
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 12 September 2022
Sec. Nutrition and Sustainable Diets
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.935425
This article is part of the Research TopicDietary Change Strategies for Sustainable Diets and their Impact on Human Health - Volume 1View all 44 articles
The consumption of probiotic foods has grown rapidly, and these are generally found in dairy matrices where their growth is favored. Therefore, this study aimed to develop a new probiotic snack made from quinoa and added with spore-forming probiotic bacteria in two concentrations of 0.3 and 0.35%. The probiotic was added by spraying, after the extrusion process, together with salt and oil, at 70°C under dry conditions. Bacterial viability, resistance to simulated gastric juice, physical, chemical, and sensory tests were then evaluated during 120 days of storage at room temperature (20°C) and compared to a controlled snack without probiotic. The probiotic Bacillus coagulans was tested for the molecular identification and inhibition of pathogenic bacteria. Viability assessment was remained above 107 CFU/g of snacks. The intestinal tract simulation resistance test showed a viability of 70%. The physicochemical and sensory properties evaluated had no significant changes during storage time compared to control snack. The results of the taxonomic analysis indicate that the analyzed strain has, on average, 98% identity in 98% of its length belonging to Bacillus coagulans and Bacillus badius species. The probiotic showed inhibition against pathogenic bacteria. The new snack with probiotic is stable during storage.
Quinoa is considered a pseudograin rich in protein (13.8–16.5%), carbohydrates (52–69%), lipids (2–9.5%), fiber (7–9.7%), vitamins and minerals (3.4%), and has a good balance of essential amino acids (Abugoch, 2009). In addition to the macronutrient content, according to a study realized by Pereira et al. (2019), three varieties of quinoa (red, black, and white color), high contents of proteins, carbohydrates, fat-soluble compounds, the presence of tocopherols, and fatty acids were evidenced, being γ-tocopherol the main isoform and linoleic acid the one found in higher concentrations. These are bioactive compounds that can generate benefits to human health, especially preventing cancer, reducing the risk of cardiovascular, inflammatory, gastrointestinal diseases, and improving metabolic health (Navruz-Varli and Sanlier, 2016). Therefore, quinoa-based foods are of great interest to the food industry.
This grain can be used to produce an innovative probiotic food, as there is a growing interest in the consumption of non-dairy probiotic foods, due to milk shortages in some countries, vegetarian and vegan trends, problems with cholesterol, allergen content, and lactose intolerances (Konuray and Erginkaya, 2020). The snacks that exist in the market are mainly made from corn or rice starch, and these present low nutritional quality due to their high-calorie content and scarcity of nutrients (Saldanha do Carmo et al., 2019). Therefore, a new quinoa probiotic food can be a healthier snack option. In the category of functional foods, those containing probiotic microorganisms represent the largest segment in the market (de Almada et al., 2016). This is because several health benefits have been demonstrated such as: facilitating lactose digestion, protecting against gastrointestinal diseases, balancing the immune system, preventing and treating dermatological diseases, and protecting against colon cancer (Sucupira and Souza, 2019).
Probiotic microorganism strains must present and maintain characteristics that guarantee their growth and survival in the food that contains them or to which they are added, as well as during their transit through the stomach and small intestine, and their ability to adhere to the mucosa of the large intestine; among the main characteristics are: viability during food processing and storage, stability against gastric acids and bile, adherence to the intestinal mucosa, and production of antimicrobial substances (Cao et al., 2020).
This type of lactic acid bacteria is generally present in dairy foods, which presumes a challenge for their incorporation in dry matrices such as cookies, snacks, and pasta among others, because most probiotic microorganisms cannot survive after heat treatment such as Lactobacillus and Bifidobacterium and some Saccharomyces species (Tripathi and Giri, 2014), for this reason, spore-forming probiotics are a viable alternative.
Bacillus coagulans is a Gram-positive, facultative, anaerobic, non-pathogenic, spore-forming, and lactic acid-producing bacterium (Lee et al., 2019). It is heat resistant; the optimal growth temperature for B. coagulans is 35 to 57°C and the optimal growth pH is 4 to 7 (Šipailiene and Petraityte, 2018). Furthermore, Soares et al. (2019) compared the resistance to gastrointestinal fluids of three probiotic strains Lactobacillus, Bifidobacterium, and Bacillus that were included in solid and liquid foods, and found that the latter had survivals of over 80%. The probiotic strain Bacillus coagulans has a good antibacterial activity due to the production of bacteriocins; in this respect, Gu et al. (2015) found that Bacillus coagulans CGMCC 9951 showed good antibacterial activity against several indicator bacteria, namely, Escherichia coli, Pasteurella multocida, Staphylococcus aureus, Streptococcus suis, and Listeria monocytogenes. Although B. coagulans produces acid, it does not produce gas from maltose, raffinose, mannitol, and sucrose fermentation; in addition to lactic acid production, some strains also produce thermostable α-amylase. For this reason, B. coagulans is important from an industrial point of view (Konuray and Erginkaya, 2018).
Several studies report Bacillus coagulans counts in food between 106 and 109 CFU in food. Bacillus coagulans GBI-30, 6086 incorporated into a wheat flour-based functional pasta remained viable during the pasta making and cooking processes (~109 CFU/100 g) (Marcial-Coba et al., 2019). Majeed et al. (2016a) in their study prepared a series of foods incorporated with Bacillus coagulans, obtaining counts above 107 CFU/g. In their research, Almada-Érix et al. (2022) proved that the probiotic Bacillus coagulans added to bread showed high resistance to the baking process and was above 107 CFU/g. Majzoobi et al. (2019) in the results of their study showed that in the production of symbiotic bread an acceptable number of GanedenBC30 (more than 106 CFU/g) was obtained even after storage for 3 days at room temperature.
Bacillus coagulans BC30 strain is commercial and proprietary, and it is recommended to investigate molecular identification because the commercial probiotic strain may undergo several changes during the continuous and multiple years of commercial production (Sanders et al., 2014), which may alter physiological characteristics due to the living nature of probiotics. For this reason, genetic and phenotypic changes, by accident or design, can affect the efficacy and/or safety of commercial probiotics.
This work seeks to determine the stability and quality of Bacillus coagulans reference BC30 incorporated in an expanded snack made from quinoa during processing and storage at room temperature in a modified atmosphere for 120 days. In addition, this research sought to evaluate the resistance of the snack with probiotics to simulated intestinal transit conditions. To validate the commercial strain of Bacillus coagulans, molecular identification and inhibition of pathogenic bacteria was performed.
Bacterial cultures. The lyophilized strain of Bacillus coagulans GBI-30, 6086 was acquired through a commercial supplier (Ganeden BC30, Kerry USA). The quinoa was provided by Segalco S.A.S. (Popayán, Colombia). The microorganisms used—Listeria monocytogenes ATCC 49594, Bacillus cereus ATCC 14579, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 25922, and Staphylococcus aureus ATCC 6538—were obtained from the von Humboldt gene bank of the Microbiology and Applied Biotechnology Research Group (MIBIA) of the Universidad del Valle (Cali, Colombia).
The snacks were provided by Seguridad Alimentaria de Occidente SEGALCO S.A.S., a company located in the city of Popayán, Colombia. All snacks were prepared following the company's methodology (probiotic concentration was selected by the company based on sensory analysis and production cost studies). The probiotic snacks were added by spray with two concentrations of B. coagulans spores at 0.3% (w/w) snack with probiotic 1 (SP1) and 0.35% snack with probiotic 2 (SP2), together with salt and oil, after the extrusion process. A control snack (SC) was used without the addition of probiotics. This concentration was based on the manufacturer's recommendations, preliminary studies, and other research (Adibpour et al., 2019). The samples were packed in a modified atmosphere packaging containing nitrogen, and the packaging was multilayer laminated, and water vapor permeability WVTR and oxygen transmission rate OTR of the packaging were 0.7 g/m2.day and 1.0 cc/m2.day, respectively, and stored at room temperature (20°C) for 120 days. A snack without SC probiotic was used as a control, during which time physical and chemical tests were measured. Furthermore, the viability of the probiotic was verified.
Molecular identification was performed to corroborate the strain as a probiotic belonging to the Bacillus species and its antimicrobial activity against Gram-positive and Gram-negative bacteria.
Total DNA was extracted using lysozyme (10 mg mL−1) followed by the addition of the DNAzol reagent (Thermo Fisher Scientific). PCR was performed using a Gene Cycler (Bio-Rad). Purification of PCR fragments and sequencing by Sanger method, using primers 337F, 518F, 800R, and 1100R of the 16S ribosomal gene. Taxonomic analysis of the problem sequence used the BLAST tool (Basic Local Alignment Search Tool), from NCBI (National Center for Biotechnology Information), compared against the reference RNA database “refseq_rna.” Taxonomic analysis of the problem sequence using the “Classifier” and “SeqMatch” tools hosted on the RDP (Ribosomal Data Project) website. The first tool is used to determine the taxonomy of the problem sequence; the second tool is used to identify the most similar sequences, in the RDP database, to the problem sequence. Both perform the comparison using RDP's own “16S rRNA training set 18” database.
Multiple alignments, using the multiple sequence comparison by log-expectation (MUSCLE) algorithm, of the problem sequence with the 30 most similar sequences were reported by BLAST. Generation of a phylogenetic tree was performed using the Tamura-Nei genetic distance model (TN93), with the “Neighbor-Joining” method and the “Bootstrap” method with 1000 replicates. The taxonomic analysis and classification of the sequence was based on that presented by Majeed et al., 2016b with some modifications.
Antimicrobial activity was performed according to the methodology by Sen et al. (2010) with slight modifications. Bacillus coagulans commercial strain was seeded in Man, Rogosa and Sharpe (MRS) broth (Scharlau, Spain) for 12 hours, cells were obtained by centrifugation of the culture broth at 3.800 rpm for 15 min and seeded by depth on glucose yeast extract (GYE) agar then the agar was allowed to dry for 15 min, then discs of approximately 0.6 mm in diameter were obtained and placed in a 15 mL layer of Mueller Hinton (Merck, Germany), containing approximately 106 CFU/mL of the indicator strains previously grown in Tryptone Soy broth (TSA) (Scharlau, Spain) either Salmonella typhimurium ATCC 14028 or Listeria monocytogenes ATCC 49594 or Bacillus cereus ATCC 14579 or Bacillus subtilis ATCC 6633 or Escherichia coli ATCC 25922 or Staphylococcus aureus ATCC 6538. The plates were allowed to stand for 30 min to allow the sample to diffuse into the agar and were subsequently taken to incubation at 37°C for 24 h. After incubation, the zone of inhibition was measured and recorded in mm.
Water activity was determined in triplicate at 20°C, using a dew point water activity meter (AquaLab Series 3TE, Decagon Devices, Inc. USA).
pH values of SP1, SP2, and SC were determined in triplicate, each replicate in quintuplicate according to the AOAC Method No. 943.02, using an Orion pH meter, model Three Stars (Thermo Fisher Scientific, Waltham, MA, USA), equipped with a model 2A04 penetration electrode (Analizador, São Paulo, Brazil).
Moisture analysis of the snacks was performed by loss on drying (130°C/3 h), based on (AOAC Official Method, 930.15, 2005).
The color of the extrudate was determined using Konica Minolta Spectrophotometer CM-5, controlled by SpectraMagic NX software, with D65 illuminant and 10° observer angle. The samples were conditioned with a food processor. Samples were analyzed in triplicate, taking ~5 g of sample. Luminosity (L) and chromaticity parameters a* (−a * = green and + a * = redness) and b * (−b * = blue and + b * = yellow) and color variation (ΔE) were measured according to the Equation (1).
Texture properties were measured as described by Li et al. (2019) with some modifications using a texture analyzer (Shimadzu EZ-L, USA) with a 5 kN load cell and a 5 mm flat-head cylindrical probe. The SP1, SP2, and SC extrudates were randomly divided into three groups. Five measurements were performed for each group and the results were reported as the average of these three groups for each extrusion series. Three texture quality parameters, hardness, crispness, and crunchiness, were obtained from the texture analysis test curve with time (s) on the x-axis and force (N) on the y-axis. Hardness (N) is defined as the maximum force, crispness is the total number of positive peaks, and crunchiness (N s) is the linear distance from the test curve.
Viable count of B. coagulans was performed according to the methodology of the probiotic supplier and Majeed et al. (2016a), with some modifications. About 1 g of SP1 or SP2 was dissolved in 199 ml of sterile saline (0.9% NaCl, w/v). Then 30 ml was taken in a sterile tube and brought for 30 min at 70°C in a water bath, then, cooled to approximately 45°C before pipetting, cultured on GYE agar at 37°C for 48 h, under aerobic conditions. Survival was determined in duplicate under two concentrations 0.3 and 0.35: w/w, at days 0, 10, 20, 40, 60, and 120.
The evaluation of the survival of the probiotic Bacillus coagulans included in snack SP1 subjected to simulated gastric and enteric conditions was performed according to Bedani et al. (2013) with some modifications.
One gram of SP1 was dissolved in 199 mL of sterile phosphate buffered saline (PBS) pH 7.4, 10 mL of this solution was taken in triplicate into flasks, then adjusted to pH 2 with 1 N HCl, and pepsin (from porcine stomach mucosa, Sigma-Aldrich) was added to the samples to a concentration of 3 g/L. The flasks were incubated at 37°C, with shaking at 150 rpm (Thermo Scientific MAxQ-4450 Shaker, USA) for 3 h, giving rise to the simulated gastric phase. In this phase, 1 mL was taken and seeding was performed on GYE agar at 37°C for 48 h under aerobic conditions. Subsequently, the pH of the samples was increased to 6.8 using an alkaline 1N NaOH solution and 30 mL of sterile phosphate buffer solution (150 ml of 1 N NaOH 14 g of PO4H2Na.2 H2O and distilled water up to 1 L), then Bile (bovine bile, Sigma-Aldrich) and pancreatin (porcine pancreas, Sigma-Aldrich) were added until reaching a concentration of 0.6 g/L and 1 g/L, respectively, in this phase, the three samples were left for 3 hours. About 1 ml of sample was taken for seeding, similar to the previous phase. Counts are expressed in log CFU/g of SP1. Survival rate at t0 and tf is expressed according to Equation (2) (Guo et al., 2009).
Where, N is the number of CFU (colony forming units).
The sensory evaluation was carried out using the hedonic test methodology with 14 panelists from the SEGALCO S.A.S. factory, composed of five women and nine men between 25 and 35 years of age. The panelists evaluated the perception parameters of general appearance, color, texture, aroma, flavor, chewiness, and acidity of the samples, giving scores from 5 to 1 (5 points, extremely good and 1 point, extremely low). For the acidity parameter, 1 is not perceived and 5 is perceived as high acidity. The study was reviewed and approved by the ethics committee of the University of Cauca and informed consent was obtained from each subject before they participated in the study.
Statistical analyses were performed using Minitab V 18 statistical software. Viability assessment during storage was performed in duplicate and in vitro gastric simulation experiments were performed in triplicate. Results were expressed as means ± standard deviation. One-way ANOVA was used to compare the viability of B. coagulans, after 0, 10, 20, 40, 40, 60, and 120 days of storage at 20°C. For sensory analysis, a Kruskal–Wallis non-parametric test was performed. Significance was set at p < 0.05. Tukey was used to compare the concentrations with a snack control without probiotics. The graphs were made in the Oring program.
Multifocus sequence typing was performed on the B. coagulans BC30 samples. The RDP classifier determined that this is a sequence of a microorganism belonging to the genus Bacillus.
However, the resolving power of this classifier does not allow assigning a genus or species (S1-A). Comparison with the RDP 16S sequence database using the SeqMatch tool (S1-B) against the cultured isolate indicates that the assembled problem sequence (Scheme 1-S1-C) shows higher homology with sequences of Bacillus coagulans species. The results of the taxonomic analysis of the assembled 1525 bp problem sequence against the NCBI ref_seq database (S1-C) indicate that it has, on average, 98% identity over 98% of its length with 16S ribosomal gene sequences belonging to Bacillus coagulans and Bacillus badius species. The distance tree (Figure 1) constructed from the 30 closest available sequences of culturable microorganisms in the NCBI RefSeq_RNA database shows the analyzed sequence clusters with sequences of the species Bacillus coagulans (S1-E). In the case of this isolate, according to the results of searches in the different databases and the origin of the sample, it is concluded that it belongs to the species Bacillus coagulans (S1-E).
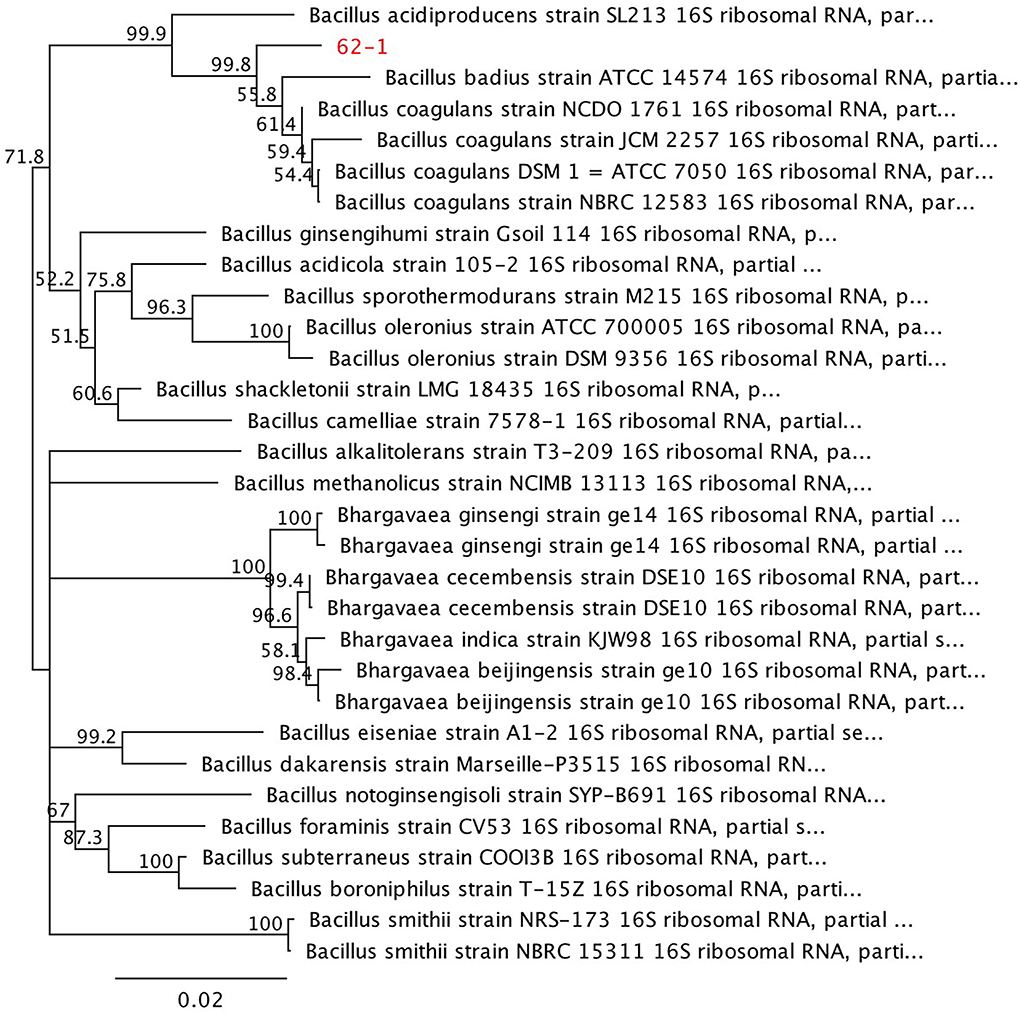
Figure 1. Phylogenetic tree by distances constructed from the thirty closest available sequences of culturable microorganisms according to the NCBI RefSeq_RNA database.
Probiotics of the genus Bacillus produce bioactive microbial secondary metabolites (bacteriocins), which regulate growth processes, applications, and/or exhibit response (regulation, inhibition, stimulation), in addition to bacteriocins, B. coagulans can also secrete other antimicrobial substances such as lactic acid (an important substance in the human intestine) and acetic acid (Cao et al., 2020).
As shown in Table 1, Bacillus coagulans generates an inhibitory halo against the growth of the pathogens under study. According to Bernardeau et al. (2017), the metabolites produced by the Bacillus genus are bacteriocins and inhibitory substances (e.g., subtilin and coagulin), an anionic antibacterial substance produced by B. coagulans that is a broad-spectrum agent against Gram-positive bacteria, which are involved in the transmission of foodborne diseases.
Furthermore, Bacillus can generate metabolites as antibiotics (e.g., surfactin, iturin, and fengycin) which may have an antibacterial or antifungal effect (Mondol et al., 2013). In their study, Abdhul et al. (2015) found that Bacillus coagulans strain [BDU3] generates antimicrobial inhibition on bacteria such as Bacillus cereus MTCC 430, Staphylococcus aureus MTCC 3160, and Enterococcus sp. MTCC 9728.
Moreover, as mentioned by Majeed et al. (2016b), commercial probiotic strains may have phenotypic changes during the continuous and multiple years of commercial production affecting their efficacy or safety; therefore, they evaluated the probiotic potential in vitro, finding that the Bacillus coagulans strain studied produces antimicrobial agents that inhibit the growth of pathogenic Gram-positive and Gram-negative bacteria.
As shown in Table 2, according to the analysis of variance, the two concentrations and storage time have no significant influence on the pH of snack samples. B. coagulans is a lactic acid-producing probiotic; however, during stress conditions such as low humidity, it is in the spore state, i.e., during the 120 days of storage, it has not initiated metabolic activity. According to Šipailiene and Petraityte (2018) the pH required by Bacillus coagulans strain is between 4 and 7, in snack samples SP1 and SP2 it was maintained between 6.01 and 5.52 during days 0 and 120, respectively. In order to survive and multiply, microorganisms have to maintain a stable pH in the cytoplasm, which ensures optimal functionality and integrity of the cytoplasm structure. In our study, the modified atmosphere packaging maintains the stable characteristics of the facultative anaerobic probiotic, since for this type of bacteria oxygen is a toxic element (Šipailiene and Petraityte, 2018).
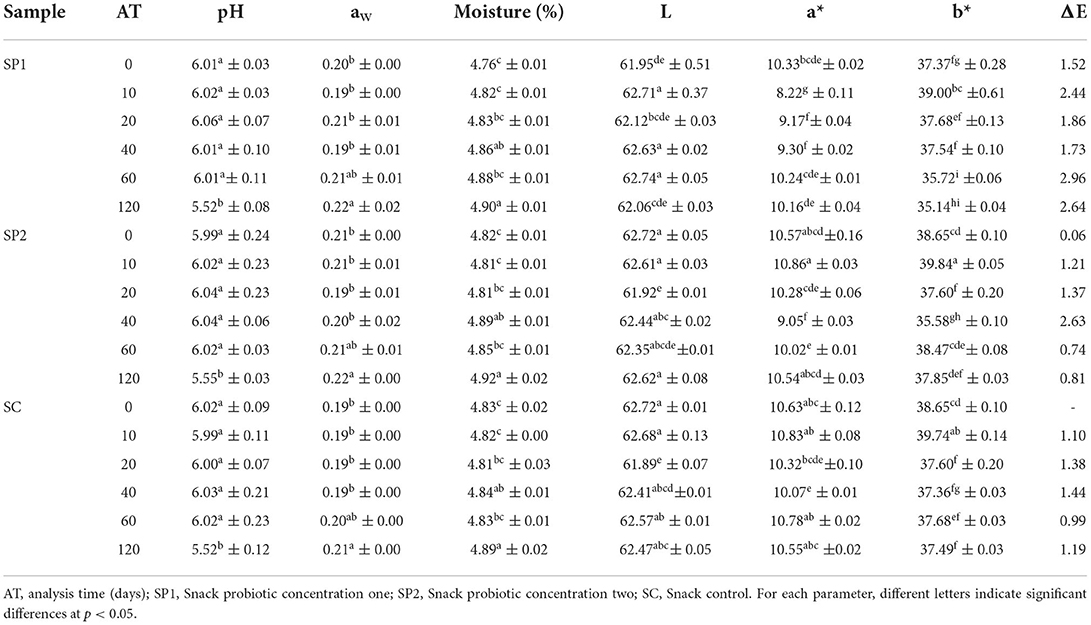
Table 2. Evaluation of the physicochemical characteristics of snacks with and without probiotic addition.
Color is one of the important characteristics of the acceptability of a food product. Table 2 shows the parameters of L* (Brightness), a* (Red/Green), and b* (blue/yellow). According to the analysis of variance, the color parameter L* is only affected by time and the parameters of a* and b* are influenced by concentration and by time (p < 0.05). The total color change (ΔE), which shows the color variation compared to the snack without probiotic addition and at day 0, was between 0.06 and 2.96, these values indicate no apparently perceptible changes during the storage time and the concentration of probiotic added. According to studies by Muñoz et al. (2022), in cereal mixtures extruded from quinoa, most of the population perceives a total color difference >3. Therefore, as shown in Figure 2, the addition of probiotics to the snack does not result in a significant color change. According to the results of Konuray and Erginkaya (2020), they had no change in color parameters over time in pasta added with probiotic lactic acid bacteria. Likewise, Akman et al. (2019) reported in their study that the enrichment of apple slices with Lacticaseibacillus paracasei had no significant effect on color changes in all dried apple samples.
Figure 3 shows the effect of the two concentrations and days of storage with respect to the control. According to the analysis of variance, the factors of concentration and time have no significant effect p > 0.05 on the strength (N), crunchiness, and crispness (NS) during the 120 days of storage, that is to say, that the SP1, SP2, and SC matrices do not have significant changes over time, due to the fact that the packaging maintains the humidity and water activity. According to Ananta et al. (2005), to maintain stability, water activity should not exceed 0.25 and moisture content should be 4–7%. In our study, aw and moisture contents were maintained between 0.19–0.22 and 4.76–4.92, respectively, during the 120 days of storage.
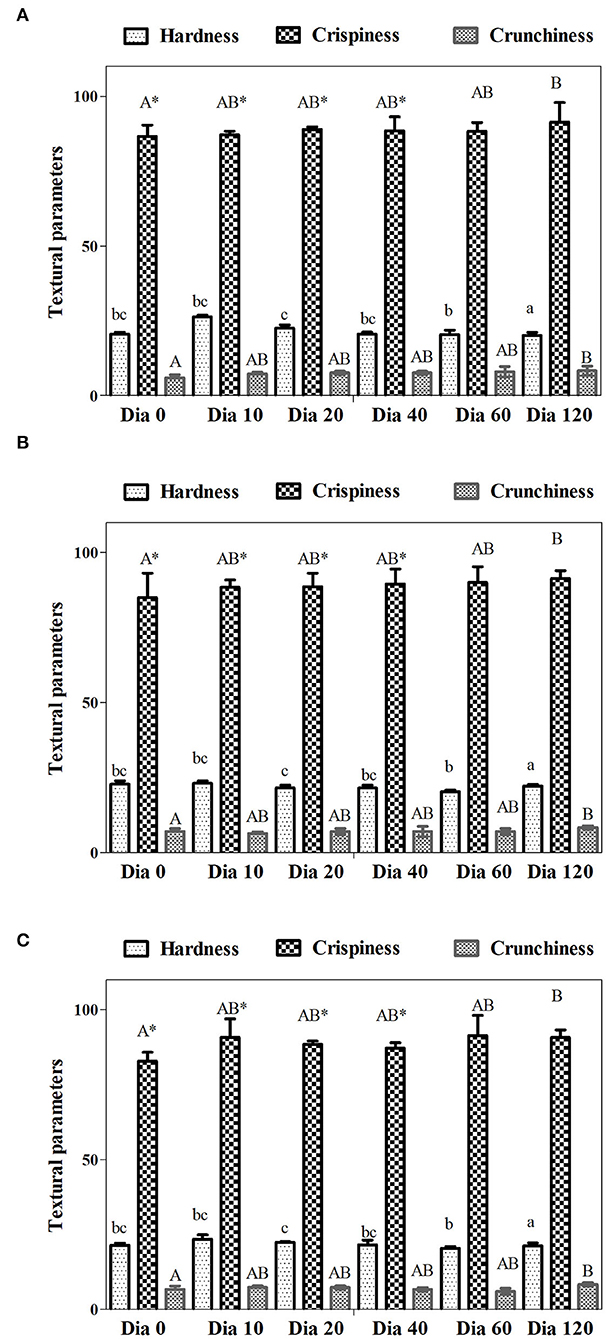
Figure 3. Textural properties (hardness, crunchiness, and crispiness) of quinoa flour extrudates obtained at different amounts of probiotics. (n = 3 for extrusion runs, n = 3 for texture measurements). (A) SP1; (B) SP2; (C) SC. Within hardness, crunchiness, and crispiness, values followed by different letters are significantly different (p < 0.05).
The extrusion process is a versatile and low-cost technology used for the production of cereal-based snacks, which transforms starch and protein-based solid materials into a viscoelastic fluid under high pressure and temperature conditions (Li et al., 2019). The addition of the probiotic does not significantly alter the texture parameters, which is corroborated in the sensory analysis, where no significant changes are perceived.
The analysis is performed at room temperature, to prove that the probiotics incorporated in snacks made from quinoa remain under shelf-life conditions.
According to the analysis of variance, the two concentrations analyzed, do not present effect on the counts (p > 0.05), however, the different days analyzed do present effect on the count (p < 0.05). As shown in Table 3, the counts from 0 to 120 days are between 7.39 log CFU/g and 7.95 log CFU/g, respectively. During the period studied, the viability of the probiotic increased by 7.57% with respect to day 0, due to the fact that during the probiotic inclusion process no extreme drying or pressure processes are performed. It is possible that the sporulated probiotic strain does not affect its viability and remains during the period of analysis, without changing the organoleptic properties of the snack as corroborated in the sensory analysis, because the packaging maintains a water activity lower than 0.25, where the molecular mobility of the matrix is limited, an aspect that discourages metabolism; in this sense, according to studies by Ananta et al. (2005) in encapsulated bacteria, where the water activity was 0.2, bacterial viability after 45 days was higher than 105 CFU/g. During the 120 days, the probiotic remained stable, i.e., it did not grow exponentially, an aspect that allows preserving the physico-chemical characteristics in the snack, and similar results were obtained by Almada-Érix et al. (2022) in samples of bread added with Bacillus coagulans GBI-30 6086.

Table 3. Evaluation of the viability of spore-forming bacteria in a quinoa snack during storage time at room temperature (20°C) and dry place.
Furthermore, an increase in the count on days 60 and 120 is possibly due to some of the latent probiotics becoming viable and active. According to Zendeboodi et al. (2020), probiotics such as Bacillus coagulans are inactive probiotics. Although they are viable, they are not active and the rate of growth and/or generation of metabolites are almost stopped, they are called “pseudoprobiotics.”
The Bacillus coagulans strain retained its viability during the 120 days of storage above 6 log CFU/g, which is the permitted limit for probiotic foods (Konuray and Erginkaya, 2020). Bacillus coagulans is a strain labeled as a GRAS food ingredient by the US FDA, it is a Gram-positive, microaerophilic, spore-producing Bacillus coagulans, which makes it heat resistant, and therefore a good choice for products with low humidity. According to studies conducted by Majeed et al. (2016a), the stability of Bacillus coagulans MTCC 5856 was analyzed in different food matrices after 12 months they obtained viability above 80%. Likewise, Majzoobi et al. (2019) obtained baked and then frozen food, added with Ganeden BC30 that retained a viability of 7.35 log CFU/g after 56 days of storage.
Viability is the ability of these microorganisms to remain alive, both in the organism and in the consumer's intestine for a given time, in order to achieve the benefits of such foods.
The survival of freeze-dried B. coagulans in spore present in the SP1 subjected to simulated gastrointestinal conditions in vitro is shown in Table 4. Based on the results, the probiotic included in the snack resists the passage through the simulation. According to Casula and Cutting (2002), spores of the genus Bacillus can germinate and proliferate in the gut of mice, indicating that spores can germinate, grow and then sporulate in the gut. According to Cao et al. (2020), the life cycle of Bacillus coagulans in the intestinal tract is described as follows: initially, it enters as a spore and continues as such during the time it remains in the stomach for almost 3 h, later the spores germinate in the small intestine, and then the live bacteria will travel down to the large intestine and sporulate in the lower part.
In future studies, it is valuable to perform in vivo tests to demonstrate the resistance of snacks with probiotic addition.
According to the above, the population of the spore-forming probiotic showed a reduction from 0 to 3 h test of 1.76 and between the 3 to 6 h period the reduction was 0.26 log CFU/g. The greatest decrease in viability of this strain was observed in the gastric phase, suggesting its high sensitivity to simulated gastric juice containing HCl and pepsin. In studies performed with a similar strain, Sui et al. (2020) found higher survival values of the sporulated probiotic above 80%, and in our assay, the percentage is almost 70%, i.e., more than half passed the gastric phase. According to Marcial-Coba et al. (2019) in order for probiotics to provide a benefit, they must pass through the acidic conditions of the stomach, and the spores of B. coagulans and other Bacillus species remain in their spore form during passage through the stomach and duodenum then germinate in the jejunum and ileum, with subsequent transient colonization of the small intestine, where vegetative cells can produce active metabolites such as L (+) lactic acid and interact with the host and intestinal microbiota. Maathuis et al. (2010) investigated the survival of the GanedenBC30 spore during its passage through the upper gastrointestinal tract in a dynamic, validated, in vitro model of the stomach and small intestine. In their study, they also found that the survival of GanedenBC30 was 70%.
A 5-point scale, 1 lowest and 5 highest, was used in this study. As shown in Figure 4, the panelists rated the SC, SP1, and SP2 between 4 and 5 for the quality parameters analyzed, with the exception of acidity, which for the three samples is 1, i.e., it is not perceived. There are no significant differences between the storage time and the control snack without probiotics. Our results corroborate that the physicochemical properties remain stable during the 120 days of analysis in metalized packaging in a modified atmosphere, so the panelists rated the snacks with high quality, both the control and those added with probiotics.
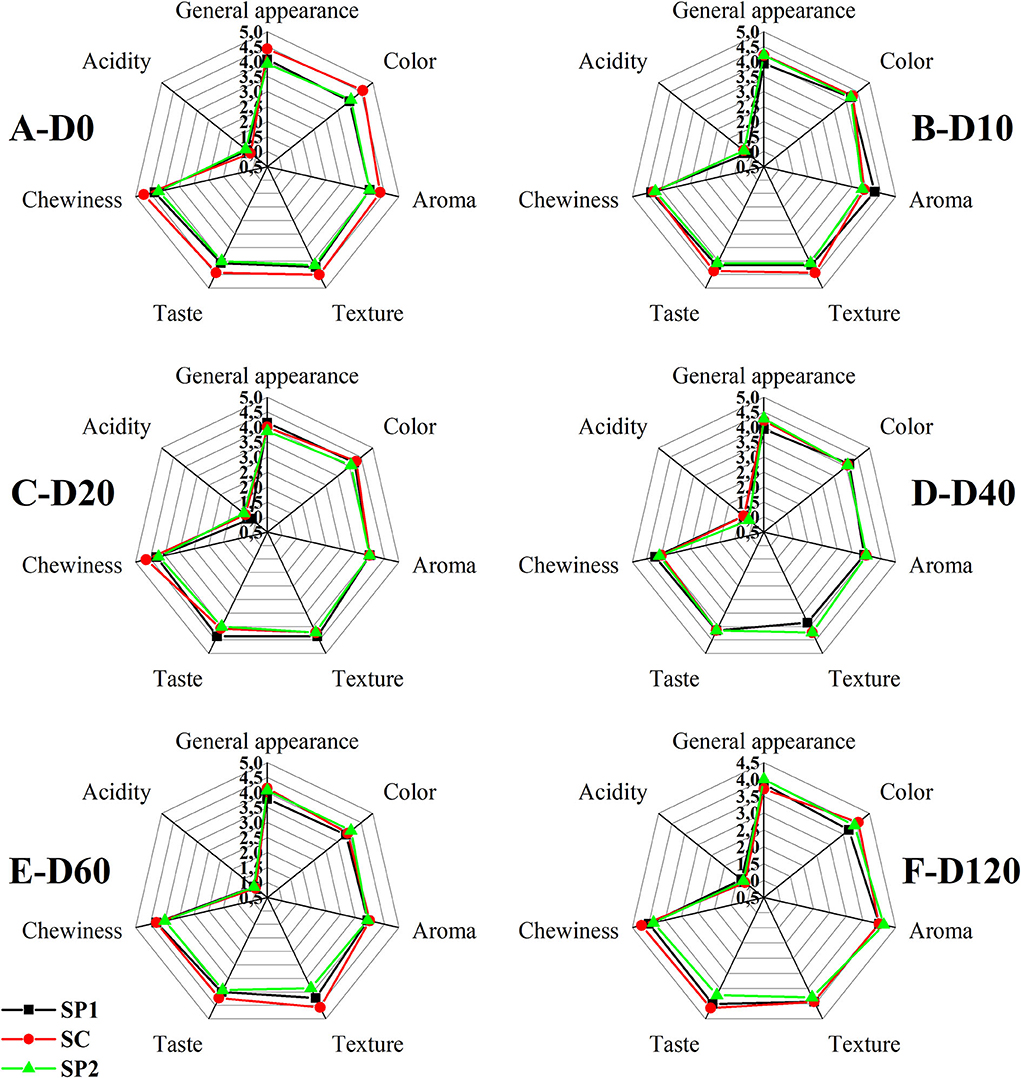
Figure 4. Sensory evaluation of samples (A, day 0; B, day 10; C, day 20; D, day 40; E, day 60; F, day 120; SP1, snack probiotic concentration one; SP2, probiotic concentration two, SC, control).
In studies conducted by Adibpour et al. (2019), they found no differences between the control and a product made with caramel and sporulated probiotic. Konuray and Erginkaya (2020) in their study with Bacillus coagulans-incorporated pasta found that sensory attributes were not affected during 6 months of storage.
The results of the study showed that the SP1 and SP2 quinoa probiotic snacks made with the addition of B. coagulans GBI-30 have a final concentration of approximately 107 UFC/g, and can be stored for 120 days at room temperature, low humidity, and aw: 0.2 ± 0.2 without affecting their quality. Sporulated B. coagulans bacteria showed inhibition against the pathogenic bacteria tested. Survival during simulated intestinal tract conditions in the food matrix SP1 showed 70%. The addition of the probiotic GBI-30 showed no statistically significant effect on the sensory characteristics, texture, color, acidity, and overall appearance of the samples during storage compared to the control snack. The probiotic snacks produced in our study can be considered an acceptable probiotic food after 120 days of storage, which can be realized at the industrial level. Due to the growing consumer interest in non-dairy probiotic food alternatives, the quinoa-based snack produced in this study is a viable alternative to a vegan probiotic food.
The original contributions on the genetic identification test presented in the study are included in the article/Supplementary material, additional inquiries can be directed to the corresponding author(s).
JS and KM: methodology, formal analysis, investigation, and writing original draft. JH: methodology, formal analysis, and supervision. All authors contributed to the article and approved the submitted version.
The authors would like to thank the Universidad del Cauca and SEGALCO S.A.S. for their support during the development of our research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2022.935425/full#supplementary-material
Abdhul, K., Ganesh, M., Shanmughapriya, S., Vanithamani, S., Kanagavel, M., Anbarasu, K., et al. (2015). Bacteriocinogenic potential of a probiotic strain Bacillus coagulans [BDU3] from Ngari. Int. J. Biol. Macromol. 79, 800–806. doi: 10.1016/j.ijbiomac.2015.06.005
Abugoch, J. L. E. (2009). “Quinoa (Chenopodium Quinoa Willd.): composition, chemistry, nutritional, and functional propertiesm,” in Advances in Food and Nutrition Research, 1st Edn, Vol. 58. Santiago de Chile: Elsevier Inc. doi: 10.1016/S1043-4526(09)58001-1
Adibpour, N., Hosseininezhad, M., and Pahlevanlo, A. (2019). Application of spore-forming probiotic Bacillus in the production of Nabat - a new functional sweetener. LWT. Food Sci. Technol. 113, 108277. doi: 10.1016/j.lwt.2019.108277
Akman, P. K., Uysal, E., Ozkaya, G. U., Tornuk, F., and Durak, M. Z. (2019). Development of probiotic carrier dried apples for consumption as snack food with the impregnation of Lactobacillus paracasei. LWT. Food Sci. Technol. 103, 60–68. doi: 10.1016/j.lwt.2018.12.070
Almada-Érix, C. N., Almada, C. N., Souza Pedrosa, G. T., Paulo Biachi, J., Bonatto, M. S., Schmiele, M., et al. (2022). Bread as probiotic carriers: resistance of Bacillus coagulans GBI-30 6086 spores through processing steps. Food Res. Int. 155, 111040. doi: 10.1016/j.foodres.2022.111040
Ananta, E., Volkert, M., and Knorr, D. (2005). Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. Int. Dairy J. 15, 399–409. doi: 10.1016/j.idairyj,.2004.08.004
AOAC Official Method, 930.15 (2005). Official Methods of Analysis of AOAC INTERNATIONAL, 18th Edn. Gaithersburg, MD: AOAC INTERNATIONAL.
Bedani, R., Rossi, E. A., and Saad, S. M. I. (2013). Impact of inulin and okara on Lactobacillus acidophilus La-5 and Bifidobacterium animalis Bb-12 viability in a fermented soy product and probiotic survival under in vitro simulated gastrointestinal conditions. Food Microbiol. 34, 382–389. doi: 10.1016/j.fm.2013.01.012
Bernardeau, M., Lehtinen, M. J., Forssten, S. D., and Nurminen, P. (2017). Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 54, 2570–2584. doi: 10.1007/s13197-017-2688-3
Cao, J., Yu, Z., Liu, W., Zhao, J., Zhang, H., and Zhai, Q. (2020). Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 64, 103643. doi: 10.1016/j.jff.2019.103643
Casula, G., and Cutting, S. M. (2002). Bacillus probiotics: spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 68, 2344–2352. doi: 10.1128/AEM.68.5.2344-2352.2002
de Almada, C. N., Almada, C. N., Martinez, R. C. R., and Sant'Ana, A. S. (2016). Paraprobiotics: evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 58, 96–114. doi: 10.1016/j.tifs.2016.09.011
Gu, S., Bin, Z.hao, L. N., Wu, Y., Li, S. C., Sun, J. R., Huang, J. F., et al. (2015). Potential probiotic attributes of a new strain of Bacillus coagulans CGMCC 9951 isolated from healthy piglet feces. World J. Microbiol. Biotechnol. 31, 851–863. doi: 10.1007/s11274-015-1838-x
Guo, Z., Wang, J., Yan, L., Chen, W., Liu, X., ming, Zhang, H., et al. (2009). In vitro comparison of probiotic properties of Lactobacillus casei Zhang, a potential new probiotic, with selected probiotic strains. LWT Food Sci. Technol. 42, 1640–1646. doi: 10.1016/j.lwt.2009.05.025
Konuray, G., and Erginkaya, Z. (2018). Potential use of Bacillus coagulans in the food industry. Foods. 7, 92. doi: 10.3390/foods7060092
Konuray, G., and Erginkaya, Z. (2020). Quality evaluation of probiotic pasta produced with Bacillus coagulans GBI-30. Innovative Food Sci. Emerg. Technol. 66, 102489. doi: 10.1016/j.ifset.2020.102489
Lee, N. K., Kim, W. S., and Paik, H. D. (2019). Bacillus strains as human probiotics: characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 28, 1297–1305. doi: 10.1007/s10068-019-00691-9
Li, X., Masatcioglu, M. T., and Koksel, F. (2019). Physical and functional properties of wheat flour extrudates produced by nitrogen injection assisted extrusion cooking. J. Cereal Sci. 89, 102811. doi: 10.1016/j.jcs.2019.102811
Maathuis, A. J. H., Keller, D., and Farmer, S. (2010). Survival and metabolic activity of the GanedenBC30 strain of Bacillus coagulans in a dynamic in vitro model of the stomach and small intestine. Beneficial Microbes. 1, 31–36. doi: 10.3920/BM2009.0009
Majeed, M., Majeed, S., Nagabhushanam, K., Natarajan, S., and Ali, F. (2016a). Original article Evaluation of the stability of Bacillus coagulans MTCC 5856 during processing and storage of functional foods. Int. J. Food Sci. Technol. 32, 1–8. doi: 10.1111/ijfs.13044
Majeed, M., Nagabhushanam, K., Natarajan, S., Sivakumar, A., Eshuis-de Ruiter, T., Booij-Veurink, J., et al. (2016b). Evaluation of genetic and phenotypic consistency of Bacillus coagulans MTCC 5856: a commercial probiotic strain. World J. Microbiol. Biotechnol. 32, 1–12. doi: 10.1007/s11274-016-2027-2
Majzoobi, M., Aghdam, M. B. K., Eskandari, M. H., and Farahnaky, A. (2019). Quality and microbial properties of symbiotic bread produced by straight dough and frozen part-baking methods. J. Texture Stud. 50, 165–171. doi: 10.1111/jtxs.12386
Marcial-Coba, M. S., Pjaca, A. S., Andersen, C. J., Knøchel, S., and Nielsen, D. S. (2019). Dried date paste as carrier of the proposed probiotic Bacillus coagulans BC4 and viability assessment during storage and simulated gastric passage. LWT Food Sci. Technol. 99, 197–201. doi: 10.1016/j.lwt.2018.09.052
Mondol, M. A. M., Shin, H. J., and Islam, M. T. (2013). Diversity of secondary metabolites from marine bacillus species: chemistry and biological activity. Marine Drugs 11, 2846–2872. doi: 10.3390/md11082846
Muñoz, K. S., Parra, A. S., Roa, D. F., Hoyos, J. L., and Bravo, J. E. (2022). Physical and paste properties comparison of four snacks produced by high protein quinoa flour extrusion Cooking 6, 1–10. doi: 10.3389/fsufs.2022.852224
Navruz-Varli, S., and Sanlier, N. (2016). Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 69, 371–376. doi: 10.1016/j.jcs.2016.05.004
Pereira, E., Encina-Zelada, C., Barros, L., Gonzales-Barron, U., Cadavez, V., and, C. F. R., et al. (2019). Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: a good alternative to nutritious food. Food Chem. 280, 110–114. doi: 10.1016/j.foodchem.2018.12.068
Saldanha do Carmo, C., Varela, P., Poudroux, C., Dessev, T., Myhrer, K., Rieder, A., et al. (2019). The impact of extrusion parameters on physicochemical, nutritional and sensorial properties of expanded snacks from pea and oat fractions. LWT Food Sci. Technol. 112, 108252. doi: 10.1016/j.lwt.2019.108252
Sanders, M. E., Klaenhammer, T. R., Ouwehand, A. C., Pot, B., Johansen, E., Heimbach, J. T., et al. (2014). Effects of genetic, processing, or product formulation changes on efficacy and safety of probiotics. Ann. N. Y. Acad. Sci. 1309, 1–18. doi: 10.1111/nyas.12363
Sen, R., Pal, D., Kodali, V. P., Das, S., and Ghosh, S. K. (2010). Molecular characterization and in vitro analyses of a sporogenous bacterium with potential probiotic properties. Probiotics Antimicrob. Proteins 2, 152–161. doi: 10.1007/s12602-010-9049-0
Šipailiene, A., and Petraityte, S. (2018). Encapsulation of probiotics: proper selection of the probiotic strain and the influence of encapsulation technology and materials on the viability of encapsulated microorganisms. Probiotics Antimicrob. Proteins 10, 1–10. doi: 10.1007/s12602-017-9347-x
Soares, M. B., Martinez, R. C. R., Pereira, E. P. R., Balthazar, C. F., Cruz, A. G., Ranadheera, C. S., et al. (2019). The resistance of Bacillus, Bifidobacterium, and Lactobacillus strains with claimed probiotic properties in different food matrices exposed to simulated gastrointestinal tract conditions. Food Res. Int. 125, 108542. doi: 10.1016/j.foodres.2019.108542
Sucupira, M. I., and Souza, M. M. B. (2019). Prebiotics and probiotics - potential benefits in human nutrition and health. Intech 15, 1–15. doi: 10.5772/intechopen.89155
Sui, L., Zhu, X., Wu, D., Ma, T., Tuo, Y., Jiang, S., et al. (2020). In vitro assessment of probiotic and functional properties of Bacillus coagulans T242. Food Biosci. 36, 100675. doi: 10.1016/j.fbio.2020.100675
Tripathi, M. K., and Giri, S. K. (2014). Probiotic functional foods: survival of probiotics during processing and storage. J. Funct. Foods 9, 225–241. doi: 10.1016/j.jff.2014.04.030
Keywords: spore-forming bacteria, gastrointestinal tract, stability, Bacillus coagulans, non-dairy probiotics, quinoa snack
Citation: Muñoz Pabon KS, Hoyos Concha JL and Solanilla Duque JF (2022) Quinoa extruded snacks with probiotics: Physicochemical and sensory properties. Front. Sustain. Food Syst. 6:935425. doi: 10.3389/fsufs.2022.935425
Received: 03 May 2022; Accepted: 02 August 2022;
Published: 12 September 2022.
Edited by:
Fatih Ozogul, Çukurova University, TurkeyReviewed by:
Jayesh Jagannath Ahire, Unique Biotech Limited, IndiaCopyright © 2022 Muñoz Pabon, Hoyos Concha and Solanilla Duque. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karen Sofia Muñoz Pabon, a3BhYm9uQHVuaWNhdWNhLmVkdS5jbw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.