
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 17 February 2023
Sec. Aquatic Foods
Volume 7 - 2023 | https://doi.org/10.3389/fsufs.2023.1013319
 Sk Farzana Islam1,2,3
Sk Farzana Islam1,2,3 Md. Humayun Kabir2
Md. Humayun Kabir2 Sabina Yasmin2
Sabina Yasmin2 Mohammad Jahangir Alam3
Mohammad Jahangir Alam3 Shamim Ahmed2
Shamim Ahmed2 Mohammad Shamsur Rahman1*
Mohammad Shamsur Rahman1*A modified QuEChERS method was developed for the simultaneous analysis of tylosin (Tyl) and metronidazole (MNZ) residues in shrimp samples using LC-ESI-MS/MS. The sample extraction procedure was based on modified QuEChERS, and the cleanup method was dispersive solid-phase extraction (dSPE). Octadecyl (C18) and primary secondary amine (PSA) sorbents were used in the dSPE cleanup. Analyte chromatographic separations were carried out using a ZORBAX RRHD Eclipse Plus C18 (100 × 2.1 mm, particle size 1.8 μm) column. The mobile phase consisted of dilluting 0.1% of formic acid with water and acetonitrile. The analyte was identified with multiple reaction monitoring and positive electrospray ionization. The analyte showed good linearity in the range of 0.5–50 μg/L for both analytes, and correlation coefficients (R2) were 0.9997 and 0.9998 for Tyl and MNZ, respectively. For the recovery study, three different concentration levels were spiked in triplicate. The recovery obtained a good result in the range of 81–85 % for Tyl with relative standard deviation (RSD) ≤ ± 4.9% and in the range of 85–88% for MNZ with RSD ≤ ± 4.07 %. The limit of detection (LOD) was estimated at 0.4 μg/kg for Tyl and 0.3 μg/kg for MNZ, and the limit of quantification (LOQ) was estimated at 1 μg/kg for Tyl and 0.9 μg/kg for MNZ. The linearity and recovery study showed that the method is validated and can be used to determine the Tyl and MNZ residues in shrimp. Finally, the method was applied to 25 real samples, which were collected from local markets and super shops in Dhaka and Khulna districts of Bangladesh, and only traces of Tyl were detected in one sample. This method is suitable for the regular analysis of Tyl and MNZ antibiotic residues in shrimp samples and can be used to ensure food safety in Bangladesh.
Shrimp are a profitable aquaculture species that are widely traded around the world (Davis et al., 2021). Global Penaeid shrimp production is roughly 6 million metric tons, with most of it coming from Southeast Asia (FAO, 2020). However, intensive shrimp farming is very susceptible to bacterial diseases (Bermdez-Almada and Espinosa-Plascenci, 2012). In recent years, shrimp farming has been afflicted with outbreaks of bacterial diseases that have greatly undermined the profitability and sustainability of operations (Hossain et al., 2013). Farmers frequently use antibiotics in aquaculture, particularly in intensive farming, to promote growth (Thornber et al., 2020) and prevent diseases (Rakesh et al., 2018). However, due to the limited number of antibiotics allowed in aquaculture, some producers use veterinary medicine (Defoirdt et al., 2011). Tylosin (Tyl) is a macrolide antibiotic and a highly effective antimicrobial drug used in veterinary medicine to treat a wide range of Gram-positive and Gram-negative bacteria (Joo et al., 2020), and metronidazole (MNZ) is an antibacterial and antiprotozoal drug used in veterinary and human medicine (Wagil et al., 2015). The incorrect use of antimicrobials or disregarding withdrawal times can lead to the accumulation of antibiotic residues in animal foods (Wang, 2009). Antimicrobial residues in muscle food are hazardous to human health because they are acutely or cumulatively allergic, organotoxic, mutagenic, or carcinogenic (Falowo and Akimoladun, 2019). To reduce the risk to human health posed by the use of antibiotics in animal food production, the European Community established a legal framework to regulate and control the use of veterinary drugs in animal-derived products (Samanidou and Evaggelopoulou, 2007). Monitoring antibiotic residue in edible animal tissues requires sensitive and selective analytical methods to ensure food safety and boost consumer confidence. Numerous analytical methods were developed to determine Tyl and MNZ residues in a variety of matrices using liquid chromatography tandem mass spectrometry (LC-MS/MS). Chen et al. (2019) described an effective method for determining Tyl and other five macrolide antibiotics (MACs) in chicken samples using LC-MS/MS followed by a self-built porous aromatic framework (PAF-)based solid-phase sorbent. Sismotto et al. (2014) developed a method for the identification of Tyl and other macrolides in tilapia filets by liquid chromatography coupled to quadrupole time-of-flight mass (LC–QToF). Samples were extracted with ethanol and hexane. A sensitive and rapid method was developed for the determination of MNZ and other nitroimidazole residues in tilapia, salmon, and shrimp samples using ultra-performance-tandem spectrometry (UPLC-MS/MS). Acetonitrile and a C18 SPE cartridge were used for sample extraction and cleanup (Watson et al., 2014). Guidi et al. (2017) determined the screening method for six groups of antibiotics (tetracyclines, quinolones, beta-lactams, macrolides, sulfonamides, and aminoglycosides) in fish muscle using LC-ESI-MS/MS, and they used trichloroacetic acid for sample preparation. Despite the benefits of employing detection techniques, real complex samples (such as foods and edible animal tissues) require proper sample preparation to reduce interferences and prevent potential matrix effects (Huerta et al., 2013). Several extraction techniques were used to analyze antibiotic residues in food matrices, including solid-phase extraction (SPE) (Jo et al., 2011), pressurized liquid extraction (PLE) (Juan et al., 2010), and liquid–liquid extraction (LLT) (Jank et al., 2015). However, some of these procedures are lengthy and laborious, and they require a cleanup step (Stolker and Brinkman, 2005). In this context, the QuEChERS (quick, easy, cheap, effective, rugged, and safe) approach is a simple extraction procedure for multiresidue analysis (Zhang et al., 2019). However, this method was most commonly used to analyze pesticide residues in fruits and vegetables and fish samples (Lazartigues et al., 2011; Wilkowska and Biziuk, 2011). A few studies have been conducted on the detection of veterinary drugs in edible tissue matrices (Stubbings and Bigwood, 2009). It has not been used frequently to determine Tyl and MNZ antibiotics in fish samples, and the recoveries for these antibiotics at low concentration levels have not been satisfactory (Wagil et al., 2015; Susakate et al., 2019). In this study, a modified QuEChERS method was developed for simultaneously determining Tyl and MNZ antibiotic residues in shrimp muscle using LC-ESI-MS/MS. The samples were extracted with acetonitrile containing 0.1% formic acid. To clean up the sample, dispersive solid-phase extraction (dSPE) sorbents were used. As dSPE sorbents, primary secondary amine (PSA) and octadecyl (C18) sorbents were employed. High extraction efficiency and minimal matrix effect were obtained after modifying the sample treatment conditions of the QuEChERS method. This method offers several benefits, including fewer extraction steps, rapid extraction, lower waste volume, satisfactory recovery, and a lower detection limit. The developed method was validated in accordance with the criteria of Commission Decision 2002/657/EC (European Commission, 2002).
Therefore, the present study aimed to develop and validate a modified QuEChERS method using LC-ESI-MS/MS for the simultaneous determination and quantification of MNZ and Tyl residues in shrimp and to use this developed and validated method to analyze shrimp collected from local markets and super shops in Dhaka and Khulna districts of Bangladesh to monitor the Tyl and MNZ contamination level.
Tyl and MNZ standards (purity 99.5%) were procured from Sigma-Aldrich, Germany. LC-MS-grade acetonitrile and formic acid (purity 99.5%) were collected from Sigma-Aldrich, Germany. Dispersive sorbent octadecyl (C18), graphitized carbon black (GCB), and primary secondary amine (PSA) were procured from Agilent Technologies, Germany. A Milli-Q apparatus (Millipore, Bedford, USA) was used to obtain deionized water.
A liquid chromatography mass spectrometer with ESI (LC-ESI MS/MS) and triple quadrupole (QQQ) mass analyzer were used for analysis. Chromatographic separation of Tyl and MNZ was carried out using an Agilent 1290 infinity (Agilent Technologies, Waldbronn, Germany), and separations were achieved using a ZORBAX RRHD Eclipse Plus C18 column (100 × 2.1 mm, 1.8 μm particle size). The analytes were separated using mobile phase A (0.1% formic acid in water) and mobile phase B (ACN). The mobile phase flow rate was 0.2 mL/min, and the injection volume was 5 μL. The retention times of Tyl and MNZ were 2.85 and 3.1 min, respectively, and the total chromatographic run time was 7 min. The mass spectrometer consisted of a triple quadrupole analyzer (6420 triple, Agilent Technologies, Waldbronn, Germany). The triple quadrupole (QQQ) mass spectrometer was operated in an MRM mode, monitoring two precursor ion transitions, where all target analytes yielded [M + H] + precursor ions. Quantification was performed on the more intense of the two mass transitions, while confirmation was performed on the other. Standard solutions of Tyl (0.2 mg/L) and MNZ (0.2 mg/L) were used to optimize the collision energy (CE), the precursor ion, and the product ion. Ionization source conditions were as follows: capillary voltage 3,500 V, nebulizer pressure 45 psi, gas flow rate 8 L/min, gas temperature 300 °C, and nebulizer pressure 45 psi. The mass spectrometer settings were tuned as stated in Table 1.
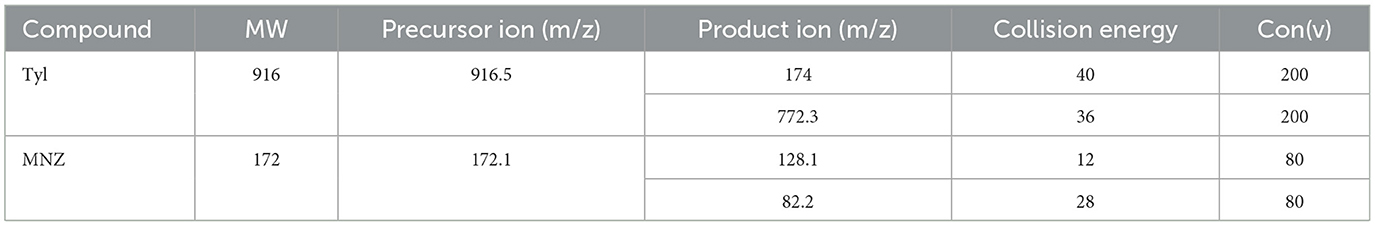
Table 1. Mass transition parameters of Tyl and MNZ in shrimp using liquid chromatography mass spectrometry.
Control shrimp (Penaeus monodon) samples were collected from the Quality Control Laboratory, Department of Fisheries, Chattogram, Bangladesh. They collected the control sample from deep sea. Internally, samples were checked to ensure that they were free of Tyl and MNZ contamination. Marketed shrimp samples (Penaeus monodon) (n = 25) was collected from local supermarkets and super shops of Dhaka and Khulna districts of Bangladesh. Samples were brought to the laboratory in an ice box from the market. After removing the shells from the shrimp, the samples were blended and stored in a zip bag at −20°C until further analysis.
Stock solutions (100 mg/L) were prepared by taking exactly 10.0 mg of MNZ and Tyl standard solution in a 100-ml volumetric flask, separately. The volumetric flask was filled up to the mark with ACN. To prepare 50 mg/L MNZ and Tyl intermediate working standard solutions, 12.5 ml of the solution was taken from standard stock solutions of MNZ (100 mg/L) and Tyl (100 mg/L) and transferred to a 25-ml volumetric flask, and the same solvent was used to fill each solution to the mark. The working standard solutions of MNZ and Tyl (10, 5, and 1 mg/L) were prepared to construct a calibration curve. Finally, calibration curves were constructed using a series of dilution of 0.2, 0.5, 1, 2, 5, 10, 20, and 50 μg/L. Before analysis, all MNZ and Tyl standard solutions were stored at 4°C.
The sample was extracted using the QuEChERS approach, with some modifications. Blended samples were weighed out (3.00 ± 0.01 g) into a 15-ml screw-capped polypropylene centrifuge tube by an analytical balance (4 dp) (Shimadzu AUY 220). In a centrifuge tube, 10 ml of ACN containing 0.1% formic acid was added. After being vortexed for 1 min, the samples were centrifuged at 4500 rpm for 5 min. A microcentrifuge tube (2 ml) was filled with 1.5 ml of the supernatant. Then, 10 mg of C18 and 50 mg of PSA were added to the microcentrifuge tube. The tube was centrifuged again for 5 min at 4,500 rpm after being shaken vigorously for one min. The supernatant was taken out using a syringe, and the supernatant was then filtered using a micron (μm) filter (0.20 μm). After filtering, the supernatant was transferred to a 2-ml vial for LC-MS/MS analysis.
The approach was validated in accordance with EU Commission Decision 2002/657/EC (European Commission, 2002). The specificity and selectivity of the method were determined by examining blank and spiked samples of shrimp with Tyl and MNZ. There was no peak in the control sample at or around the same retention time as the target analyte, confirming the specificity of the technique. The selectivity of the method was evaluated by the absence of any signal at the same retention time of the analytes. The coefficient of determination (R2) was calculated from an 8-point calibration curve to determine linearity. A linear regression analysis on spiked samples was used to create the calibration curves for the analytes' detection. The method's limit of detection was 3.3 times the standard error of the intercept divided by the calibration curve's slope, and the limit of quantification was 10 times the standard error of the intercept divided by the slope. For the recovery study, three different concentration levels were spiked in triplicate. The precision was estimated using the relative standard deviation (RSD%), and the accuracy was calculated as a percentage of the recovery. As matrix components can increase or reduce the analytical signal, matrix-matched calibration (MMC) was used to minimize the matrix effect. The percentage of matrix effect was estimated using the peak area of the matrix in the control sample and the peak area of the standard solvent.
In a multiclass antibiotic analysis, a suitable sample extraction is necessary for accurate results. Sample extraction should be quick, inexpensive, simple, and environment friendly. The original QuEChERS approach requires two steps for the extraction of target analytes: the first is a liquid–liquid extraction through a salting out step in acetonitrile and the second is dispersive SPE (dSPE) cleanup. We followed QuEChERS extraction after some modifications. Optimization or modification of the proposed method was carried out by checking different types of extraction solvents, inorganic salts, and sorbents. ACN was regarded as the ideal extraction solvent due to its capacity to precipitate proteins and lipids, which may degrade drug residues during sample processing (Jiménez et al., 2011). Initially, we extracted Tyl and MNZ using ACN. However, the extraction efficiency was maximized when 0.1% formic acid was added to acetonitrile, resulting in satisfactory recovery values that were greater than that of ACN. Conventional QuEChERS suggests various inorganic salts during sample preparation. This step was skipped in this study since clean extracts and a constant chromatographic response were obtained, and the sample treatment time and cost were reduced. Previous research indicated that PSA, C18, and GCB sorbents can produce appropriate results with UHPLC–MS/MS (Wilkowska and Biziuk, 2011; Lombardo-Agüí et al., 2012). We evaluated various combinations of dispersive sorbents to achieve the highest recoveries. The recoveries of antibiotics decreased with the addition of GCB, while a conjugation of PSA and C18 resulted in better recoveries than C18 alone. Finally, consistent and good recovery was observed when 50 mg of PSA and 10 mg of C18 were added. Similar findings were described by Grande-Martinez et al. (2018). They optimized the QuEChERS method for the determination of tetracyclines in fish muscle by UHPLC–MS/MS. They used PSA and C18 as dSPE sorbents and obtained recoveries in the range of 80–105%. Some target analytes may be lost during multistep and sophisticated sample cleanups. Thus, a simple extraction approach, as stated earlier in Section 2.5, was used in this study, and the recovery values were higher than those in previous studies (Table 2).
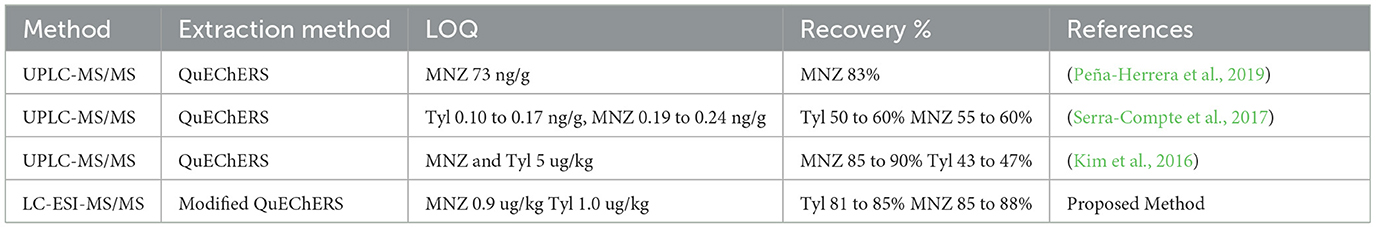
Table 2. A comparison of the proposed method with other reported methods for the determination of Tyl and MNZ in shrimp muscle.
The LC-MS/MS technique was used in this study. First, we optimized the MS parameters. The ESI (+) mode was chosen for the detection of Tyl and MNZ because this type of ionization was previously used to detect these compounds. Standard solutions (0.2 mg/L) of Tyl and MNZ prepared in the laboratory were directly injected into the mass spectrometer to adjust the parameters. For each compound, one precursor ion and two product ions were chosen for MRM analysis (Figure 1). In the MRM mode, the LC-MS/MS technique used two transitions and the retention time to achieve four identification points for each analyte (Figure 2). In positive ion mode, several parameters, such as flow rate, capillary voltage, collision energy, and nebulizer pressure, which can affect mass spectra were optimized. We also studied the LC conditions. In the LC-MS/MS technique, formic acid, acetic acid, or ammonium acetate are the best additives with water to separate antibacterial substances from biological matrices (Gavilán et al., 2015). The results of our experiments showed that 0.1% formic acid was the best additive for increasing peak resolution and sensitivity. Most published methods used acetonitrile as an organic solvent mobile phase for the analysis of an antibacterial compound (Gbylik et al., 2012). In this study, the best results were obtained utilizing a gradient elution and a chromatographic column with acetonitrile and 0.1% formic acid in Milli-Q water.
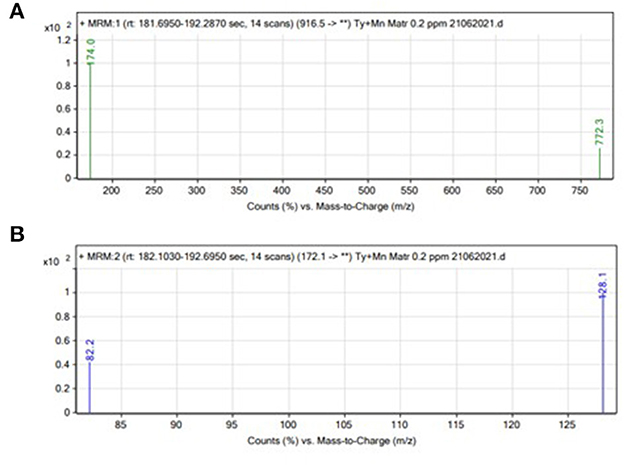
Figure 1. Liquid chromatography tandem mass spectrometry spectrums of (A) Tyl and (B) MNZ production.
Selectivity was evaluated by analyzing standard solutions of Tyl and MNZ, blank matrices, and sample matrices spiked with Tyl and MNZ simultaneously and monitoring the retention times. The absence of any signal at the same retention time as the antibiotics indicated that there were no matrix interferences that could have resulted in a false positive signal, resulting in a clean chromatogram that was free of interference. Specificity was confirmed by injecting 10 control or blank samples. No potential interference was detected. The results showed that the method was specific (Figure 3).
The linearity of the calibration curve was confirmed by plotting the concentration against the instrumental peak area. A very good linearity in the range of 0.2-50 μg/L for both analytes and correlation coefficients (R2) of 0.9997 and 0.9998 were achieved for the matrix-matched calibration curves of Tyl and MNZ, respectively.
The limit of detection (LOD) was estimated at 0.4 μg/kg for Tyl and 0.3 μg/kg for MNZ, and the limit of quantification (LOQ) was estimated at 1 μg/kg for Tyl and 0.9 μg/kg for MNZ. The LOQ was lower than the MRL.
The recovery and intraday precision (repeatability) of the method were performed by spiking blank shrimp samples at three different concentration levels (50, 10, and 5 μg/L) using three replicates for each concentration level in 1 day. The same concentration levels were studied with spike blanks over five consecutive days to assess interday precision (reproducibility). The intraday and interday precision of the method was expressed as % RSD. The recovery was obtained in the range of 81–85% for Tyl and 85–88% for MNZ. Intraday precision (RSD%) of Tyl was in the range of 0.7–4.9% and 0.9–4.07% for MNZ, and interday precision (RSD%) for Tyl was in the range of 0.8–4.1% and 1.20–3.09% for MNZ (Table 3).
The mild matrix enhancement effect for Tyl and MNZ was in the range of 80–85%. It was determined that the sample matrix hampered the detection of Tyl and MNZ. Thus, matrix-matched calibration (MMC) was used to minimize the matrix effect for quantitative analysis.
The developed method was applied to real samples that were collected from various supermarkets and local markets in the Dhaka and Khulna districts of Bangladesh. A matrix-matched calibration, a reagent blank, and a spiked blank sample were used for quality control. Furthermore, following the criteria defined by Commission Decision 2002/657/EC, the retention time and the ion ratio of the detected antibiotic in a real sample were compared to those of matching calibration standards in the same batch to ensure the identity of the detected molecule. Twenty-five samples were analyzed for simultaneous detection using LC-ESI-MSMS. One sample of 25 shrimp samples was greater than LOQ.
A modified QuEChERS method was developed for the quantification of Tyl and MNZ antibiotic residues in shrimp muscle. Analyte extraction and cleanup were simply accomplished using a modified QuEChERS technique. Validation data for linearity, selectivity, accuracy, precession, LOD, and LOQ were satisfactory. These results demonstrated that the modified QuEChERS method is quick, inexpensive, environment friendly, and suitable for the determination of Tyl and MNZ antibiotic residues in shrimp muscle. The method was successfully applied to 25 samples from local markets and super shops in Dhaka and Khulna districts of Bangladesh, and the presence of Tyl greater than LOQ was detected in one sample out of twenty-five. However, further studies with larger samples size are required to confirm the presence of Tyl and MNZ residues in shrimp muscles. To the best of our knowledge, this is the first time in Bangladesh that a modified QuEChERS method was used to analyze Tyl and MNZ antibiotic residues in shrimp muscles. The method can be used to improve food safety in Bangladesh, which will have an impact on the country's exports.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
MR and MK conceived and designed the study. SI and MK developed the method. SI collected the sample, analyzed it, interpreted the data, and wrote the manuscript. SY, MA, and SA provided guidance on the analysis of the data. MR provided overall research guidance and support, edited, and reviewed the manuscript. All authors provided comments on the manuscript prior to submission.
This study was supported by a doctoral fellowship of the SI of the article granted from the Bangabandhu Science and Technology Fellowship Trust, Ministry of Science and Technology, Government of the People's Republic of Bangladesh.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bermdez-Almada, M. C., and Espinosa-Plascenci, A. (2012). “The use of antibiotics in shrimp farming,” in Health and Environment in Aquaculture. (Rijeka: Intech). doi: 10.5772/28527
Chen, Q, Pan, X.-D, Huang, B.-F., Han, J.-L., and Zhou, B. (2019). Screening of multi-class antibiotics in pork meat by LC-Orbitrap-MS with modified QuEChERS extraction. RSC Adv. 9, 19–25. doi: 10.1039/C9RA04853G
Davis, R. P., Davis, D. A., and Boyd, C. E. (2021). A preliminary survey of antibiotic residues in frozen shrimp from retail stores in the United States. Curr. Res. Food Sci. 4, 679–683. doi: 10.1016/j.crfs.2021.09.009
Defoirdt, T., Sorgeloos, P., and Bossier, P. (2011). Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 14, 251–258. doi: 10.1016/j.mib.2011.03.004
European Commission (2002). European Commission, Commission decision 2002/657/EC implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Official Journal European Commission: Legis. L221, 8.
Falowo, A. B., and Akimoladun, O. F. (2019). Veterinary drug residues in meat and meat products: Occurrence, detection and implications. Veter. Med. Pharmac. 3, 194. doi: 10.5772/intechopen.83616
FAO (2020). “Fishery and aquaculture statistics. Global aquaculture production 1950-2018 (FishstatJ),” in FAO Fisheries and Aquaculture Department (Rome, Italy: FAO).
Gavilán, R. E., Nebot, C., Miranda, J. M., Martín-Gómez, Y., Vázquez-Belda, B., Franco, C. M., et al. (2015). Analysis of tetracyclines in medicated feed for food animal production by HPLC-MS/MS. Antibiotics 5, 1. doi: 10.3390/antibiotics5010001
Gbylik, M., Posyniak, A., and Gajda, A. (2012). Simultaneous determinaton of fluoroquinolones in feed by liquid chromatography with fluorescence detection. Bull. Veter. Inst. Pulwy 56, 343–347. doi: 10.2478/v10213-012-0060-y
Grande-Martinez, A., Moreno-Gonzalez, D., Arrebola-Liebanas, F. J., Garrido-Frenich, A., and Garcia-Campana, A. M. (2018). Optimization of a modified QuEChERS method for the determination of tetracyclines in fish muscle by UHPLC–MS/MS. J. Pharmac. Biomed. Analy. 155, 27–32. doi: 10.1016/j.jpba.2018.03.029
Guidi, L. R., Santos, F. A., Ribeiro, A. C. S. R., Fernandes, C., Silva, L. H. M., and Gloria, M. B. A. (2017). A simple, fast and sensitive screening LC-ESI-MS/MS method for antibiotics in fish. Talanta 163, 85–93. doi: 10.1016/j.talanta.2016.10.089
Hossain, M. S., Uddin, M. J., and Fakhruddin, A. N. M. (2013). Impacts of shrimp farming on the coastal environment of Bangladesh and approach for management. Rev. Environ. Sci. Bio/Technol. 12, 313–332. doi: 10.1007/s11157-013-9311-5
Huerta, B., Jakimska, A., Gros, M., Rodríguez-Mozaz, S., and Barcel,ó, D. (2013). Analysis of multi-class pharmaceuticals in fish tissues by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A. 1288, 63. doi: 10.1016/j.chroma.2013.03.001
Jank, L., Martins, M. T., Arsand, J. B., Motta, T. M. C., Hoff, R. B., Barreto, F., et al. (2015). High-throughput method for macrolides and lincosamides antibiotics residues analysis in milk and muscle using a simple liquid–liquid extraction technique and liquid chromatography–electrospray–tandem mass spectrometry analysis (LC–MS/MS). Talanta 144, 686–695. doi: 10.1016/j.talanta.2015.06.078
Jiménez, V., Rubies, A., Centrich, F., Companyó, R, and Guiteras, J. (2011). Development and validation of a multiclass method for the analysis of antibiotic residues in eggs by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 1218, 1443–1451. doi: 10.1016/j.chroma.2011.01.021
Jo, M. R., Lee, H. J., Lee, T. S., Park, K., Oh, E. G., Kim, P. H., et al. (2011). Simultaneous determination of macrolide residues in fish and shrimp by liquid chromatography-tandem mass spectrometry. Food Sci. Biotechnol. 20, 823–827. doi: 10.1007/s10068-011-0114-6
Joo, M. S., Hwang, S. D., Choi, K. M., Kim, Y. J., Hwang, J. Y., Kwon, M. G., et al. (2020). Application of tylosin antibiotics to olive flounder (Paralichthys olivaceus) infected with Streptococcus parauberis. Fisher. Aquatic Sci. 23, 1–18. doi: 10.1186/s41240-020-00165-8
Juan, C., Molt,ó, J. C., Mañes, J., and Font, G. (2010). Determination of macrolide and lincosamide antibiotics by pressurised liquid extraction and liquid chromatography-tandem mass spectrometry in meat and milk. Food Control 21, 1703–1709. doi: 10.1016/j.foodcont.2010.05.004
Kim, J., Suh, J. H., Cho, H. D., Kang, W., Choi, Y. S., and Han, S. B. (2016). Analytical method for fast s creening and confirmation of multi-class veterinary drug residues in fish and shrimp by LC-MS/MS. Food Addit. Contamin. Part A 33, 420–432. doi: 10.1080/19440049.2016.1139752
Lazartigues, A., Wiest, L., Baudot, R., Thomas, M., Feidt, C., and Cren-Olivé, C. (2011). Multiresidue method to quantify pesticides in fish muscle by QuEChERS-based extraction and LC-MS/MS. Analyt. Bioanalyt. Chem. 400, 2185–2193. doi: 10.1007/s00216-011-4945-z
Lombardo-Agüí, M., García-Campaña, A. M., Gámiz-Gracia, L., and Cruces-Blanco, C. (2012). Determination of quinolones of veterinary use in bee products by ultra-high-performance liquid chromatography–tandem mass spectrometry using a QuEChERS extraction procedure. Talanta 93, 193–199. doi: 10.1016/j.talanta.2012.02.011
Peña-Herrera, J. M., Montemurro, N., Barceló, D., and Pérez, S. (2019). Development and validation of an analytical method for determination of pharmaceuticals in fish muscle based on QuEChERS extraction and SWATH acquisition using LC-QTOF-MS/MS system. Talanta 199, 370–379. doi: 10.1016/j.talanta.2019.01.119
Rakesh, K., Naik, G. M., Pinto, N., Dharmakar, P., Pai, M., and Anjusha, K. V. (2018). A Review on Drugs Used in Shrimp Aquaculture. Int. J. Pure Appl. Biosci. 6, 77–86. doi: 10.18782/2320-7051.6296
Samanidou, V. F., and Evaggelopoulou, E. N. (2007). Analytical strategies to determine antibiotic residues in fish. J. Separ. Sci. 30, 2549–2569. doi: 10.1002/jssc.200700252
Serra-Compte, A., Álvarez-Muñoz, D., Rodríguez-Mozaz, S., and Barceló, D. (2017). Multi-residue method for the determination of antibiotics and some of their metabolites in seafood. Food Chem. Toxicol. 104, 3–13. doi: 10.1016/j.fct.2016.11.031
Sismotto, M., Paschoal, J. A., Teles, J. A., de Rezende, R. A., and Reyes, F. G. (2014). A simple liquid chromatography coupled to quadrupole time of flight mass spectrometry method for macrolide determination in tilapia fillets. J. Food Composit. Analy. 34, 153–162. doi: 10.1016/j.jfca.2014.02.006
Stolker, A. A. M., and Brinkman, U. T. (2005). Analytical strategies for residue analysis of veterinary drugs and growth-promoting agents in food-producing animals—a review. J. Chromatogr. A 1067, 15–53. doi: 10.1016/j.chroma.2005.02.037
Stubbings, G., and Bigwood, T. (2009). The development and validation of a multiclass liquid chromatography tandem mass spectrometry (LC–MS/MS) procedure for the determination of veterinary drug residues in animal tissue using a QuEChERS (QUick, Easy, CHeap, Effective, Rugged and Safe) approach. Analyt. Chimica Acta 637, 68–78. doi: 10.1016/j.aca.2009.01.029
Susakate, S., Poapolathep, S., Chokejaroenrat, C., Tanhan, P., Hajslova, J., Giorgi, M., et al. (2019). Multiclass analysis of antimicrobial drugs in shrimp muscle by ultra-high-performance liquid chromatography-tandem mass spectrometr y. J. Food Drug Analy. 27, 118–134. doi: 10.1016/j.jfda.2018.06.003
Thornber, K., Verner-Jeffreys, D., Hinchliffe, S., Rahman, M. M., Bass, D., and Tyler, C. R. (2020). Evaluating antimicrobial resistance in the global shrimp industry. Rev. Aquac. 12, 966–998. doi: 10.1111/raq.12367
Wagil, M., Maszkowska, J., Białk-Bielińska, A., Caban, M., Stepnowski, P., and Kumirska, J. (2015). Determination of metronidazole residues in water, sediment and fish tissue samples. Chemosphere 119, S28–S34. doi: 10.1016/j.chemosphere.2013.12.061
Wang, J. (2009). Analysis of macrolide antibiotics, using liquid chromatography-mass spectrometry, in food, biological and environmental matrices. Mass Spectr. Rev. 28, 50–92. doi: 10.1002/mas.20189
Watson, L., Potter, R., MacNeil, J. D., and Murphy, C. (2014). Development and method validation for the determination of nitroimidazole residues in salmon, tilapia and shrimp muscle. J. AOAC Int. 97, 273–281. doi: 10.5740/jaoacint.12-322
Wilkowska, A., and Biziuk, M. (2011). Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 125, 803–812. doi: 10.1016/j.foodchem.2010.09.094
Keywords: tylosin, metronidazole, shrimp, residue, QuEChERS
Citation: Islam SF, Kabir MH, Yasmin S, Alam MJ, Ahmed S and Rahman MS (2023) A modified QuEChERS method development to analyze tylosin and metronidazole antibiotics residue in shrimp (Penaeus monodon) using LC-ESI MS/MS. Front. Sustain. Food Syst. 7:1013319. doi: 10.3389/fsufs.2023.1013319
Received: 06 August 2022; Accepted: 26 January 2023;
Published: 17 February 2023.
Edited by:
Mohammad Mahfujul Haque, Bangladesh Agricultural University, BangladeshReviewed by:
C. K. Sunil, Indian Institute of Food Processing Technology, IndiaCopyright © 2023 Islam, Kabir, Yasmin, Alam, Ahmed and Rahman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Shamsur Rahman,  c2hhbXN1ckBkdS5hYy5iZA==
c2hhbXN1ckBkdS5hYy5iZA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.