
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 07 November 2022
Sec. Crop Biology and Sustainability
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.893525
This article is part of the Research TopicSystem Fertilization and Nutrient CyclingView all 5 articles
The main factors affecting phosphorus (P) availability in soils are mineralogy, acidity, and concentration of elements in the soil solution. Moreover, P fertilizer sources and amounts can affect P cycling and adsorption of this element on colloids. In this research, we hypothesized that the use of phosphate sources of different solubilities can alter soil P adsorption process, due to chemical compositions of these sources and, in ICLS, the soil-plant-animal system can change the P forms distribution on soil profile and its interactions with the soil chemical attributes. To examine these hypotheses, a field experiment was conducted over 5 years, under a Haplic Cambisol, in an incomplete factorial scheme, with the treatments being composed of three P sources (triple superphosphate, rock phosphate—Arad and magnesium thermophosphate), three doses of total P2O5 (60, 120, and 180 kg ha−1) plus a treatment without application of phosphate fertilizer. Phosphate applications occurred annually, broadcast without incorporation, at the time of sowing the annual winter forage, in a crop rotation system that included pasture in autumn-winter and grain crops in spring-summer. Soil samples were collected at depths of 0 to 5, 5 to 10, 10 to 15, 15 to 20, and 20 to 30 cm and evaluated by Hedley P fractionation and the soil chemical attributes were quantified. The use of different phosphates applied broadcast without incorporation did not influence the concentrations of soil P fractions over a 5 year study. Phosphate fertilizer doses above 120 kg ha−1 of total P2O5 increased moderately labile and non-labile P fractions. The highest concentration of labile P was found up to 15 cm soil depth. It was found that P lability is strongly associated with calcium and total organic carbon concentrations in the soil profile. High concentrations of basic cations and sulfate in the soil solution increased moderately labile and non-labile P fractions. The complexity of ICLS interactions to maintain phosphorus dynamics in the soil was shown to be an intricate P release/adsorption process associated with soil chemical attributes.
Integrated crop-livestock systems (ICLS) under no-tillage brings numerous benefits to agricultural fields, such as (i) deposition of animal and plant residues in the soil (Carvalho et al., 2010); (ii) increased nutrient cycling (Assmann et al., 2017); and (iii) conservation/increase of soil organic matter (SOM) (Alves et al., 2019). Furthermore, ICLS can contribute to increase the availability of phosphorus (P) in the soil over time (Assmann et al., 2017), maximizing the efficient phosphate fertilizer utilization. The P dynamic in soil under ICLS tend to be more complex when compared to conventional grain or pasture systems. The soil-plant-animal interaction influences nutrient cycling, changing the distribution of P forms in the soil profile (Costa et al., 2014). This complexity of ICLS can also interfere in the interaction and adsorption reactions of inorganic (Pi) and organic (Po) P with the soil chemical attributes (Arruda Coelho et al., 2019).
Soil organic matter can directly influence the P lability (Tiessen et al., 1984; Menezes-Blackburn et al., 2018), which, driven by soil management in ICLS, can become one of the main factors controlling the forms of this nutrient in the soil. Other chemical attributes also interfere with the dynamics of P forms in the soil. In this sense, exchangeable cations can influence the adsorption processes, mainly Ca (Bolan et al., 1993; Xu et al., 2010) and, therefore, the P lability. High concentrations of Ca in the soil solution can result in forms of P poorly soluble (Teng et al., 2020), depending on the pH range.
Furthermore, P added to the soils of low degree of weathering (e.g., Cambisol) as a mineral fertilizer, can be found later in the following fractions: (i) adsorbed at high energy with Fe and Al oxyhydroxides and in clay minerals type 1:1, through the exchange of ligands; (ii) precipitated as Fe and Al phosphate in tropical and subtropical, predominantly acidic soils; (iii) bound to Ca, producing compounds with varying solubility depending on the origin of the phosphate fertilizer (if water-soluble or water-insoluble); (iv) physiochemically stabilized in SOM complexes (Teng et al., 2020). Due to these reactions, P often accumulates in the soil after continuous annual application of phosphate fertilizers, resulting in an increase in the P pool (Arruda Coelho et al., 2019). Other factors related to soil P management involve the choice of phosphate fertilizer source. The most used phosphates are water-soluble, citrate soluble and phosphates with low solubility in water and citric acid (Chien et al., 2011). Phosphates that provide greater solubility, such as triple superphosphate (soluble in water + neutral ammonium citrate) and magnesium thermophosphate (soluble in citric acid), usually show better short-term performance when compared to lower solubility sources, such as rock phosphate (water-insoluble + neutral ammonium citrate and partially soluble in citric acid). This last phosphate ensures the gradual release of P to crops (Galetto et al., 2014a,b; Guera et al., 2020).
In variable charge soils, phosphate fertilization has been used in the sowing furrow (Arruda Coelho et al., 2019), below and beside the seeds, considering the crop requirement to be implanted. However, in recent years, system fertilization has been used successfully (Galetto et al., 2014a,b; Guera et al., 2020). This system fertilization practice is more commonly practiced in South Brazil (Galetto et al., 2014a; Guera et al., 2020) by the anticipation of fertilization to the cover crop phase. System fertilization can be done using several approaches around the world and it is important to put this strategy in context to a broader audience.
Additionally, there is a tendency to apply phosphate fertilizer broadcast with or without incorporation instead of applying it in sowing furrow, to maximize the use of machines and seeding (Galetto et al., 2014a; Hansel et al., 2017; Guera et al., 2020). However, application of phosphates in ICLS under no-tillage can lead to increased P concentrations near the soil surface and P immobilization due to increased adsorption on soil colloids (Hansel et al., 2017). In this context, it is expected that there will be an increase in available P fractions, over time, combined to different low solubility sources of phosphate utilization, annually applied in broadcast without incorporation and with the maintenance of SOM and greater cycling of nutrients in ICLS. It is expected that there will be an over-time increase in available P fractions, and P availability could be enhanced with more soluble P sources applied annually as broadcast without incorporation in no-tillage ICLS.
The field experiment was carried out in the county of Castro, state of Paraná, Brazil (24°51'49“S, 49°56'6”W, 1,020 m altitude), over 5 years (April 2009 in 2014). The climate is subtropical with mild summer (Cfb), according to the Köppen classification (Alvares et al., 2013). The average annual temperature is 16.7°C and the average annual precipitation of 1.565 mm, evenly distributed throughout the year. The soil was classified as clayey Haplic Cambisol (605, 225, and 170 g kg−1 of clay, silt, and sand, respectively). The predominant minerals in the clay fraction were quartz, kaolinite, and gibbsite (Galetto et al., 2014a,b). Before setting up the experiment, the field was managed for 8 years under no-tillage being cultivated with black oats (Avena strigosa Schreb.), ryegrass (Lolium multiflorum L.), wheat (Triticum aestivum L.), soybean (Glycine max L.) and corn (Zea mays L.) as rotating crops. The soil acidity correction was carried out in 2008, using dolomitic limestone (3 Mg ha−1) in broadcast without incorporation, to increase base saturation to 60%. The soil chemical attributes in the 0 to 20 cm layer, over 60 months of experimentation, are shown in Table 1.
The experimental design used was a randomized complete block, in an incomplete factorial scheme. Three phosphate sources were studied [triple superphosphate (TSP), rock phosphate—Arad (RP) and magnesium thermophosphate (MTP)], and three doses (60, 120, and 180 kg ha−1) of total P2O5, plus a control (without phosphate fertilizer application). The treatments with sources and doses of P were applied annually, broadcast without incorporation, at the time of sowing of winter forages. The chemical composition of the studied fertilizers is shown in Table 2. The crops used for the crop rotation system in the experimental period were black oat/corn/ryegrass/soybean. The experimental schedule used in ICLS is shown in Table 3. During spring-summer, grain crops were cultivated in plot represented 273 m2 totalizing fifty-two plots.
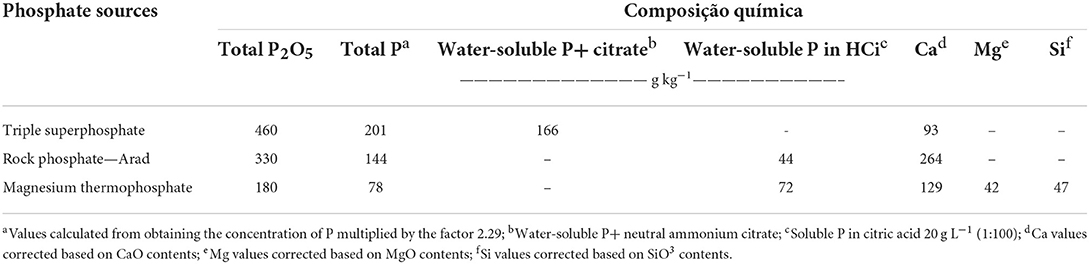
Table 2. Chemical composition of phosphate sources applied annually during 60 months of evaluation (2009 to 2014).
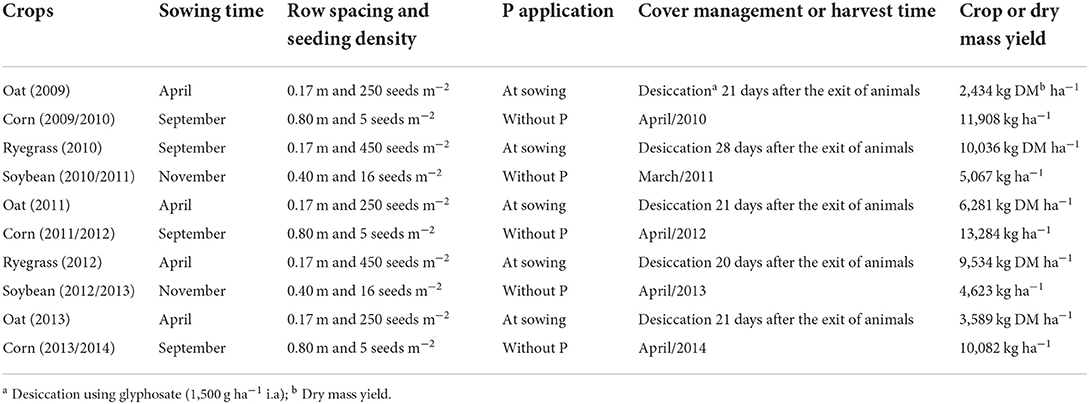
Table 3. Experimental schedule: crops, sowing time, phosphorus application, cover management (forage crops), or harvest (grain crops) and average crop yield.
At the same area, during the ideal autumn-winter forage biomass accumulation, a rotational grazing system was adopted. The field experimental was split in four paddocks with 5,525 m2 each, where the cattle remained in each paddock for 4 to 7 days, according to forage heights (30 cm for entrance and 15 cm for exits the paddock). The cattle used in the grazing period corresponded to heifers of the Dutch dairy cattle, with an average weight of 250 kg. Cattle had access to all field experiment, thus the distribution of manure was heterogeneous according to the movement of cattle throughout the field experimental. Thus, deposition of cattle manure in field experimental was considered together to gains of P soil phosphate fertilizers.
After the beginning of the experiment (April 2009), the limestone was not applied. In accordance with crop requirements, the following were used: (i) nitrogen mineral fertilizer, in the form of urea [CO(NH2)2] and potassium fertilizer, in the form of potassium chloride (KCl); (ii) inoculants containing selected strains of Bradyrhizobium japonicum (SEMIA 5079) (~3.6 × 106 cells seed−1), 2.5 g ha−1 of cobalt and 15 g ha−1 of molybdenum for seeds soybean; (iii) P, in accordance with each treatment. When necessary, other agronomic practices (seed treatment, control of weeds, pests, and diseases) were carried out to facilitate the proper growth and development of crops.
Soil samples were collected 60 months after the beginning of the experiment (April 2014), after harvesting the corn crop at depths from 0 to 5, 5 to 10, 10 to 15, 15 to 20, and 20 to 30 cm. At each depth, 12 simple samples were collected to form a composite sample. Then, the composite samples were air-dried at 40°C for 48 h and sieved through 2.0 mm sieves for chemical analysis.
The chemical fractionation of soil P was performed according to the procedures described by Hendley et al. (1982), with modifications by Condron et al. (1985). To determine the inorganic (Pi) and organic (Po) forms of P by sequential extraction (in 15 mL centrifuge tubes), 0.5 g of ground soil was weighted. At each stage, 10 mL of extractor were added (soil ratio: 1:20 solution) and the tubes were shaken horizontally at 150 rotations per minute (rpm) for 16 h, at a temperature of 25 ± 0.5°C.
The extraction sequences were: (i) anion exchange resin for extraction of inorganic P (PAER) in deionized water, using a membrane resin with a 2.0 cm2 area; (ii) 0.5 mol L−1 NaHCO3 for extraction of inorganic P (PiBIC) weakly adsorbed on the surface of crystalline compounds and organic P compounds (PoBIC) with low recalcitrance, such as ribonucleic acid and glycerophosphate; (iii) NaOH 0.1 mol L−1, for the extraction of inorganic P (Pi0.1HID) strongly adsorbed on Fe and Al minerals, clay and organic P (Po0.1HID) associated mainly with fulvic and humic acids adsorbed onto minerals and SOM surfaces; and (iv) 1 mol L−1 HCl, for extraction of inorganic P (PHCl) associated with poorly soluble Ca-bound P compounds or negatively charged oxide surfaces; (v) 0.5 mol L−1 NaOH, for extraction of inorganic P (Pi0.5HID) associated with Fe and Al oxides, clay minerals and organic P (Po0.5HID) associated with fulvic and humic acids. After each extraction, the soil suspensions were centrifuged at 4,000 rpm for 15 min to collect the supernatant. After each centrifugation and removal of the supernatant, at each stage of fractionation, the soil samples were washed with 10 mL of 1.0 mol L−1 NaCl solution to remove exchangeable cations, which could affect the subsequent solubility of P. For the alkaline extracts (PiBIC, Pi0.1HID and Pi0.5HID), 1 mL of each extract, 0.5 mL of 1:1 sulfuric acid solution, 5 mL of ammonium persulfate 7.5 mol L−1 were added, in a digestion tube. After preparation, the solutions were placed in an autoclave at 121°C and 103 kPa, for 2 h, to determine the total P (Pt) (Pi + Po). Organic P (PoBIC, Po0.1HID and Po0.5HID) in each alkaline extract was determined by the difference between Pt and Pi.
After extractions, the remaining soil samples were dried at 40°C for 72 h. A subsample of 0.1 g was taken to determine the P occluded (PRESIDUAL), through digestion with H2SO4 + H2O2 + MgCl2 according to Brookes and Powlson (1981). The inorganic P, in each acid extract (PAER, PHCl, PRESIDUAL and digestion of PtBIC, Pt0.1HID and Pt0.5HID), was determined according to Murphy and Riley (1962). The concentrations of Pi (PiBIC, Pi0.1HID and Pi0.5HID) in alkaline extracts were determined according to Dick and Tabatabai (1997). The sets of extracts containing P were quantified based on the lability predicted by the extractors. Soil labile P (LP) consisted of PAER and PBIC (Pi and Po). Moderately labile P (MLP) consisted of P0.1HID (Pi and Po) and PHCl. The non-labile P (NLP) consisted of P0.5HID (Pi and Po) and the PRESIDUAL. This approach was based on previous studies by Cross and Schlensinger (1995).
The active acidity (pH) of the soil was performed in calcium chloride (CaCl2) solution 0.01 mol L−1 (soil: solution ratio of 1:2.5). The procedure consisted of adding the 0.01 mol L−1 CaCl2 solution with agitation for 15 min, followed by 30 min of decantation and measured by a potentiometer (Silva, 2009).
The extraction of Al, Ca and Mg were obtained in a solution of potassium chloride (KCl) 1 mol L−1 (soil: solution ratio of 1: 12.5). The solution of KCl 1 mol L−1 was added to the soil and agitated for 15 min at 220 rpm, followed by decantation for 12 h. The Al concentration was determined by NaOH titration, in presence of bromothymol blue as indicator. The Ca and Mg concentrations were determined by complexometric ethylenediamine tetra acetic acid (EDTA). The K concentration in the soil solution was determined by the Mehlich-1 extractant. The extraction process follows the same procedures for the extraction of P. However, the concentrations of K were obtained by the photometer flame emission (Silva, 2009).
Sulfate extraction was performed by agitation of 10 g soil with 25 mL of extracting solution (0.5 N ammonium acetate in 0.25 N acetic acid) for 30 min at 220 rpm. Then 0.25 g of charcoal activated was added followed by agitation for 3 min at 220 rpm. After the samples were filtered and a 10 ml aliquot was transferred to the test tube. The determination was carried out by the turbidimetric method according to Silva (2009).
Total organic carbon (TOC) was determined by the Walkley-Black methodology. A soil sample of 1.0 g was transferred to a 125 mL Erlenmeyer flask. Ten milliliter of 0.167 mol L−1 potassium dichromate solution and 20 mL of concentrated sulfuric acid were added. The flasks were left to rest for 30 min to wait for the reaction between the solutions. After resting, 50 mL of deionized water, 3 mL of concentrated orthophosphoric acid and 0.5 ml of diphenylamine were added. The determination was performed by titration with 1 mol L−1 ferrous sulfate. Values were corrected to obtain complete carbon oxidation of organic matter (correction factor = 1.33).
The calculations and the statistical analysis were performed with software R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria). The data were submitted to the adjustment of generalized linear models including blocks, depth, sources, and doses as fixed factors and soil properties including P fractions as continuous variables were analyzed using the lme4 package (Bates et al., 2014). Principal component analysis was performed using the MVar.pt package ( Ossani and Cirillo,2020) to visualize the interrelationship between P fractions and soil chemical attributes. Spearman's correlation analysis (non-linear relationship of variables) (P < 0.01 and P < 0.05) was performed using the ppcor computational package (Kim, 2015), to assess the magnitude of the association between the fractions of P and the chemical attributes of the soil.
Data were also submitted to the decision tree model using the rpart package (Therneau et al., 2015). This model was used as a hierarchical method to verify the distribution of P fractions, along the profile, in relation to the other chemical attributes of the soil submitted to the treatments of sources and doses of P. The identification of the data that had the greatest effect on the association with the variables was adjusted by the Analysis of Variance.
The relationship between LP and soil chemical attributes are shown in Figure 1A. The first two axes explained 91.7% of the data variance. The pH and concentrations of TOC, Ca and K were closely and related to the P labile fraction in the soil (Figure 1A). On the other hand, the concentrations of Al, Mg and S were inversely associated with the increase in LP in the soil solution. In this context, the concentrations of Ca and TOC in the soil had a greater positive association with the LP, and the concentrations of Al negatively influenced this fraction (Table 4). The greatest availability of LP is found at depths up to 15 cm, regardless of the sources and/or doses of P applied annually in broadcast without incorporation (Figure 2). At a depth of up to 5 cm (25% of the occurrence of data), in a pH range between 4.8 and 6.0, the highest concentration of TOC (this factor being the most relevant) led to an increase in the concentration of P labile fractions. At depth between 5 and 10 cm (23% of the occurrence of data), Ca concentrations ≥to 4.1 cmolc kg−1 are determinant for the highest concentrations of LP in the soil solution, associated with TOC (Figure 2). At depths >10 cm, the concentration of Al is a key factor in the behavior of LP in the soil, associated with the presence of TOC at concentrations around 30 g kg−1. The LP, regardless of the influence of the soil chemical attributes has its availability reduced at depths >15 cm (Figure 2).
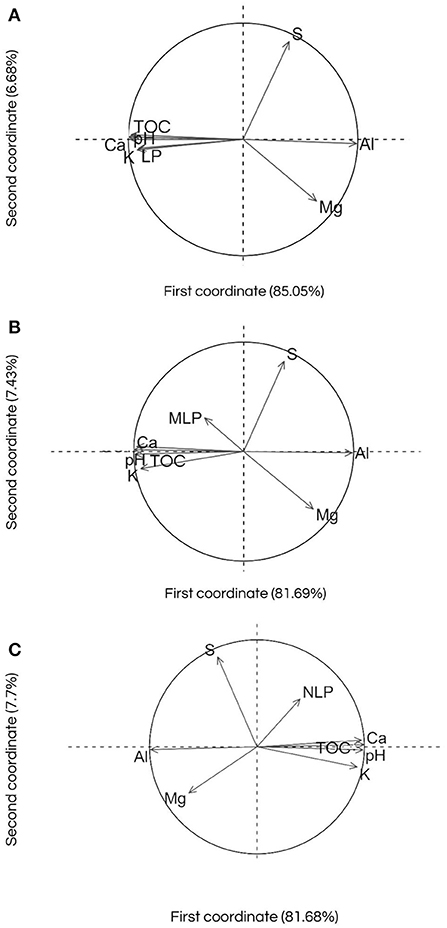
Figure 1. Analysis of principal components (A) labile phosphorus (LP), (B) moderately labile phosphorus (MLP), (C) non-labile phosphorus (NLP) and chemical attributes [total organic carbon (TOC), available sulfur (S), active acidity (pH), aluminum (Al), exchangeable calcium (Ca), exchangeable magnesium (Mg), and exchangeable potassium (K)] in a Cambisol in an integrated crop-livestock system under application of phosphates sources and doses.

Table 4. Spearman's correlation coefficients between phosphorus fractions [labile (LP), moderately labile (MLP) and non-labile (NLP)] and soil chemical attributes [total organic carbon (TOC), sulfate (S), active acidity (pH), exchangeable aluminum (Al), and basic cations (Ca, Mg and K)] under application of phosphates sources and doses.
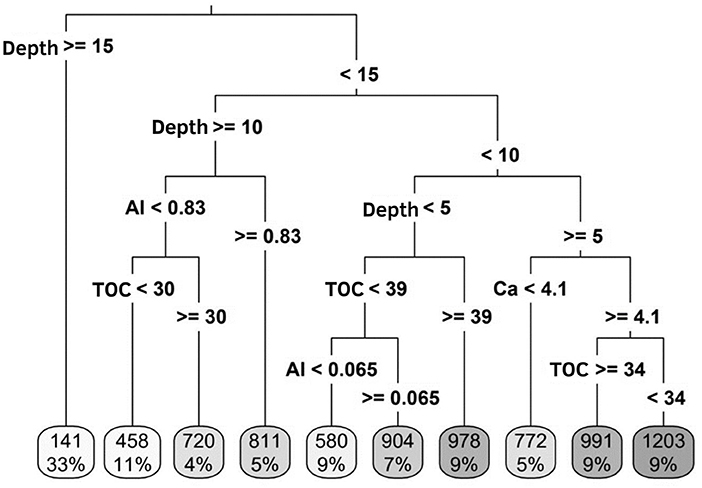
Figure 2. Interpretation of the variability of concentrations of soil chemical attributes [total organic carbon (TOC, g kg−1) sulfur (S, mg kg−1) available, active acidity (pH), aluminum (Al, cmolc kg−1), calcium (Ca, cmolc kg−1), magnesium (Mg, cmolc kg−1) and potassium (K, cmolc kg−1) exchangeable], according to P labile fraction (mg kg−1), in the depths sampled (0 to 5, 5 to 10, 10 to 15, 15 to 20, and 20 to 30 cm), in an integrated crop-livestock system under application of different sources and doses of P2O5. The values at the end of the decision tree represent the concentration (above) and percentage (below) of labile phosphorus according to the variation of chemical attributes of the soil.
The first component explained 81.7% of the data variance. In an equivalent way to what was observed for LP, the pH, and the concentrations of TOC, Ca and K showed a directly proportional relationship with the MLP in the soil (Figure 1B). These soil chemical attributes, except for K, were shown to be associated with an increase in the moderately labile fraction (Table 4). On the other hand, the concentrations of Al, Mg and S were inversely related to this fraction (Figure 1B). At a depth up to 5 cm, the annual application of more than 120 kg ha−1 of total P2O5 increased the MLP, regardless of the P-fertilizer source studied (Figure 3). The moderately labile P fraction, at depth <5 cm, increased when the P dose was <120 kg ha−1 of total P2O5 and Mg concentrations were equal or >4.4 cmolc kg−1 (Figure 3). At depths between 15 and 20 cm, the application of doses >120 kg ha−1 of total P2O5 increased the concentrations of MLP. Lower concentrations of MLP were observed at depths between 5 and 15 cm, and >20 cm (59% of data occurrence), regardless of the soil chemical attributes and the phosphate source studied (Figure 3).

Figure 3. Interpretation of the variability of concentrations of soil chemical attributes [total organic carbon (TOC, g kg−1) sulfur (S, mg kg−1) available, active acidity (pH), aluminum (Al, cmolc kg−1), calcium (Ca, cmolc kg−1), magnesium (Mg, cmolc kg−1), and potassium (K, cmolc kg−1) exchangeable], according to the concentration of the moderately labile fraction (mg kg−1), in the depths sampled (0 to 5, 5 to 10, 10 to 15, 15 to 20, and 20 to 30 cm), in an integrated crop-livestock system under application of different sources and doses of P2O5. The values at the end of the decision tree represent the concentration (above) and percentage (below) of moderately labile phosphorus according to the variation of chemical attributes of the soil.
The data variance was 81.7% explained by the first component. The concentrations of TOC, Ca and K, and pH showed a directly proportional relationship with NLP (Figure 1C). On the other hand, the concentrations of Al, Mg and S were inversely related to NLP (Figure 1C). The concentrations of TOC, Ca and pH were shown to be more associated with an increase in the NLP fraction (Table 4). The NLP fraction was not influenced by the sources and doses of P applied to the soil. However, Ca concentrations were determinant for the behavior of NLP (Figure 4). When Ca concentrations were ≥6.8 cmolc kg−1, the increase in NLP was greater in the presence of S (>3.4 mg kg−1) and K (≥0.5 cmolc kg−1) (Figure 4). When Ca concentrations were lower than 6.8 cmolc kg−1, at depths between 10 and 20 cm, the TOC was fundamental role in the distribution of NLP in the soil (36% of the occurrence of data). Furthermore, the complex dynamics of the soil chemical attributes, related to the concentrations of Mg, TOC, and Al, determined the behavior of the NLP, at depth <10 cm (Figure 4). At depths above 20 cm, the presence of Mg, at concentrations >4.8 cmolc kg−1, led to an increase in NLP fraction.
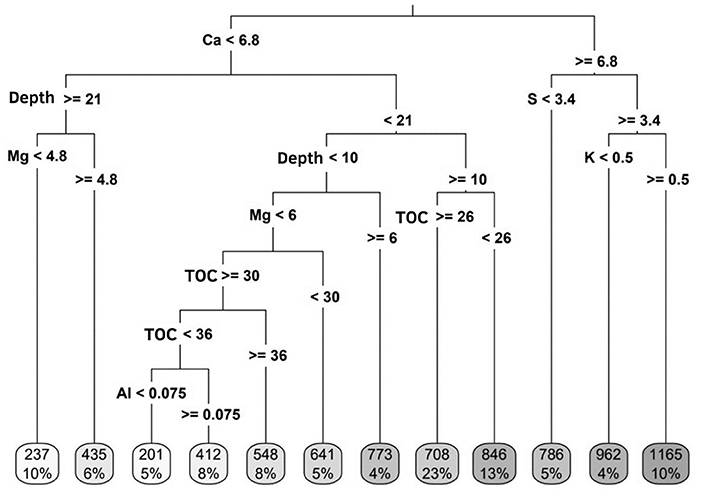
Figure 4. Interpretation of the variability of concentrations of soil chemical attributes [total organic carbon (TOC, g kg−1) sulfur (S, mg kg−1) available, active acidity (pH), aluminum (Al, cmolc kg−1), calcium (Ca, cmolc kg−1), magnesium (Mg, cmolc kg−1), and potassium (K, cmolc kg−1) exchangeable], according to the concentration of the non-labile fraction (mg kg−1), in the depths sampled (0 to 5, 5 to 10, 10 to 15, 15 to 20, and 20 to 30 cm), in an integrated crop-livestock system under application of different sources and doses of P2O5. The values at the end of the decision tree represent the concentration (above) and percentage (below) of non-labile phosphorus according to the variation of chemical attributes of the soil.
The highest concentrations of LP were associated with increasing concentration of TOC and Ca (2.1 to 6.0 cmolc kg−1, according to Sociedade Brasileira de Ciência do Solo/Núcleo Estadual do Paraná – SBCS/NEPAR, 2017) (Figures 1A, 2), at the soil depth of up to 10 cm. The lability of P is associated with several biogeochemical factors, including SOM, which stands out as one of the main factors contributing to the increase of P labile forms in the soil (Costa et al., 2014; Menezes-Blackburn et al., 2018).
Negatively charged organic functional groups (OFG) (e.g., carboxyl, phenol) can interact with positively charged minerals such as Fe and Al oxides. The OFG altered P adsorption (Liu et al., 1999), providing anions adsorption through cationic bridges and/or increased competition for adsorption sites (Hinsiger et al., 2011; Gérard, 2016). There is a tendency for OFG to adsorb P in the form of cationic bridges with Fe, Al and with Ca (Santos et al., 2017; Teles et al., 2017; Soltangheisi et al., 2020). The increase in exchangeable Ca in the soil solution favors the occurrence of a complex between Ca and OFG bound to phosphate on the surface of the colloids (Santos et al., 2017; Teles et al., 2017; Soltangheisi et al., 2020). This Ca-OFG-P complex can prevent the binding of P with Fe and Al oxides, reducing the possible adsorption by bonds aging between P-Fe and P-Al. Between 5 and 10 cm, Ca concentrations >4.0 cmolc kg−1 were determinant for the increase in LP associated with SOM (Figure 2). The increase of Ca in the soil solution, at pH between 5.0 and 6.0 (experimental condition), favors the formation of monocalcium phosphates [Ca(H2PO4)2. H2O]—fewer stable forms—(Olsen and Khasawneh, 1980), contributing to the increase of more labile forms of P bound to Ca.
Furthermore, in ICLS, for basic cations like Ca, the extraction from cattle is exceptionally low. In autumn-winter pastures in the Brazilian subtropical region (Kunrath et al., 2014; Carvalho et al., 2010; Martins et al., 2016), only 0.19 kg of Ca were exported annually, according to the animal production values by Price and Schweigert (1994). The animals act as catalysts that modify and accelerate the flow of nutrients by ingesting biomass and returning 70–95% of nutrients in the plants to the soil such as urine and manure (Haynes and Williams, 1993). Grazing provides continuous plant growth, resulting in greater production of total dry matter in the pasture (de Moraes et al., 2014) and acts in the recovery of nutrients, such as Ca, in the deeper layers, bringing them to the soil surface (Martins et al., 2016).
However, LP concentrations at a depth between 10 and 15 cm showed a reduction associated with an average level of Al concentration (0.8 to 1.5 cmolc kg−1, according to Sociedade Brasileira de Ciência do Solo/Núcleo Estadual do Paraná – SBCS/NEPAR, 2017) (Figure 2). The reaction of phosphate with Al can result in the formation of compounds with low solubility in the soil solution or there may be adsorption on the surface of the colloids, in the form of P-Al. In this case, the most relevant fact is the nature of the bond, of the covalent or ligand exchange type (Fink et al., 2016; Gérard, 2016). Thus, specifically adsorbed phosphate decreases the reversibility of the binding process and, therefore, its lability.
Increases in the MLP and NLP fractions were observed due to the distribution of extremely high Mg concentrations [>2.0 cmolc kg−1 according to SBCS/NEPAR (2017)] in the soil profile (Figures 3, 4). For MLP, this increase was associated with applications of annual doses lower than 120 kg ha−1 of total P2O5. Furthermore, Ca concentrations above 7.0 cmolc kg−1 led to an increase in NLP associated, also, with extremely high concentrations of K and S-SO42− [>0.45 cmolc kg−1 and >6.0 mg kg−1, respectively, according to SBCS/NEPAR (2017)] (Figure 4). In soils with variable charge, there is an increase in anion adsorption when cations, especially divalent, are present in the soil solution (Bolan et al., 1993). The ionic strength of the solution affects the electrostatic potential of the adsorption plane. The increase of cations on a negatively charged surface makes the electrostatic potential of the adsorption plane less negative, increasing the adsorption of P (Xu et al., 2010). The high concentrations of Ca, Mg and K in the solution may have induced greater phosphate adsorption, increasing the MLP and NLP fractions. Furthermore, the significant increase in the proportions of P and cations (e.g., K and Al) results in the binding of phosphates on the surface of the gibbsite crystals, accompanied by the formation of taranakite [K3Al5(HPO4)6(PO4)2.18(H2O)]. The formation of taranakite impacts the reduction of phosphate-free anions at a high concentration in the soil solution (Kudeyarova et al., 2016).
Also, in the presence of high concentrations of S-SO42− there was a higher concentration of NLP. Phosphate and sulfate anions compete for the same adsorption site on colloids (Geelhoeld et al., 1997). As the concentration of sulfate increases, there is an unfavorable adsorption of phosphate, making the latter more available. However, at the same concentration, phosphate is much more reactive than sulfate, with phosphate being adsorbed on inorganic colloids in the soil (Geelhoeld et al., 1997). Higher concentration of S-SO42− in the soil solution may indicate that this anion was displaced from the colloid surface and the phosphate bound more strongly to this adsorbent site, in a non-reversible way, showing an increase in NLP under these conditions.
The availability of more labile P (LP) fractions under annual phosphate application in ICLS, after 5 years, was independent of the phosphate source (Figures 1A, 2). Although the phosphates application was carried out annually, in broadcast without incorporation, the distribution of P labile was not limited only to the topsoil but was redistributed to a depth of 15 cm (Figure 2). In ICLS, the concentrations of TOC and nutrient increase over time (de Moraes et al., 2014; Lemaire et al., 2014). The greater renewal of the root system, under grazing conditions (Carvalho et al., 2010; Martins et al., 2016), promotes the formation of biopores, leading to the migration of nutrients, especially P, to deeper soil layers (Costa et al., 2014).
The dynamics of the soil-plant-animal system in ICLS can promote the greater physiological activity of the roots and accumulation of residues on the soil surface, which allow an increase in the release of low molecular weight organic acids (LMWOA) (Carvalho et al., 2010; Soussana and Lemaire, 2014; Assmann et al., 2017). The LMWOA can stimulate P release from water-insoluble phosphates (Dotaniya and Meena, 2015). Thus, the dissolution of these applied phosphates (e.g., rock phosphate and thermophosphates) and, managed under ICLS is favored, releasing P gradually for uptake by plant and presenting a greater residual effect in the soil solution, when applied annually (Galetto et al., 2014a,b; Guera et al., 2020). It appears that the use of phosphates with low solubility in ICLS, applied in broadcast without incorporation, can be an efficient alternative for the management of P fertility. It is important to note that the effects of phosphate applications can add to the benefits of ICLS. Factors such as high SOM content (Alves et al., 2019), nutrient cycling (Assmann et al., 2017), and correct pasture management (Carvalho et al., 2010) favored the increase in the P labile pool over time. However, ICLS contributions alone may not be sufficient to maintain adequate P levels for crop development. The combination of practices in ICLS and management of P fertilization can guarantee a higher concentration of P labile forms.
The annual broadcast application and without incorporation of P doses higher than 120 kg P2O5 ha−1 increased the fractions of moderately labile P (MLP) regardless of the phosphate sources applied to the soil surface (Figure 3). Furthermore, for the same dose of total P2O5, the MLP showed an increase in depth (15 to 20 cm). Among the mechanisms of MLP formation, from a phosphate source applied, is the occurrence of two coordinated bonds with the adsorbent surface of colloids (Tiessen et al., 1984). These two coordinated bonds, unlike one, would not allow the desorption of P (Parfitt, 1978) and would therefore cease to be useful for the immediate uptake of plants. Thus, the application of annual doses of total P2O5 above 120 kg ha−1 may have maximized the occurrence of higher energy bonds between P and colloids, resulting in an increase in the MLP.
The P dynamics in a soil conservation system under annual phosphate fertilization can increase the concentrations of this nutrient in depth, as P inorganic (Rodrigues et al., 2016), due to root decomposition and P cycling (Costa et al., 2010). This increase in inorganic P, resulting from the soil management system, is more susceptible to adsorption reactions, especially in soils with variable charge (Cross and Schlensinger, 1995), transforming the MLP pool—an important source of P for plant nutrition in soil conservation systems (Costa et al., 2010, 2014; Rodrigues et al., 2016; Arruda Coelho et al., 2019). Practices such as SOM addition and accumulation suggest sufficient energy demand for the MLP to become reversible, at least partially (Menezes-Blackburn et al., 2018).
There is a tendency for the pool of MLP in ICLS to have higher concentrations compared to LP (Costa et al., 2014). Changes in the P lability, with the migration of P to less labile forms, have already been reported by Tiessen et al. (1984), due to variations in the SOM content. Increases in MLP are desired to maintain animal and/or agricultural production, to reduce P inputs, besides maintaining a reserve of this nutrient in the soil (Costa et al., 2014; Arruda Coelho et al., 2019), increasing the sustainability of the production system. Thus, the understanding of the pools and processes of P dynamics in the soil can form a solid basis for more efficient management of this nutrient in the soil.
Higher concentrations of phosphorus labile and poorly labile forms in the soil were shown to be strongly associated with the soil organic component—total organic carbon. The phosphorus labile fractions readily available for plant uptake, are linked to soil management aimed at the accumulation of soil organic carbon, being directly proportional. This shows us the importance of cultivating the soil under conservationist practices. After 5 years of study, soil organic carbon proved to be a master key for the balance between nutrients in the soil solution. High concentrations of calcium, associated with soil total organic carbon, increased the phosphorus reserve through easily reversible organic bonds (cationic bridges). The biochemical balance and accumulation of total organic carbon, coming from soil management in an integrated crop-livestock system, also showed greater activity of labile phosphorus in the most topsoil layer, maintaining a high concentration of phosphorus for the roots in the first 15 cm, regardless of the solubility of the phosphorus source used. Thus, greater root development in this integrated system led to an increase in labile phosphorus in the uppermost soil layer. Phosphorus enrichment in the soil can also promote the reduction of the doses of phosphate fertilizers used. This study showed that doses of phosphate fertilizers above 120 kg P2O5 ha−1 increased phosphorus non-labile fractions. However, the moderately labile fraction proved to be an interesting source of phosphorus in an integrated crop-livestock system, since this fraction tends to increase because it is linked to total organic carbon. Thus, the bonds of P with the organic radicals are reversible to become labile again. In this way, reducing fertilizer inputs and making the food production more sustainable.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AF was a project coordinator and advisor for KG. AF is a professor and researcher of Plant Nutrition, Soil Fertility and Fertilizers. This manuscript is part of KG's doctoral thesis. All authors contributed to the article and approved the submitted version.
This research was funded by the National Council for Scientific and Technological Development (CNPq), Grant Numbers 484291/2011-4 and 310903/2018-1; and Araucaria Foundation, Grant Number 405/2009.
The authors thank the staff of the ABC Foundation for their valuable contribution to this study. Also, the International Plant Nutrition Institute (IPNI), Mineração Curimbaba, Coordination for the Improvement of Higher Education Personnel (CAPES), and National Council for Scientific and Technological Development (CNPq) for funding.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alvares, C. A., Stape, J. L., Sentelhas, P. C., Gonçalves, J. L. M., and Sparovek, G. (2013). Köppen's climate classification map for Brazil. Meteorol. Zeitschrift. 22, 711–728. doi: 10.1127/0941-2948/2013/0507
Alves, L. A., Denardin, L. G., de, O., Martins, AP, Anghinoni, I., Carvalho, P. C., de, F., et al. (2019). Soil acidification and P, K, Ca and Mg budget as affected by sheep grazing and crop rotation in a long-term integrated crop-livestock system in southern Brazil. Geoderma 351, 197–208. doi: 10.1016/j.geoderma.2019.04.036
Arruda Coelho, M. J., Diaz, D. R., Hettiarachchi, G. M., and Hansel, F. D. (2019). Soil phosphorus fractions and legacy in a corn-soybean rotation on Mollisols in Kansas, USA. Geoderma Reg. 18, e00228. doi: 10.1016/j.geodrs.2019.e00228
Assmann, J. M., Martins, A. P., Anghinoni, I., Denardin, L. G. O., Nichel, G. H., Costa, S. E. V. G.A., et al. (2017). Phosphorus and potassium cycling in a long-term no-till integrated soybean-beef cattle production system under different grazing intensities in subtropics. Nutr. Cycl. Agroecosyst. 108, 21–33. doi: 10.1007/s10705-016-9818-6
Bates, D., Mächler, M., Bolker, B. M., and Walker, S. C. (2014). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67. doi: 10.18637/jss.v067.i01
Bolan, N. S., Syers, J. K., and Sumner, M. E. (1993). Calcium-induced sulfate adsorption by soils. Soil Sci. Soc. Am J. 57, 691–696. doi: 10.2136/sssaj1993.03615995005700030011x
Brookes, P. C., and Powlson, D. S. (1981). Preventing phosphorus losses during perchloric acid digestion of sodium bicarbonate soil extracts. J. Sci. Food Agric. 32, 671–674. doi: 10.1002/jsfa.2740320707
Carvalho, P. C. F., Anghinoni, I., Moraes, A., Souza, E. D., Sulc, R. M., Lang, C. R., et al. (2010). Managing grazing animals to achieve nutrient cycling and soil improvement in no-till integrated systems. Nutr. Cycl. Agroecosyst. 88, 259–273. doi: 10.1007/s10705-010-9360-x
Chien, S. H., Prochnow, L. I., Tu, S., and Snyder, C. S. (2011). Agronomic and environmental aspects of phosphate fertilizers varying in source and solubility: an update review. Nutr. Cycl. Agroecosyst. 89, 229–255. doi: 10.1007/s10705-010-9390-4
Condron, L. M., Goh, K. M., and Newman, R. H. (1985). Nature and distribution of soil phosphorus as revealed by a sequential extraction method followed by 31P nuclear magnetic resonance analysis. J. Soil. Sci. 36, 199–207. doi: 10.1111/j.1365-2389.1985.tb00324.x
Costa, S. E. V. G.A., Anghinoni, I., Flores, J., Vieira, F. C. B., Martins, A. P., and Ferreira, E. (2010). Patterns in phosphorus and corn root distribution and yield in long-term tillage systems with fertilizer application. Soil Tillage Res. 109, 41–49. doi: 10.1016/j.still.2010.04.003
Costa, S. E. V. G.A., Souza, E. D., Anghinoni, I., Carvalho, P. C. F., Martins, A. P., Kunrath, T. R., et al. (2014). Impact of an integrated no-till crop–livestock system on phosphorus distribution, availability and stock. Agric. Ecosyst Environ. 190, 43–51. doi: 10.1016/j.agee.2013.12.001
Cross, A. F., and Schlensinger, W. H. (1995). A literature review and evaluation of the. Hedley fractionation: applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 64, 197–214. doi: 10.1016/0016-7061(94)00023-4
de Moraes, A., de Faccio Carvalho, P. C., Anghinoni, I., Lustosa, S. B. C., Costa, S. E. V. G.A., and Kunrath, R. T. (2014). Integrated crop–livestock systems in the Brazilian subtropics. Eur. J. Agron. 57, 4–9. doi: 10.1016/j.eja.2013.10.004
Dick, W. A., and Tabatabai, M. A. (1997). Determination of orthophosphate in aqueous solutions containing labile organic and inorganic phosphorus compounds. J. Environ. Qual. 6, 82–85. doi: 10.2134/jeq1977.00472425000600010018x
Dotaniya, M. L., and Meena, V. D. (2015). Rhizosphere effect on nutrient availability in soil and its uptake by plants: a review. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 85, 1–12. doi: 10.1007/s40011-013-0297-0
Fink, J. R., Inda, A. V., Bavaresco, J., Barrón, V., Torrent, J., and Bayer, C. (2016). Adsorption and desorption of phosphorus in subtropical soils as affected by management system and mineralogy. Soil Tillage Res. 155, 62–68. doi: 10.1016/j.still.2015.07.017
Galetto, S. L., Fonseca, da, A. F., Harkatin, S, Auler, A. C., and Carvalho, I. Q. (2014a). Availability of phosphorus for maize in crop-livestock integration system. Rev. Ciênc. Agron. 45, 956–967. doi: 10.1590/S1806-66902014000500011
Galetto, S. L., Fonseca, da, A. F., Harkatin, S, Auler, A. C., and Carvalho, I. Q. (2014b). Grain crops and forage yield resulting from the use of phosphates in integrated production system. Rev. Ciênc. Agron. 45, 931–945. doi: 10.1590/S1806-66902014000500009
Geelhoeld, J. S., Hiemstra, T., and Van Riemsdijk, W. H. (1997). Phosphate and sulfate adsorption on goethite: single anion and competitive adsorption. Geochim. Cosmochim. Acta 61, 2389–2396. doi: 10.1016/S0016-7037(97)00096-3
Gérard, F. (2016). Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils – a myth revisited. Geoderma 262, 213–226. doi: 10.1016/j.geoderma.2015.08.036
Guera, K. C. S., da Fonseca, A. F., and Harkatin, S. (2020). Phosphorus use in soybean in integrated production system under anticipation of phosphate fertilization. Rev. Ciênc. Agron. 51, 1–7. doi: 10.5935/1806-6690.20200044
Hansel, F. D., Diaz, D. R., Amado, T. J. C., and Rosso, L. H. M. (2017). Deep banding increases phosphorus removal by soybean grown under no-tillage production systems. Agron. J. 109, 1091–1098. doi: 10.2134/agronj2016.09.0533
Haynes, R. J., and Williams, P. H. (1993). Nutrient cycling and soil fertility in the grazed pasture ecosystem, in Advances in Agronomy, eds D. L. Sparks (Academic Press). doi: 10.1016/S0065-2113(08)60794-4
Hendley, M. J., Stewart, J. W. B., and Chauhan, B. S. (1982). Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 46, 970–976. doi: 10.2136/sssaj1982.03615995004600050017x
Hinsiger, P., Brauman, A., Devau, N., Gérard, F., Jourdan, C., Laclau, J., et al. (2011). Acquisition of phosphorus and other poorly mobile nutrients by roots. Where do plant nutrition models fail?. Plant Soil 348, 29–61. doi: 10.1007/s11104-011-0903-y
Kim, S. (2015). ppcor: an R package for a fast calculation to semi-partial correlation coefficients. Commun. Stat. Appl. Methods 22, 665–674. doi: 10.5351/CSAM.2015.22.6.665
Kudeyarova, A. Y., Alekseeva, T. V., and Elfimov, E. I. (2016). Dissolution of gibbsite and its transformation to taranakite depending on the concentration of phosphate anions in the solution. Eurasian Soil Sci. 49, 180–193. doi: 10.1134/S106422931602006X
Kunrath, T. S., Brambilla, M. C. D. M, Anghinoni, I, Moraes, A., Barro, R. S., and Carvalho, P. C. F. (2014). Management targets for continuously stocked mixed oat × annual ryegrass pasture in a no-till integrated crop-livestock system. Eur. J. Agron. doi: 10.1016/j.eja.2013.09.013
Lemaire, G., Franzluebbers, A., de Faccio Carvalho, P. C., and Dedieu, B. (2014). Integrated crop–livestock systems: strategies to achieve synergy between agricultural production and environmental quality. Agric. Ecosyst. Environ. 190, 4–8. doi: 10.1016/j.agee.2013.08.009
Liu, F., He, J., and Colombo, C. (1999). Competitive adsorption of sulfate and oxalate on goethite in the abscense or presence of phosphate. Soil Sci. 164, 180–189. doi: 10.1097/00010694-199903000-00004
Martins, A. P., Cecagno, D., Borin, J. B. M., Arnuti, F., Lochmann, S. H., Anghinoni, I., et al. (2016). Long-, medium-, and short-term dynamics of soil acidity in na integrated crop-livestock system under different grazing intensities. Nutr. Cycl. Agroecosyst. 104, 67–77. doi: 10.1007/s10705-015-9759-5
Menezes-Blackburn, D., Giles, C., Darch, T., Blackwell, M., Stutter, M., Shand, C., et al. (2018). Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: a review. Plant Soil 427, 5–16. doi: 10.1007/s11104-017-3362-2
Murphy, J., and Riley, J. P. (1962). A modified single solution method for the determination of phosphates in natural waters. Anal. Chim. Acta 27, 31–36. doi: 10.1016/S0003-2670(00)88444-5
Olsen, S. R., and Khasawneh, F. E. (1980). “Use and limitations of physical-chemical criteria for assessing the status of phosphorus in soils,” in The Role of Phosphorus in Agriculture, Eds F. E. Khasawneh, E. C. Samples, and E. J. Kamprath (New Jersey, NY: Wiley Online Library), 361–410.
Ossani, P. C., and Cirillo MVar, M. A. (2020). pt-package: Analise Multivariada (Brazilian Portuguese). Available online at: https://cran.r-project.org/web/packages/MVar.pt/MVar.pt.pdf (accessed July 24, 2020).
Parfitt, R. L. (1978). Anion adsorption by soils and soil materials. Adv. Agron. 30, 1–50. doi: 10.1016/S0065-2113(08)60702-6
Price, J., and Schweigert, B. (1994). The Science of Meat and Meat Products, 3rd Edn. Connecticut: Food and Nutrition Press.
Rodrigues, M., Pavinato, P. S., Withers, P. J. A., Teles, A. P. B., and Herrera, W. F. B. (2016). Legacy phosphorus and no tillage agriculture in tropical oxisols of the Brazilian savanna. Sci. Total Environ. 542, 1050–1061. doi: 10.1016/j.scitotenv.2015.08.118
Santos, S. R., Silva, E. B., Alleoni, L. R. F., and Grazziotti, P. H. (2017). Citric acid influence on soil phosphorus availability. J. Plant. Nutr. 40, 2138–2145. doi: 10.1080/01904167.2016.1270312
Silva, F. C. (2009). Manual de Analises Químicas de Solos, Plantas e Fertilizantes. Brasília: EMBRAPA.
Sociedade Brasileira de Ciência do Solo/Núcleo Estadual do Paraná – SBCS/NEPAR (2017). Manual nde Adubação e Calagem do Estado do Paraná. Curitiba: SBCS/NEPAR.
Soltangheisi, A., Teles, A. P. B., Sartor, L. R., and Pavinato, P. S. (2020). Cover cropping may alter legacy phosphorus dynamics under long-term fertilizer addition. Front. Environ. Sci. 13, 1–12. doi: 10.3389/fenvs.2020.00013
Soussana, J. F., and Lemaire, G. (2014). Coupling carbon and nitrogen cycles for environmentally suitable intensification of grasslands and crop-livestock systems. Agric. Ecosyst Environ. 190, 9–17. doi: 10.1016/j.agee.2013.10.012
Teles, A. P. B., Rodrigues, M., Herrera, W. F. B., Soltangheisi, A., Sartor, L. R., Withers, P. J. A., et al. (2017). Do cover crops change the lability of phosphorus in a clayey subtropical soil under different phosphate fertilizers?. Soil Use Manag. 33, 34–44. doi: 10.1111/sum.12327
Teng, Z., Zhu, J., Shao, W., Zhang, K., Li, M., and Whelan, M. J. (2020). Increasing plant availability of legacy phosphorus in calcareous soils using some phosphorus activators. J. Environ. Manage. 256, 109952. doi: 10.1016/j.jenvman.2019.109952
Therneau, T., Atkinson, B., and Ripley, B. (2015). Package ‘Rpart'. 2015. Available online at: https://cran.r-project.org/web/packages/rpart/rpart.pdf> (accessed July 24, 2020).
Tiessen, H. J. W. B., Stewart, J. W. B., and Cole, C. V. (1984). Pathways of phosphorus transformations in soils of differing pedogenesis. Soil Sci. Soc. Am J. 48, 853–858. doi: 10.2136/sssaj1984.03615995004800040031x
Xu, R., Jiang, J., and Cheng, C. (2010). “Effect of ionic strength on specific adsorption of ions by variable charge soils: experimental testification on the adsorption model of Bowden et al.,” in Molecular Environmental Soil Science in Interfaces in the Earth's Critical Zone, Eds. J. Xu and P. M. Huang (Berlin: Springer), 78–80.
Keywords: P fertilization, nutrient management, soil P pools, soil chemical attributes, soil acidity
Citation: Guera KCS and da Fonseca AF (2022) Phosphorus fractions and their relationships with soil chemical attributes in an integrated crop-livestock system under annual phosphates fertilization. Front. Sustain. Food Syst. 6:893525. doi: 10.3389/fsufs.2022.893525
Received: 10 March 2022; Accepted: 21 October 2022;
Published: 07 November 2022.
Edited by:
Leonardo Deiss, The Ohio State University, United StatesReviewed by:
Nirmalendu Basak, Central Soil Salinity Research Institute (ICAR), IndiaCopyright © 2022 Guera and da Fonseca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keli Cristina Silva Guera, a2VsaWd1ZXJhQG91dGxvb2suY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.