible mixtures of bacterial antagonists improve biological control of fire blight of pear. Phytopathology 101, 113–123. doi: 10.1094/PHYTO-03-10-0098
Tsavkelova, E. A., Cherdyntseva, T. A., Botina, S. G., and Netrusov, A. I. (2007). Bacteria associated with orchid roots and microbial production of auxin. Microbiol. Res. 162, 69–76. doi: 10.1016/j.micres.2006.07.014
Uddin, A. B. M., Khalid, R. S., Alaama, M., Abdualkader, A. M., and Kasmuri, A. (2016). Comparative study of three digestion methods for elemental analysis in traditional medicine products using atomic absorption spectrometry. J. Anal. Sci. Technol. 7, 1–7. doi: 10.1186/s40543-016-0085-6
Whitelaw, M. A. (1999). Growth promotion of plants inoculated with phosphate-solubilizing fungi. Adv. Agron. 69, 99–151. doi: 10.1016/S0065-2113(08)60948-7
Keywords: Anethum graveolens L., medicinal plants, microbial consortia, plant growth promoting rhizobacteria (PGPR), two species consortia
Citation: Joshi N, Saraf M, Jha CK, Sudha A, Alharbi SA, Alfarraj S and Datta R (2023) Harnessing the efficacy of multifunctional rhizobacterial consortia for promoting the growth of Anethum graveolens L. Front. Sustain. Food Syst. 7:1126621. doi: 10.3389/fsufs.2023.1126621
Received: 18 December 2022; Accepted: 13 February 2023;
Published: 08 March 2023.
Edited by:
Dilfuza Egamberdieva, Leibniz Center for Agricultural Landscape Research (ZALF), GermanyReviewed by:
Sowmyalakshmi Subramanian, McGill University, CanadaHumaira Yasmin, COMSATS University Islamabad, Pakistan
Copyright © 2023 Joshi, Saraf, Jha, Sudha, Alharbi, Alfarraj and Datta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaitanya Kumar Jha, Y2hhaXRhbnlha2poYUBnbWFpbC5jb20=
 Nishra Joshi1,2
Nishra Joshi1,2 Meenu Saraf
Meenu Saraf Chaitanya Kumar Jha
Chaitanya Kumar Jha