
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Sustain. Food Syst. , 10 August 2022
Sec. Sustainable Food Processing
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.940900
This article is part of the Research Topic Food Bioactives: Implications for Meeting Sustainable Development Goals View all 8 articles
 Lingyu Wang1†
Lingyu Wang1† Ruolan Li1†
Ruolan Li1† Qing Zhang1
Qing Zhang1 Jia Liu1
Jia Liu1 Ting Tao1
Ting Tao1 Ting Zhang1
Ting Zhang1 Chunjie Wu1
Chunjie Wu1 Qiang Ren2*
Qiang Ren2* Xufeng Pu3*
Xufeng Pu3* Wei Peng1*
Wei Peng1*Pyracantha fortuneana (Maxim.) Li has been used as a herbal medicine in China in its long history. Since ancient times, the fruits of P. fortuneana has been considered a functional food to improve various diseases. Many bioactive substances, including proanthocyanidins, phenols, polysaccharides, and dietary fibers, have been isolated and identified from the P. fortuneana, which possess diverse biological properties both in vitro and in vivo. Although the researches on the P. fortuneana have achieved extensive progress, the systematic study of its biological activities is still relatively lacking. In addition, accumulating researches focus on the landscape value of the P. fortuneana and the development of its by-products. The by-products of P. fortuneana, which show good development potentials in the field of agricultural production and environmental protection, are important for improving the economic value of P. fortuneana and its significance. After extensive reviewing and analyzing the existing published articles, books, and patents, this study aims to a systematic and summarized research trends of P. fortuneana and its phytochemical compositions, nutritional values, pharmacological effects and health benefits of its extracts/monomers, which would be beneficial for the future development of this medicinal plant as functional food or drugs.
Pyracantha fortuneana (Maxim.) Li is an evergreen wild shrub and fruit tree of the genus Pyracantha in the Rosaceae apple subfamily, distributed from eastern Asia to southern Europe. So far, 10 species of Pyracantha have been reported in the world, while seven species have been found in China, and mainly produced in the southeastern and southwestern provinces of China (Jiang et al., 2007; Nai et al., 2020).
In the recent years, accumulating scientific research related to P. fortuneana has carried out a lot of researches on the nutritional components, efficacy, and processing application of P. fortuneana, and found that P. fortuneana contains many biological active substances, including proanthocyanidins, phenols, natural pigments, polysaccharides, dietary fiber, etc. Furthermore, the previous literatures also revealed that P. fortuneana and its bioactive substances possess various pharmacological activities, including anti-tumor effects, immune regulation effects, antioxidative effects, blood lipid-lowering effects, regulating effects on intestinal flora, and protecting effects on kidney (Chen et al., 2011). In addition, the fruits of P. fortuneana can be used as nutritional supplement. It is reported that the moisture content of fresh fruits of P. fortuneana is above 60%, which is close to the moisture content of most fruits on the market (>65%), the ash content is 1.83%, the amino acid content is 2.80 g/kg. The crude fat content of the fruits of P. fortuneana is 1.30%, higher than apples and macaques (Chen and Tan, 2021). Essential trace elements are required by man in amounts ranging from 50 μg to 18 mg per day. Acting as catalytic or structural components of larger molecules, they have specific functions and are indispensable for life (Mertz, 1981). In He' study, it has determined the trace elements contents in the fruits of P. fortuneana by ICP-MS, and found that 16 trace elements were detected, including Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu (He et al., 2018). What is more prevalent in the case is that the contents of trace elements in the leaves of P. fortuneana were higher than that in the fruits of P. fortuneana (592 vs. 104 μg/kg) (He et al., 2019). Compared with white radish, the presence of Cu in the fruits of P. fortuneana is 9 times and that of Zn is 3 times, Fe is 2 times, and Mn is 2 times (Huang, 2014). The metal elements in the fruits of P. fortuneana were determined by flame atomic absorption spectrometry, and the content of metal elements was Mg > Fe > Zn, while another experiment showed a different result of Fe > Mg > Zn (Chu et al., 2015; Gao Z. Y. et al., 2019). It is reported that the fruits of P. fortuneana contains 149.40 mg/g soluble sugar, 105.00 mg/g soluble protein, 0.32 mg/g Vitamin C (Vc), 3.34 μg/g anthocyanin, 11.88 mg/g polyphenol, 2.10 mg/g total flavonoid, and the peroxidase activity was 0.16 μg/min (Han and Zhang, 2019). Four species of P. fortuneana in Guizhou were analyzed by the anthrone colorimetric method, 2,6-dichlorophenol-indophenol titration method and phenolphthalein indicator method. The related results showed that the soluble sugar content in the fruits of Pyracantha crenulata variant was the highest (13.02%), and the results also showed that the Vc content and organic acid content in the fruits of P. crenulata were the highest (696.9 mg/kg and 0.84%) (Fu, 2014). The nutritional value of P. fortuneana leaves varies greatly in different seasons. The newly sprouted leaves in spring has the best nutritional value, with a crude protein content of 15.5%, the crude fat content of 3.5%, Ca content of 1.01%, total phosphorus content of 0.83%, and carotene content of 1,025 μg/kg (Zhou et al., 2017). It has many functions contributing to nutritional and healthy food, medicine, cosmetics, daily necessities, bonsai cultivation, and ecology, etc., and its comprehensive utilization value is high and has a broad market value (Huang and Fu, 2014).
This study summarized the research progress of the functional components of P. fortuneana and their extraction process, pharmacological effects, industrial development, and utilization discovered in the recent years, which would provide research basis and reference as an important raw material for many functional foods and medicines that can be prepared from this plant.
The edible and medicinal properties of P. fortuneana have long been proven by practice in the producing areas. The Yi people in Liangshan have a tradition of using the fruits of P. fortuneana as a medicine and food (Wang et al., 2020). The fruit of P. fortuneana, first recorded in “Southern Yunnan Materia Medica,” has been used in folk medicine with various medicinal functions, such as “invigorating the spleen and eliminating accumulation, promoting body fluid and quenching thirst, clearing heat and detoxifying, promoting blood circulation, and stopping bleeding” (Zeng et al., 2016), and it can be used to treat lumps in the chest, food accumulation, metrorrhagia, postpartum blood stasis, and other symptoms, but also eliminates insects and boost eyesight (Hou et al., 2002). Besides, the root of P. fortuneana can also be used as medicine in folk medicine with the functions of “upward diarrhea, hemostasis, dispersing stasis, and eliminating accumulation.” It can be also used for hemorrhoids bleeding, red eyes, swelling and pain, wind-fire toothache, bruises, and other symptoms (Luo et al., 2014). In some areas of China, the leaves of P. fortuneana can be used as tea, which can “clear heat and detoxify, promote body fluid and quench thirst, and relieve diarrhea” (Wang and Deng, 1988).
The P. fortuneana is an evergreen shrub, up to 3-m tall; lateral branches are short thorn-like at the apex; young branches are covered with rust-colored pubescent hairs; old branches are dark brown and glabrous; and buds are small and covered with pubescent hairs (Tang and Liu, 2002). Leaf–blade is obovate or obovate–oblong, 1.5–6 cm long, 0.5–2 cm wide, apex rounded or slightly concave, sometimes with short cusps, base cuneate, extending down to petiole, margin with blunt serrations, teeth of the tip is curved inward, nearly the base is entire, and both surfaces are glabrous; the petiole is short, glabrous, or pubescent when young (Su, 2019). Flowers are integrated into compound corymbs, 3–4 cm in diameter pedicels and common pedicels nearly glabrous, pedicels about 1-cm long; flowers about 1 cm in diameter; calyx tube campanulate, glabrous; sepals triangular-ovate, apex obtuse; petals are white, nearly round, about 4-mm long, and 3-mm wide; stamens are 20 in number, filaments 3–4-mm long, anther is in yellow color; styles 5 in number and free, as long as stamens, densely white pubescent on the upper part of the ovary. The fruit is nearly spherical, about 5 mm in diameter, orange–red or dark red. The flowering period is during March–May, and the fruiting period is during August–November (Editorial Committee of Flora of China and Chinese Academy of Sciences, 1973). The flowers and fruits of P. fortuneana are shown in Figure 1.
The mature fruits of P. fortuneana has a strong aroma, sweet and sour, and a unique flavor. It can be eaten fresh, or it can be dried and ground to replace grain. It is often called “military grain” and “life-saving grain.” Because of its dense branches and leaves, orange or bright red cones are densely packed and covered with branches. Distributed in groups by the hillside ditch, it is also known as “red son” and “torch fruit” (Li et al., 2012; Gao, 2020). The general geographical distribution of P. fortuneana shows in Figure 2.
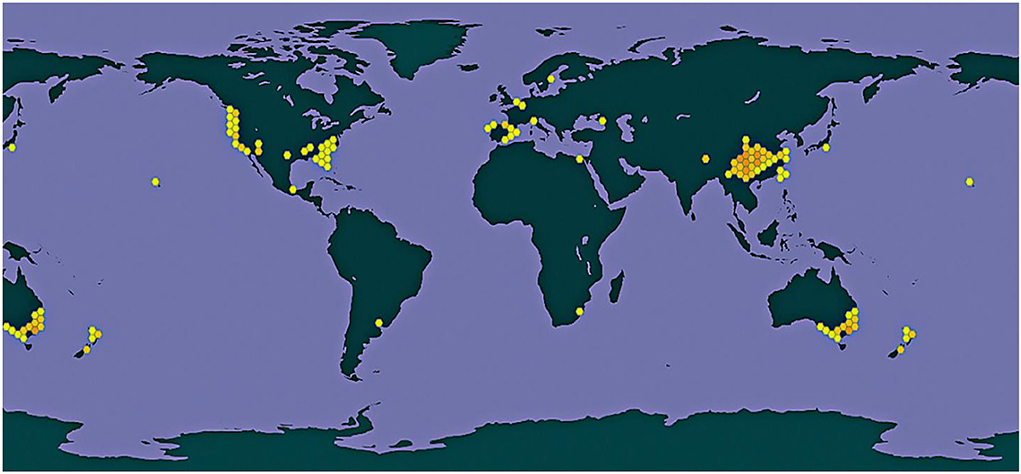
Figure 2. General geographical distribution of P. fortuneana (Maxim.) Li in the world [Color figure can be viewed at wileyonlinelibrary.com].
The recent researches shows that P. fortuneana is rich in nutrients, mainly including flavonoids, terpenes, phenolic acids, glycosides, carbohydrates, and steroids (Sharifi-Rad et al., 2020). Until now, more than 220 compounds have been isolated and identified from this plant. In this review, the constituents in this plant were comprehensively reported, including phenolic acids, flavonoids, anthocyanins, carbohydrates, terpenes, steroids, alkaloids, alkanes, esters, and phenylpropanoids. In this section, the identified constituents of P. fortuneana are listed in the tables and the corresponding structures are also comprehensively presented (Tables 1–4, Figures 3–6).
Phenolic compounds are the secondary metabolites synthesized by plants, they are present in everyday foods such as fruits and vegetables, contributing to the unique sensory and organoleptic properties; for instance, color, astringency, and taste of the fruits and vegetables. Phenolic compounds are associated with atherosclerosis, cardiovascular disease, antioxidants, cancer prevention, and neurodegenerative diseases (Chhikara et al., 2019; Laganà et al., 2020). Dai et al. isolated a series of biphenyl glycosides and acylphloroglucinol glycosides from the fruits of P. fortuneana, of which seven glycosides had mild tyrosinase (TYR) inhibitory activity (Dai et al., 2006, 2008, 2009). The inhibitory activity of biphenyl glycosides possibly arose from the chelating to copper ion in the active site of the TYR (Yuan et al., 2015b). It is reported that the fruits of P. fortuneana also contains 9-hydroxyeriobofuran (12), 5,7-dihyroxychromone7-β-D-glucoside (13), arbutin (14), cimidahurinine (15), eugenol (16), Vanillin (17), and 3-hydroxyphloretin 2'-O-glucoside (18) (Zhu et al., 2013; Ge et al., 2014; Shim et al., 2020; Sharifi-Rad et al., 2021). Among them, arbutin treatment fends off glucocorticoid-induced osteoporosis, partly through promoting differentiation and mineralization of osteoblasts by autophagy activation (Zhang Y. et al., 2021). Eugenol inhibited the complex I activity of the mitochondrial respiratory chain in the oxidative phosphorylation pathway by binding to nicotinamide adenine dinucleotide dehydrogenase chain 2 and resulted in the death of mites (Shang et al., 2021). Vanillin enhanced liver regeneration in thioacetamide-induced liver damage model, targeting growth factors of hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF), and cellular proliferation marker of cyclin D1 (Ghanim et al., 2021).
It is reported that the fruits of P. fortuneana contains both hydroxybenzoic and hydroxycinnamic acid derivatives, which are the two major categories of phenolic acids in plants. These phenolic acids are present in free or conjugated form, commonly as simple esters with quinic acid or glucose (Mattila and Kumpulainen, 2002; Russell et al., 2009). Among these hydroxybenzoic acids, gallic acid (20), 4-hydroxybenzoic acid (21), 3-O-methylgallic acid (23), 3-hydroxybenzoic acid (27), 2,3-dihydroxybenzoic acid (29), veratric acid (39), and their glycoside derivatives have been reported in the fruits of P. fortuneana. Hydroxycinnamic acids include m-coumaric acid (24), p-coumaric acid (25), caffeic acid (31), ferulic acid (33), 5-p-coumaroylquinic acid (41), and their glycoside derivatives. In addition, phenolic acids formed by the condensation of hydroxycinnamic acid and hydroxybenzoic acid have also been found, such as chlorogenic acid (19), 1,5-dicaffeoylquinic acid (30), and 3-p-coumaroylquinic acid (35) (Otsuka et al., 1981; Gan et al., 2012; Xu et al., 2016; Belwal et al., 2019; Wang et al., 2019; Sharifi-Rad et al., 2021). Chlorogenic acid attenuates dextran sulfate-induced ulcerative colitis in mice by reducing tissue inflammation and apoptosis (Gao W. et al., 2019). Ellagic acid protects against 3-nitropropionic acid induced mitochondrial dysfunction and oxide-nitrosative stress in the brain (Sharma et al., 2021; Zhang Q. et al., 2021). Ferulic acid potently improved hepatic fibrosis via inhibition of the TGF-β1/Smad pathway in vitro and in vivo (Mu et al., 2018).
Proanthocyanidins are also important components of P. fortuneana polyphenols. Using the fruits of the wild P. fortuneana as material, proanthocyanidins were extracted with an ethanol solution, the yield could reach 93.446 mg/g (Yang et al., 2011). The highest yields of flavonoids and proanthocyanidins were 3.47 and 4.63%, and the yield of polyphenols was 3.86% by using the fruits of P. fortuneana from different origins and different harvest periods as raw materials (Li et al., 2008, 2020). Proanthocyanidins isolated from P. fortuneana were predominantly constituted of procyanidin with A-type and B-type linkages, including (+)-catechin (47), (+)-epicatechin (51), gallocatechin (49), and epigallocatechin (50). Besides, P. fortuneana also contains proanthocyanidins glycosides such as catechin–glucosides (48) and epicatechin-3-glucosides (52) (Zhao et al., 2015; Wei et al., 2017). The content of polyphenols in unripe fruits is high, and as the fruit matures, the phenolic substances are oxidized and the level of anthocyanin increases; for example, cyanin (45) and delphinidin (46) (Wei et al., 2017; Peng et al., 2019). The stability of proanthocyanidins in P. fortuneana during processing and storage was investigated. The processing and storage temperature was lower than 80°C, and the stability of proanthocyanidins was good in the pH value ranging from 3 to 7; VC, sodium citrate and sodium bisulfite were helpful to maintain the stability of proanthocyanidins; Cu2+ is not conducive to its stability, and Fe3+ has an obvious destructive effect; natural light is not conducive to the stability of proanthocyanidins, and ultraviolet light is very destructive (Liu et al., 2015). The chemical constituents of phenols and their corresponding structures are depicted in Table 1 and Figure 3.
Flavonoids, commonly found in plants, are a class of polyphenolic compounds with the basic structural unit of 2-phenylchromone, and flavonoids have attracted much attention due to their wide range of biological applications (Wen et al., 2021). Modern studies have shown that flavonoids have pharmacological effects such as the treatment of diabetic retinopathy, anti-tumor, and hypolipidemic effects (Luo et al., 2021; Xuan et al., 2021; Xu et al., 2021). To date, more than 30 flavonoids have been isolated from the P. fortuneana. The flavonoid profiles of P. fortuneana at different ages showed obviously differences in aerial and hypogeal parts. In the vegetative phase, there are flavonoids (flavanones, flavones, and flavonols) only in the aerial parts and they appear gradually during the plant life (Fico et al., 2000).
The flavonoids reported in P. fortuneana include quercetin (53), apigenin (54), kaempferol (55), chrysin (57), hyperoside (59), vitexin (68), naringenin (71), pyracanthoside (75), eriodictyol (76), and hexaacetylpyracanthoside (87) in the leaves; the 5,7,2',5'-tetrahydroxyfavanone (60), myricetin (62), isoorientin (69), dihydrokaempferol (72), dihydroquercetin (74), sakuranetin (77), pinocembrin (78), sakuranin (80), and dihydrowogonin (81) in the roots (Fico et al., 2000). The 7,4'-dihydroxyflavone (66), cosmosiin (70), phloridzin (84), and their glycoside derivatives in the leaves of P. fortuneana also reported in small quantities (Table 2, Figure 4; He et al., 2011; Shim et al., 2020; Sharifi-Rad et al., 2021).
Quercetin can be used to treat diabetes, hyperlipidemia, and non-alcoholic fatty liver disease (Yi et al., 2021). The content of quercetin in different parts of P. fortuneana varies greatly. It was reported that no Quercetin was detected in the root of P. fortuneana, while the content of quercetin in the fruit was 4 times that of the leaves (Gan et al., 2012). Apigenin could suppress neovascularization, and it has antiapoptotic and antioxidative effects in an oxygen-induced retinopathy mouse model, and can be considered as a promising agent for treating ocular neovascular diseases (Sarigul Sezenoz et al., 2021). Eriodictyol can produce antidepressant-like effects and ameliorate cognitive impairments induced by chronic stress (Zhang et al., 2020). Chrysin has also been proven in various diabetic complications, such as retinopathy, nephropathy, neuropathy, and cardiomyopathy, with the pathogenesis generally linked to hyperglycemia-induced oxidative stress and inflammation and apoptosis (Farkhondeh et al., 2019). Kaempferol supplementation showed bone-sparing effects in newborn rats, glucocorticoid-induced and ovariectomy-induced osteoporotic models as well as bone fracture models (Wong et al., 2019).
The volatile components of P. fortuneana are diverse in different precious investigations, mainly including aldehydes, ketones, alcohols, alkanes, alkenes, esters, and terpenes. More than 60 compounds were identified from the flower volatile oil of P. fortuneana by gas chromatograph–mass spectrometer (GC–MS), accounting for 83.77% of the total volatile oil, containing a variety of biologically active components, including terpenes and their oxygenated derivatives (50.31%), alkanes (18.52%), and aldehydes (5.54%) (Ge et al., 2014). A total of 32 compounds were identified from the volatile oil of fruits of P. fortuneana, accounting for 71.97% of the total volatile oil, mainly terpenes and alkanes (Wang et al., 2013). A total of 69 compounds were identified from the volatile oil of leaves of P. fortuneana, accounting for 80.52% of the total volatile oil (Zhu et al., 2013).
Terpenoids refer to olefinic compounds whose molecular formula is an integer multiple of isoprene. They are natural hydrocarbons that widely exist in plants. Many terpenoids have important physiological activities and are important for the research of natural products and the development of new drugs source (González-Burgos and Gómez-Serranillos, 2012; Peng et al., 2022). The previous studies have showed that the different growing parts of the P. fortuneana contain different terpenoids. Terpenoids in flowers of the P. fortuneana include isoledene (91), isophorone (92), farnesol (132), farnesyl acetone (133), and trans-nerolidol (152) (Ge et al., 2014). Terpenoids endemic to the fruits of P. fortuneana include isoterpinolene (101), menthol (102), and bicyclosesquiphellandrene (103) (Wang et al., 2013). It is reported that the bright color of the fruits of P. fortuneana is mainly due to its rich content of carotene (100) and lycopene (140) (Humbeck et al., 1989). In addition, the leaves of P. fortuneana also contains megastigmatrienone (104), α-calacorene (105), α-ionone (106), α-muurolene (107), α-pinene (108), α-terpinene (109), β-cubebene (110), and isophytol (148) (Zhu et al., 2013). It is reported that Jasmone (94), trans-squalene (101), and geraniol (151) present in both flowers and leaves of P. fortuneana (Zhu et al., 2013; Ge et al., 2014). The terpenes present in flowers, fruits and leaves of the P. fortuneana are mainly α-Cubebene (93), α-terpineol (96) and β-ionone (97), respectively (Wang et al., 2013; Zhu et al., 2013; Ge et al., 2014). Co-administration of geraniol with methotrexate may attenuate methotrexate-induced acute kidney injury (Younis et al., 2021). The β-ionone could prevent stress-induced skin aging via inhibition of GR signaling in human dermal fibroblasts (Choi et al., 2021). The α-pinene activates natural killer (NK) cells and increases NK cells cytotoxicity, suggesting it is a potential compound for cancer immunotherapy (Jo et al., 2021).
The alkanes in P. fortuneana include octane (116), n-nonane (117), decane (118), n-pentadecane (119), docosane (120), tricosane (121), pentacosane (123), tetracosane (127), and other straight-chain alkanes (Wang et al., 2013; Zhu et al., 2013; Ge et al., 2014; Wang C. F. et al., 2016). Among these aldehydes, ketones, and alcohol components, benzyl alcohol is mostly as fragrance ingredients and preservatives in cosmetic products (Johnson et al., 2017). It was reported that linalool odor-induced analgesia was triggered through a TRPA1-independent pathway in mice (Kashiwadani et al., 2021). Decanal could protect human dermal fibroblasts against UVB-induced photoaging via the cAMP pathway (Kang et al., 2020), and the 2-hexenal inhibit Aspergillus flavus spore germination involving disruption of mitochondrial energy metabolism and induction of early apoptosis (Ma et al., 2019). The volatile esters in P. fortuneana are mainly benzoates, such as benzyl benzoate (154), benzyl salicylate (155), hexyl benzoate (156), and methyl salicylate (157), and fatty acid derivatives, such as ethyl linolenate (161), octyl formate (165), and heptacosanoic acid methyl ester (166) (Zhu et al., 2013; Ge et al., 2014; Wang C. F. et al., 2016). The main components of fruit oil in P. fortuneana are methyl linoleate (158) (18.7%), methyl linolenate (159) (22.8%), methyl palmitate (162) (20.3%), and methyl stearate (163) (4.6%), and interestingly, the content is higher than other common edible oils and fats currently on the market (Wang C. F. et al., 2016). The chemical constituents of volatile constituents and their corresponding structures are exhibited in Table 3 and Figure 5.
Besides compounds mentioned above P. fortuneana also contains other compounds such as polysaccharides, dietary fiber, phytosterols, and organic acids (Table 4, Figure 6). The polysaccharides in P. fortuneana have various bioactivities such as antimutagenic, immunomodulatory, and antioxidant effects. It was reported that PP-A2, PP-A3, and PP-A4; and PP-B1, PP-B2, and PP-B36 fractions were separated from the fruits of P. fortuneana by fractional precipitation, DE-52 column chromatography. Separated by SephadexG-200 column analysis and identification, PP-A2, PP-A3, and PP-B2 are homogeneous polysaccharides (Yang et al., 2004). The molecular mass of PP-A2 is 30 kDa, and its monosaccharide component is mainly a heteropolysaccharide composed of arabinose (168) and rhamnose (170), which also contains a small amount of mannose (171), galactose (173), and fructose (175). The ratio of the PP-A2 substances is as follows: (Arabinose + Rhamnose):Fructose:Galactose:Mannose = 9.56:3.37:1.88:1 (Huang et al., 2007b). Also, PP-A3 is a glycoprotein with a molecular mass of 210 kDa, mainly composed of arabinose, which contains a small amount of glucose (172), and fructose. The ratio of the amount of PP-A3 is Arabinose:Glucose:Fructose = 1:62:1:1.59, in which the total amino acid content is 0.5% (Huang et al., 2007a). High-performance liquid chromatography (HPLC) analysis revealed that Se-conjugated polysaccharides from P. fortuneana (Se-PFPs) were heteropolysaccharides composed of xylose (167) (29.8%), arabinose (23.2%), fucose (169) (25.7%), mannose (4.1%), glucuronic acid (176) (2.9%), galacturonic acid (177) (3.2%), ribose (178) (1.8%), rhamnose (3.8%), glucose (2.2%), and galactose (3.3%), respectively (Yuan C. et al., 2016).
Dietary fiber is described as supporting laxation, attenuating blood glucose responses and assisting with cholesterol-lowering. The different types of dietary fiber have various effects (Fuller et al., 2016). The monosaccharide components of the dietary fiber from P. fortuneana are mainly xylose, arabinose, rhamnose, and sorbose (174), and also contain a small amount of fructose and mannose, of which the content of xylose is the highest (33.56%). The physicochemical properties of dietary fiber of P. fortuneana fruit showed that P. fortuneana dietary fiber exhibited good water and oil holding capacity, and also had strong absorption capacity for glucose, while in terms of solubility and swelling were relatively insufficient (Han et al., 2015).
The lipid-soluble vitamins and phytosterol levels of P. fortuneana extracts were δ-Tocopherol (179) (0.13 mg/kg), α-tocopherol (180) (31.02 mg/kg), vitamin K (181) (0.68 mg/kg), and vitamin D (182) (4.13 mg/kg). Moreover, β-sitosterol (183) (122.25 mg/kg), ergosterol (184) (4.57 mg/kg), stigmasterol (185) (7.25 mg/kg), 4,5-epoxycholestane (186), cholesterol (187), cholic acid ethyl ester (188), and stanozolol (189) are the phytosterols found in P. fortuneana (Keser, 2014; Wang C. F. et al., 2016). Vitamin E in P. fortuneana seed oil is positively correlated with soil Ca and Mg content, as well as linoleic acid and soil phosphorus content (Tang et al., 2007).
Lipids are one of the important nutrients that the human body needs, and they provide the energy and essential fatty acids that the human body needs (Yoon et al., 2021). The major fatty acids in the P. fortuneana extract were linolenic acid (196) (45.91%), quinic acid (202), malic acid (203), palmitic acid (206) (29.10%), caproic acid (208), decanoic acid (209), myristic acid (211), and trans-2-hexenoic acid (215) (Wang et al., 2013; Zhu et al., 2013; Ge et al., 2014). Phospholipids have the functions of improving nerve dysfunction, enhancing memory and anti-aging (Tayebati, 2018). A total of six phospholipid components were detected from the fruits of P. fortuneana, and phosphatidic acid (190) was the main component that is accounting for more than 80% of the total phospholipids (Dong, 2002).
Besides, P. fortuneana also contains a small number of phenylpropanoid compounds such as coumarin (216), 4-hydroxybenzaldehyde (217), and Schisandrol B (218) (Sharifi-Rad et al., 2021). Alkaloids contain Cinchonain I (220), indole (221), and stearic acid hydrazide (222) (Wang et al., 2013, 2019; Ge et al., 2014).
The pharmacological effects of P. fortuneana are mainly reflected in the aspects of immune regulation, TYR inhibition, anti-oxidation, anti-tumor, inflammatory, and antimutagenic effects (Table 5, Figures 7–9).
The peroxide value (POV) of oil determined by the oven storage method showed that the antioxidant activity of total flavonoids of P. fortuneana fruit was higher than Vc and citric acid (Han and Zhang, 2019). Both crude extracts and purified flavonoids from the fruits of P. fortuneana could increase the antioxidant capacity of various vegetable and animal oils and all had a dose–effect relationship with the concentration. The flavonoids from P. fortuneana can inhibit the hydrolysis of oil rancidity into free fatty acids (Wang et al., 2015a). The same effect was also observed in the polysaccharide from P. fortuneana (Wang X. J. et al., 2016). The content of flavonoids in the fruits of P. fortuneana with lower maturity is higher. In the auto-oxidation system of 2,2-diphenyl-1-picrylhydrazyl (DPPH) and pyrogallol, the flavonoids from P. fortuneana have a significant scavenging effect on DPPH• and superoxide free radicals(). The antioxidant activity of the purified flavonoids from fruits of P. fortuneana was enhanced compared with that before purification (Li and Chen, 2013). Also, P. fortuneana flavonoids have stronger scavenging effects on 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and DPPH• than propyl gallate (Li W. et al., 2013). The statistical analysis shows that flavonoids from P. fortuneana and VC compounds have synergistic antioxidant effects in inhibiting lipid oxidation, scavenging hydroxyl radicals (OH−) and . In addition, the combination of Vc and flavonoids from P. fortuneana have superior antioxidant activity than VC or flavonoids alone. When the mass concentration was 0.25 mg/ml, the protection rates of flavonoids from P. fortuneana, flavonoids from P. fortuneana + VC were 93.20 and 93.62% for vegetable oils; and 70.20 and 75.28% for animal oils, respectively. When the mass concentration is 0.65 mg/ml, the scavenging rates of the compound solution to OH− and were 27.61 and 31.02% (Wang et al., 2015b).
The extracts of different polar parts from the fruits of P. fortuneana have antioxidant activities, and the antioxidant activities of the extracted parts have a good correlation with the total polyphenol content. Among them, the water fraction extract DPPH• and ABTS+ had the highest free radical scavenging rates, with IC50 values of 0.76 ± 0.03 mg/ml and 1.71 ± 0.10 mg/ml, respectively, the ferric ion reducing antioxidant power (FRAP) value of the ethyl acetate site was the highest, 382.20 ± 4.72 μmol Fe2+/g dry sample; The total phenolic content of the n-butanol was the highest, which was 2,763 ± 3.91 mg GAE/100 g dry sample (Weng and Gao, 2015). The scavenging activity of the ethanol, methanol and acetone extracts of P. fortuneana on ABTS+ is higher than butylated hydroxytoluene (BHT). The scavenging activity of the methanol and ethanol extracts of P. fortuneana on DPPH• and OH− is higher than BHT (Keser, 2014). The strongest DPPH•, ABTS+ scavenging activity and Fe3+ reducing power were observed in the 50% acetone extract of P. fortuneana with IC50 values of 0.61, 0.74, and 2.98 mg DM/ml (Wang et al., 2019).
As shown in Figure 8, the extracts from the fruits of P. fortuneana extract alleviates oxidative stress in the D-gal and bromobenzene-injured model mouse via enhancing endogenous antioxidant activity. It can enhance the activity of glutathione peroxidase (GPx) and superoxide dismutase (SOD), inhibit the increase of alanine aminotransferase (ALT), malondialdehyde (MDA), and the aspartate aminotransferase (AST) (Xu et al., 2016). Another experiment showed adding the fruit powder of P. fortuneana to the diet can reduce the increase of MDA content in heart, liver and brain of D-gal induced mice. In addition, the fruit administration oraly also enhanced SOD activity of red blood cells, liver and brain, increased GPx, and glutathione (GSH) in whole-blood and liver, and improved thymus gland index (Hou et al., 2003b). In addition, the fruit powder treatment can also reduce the activity of monoamine oxidase (MAO) in the hippocampus of mice (Qin and Wei, 2011). The P. fortuneana fruit extract (PFE) has a certain protective effect on liver injury caused by restraint stress treatment via scavenging reactive oxygen species (ROS) and inhibiting lipid peroxidation (Xu et al., 2007).
The polysaccharides from P. fortuneana (PFPs) (10 mg/ml) had a scavenging rate of 67.13% against metal ions; the scavenging effect of PFPs against DPPH was 93.5% at 8 mg/ml; in addition, the PFPs also showed scavenging activity against ABTS+ radical and at concentration of 1–4 mg/ml (Yao et al., 2020). Yuan et al. reported that PFPs significantly increase splenocyte GPx and SOD activities and reduce MDA levels (Yuan et al., 2010). In addition, it is reported that PFPs can effectively scavenge and OH−, and inhibit Fe2+-H2O2-induced lipid peroxidation of liver homogenate in healthy mice, and significantly reduce the level of MDA in the liver of CCL4 liver-injured mice. The liver homogenate of the mice in the PFPs-treated group can significantly inhibit the lipid peroxidation induced in vitro, indicating that PFPs has the effect of scavenging oxygen free radicals and resisting lipid peroxidation (Zhao et al., 2012).
The antioxidant activity of P. fortuneana is also related to the non-extractable polyphenol (NEPP). The proportion of NEPP in total extractable polyphenol (TEPP) from P. fortuneana was relatively high as to 80.55%. The antioxidant capacity of ABTS+ and FRAP was positively correlated with the content of NEPP (Xu et al., 2015). The antioxidant capacity contributed by the proanthocyanidins in the fruits of P. fortuneana (PYFP) accounts for a large proportion of the total antioxidant capacity of its antioxidant extract (Yan et al., 2015). The previous reports showed that PYFP had strong scavenging effects on DPPH• and ABTS+ cationic free radicals (Zhang et al., 2014). In addition, PYFP can enhance the stability of small molecular polyphenols such as quercetin, improve bioavailability, and thus exert the antioxidant effect of co-ketones (Zhao et al., 2015). The red pigment from the fruits of P. fortuneana (RPP) purified by C18 Sep–Pak column has IC50 of 1.43, 3.13 mg/ml, and 3.43 g/ml for scavenging OH–, , and DPPH, respectively (Li P. X. et al., 2013).
Also, volatile oil from the fruits of P. fortuneana (VOPF) also has obvious scavenging effects on DPPH, and the scavenging effect of VOPF on sodium nitrite is better than BHT (Ge et al., 2014). According to the total reducing power and the scavenging ability of ABTS+ free radicals, the VOPF has a certain antioxidant activity, and the antioxidant activity has a significant dose-effect relationship with the sample amount (Wang et al., 2013). In addition, P. fortuneana oil has good Fe2+ reducing ability, and its antioxidant activity increases with the increase of sample concentration (Wang C. F. et al., 2016).
As shown in Figure 9, the anticancer effect of P. fortuneana is mainly accomplished by promoting the apoptosis of cancer cells and inhibiting the migration of cancer cells (Figure 9). It was reported that polysaccharides from the fruits of P. fortuneana (PFPs) possessed cytoxcities on the human ovarian carcinoma Skov3 cells via induction of apoptosis by upregulating Bax and caspase-3. Furthermore, PFPs can increase ROS, decrease mitochondrial membrane potential (MMOP), and damage DNA (detected as γ-H2AX and RAD51 foci) (Yao et al., 2020). Another report by Sun et al. reported that selenium (Se)-PFPs can induce the apoptosis of ovarian cancer cell lines (HY and Skov3) and inhibit their migration and invasion via downregulating cyclin D1, Bcl-2, and matrix metalloproteinase (MMP) 9; upregulating the cleavage (C) of poly ADP-ribose polymerase (PARP) and C-caspase-3; and enhancing the activities of caspase-3 and−9. Furthermore, Se-PFPs inhibited epithelial to mesenchymal transition (EMT) of cancer cells by upregulating E-cadherin and cytokeratin 19, and downregulating N-cadherin, vimentin, zinc finger E-box binding homeobox (ZEB1)-1, and ZEB2. The further in vivo experiments also showed that Se-PFPs can reduce the β-catenin both in cytoplasmic and nuclear, and increased the phosphorylation of β-catenin (Sun et al., 2016). Li et al. reported that PFPs-iron complex (PPI) can induce apoptosis of Skov3 cells via reducing MMOP and increasing ROS, downregulating Bcl-2 and upregulating Bax. What's more, PPI can also result in DNA damage via upregulating γ-H2AX and RAD51 (Li et al., 2021).
Another experimental result showed that Se-PFPs (containing 93.7% of PFPs, 2.1% of uronic acid, and 3.7 μg/g of Se) can inhibit triple negative breast cancer MDA-MB-231 cell growth dose-dependently by inducing G2 phase cell arrest via inhibiting CDC25C-CyclinB1/CDC2 pathway. In addition, Se-PFPs caused cancer cell apoptosis via upregulating p53, Bax, Puma, and Noxa, downregulating Bcl2, and increasing activities of caspases-3 and−9. Furthermore, the antitumor effects of the Se-PFPs were confirmed using a in vivo mouse xerograph model (Yuan C. et al., 2016).
Cyclophosphamide (CTX) was commonly used to induce experimental animal immune system damage. It is found that the red pigment from the fruits of P. fortuneana (RPP) has a good protective effect on the immune organs of the immunosuppressed mice induced by CTX, and can improve the phagocytic function of the peritoneal macrophages in immunosuppressed mice. What is more interesting is that RPP can also promote the formation of serum hemolysin and hemolytic plaques (Xu and Zha, 2013). Furthermore, PFPs can significantly increase the thymus and spleen index of mice, and promote splenocyte proliferation and increase the NK cell activity. The PFPs can increase the number of CD4 T cells and CD4+/CD8+ ratio, as well as the interleukin-2 (IL-2) levels, reduce the levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). In addition, PFPs could upregulate the nuclear factor E2-related factor (Nrf2) in splenocytes (Yuan et al., 2010). The combined use of Se-enriched green tea polysaccharides and PFPs (Se-GTP+PFPs) has stronger immunomodulatory effects than PFPs alone. Besides increasing indices of spleen and thymus and NK cell activity and upregulating Nrf2, Se-GTP+PFPs can decrease the IL-6 and TNF-α. The Se-GTP+PFPs can also enhance the activities of GPx and SOD, and reduce the content of MDA in mice, suggesting that the combined administration of Se-GTP and PFPs can synergistically improve immune function and decrease oxidative stress by enhancing the clearance of free radicals (Yuan et al., 2015a).
Extracts from the fruits of P. fortuneana (PFE) can significantly inhibit TYR activity (IC50 = 32.28 μg/ml) and histamine release from rat abdominal mast cells. The PFE can reduce the content of melanin in mouse B16 cells (IC50 = 82.47 μg/ml), and have an inhibitory effect on TYR activity in B16 cells (IC50 = 53.48 μg/ml) (He et al., 2011). Using bioactivity-guided fractionation to screen constituents with alleviating effects against melanogenesis and oxidation from the fruits of P. fortuneana, seven compounds with inhibitory melanin production and TYR activity, ABTS+ and DPPH scavenging activities were isolated from the n-butanol extract of the fruits of P. fortuneana. Among them, p-hydroxybenzoic acid β-D-glucosylester (HG), and cimidahurinine (CH) have strong inhibitory effects on melanogenesis and TYR activity as well as ABTS+ and DPPH• scavenging activities. Furthermore, HG and CH can also inhibit the ROS production of B16F10 cells induced by tert-butyl hydroperoxide (TBHP), and western blot analysis revealed HG and CH downregulated the tyrosinase-related protein (TYRP)-1 and−2 (Shim et al., 2020). In 2015, Lin et al. reported that a new compound 13, named 3,4-dihydroxy-5-methoxybiphenyl-21-O-β-D-glucopyranoside, isolated from the P. fortuneana had powerful TYR inhibitory activity in human epidermal melanocytes to achieve the effect of skin whitening via downregulating the expressions of TYRP, microphthalmia-associated transcription factor (MITF), and paired box 3 (PAX3) (Lin et al., 2015).
The Se-PFPs have protective effects on CCl4-induced liver injury, and reduced the CCL4 induced increase of ALT, AST, lactic dehydrogenase (LDH), cholesterol, and triglycerides in serum of mice. The Se-PFPs treatment elevated the activities of SOD, GPx, and levels of GSH in liver, and decreased the levels of thiobarbituric acid reactive substances (TBAR) and H2O2 in liver which served as lipid peroxidation biomarkers, indicating that Se-PFPs can attenuate the CCl4-induced liver injury. The mechanism underlying this effect mentioned above may be attributed to the reduction of oxidative stress and inflammation in the liver by Se-PFPs through increasing antioxidant system (Yuan et al., 2015b). Also, it is reported that PFPs can increase GPx and total antioxidative capacity (T-AOC) in liver tissue of ClC4-induced mice, reducing MDA, and upregulating Nrf2 (Yuan C. et al., 2016).
It was reported that proanthocyanidins from the fruits of P. fortuneana (PYFP) had an inhibitory effect against α-glucosidase (IC50 = 0.15 ± 0.01 μg/ml) in a non-competitive type (Wei et al., 2017). Wang et al. investigated the inhibitory activities of different fractions from the fruits of P. fortuneana, and found that 50 and 70% acetone extracts of the fruits of P. fortuneana had the highest inhibitory activity against α-glucosidase with the IC50 values of 0.37 and 0.35 mg DM/ml, respectively (Wang et al., 2019).
Ke et al. reported that PFE can alleviate acute nephrotoxicity induced by cadmium chloride (CdCl2) in rats. The PFE (250 mg/kg) increased the CdCl2 reduced body weight, antioxidant enzymes in kidney tissue including glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione peroxidase (GR) in rats. The PFE treatment alleviated inflammation and apoptosis in renal tissue of rats induced by CdCl2 via increasing Bcl-2, NQO1, γ-GCS, HO-1, and Nrf2, and decreasing Bax, Keap-1, and TNF-α in kidney tissue, indicating protective activities of PFE in renal tissues might be achieved through the Nrf2/Keap-1 pathway (Ke et al., 2019). Xu et al. reported that 5% PFE treatment reduced body weight, triglyceride (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL) and increases high-density lipoprotein cholesterol (HDL) in high-fat and high-cholesterol diet-fed rats. The PFE exerted higher LDL receptor (LDLr)-binding activity than single compounds in HepG2 cells (Xu et al., 2016). The intragastric administration of PFE can significantly shorten the glass slide blood coagulation time of mice. In vitro, the PFE fractions can significantly shorten the plasma recalcification time and plasma prothrombin time, and the chloroform fraction of PFE has the most significant effect in PFE (Mei et al., 2001). The PFE can inhibit the water absorption of the small intestine in normal rats, and promote the secretion of bile in rats. The results showed that PFE can increase the solids content in bile, promote the secretion of gastric juice in rats, and improve the activity of pepsin, indicating the PFE has a strong function in improving digestion and invigorating the spleen (Hou et al., 2003a). Peng et al. explored the effects of Se-PFPs on CTX-induced micronucleus formation in both bone marrow and peripheral blood, and found that Se-PFPs enhanced the activities of SOD and GPx and reduced the activity and expression of cytochrome P450 1A (CYP4501A) in mouse liver, with a dose-dependent manner. This study indicated that the Se-PFPs may provide an alternative strategy for cancer therapy by targeting CYP1A (Peng et al., 2016).
Taheri et al. reported that the oral administration of ethanol extract from the fruits of P. fortuneana (ETFP) can reduce the formalin-induced pain, and carrageenan caused paw edema in rats (Taheri et al., 2021). The extracted hydro-alcohol polyphenols of P. fortuneana (PPFE) showed excellent antibacterial potentials against both Staphylococcus aureus and Escherichia coli with the minimum inhibitory concentration (MIC) of 10 and 20 mg/ml (Sun et al., 2019). The PFE intervention could ameliorated intestinal barrier dysfunction in HFD fed rats by protecting structure integrity of intestinal barrier, reducing lactulose/mannitol ratio, inhibiting digestive enzyme activities and upregulating the tight junction proteins, as well as downregulating glucose transporter 2 (GLUT2). Furthermore, the ameliorations in intestinal barrier function were also associated with modulating Intestinal flora (Xu et al., 2019).
So far, many compounds contained in the P. fortuneana have shown certain therapeutic effects in clinical studies. Among the phenolic components, arbutin have been used to treat the uncomplicated urinary tract infections (UTIs) in clinical research; the results showed arbutin may reduce UTI symptoms and reduce antibiotic use for treating UTI (Afshar et al., 2018). Dental caries can occur on crowns and roots of the teeth, and it can appear in early childhood as an aggressive form of tooth decay. Zinc oxide eugenol has been a conventional root canal filling material for pulpectomy of primary teeth since 1930 (Najjar et al., 2019). For overweight men with diabetes, chlorogenic acid (1 g) significantly reduced glucose and insulin concentrations (−0.7 mmol/L and −73 pmol/L, respectively) (van Dijk et al., 2009). Gallic acid can prevent oxidative DNA damage and inflammation in T2D patients, and increase risk of cancer and cardiovascular diseases (Ferk et al., 2018). Ellagic acid has beneficial effects for improving sleep quality and gastrointestinal functions in irritable bowel syndrome patients (Mirzaie et al., 2021). In addition, caffeic acid tablets could be used to treat the primary immune thrombocytopenia with less side effects (Qin et al., 2015). Ferulic acid can improve lipid profile, oxidative stress, oxidized LDL-C, and inflammation in hyperlipidemia patients, and reduce cardiovascular disease risk factors (Bumrungpert et al., 2018). Caffeic acid and ferulic acid can be used for alleviating UV caused skin damage (Saija et al., 2000). Oleanolic acid treatment could reduce serum TC, TG, and HDLC in hyperlipidemia patients (Luo et al., 2018). Interestingly, the Quercetin Phytosome® was reported to be safe drug can be applied to control infection with coronavirus disease 2019 (COVID-19) in the early stages (Di Pierro et al., 2021). A long-term treatment with the mixture of apigenin and epigallocathechin gallate (1:1) can reduce the recurrence rate of colon tumors (Hoensch et al., 2008). Chrysin can reduce the irinotecan (CPT-11) induced diarrhea in cancer patient with metastatic colon cancer (Tobin et al., 2006). Vitexina has radioprotective effects on breast cancer patients receiving Co-60 radiation therapy (Hien et al., 2002). Naringenin can inhibit the inflammation of bronchitis in children, shorten clinical symptoms time, reduce complications incidence, and related adverse reactions (Yao et al., 2021). Rutin can increase dermal density and skin elasticity in women and reduce wrinkles (Choi et al., 2016). The β-eudesmol inhibits sympathetic activity in response to the acute mental stress (Ohara et al., 2018).
The fruits of P. fortuneana can be prepared into a variety of agricultural and sideline products through fermentation technology (Figure 10). The fruit wine of P. fortuneana (FFW) has the unique style with bright red color and full-bodied flavor (Nie et al., 2018; Shi et al., 2020). During the fermentation process of P. fortuneana fruit, the changing trends of different chemical components were different. The contents of polysaccharides gradually decreased, whereas the alcohol and free amino acids increased gradually. The contents of flavonoids and polyphenols first increased and then gradually decreased during the fermentation. In addition to its unique flavor, FFW also has a certain antioxidant and α-glucosidase inhibitory activities effect (Cakar et al., 2017; Duan et al., 2020). It is reported that the fermentation broth of the FFW possesses good scavenging effects on free radical of ABTS+, and the scavenging effects of FFW would be increased gradually with the prolongation of fermentation time (Jiang et al., 2013). The fruits of P. fortuneana can be also fermented with glutinous rice to obtain rice wine. For the rice wine fermented with the fruits of P. fortuneana and glutinous rice, the content of sugar, ascorbic acid, total phenols, total flavonoids, and anthocyanins was higher, and the scavenging activity of DPPH• and ABTS+ was also higher than that of rice wine fermented with glutinous rice alone (Wang et al., 2021).
Pectin is a general term for protopectin and pectate, a hydrophilic vegetable gum that widely exists in the roots, stems, leaves, and fruit cell walls of plants, such as the lacturonic acid, which is a polylinear polysaccharide with an average molecular weight between 2,000 and 40,000 (Thakur et al., 1997). Pectin has good gelling properties, emulsification stability, and high health care effects (Chan et al., 2017). The pectin can be used as a gelling and stabilizing polymer in various food and specialty products, and therefore pectin is also an excellent matrix for pharmaceutical preparations (Mohnen, 2008). It was reported that the fruits of P. fortuneana has abundant pectin, and Chen et al. extracted the pectin with a yield of 83.7% using ultrasonic extraction from the residue of the fruits of P. fortuneana after pigment extraction (Chen et al., 2009). Another research by Xiong et al. reported that pectin was extracted from the fruits of P. fortuneana by microwave method using dilute hydrochloric acid solution as extractant, and the yield was 7.5% (Xiong et al., 2014).
What is more interesting is, the fruits of P. fortuneana can be commonly prepared as drinks such as fruit juice, which possesses good taste and flavor (Cai and Ding, 1996). Besides, it is also reported that the fruit juice of P. fortuneana can alleviate liver damage in arsenic poisoned mice by scavenging free radicals (Wei et al., 2006). For the fruit juice drinks, it is reported that original juice content is above 35%, soluble solid content (Brix) is above 8%, total acid is below 0.3% (Chen et al., 2007). Interestingly, the fruits of P. fortuneana can be also prepared as lactic acid beverage with abundant nutrition. The protein content of the fruits of P. fortuneana lactobacillus beverage obtained by fermentation technology is more than or 1%, and the polysaccharide content is more than or 0.035% (Liu and Wang, 2011). In addition, the fruits of P. fortuneana can be also combined with other herbal medicines or fruits to prepare mixed beverages, such as Ganoderma lucidum and kiwi fruit (Li et al., 2016a). It is reported that the mixed drink with G. lucidum and the fruits of P. fortuneana can enhance the SOD activity (13.9%) and decrease the MDA contents (17.4) in Drosophila melanogaster, and extend the lifespan of D. melanogaster, compared to the control D. melanogaster (Li et al., 2016b). In addition, for the mixed drink of kiwi fruit and the fruits of P. fortuneana, the sensory evaluation results showed that the taste and flavor is good, and it is rich in flavonoids, polyphenols, Vc and other functional ingredients (Zhang et al., 2017).
Besides, the fruits of P. fortuneana is often used to prepare fruit vinegar, which has lots of biological activities such as anti-fatigue, lipid-lowering, and anti-oxidative stress (Halima et al., 2018; Choi et al., 2020; Kim et al., 2020). For the fruit vinegar of P. fortuneana fermented by immobilized acetic bacteria, the contents of total acid (calculated as acetic acid) and total sugar (calculated as glucose) of were 4.9 g/100 ml and 3.6 g/100 ml, respectively (Li, 2012). In addition, the acetic acid content of the fruit vinegar of P. fortuneana fermented by acetic shake flask can achieve the high value of 60.3 mg/ml (Zhou et al., 2008). The average content of γ-aminobutyric acid in the compound fruit vinegar of P. fortuneana and sprouted brown rice is 331.2 mg/L, and it also contains nine amino acids necessary for the human body, including leucine (32.42 mg/100 ml), isoleucine (15.46 mg/100 ml), lysine (3.91 mg/100 ml), methionine (8.25 mg/100 ml), phenylalanine (21.82 mg/100 ml), threonine (14.79 mg/100 ml), tryptophan (2.95 mg/100 ml), valine (923.60 mg/100 ml), and histidine (11.06 mg/100 ml) (Yuan et al., 2006). Furthermore, the food industry also often uses fruit vinegar to improve the bioavailability of fruit by-products (Luzón-Quintana et al., 2021). The fruit vinegar of P. fortuneana is rich in natural and harmonious aroma, sweet and sour taste, and is a health care fruit vinegar with nutrition (Gao, 2012).
Owing to the continuous improvement of people's living environment and the gradual improvement of urban landscaping grades, people pay increasing attention to the color beauty of landscaping plants. Color plants can achieve a harmonious unity between man and nature, which is a manifestation of people's longing for colorful plants. Also, P. fortuneana has strong adaptability and is easy to prune. It can be made into hedges, planted in green belts on roads, used in lawn arrangements, and can be also used for potted plants (Zhang and Li, 2020). For example, leaves of the P. fortuneana “Harlequin” are white, with good ornamental and ecological properties, and are commonly used in garden landscaping (Xu, 2017). Furthermore, P. fortuneana leaves can absorb and retain atmospheric particles and are also important for remediating environmental pollution (Sun et al., 2018).
Stony desertification refers to the destruction of surface vegetation by human activities under the tropical and subtropical humid and semi-humid climatic conditions and the natural background of extremely developed karst. Stony desertification resulted in serious soil loss and large areas of exposed bedrock or karst (Zhang et al., 2018). Moreover, P. fortuneana has strong adaptability and vitality and can be propagated by cuttings, sowing, and layering, and its fruit ripening in winter also maintains species diversity. In addition, it has many functions such as edible, medicinal, feed, ornamental, and soil and water conservation, it consequently has high development and utilization value and broad market prospects. It is reported that P. fortuneana is the preferred species for controlling rocky desertification, and it should be developed and utilized rationally (He, 2014; Rojas et al., 2019). Although P. fortuneana has a tenacious vitality and can adapt to various environments, more attention should be also paid to the protection of diseases, especially anthracnose (Peng and Xie, 2020). In addition, further works should be also paid to pests such as pear crown stink bug, boat caterpillar, and cinnabar spider mite, as well as diseases such as powdery mildew, leaf spot and rust (Pu, 2014).
The endophytic fungi isolated from P. fortuneana can produce anti-bacterial effects on nine kinds of crop pathogenic fungi, such as wheat root rot, wheat scab, tomato early blight, etc. It has the characteristics of remarkable anti-bacterial effect with broad anti-bacterial spectrum and can be developed as an effective pesticide of microbial origin (Tian and Chen, 2012).
Aflatoxin is a mycotoxin produced by Aspergillus and is present in various foods. Eating foods contaminated with aflatoxin can cause adverse health effects (Saha Turna and Wu, 2019). The biomass in the aqueous extracts of P. fortuneana has an adsorption effect on aflatoxins (Zavala-Franco et al., 2018). The adsorption mechanism is mainly regarding the electrostatic interaction between the negatively charged functional groups and the positively charged aflatoxin molecules. Further studies showed that hydroxyl, amino, carboxyl, amide, phosphate, and ketone play important roles in the adsorption process, which can be used as a substitute for traditional aflatoxin removal (Ramales-Valderrama et al., 2016; Méndez-Albores et al., 2020).
Besides, it is reported that P. fortuneana extract has high bioabsorption performance for the dye methylene blue at the pH around 6, and P. fortuneana can be used as a natural, economical, abundant, and effective bio adsorbent in wastewater treatment (Akar et al., 2009). Alkyl benyzldimethyl ammonium chloride (ABDAC) modification significantly increased the biosorption yield of P. fortuneana to 97.27%, which was 3.88 times higher than that of natural biomass. The prepared biosorbent was effectively used for the decolorization of reactive Red 45 contaminated solutions after the optimization of biosorption conditions (Akar et al., 2013). Next, modification of the biosorbent obtained from P. fortuneana with anionic surfactants was successfully employed for decolorization of methyl violet (MV)-contaminated solutions (Akar et al., 2014).
From the ancient times to the present, the P. fortuneana is commonly considered as a safe nutritious plant without toxicity, and there are few reports regarding the side effects of this plant so far. In 1992, Wang et al. evaluated the oral toxicity of the fruit juice from P. fortuneana (containing 60% solids) in mice, and the results showed that the median lethal dose (LD50) value was higher than 28 g/kg, indicating that the toxicological classification of the fruit juice of P. fortuneana could belong to “non-toxic” (Wang et al., 1992). However, it is reported that ursolic acid, which is a compound reported in the fruits of P. fortuneana, would lead to transient relief of metabolic syndrome, weight loss, waist circumference and fasting blood glucose, and increased insulin sensitivity (Ramírez-Rodríguez et al., 2017).
In conclusion, the fruits, leaves, and roots of P. fortuneana are rich in bioactive components, mainly including proanthocyanidins, polyphenols, natural pigments and polysaccharides, etc. The previous in vitro and in vivo studies have revealed that extracts or compounds in P. fortuneana have potential biological and pharmacological properties, such as antioxidant, antitumor, hypolipidemic, gut microbiota regulation, and immunomodulatory activities. However, the key problem in the products development of P. fortuneana is that the fruit is astringent in taste and small in size, which leads to the low acceptance of this fruit and greatly affects the industrialization of its related products. Therefore, more research could be carried out to the appropriate increase of production of the fruits of P. fortuneana, and solve the astringent taste of the fruit. Although researchers have successively developed a series of products such as fruit juice, beverages, fruit vinegar, and health care wine, these products mentioned above are still in the relatively primary development stage, and no large-scale industrial production has been seen (Teng et al., 2017; Zhou et al., 2018). Consequently, further works should be devoted to development of more high-end products of this plant, such as clinical drugs, functional foods, and biopesticide, etc. Furthermore, the available current quality controlling of the fruits of P. fortuneana is lacking, especially for the medicinal use, which seriously limited the development of the high-end products of the fruits of P. fortuneana. So, the quality controlling standard of the fruits of P. fortuneana for food and medicinal use should be constructed based on the studies of characteristic bio-active compounds of this plant. Lastly, although lots of the activities of the fruit have been reported, most of them are studied superficially, and most or the previous studies mainly focused on the preliminary efficacy without a systemic research on molecular mechanisms, targets, and target organs. Thus, the future investigations should be devoted to explore the profound mechanisms of the extracts/compounds in the fruit of this plant. This study systematically summarized the research trends of the P. fortuneana and its phytochemical compositions, nutritional values, pharmacological effects, and health benefits of its extracts/monomers, which would be beneficial for the future development of this medicinal plant as functional food or drugs.
WP, XP, and CW organized and supervised this study. LW wrote the manuscript and prepared tables and figures. RL wrote and contributed to the revision of the manuscript. QZ contributed to the check of tables. TT performed the revisions of figures. TZ and QR participated in the revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Project of Sichuan Science and Technology Program (Grant Nos. 2022NSFSC0720 and 2019JDRC0074) and State Administration of Traditional Chinese Medicine of Sichuan Province of China (Grant No. 2021MS460).
Author XP is employed by Chengdu Medical and Health Investment Group Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Afshar, K., Fleischmann, N., Schmiemann, G., Bleidorn, J., Hummers-Pradier, E., Friede, T., et al. (2018). Reducing antibiotic use for uncomplicated urinary tract infection in general practice by treatment with uva-ursi (REGATTA) - a double-blind, randomized, controlled comparative effectiveness trial. BMC Complement. Altern. Med. 18, 203. doi: 10.1186/s12906-018-2266-x
Akar, S. T., Sayin, F., Turkyilmaz, S., and Akar, T. (2014). Multivariate optimization of the decolorization process by surface modified biomaterial: Box-Behnken design and mechanism analysis. Environ. Sci. Pollut. Res. Int. 21, 13055–13068. doi: 10.1007/s11356-014-3245-5
Akar, T., Anilan, B., Gorgulu, A., and Akar, S. T. (2009). Assessment of cationic dye biosorption characteristics of untreated and non-conventional biomass: Pyracantha coccinea berries. J Hazard Mater. 168, 1302–1309. doi: 10.1016/j.jhazmat.2009.03.011
Akar, T., Ozkara, E., Celik, S., Turkyilmaz, S., and Akar, S. T. (2013). Chemical modification of a plant origin biomass using cationic surfactant ABDAC and the biosorptive decolorization of RR45 containing solutions. Colloids Surf. B Biointerfaces 101, 307–314. doi: 10.1016/j.colsurfb.2012.06.016
Belwal, T., Pandey, A., Bhatt, I. D., Rawal, R. S., and Luo, Z. (2019). Trends of polyphenolics and anthocyanins accumulation along ripening stages of wild edible fruits of Indian Himalayan region. Sci. Rep. 9, 5894. doi: 10.1038/s41598-019-42270-2
Bumrungpert, A., Lilitchan, S., Tuntipopipat, S., Tirawanchai, N., and Komindr, S. (2018). Ferulic acid supplementation improves lipid profiles, oxidative stress, and inflammatory status in hyperlipidemic subjects: a randomized, double-blind, placebo-controlled clinical trial. Nutrients. 10, 713. doi: 10.3390/nu10060713
Cai, J. T., and Ding, Z. H. (1996). The processing technology of the Pyracantha fruit juice drinks. Sci. Technol. Food Indu. 2, 40–42.
Cakar, U., Grozdanic, N., Petrovic, A., Pejin, B., Nastasijevic, B., Markovic, B., et al. (2017). Fruit wines inhibitory activity against α-glucosidase. Curr. Pharm. Biotechnol. 18, 1264–1272. doi: 10.2174/1389201019666180410112439
Chan, S. Y., Choo, W. S., Young, D. J., and Loh, X. J. (2017). Pectin as a rheology modifier: origin, structure, commercial production and rheology. Carbohydr. Polym. 161, 118–139. doi: 10.1016/j.carbpol.2016.12.033
Chen, K., and Tan, Q. Q. (2021). Analysis of nutrient components of Pyracantha fruit. Chin. Food Saf. Mag. 15, 94–96. doi: 10.16043/j.cnki.cfs.2021.15.057
Chen, Q., Li, Z., and Sun, M. (2011). Research advance in Pyracantha rosa L. of rosaceae. Food Eng. 3, 11–13. doi: 10.3969/j.issn.1673-6044.2011.03.006
Chen, Y., Huang, Z. L., Jiang, L. H., Xiong, Y. F., Wen, Z. Y., and Zhu, H. F. (2009). Studies on extraction of pectin in Pyracantha fotuneanas with ultrasonicwave. China Food Addit. 6, 54–58. doi: 10.3969/j.issn.1006-2513.2009.06.008
Chen, Y., Lu, Z. M., and Li, Z. X. (2007). Study on processing and HACCP controlling of Pyracantha fortuneana beverage. Food Sci. 8, 598–601. doi: 10.3321/j.issn:1002-6630.2007.08.152
Chhikara, N., Kushwaha, K., Sharma, P., Gat, Y., and Panghal, A. (2019). Bioactive compounds of beetroot and utilization in food processing industry: a critical review. Food Chem. 272, 192–200. doi: 10.1016/j.foodchem.2018.08.022
Choi, D., Kang, W., Park, S., Son, B., and Park, T. (2021). β-Ionone attenuates dexamethasone-induced suppression of collagen and hyaluronic acid synthesis in human dermal fibroblasts. Biomolecules 11, 619. doi: 10.3390/biom11050619
Choi, J. H., Kim, M. K., Yeo, S. H., and Kim, S. (2020). Short-term Cudrania tricuspidata fruit vinegar administration attenuates obesity in high-fat diet-fed mice by improving fat accumulation and metabolic parameters. Sci Rep. 10, 21102. doi: 10.1038/s41598-020-78166-9
Choi, S. J., Lee, S. N., Kim, K., Joo da, H., Shin, S., Lee, J., et al. (2016). Biological effects of rutin on skin aging. Int J Mol Med. 38, 357–363. doi: 10.3892/ijmm.2016.2604
Chu, H. Y., Wei, J. H., and Li, Y. (2015). Determining trace elements in gleditsia sinensis Lam. Stings and Pyracantha fortuneana with atomic absorption spectrometry. Hubei Agric. Sci. 54, 171–174. doi: 10.14088/j.cnki.issn0439-8114.2015.01.044
Dai, Y., He, X. J., Zhou, G. X., Kurihara, H., Ye, W. C., and Yao, X. S. (2008). Acylphloroglucinol glycosides from the fruits of Pyracantha fortuneana. J. Asian Nat. Prod. Res. 10, 111–117. doi: 10.1080/10286020601106018
Dai, Y., Zhou, G. X., Kurihara, H., Ye, W. C., and Yao, X. S. (2006). Biphenyl glycosides from the fruit of Pyracantha fortuneana. J. Nat. Prod. 69, 1022–1024. doi: 10.1021/np0600853
Dai, Y., Zhou, G. X., Kurihara, H., Ye, W. C., and Yao, X. S. (2009). A biphenyl glycoside from Pyracantha fortuneana. Nat. Prod. Res. 23, 1163–1167. doi: 10.1080/14786410802213985
Di Pierro, F., Derosa, G., Maffioli, P., Bertuccioli, A., Togni, S., Riva, A., et al. (2021). Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study. Int. J. Gen. Med. 14, 2359–2366. doi: 10.2147/IJGM.S318720
Dong, L. S. (2002). Analysis of phospholipids in Pyracantha fortuneana fruit. Acta Nutr. Sin. 2, 209–211. doi: 10.13325/j.cnki.acta.nutr.sin.2002.02.027
Duan, Q. X., Li, D. J., Duan, Z. H., Chen, Y., Tang, M. L., and Wu, L. M. (2020). Study on changes of antioxidant activity of red heart Pitaya wines during storage. Food Res. Dev. 41, 43–49. doi: 10.12161/j.issn.1005-6521.2020.24.008
Editorial Committee of Flora of China and Chinese Academy of Sciences (1973). Pyracantha fortuneana (Maxim.) Li. Flora China 36, 180.
Farkhondeh, T., Samarghandian, S., and Roshanravan, B. (2019). Impact of chrysin on the molecular mechanisms underlying diabetic complications. J. Cell Physiol. 234, 17144–17158. doi: 10.1002/jcp.28488
Ferk, F., Kundi, M., Brath, H., Szekeres, T., Al-Serori, H., Mišík, M., et al. (2018). Gallic acid improves health-associated biochemical parameters and prevents oxidative damage of DNA in type 2 diabetes patients: results of a placebo-controlled pilot study. Mol. Nutr. Food Res. 62, 1–30. doi: 10.1002/mnfr.201700482
Fico, G., Billam, A. R., Morelli, I. I., and Tomè, F. (2000). Flavonoid distribution in Pyracantha coccinea plants at different growth phases. Biochem. Syst. Ecol. 28, 673–678. doi: 10.1016/S0305-1978(99)00109-X
Fu, Y. (2014). Analysis and evaluation on nutritional components of fruit of 4 germplasms in Pyracantha fortuneana. J. Anhui Agric. Sci. 42, 2. doi: 10.13989/j.cnki.0517-6611.2014.06.083
Fuller, S., Beck, E., Salman, H., and Tapsell, L. (2016). New horizons for the study of dietary fiber and health: a review. Plant Foods Hum. Nutr. 71, 1–12. doi: 10.1007/s11130-016-0529-6
Gan, X. H., Zhao, Y., Zhou, X., Zhao, C., and Liang, Z. Y. (2012). Comparison of the contents of quercitin in various medicinal parts of Pyracantha fortuneana. Chin. J. Exp. Trad. Med. Formulae. 18, 100–102. doi: 10.13422/j.cnki.syfjx.2012.11.036
Gao, L. (2012). Application of immobilized technology in the fermentation process of Pyracantha fortuneana vinegar. Storage Process. 12, 35–38. doi: 10.3969/j.issn.1009-6221.2012.05.008
Gao, W., Wang, C., Yu, L., Sheng, T., Wu, Z., Wang, X., et al. (2019). Chlorogenic acid attenuates dextran sodium sulfate-induced ulcerative colitis in mice through MAPK/ERK/JNK pathway. Biomed. Res. Int. 2019, 6769789. doi: 10.1155/2019/6769789
Gao, Y. L. (2020). Pyracantha fortuneana. Flowers 9, 47. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=HUHU202009019&DbName=CJFN2020
Gao, Z. Y., Liu, S. L., Zhang, H. L., and Xie, H. X. (2019). Determination of contents of metal elements in Pyracantha fortuneana fruit by flame atomic absorption spectrometry. Chem. Bioeng. 36, 65–68. doi: 10.3969/j.issn.1672-5425.2019.04.015
Ge, L. N., Han, X., Ren, K. K., Zhang, P., Peng, Y. Y., and Bi, S. F. (2014). GC-MS analysis on chemical constituents and antioxidant activity of volatile oil from Pyracantha fortuneana flowers. Bull. Bot. Res. 34, 276–281. doi: 10.7525/j.issn.1673-5102.2014.02.022
Ghanim, A. M. H., Younis, N. S., and Metwaly, H. A. (2021). Vanillin augments liver regeneration effectively in thioacetamide induced liver fibrosis rat model. Life Sci. 286, 120036. doi: 10.1016/j.lfs.2021.120036
González-Burgos, E., and Gómez-Serranillos, M. P. (2012). Terpene compounds in nature: a review of their potential antioxidant activity. Curr. Med. Chem. 19, 5319–5341. doi: 10.2174/092986712803833335
Halima, B. H., Sonia, G., Sarra, K., Houda, B. J., Fethi, B. S., and Abdallah, A. (2018). Apple cider vinegar attenuates oxidative stress and reduces the risk of obesity in high-fat-fed male wistar rats. J. Med. Food 21, 70–80. doi: 10.1089/jmf.2017.0039
Han, L., Wu, Y. M., Wang, K. T., Wang, Z. D., Tang, H. L., and Gao, Y. J. (2015). Study on the monosaccharide compositions, physico-properties and preparation technology of soluble dietary fiber by enzymatic method from Pyracantha fortuneana. Sci. Technol. Food Ind. 36, 215–219. doi: 10.13386/j.issn1002-0306.2015.17.035
Han, W. Q., and Zhang, G. F. (2019). Study on nutritional components and antioxidant activity of total flavonoids in Pyracantha fruit. Agric Sci. 9, 399–404. doi: 10.12677/HJAS.2019.96059
He, R. F. (2014). Development and utilization of Pyracantha fortuneana in rocky desertification control. Agric Sci. 34, 128–133. doi: 10.3969/j.issn.1671-962X.2014.06.098
He, R. R., Li, W. X., Li, Y. F., and Li, Y. B. (2011). Whitening effects of fruit extract of Pyracantha fortuneana. Chin. J. Exp. Tradit. Med. Formul. 17, 184–188. doi: 10.13422/j.cnki.syfjx.2011.02.052
He, Y. F., Huang, Y. B., Li, Y. M., Yang, M., Yin, C. R., Liao, Y. Q., et al. (2018). Simultaneous determination of multiple trace elements in Pyracantha fortuneana by ICP-MS. J. A. Agric. Sci. 46, 177−178+218. doi: 10.13989/j.cnki.0517-6611.2018.13.053
He, Y. F., Yang, S. J., Zhang, W., Zhou, Y., Duan, Y. J., and Wu, J. W. (2019). Determination of the contents of earth elements in different tissues of Pyracantha fortuneana. Sci. Tech. Food Ind. 40, 257–265. doi: 10.13386/j.issn1002-0306.2019.04.042
Hien, T. V., Huong, N. B., Hung, P. M., and Duc, N. B. (2002). Radioprotective effects of vitexina for breast cancer patients undergoing radiotherapy with cobalt-60. Integr. Cancer Ther. 1, 38–34; discussion 42–33. doi: 10.1177/1534735402001001003
Hoensch, H., Groh, B., Edler, L., and Kirch, W. (2008). Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J. Gastroenterol. 14, 2187–2193. doi: 10.3748/wjg.14.2187
Hou, J. J., Liu, X. L., Wei, W. K., and Wu, M. G. (2003a). Animal test on the efficacy of pyracantha to dissipate food and invigorate the spleen. Hubei Agric. Sci. 84–86. doi: 10.3969/j.issn.0439-8114.2003.04.035
Hou, J. J., Wei, W. K., Huang, H., and Wu, M. G. (2003b). Antioxidation effects of pyracantha on aging mice model induced by overdose of D-galactose. Chin. J. Public Health 19, 944–945. doi: 10.11847/zgggws2003-19-08-31
Hou, J. J., Wei, W. K., Xue, H., and Zhang, H. (2002). Research progress of wild plant Pyracantha fortuneana. J. Hubei Minzu Univ. 15–18. doi: 10.3969/j.issn.1008-8423.2002.01.005
Huang, P. A. (2014). Determination of trace elements in Pyracantha fortuneana. Edu. Teach. Forum 31, 156–157. doi: 10.3969/j.issn.1674-9324.2014.31.107
Huang, R., and Fu, X. H. (2014). Advances on research of functional composition of Pyracantha fortuneana. Chin. Wild Plant Res. 33, 37–41. doi: 10.3969/j.issn.1006-9690.2014.05.011
Huang, Y. C., Yang, F., Xie, Q. R., and Duan, Y. F. (2007a). Study on physicochemical property of PP-A3 fromwater-soluble Pyracantha fortuneana polysaccharides. Food Res. Dev. 8, 75–79. doi: 10.3969/j.issn.1005-6521.2007.08.023
Huang, Y. C., Yang, F., Xie, Q. R., and Duan, Y. F. (2007b). Study on physicochemical property of PP-A2 from water-soluble Pyracantha fortuneana polysaccharides. Food Sci. Technol. 6, 116–119. doi: 10.13684/j.cnki.spkj.2007.06.037
Humbeck, K., Römer, S., and Senger, H. (1989). Evidence for an essential role of carotenoids in the assembly of an active photosystem II. Planta. 179, 242–50. doi: 10.1007/bf00393695
Jiang, C. L., Zhuang, Y., Zhu, D. G., Cheng, C., and Li, W. (2013). Changes of chemical contents and their antioxidant activities during wine processing of Pyracantha fortuneana (Maxim.) Li. China Brew. 32, 80–83. doi: 10.3969/j.issn.0254-5071.2013.08.019
Jiang, L. H., Xiong, Y. F., Li, X., Wen, Z. Y., and Liu, W. (2007). Research progress on active ingredients of wild Pyracantha fortuneana. Chin Wild Plant Res. 26, 8–10. doi: 10.3969/j.issn.1006-9690.2007.02.003
Jo, H., Cha, B., Kim, H., Brito, S., Kwak, B. M., Kim, S. T., et al. (2021). α-Pinene enhances the anticancer activity of natural killer cells via ERK/AKT pathway. Int. J. Mol. Sci. 22, 656. doi: 10.3390/ijms22020656
Johnson, W., Bergfeld, W. F., Belsito, D. V., Hill, R. A., Klaassen, C. D., Liebler, D. C., et al. (2017). Safety assessment of benzyl alcohol, benzoic acid and its salts, and benzyl benzoate. Int. J. Toxicol. 36 (3 Suppl), 5s−30s. doi: 10.1177/1091581817728996
Kang, W., Choi, D., and Park, T. (2020). Decanal protects against UVB-induced photoaging in human dermal fibroblasts via the cAMP pathway. Nutrients 12, 1214. doi: 10.3390/nu12051214
Kashiwadani, H., Higa, Y., Sugimura, M., and Kuwaki, T. (2021). Linalool odor-induced analgesia is triggered by TRPA1-independent pathway in mice. Behav. Brain Funct. 17, 3. doi: 10.1186/s12993-021-00176-y
Ke, Y., Yu, K., Zeng, W., and Lian, G. (2019). Protective roles of Pyracantha fortuneana extract on acute renal toxicity induced by cadmium chloride in rats. Acta Cir. Bras. 34, e201900706. doi: 10.1590/s0102-865020190070000006
Keser, S. (2014). Antiradical activities and phytochemical compounds of firethorn (Pyracantha coccinea) fruit extracts. Nat. Prod. Res. 28, 1789–1794. doi: 10.1080/14786419.2014.942304
Kim, J. H., Cho, H. D., Won, Y. S., Hong, S. M., Moon, K. D., and Seo, K. I. (2020). Anti-fatigue effect of Prunus mume vinegar in high-intensity exercised rats. Nutrients 12, 1205. doi: 10.3390/nu12051205
Laganà, P., Coniglio, M. A., Fiorino, M., Delgado, A. M., Chammen, N., Issaoui, M., et al. (2020). Phenolic substances in foods and anticarcinogenic properties: a public health perspective. J. AOAC Int. 103, 935–939. doi: 10.1093/jaocint/qsz028
Li, J. X., Huang, S. E., and Liang, X. Z. (2012). Progressinresearch and development of Pyracantha fortuneana. Food. Mchnr. 28, 260–263. doi: 10.3969/j.issn.1003-5788.2012.06.065
Li, J. Z., Wang, D. Z., and Huang, Y. Q. (2016a). Compound health beverage of Ganoderma lucidum and Pyracantha fortuneana developed by response surface method. Sci. Technol. Food Ind. 37, 238–242. doi: 10.13386/j.issn1002-0306.2016.21.037
Li, J. Z., Wang, D. Z., and Huang, Y. Q. (2016b). Effect of Pytacantha crenulata-Ganoderma lucidum beverage on the growth and antioxidant properties of drosophila melanogaster. Acta Edulis Fungi 23, 40–43. doi: 10.16488/j.cnki.1005-9873.2016.04.008
Li, P. X., Mao, G. H., Zhao, T., Zhou, Y., Ren, Y. N., Bai, S. Q., et al. (2013). Extraction and antioxidant activity of red pigments from Pyracantha fortuneana. Food Sci. 34, 116–119. doi: 10.7506/spkx1002-6630-201317026
Li, R. L., Zhang, Q., Liu, J., Sun, J. Y., He, L. Y., Duan, H. X., et al. (2020). Hydroxy-α-sanshool possesses protective potentials on H2O2-stimulated PC12 cells by suppression of oxidative stress-induced apoptosis through regulation of PI3K/Akt signal pathway. Oxid. Med. Cell Longev. 2020, 3481758. doi: 10.1155/2020/3481758
Li, W., and Chen, C. (2013). The antioxidation effect in vitro of flavonoides from different Pyracantha fortuneana fruits. J. Hubei Minzu Univ. 31, 382–385. doi: 10.3969/j.issn.1008-8423.2013.04.005
Li, W., Cheng, C., Zhang, Y. T., Yan, L., and Mo, K. J. (2008). Clustering analysis of functional components in different Pyracantha fortuneana (Maxim.) Li. fruits. Food Sci. 207–210. doi: 10.3321/j.issn:1002-6630.2008.09.043
Li, W., Tian, C., Wang, M., and Cheng, C. (2013). Extraction optimization and its antioxidant activities of flavonoides from Pyracantha fortuneana (Maxim.) Li. fruits. J. Hubei Minzu Univ. 31, 145–148. doi: 10.3969/j.issn.1008-8423.2013.02.007
Li, W. F., Ma, H. H., Yuan, S., and Zhang, X. F. (2021). Production of pyracantha polysaccharide-iron(III) complex and its biologic activity. Molecules 26, 1949. doi: 10.3390/molecules26071949
Li, Y. H. (2012). Study on the technology of immobilized acetobacter for brewing Pyracantha vinegar. J. Shaanxi Inst. Edu. 28, 90–94. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=SHAA201203020&DbName=CJFQ2012
Lin, R. D., Chen, M. C., Liu, Y. L., Lin, Y. T., Lu, M. K., Hsu, F. L., et al. (2015). New whitening constituents from Taiwan-native Pyracantha koidzumii: structures and tyrosinase inhibitory analysis in human epidermal melanocytes. Int. J. Mol. Sci. 16, 28598–28613. doi: 10.3390/ijms161226115
Liu, J., Xu, Y. F., Lu, H. C., Wang, H. J., Gui, J., Chen, X. Z., et al. (2015). Study on the stability of proanthocyanidins from Pyracantha fortuneana fruit. Food Res. Dev. 36, 29–32. doi: 10.3969/j.issn.1005-6521.2015.07.008
Liu, M. H., and Wang, D. H. (2011). Development of Pyracantha fortuneana lactobacillus beverage. China Brew. 6, 92–196. doi: 10.3969/j.issn.0254-5071.2011.06.055
Luo, B. F., Wang, J. N., Huang, Y. M., and Huang, Z. L. (2014). Advances in the wild Pyracantha fortuneana research. China Brew. 33, 1–4. doi: 10.3969/j.issn.0254-5071.2014.02.001.y
Luo, H. Q., Shen, J., Chen, C. P., Ma, X., Lin, C., Ouyang, Q., et al. (2018). Lipid-lowering effects of oleanolic acid in hyperlipidemic patients. Chin. J. Nat. Med. 16, 339–346. doi: 10.1016/S1875-5364(18)30065-7
Luo, X., Li, T. J., Bao, Y. R., Wang, S., and Meng, X. S. (2021). Extraction of active components from Fructus aurantii for colon cancer based on HT-29 cells. Cent. South Pharm. 19, 455–459. doi: 10.7539/j.issn.1672-2981.2021.03.015
Luzón-Quintana, L. M., Castro, R., and Durán-Guerrero, E. (2021). Biotechnological processes in fruit vinegar production. Foods 10, 945. doi: 10.3390/foods10050945
Ma, W., Zhao, L., Zhao, W., and Xie, Y. (2019). (E)-2-Hexenal, as a potential natural antifungal compound, inhibits aspergillus flavus spore germination by disrupting mitochondrial energy metabolism. J. Agric. Food Chem. 67, 1138–1145. doi: 10.1021/acs.jafc.8b06367
Mattila, P., and Kumpulainen, J. (2002). Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J. Agric. Food Chem. 50, 3660–3667. doi: 10.1021/jf020028p
Mei, X. G., Wan, G. H., Zhou, Z. Q., Chang, J. L., and Wu, H. L. (2001). Effects of Pyracantha fortunezna extract on blood coagulation. J. Chin. Med. Mater. 24, 874–876. doi: 10.3321/j.issn:1001-4454.2001.12.014
Méndez-Albores, A., Escobedo-González, R., Aceves-Hernández, J. M., García-Casillas, P., Nicolás-Vázquez, M. I., and Miranda-Ruvalcaba, R. (2020). A theoretical study of the adsorption process of aflatoxins using Pyracantha koidzumii (Hayata) rehder biomasses. Toxins 12, 283. doi: 10.3390/toxins12050283
Mertz, W. (1981). The essential trace elements. Science. 213, 1332–1338. doi: 10.1126/science.7022654
Mirzaie, Z., Bastani, A., Hesami, S., Pouryousefi, E., Kavianpour, M., and Haghighian, H. K. (2021). Improving effect of ellagic acid on sleep quality and gastrointestinal symptoms in patient with irritable bowel syndrome: randomized double-blind clinical trial. Turk. J. Gastroenterol. 32, 937–944. doi: 10.5152/tjg.2021.20344
Mohnen, D. (2008). Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11, 266–277. doi: 10.1016/j.pbi.2008.03.006
Mu, M., Zuo, S., Wu, R. M., Deng, K. S., Lu, S., Zhu, J. J., et al. (2018). Ferulic acid attenuates liver fibrosis and hepatic stellate cell activation via inhibition of TGF-β/Smad signaling pathway. Drug Des. Dev. Ther. 12, 4107–4115. doi: 10.2147/DDDT.S186726
Nai, G. R. Y., Li, P. H., Peng, Z. S., Li, S. Q., Peng, R., and Zhao, H. (2020). Research progress of Pyracantha fortuneana. Sichuan Agric. Sci. Tech. 10, 20–22. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=SNYK202010007&DbName=CJFQ2020
Najjar, R. S., Alamoudi, N. M., El-Housseiny, A. A., Al Tuwirqi, A. A., and Sabbagh, H. J. (2019). A comparison of calcium hydroxide/iodoform paste and zinc oxide eugenol as root filling materials for pulpectomy in primary teeth: a systematic review and meta-analysis. Clin. Exp. Dent. Res. 5, 294–310. doi: 10.1002/cre2.173
Nie, Z. L., Xu, Y. Q., Liu, J., and Wang, J. H. (2018). Study on the brewing method of wild Pyracantha fortuneana fruit wine. Food Res. Dev. 39, 104–108. doi: 10.3969/j.issn.1005-6521.2018.24.017
Ohara, K., Misaizu, A., Kaneko, Y., Fukuda, T., Miyake, M., Miura, Y., et al. (2018). β-Eudesmol, an oxygenized sesquiterpene, reduces the increase in saliva 3-methoxy-4-hydroxyphenylglycol after the “trier social stress test” in healthy humans: a randomized, double-blind, placebo-controlled cross-over study. Nutrients 11, 9. doi: 10.3390/nu11010009
Otsuka, H., Fujioka, S., Komiya, T., Goto, M., Hiramatsu, Y., and Fujimura, H. (1981). Studies on anti-inflammatory agents. V. A new anti-inflammatory constituent of Pyracantha crenulata roem. Chem. Pharm Bull. 9, 3099–3104. doi: 10.1248/cpb.29.3099
Peng, F., Guo, X., Li, Z., Li, C., Wang, C., Lv, W., et al. (2016). Antimutagenic effects of selenium-enriched polysaccharides from Pyracantha fortuneana through suppression of cytochrome P450 1A subfamily in the mouse liver. Molecules 21, 1731. doi: 10.3390/molecules21121731
Peng, W., Chen, Y., Tumilty, S., Liu, L., Luo, L., Yin, H., et al. (2022). Paeoniflorin is a promising natural monomer for neurodegenerative diseases via modulation of Ca and ROS homeostasis. Curr. Opin. Pharmacol. 62, 97–102. doi: 10.1016/j.coph.2021.11.009
Peng, W., Du, H., Liu, G., Zhang, Q., Kuang, T., Wang, Z., et al. (2019). Antistress effects of San-Huang-Xie-Xin decoction on restraint-stressed mice revealed by 1H NMR-Based metabolomics and biochemistry analysis. Oxid. Med. Cell Longev. 2019, 5897675. doi: 10.1155/2019/5897675
Peng, Z. Z., and Xie, F. G. (2020). Transmission route and control measures of Pyracantha anthracnose in Jiuzhaigou. Rural Pract. Tech. 2, 76.
Pu, S. L. (2014). Pyracantha propagation and cultivation management technology. For. Pro. Spec. Chin. 6, 56–57.
Qin, H. B., and Wei, L. (2011). Experimental study on the antioxidant effect of pyracantha on D-galactose-induced aging mice. J. Heze. Med. Colg. 23, 6–8. doi: 10.3969/j.issn.1008-4118.2011.04.03
Qin, P., Wei, Y., Hou, M., Zhao, C., and Shen, Z. (2015). [A multicenter clinical trial of caffeic acid tablet in treatment of 103 primary immune thrombocytopenia patients]. Zhonghua Xue Ye Xue Za Zhi 36, 103–106. doi: 10.3760/cma.j.issn.0253-2727.2015.02.004
Ramales-Valderrama, R. A., Vázquez-Durán, A., and Méndez-Albores, A. (2016). Biosorption of B-aflatoxins using biomasses obtained from formosa firethorn [Pyracantha koidzumii (Hayata) Rehder]. Toxins 8, 218. doi: 10.3390/toxins8070218
Ramírez-Rodríguez, A. M., González-Ortiz, M., Martínez-Abundis, E., and Acuña Ortega, N. (2017). Effect of ursolic acid on metabolic syndrome, insulin sensitivity, and inflammation. J. Med. Food 20, 882–886. doi: 10.1089/jmf.2017.0003
Rojas, T. N., Vergara-Tabares, D. L., Valdez, D. J., Ponzio, M. F., and Peluc, S. I. (2019). Food supplementation by an invasive fleshy-fruited shrub sustains body condition of a native frugivorous bird during winter. Integr. Zool. 14, 259–269. doi: 10.1111/1749-4877.12353
Russell, W. R., Labat, A., Scobbie, L., Duncan, G. J., and Duthie, G. G. (2009). Phenolic acid content of fruits commonly consumed and locally produced in Scotland. Food Chem. 115, 100–104. doi: 10.1016/j.foodchem.2008.11.086
Saha Turna, N., and Wu, F. (2019). Risk assessment of aflatoxin-related liver cancer in Bangladesh. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 36, 320–326. doi: 10.1080/19440049.2019.1567941
Saija, A., Tomaino, A., Trombetta, D., De Pasquale, A., Uccella, N., Barbuzzi, T., et al. (2000). In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int. J. Pharm. 199, 39–47. doi: 10.1016/S0378-5173(00)00358-6
Sarigul Sezenoz, A., Akkoyun, I., Helvacioglu, F., Haberal, N., Dagdeviren, A., Bacanli, D., et al. (2021). Antiproliferative and mitochondrial protective effects of apigenin in an oxygen-induced retinopathy in vivo mouse model. J. Ocul. Pharmacol. Ther. 37, 580–590. doi: 10.1089/jop.2021.0046
Shang, X. F., Dai, L. X., Yang, C. J., Guo, X., Liu, Y. Q., Miao, X. L., et al. (2021). A value-added application of eugenol as acaricidal agent: the mechanism of action and the safety evaluation. J. Adv. Res. 34, 149–158. doi: 10.1016/j.jare.2020.12.010
Sharifi-Rad, J., Song, S., Ali, A., Subbiah, V., Taheri, Y., and Suleria, H. A. R. (2021). LC-ESI-QTOF-MS/MS characterization of phenolic compounds from Pyracantha coccinea M.Roem. and their antioxidant capacity. Cell Mol. Biol. 67, 201–211. doi: 10.14715/cmb/2021.67.1.29
Sharifi-Rad, J., Taheri, Y., Ayatollahi, S. A., Naderi, N., Kumar, N. V. A., Koirala, N., et al. (2020). Biological activities and health-promoting effects of pyracantha genus: a key approach to the phytochemical's potential. Cell Mol. Biol. 66, 20–27. doi: 10.14715/cmb/2020.66.4.4
Sharma, P., Kumar, M., and Bansal, N. (2021). Ellagic acid prevents 3-nitropropionic acid induced symptoms of Huntington's disease. Naunyn Schmiedebergs Arch. Pharmacol. 394, 1917–1928. doi: 10.1007/s00210-021-02106-1
Shi, W., Fu, G. H., Zhng, K., and Wu, D. G. (2020). Optimization of fermentation technology of wild Pyracantha fortuneana health wine by response surface methodology. China Brew. 39, 109–114. doi: 10.11882/j.issn.0254-5071.2020.03.022
Shim, S. Y., Lee, Y. E., Song, H. Y., and Lee, M. (2020). p-hydroxybenzoic acid β-d-glucosyl ester and cimidahurinine with antimelanogenesis and antioxidant effects from Pyracantha angustifolia via bioactivity-guided fractionation. Antioxidants 9, 258. doi: 10.3390/antiox9030258
Su, K. Q. (2019). The research status and development prospect of three kinds of characteristic wild fruit resources in yunnan. Farm Prod. Proc. 4, 90–95. doi: 10.16693/j.cnki.1671-9646(X).2019.02.058
Sun, H., Wang, X., Zhou, Z., and Wang, R. (2019). Extraction optimization of polyphenols from fruits of Pyracantha fortuneana (Maxim.) Li by ultrasonic assistant method and their antibacterial activity. Pak. J. Pharm Sci. 32, 1635–1641.
Sun, Q., Dong, M., Wang, Z., Wang, C., Sheng, D., Li, Z., et al. (2016). Selenium-enriched polysaccharides from Pyracantha fortuneana (Se-PFPs) inhibit the growth and invasive potential of ovarian cancer cells through inhibiting β-catenin signaling. Oncotarget 7, 28369–28383. doi: 10.18632/oncotarget.8619
Sun, X., Li, H., Guo, X., Sun, Y., and Li, S. (2018). Capacity of six shrub species to retain atmospheric particulates with different diameters. Environ. Sci. Pollut. Res. Int. 25, 2643–2650. doi: 10.1007/s11356-017-0549-2
Taheri, Y., Naderi, N., Ayatollahi, S. A., Baghalpour, N., Mahroo-Bakhtiyari, J., and Sharifi-Rad, J. (2021). High-performance thin-layer chromatography fingerprinting and anti-inflammatory and antinociceptive activities of Pyracantha coccinea M.Roem.: a laboratory-based study. Cell Mol. Biol. 67, 106–111. doi: 10.14715/cmb/2021.67.1.16
Tang, K. H., Xun, Y., Ding, W., Li, Y. F., and Chen, G. X. (2007). A oil content of pyracantha seeds and its relations to soil nutrients in northwestern Hunan province. Chin. J. Appl. Ecol. 18, 1903–1907.
Tang, Y., and Liu, J. L. (2002). Development, utilization and sustainable development of Pyracantha fortuneana resource in Liangshan prefecture. Sichuan Agric. Sci. Tech. 9, 6. doi: 10.3969/j.issn.1004-1028.2002.09.004
Tayebati, S. K. (2018). Phospholipid and lipid derivatives as potential neuroprotective compounds. Molecules 23, 2257. doi: 10.3390/molecules23092257
Teng, J. T., Cheng, Y. H., Xue, J. P., and Sheng, W. (2017). Research progress of effective components and resources development of Pyracantha fortuneana. J. Huaibei Norm. Univ. Nat. Sci. Educ. 38, 43–48.
Thakur, B. R., Singh, R. K., and Handa, A. K. (1997). Chemistry and uses of pectin–a review. Crit. Rev. Food Sci. Nutr. 37, 47–73. doi: 10.1080/10408399709527767
Tian, X. M., and Chen, K. M. (2012). Screening of Pyracantha fortuneana endophytic fungus and function examination of antagonism to plant pathogenic fungi. J. N. W. For. Univ. 27, 117–120. doi: 10.3969/j.issn.1001-7461.2012.05.22
Tobin, P. J., Beale, P., Noney, L., Liddell, S., Rivory, L. P., and Clarke, S. (2006). A pilot study on the safety of combining chrysin, a non-absorbable inducer of UGT1A1, and irinotecan (CPT-11) to treat metastatic colorectal cancer. Cancer Chemother. Pharmacol. 57, 309–316. doi: 10.1007/s00280-005-0053-0
van Dijk, A. E., Olthof, M. R., Meeuse, J. C., Seebus, E., Heine, R. J., and van Dam, R. M. (2009). Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 32, 1023–1025. doi: 10.2337/dc09-0207
Wang, C. F., Gao, X., and Wang, X. M. (2016). Chemical composition and antioxidant activity of the oil of Pyracantha atalantioides ftuits. Sci. Technol. Food Ind. 37, 81–88. doi: 10.13386/j.issn1002-0306.2016.09.007
Wang, H., Ye, Y. H., Wang, H. H., Liu, J., Liu, Y. J., and Jiang, B. W. (2019). HPLC-QTOF-MS/MS profiling, antioxidant, and α-glucosidase inhibitory activities of Pyracantha fortuneana fruit extracts. J. Food Biochem. 43, e12821. doi: 10.1111/jfbc.12821
Wang, J., Seyler, B. C., Ticktin, T., Zeng, Y., and Ayu, K. (2020). An ethnobotanical survey of wild edible plants used by the Yi people of Liangshan prefecture, Sichuan province, China. J Ethnobiol Ethnomed. 16, 10. doi: 10.1186/s13002-019-0349-5
Wang, J. M., Liao, D. S., Li, Q. G., and Wang, Z. L. (1992). Study on nutritional components and pectin of Pyracantha fortuneana. Food Sci. 4, 40–42.
Wang, R. G., Xue, C. B., Wei, M. X., Bi, F. X., and Bi, S. F. (2013). GC-MS analysis and antioxidant activity of essential oil from Pyracantha fortuneana fruits. Sci. Technol. Food Ind. 34, 95–97. doi: 10.13386/j.issn1002-0306.2013.07.018
Wang, S. G., and Deng, R. F. (1988). Development, utilization and physiological ecology of wild plant Pyracantha fortuneana. Chin Wild Plant Res. 3, 13–15.
Wang, X., Yang, H., Tian, R., Mo, Y., Dong, L., Shen, C., et al. (2021). Effect of the joint fermentation of pyracantha powder and glutinous rice on the physicochemical characterization and functional evaluation of rice wine. Food Sci. Nutr. 9, 6099–6108. doi: 10.1002/fsn3.2560
Wang, X. J., Chen, L. H., and Huang, Y. L. (2015a). Antioxidant activity of flavonoids from Pyracantha fortuneana to oils and fats. Nat. Prod. Res. Dev. 27, 909–914. doi: 10.16333/j.1001-6880.2015.05.031
Wang, X. J., Chen, L. H., and Xiang, M. F. (2016). Analysis for lipid antioxidant activity of polysaccharides from Pyracantha fortuneana. Food Ferment. Ind. 42, 175–179. doi: 10.13995/j.cnki.11-1802/ts.20160530
Wang, X. J., Zhang, L., Chen, L. H., and Liu, M. (2015b). Cooperative antioxidation activity of Pyracantha fortuneana flavonoids extracts and VC. China Oils Fats 40, 36–40. doi: 10.3969/j.issn.1003-7969.2015.09.011
Wei, M., Chai, W. M., Yang, Q., Wang, R., and Peng, Y. (2017). Novel insights into the inhibitory effect and mechanism of proanthocyanidins from Pyracantha fortuneana fruit on α-Glucosidase. J. Food Sci. 82, 2260–2268. doi: 10.1111/1750-3841.13816
Wei, Z. H., Hu, L., and Huang, J. X. (2006). Effects of pyracantha syrup on the indexes related to lesion induced by arsenic poisoning in mice. Chin. J. Tissue Eng. Res. 10, 99–101.
Wen, K., Fang, X., Yang, J., Yao, Y., Nandakumar, K. S., Salem, M. L., et al. (2021). Recent research on flavonoids and their biomedical applications. Curr. Med. Chem. 28, 1042–1066. doi: 10.2174/0929867327666200713184138
Weng, W. J., and Gao, X. (2015). Study on antioxidant activity in vitro about different solvent extracts of fruits of Pyracantha fortuneana. Sci. Technol. Food Ind. 36, 77–96. doi: 10.13386/j.issn1002-0306.2015.01.008
Wong, S. K., Chin, K. Y., and Ima-Nirwana, S. (2019). The osteoprotective effects of kaempferol: the evidence from in vivo and in vitro studies. Drug Des. Dev. Ther. 13, 3497–3514. doi: 10.2147/DDDT.S227738
Xiong, H. R., Xia, L., Wen, Z. Y., Xiong, Y. F., Wang, J., and Liu, J. (2014). Studies on microwave extraction and quality of pectin in Pyracantha fortuneanas fruit. Chin. Agric. Sci. Bull. 30, 271–275. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZNTB201403050&DbName=CJFQ2014
Xu, F., and Zha, C. C. (2013). In vivo immune activity of Pyracantha fruit red pigment extract. Guide Chin. Med. 11, 97–98. doi: 10.15912/j.cnki.gocm.2013.18.071
Xu, G. F. (2017). The cultivation and care of clown Pyracantha fortuneana 'harlequin'. Mod. Agric. Sci. Tech. 159–160. doi: 10.3969/j.issn.1007-5739.2017.14.102
Xu, H., Zhao, C., Li, Y., Liu, R., Ao, M., Li, F., et al. (2019). The ameliorative effect of the Pyracantha fortuneana (Maxim.) H. L. Li extract on intestinal barrier dysfunction through modulating glycolipid digestion and gut microbiota in high fat diet-fed rats. Food Funct. 10, 6517–6532. doi: 10.1039/C9FO01599J
Xu, H., Zhao, C. F., Tian, G. G., Qian, L. R., and Yu, L. J. (2016). Characterization of active constituents in Pyracantha fortuneana fruit extract and their effects on hyperlipidaemia, obesity, and oxidative stress in rodents. J. Funct. Foods 22, 278–290. doi: 10.1016/j.jff.2016.01.028
Xu, J. K., Yao, X. S., Zheng, J. J., Dai, Y., and Li, Y. B. (2007). Protection of Pyracantha fortuneana fruit extract on liver injury in mice loaded with restraint stress. Chin. Trad. Herb. Drugs 12, 1849–1853. doi: 10.3321/j.issn:0253-2670.2007.12.031
Xu, Y. F., Liu, S., Li, C. C., Fang, T. R., Qiu, F., Tao, X. Z., et al. (2015). Extraction and antioxidant activity of non-extractable polyphenols from Pyracantha fortuneana. Nat. Prod. Res. Dev. 27, 2109–2133. doi: 10.16333/j.1001-6880.2015.12.021
Xu, Z. G., Bai, R. Y., Yan, X. M., Li, Y., Zhou, Y., Zhang, J., et al. (2021). Effect of total flavonoids from Apocynum venetum L. Leaves on blood lipid metabolism in hyperlipidemic rats. Tradit. Chin. Drug Res. Clin. Pharmacol. 32, 208–213. doi: 10.19378/j.issn.1003-9783.2021.02.008
Xuan, S. Y., Liang, J. N., Liang, H. S., Wang, X. H., Liang, J. G., and Chen, L. (2021). Utilizing network pharmacology to explore the mechanism of flavonoids from sophora flavescens in diabetic retinopathy. Guangdong Chem. Ind. 48, 86–88. doi: 10.3969/j.issn.1007-1865.2021.08.032
Yan, Y. Y., Shen, D. H., Wang, H. J., Wu, P., Zhao, C. F., and Yu, L. J. (2015). Pyracantha fortuneana procyanidins preparation and their correlation research with antioxidant activity. Food Res. Dev. 36, 35–148. doi: 10.3969/j.1005-6521.2015.04.010
Yang, B., Yan, Y. Y., Zhong, F. X., and Peng, Y. R. (2011). Optimization of extraction process of proanthocyanidins from pyracantha. Food. Nutr. Chin. 17, 60–66. doi: 10.3969/j.issn.1006-9577.2011.05.015
Yang, F., Duan, Y. F., and Zhou, F. (2004). Isolation & purification and antioxidation of polysaccharides in Pyracantha fortuneana. Acta Bot. Boreali Occident. Sin. 26, 2126–2130. doi: 10.3321/j.issn:1000-4025.2004.11.028
Yao, W., Zhang, X., Xu, F., Cao, C., Liu, T., and Xue, Y. (2021). The therapeutic effects of naringenin on bronchial pneumonia in children. Pharmacol. Res. Perspect. 9, e00825. doi: 10.1002/prp2.825
Yao, Y. L., Shu, C., Feng, G., Wang, Q., Yan, Y. Y., Yi, Y., et al. (2020). Polysaccharides from Pyracantha fortuneana and its biological activity. Int. J. Biol. Macromol. 150, 1162–1174. doi: 10.1016/j.ijbiomac.2019.10.125
Yi, H., Peng, H., Wu, X., Xu, X., Kuang, T., Zhang, J., et al. (2021). The therapeutic effects and mechanisms of quercetin on metabolic diseases: pharmacological data and clinical evidence. Oxid. Med. Cell. Longev. 2021, 6678662. doi: 10.1155/2021/6678662
Yoon, H., Shaw, J. L., Haigis, M. C., and Greka, A. (2021). Lipid metabolism in sickness and in health: emerging regulators of lipotoxicity. Mol. Cell 81, 3708–3730. doi: 10.1016/j.molcel.2021.08.027
Younis, N. S., Elsewedy, H. S., Shehata, T. M., and Mohamed, M. E. (2021). Geraniol averts methotrexate-induced acute kidney injury via Keap1/Nrf2/HO-1 and MAPK/NF-κB pathways. Curr. Issues Mol. Biol. 43, 1741–1755. doi: 10.3390/cimb43030123
Yuan, C., Li, Z., Peng, F., Xiao, F., Ren, D., Xue, H., et al. (2015a). Combination of selenium-enriched green tea polysaccharides and Huo-ji polysaccharides synergistically enhances antioxidant and immune activity in mice. J. Sci. Food Agric. 95, 3211–3217. doi: 10.1002/jsfa.7287
Yuan, C., Li, Z., Yi, M., Wang, X., Peng, F., Xiao, F., et al. (2015b). Effects of polysaccharides from selenium-enriched Pyracantha fortuneana on mice liver injury. Med. Chem. 11, 780–788. doi: 10.2174/1573406411666150602153357
Yuan, C., Wang, C., Bu, Y., Xiang, T., Huang, X., Wang, Z., et al. (2010). Antioxidative and immunoprotective effects of Pyracantha fortuneana (Maxim.) Li polysaccharides in mice. Immunol Lett. 133, 14–18. doi: 10.1016/j.imlet.2010.04.004
Yuan, C., Wang, C., Wang, J., Kumar, V., Anwar, F., Xiao, F., et al. (2016). Inhibition on the growth of human MDA-MB-231 breast cancer cells in vitro and tumor growth in a mouse xenograft model by Se-containing polysaccharides from Pyracantha fortuneana. Nutr. Res. 36, 1243–1254. doi: 10.1016/j.nutres.2016.09.012
Yuan, C. F., Li, Z. H., Peng, F., and Xiao, F. X. (2016). Hepatoprotective effect of Pyracantha fortuneana polysaccharides on liver injury induced by CCI4 in rats. Chin. J. Public Health 32, 1485–1487. doi: 10.11847/zgggws2016-32-11-09
Yuan, Z. C., Hu, Z. Z., Gao, B., Chen, P., and Wang, F. A. (2006). Development of Pyracantha fortuneana fruit and germinated brown rice compound fruit vinegar. Food Ferment. Ind. 11, 160–161. doi: 10.13995/j.cnki.11-1082/fs.2006.11.041
Zavala-Franco, A., Hernández-Patlán, D., Solís-Cruz, B., López-Arellano, R., Tellez-Isaias, G., Vázquez-Durán, A., et al. (2018). Assessing the aflatoxin B1 adsorption capacity between biosorbents using an in vitro multicompartmental model simulating the dynamic conditions in the gastrointestinal tract of poultry. Toxins 10, 484. doi: 10.3390/toxins10110484
Zeng, J., Yang, R., Li, Y. J., Chen, T., Yan, J. Y., Li, J., et al. (2016). Research progress on chemical constituents and pharmacological effects of Pyracantha fortuneana. Hunan J. Trad. Chin. Med. 32, 226–228. doi: 10.16808/j.cnki.issn1003-7705.2016.10.098
Zhang, B. P., and Li, L. (2020). Pyracantha planting skills and application in garden landscape. Rural Prac. Tech. 4, 48–49.
Zhang, H., Mao, Y. T., Ma, M. X., Ma, K., Wang, M. L., and Tao, G. C. (2017). Development of compound beverage of Pyracantha fortuneana and Actinidia chinensis. China Brew. 36, 177–181. doi: 10.11882/j.issn.0254-5071.2017.08.039
Zhang, L., Liu, C., and Yuan, M. (2020). Eriodictyol produces antidepressant-like effects and ameliorates cognitive impairments induced by chronic stress. Neuroreport 31, 1111–1120. doi: 10.1097/WNR.0000000000001525
Zhang, Q., Duan, H. X., Li, R. L., Sun, J. Y., Liu, J., Peng, W., et al. (2021). Inducing apoptosis and suppressing inflammatory reactions in synovial fibroblasts are two important ways for Guizhi-Shaoyao-Zhimu decoction against rheumatoid arthritis. J. Inflamm. Res. 14, 217–236. doi: 10.2147/JIR.S287242
Zhang, Y., Li, M., Liu, Z., and Fu, Q. (2021). Arbutin ameliorates glucocorticoid-induced osteoporosis through activating autophagy in osteoblasts. Exp. Biol. Med. 246, 1650–1659. doi: 10.1177/15353702211002136
Zhang, Y. Z., Huang, M., and Liu, S. (2014). Extraction and antioxidant effect of proanthocyanidins from pyracantha. Asia Pac. Trad. Med. 10, 26–28.
Zhang, Z., Zhou, Y., Wang, S., and Huang, X. (2018). Spatial distribution of stony desertification and key influencing factors on different sampling scales in small karst watersheds. Int. J. Environ. Res. Public Health 15, 743. doi: 10.3390/ijerph15040743
Zhao, C. F., Lei, D. J., Song, G. H., Zhang, H., Xu, H., and Yu, L. J. (2015). Characterisation of water-soluble proanthocyanidins of Pyracantha fortuneana fruit and their improvement in cell bioavailable antioxidant activity of quercetin. Food Chem. 169, 484–491. doi: 10.1016/j.foodchem.2014.07.091
Zhao, J., Sun, S. Z., Guan, S. T., Zhang, H. M., and Ding, A. L. (2012). Effects of Pyracantha fortuneana polysaccharide on scavenging oxygen free radical and anti-lipid peroxidation. J. Hubei Univ. Med. 31, 464–467.
Zhou, W. B., Wang, X. J., and Gan, X. H. (2008). Study on the fermentation conditions of Pyracantha fortuneana vinegar in shake flasks. J. Southwest Univ. Nat. Sci. Educ. 30, 152–155. doi: 10.13718/j.cnki.xdzk.2008.12.014
Zhou, Y., Shan, H. T., and Wen, Y. (2017). Nutritional components analysis of Pyracantha fortuneana leaves in different seasons. Feed Rev. 10, 11–14.
Zhou, Y. J. J., Zhou, W., Long, B., Chen, Q. S., Huang, K. X., and Zhu, Y. W. (2018). Research progresses on functional components and extraction technologies of Pyracantha fruit. Farm Prod. Proc. 22, 65–68. doi: 10.16693/j.cnki.1671-9646(X).2018.11.048
Keywords: Pyracantha fortuneana (Maxim.) Li, phytochemistry, botany, pharmacological properties, product development
Citation: Wang L, Li R, Zhang Q, Liu J, Tao T, Zhang T, Wu C, Ren Q, Pu X and Peng W (2022) Pyracantha fortuneana (Maxim.) Li: A comprehensive review of its phytochemistry, pharmacological properties, and product development. Front. Sustain. Food Syst. 6:940900. doi: 10.3389/fsufs.2022.940900
Received: 10 May 2022; Accepted: 14 July 2022;
Published: 10 August 2022.
Edited by:
Chibuike C. Udenigwe, University of Ottawa, CanadaReviewed by:
Baskaran N, Indian Institute of Food Processing Technology, IndiaCopyright © 2022 Wang, Li, Zhang, Liu, Tao, Zhang, Wu, Ren, Pu and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Ren, NDAxNjI3NDA2QHFxLmNvbQ==; Xufeng Pu, cHhmNjhAMjYzLm5ldA==; Wei Peng, cGVuZ3dlaUBjZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.