- 1Department of Agricultural Technology, Faculty of Technology, University of Colombo, Colombo, Sri Lanka
- 2Department of Food Science and Technology, Faculty of Agriculture, University of Peradeniya, Peradeniya, Sri Lanka
- 3Department of Biosystems Technology, Faculty of Technology, University of Sri Jayewardenepura, Nugegoda, Sri Lanka
- 4Industrial Technology Institute (ITI), Colombo, Sri Lanka
- 5Department of Basic Science and Social Science, Faculty of Nursing, University of Colombo, Colombo, Sri Lanka
- 6Department of Agriculture, Field Crop Research and Development Institute, Mahailluppallama, Sri Lanka
Methanolic extracts of whole grains of five millet types and two sorghum varieties were evaluated for anti-lipidemic, anti-inflammatory, and a range of anti-oxidant properties in vitro (n = 3 each). Furthermore, proximate composition (n = 3 each) was also studied. Results showed significant differences (P < 0.05) among the selected samples for studied parameters. Pancreatic lipase and cholesterol esterase inhibitory activities of selected samples (2 mg/ml) ranged from 21.16 ± 1.58 to 66.65 ± 3.30 and 17.43 ± 0.60 to 52.09 ± 1.61%, respectively. Nitric oxide inhibitory activity of selected samples (2 mg/ml) ranged from −1.17 ± 0.32 to 13.56 ± 0.93%. Total polyphenolic content (TPC), total flavonoid content (TFC), and total proanthocyanidin content (TPAC) were in the range of 0.19 ± 0.01–12.50 ± 0.87 mg gallic acid equivalents/g, 0.05 ± 0.00–1.57 ± 0.01 mg quercetin equivalents/g, and 0.35 ± 0.01–12.87 ± 0.25 mg cyaniding equivalents/g of samples, respectively. Ferric reducing anti-oxidant power, oxygen radical absorbance capacity, ferrous ion chelating activity, and ABTS and DPPH anti-oxidant properties ranged from 0.15 ± 0.00 to 4.56 ± 0.03 mg of Trolox equivalents (TEs)/g, 0.19 ± 0.01 to 8.50 ± 0.72 mg of TEs/g, 0.13 ± 0.00 to 0.79 ± 0.03 mg EDTA equivalents/g, 0.22 ± 0.00 to 25.57 ± 0.35 mg of TEs/g, and 0.07 ± 0.00 to 22.97 ± 0.83 mg of TEs/g of samples, respectively. Among the studied samples, pigmented sweet sorghum exhibited the highest activities for all the tested parameters. The observed activities were moderate compared to the reference standards used. The highest values for proximate composition parameters tested varied with the different samples studied. In conclusion, the consumption of especially pigmented millet and sorghum in Sri Lanka may play an important role in the prevention and management of oxidative stress–associated chronic diseases. This is the first study to report pancreatic lipase and cholesterol esterase inhibitory activities of any millet types and sorghum varieties in Sri Lanka and the first report of cholesterol esterase inhibitory activity of millet and sorghum the world over.
Introduction
Plant-based materials have long been used for treating a variety of diseases, improving lipid profiles, and managing inflammation (Singh et al., 2016; Yousuf et al., 2016; Pohl and Kong Thoo Lin, 2018). They are potential sources for the development of functional foods, nutraceuticals, and modern drugs (Singh and Sashidhara, 2017; Wilson et al., 2017; Jain et al., 2018). Millets and sorghum are small-seeded grasses and are dietary staples for millions of people living in the semi-arid tropics of Asia and Africa (Vila-Real et al., 2017; Chisi and Peterson, 2019). Whole grains of millets and sorghum are reported to have a variety of health benefits including anti-lipidemic, anti-inflammatory, and anti-oxidant activities worldwide (Chandra et al., 2016; Sarita and Singh, 2016; Simnadis et al., 2016; Bhat et al., 2018; Jayawardana et al., 2021). Currently, there is a growing interest to explore and identify functional dietary staples which could be effectively used as vehicles for the prevention and management of hyperlipidemia and oxidative stress–associated chronic diseases, namely, diabetes, cancer, cardiovascular diseases, and other inflammatory diseases (Jew et al., 2009; Senevirathne et al., 2021).
Although there are reported studies on the health benefits of millets and sorghum the world over (Chandra et al., 2016; Sarita and Singh, 2016; Simnadis et al., 2016; Bhat et al., 2018; Jayawardana et al., 2021), studies addressing anti-inflammatory (Burdette et al., 2010; Shi et al., 2017; Jayawardana et al., 2021) and lipase inhibitory activity (Irondi et al., 2019; Jakubczyk et al., 2019) which are related to the anti-lipidemic property of sorghum and millet are scanty to date. Furthermore, reported findings have shown variation in activity depending on the genotype and availability of pigments in the grains (Awika et al., 2003; Dykes et al., 2005; Chandrasekara and Shahidi, 2010). To the best of our knowledge, there are no previous reports on cholesterol esterase inhibitory activity of millet and sorghum worldwide.
In Sri Lanka, millets and sorghum are important cereals (Kumari et al., 2017, 2020; Jayawardana et al., 2018, 2021; Senevirathne et al., 2021) and a range of millet types and sorghum varieties are being cultivated island-wide. Recent research conducted in the country was able to report glycemic regulatory properties of a range of millet types and sorghum varieties in Sri Lanka through anti-amylase, anti-glucosidase, antiglycation, and glycation-reversing activities (Senevirathne et al., 2021; Jayawardana et al., 2022). Furthermore, anti-oxidant (Chandrasekara and Shahidi, 2010; Kumari et al., 2017; Jayawardana et al., 2018), anti-microbial (Jayawardana et al., 2020), and anti-inflammatory properties (Jayawardana et al., 2021) of some millet types in Sri Lanka have also been reported. However, the anti-oxidant properties of sorghum varieties and anti-lipidemic properties of any of the millet types and sorghum varieties in Sri Lanka have not been previously reported. Furthermore, a comparative study addressing a range of anti-oxidant, anti-inflammatory, and anti-lipidemic properties for a wide range of millet types and sorghum varieties cultivated in Sri Lanka is not reported to date. Thus, the present study was planned to evaluate the anti-lipidemic, anti-inflammatory, and anti-oxidant properties, and proximate the composition of a range of millet types and sorghum varieties in Sri Lanka.
Materials and methods
Chemicals and reagents
Pancreatic lipase (Porcine pancreatic lipase, type II), cholesterol esterase (cholesterol esterase porcine), 4-nitrophenyl butyrate (p-NPB), sodium taurocholate hydrate, orlistat, simvastatin, gallic acid, quercetin, 6-hydroxy-2-5-7-8-tetramethylchroman-2-carboxylic acid (Trolox), rutin, ethylenediamine tetra acetic acid (EDTA), 2,4,6-tripyridyl-s-triazine (TPTZ), 1,1-diphenyl-2-picryl-hydrazyl (DPPH), 2,2'-azobis (2-amidinopropane) dihydrochloride (AAPH), 2,2 azinobis (3-ethylbenzothia zoline-6-sulfonate diammonium salt) (ABTS), 4,4-disulfonic acid sodium salt (Ferrozine), fluorescein, Folin Ciocalteus reagent, dimethyl sulfoxide (DMSO), aluminum chloride (AlCl3), sodium acetate tetrahydrate, glacial acetic acid, sodium hydroxide (NaOH), hydrochloric acid (HCl), methanol, monosodium dihydrogen orthophosphate, disodium monohydrogen orthophosphate, N-(1-Naphthyl ethylene diamine dihydrochloride), phosphoric acid, sulfanilamide, sodium nitroprusside, ferrous sulfate, acetonitrile, sodium phosphate, sodium chloride (NaCl) were purchased from Sigma-Aldrich, St Louis, USA. All the other chemicals and reagents used were of analytical grade.
Sample collection
Five millet types, namely, foxtail millet (Setaria italica), finger millet (Eleusine coracana) [Oshadha, Rawana, and white finger millet varieties], proso millet (Panicum miliaceum), Kodo millet (Paspalum scrobiculatum), and two sorghum varieties (Sorghum bicolor), namely, sweet sorghum and sorghum ICSV 112 (see Figure 1) were collected from Field Crop Research and Development Institute, Mahailluppallama, Sri Lanka.

Figure 1. Millet types and sorghum varieties selected for the study (A) millet types (B) sorghum varieties.
Preparation of samples
Whole grains of selected samples were milled using a laboratory mill (Fritsch, Pulverisette 14, Germany) and passed through a 0.5-mm sieve to obtain flour. Then, the whole grain flour of each millet type and sorghum variety was kept at 4°C in a laboratory refrigerator until the analysis.
Preparation of extracts
One gram of flour from each selected millet type and sorghum variety was extracted in 100 ml of methanol overnight at 160 rpm using a laboratory shaker (Orbital laboratory shaker, Stuart, UK) at room temperature (28 ± 2°C). Extracts were then centrifuged (825 g) for 10 min, filtered, and evaporated under vacuum using a rotary evaporator (Büchi® Rotavapor® R-210 evaporator, Switzerland) and freeze-dried (Christ-Alpha 1-4 Freeze dryer, Biotech International, Germany).
Anti-lipidemic properties
Lipase inhibitory activity
Pancreatic lipase inhibitory activity of selected samples was performed according to the method of Kim et al. (2010) and Abeysekera et al. (2017a) with some modifications using 96-well microplates (n = 3 each). Reaction volume of 200 μl containing 100 μl of the sample (2 mg/ml) in 0.1 M Tris HCl buffer with 5 mM CaCl2 and 50 μl of 5 mg/ml porcine pancreatic lipase were pre-incubated at 37°C for 15 min. Then, 10 μl of 10 mM 4-nitrophenyl butyrate (p-NPB) in dimethylformamide was added and incubated at 37°C for 30 min and the absorbance readings were measured at 405 nm using a 96-well microplate reader (SpectraMax Plus384, Molecular Devices, USA). Orlistat was used as the positive control. Inhibition of lipase activity was expressed as the % of lipase inhibitory activity and IC50 values (μg/ml).
Anti-cholesterol esterase activity
Pancreatic cholesterol esterase inhibitory activity of samples was performed according to the method of Pietsch and Gütschow (2005) and Abeysekera et al. (2017a) with some modifications using 96-well microplates (n = 3 each). A reaction volume of 200 μl containing 50 μl of 24 mM taurocholic acid, 10 μl of 8 mM p-NPB in 0.1M sodium phosphate buffer, and 0.1M NaCl, and 100 μl of the sample (2 mg/ml) were pre-incubated at 25°C for 10 min. Then, 30 μl of (1 μg/ml) cholesterol esterase was added and incubated at 25°C for 6 min and absorbance readings were monitored at 405 nm using a 96-well microplate reader (SpectraMax Plus384, Molecular Devices, USA). Simvastatin was used as the positive control. Results were expressed as the % of cholesterol esterase inhibitory activity inhibition and IC50 values (μg/ml).
Anti-inflammatory activity
Nitric oxide inhibitory activity
The nitric oxide inhibition assay was performed according to the method of Patel Rajesh and Patel Natvar (2011) with some modifications to the 96-well microplates (n = 3). The reaction volume of 100 μl containing 50 μl of the sample (2 mg/ml) and 50 μl of 10 mM sodium nitroprusside (30 mg/ml) was incubated at room temperature (25 ± 2°C) for 90 min. Then, 60 μl of 0.1% N-(1-Naphthyl ethylene diamine dihydrochloride) in 2.5% phosphoric acid and 60 μl of 1% sulfanilamide in 2.5% phosphoric acid were added and incubated at room temperature (25 ± 2°C) for 30 min. Absorbance readings were then recorded at 550 nm using a 96-well microplate reader (SpectraMax Plus384, Molecular Devices, USA). Rutin was used as the standard. Results were expressed as % of nitric oxide inhibitory activity.
Anti-oxidants and anti-oxidant properties
Total polyphenolic content
TPC of selected samples was determined using the Folin-Ciocalteu method (Singleton et al., 1999) with the 96-well microplates (n = 3 each). Reaction volume of 200 μl containing 20 μl of the sample (2 mg/ml), 110 μl of 10 times diluted Folin-Ciocalteu reagent, and 70 μl of 10% sodium carbonate were incubated at room temperature (25 ± 2°C) for 30 min, and the absorbance readings were recorded at 765 nm using a 96-well microplate reader (SpectraMax Plus384, Molecular Devices, USA). Gallic acid was used as the standard. TPC was expressed as mg gallic acid equivalents (GAEs) per g of sample on a dry weight basis.
Total flavonoid content
TFC of selected samples was determined using the aluminum chloride method (Siddhuraju and Becker, 2003) using 96-well microplates (n = 3 each). A reaction volume of 200 μl containing 100 μl of 2% aluminum chloride in methanol and 100 μl of each sample (2 mg/ml) were incubated at room temperature (25 ± 2°C) for 10 min and absorbance readings were taken at 415 nm using a 96-well microplate reader (SpectraMax Plus384, Molecular Devices, USA). Quercetin was used as the standard. TFC was expressed as mg Quercetin equivalents (QEs) per g of sample on a dry weight basis.
Total proanthocyanidin content
TPAC of samples was determined by HCl-butanol assay as described by Porter et al. (1985) with minor modifications. A reaction volume of 3.6 ml containing 100 μl of 2% ammonium iron (III) sulfate dodecahydrate in 2M HCl, 3 ml of HCl butanol reagent, and 0.5 ml of sample (0.5 mg/ml in methanol) were allowed to react in screw-capped test tubes in a water bath at 95°C for 40 min. The same reaction mixture without heating in a water bath was used as the sample negative control. Blank was prepared by replacing the extract with methanol. The mixtures were allowed to cool to room temperature (25 ± 2°C), and absorbance readings were taken at 550 nm using a 96-well microplate reader (SpectraMax Plus384, Molecular Devices, USA). Cyanidin chloride was used as the standard. Results were expressed as mg of cyanidin equivalents per g of the sample on a dry weight basis.
Ferric reducing anti-oxidant power
Ferric reducing anti-oxidant power (FRAP) of selected samples was performed according to the method of Benzie and Szeto (1999) with modifications using 96-well microplates (n = 3 each). The FRAP reagent was prepared by mixing 300 mM of acetate buffer (pH-3.6), 20 mM of ferric chloride solution (FeCl3.6H2O), and 10 mM of 2,4,6-tripyridyl-s-triazine (TPTZ) in a ratio of 10:1:1 just before use and incubated at 37°C for 10 min. Reaction volume of 200 μl containing 150 μl of working FRAP reagent, 30 μl of acetate buffer, and 20 μl of the sample (2 mg/ml) were incubated at room temperature (25 ± 2°C) for 8 min, and the absorbance readings were taken at 600 nm using a 96-well microplate reader (SpectraMax Plus384, Molecular Devices, USA). Trolox was used as the standard. Results were expressed as mg of Trolox equivalents (TEs) per g of sample on a dry weight basis.
Oxygen radical absorbance capacity
Oxygen radical absorbance capacity (ORAC) of samples was performed according to the method described by Ou et al. (2001) with some modification in 96-well microplates (n = 3 each). Reaction volume of 200 μl containing 100 μl fluorescein (4.8 μM), 50 μl of the sample (1 mg/ml), or Trolox (1.5 and 0.75 μg/ml), and 50 μl AAPH (40 mg/ml), or 50 μl phosphate buffer for control (75 mM, pH 7.4) were incubated at 37°C 5 min. The plate was placed on the fluorescent microplate reader (SPECTRAmax- Gemini EM, Molecular Devices Inc, USA) set with excitation and emission at 494 and 535 nm, respectively, and decay of fluorescein was recorded in 1 min interval for 35 min. Trolox was used as the standard. Results were expressed as mg of TEs per g of sample on a dry weight basis.
Ferrous ion chelating activity
Ferrous ion chelating activity (FICA) of selected samples was determined according to the method of Chandrasekara and Shahidi (2010) with some modifications using 96-well microplates (n = 3 each). Reaction volume of 200 μl containing 100 μl of the sample (2 mg/ml), 20 μl of 1 mM ferrous sulfate solution, 40 μl of distilled water, and 40 μl of 1 mM ferrozine solution was incubated at room temperature (25 ± 2°C) for 10 min. The absorbance readings were measured at 562 nm using a 96-well microplate reader (SpectraMax Plus384, Molecular Devices, USA). EDTA was used as the standard. Results were expressed as mg of EDTA equivalents per g of sample on a dry weight basis and IC50 values (μg/ml).
ABTS radical scavenging activity
The ABTS radical scavenging activity was performed according to the method by Re et al. (1999) with modifications in 96-well microplates (n = 3 each). ABTS radical was generated by incubating 10 mg ABTS in 2.5 ml of 2.5 mM potassium persulfate at 37°C for 16 h under dark conditions. Reaction volume of 200 μl containing 55 μM of ABTS radical, 50 μl of the sample (2 mg/ml), and 95 μl of buffer were incubated at room temperature (25 ± 2°C) for 10 min. Then, absorbance readings were recorded at 734 nm using a 96-well microplate reader (SpectraMax Plus384, Molecular Devices, USA). For dose-response studies, 7.8125, 15.625, 31.25, 62.5, 125, and 250 μg/ml concentrations of selected extracts were used. Trolox was used as the standard. Results were expressed as mg of TEs per g of sample on a dry weight basis and IC50 values (μg/ml).
DPPH radical scavenging activity
The DPPH radical scavenging activity was determined according to the method of Blois (1958) with modifications using 96-well microplates (n = 3 each). Reaction volume of 200 μl containing 60 μl of DPPH (0.2 mg/1 ml), 90 μl of methanol, and 50 μl of the sample (2 mg/ml) was incubated at room temperature (25 ± 2°C) for 10 min and the absorbance readings were measured at 517 nm using a 96-well microplate reader (SpectraMax Plus384, Molecular Devices, USA). For dose-response studies, 7.8125, 15.625, 31.25, 62.5, 125, 250, 500, and 1,000 μg/ml concentrations of selected extracts were used. Trolox was used as the standard. Results were expressed as mg TEs per g of the sample on a dry weight basis and IC50 values (μg/ml).
Proximate composition
As a proximate composition, whole grain flour of selected samples was analyzed for moisture content (oven dry method), crude ash (dry ashing method), crude protein (Kjeldahl method), crude fat (Soxhlet method), and crude fiber contents (n = 3 each) using methods described in the Association of Official Analytical Chemists (AOAC, 2000).
Statistical analysis
Data were analyzed using Minitab software (Version 17.3.1, Minitab, Inc, Pennsylvania, USA). Experiments were carried out in triplicates (n = 3) and results were expressed as mean ± standard deviation (SD). The differences in mean values among samples were determined using the one-way analysis of variance (ANOVA) followed by Tukey's Honestly Significant Difference (HSD) multiple rank tests at a P ≤ 0.05 significance level.
Results and discussion
Anti-lipidemic activity
Lipase inhibitory activity
Pancreatic lipase is a key enzyme involved in the digestion of fats into free fatty acids and glycerol in the intestine (Birari and Bhutani, 2007). Percent lipase inhibitory activity of selected millet types and sorghum varieties screened at 2 mg/ml is given in Figure 2A. All the selected millet types and sorghum varieties showed lipase inhibitory activity, and it ranged from 21.16 ± 1.58 to 66.65 ± 3.30%. Among the studied samples, sweet sorghum exhibited the highest inhibitory activity (P < 0.05) at the tested concentration. Furthermore, it showed concentration-dependent relationship (r2 = 0.98) in dose-response studies. The IC50 value was 927.35 ± 5.57 μg/ml, and the results are given in Figure 2B. However, the observed lipase inhibitory activity of sweet sorghum was significantly low (P < 0.05) compared to the reference drug orlistat (26.78 ± 2.45 μg/ml) used in this study. The order of potency of samples in terms of lipase inhibitory activity was sweet sorghum > Kodo millet > Oshadha > sorghum ICSV112 = Rawana = foxtail millet > white finger millet = proso millet.

Figure 2. Lipase inhibitory activity of selected samples. (A) Screening of selected millet types and sorghum varieties for lipase inhibitory activity at 2 mg/ml concentration. (B) Dose-response relationship of sweet sorghum for lipase inhibitory activity: IC50: 927.35 ± 5.57 μg/ml. Different letters on bar graphs are significantly different at P < 0.05.
Worldwide reports on the lipase inhibitory activity of millets and sorghum are extremely limited. Irondi et al. (2019) have reported lipase inhibitory activity (IC50 value) of raw and roasted red sorghum as 12.72 ± 1.1 and 14.13 ± 1.42 μg/ml, respectively. In a study by Jakubczyk et al. (2019), lipase inhibitory activity (IC50 value) of heat-treated millet grains was reported as 0.03 mg/ml. The observed lipase inhibitory activities of selected millet types and sorghum varieties in Sri Lanka were lower than the findings of Irondi et al. (2019) and Jakubczyk et al. (2019). This might be due to the differences in genotypes and extraction protocols used in the studies. It has been shown that lipase inhibitory activity has a positive relationship with the presence of anti-oxidants such as a variety of polyphenolic compounds and anti-oxidant activities, namely, DPPH and ABTS radical scavenging activities and FRAP and ORAC in natural products (Quiroga et al., 2013; Huang et al., 2020). Interestingly, Irondi et al. (2019) have also observed higher lipase inhibitory activity in sorghum samples containing high anti-oxidant activity. In the present study, we also observed high lipase inhibitory activity in millet and sorghum samples (Oshadha and sweet sorghum) containing high amounts of TPC, TPAC, and high DPPH, and ABTS, FRAP, and ORAC anti-oxidant properties. Therefore, the observed lipase inhibitory activity of samples may be at least partly due to the presence of anti-oxidants and their mode of action. However, the exact mechanisms in mediating the lipase inhibitory activity of millet and sorghum in Sri Lanka should be evaluated in future research studies.
Cholesterol esterase inhibitory activity
Dietary cholesterol contains free and esterified cholesterols, and pancreatic cholesterol esterase is the enzyme responsible for the hydrolysis of esterified cholesterols. Therefore, inhibition of cholesterol esterase activity plays a key role to bring down dietary cholesterol absorption (Kumar et al., 2011). All the selected millet types and sorghum varieties showed cholesterol esterase inhibitory activity, and it ranged from 17.43 ± 0.60 to 52.09 ± 1.61% (2 mg/ml), and the results are given in Figure 3A. Sweet sorghum exhibited the highest inhibitory activity (52.09 ± 1.61%) among the samples tested and the IC50 value was 1861.71 ± 20.74 μg/ml. The dose-response relationship (r2 = 0.99) of sweet sorghum for cholesterol esterase inhibitory activity is given in Figure 3B. However, the observed activity of sweet sorghum was significantly low (P < 0.05) compared to the reference drug Simvastatin (IC50 value: 18.56 ± 0.68 μg/ml). Among the millets studied, Oshadha showed the highest cholesterol esterase inhibitory activity. The order of potency of tested samples was sweet sorghum > Oshadha > sorghum ICSV112 = proso millet = foxtail millet > Kodo millet > Rawana = white finger millet.
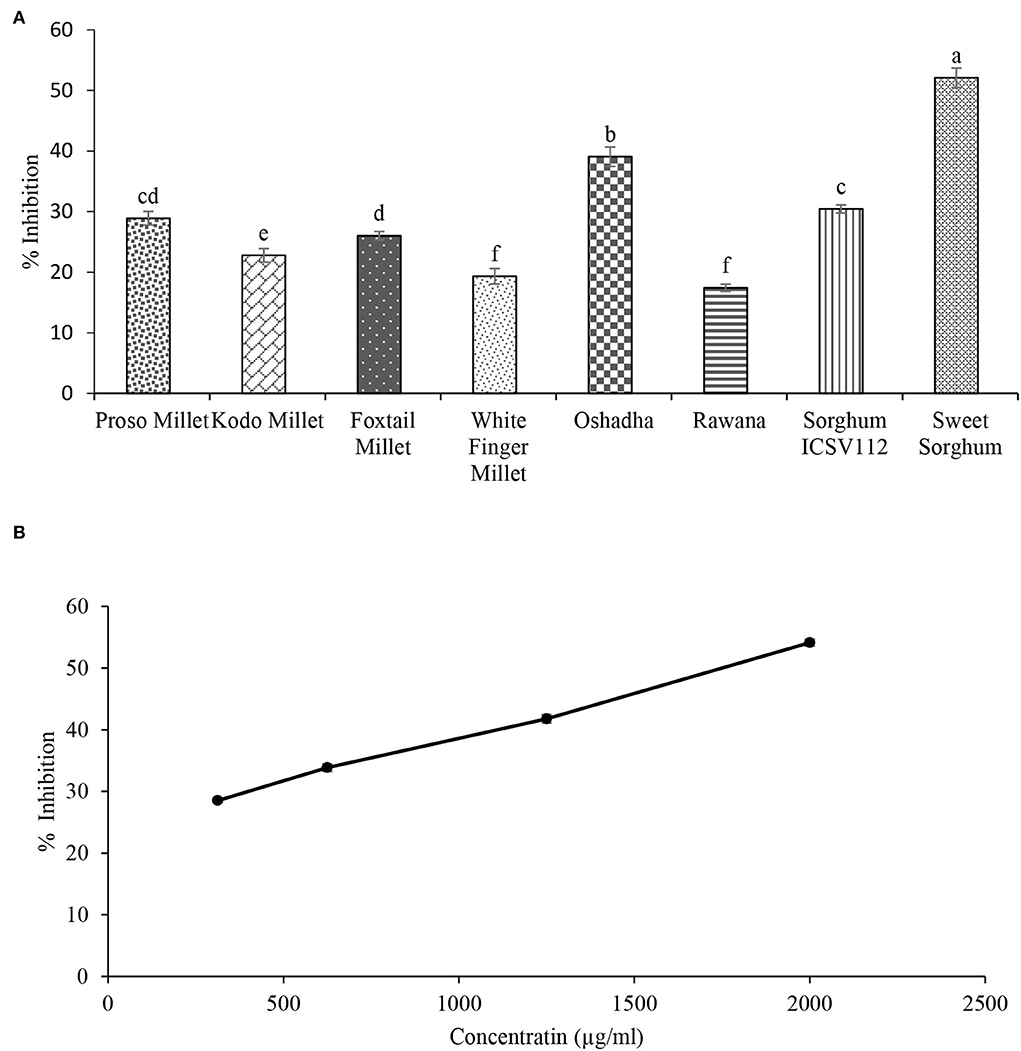
Figure 3. Cholesterol esterase inhibitory activity of selected samples. (A) Screening of selected millet types and sorghum varieties for cholesterol esterase inhibitory activity at 2 mg/ml concentration. (B) Dose-response relationship of sweet sorghum for cholesterol esterase inhibitory activity: IC50: 1861.71 ± 20.74 μg/ml. Different letters on bar graphs are significantly different at P < 0.05.
The presence of anti-oxidants such as flavonoids, phenolic compounds, and anti-oxidant properties, namely, free radical scavenging ability (ABTS and DPPH radical scavenging activity), reducing ability (FRAP) have shown a positive relationship in mediating cholesterol esterase inhibitory activity of some natural products (Adisakwattana et al., 2012; Chatatikun and Kwanhian, 2020). In the present study, we too observed high cholesterol esterase inhibitory activities in Oshadha and sweet sorghum samples containing the greatest anti-oxidants (TPC and TPAC) and anti-oxidant activities (ABTS, DPPH, ORAC, and FRAP). Therefore, the observed cholesterol esterase inhibitory activity of millet types and sorghum in Sri Lanka might be due to the presence of anti-oxidants and anti-oxidant activities through multiple mechanisms. As there are no previous reports of cholesterol esterase inhibitory activity in millet and sorghum the world over, a comparison is impossible.
Anti-inflammatory activity
Nitric oxide inhibitory activity
Plant extracts and plant-derived formulations have been used to control inflammatory disorders and related diseases since ancient times (Salminen et al., 2008; Mueller et al., 2010). The % nitric oxide inhibitory activity of selected millet types and sorghum varieties ranged from−1.17 ± 0.32 to 13.56 ± 0.93 at 2 mg/ml, and the results are shown in Figure 4. Oshadha (13.56 ± 0.93%) and sweet sorghum (12.99 ± 1.27%) exhibited significantly high (P < 0.05) nitric oxide inhibitory activity among the samples tested in this study. Next, the highest nitric oxide inhibitory activities were observed in white finger millet (4.76 ± 0.80%) and Rawana (3.61 ± 1.80%) varieties. Kodo millet and sorghum ICSV112 did not show nitric oxide inhibitory activity at the tested concentration. The concentrations higher than the tested concentration were not evaluated as the tested concentration is an agreeable concentration for a natural product to present a biological activity of interest. Results clearly showed that observed nitric oxide inhibitory activities of the tested samples were very low compared to the reference drug rutin (IC50: 17.62 ± 0.01 μg/ml) used in this study. To the best of our knowledge, NO scavenging activity of millet is extremely limited, and this highlights the need for conducting research on this topic. A recent study (Ajiboye et al., 2017) has reported NO scavenging activity of ethanolic and methanolic extracts of finger millet and the highest activity has been observed in the ethanolic extract with IC50 value of nearly 10 mg/ml. In the present study, we observed the highest activity of 13.56 ± 0.93 at 2 mg/ml and this indicates finger millets might have less NO activity. NO scavenging activity of sorghum has been reported in few studies (Dia et al., 2016; Hong et al., 2020). Dia et al. (2016) have reported that methanolic extracts of sorghum inhibited NO production from 16.8% to 51.5% while ethanolic extracts inhibited NO production from 33.8 to 61.7%. In the present study, we observed NO inhibitory activity for sweet sorghum as 12.99 ± 1.27% at 2 mg/ml concentration. Hong et al. (2020) have reported NO scavenging activity using cell-based assays and thus comparison with the present study is quite difficult.
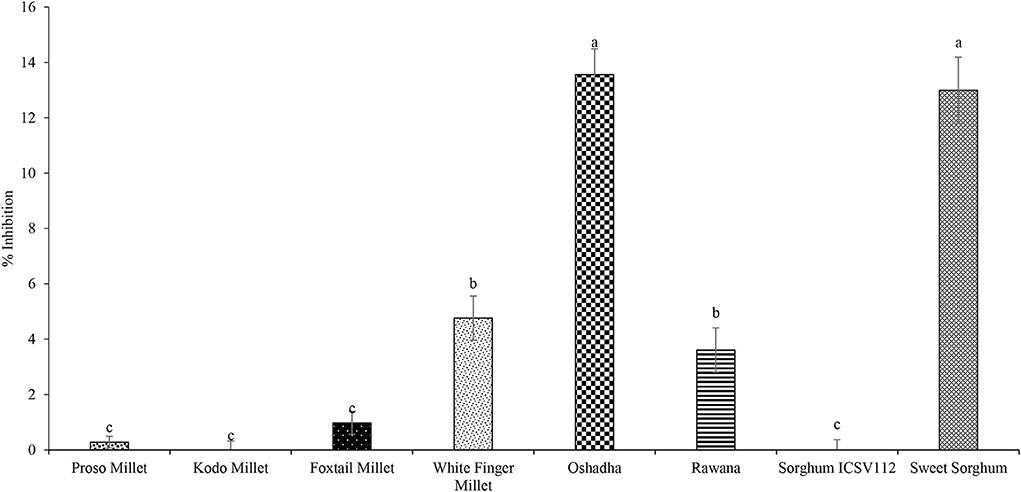
Figure 4. Nitric oxide inhibitory activity of selected millet types and sorghum varieties at 2 mg/ml concentration. Different letters on bar graphs are significantly different at P < 0.05.
Jayawardana et al. (2021) have reported anti-inflammatory activity of methanolic and ethanolic extracts of Oshadha and Rawana Sri Lankan finger millet varieties through inhibition of Arachidonate 5-lipoxygenase enzyme and have shown strong positive correlation with anti-oxidant activities, namely, DPPH and ABTS radical scavenging activities. In the present study, we observed high nitrogen oxide inhibitory activity in millet (Oshadha) and sorghum samples (Sweet sorghum) containing greatest amounts of TPC & TPAC and anti-oxidant activities, namely, FRAP, ABTS and DPPH radical scavenging abilities and ORAC.
Anti-oxidant activity
Total phenolic content
Phenolic compounds present in plants play a vital role in exhibiting anti-oxidant activities through multiple pathways (Tungmunnithum et al., 2018). In this study, the TPC of selected millet types and sorghum varieties in Sri Lanka ranged from 0.19 ± 0.01 to 12.50 ± 0.87 mg of GAEs/g of the sample, and the results are presented in Figure 5. Sweet sorghum showed significantly high (P < 0.05) TPC (12.50 ± 0.87 mg of GAEs/g) among the samples tested in this study. The order of samples in term of TPC was sweet sorghum > Oshadha > Kodo millet = Rawana > foxtail millet = sorghum ICSV112 = proso millet = white finger millet.
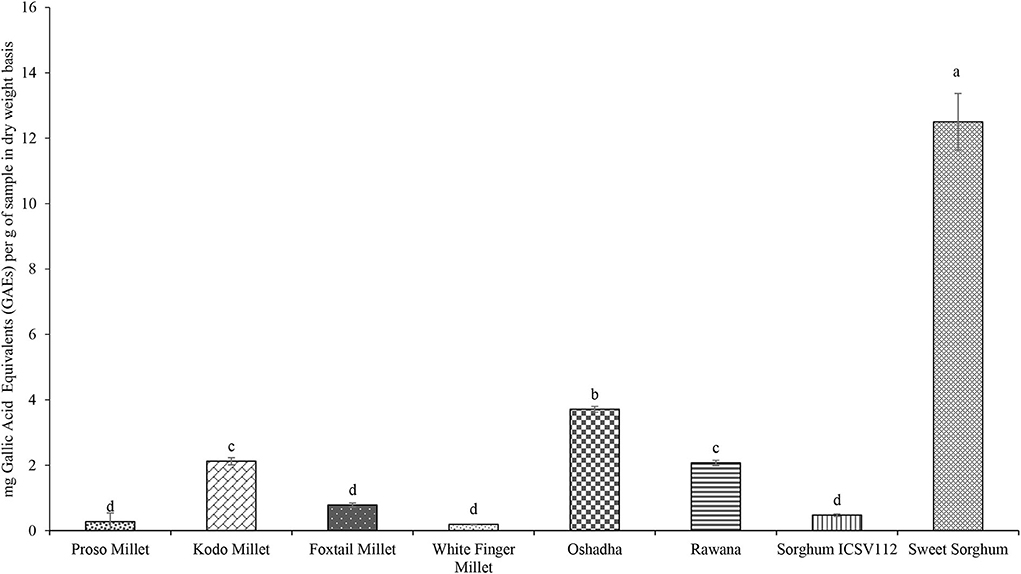
Figure 5. Total Phenolic Content (TPC) of selected millet types and sorghum varieties. Different letters on bar graphs are significantly different at P < 0.05.
The TPC of a range of millet types in Sri Lanka has been reported in few studies conducted in the country (Chandrasekara and Shahidi, 2010, 2011a,b; Kumari et al., 2017; Jayawardana et al., 2018, 2021). The reported studies have clearly shown that TPC was highest in pigmented millet types (Finger millet variety Ravi and Kodo millet) compared to the non-pigmented millet types such as pearl, proso, foxtail, and little millet (Chandrasekara and Shahidi, 2010). Comparison of reported results with the present study is quite difficult as in most of the reported studies the sample preparation and extraction protocols and the expression of results (mostly presented as ferulic acid equivalents/g of dry matter) are different from the present study. However, the results of the present study are at least in agreement with the previous research findings with respect to the highest TPC in pigmented millet types (Oshadha, Rawana, and Kodo millet) compared to the non-pigmented millet types. To the best of our knowledge, there are no previous reports on the TPC of sorghum in Sri Lanka. However, there are a number of studies on the TPC of sorghum varieties the world over (Dykes et al., 2005; Wu et al., 2016; Rao et al., 2018). According to Dykes et al. (2005), the purple and red color sorghum grain samples had high levels of total phenolics (3.1–8.9 mg GAEs/g) than those from tan-colored sorghum grain samples (2.1–2.6 mg GAEs/g). Similarly, the same results were observed in a study conducted by Rao et al. (2018). In the present study, we observed that TPC was highest in pigmented sorghum variety, namely, sweet sorghum compared to the non-pigmented variety, sorghum ICSV112. Thus, our results are in agreement with the previous research findings.
Total flavonoid content
Flavonoids are a group of polyphenolic compounds that exert anti-oxidant properties through multiple mechanisms (Hendrich, 2006). The TFC of selected millet types and sorghum varieties in Sri Lanka is given in Figure 6. The TFC of selected millet types and sorghum varieties ranged from 0.05 ± 0.00 to 1.57 ± 0.01 mg of QEs/g of the sample. Among the samples studied, sweet sorghum exhibited the highest (P < 0.05) TFC. The order of samples for TFC was sweet sorghum > Kodo millet > foxtail millet > Oshadha > Rawana > sorghum ICSV112 = white finger millet = proso millet. According to few reported studies in Sri Lanka (Chandrasekara and Shahidi, 2010, 2011a,b; Kumari et al., 2017; Jayawardana et al., 2021) and the world over (Upadhyay et al., 2015), TFC of millet types and sorghum varieties had shown vast differences. This might be due to differences in genotypes, sample preparation protocols, extraction protocols, processing conditions, and the optimized assay conditions used in such studies (Abeysekera et al., 2017a). Therefore, the comparison of the results of the present study with the previous studies is quite difficult. To the best of our knowledge, this is the first report of TFC of sorghum varieties in Sri Lanka.
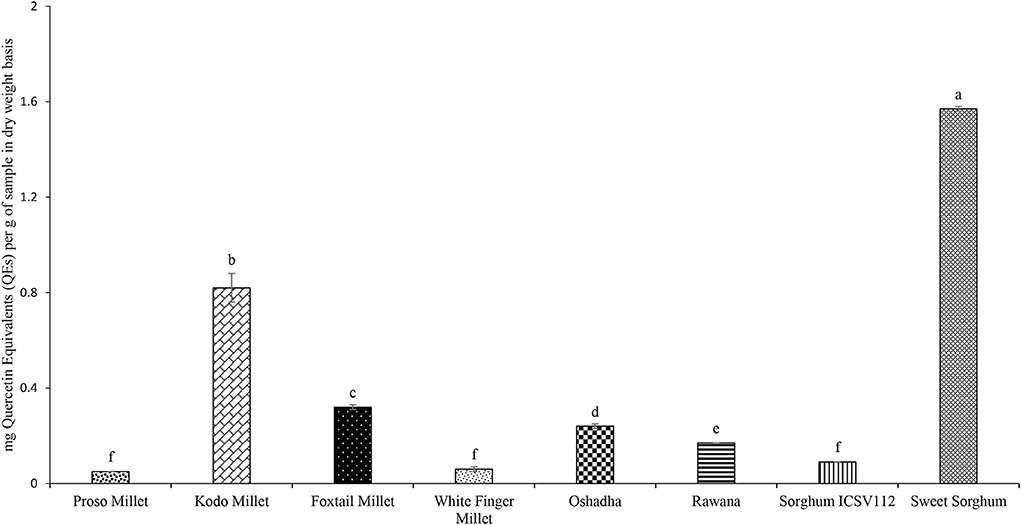
Figure 6. Total Flavonoid Content (TFC) of selected millet types and sorghum varieties. Different letters on bar graphs are significantly different at P < 0.05.
Total proanthocyanidin content
Proanthocyanidins or condensed tannins are a group of phytochemicals that are present in various plants exerting a wide range of biological activities including anti-oxidant properties (Smeriglio et al., 2017). The total TPAC of the selected millet types and sorghum varieties in Sri Lanka is given in Figure 7. Significant differences (P < 0.05) were observed among the samples for TPAC, and they ranged from 0.35 ± 0.01 to 12.87 ± 0.25 mg of cyanidin equivalents (CEs)/g of the sample. The highest (P < 0.05) TPAC was observed in sweet sorghum while the second highest TPAC was observed in sorghum variety ICSV 112. The order of samples for TPAC was sweet sorghum > Oshadha > Kodo millet = foxtail millet = Rawana = sorghum ICSV112 = white finger millet = proso millet.
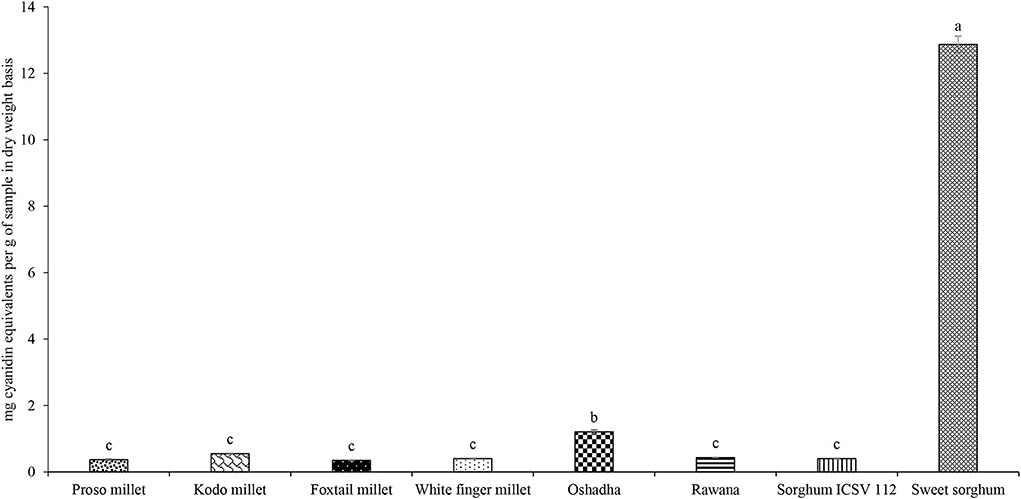
Figure 7. Total Proanthocyanidin Content (TPAC) of selected millet types and sorghum varieties. Different letters on bar graphs are significantly different at P < 0.05.
Few reports are available on the proanthocyanidin content of millet types cultivated in Sri Lanka. According to a study conducted by Kumari et al. (2017), the Rawana finger millet variety had the highest TPAC compared to Oshadha and Ravi finger millet varieties. Chandrasekara and Shahidi (2010) have shown that TPAC was highest in a local finger millet variety (311.28 ± 3.0 μmol of CEs/g of defatted meal) followed by finger millet variety Ravi, foxtail millet, little millet, pearl millet, and proso millet. The results of both studies mentioned above have been presented in different units hence the comparison of results with the present study makes it impossible. Furthermore, international reports on TPAC of millets and sorghum are extremely limited. According to a study by Bvochora et al. (1999), malting and lactic acid fermentation could decrease the TPAC of high proanthocyanidin-containing sorghum cultivars indicating that there is an effect of processing conditions on the TPAC of sorghum cultivars. Thus considering the reported findings, it is clear that the TPAC of millet and sorghum varies based on a number of factors. This is the first study to report TPAC of sorghum varieties in Sri Lanka.
Ferric reducing anti-oxidant power
The FRAP assay is widely used in assessing the reducing power of plasma. However, currently, this assay is extensively used for evaluating anti-oxidant activity of various foods and other natural products (Benzie and Strain, 1996). All selected millet types and sorghum varieties in Sri Lanka showed FRAP and results varied significantly (P < 0.05) among the samples. The FRAP of tested samples ranged from 0.15 ± 0.00 to 4.56 ± 0.03 mg of TEs/g of the sample, and the results are given in Figure 8. Sweet sorghum (4.56 ± 0.03 mg of TEs/g) exhibited the highest FRAP among all the studied millet and sorghum samples. The order of potency of samples for FRAP was sweet sorghum > Oshadha > Rawana > Kodo millet > foxtail millet = proso millet = sorghum ICSV112 = white finger millet.
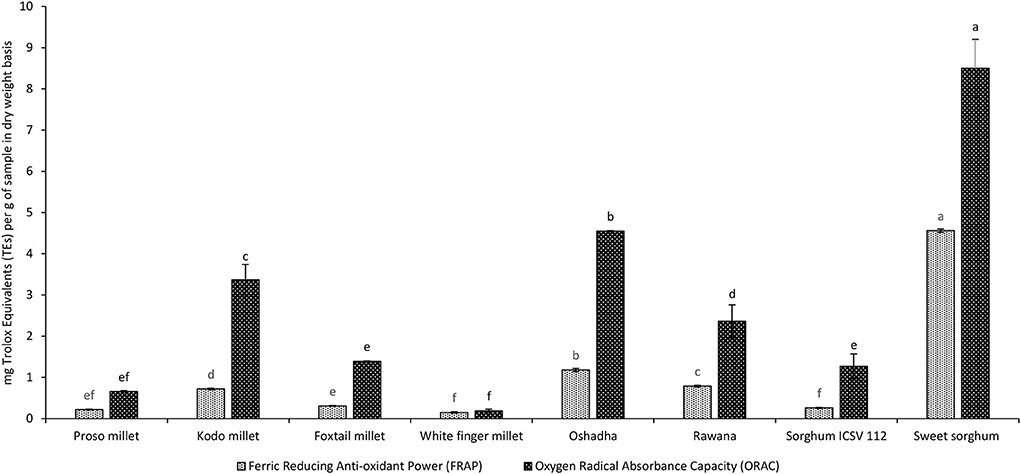
Figure 8. Ferric Reducing Anti-oxidant Power (FRAP) and Oxygen Radical Absorbance Capacity (ORAC) of selected millet types and sorghum varieties. FRAP and ORAC have been statistically analyzed separately. Different letters on bar graphs are significantly different at P < 0.05.
There are few reported studies on the FRAP of millet and sorghum the world over (Sreeramulu et al., 2009; Abeysekera et al., 2017b; Kumari et al., 2017; Jayawardana et al., 2018). However, none of these studies have addressed FRAP of a range of millet types and sorghum varieties as we report in the present study. Furthermore, the results of such studies have shown wide variations for FRAP of millet and sorghum varieties tested. Kumari et al. (2017) have reported that the FRAP of the Rawana millet variety was higher than that of the Oshadha millet variety, and the results are different from the findings of the present study. The observed variations might be due to the differences in the sample preparation and extraction protocols used in these two studies. According to a study by Jayawardana et al. (2018), the FRAP of finger millet (43.81–58.18 mg GAEs/100 g) was higher than the FRAP of foxtail millet (21.36–25.78 mg GAEs/100 g). Furthermore, they have observed a strong positive correlation between FRAP and TPC. In the present study, we also observed that samples that showed the highest TPC, TFC, and TPAC exhibited the highest FRAP. Thus, phenolic compounds might play an important role in exerting FRAP of millet and sorghum varieties. Abeysekera et al. (2017b) have reported that FRAP of methanolic extract (1413.69 ± 1.61 TEs/100 g whole grain) of finger millet sample collected from the local market was significantly (P < 0.05) higher than the ethanolic extract (606.34 ± 5.00 mg of TEs/100 g whole grain) of the same sample showing that extraction protocols have a major influence on the FRAP of cereals including millets. There are an extremely limited number of studies on the FRAP of sorghum the world over (Rao et al., 2018). Results of reported studies (Abeysekera et al., 2017b; Kumari et al., 2017; Jayawardana et al., 2018) have clearly shown that the FRAP of pigmented sorghum varieties is much greater than the non-pigmented sorghum varieties. Thus, the findings are in accordance with the findings of the present study.
Oxygen radical absorbance capacity
Peroxyl radicals play a major role in lipid peroxidation in foods and create oxidative stress in biological systems under physiological conditions. The ORAC assay evaluates the ability of an anti-oxidant to inhibit the peroxyl radical-induced oxidation. Thus, ORAC values are considered to be more biologically relevant as a reference for anti-oxidant effectiveness (Cao et al., 1993).
ORAC of selected millets and sorghum samples varied from 0.19 ± 0.01 to 8.50 ± 0.72 mg of TEs/g of the sample, and the results are given in Figure 8. Among the samples tested sweet sorghum exhibited significantly high (P < 0.05) ORAC compared to all the other samples studied. The order of potency of samples for ORAC was sweet sorghum > Oshadha > Kodo millet > Rawana > foxtail millet = sorghum ICSV112 = proso millet = white finger millet.
There are a limited number of reported studies on ORAC of millet and sorghum varieties in the world (Awika et al., 2003; Chandrasekara and Shahidi, 2011a,b; Abeysekera et al., 2017b). Chandrasekara and Shahidi (2011a) have reported ORAC of Kodo millet as 95.7 ± 2.37 μmol/g for defatted meal. Furthermore, in another study, they have shown that ORAC was high in millet varieties with high TPC (Chandrasekara and Shahidi, 2011b). Similarly, in the present study, we observed that TPC might have a role in exerting ORAC of millet and sorghum. Abeysekera et al. (2017b) have reported that ORAC of market collected finger millet samples as 1240.45 ± 67.38 and 1112.52 ± 24.38 mg TEs/100 g in methanolic and ethanolic extracts of samples, respectively. Comparison of results with the present study is quite difficult without knowing the extract variety used in the study of Abeysekera et al. (2017b). Awika et al. (2003) have clearly shown that ORAC values become low with the low levels of tannins, anthocyanins, and phenolic compounds in sorghum. Furthermore, in this study, they reported pigmented sorghums (brown sorghum and black sorghum) with greater ORAC values (140–870 mg of TEs/g grains and 710–3,100 mg of TEs/g brans) compared to non-pigmented sorghum (white sorghum) tested. In the present study, we also observed the same finding. This is the first study to report ORAC of sorghum in Sri Lanka.
Ferrous ion chelating activity
Ferrous ions are identified as an important catalyst to generate free radicals and initiate radical-mediated lipid peroxidation. Chelating agents inhibit the free radical generation through stabilizing transition metal ions and this process subsequently reduces free radical-induced damage to the biologically important macromolecules. Therefore, compounds which can play a role in chelating transitional metal irons are important in the management of oxidative stress-associated chronic diseases (Patel, 2013).
The FICA of selected millet types and sorghum varieties is varied significantly (P < 0.05) among the samples and the results are given in Figure 9. The FICA of tested samples varied from 0.13 ± 0.00 to 0.79 ± 0.03 mg of EDTA equivalents/g of the sample. Among the samples studied, sweet sorghum (0.79 ± 0.03 mg of EDTA equivalents/g) showed the highest FICA. The order of potency of samples for FICA was sweet sorghum > Kodo millet > Oshadha = proso millet = foxtail millet > sorghum ICSV112 = Rawana = white finger millet. However, all the tested samples showed low FICA at the tested (2 mg/ml) sample concentration. The concentrations higher than the tested concentration were not tested in the present study as pigments in the millet and sorghum samples interfere with the assay condition. Furthermore, a tested concentration is an agreeable concentration for a natural product to present a biological activity of interest.
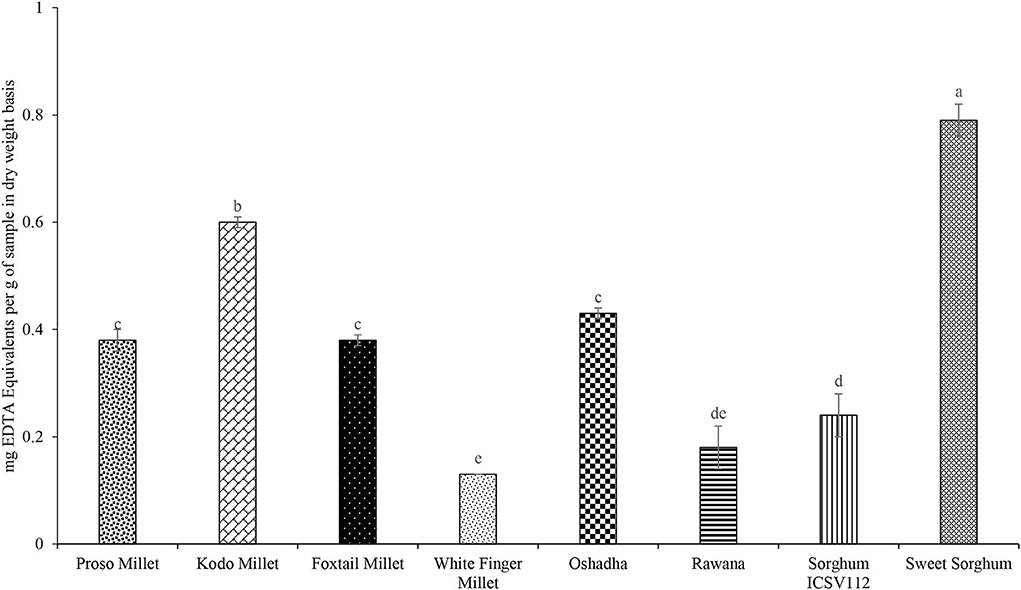
Figure 9. Ferrous Ion Chelating Activity (FICA) of selected millet types and sorghum varieties. Different letters on bar graphs are significantly different at P < 0.05.
Studies on FICA of millets and sorghum are extremely limited. Kumari et al. (2017) have reported that the Rawana millet variety (4.3 ± 0.2 μmol of EDTA equivalents/g dry matter) had a higher FICA than the Oshadha millet variety (3.5 ± 0.1 μmol of EDTA equivalents/g dry matter). Furthermore, the above-stated study has also shown that foxtail millet and proso millet with lower FICA than finger millet, and the results are not in agreement with the present study. The observed variations might be due to the differences in sample preparation and extraction protocols used in the above-stated study and the present study. To the best of our knowledge, there are no other reported studies on FICA of millet and sorghum the world over.
ABTS radical scavenging activity
ABTS radical scavenging assay is extensively used to evaluate the anti-oxidant activity of a variety of foods and natural products (Re et al., 1999). The screening of selected millet types and sorghum varieties in Sri Lanka for ABTS radical scavenging activity is given in Figure 10. Furthermore, the dose-response relationship of selected samples is given in Figures 11A,B. Results showed that ABTS radical scavenging activity of selected millet types and sorghum varieties ranged from 0.22 ± 0.00 to 25.57 ± 0.35 mg of TEs/g of the sample at the screening. Among the samples studied, sweet sorghum exhibited significantly high (P < 0.05) ABTS radical scavenging activity compared to all the other samples tested. The order of potency of samples at screening (2 mg/ml) for ABTS radical scavenging activity was sweet sorghum > Oshadha > Rawana > Kodo millet > sorghum ICSV112 = foxtail millet = proso millet > white finger millet.
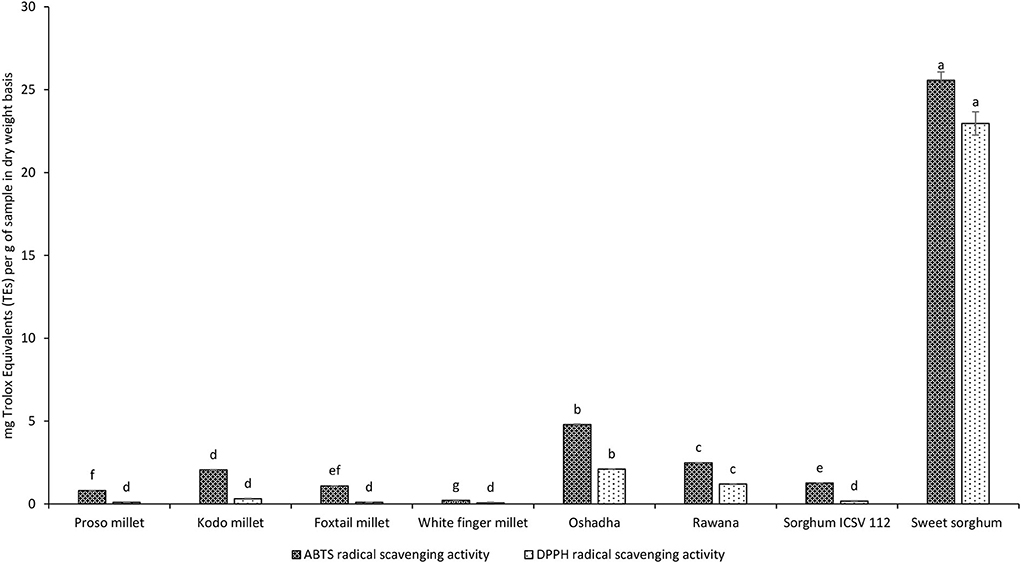
Figure 10. Screening of selected millet types and sorghum varieties for ABTS and DPPH radical scavenging activities. ABTS and DPPH radical scavenging activities have been separately analyzed. Different letters on bar graphs are significantly different at P < 0.05.
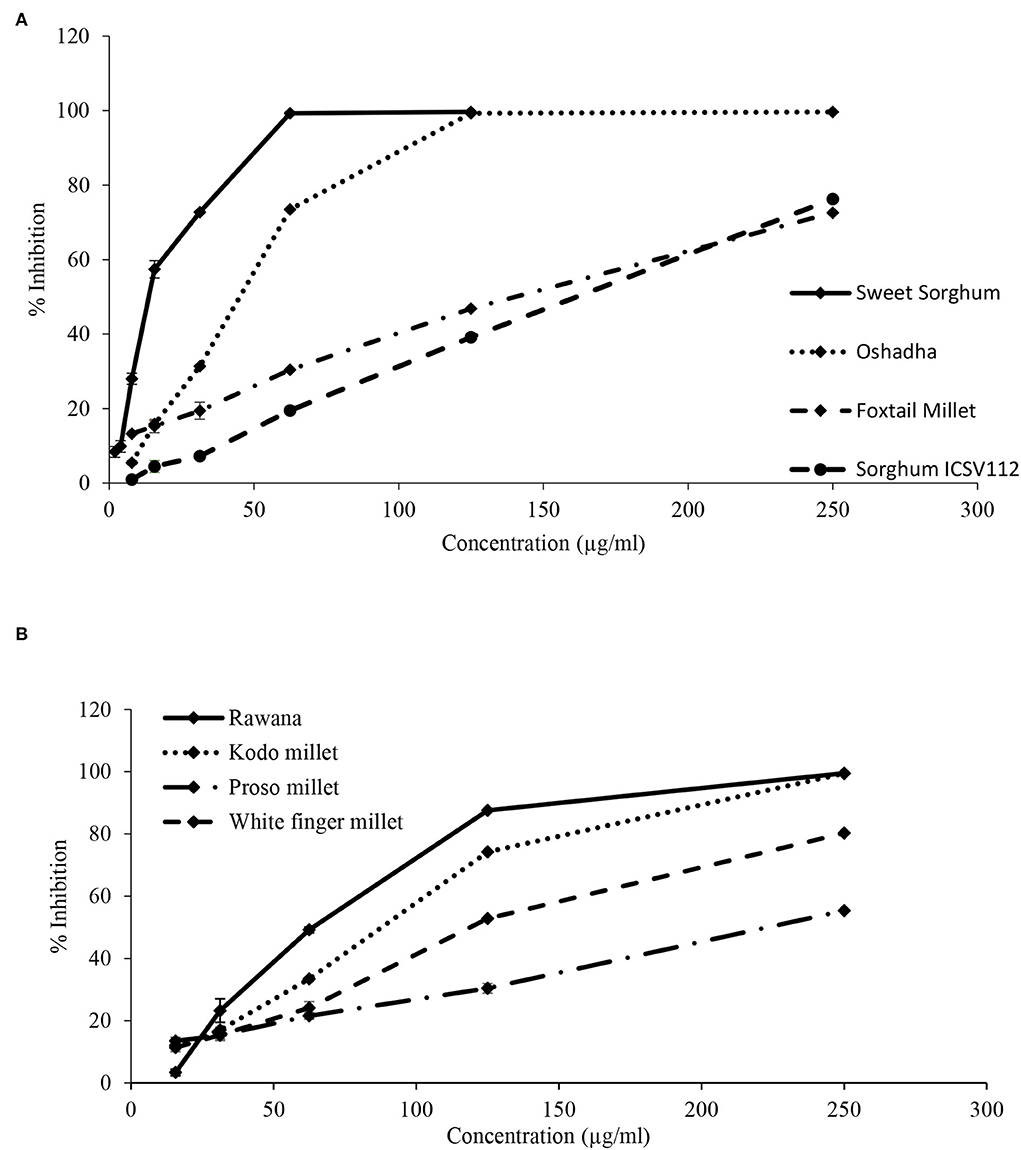
Figure 11. Dose-response relationship of selected samples for ABTS radical scavenging activity. (A) Dose-response relationship of sorghum ICSV112, foxtail millet, Oshadha, and sweet sorghum: IC50 values: 163.24 ± 1.11b, 136.53 ± 6.93c, 44.33 ± 0.19f and 13.75 ± 0.17g μg/ml, respectively. (B) Dose-response relationship of proso millet, white finger millet, Kodo millet, and Rawana: IC50: 222.22 ± 9.46a, 147.41 ± 0.39c, 86.07 ± 1.64d and 61.87 ± 0.66e μg/ml, respectively. IC50 values superscripted by different letters are significantly different at p < 0.05.
In dose-response studies, all the selected samples, namely, sweet sorghum (r2 = 0.96), Oshadha (r2 = 0.94), Rawana (r2 = 0.98), Kodo millet (r2 = 0.94), sorghum ICSV 112 (r2 = 0.99), foxtail millet (r2 = 0.99), proso millet (r2 = 0.99), and white finger millet (r2 = 0.97) exhibited good dose-response relationship (Figures 11A,B). The observed ABTS radical scavenging activities were significantly (P < 0.05) different among the samples and IC50 values ranged from 13.75 ± 0.17 to 222.22 ± 9.46 μg/ml. Interestingly, ABTS radical scavenging activity of sweet sorghum (13.75 ± 0.17 μg/ml) was comparable with the reference drug Trolox (IC50:7.73 ± 0.11 μg/ml) used in this study. Thus, sweet sorghum shows its potential to be used in functional foods, medic foods, and nutraceutical industries. The order of potency of tested samples for ABTS radical scavenging activity in terms of IC50 values was proso millet > sorghum ICSV112 > white finger millet > foxtail millet > Kodo millet > Rawana > Oshadha > sweet sorghum.
The ABTS radical scavenging activities of millet and sorghum have been reported in a number of studies (Ragaee et al., 2006; Choi et al., 2007; Abeysekera et al., 2017b). Abeysekera et al. (2017b) have reported that ABTS radical scavenging activity of market collected finger millet samples was 100.47 ± 0.19% and 88.52 ± 4.32% at 50 μg/ml in methanolic and ethanolic extracts, respectively. According to a study by Ragaee et al. (2006), the ABTS scavenging capacity of sorghum at 3 min time period was 51.7 ± 0.57 (μmole/g). However, in all the above-reported studies, the presentation of results, optimized assay conditions, sample preparation, and extraction protocols used are quite different from the present study. Therefore, the comparison of results with the present study is quite difficult.
DPPH radical scavenging activity
DPPH radical is a synthetic stable-free radical that can be scavenged by the presence of anti-oxidant compounds in various food samples (Pyrzynska and Pekal, 2013). The screening of selected millet types and sorghum varieties in Sri Lanka for DPPH radical scavenging activity is given in Figure 10. Furthermore, the dose-response relationship of samples for DPPH radical scavenging activity is given in Figures 12A,B. Results showed that the DPPH radical scavenging activity of selected millet types and sorghum varieties ranged from 0.07 ± 0.00 to 22.97 ± 0.83 mg of TEs/g of the sample at the screening. Among the samples studied, sweet sorghum exhibited significantly high (P < 0.05) DPPH radical scavenging activity compared to all the other samples tested. The order of potency of samples at screening (7.8125, 15.625, 31.25, 62.5, 125, 250, 500, and 1,000 μg/ml) for DPPH radical scavenging activity was sweet sorghum > Oshadha > Rawana > Kodo millet = sorghum ICSV112 = foxtail millet = proso millet = white finger millet.
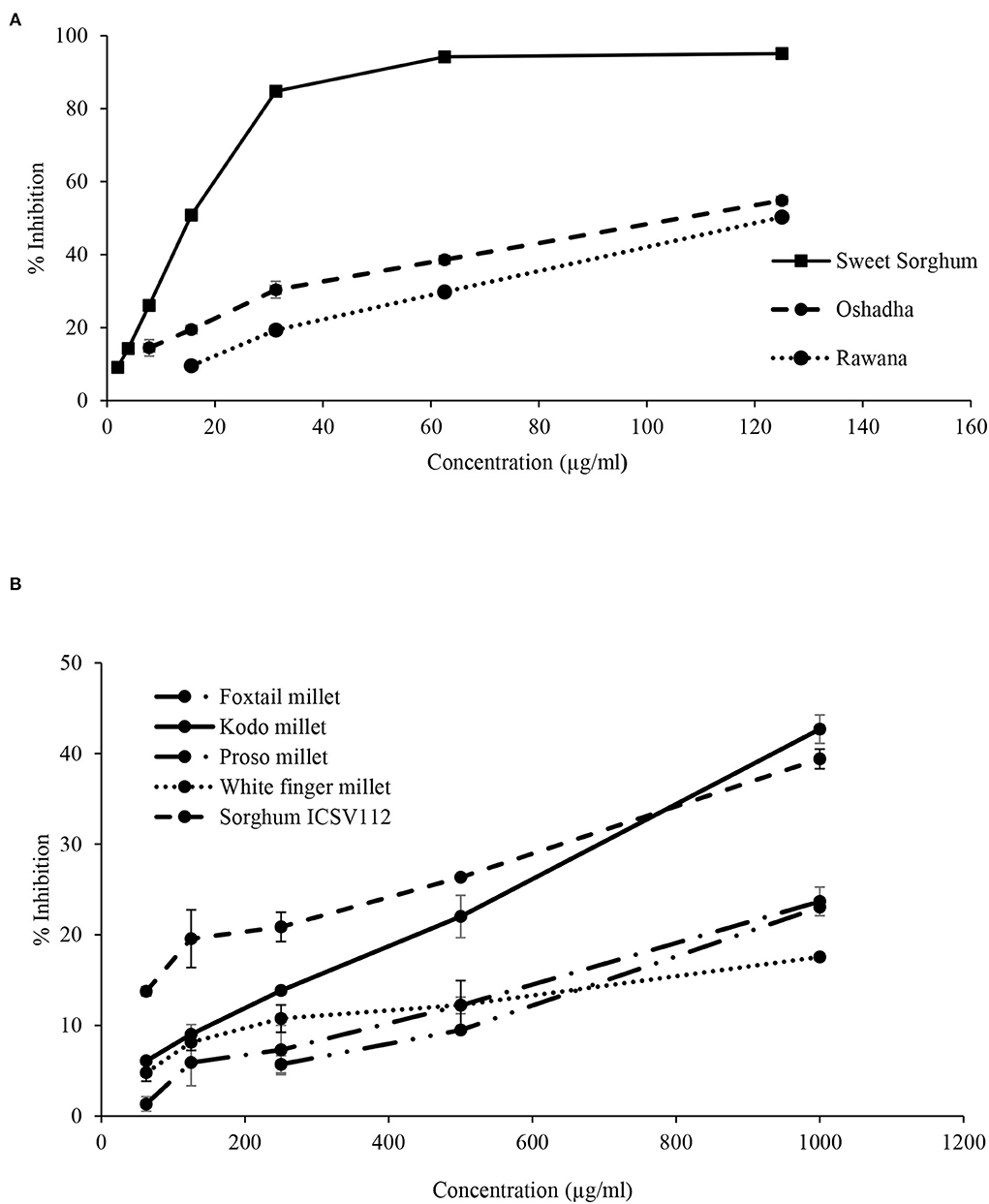
Figure 12. Dose-response relationship of selected samples for DPPH radical scavenging activity. (A) Dose-response relationship of sweet sorghum, Oshadha, and Rawana: IC50 values: 15.45 ± 0.21e, 85.10 ± 3.13e and 120.93 ± 2.15e μg/ml respectively. (B) Dose-response relationship of white finger millet, foxtail millet, proso millet, sorghum ISCV112, and Kodo millet: IC50: 3455.2 ± 55.4a, 2170.7 ± 97.7b, 2168.0 ± 55.9b, 1383.4 ± 48.1c and 1075.6 ± 56.3d μg/ml, respectively. IC50 values superscripted by different letters are significantly different at p < 0.05.
In dose-response studies, all the selected samples, namely, sweet sorghum (r2 = 0.94), Oshadha (r2 = 0.97), Rawana (r2 = 0.99), Kodo millet (r2 = 0.99), sorghum ICSV 112 (r2 = 0.97), foxtail millet (r2 =0.98), proso millet (r2 = 0.98), and white finger millet (r2 = 0.96) exhibited good dose-response relationship (Figures 12A,B). The observed DPPH radical scavenging activities were significantly (P < 0.05) different among the samples and IC50 values ranged from 15.45 ± 0.21 to 3455.2 ± 55.4 μg/ml. Interestingly, the DPPH radical scavenging activity of sweet sorghum (15.45 ± 0.21 μg/ml) was comparable with the reference drug Trolox (IC50:9.33 ± 0.21 μg/ml) used in this study. Thus, sweet sorghum shows its potential to be used in functional foods, medic foods, and nutraceutical industries. The order of potency of tested samples for DPPH radical scavenging activity in terms of IC50 values was white finger millet > foxtail millet > proso millet > sorghum ICSV112 > Kodo millet > Rawana > Oshadha > sweet sorghum.
There are a number of reported studies on the DPPH radical scavenging activity of millet and sorghum varieties cultivated throughout the globe (Dykes et al., 2005; Sreeramulu et al., 2009; Chandrasekara and Shahidi, 2011b; Wu et al., 2016; Abeysekera et al., 2017b; Kumari et al., 2017; Rao et al., 2018). A study conducted by Kumari et al. (2017) has reported that Rawana (10.3 ± 0.7 μmol of TEs/g of dry matter) and Oshadha (8.8 ± 0.2 μmol of TEs/g of dry matter) millet varieties cultivated in Mahailluppallama, Sri Lanka, had higher DPPH radical scavenging activity than foxtail millet and proso millet, and results are accordance with the present study. According to the findings of Abeysekera et al. (2017b), the DPPH radical scavenging activities of finger millet collected from the local market were 67.05 ± 1.24% and 39.97 ± 0.27% in methanolic and ethanolic extracts, respectively, at 50 μg/ml. However, in the present study, finger millet samples [(Oshadha: 2.10 ± 0.09 mg of TEs/g of the sample) and (Rawana: 1.20 ± 0.02 mg of TEs/g of the sample)] and white finger millet (0.07 ± 0.00 mg of TEs/g of the sample)] showed lower DPPH radical scavenging activity at the same concentration. A study conducted by Sreeramulu et al. (2009) has reported DPPH radical scavenging activity of finger millet as 1.73 ± 0.03 TEs mg/g, and the results are in accordance with the present study. A number of studies have reported a positive correlation between DPPH radical scavenging activity and the TPC (Dykes et al., 2005; Wu et al., 2016; Rao et al., 2018). In the present study, we also observed higher DPPH radical scavenging activity in the samples containing more TPC. This is the first report of the DPPH radical scavenging activity of sorghum in Sri Lanka.
Proximate composition
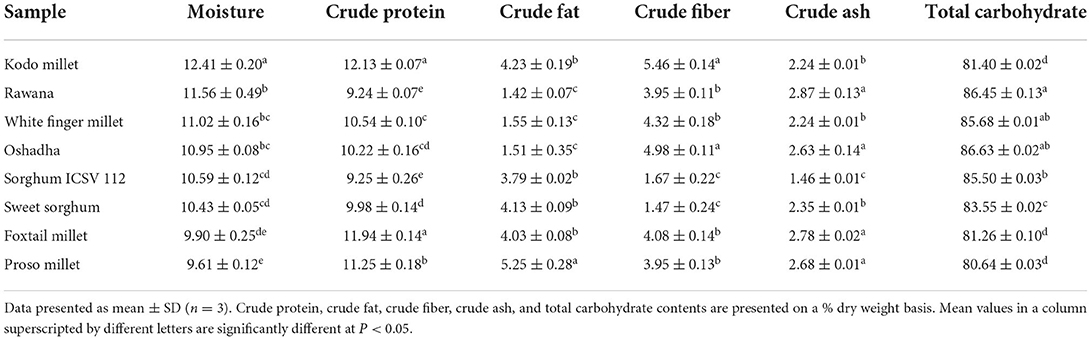
Moisture, crude protein, crude fat, crude fiber, crude ash, and total carbohydrate contents of selected millet types and sorghum varieties in Sri Lanka are presented in Table 1. Moisture, crude protein, crude ash, crude fat, crude fiber, and total carbohydrate contents of selected millet types and sorghum varieties ranged from 10.43 ± 0.05 to 12.41 ± 0.20, 9.24 ± 0.07 to 12.13 ± 0.07, 1.46 ± 0.01 to 2.87 ± 0.13, 1.42 ± 0.07 to 5.25 ± 0.28, 1.47 ± 0.24 to 5.46 ± 0.14 and 80.64 ± 0.03 to 86.63 ± 0.02%, respectively. Kodo millet (12.13 ± 0.07%) and foxtail millet (11.94 ± 0.14%) showed significantly high (P < 0.05) crude protein contents compared to the other samples tested. Furthermore, Kodo millet also showed significantly high (P < 0.05) crude fiber (5.46 ± 0.14%) content while proso millet was highest in crude fat content (5.25 ± 0.28%). For crude ash content, foxtail millet, proso millet, and finger millet varieties, Rawana and Oshadha showed comparable and highest (P < 0.05) ash contents compared to the other samples. Total carbohydrate content was highest (P < 0.05) in Rawana, Oshadha, white finger millet, and ICSV 112 sorghum samples.
Proximate composition of millets and sorghum in Sri Lanka has been reported in several studies (Jayawardana et al., 2018, 2019). According to a study by Jayawardana et al. (2018), moisture content, protein content, crude fat content, ash content, total carbohydrate content, and crude fiber content of Sri Lankan finger millet varieties, namely, Ravi, Oshadha, and Rawana were in the ranges of 10.60–13.16, 8.13–8.74, 1.40–1.31, 2.95–3.22, 86.92–87.24, and 3.73–3.82%, respectively. Analysis of the nutritional composition of finger millet, common millet, foxtail millet, and Kodo millet by the Department of Agriculture, Sri Lanka, has shown that the moisture, protein, fat, and carbohydrate contents were as 11.09–18.1, 7.3–11.43, 1.3–4.83, and 59.75–72%, respectively. The results of the present study are more or less in accordance with the findings of the above-stated national studies. The slight deviations observed might be due to the effect of agro-climatic factors on crop production. Interestingly, the findings of the present study are also comparable with the findings of international studies conducted by Chinenye et al. (2017), Ahmad et al. (2018), Al-Juhaimi et al. (2019), and Mohapatra et al. (2019).
Conclusion
In conclusion, proximate composition, anti-lipidemic, anti-inflammatory, and anti-oxidant properties of millet and sorghum in Sri Lanka varied significantly depending on the variety. Among the studied millet types and sorghum varieties, the pigmented sweet sorghum showed the highest activities for all the investigated biological activities. Overall, the pigmented millet and sorghum samples showed the highest biological activities compared to the non-pigmented millet and sorghum samples studied. Interestingly, this relationship was not observed for the proximate composition of millet and sorghum samples studied. This indicates that nutritional composition does not vary much depending on pigmented or non-pigmented varieties; however, biological activities vary significantly due to the presence of pigments in millet and sorghum. Thus, for the prevention and management of non-communicable diseases and their related complications, pigmented millet and sorghum varieties might be useful and could be used in developing value-added functional foods and nutraceuticals. However, nutritional improvement in healthy individuals could be performed with the enhanced consumption of millet and sorghum with high nutrient contents irrespective of the presence of pigments in the grain. Interestingly, this is the first comparative study conducted in Sri Lanka covering a wide range of anti-oxidant, anti-inflammatory, and anti-lipidemic properties for millet types and sorghum varieties in the country. Furthermore, this is the first report of cholesterol esterase inhibitory activity of millet and sorghum the world over.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WKA and WPA served as principal supervisors in the research study, contributed to research designing, planning, data analysis, and manuscript preparation. WKA initiated the research collaboration between Industrial Technology Institute (ITI) and Field Crop Research and Development Institute, Mahailuppallama, Sri Lanka. SJ conducted whole laboratory experiments and contributed to data analysis and manuscript writing. IS contributed to the experiments of proximate analysis of samples. NJ served as a research supervisor. GP helped in building the research collaboration between ITI and Field Crop Research and Development Institute. DW provided authenticated millet and sorghum samples for the research study. All authors contributed to the article and approved the submitted version.
Funding
This research work was conducted at the Food & Herbal Technology Sections of the Industrial Technology Institute (ITI), Sri Lanka. The research work was funded by the Treasury, Sri Lanka.
Acknowledgments
The authors would like to acknowledge ITI and Treasury, Sri Lanka for the greatest support provided for the research study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abeysekera, W. K. S. M., Jayawardana, S. A. S., Abeysekera, W. P. K. M., Yathursan, S., Premakumara, G. A. S., and Ranasinghe, P. (2017b). Antioxidant potential of selected whole grain cereals consumed by Sri Lankans: a comparative in vitro study. Sri Lankan J. Biol. 2:9. doi: 10.4038/sljb.v2i2.9
Abeysekera, W. P. K. M., Arachchige, S. P. G., and Ratnasooriya, W. D. (2017a). Bark extracts of ceylon cinnamon possess antilipidemic activities and bind bile acids in vitro. Evid. Based Complement. Alternat. Med. 2017:7347219. doi: 10.1155/2017/7347219
Adisakwattana, S., Intrawangso, J., Hemrid, A., Chanathong, B., and Mäkynen, K. (2012). Extracts of edible plants inhibit pancreatic lipase, cholesterol esterase and cholesterol micellization, and bind bile acids. Food Technol. Biotechnol. 50, 11–16.
Ahmad, F., Pasha, I., Saeed, M., and Asgher, M. (2018). Biochemical profiling of Pakistani sorghum and millet varieties with special reference to anthocyanins and condensed tannins. Int. J. Food Propert. 21, 1586–1597. doi: 10.1080/10942912.2018.1502198
Ajiboye, A. A., Dedeke, O. A., and Adeyemo, F. C. (2017). Investigation on antioxidants, free radical scavenger and lipid peroxidation activities of whole grains finger millet (Eleusine coracana L.). Int. J. Plant Biol. 8, 6684. doi: 10.4081/pb.2017.6684
Al-Juhaimi, F., Simşek, S., Ghafoor, K., Babiker, E.E., Özcan, M. M., Ahmed, I. A. M., et al. (2019). Effect of varieties on bioactive properties and mineral contents of some sorghum, millet and lupin seeds. J. Oleo Sci. 2019, 19113. doi: 10.5650/jos.ess19113
AOAC (2000). Official methods of analysis of AOAC international (17th ed.). Gaithersburg, MD: Association of Official Analytical Chemists (AOAC) International.
Awika, J. M., Rooney, L. W., Wu, X., Prior, R. L., and Cisneros-Zevallos, L. (2003). Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agric. Food Chem. 51, 6657–6662. doi: 10.1021/jf034790i
Benzie, I. F., and Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239, 70–76. doi: 10.1006/abio.1996.0292
Benzie, I. F., and Szeto, Y. T. (1999). Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 47, 633–636. doi: 10.1021/jf9807768
Bhat, S., Nandini, C., and Tippeswamy, V. (2018). Significance of small millets in nutrition and health-A review. Asian J. Dairy Food Res. 37, 35–40. doi: 10.18805/ajdfr.DR-1306
Birari, R. B., and Bhutani, K. K. (2007). Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov. Today 12, 879–889. doi: 10.1016/j.drudis.2007.07.024
Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nature 181, 1199–1200. doi: 10.1038/1811199a0
Burdette, A., Garner, P. L., Mayer, E. P., Hargrove, J. L., Hartle, D. K., and Greenspan, P. (2010). Anti-inflammatory activity of select sorghum (Sorghum bicolor) brans. J. Med. Food 13, 879–887. doi: 10.1089/jmf.2009.0147
Bvochora, J. M., Reed, J. D., Read, J. S., and Zvauya, R. (1999). Effect of fermentation processes on proanthocyanidins in sorghum during preparation of Mahewu, a non-alcoholic beverage. Process Biochem. 35, 21–25. doi: 10.1016/S0032-9592(99)00027-8
Cao, G., Alessio, H. M., and Cutler, R. G. (1993). Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 14, 303–311. doi: 10.1016/0891-5849(93)90027-R
Chandra, D., Chandra, S., and Sharma, A. K. (2016). Review of Finger millet [Eleusine coracana (L.) Gaertn]: a power house of health benefiting nutrients. Food Sci. Human Wellness 5, 149–155. doi: 10.1016/j.fshw.2016.05.004
Chandrasekara, A., and Shahidi, F. (2010). Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agric. Food Chem. 58, 6706–6714. doi: 10.1021/jf100868b
Chandrasekara, A., and Shahidi, F. (2011a). Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSn. J. Funct. Foods 3, 144–158. doi: 10.1016/j.jff.2011.03.007
Chandrasekara, A., and Shahidi, F. (2011b). Bioactivities and antiradical properties of millet grains and hulls. J. Agric. Food Chem. 59, 9563–9571. doi: 10.1021/jf201849d
Chatatikun, M., and Kwanhian, W. (2020). Phenolic profile of nipa palm vinegar and evaluation of its antilipidemic activities. Evid. Based Complement. Alternat. Med. 2020:e6769726. doi: 10.1155/2020/6769726
Chinenye, O. E., Ayodeji, O. A., and Baba, A. J. (2017). Effect of fermentation (natural and starter) on the physicochemical, anti-nutritional and proximate composition of pearl millet used for flour production. Am. J. Biosci. Bioeng. 5, 12–16. doi: 10.11648/j.bio.20170501.13
Chisi, M., and Peterson, G. (2019). Breeding and Agronomy. In Sorghum and Millets. Oxford: AACC International Press. doi: 10.1016/B978-0-12-811527-5.00002-2
Choi, Y., Jeong, H. S., and Lee, J. (2007). Antioxidant activity of methanolic extracts from some grains consumed in Korea. Food Chem. 103, 130–138. doi: 10.1016/j.foodchem.2006.08.004
Dia, V. P., Pangloli, P., Jones, L., McClure, A., and Patel, A. (2016). Phytochemical concentrations and biological activities of Sorghum bicolor alcoholic extracts. Food Funct. 7, 3410–3420. doi: 10.1039/C6FO00757K
Dykes, L., Rooney, L. W., Waniska, R. D., and Rooney, W. L. (2005). Phenolic compounds and antioxidant activity of sorghum grains of varying genotypes. J. Agric. Food Chem. 53, 6813–6818. doi: 10.1021/jf050419e
Hendrich, A. B. (2006). Flavonoid-membrane interactions: possible consequences for biological effects of some polyphenolic compounds 1. Acta Pharmacol. Sin. 27, 27–40. doi: 10.1111/j.1745-7254.2006.00238.x
Hong, S., Pangloli, P., Perumal, R., Cox, S., Noronha, L. E., Dia, V. P., et al. (2020). A comparative study on phenolic content, antioxidant activity and anti-inflammatory capacity of aqueous and ethanolic extracts of sorghum in lipopolysaccharide-induced RAW 264.7 macrophages. Antioxidants 9:1297. doi: 10.3390/antiox9121297
Huang, R., Zhang, Y., Shen, S., Zhi, Z., Cheng, H., Chen, S., et al. (2020). Antioxidant and pancreatic lipase inhibitory effects of flavonoids from different citrus peel extracts: An in vitro study. Food Chem. 326:126785. doi: 10.1016/j.foodchem.2020.126785
Irondi, E. A., Adegoke, B. M., Effion, E. S., Oyewo, S. O., Alamu, E. O., and Boligon, A. A. (2019). Enzymes inhibitory property, antioxidant activity and phenolics profile of raw and roasted red sorghum grains in vitro. Food Sci. Human Wellness 8, 142–148. doi: 10.1016/j.fshw.2019.03.012
Jain, S., Buttar, H. S., Chintameneni, M., and Kaur, G. (2018). Prevention of cardiovascular diseases with anti-inflammatory and anti-oxidant nutraceuticals and herbal products: An overview of pre-clinical and clinical studies. Recent Pat. Inflamm. Allergy Drug Discov. 12, 145–157. doi: 10.2174/1872213X12666180815144803
Jakubczyk, A., Szymanowska, U., Karaś, M., Złotek, U., and Kowalczyk, D. (2019). Potential anti-inflammatory and lipase inhibitory peptides generated by in vitro gastrointestinal hydrolysis of heat treated millet grains. CyTA-J. Food 17, 324–333. doi: 10.1080/19476337.2019.1580317
Jayawardana, N., Wimalasiri, K. M. S., Samarasinghe, G., and Madhujith, T. (2018). Bound and total phenolic contents and antioxidant potential of selected Sri Lankan millet varieties. Trop. Agri. Res. 29, 316–321. doi: 10.4038/tar.v29i3.8270
Jayawardana, S. A. S., Samarasekera, J. K. R. R., and Hettiarachchi, G. H. C. M. (2020). Antimicrobial properties of ethanolic and methanolic extracts of finger millet [Eleusine coracana (L.) Gaertn.] varieties cultivated in Sri Lanka. Int. J. Multidiscipl. Stud. 7, 78–94. doi: 10.4038/ijms.v7i1.123
Jayawardana, S. A. S., Samarasekera, J. K. R. R., Hettiarachchi, G. H. C. M., Gooneratne, J., Choudhary, M. I., and Jabeen, A. (2021). Anti-inflammatory and antioxidant properties of finger Millet [Eleusine coracana (L.) Gaertn.] varieties cultivated in Sri Lanka. BioMed Res. Int. 2021:10. doi: 10.1155/2021/7744961
Jayawardana, S. A. S., Samarasekera, J. K. R. R., Hettiarachchi, G. H. C. M., Gooneratne, J., Mazumdar, S. D., and Banerjee, R. (2019). Dietary fibers, starch fractions and nutritional composition of finger millet varieties cultivated in Sri Lanka. J. Food Composit. Analy. 82:103249. doi: 10.1016/j.jfca.2019.103249
Jayawardana, S. A. S., Samarasekera, J. K. R. R., Hettiarachchi, G. H. C. M., and Gooneratne, M. J. (2022). Antidiabetic properties of finger millet (Eleusine coracana (L.) Gaertn.) varieties cultivated in Sri Lanka. J. Herbal Med. 32:100534. doi: 10.1016/j.hermed.2022.100534
Jew, S., AbuMweis, S. S., and Jones, P. J. (2009). Evolution of the human diet: linking our ancestral diet to modern functional foods as a means of chronic disease prevention. J. Med. Food 12, 925–934. doi: 10.1089/jmf.2008.0268
Kim, Y. S., Lee, Y. M., Kim, H., Kim, J., Jang, D. S., Kim, J. H., et al. (2010). Anti-obesity effect of Morus bombycis root extract: anti-lipase activity and lipolytic effect. J. Ethnopharmacol. 130, 621–624. doi: 10.1016/j.jep.2010.05.053
Kumar, A. P., Sivashanmugam, A. T., Umamaheswari, M., Subhadradevi, V., and Jagannath, P. (2011). Cholesterol esterase enzyme inhibitory and antioxidant activities of leaves of Camellia sinensis (L.) Kuntze. using in vitro models. Int. J. Pharm. Sci. Res. 2:2675.
Kumari, D., Chandrasekara, A., Athukorale, P., and Shahidi, F. (2020). Finger millet porridges subjected to different processing conditions showed low glycemic index and variable efficacy on plasma antioxidant capacity of healthy adults. Food Product. Process. Nutr. 2, 1–11. doi: 10.1186/s43014-020-00027-9
Kumari, D., Madhujith, T., and Chandrasekara, A. (2017). Comparison of phenolic content and antioxidant activities of millet varieties grown in different locations in Sri Lanka. Food Sci. Nutr. 5, 474–485. doi: 10.1002/fsn3.415
Mohapatra, D., Patel, A. S., Kar, A., Deshpande, S. S., and Tripathi, M. K. (2019). Effect of different processing conditions on proximate composition, anti-oxidants, anti-nutrients and amino acid profile of grain sorghum. Food Chem. 271, 129–135. doi: 10.1016/j.foodchem.2018.07.196
Mueller, M., Hobiger, S., and Jungbauer, A. (2010). Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 122, 987–996. doi: 10.1016/j.foodchem.2010.03.041
Ou, B., Hampsch-Woodill, M., and Prior, R. L. (2001). Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 49, 4619–4626. doi: 10.1021/jf010586o
Patel Rajesh, M., and Patel Natvar, J. (2011). In vitro antioxidant activity of coumarin compounds by DPPH, Super oxide and nitric oxide free radical scavenging methods. J. Adv. Pharm. Educ. Res. 1, 52–68.
Patel, R. M. (2013). Ferrous ion chelating activity (FICA)-a comparative antioxidant activity evaluation of extracts of eleven naturally growing plants of Gujarat, India. Int. J. Sci. Res. 2, 426–428. doi: 10.15373/22778179/AUG2013/142
Pietsch, M., and Gütschow, M. (2005). Synthesis of tricyclic 1, 3-oxazin-4-ones and kinetic analysis of cholesterol esterase and acetylcholinesterase inhibition. J. Med. Chem. 48, 8270–8288. doi: 10.1021/jm0508639
Pohl, F., and Kong Thoo Lin, P. (2018). The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: in vitro, in vivo and clinical trials. Molecules 23:3283. doi: 10.3390/molecules23123283
Porter, L. J., Hrstich, L. N., and Chan, B. G. (1985). The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25, 223–230. doi: 10.1016/S0031-9422(00)94533-3
Pyrzynska, K., and Pekal, A. (2013). Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Analyt. Methods 5, 4288–4295. doi: 10.1039/c3ay40367j
Quiroga, P. R., Grosso, N. R., Lante, A., Lomolino, G., Zygadlo, J. A., and Nepote, V. (2013). Chemical composition, antioxidant activity and anti-lipase activity of Origanum vulgare and L ippia turbinata essential oils. Int. J. Food Sci. Technol. 48, 642–649. doi: 10.1111/ijfs.12011
Ragaee, S., Abdel-Aal, E. S. M., and Noaman, M. (2006). Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 98, 32–38. doi: 10.1016/j.foodchem.2005.04.039
Rao, S., Santhakumar, A. B., Chinkwo, K. A., Wu, G., Johnson, S. K., and Blanchard, C. L. (2018). Characterization of phenolic compounds and antioxidant activity in sorghum grains. J. Cereal Sci. 84, 103–111. doi: 10.1016/j.jcs.2018.07.013
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 26, 1231–1237. doi: 10.1016/S0891-5849(98)00315-3
Salminen, A., Lehtonen, M., Suuronen, T., Kaarniranta, K., and Huuskonen, J. (2008). Terpenoids: natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell. Mol. Life Sci. 65, 2979–2999. doi: 10.1007/s00018-008-8103-5
Sarita, E. S., and Singh, E. (2016). Potential of millets: nutrients composition and health benefits. J. Sci. Innovative Res. 5, 46–50. doi: 10.31254/jsir.2016.5204
Senevirathne, I. G. N. H., Abeysekera, W. K. S. M., Abeysekera, W. P. K. M., Jayanath, N. Y., Galbada Arachchige, S. P., and Wijewardana, D. C. M. S.I. (2021). Antiamylase, antiglucosidase, and antiglycation properties of millets and sorghum from Sri Lanka. Evid. Based Complement. Alternat. Med. 2021:e5834915. doi: 10.1155/2021/5834915
Shi, J., Shan, S., Li, H., Song, G., and Li, Z. (2017). Anti-inflammatory effects of millet bran derived-bound polyphenols in LPS-induced HT-29 cell via ROS/miR-149/Akt/NF-κB signaling pathway. Oncotarget 8:74582. doi: 10.18632/oncotarget.20216
Siddhuraju, P., and Becker, K. (2003). Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 51, 2144–2155. doi: 10.1021/jf020444+
Simnadis, T. G., Tapsell, L. C., and Beck, E. J. (2016). Effect of sorghum consumption on health outcomes: a systematic review. Nutr. Rev. 74, 690–707. doi: 10.1093/nutrit/nuw036
Singh, B., Singh, J. P., Kaur, A., and Singh, N. (2016). Bioactive compounds in banana and their associated health benefits–A review. Food Chem. 206, 1–11. doi: 10.1016/j.foodchem.2016.03.033
Singh, S. P., and Sashidhara, K. V. (2017). Lipid lowering agents of natural origin: An account of some promising chemotypes. Eur. J. Med. Chem. 140, 331–348. doi: 10.1016/j.ejmech.2017.09.020
Singleton, V. L., Orthofer, R., and Lamuela-Raventós, R. M. (1999). [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 299, 152–178. doi: 10.1016/S0076-6879(99)99017-1
Smeriglio, A., Barreca, D., Bellocco, E., and Trombetta, D. (2017). Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 174, 1244–1262. doi: 10.1111/bph.13630
Sreeramulu, D., Reddy, C., and Raghunath, M. (2009). Antioxidant Activity of Commonly Consumed Cereals, Millets, Pulses and Legumes in India. Hyderabad: Indian Journal of Biochemistry and Biophysics.
Tungmunnithum, D., Thongboonyou, A., Pholboon, A., and Yangsabai, A. (2018). Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 5:93. doi: 10.3390/medicines5030093
Upadhyay, R., Jha, A., Singh, S. P., Kumar, A., and Singh, M. (2015). Appropriate solvents for extracting total phenolics, flavonoids and ascorbic acid from different kinds of millets. J. Food Sci. Technol. 52, 472–478. doi: 10.1007/s13197-013-0976-0
Vila-Real, C., Pimenta-Martins, A., Maina, N., Gomes, A., and Pinto, E. (2017). Nutritional value of indigenous whole grain cereals millet and sorghum. Nutr. Food Sci. Int. J. 4:e555628. doi: 10.19080/NFSIJ.2017.04.555628
Wilson, D. W., Nash, P., Buttar, H. S., Griffiths, K., Singh, R., De Meester, F., et al. (2017). The role of food antioxidants, benefits of functional foods, and influence of feeding habits on the health of the older person: an overview. Antioxidants 6:81. doi: 10.3390/antiox6040081
Wu, G., Johnson, S. K., Bornman, J. F., Bennett, S., Singh, V., and Fang, Z. (2016). Effect of genotype and growth temperature on sorghum grain physical characteristics, polyphenol content, and antioxidant activity. Cereal Chem. 93, 419–425. doi: 10.1094/CCHEM-01-16-0003-R
Keywords: millet, sorghum, anti-lipidemic, anti-inflammatory, anti-oxidant, proximate composition
Citation: Abeysekera WKSM, Jayathilaka SI, Abeysekera WPKM, Senevirathne IGNH, Jayanath NY, Premakumara GAS and Wijewardana DCMSI (2022) In vitro determination of anti-lipidemic, anti-inflammatory, and anti-oxidant properties and proximate composition of range of millet types and sorghum varieties in Sri Lanka. Front. Sustain. Food Syst. 6:884436. doi: 10.3389/fsufs.2022.884436
Received: 26 February 2022; Accepted: 15 August 2022;
Published: 16 September 2022.
Edited by:
Nitya Sharma, Indian Institute of Technology Delhi, IndiaReviewed by:
Gamage Anoma Chandrasekara, Wayamba University of Sri Lanka, Sri LankaSneh Punia, Clemson University, United States
Prasad Rasane, Lovely Professional University, India
Copyright © 2022 Abeysekera, Jayathilaka, Abeysekera, Senevirathne, Jayanath, Premakumara and Wijewardana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Walimuni Kanchana Subhashini Mendis Abeysekera, a2FuY2hhbmFAYXQuY21iLmFjLmxr
 Walimuni Kanchana Subhashini Mendis Abeysekera
Walimuni Kanchana Subhashini Mendis Abeysekera Sewwandi Indrachapa Jayathilaka
Sewwandi Indrachapa Jayathilaka Walimuni Prabhashini Kaushalya Mendis Abeysekera3
Walimuni Prabhashini Kaushalya Mendis Abeysekera3 Galbada Arachchige Sirimal Premakumara
Galbada Arachchige Sirimal Premakumara