
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 15 April 2022
Sec. Water-Smart Food Production
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.836275
This article is part of the Research TopicSustainable Feed for AquacultureView all 6 articles
Palm oil has been recognized as a high potential alternative dietary lipid source to reduce the reliance on expensive fish oil in aquaculture feeds. Unfortunately, most research studies were focusing on the juvenile or grow-out stage of aquatic species. This study was designed to develop weaning microdiets for Asian seabass larvae with dietary fish oil being replaced with crude palm oil (CPO) at 25, 50, and 75% (CPO25, CPO50, and CPO75) and refined bleached deodorized palm olein, refined palm oil (RPO) at 50 and 75% (RPO50 and RPO75) replacement levels. A fish-oil-based microdiet was used as a control treatment (FO100). The triplicate groups of fish larvae with initial weight and length of 1.71 ± 0.13 mg and 5.54 ± 0.34 mm, respectively, were stocked at 150 larvae/tank and co-fed with the experimental microdiets and live feeds (L-type rotifer and artemia). The final body weight (0.54–0.63 g) and specific growth rate (SGR) (12.8–13.13%/d) of fish-fed palm oil-based diets were significantly better than the control diet (0.42 g; 12.21%/day, respectively). In particular, RPO75 yielded the best SGR followed by RPO50, CPO75, CPO50, and CPO25. The feeding intake and feed conversion ratio (FCR) were not statistically different from other treatments (0.2–0.3 g/fish/d and 1.06–1.63, respectively). The survival rate of larvae-fed palm oil-based diets (33.11–46.67%) during the feeding trial was comparable to the control diet (39.33%). In the 65 ppt-salinity stress test at 25 DPH, there was no significant difference in terms of the survival rate of larvae fed the control diet and the CPO-based diets, but the lowest survival rate was observed in the RPO-based diets than the control diet. Higher final whole-body protein and lipid contents (15.3 ± 0.4 and 3.7 ± 0.0%, respectively) were observed in fish-fed CPO50 compared to other treatments. Generally, the replacement of fish oil with palm oil increased the palmitic acid (C:16:0) and oleic acid (C18:1n9) and significantly reduced the eicosapentaenoic acid (EPA) (C20:5n3) and docosahexaenoic acid (DHA) (C22:6n3) contents in both the microdiets and larval body, a common observation in this kind of investigation. Considering the good growth and survival of Asian seabass larvae in this study, availability of palm oil, and its competitive price compared to fish oil, it is suggested that weaning diets for Asian seabass larvae can be developed using palm oil as a partial source of dietary lipid.
Asian seabass (Lates calcarifer) aquaculture is well-established in the Indo-Pacific region with ongoing efforts to increase the production efficiency. It has a good market price and is preferred by consumers due to its good taste and white flesh (Szucs et al., 2018). Current Asian seabass global production is around 130,000 metric tons (MTs) and the global market is forecasted to expand at 5.5% compound annual growth rate (CAGR) for the period 2021–2031 (Future Market Insight, 2021). In Malaysia, Asian seabass has contributed the largest production rate at 48.23% (23,033.12 tons) under the category of marine/brackish water culture system by species (Department of Fisheries Malaysia, 2021). One of the focuses in the industry is to develop cost-effective diets that can support the profitable production of this carnivorous species. Most research on feed development for Asian seabass was concentrated on the juvenile and grow-out stages considering the availability of the fish and higher survival rate at these stages (Booth et al., 2010; Glencross, 2011; Tu et al., 2012; Salini et al., 2015; Glencross et al., 2016). The limited effort was done on the larval feed development, especially for weaning purposes. The larval phase is the most sensitive stage in a life cycle and one of the main challenges is to provide adequate nutritional balance to support the fast growth and development. Under the hatchery feeding protocol, marine fish highly rely on the expensive live feed (rotifer and artemia) and are slowly weaned to microdiet. Most importantly, the weaning or transition period to inert diets adaptation should not bring any detrimental effects on fish performance, provide chemical stimulation to facilitate the larval ingestion, and contain numerous nutritional factors stimulating the digestive system (Cahu and Zambonino-Infante, 2001). Currently, the available weaning or larval diets for marine fishes in the Malaysia's market are mostly fish-based products, imported, and very costly at about US$ 30–45/kg. Several studies on co-feeding microdiets with live feeds have shown improved larval performances (Baskerville-Bridges and Kling, 2000; Cahu and Zambonino-Infante, 2001; Kolkovski et al., 2010; Bonaldo et al., 2011; Süzer et al., 2011; Hauville et al., 2014; Hu et al., 2017).
Exploring the potential of alternative ingredients to lower the cost of weaning diets is an important aspect to be studied. Fish oil has been extensively used in marine larval nutrition as an exogenous marine lipid source. However, the global fish oil supply is insufficient for aquaculture due to a decrease in marine capture fishery, a rise in the total global consumption, and robust industrial demand for fish oil in the major consumption countries (Future Market Insight, 2021). As Asian seabass aquaculture is expanding, sustainable alternatives to fish oil are needed to supply this fast-growing industry (Alhazzaa, 2012). Plant oils are a good candidate for fish nutrition, which can be used sustainably to replace fish oil in weaning diets (Moldal et al., 2014; Gisbert et al., 2016). Many research studies have highlighted the successful use of palm oil as a dietary lipid source and also to reduce diet cost in grow-out marine fish diets (Bell et al., 2002; Shapawi et al., 2008, 2011a,b). Palm oil is the most consumed vegetable oil in the world and has been highlighted as the main agricultural commodity in Malaysia (Malaysia Palm Oil Council, 2021). The world production of palm oil was 75.50 million MTs in the marketing year 2020/2021 (U.S. Department of Agriculture, 2021). Malaysia is the second largest producer and exporter of palm oil worldwide, accounting for 25.8 and 34.3% of the world's production and export, respectively (Malaysia Palm Oil Council, 2021). The nutritional value and nutrient composition of palm oil have been discovered with great findings. Palm oil contains high vitamin E, carotenoids and tocotrienols, natural antioxidants, is free from dioxins, and is able to prolong the shelf-life of fish products (Ng et al., 2007; Han et al., 2012). Palm oil has been successfully included in practical formulated diets with promising results on cultured marine fishes (Bell et al., 2002; Richard et al., 2006; Shapawi et al., 2008, 2011b; Wan Ahmad et al., 2013). However, the acceptability of palm oil in fish is species specific and stage dependent. In our previous study using Asian seabass larvae, palm oil was successfully replaced by fish oil in the enrichment diets for the live feed, rotifer (Safiin et al., 2021). More efforts are needed to explore the potential of this dietary lipid source. Research on feed ratio at the weaning period is beneficial in the Asian seabass development as manipulation in feeding regime may be altered to reduce feeding cost impact at early stages (Williams and Barlow, 1999; Herbert, 2005). In this study, alternative lipid sources from two palm oil products were incorporated in the microdiets and co-fed with live feeds and fed to Asian seabass up to the late larval stage and their effects on growth, feed utilization, whole-body proximate composition, salinity, and tolerance were investigated.
Crude palm oil (CPO) and refined bleach deodorized palm olein (RPO) were obtained from a local palm oil factory, Lumadan Palm Oil Mill (Sawit Kinabalu Sdn. Bhd) in Beaufort, Sabah. Six experimental microdiets were formulated to be isonitrogenous (50% protein) and isolipidic (15% lipid) to meet the nutritional requirement of Asian seabass larvae (Catacutan and Coloso, 1995; Nankervis and Southgate, 2006). The microdiets were prepared using different inclusion levels of two palm oil types, which were CPO (25, 50, and 75%) and RPO (50 and 75%) to substitute fish oil. Diet without palm oil (FO100) was served as a control microdiet. Danish fish meal, soy-protein concentrated (SPC), and shrimp meal (SHM) were used as the main protein sources. All the ingredients were ground using a CyclotecTM Mill (FOSS Analytical Corporation Ltd.) and sieved to pass through at 50–100 μm. All the homogenized and dried ingredients were well-mixed and subsequent palm oil, fish oil, and water were added (Table 1). The dough was screw-passed through a 3-mm die and the feed strands were dried in an oven at 40°C for 6 h. The microdiets were grounded and sieved according to different sizes: 70–200 μm for 13 DPH to 20 DPH larvae, 180–250 μm for 21 to 25 DPH larvae, 230–380 μm for 26–35 DPH larvae, and 350–400 μm for 36–45 DPH larvae.
The weaning microdiets and fish bodies were analyzed for proximate composition following methods described in the Association of Official Analytical Chemists AOAC (1997). Crude protein (N ×6.25) was determined using the KjeldahlTM 2300 Protein Analyzer (FOSS Tecator, Sweden). Crude lipid was determined gravimetrically by petroleum benzene-extraction method using the SoxtecTM 2043 Hot Extraction (FOSS Tecator, Sweden). The crude fiber was analyzed by sulfuric acid and sodium hydroxide digestion through the Fibertec System 1021 Cold and Hot Extractor (FOSS Tecator, Sweden). Total ash content was calculated gravimetrically following ignition of samples in a muffle furnace at 550°C. Dry matter was calculated by oven drying at 105°C until constant weight. The proximate composition of the experimental diets is given in Table 1. For fatty acid analysis, the microdiets and fish bodies were subjected to lipid extraction by using chloroform: methanol (1: 1, v/v) following Bligh and Dyer (1959) method, transmethylation, and fatty acid determination. The lipid extract was then fractionated by a short column filled with silica gel 60 F254 (Merck, Darmstadt, Germany) with a mesh size of 0.063–0.2 mm in a hexane:ethyl acetate solvent system (9:1, v/v). Methylation of the extract was carried out inside a reaction vessel for 2 h using sodium methylate. The extract was purified using the silica gel column system before the fatty acid methyl esters were analyzed in a gas chromatography (Shimadzu GC-2010, Shimadzu Corporation, Kyoto, Japan) equipped with a flame ionization detector and an autoinjector. The esters were separated using a capillary column (60 m ×0.25 mm ID; BPX70 column, SGE Australia Pty. Ltd., Ringwood, Victoria, Australia) and chromatography peaks were identified by comparing retention times with known standards (Supelco™ 37 Component FAME mix, Supelco Incorporation, Bellefonte, USA).
Asian seabass larvae were obtained from the Marine Fish Hatchery of the Sabah Fisheries Department (Tanjung Badak Station, Tuaran). During the acclimatization period, experimental larvae were fed L-type rotifer (Brachionus plicatilis) at the density of 20 rotifer/ml. When larvae reached 13 DPH, a total of 2,700 Asian seabass larvae with an initial length 5.54 ± 0.34 mm (mean ± SE) and weight 1.71 ± 0.13 mg (mean ± SE) were randomly distributed into 18 conical fiber-reinforced plastic (FRP) tanks (150 L) with initial stocking number of 150 larvae/tank, in triplicate treatments. The experimental tanks were randomly arranged using a closed culture system, supplied with continuous mild aeration (250 ml/s). Starting from 13 DPH, the larvae were co-fed with mixed diets (microdiets, rotifer, and artemia nauplii) following the feeding regime in Figure 1. Nannochloropsis oculata was provided in the larval tank with the concentration of 0.5 × 106 cell/ml. Meanwhile, weaning microdiets were given four times/day; in the morning, before rotifer was added at 08:00, 11:00, 14:00, and 16:00 h. The density of enriched rotifer was monitored and maintained twice daily at 08:30 and 14:00 h. The enriched artemia nauplii were given at 16:30 everyday starting from 13 to 25 DPH (13 days) at the density of 1–2 artemia/ml before the microdiets were used alone until 45 DPH to observe the larval performance and reach nursery stage. Water was routinely exchanged at about 10% daily. The physicochemical parameters of water temperature, dissolved oxygen (DO), and pH throughout the experiment were kept at 29.31 ± 0.80°C, 5.54 ± 0.11 mg/L, and 8.12 ± 0.04, respectively. The rearing protocol followed a study by Safiin et al. (2021) and the experiment duration lasted for 33 days.
High-salinity stress test (sensitivity index) was carried out by modifying the methods described in Dhert et al. (1990) and Bonaldo et al. (2011). Triplicate samples of ten 25 DPH Asian seabass larvae were randomly selected from each treatment and transferred to a square mesh net inside a 7-L transparent tank containing 6 L seawater. The salinity was refractometrically measured at 65 ppt. The experimental tanks were provided with mild aeration. Mortality was monitored at 10 min intervals during the 60 min observations. Larvae with opaque bodies that do not respond to a gentle touch using pipette were considered dead. The larval sensitivity to the stress was expressed as mortality rate, which was determined by the end of 60 min exposure.
For larval weight, triplicate pooled samples of initial and 13 DPH larvae (n = 100) were collected and measured using an analytical balance. Every 10 days interval up to 45 DPH, 10 larvae (n = 10) from each treatment were sampled, anesthetized with Transmore (alpha-methylquinoline) (Nika Trading, Puchong, Malaysia), and measured for morphometric observation on total length (TL), standard length (SL), body height (BH), head length (HL), head depth (HD), and eye diameter (ED). The larval gut content was observed under a light microscope (Nikon, Eclipse E600, Japan). Larvae were sampled (n = 5) daily for feed incidence (%) after microdiets were introduced in the culture system first as morning first feeding. Gut contents were calculated from the appearance of microdiets ingested in the gut from the total larvae were sampled. The condition factor (K), the ratio of HL to TL (HL: TL), and the ratio of TL to body weight (TL: BW) (Chatain, 1994) were identified at the end of the experiment.
Calculations of larval performances were described below:
IBM Statistical Package of Social Sciences version 23.0 was used for all the statistical analyses. All the presented data in percentage were arcsine transformed prior to data analysis. One-way ANOVA was analyzed to determine the mean differences among treatments at a significant level of 0.05. The homogeneity of variance was tested using Levene's test and multiple comparisons among treatments were presented using a Duncan's multiple range test. The effects of oil types, levels and interaction of oil types, and levels were performed by two-way ANOVA.
The growth performance of experimental fish is shown in Table 2. The final body weight, final TL, and SGR of fish-fed palm oil-based microdiets were significantly higher than the control treatment (FO100). Final mean body weight (0.63 ± 0.07 g), final mean TL (35.33 ± 1.29 mm), and SGR (13.13 ± 0.26%/day) of Asian seabass larvae-fed RPO75 were not statistically higher (Duncan's test, P > 0.05) than other palm oil-based diets, but significantly higher (P < 0.05) compared to control treatment (0.42 ± 0.06 g, 30.33 ± 0.77 mm, and 12.21 ± 0.33%/day, respectively). Palm oil levels are significantly affected the final body weight and TL (two-way ANOVA, P = 0.03 and P = 0.01, respectively) at the end of the experiment. The final survival of Asian seabass within the 33 days experimental period was not significantly affected by palm oil type, level, and interaction (two-way ANOVA, P > 0.05). The survival of Asian seabass-fed RPO50 (46.67 ± 6.36%) was significantly higher than those fed CPO25 and CPO75 (33.11 ± 3.67 and 33.11 ± 0.77%, respectively), but not statistically different (Duncan's test, P > 0.05) from RPO75, FO100, and CPO50 (39.11 ± 8.28, 39.33 ± 8.08, and 40.67 ± 5.46%, respectively. The survival rate of Asian seabass (25 DPH) after 60 min SST is shown in Table 2 and the palm oil type was significantly affected (two-way ANOVA, P = 0.04) by high salinity exposure. A significant higher value of survival was observed in FO100 (60.00 ± 17.32%) than in other diets, but this value was not significantly different (Duncan's test, P > 0.05) from the survival rate in CPO-based diets. Meanwhile, the survival of RPO-based larvae shows significantly (Duncan's test, P < 0.05) lowest survival compared to the control diet.
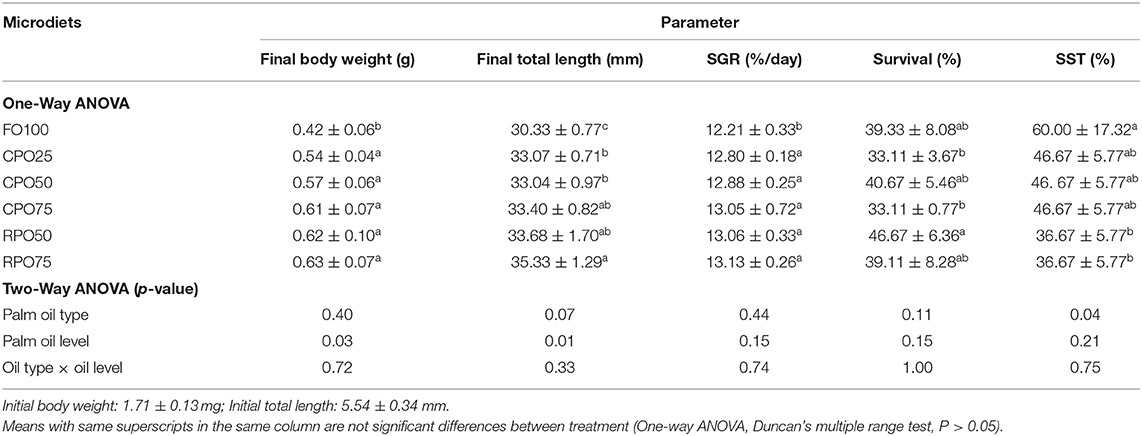
Table 2. Growth and survival performance of Asian seabass Lates calcarifer fed different microdiets for 33 days.
There was no significant difference (Duncan's test, P > 0.05) in feed intake and FCR among all the treatments (0.2–0.3 g/fish/d and 1.06–1.63, respectively), as shown in Table 3. The palm oil type, level, and interaction (two-way ANOVA, P > 0.05) were not significantly affected the feed intake and FCR. The feeding incidence (%) of Asian seabass fed different palm oil-based microdiets within 20 days experimental periods is shown in Figure 2. The feeding incidence increased as the gut capacity increased with fish growth. All the fishes fully ingested the microdiets at 18 DPH and showed no significant difference (Duncan's test, P > 0.05) in feed consumption among treatments indicating that the fish accepted the microdiets.
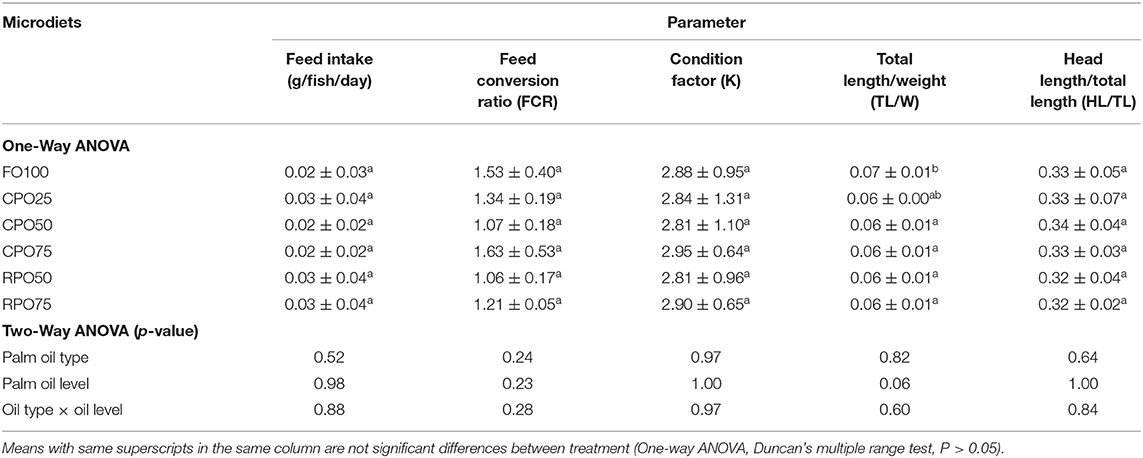
Table 3. Feeding performances and body indices of Asian seabass Lates calcarifer fed different microdiets for 33 days.
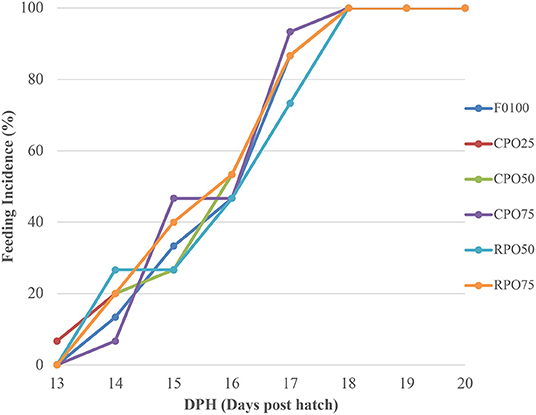
Figure 2. Feeding incidence (%) of Asian seabass fed different microdiets within 20 days experimental periods.
The morphometric variables of Asian seabass at the end of the experiment are shown in Figure 3. The morphometric value of each parameter was unaffected (two-way ANOVA, P > 0.05) by the palm oil type and level. RPO75 shows higher (Duncan's test, P > 0.05) SL and HL (28.46 ± 1.81 and 11.32 ± 0.58 mm, respectively) than other treatments. Insignificant higher (Duncan's test, P > 0.05) BH, HD, and DH (10.17 ± 1.09, 7.53 ± 0.68, and 6.02 ± 0.67 mm, respectively), were observed in CPO50 compared to other treatments. Meanwhile, the highest (Duncan's test, P > 0.05) ED (2.62 ± 0.22 mm) morphometric value was shown in fish-fed CPO75 and lower morphometric values (Duncan's test, P > 0.05) were observed in the control treatment, FO100 (2.38 ± 0.18 mm). The body indices are shown in Table 3. The condition factor (K), TL/W, and HL/TL were not significantly (Duncan's test, P > 0.05) different among treatments (ranging from 2.81 ± 1.1 to 2.95 ± 0.64; 0.06 ± 0.00 to 0.07 ± 0.01; and 0.32 ± 0.02 to 0.34 ± 0.04, respectively). The body indices parameter was not significantly affected by the palm oil type, level, and interaction (two-way ANOVA, P > 0.05). Overall, the body conditions show that Asian seabass is in normal condition.
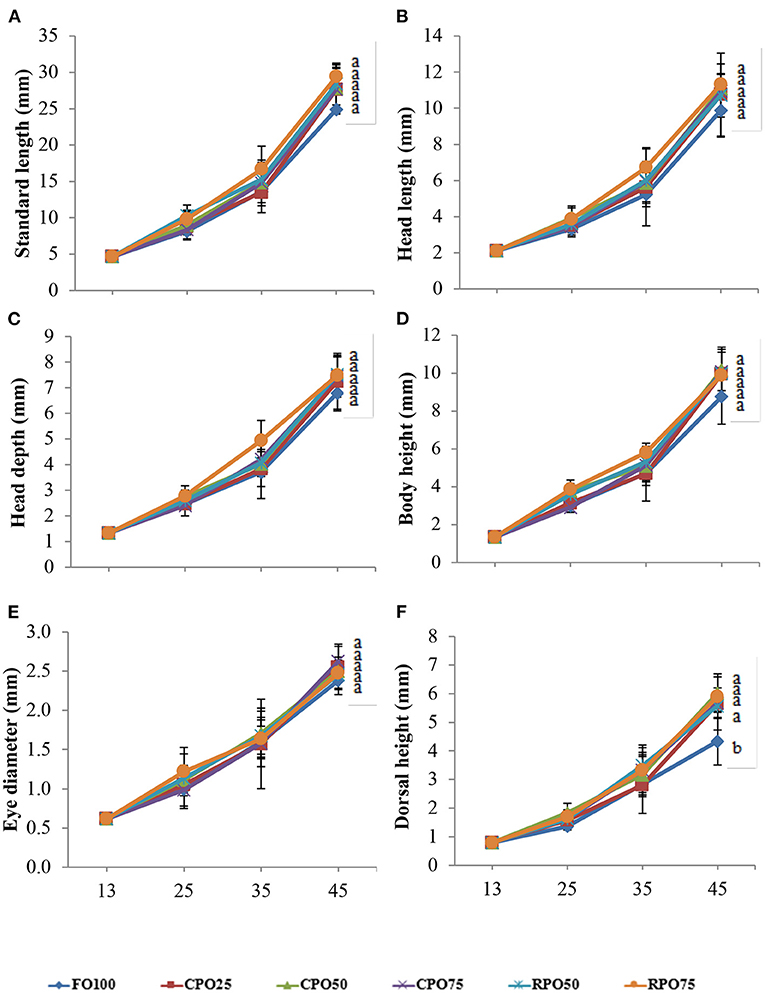
Figure 3. Morphometric changes of Asian seabass fed different microdiets within 33 days experiment period. (A) Standard length (mm), (B) body height (mm), (C) head length (mm), (D) head depth (mm), (E) eye diameter (mm), (F) dorsal height (mm), means with different superscripts are significantly difference between treatments (Duncan's multiple range test, P < 0.05).
The whole-body proximate composition of the larvae is shown in Table 4. Higher whole-body protein and lipid contents (15.3 ± 0.4 and 3.7 ± 0.0%, respectively), were observed in fish-fed CPO50 compared to other treatments. Meanwhile, the highest crude ash content was observed in CPO75 (5.1 ± 0.2%) and the lowest crude ash content was observed in CPO50 (4.1 ± 1.4%). Whole-body moisture was affected by palm oil type and interaction (two-way ANOVA, P = 0.04 and P = 0.02, respectively) and whole-body lipid was affected by palm oil level and interaction (two-way ANOVA, P = 0.00) at the end of the feeding trial.
Table 5 shows the fatty acid composition of different microdiets. Generally, the replacement of fish oil with palm oil increased the palmitic acid (C:16:0) and oleic acid (C18:1n9) and significantly (Duncan's test, P < 0.5) reduced the EPA (C20:5n3) and DHA (C22:6n3) contents in both the microdiets and larval body. The microdiets treatment of CPO75 shows significantly high C16:0 and C18:1n9 (41.3 ± 0.1 and 51.4 ± 0.1%, respectively), compared to other treatments. Palm oil level affected the total saturates, monoenes, polyunsaturated fatty acids (PUFA), n−3, and n−3/n−6. Palm oil level significantly affected (two-way ANOVA, P = 0.00) the total saturates levels. The total saturates in the palm oil-based microdiets were significantly (Duncan's test, P < 0.05) higher than in the control diets, ranging from 40.6 ± 0.3 to 47.7 ± 0.1%. The total PUFA (18.8 ± 0.6%), n-3 PUFA (14.7 ± 1.2%), and n-3/n-6 (3.6 ± 0.8%) ratios were significantly (Duncan's test, P < 0.05) higher in the control microdiet.
Generally, the total fatty acids body composition of Asian seabass shows the same pattern as the total fatty acids of microdiets as shown in Table 6. Total saturates in fish-fed palm oil-based microdiets were significantly (Duncan's test, P < 0.05) higher than control diets (FO100). The highest value of saturates was observed in fish-fed CPO50 and RPO75 (43.5 ± 0.8 and 43.9 ± 1.0%, respectively). The replacement of fish oil with dietary palm oil produced a significant effect on the monoenes level of fish-fed dietary CPO75 (50.5 ± 0.7%) and FO100 (36.8 ± 0.2%). As expected, the total PUFA, n−3 PUFA, n−3: n−6 ratios, EPA (20:5n−3), and DHA (22:6n−3) levels were significantly higher in the control microdiet (FO100) than in other treatments. Meanwhile, the lowest PUFA value was observed in CPO50 (7.2 ± 0.3%) and CPO75 (6.3 ± 1.1%). The palm oil level was significantly affected the DHA//EPA and other total fatty acids (two-way ANOVA, P = 0.00, and P = 0.01, respectively). Meanwhile, palm oil level affected the total saturates, monoenes, PUFA, and n-3 levels (two-way ANOVA, P = 0.00) and interaction affected the saturates and monoenes (two-way ANOVA, P = 0.00, and P = 0.01, respectively).
The use of palm oil-based weaning microdiet in this study resulted in positive effects on survival, growth, and feed utilization performances. The good performance of palm oil-based microdiets in Asian seabass indicated that fish properly ingested the available dietary nutrients under the practiced feeding regime. In a study by Curnow et al. (2006), commercial microdiets were also introduced as early as 13 DPH, co-feeding with either rotifer or artemia and well-accepted by the larvae. It is well-documented that the weaning of marine fish larvae can significantly affect their growth performances (Engrola et al., 2007; Hauville et al., 2014; Ma et al., 2015). Most aquatic animals are able to utilize dietary lipid with high levels of saturated and monoenoic fatty acids as an energy source, especially when the essential fatty acid requirements are met (Ng et al., 2007). In addition, there is a possibility of substrate preference in fish for saturated and monosaturated fatty acids over PUFA, which may explain the good performance of palm oil-based diets in the previous study (Fonseca-Madrigal et al., 2005). Palm oil is well-known for its nutritional advantages and positive attributes from β-carotene, α-tocopherol, and tocotrienols and serves as provitamin A (Ng and May, 2012). In particular, tocotrienol serves as potent inhibitors of lipid peroxidation and protein oxidation due to its ability to penetrate into tissue with saturates layer more efficiently which means it can attach to the cell membranes and exerts its antioxidant property (Ng et al., 2007; Saher and Owen, 2015). The α-tocotrienol reported 40–60% times more potent that α-tocopherol in preventing the lipid peroxidation (Serbinova et al., 1991) and protecting against free radical-induced oxidative stress. The presence of these components in the weaning diet formulation may reduce the lipid peroxidation in the tissue of Asian seabass larvae (Tocher et al., 2003; Jalali et al., 2008). It was also well-documented that the excellent natural antioxidants may confer beneficial effects to growth and flesh quality when fish are fed high levels of palm oil in their diets (Ng et al., 2007). The supplementation of antioxidants is necessary with the incorporation of n-3 PUFA in larval diets to prevent adverse effects and improve beluga (Huso huso) larval performances, as emphasized by Jalali et al. (2008). Not many past studies were conducted on alternative dietary oils in weaning diets for marine fishes. In a related recent study by Safiin et al. (2021), Asian seabass was successfully fed with palm oil-based enriched rotifer at a replacement level of 75% fish oil with RPO performed better than other palm oil-based enriched diets and the control. Juvenile Asian seabass is well-documented to effectively utilize formulated feeds, but little success has been previously documented for the use of weaning diets based on palm oil. Shapawi et al. (2011a) reported that a palm oil-based diet enhances the growth performance of Asian seabass juveniles when at least 65% RPO was replaced the dietary lipid in diets. Wan Ahmad et al. (2013) demonstrated that replacement of dietary fish oil with either palm or poultry oil in Asian seabass juvenile did not have negative effects on growth or hepatosomatic index. Palm oil was also reported to have the ability to improve protein efficiency when added to oxidize fish diets as well as a positive trend on growth performance in juvenile Japanese seabass, Lateolabrax japonicus (Han et al., 2012). Other studies reported the successful partial replacement of RPO as an excellent lipid source to replace fish oil over the other vegetable oils on hybrid grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus) juvenile (Yong et al., 2019) and up to 100% replacement with CPO resulted no negative effects on the fish performance and the sensory attribute at grow-out stage (Gudid et al., 2020).
Unsuitable weaning time and protocol can cause starvation of fish larvae (Ma et al., 2014). In this study, the lowest growth was obtained in the control diet. A high level of fish oil in diet may lead to poor retention efficiency of fatty acids, reduced acceptability and efficiency of nutrient uptake in the larval gut, and consequently suppress the growth (Bell et al., 2002). Within the weaning period, the slowest growth fish group may use their body energy to maintain basic metabolism for body maintenance and allocate less energy to growth when fish cannot obtain enough nutrition from available weaning diets as reported by Hu et al. (2017). This phenomenon has been observed in the larvae of Senegalese sole, Solea senegalensis (Engrola et al., 2007), spotted sand bass, Paralabrax maculatofasciatus (Civera-Cerecedo et al., 2008) and yellowtail amberjack, Seriola lalandi (Hu et al., 2017).
Feeding incidence of Asian seabass during the weaning period showed an increasing pattern starting from 13 to 17 DPH as indicated in the observed gut content. Fish showed slow attraction to microdiets at an early stage. The slower adaptation is relatively related to the variable acceptance and attraction of inert particles compounded by inadequate ingestion, digestion, and assimilation ability (Bonaldo et al., 2011). Asian seabass larvae were observed to fully ingest the microdiets at 18 DPH. Asian seabass has been reported to develop functional stomach and enzymatic enzymes such as pepsin at 17 DPH as described by Walford and Lam (1993).
Salinity stress test was developed to identify the differences in physiological conditions between the treatment groups for nutritional studies (Jalali et al., 2008; Nhu et al., 2010). Dhert et al. (1990) discovered that the larvae received n3-HUFA-fortified artemia had a superior physiological condition, which was reflected in significantly lower mortality during the stress test. The nutritional importance of essential fatty acids is well-recognized to increase the stress resistance of marine fish as reported by previous studies (Noori et al., 2011). In this study, the control diet (FO100) containing high essential fatty acid particularly DHA and EPA yielded the highest survival rate than the other treatments, but not a significant difference to the CPO-based microdiets.
The fatty acid profile of the Asian seabass larvae at the end of the trial is strongly influenced by the experimental diets. Replacement of fish oil with palm oil increased the palmitic acid (C16:0) and oleic acid (C18:1n9) and significantly reduced the EPA (C20:5n3) and DHA (C22:6n3) contents in both the microdiets and larval body. It appears that the palm oil-based microdiets used in this study contained a sufficient amount of essential fatty acids (2.3–6.4% EPA, 2.3–5.1% DHA, and ratio of 1.2–3.0: 1 n-3 to n-6 ratio) for normal development. A previous study by Safiin et al. (2021) on palm oil replacement on enriched rotifer at the early larval stage of Asian seabass reported that 50–75% of RPO contained n3:n9 of 1.1–2.1, which can promote better growth. It was reported that Asian seabass larvae required at least 0.75%, 1.15%, and 1.5–1.8: 1 of EPA, DHA, and n−3: n−6, respectively, to support their normal growth (Boonyaratpalin and Williams, 2002). The nutrient requirement is normally species specific and stage dependent. Fishes have the ability to synthesize essential fatty acids and selectively absorb and metabolize dietary fatty acid including n-3 PUFA (Bell et al., 2002) in order to obtain an optimal fatty acid composition (Alhazzaa, 2012). The positive attributes of palm oil-based diets particularly available saturates and monoenes were well-utilized by Asian seabass larvae to support the normal development as the fatty acids are preferred over PUFA for mitochondrial β-oxidation in fish as a source of metabolic energy (Fonseca-Madrigal et al., 2005; Bami et al., 2017).
Palm oil is available in the world market with a steady supply, competitive price, and most importantly, is much cheaper than fish oil. The findings from this study will provide a new insight into marine larval fish feed development, which normally utilize a high level of fish-based ingredients in the formulation due to the perception of high essential fatty acid requirements. CPO would be the preferred palm oil source, given its lower cost compared with RPO and the presence of a relatively higher level of natural antioxidants such as vitamin E and carotenoids, which can be destroyed during the oil-refining process.
Weaning microdiets formulated for Asian seabass larvae using either RPO or CPO performed significantly better than the control (FO-based diet) in terms of growth performance. In particular, replacement of fish oil with palm oil of up to 75% is recommended. Apart from the growth benefits observed in this study, the use of palm oil in the microdiet formulation will be able to reduce the feed cost due to the much cheaper price of this commodity, especially the CPO. It can be concluded that the current feeding regime of Asian seabass larvae with live feeds and palm oil-based microdiets can be successfully practiced in the hatchery.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Researcher's guidelines on code of practice for the care and use of animals for scientific purposes.
RS is the key person for this research, designed the experimental study, involved in the data interpretation & analysis, and manuscript write-up. NS under the supervision of RS and FC, executed the experiments and collected the data, analyzed the results, and prepared the manuscript draft. FC involved in the design of the culture facility and feeding protocol of Asian seabass larvae. All authors contributed to the article and approved the submitted version.
This research project was partially funded by a research grant awarded by Universiti Malaysia Sabah UMSGreat (GUG0270-2/2018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alhazzaa, R (2012). Alternative Sources of Omega-3 Oils for Barramundi, Lates calcarifer, Aquaculture. (Ph.D. thesis), University of Tasmania, Hobart, TAS (Australia).
AOAC (1997). Association of Official Analytical Chemists International Official Methods of Analysis, 16th Edn. Arlington, VA: AOAC.
Bami, M. L., Kamarudin, M. S., Saad, C. R., and Arshad, A. (2017). Effects of palm oil products on growth performance, body composition and fatty acid profile of juvenile Malaysian mahseer (Tor tambroides). J. Oil Palm Res. 29, 387–400. doi: 10.21894/jopr.2017.2903.12
Baskerville-Bridges, B., and Kling, L. J. (2000). Development and evaluation of microdiets for early weaning of Atlantic cod (Gadus morhua) larvae. Aquac. Nutr. 6, 171–182. doi: 10.1046/j.1365-2095.2000.00149.x
Bell, J. G., Henderson, R. J., Tocher, D. R, McGhee, F., Dick, J. R., Porter, A., et al. (2002). Substituting fish oil with crude palm oil in the diet of Atlantic salmon (Salmo salar) affects tissue fatty acid compositions and hepatic fatty acid metabolism. J. Nutr. 132, 222–230. doi: 10.1093/jn/132.2.222
Bligh, E. G., and Dyer, W. J. (1959). A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. doi: 10.1139/y59-099
Bonaldo, A., Parma, L., Badiani, A., Serratore, P., and Gatta, P. (2011). Very early weaning of common sole (Solea solea l.) larvae by means of different feeding regimes and three commercial microdiets: influence on performances, metamorphosis development and tank hygiene. Aquaculture 321, 237–244. doi: 10.1016/j.aquaculture.2011.09.007
Boonyaratpalin, M., and Williams, K. (2002). “Asian Seabass, Lates calcarifer,” in Nutrient Requirements and Feeding of Finfish for Aquaculture, eds C. D. Webster, and C. E. Lim (New York, NY: CABI), 40–50. doi: 10.1079/9780851995199.0040
Booth, M. A., Allan, G. L., and Russell, I. (2010). “Development of aquafeeds containing optimal inclusion levels of SBM and SPC for Asian seabass Lates calcarifer,” in Final Report Submitted to The United Soybean Board (USB) New Uses Committee USB Project FY2010 SB0463 (New South Wales: Industry and investment NSW Port Stephens Fisheries Institute, PSFI), 59.
Cahu, C., and Zambonino-Infante, J. L. (2001). Substitution of live food by formulated in marine fish larvae. Aquaculture 200, 161–180. doi: 10.1016/S0044-8486(01)00699-8
Catacutan, M. R., and Coloso, R. M. (1995). Effect of dietary protein to energy ratios on growth, survival and body composition of juvenile Asian seabass, Lates calcarifer. Aquaculture 131, 125–133. doi: 10.1016/0044-8486(94)00358-U
Chatain, B (1994). “Quality assessment of marine fish larvae and juveniles,” in Measures for Success, eds P. Kestemont, J. Muir, F. Sevila, and P. Williot (Bordeaux: CEMAGREF), 181–183.
Civera-Cerecedo, R., Alvarez-Gonzalez, C. A., García-Gómez, R. E., Carrasco-Chavez, V., Ortiz-Galindo, J. L., Rosales-Velazquez, M. O., et al. (2008). Effect of microparticulate diets on growth and survival of spotted sand bass larvae, Paralabrax maculatofasciatus, at two early weaning times. J. World Aquac. Soc. 39, 22–36. doi: 10.1111/j.1749-7345.2007.00132.x
Curnow, J., King, J., Patridge, G., and Kolkovski, S. (2006). Effects of two commercial microdiets on growth and survival of barramundi (Lates calcarifer Bloch) larvae within various early weaning protocols. Aquac. Nutr. 12, 247–255. doi: 10.1111/j.1365-2095.2006.00399.x
Department of Fisheries Malaysia (2021). Fisheries Statistics I 2020. Department of Fisheries Malaysia. Available online at: https://www.dof.gov.my/en/resources/fisheries-statistics-i/ (accessed January 10, 2022).
Dhert, P., Lavens, P., Duray, M., and Sorgeloos, P. (1990). Improved larval survival at metamorphosis of Asian seabass (Lates calcarifer) using ω3-HUFA-enriched live food. Aquaculture 90, 63–74. doi: 10.1016/0044-8486(90)90283-S
Engrola, S., Conceição, L. E. C., Dias, L., Pereira, R., Ribeiro, L., and Dinis, M. T. (2007). Improving weaning strategies for Senegalese sole: effects of body weight and digestive capacity. Aquac. Res. 38, 696–707. doi: 10.1111/j.1365-2109.2007.01701.x
Fonseca-Madrigal, J., Karalazos, V., Campbell, P. J., Bell, J. G., and Tocher, D. R. (2005). Influence of dietary palm oil on growth, tissue fatty acid compositions, and fatty acid metabolism in liver and intestine in rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 11, 241–250. doi: 10.1111/j.1365-2095.2005.00346.x
Future Market Insight (2021). Asian Sea Bass Market-Analysis, Outlook, Growth, Trends, Forecast. Future Market Insight. Available online at: https://www.futuremarketinsights.com/reports/sea-bass-market (accessed January 10, 2022).
Gisbert, E., Mozanzadeh, M. T., Kotzamanis, Y., and Estévez, A. (2016). Weaning wild flathead grey mullet (Mugil cephalus) fry with diets with different levels of fish meal substitution. Aquaculture 462, 92–100. doi: 10.1016/j.aquaculture.2016.04.035
Glencross, B., Blyth, D., Irvin, S., Bourne, N., Campet, M., Boisot, P., et al. (2016). An evaluation of the complete replacement of both fish meal and fish oil in diets for juvenile Asian seabass, Lates calcarifer. Aquaculture 45, 298–309. doi: 10.1016/j.aquaculture.2015.09.012
Glencross, B. D (2011). A comparison of the digestibility of diets and ingredients fed to rainbow trout (Oncorhynchus mykiss) or barramundi (Lates calcarifer)—the potential for inference of digestibility values among species. Aquac. Nutr. 17, 207–215. doi: 10.1111/j.1365-2095.2010.00752.x
Gudid, S. N., Seng, L. L., Kian, A. Y. S., Yanuhar, U., Mustafa, S., and Shapawi, R. (2020). Growth performance and organoleptic quality of hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) fed palm-oil based diets at grow-out stage. Sains Malays 49 1567–1576. doi: 10.17576/jsm-2020-4907-09
Han, Y. Z., Ren, T. J., Jiang, Z. Q., Jiang, B. Q., Gao, J., Koshio, S., et al. (2012). Effects of palm oil blended with oxidized fish oil on growth performances, hematology and several immune parameters in juvenile Japanese seabass, Lateolabrax japonicas. Fish Physiol. Biochem. 38, 1785–1794. doi: 10.1007/s10695-012-9675-4
Hauville, M. R., Zambonino-Infante, J. L., Bell, G., Migaud, H., and Main, K. L. (2014). Impacts of three different microdiets on Florida pompano, Trachinotus carolinus, weaning success, growth, fatty acid incorporation and enzyme activity. Aquaculture 422–423, 268–276. doi: 10.1016/j.aquaculture.2013.12.006
Herbert, B (2005). Feeding and Growth of Golden Perch (Macquaria ambigua) and Assessment of Its Potential for Aquaculture. (Ph.D. thesis), James Cook University. Available online at: https://researchonline.jcu.edu.au/1332/ (accessed December 1, 2021).
Hu, J., Yang, R., Chen, X., Yang, Q., Ma, Z., and Yu, G. (2017). Early weaning of yellowtail amberjack seriola lalandi dorsalis (Gill 1863) larvae. J. Coast. Life Med. 5, 187–190. doi: 10.12980/jclm.5.2017J7-62
Jalali, M. A., Hosseini, S. A., and Imanpour, M. R. (2008). Effect of vitamin E and highly unsaturated fatty acid-enriched artemia urmiana on growth performance, survival and stress resistance of beluga (Huso huso) larvae. Aquac. Res. 39, 1286–1291. doi: 10.1111/j.1365-2109.2008.01992.x
Kolkovski, S., Curnow, J., and King, J. (2010). Development Towards Commercialization of Marine Fish Larvae Feeds-Microdiets. Project No. 004/58. Fisheries research Report No. 198. Department of Fisheries, Western Australia, 180.
Ma, Z., Qin, J. G., Hutchinson, W., Chen, B. N., and Song, L. (2014). Responses of digestive enzymes and body lipids to weaning times in yellowtail kingfish Seriola lalandi (Valenciennes, 1833) larvae. Aquac. Res. 45, 973–982. doi: 10.1111/are.12039
Ma, Z., Zheng, P., Guo, H., Zhang, N., Wang, L., and Jiang, S. (2015). Effect of weaning time on the performance of Trachinotus ovatus (Linnaeus 1758) larvae. Aquac. Nutr. 21, 670–678. doi: 10.1111/anu.12183
Malaysia Palm Oil Council (2021). Malaysian Palm Oil Industry. Malaysia Palm Oil Council. Available online at: https://mpoc.org.my/malaysian-palm-oil-industry/ (accessed January 09, 2022).
Moldal, T., Løkka, G., Wiik-Nielsen, J., Austbø, L., Torstensen, B. E., Rosenlund, G., et al. (2014). Substitution of dietary fish oil with plant oils is associated with shortened mid intestinal folds in Atlantic salmon (Salmo salar). BMC Vet. Res. 10, 60. doi: 10.1186/1746-6148-10-60
Nankervis, L., and Southgate, P. C. (2006). An integrated assessment of gross marine protein sources used in formulated microbound diets for barramundi (Lates calcarifer) larvae. Aquaculture 257, 453–464. doi: 10.1016/j.aquaculture.2006.03.006
Ng, M. H., and May, C. Y. (2012). Chromatographic analyses of tocopherols and tocotrienols in palm oil. J. Chromatogr. Sci. 50, 283–286. doi: 10.1093/chromsci/bms002
Ng, W. K., Tocher, D. R., and Bell, J. G. (2007). The use of palm oil in aquaculture feeds for salmonid species. Eur. J. Lipid Sci.Technol. 109, 394–399. doi: 10.1002/ejlt.200600209
Nhu, V. C., Dierckens, K., Nguyen, H. T., and Hoang, T. M. (2010). Effect of early co-feeding and different weaning diets on the performance of cobia (Rachycentron canadum) larvae and juveniles. Aquaculture 305, 52–58. doi: 10.1016/j.aquaculture.2010.04.010
Noori, F., Takami, G. A., Van, S. M., Van, S. G., and Sorgeloos, P. (2011). Feeding Acipenser persicus and Huso huso (Acipenseriformes) larvae with Artemia urmiana nauplii enrich with HUFA and vitamin C: II. Effect on tolerance to shock exposure of environmental factors. J. Appl. Ichthyol. 27, 781–786. doi: 10.1111/j.1439-0426.2010.01647.x
Richard, N., Mourente, G., Kaushik, S., and Corraze, G. (2006). Replacement of a large portion of fish oil by vegetable oils does not affect lipogenesis, lipid transport and tissue lipid uptake in European seabass (Dicentrarchus labrax L.). Aquaculture 261, 1077–1087. doi: 10.1016/j.aquaculture.2006.07.021
Safiin, N. S. Z., Mustafa, S., Ching, F. F., and Shapawi, R. (2021). Palm oil-based enriched diets for the rotifer, Brachionus plicatilis, improved the growth of Asian seabass (Lates calcarifer) larvae. Front. Mar. Sci. 8, 613312. doi: 10.3389/fmars.2021.613312
Saher, F. A., and Owen, L. W. (2015). Tocotrienol rich palm oil extract is more effective than pure tocotrienols at improving endothelium-dependent relaxation in the presence of oxidative stress. Oxid. Med. Cell. Longev. 2015, 150829. doi: 10.1155/2015/150829
Salini, M., Irvin, S., Bourne, N., Blyth, D., Cheers, S., Habilay, N., et al. (2015). Marginal efficiencies of long chain-polyunsaturated fatty acid use by barramundi (Lates calcarifer) when fed diets with varying blends of fish oil and poultry fat. Aquaculture 449, 48–57. doi: 10.1016/j.aquaculture.2015.02.027
Serbinova, E., Kagan, V., Han, D., and Pecker, L. (1991). Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 10, 263–275. doi: 10.1016/0891-5849(91)90033-Y
Shapawi, R., Mohd Zain, M. A., and Senoo, S. (2011a). palm oil based-diet enhances growth performance of asian seabass (Lates calcarifer, Centropomidae) juveniles. Asian Fish. Sci. 24, 218–223. doi: 10.33997/j.afs.2011.24.2.010
Shapawi, R., Mustafa, S., and Ng, W. K. (2008). Effects of dietary fish oil replacement with vegetable oils on growth and tissue fatty acid composition of humpback grouper, Cromileptes altivelis (Valenciennes). Aquac. Res. 39, 315–323. doi: 10.1111/j.1365-2109.2007.01882.x
Shapawi, R., Mustafa, S., and Ng, W. K. (2011b). A comparison of the growth performance and body composition of the humpback grouper, Cromileptes altivelis fed on farm-made feeds, commercial feeds and trash fish. J. Fish. Aquat. Sci. 6, 523–534. doi: 10.3923/jfas.2011.523.534
Süzer, C., Kamaci, H., Çoban, D., Saka, S., Firat, K., and Karacaoglan, A. (2011). Early weaning of seabasss (D. labrax) larvae: effects on growth performance and digestive enzyme activities. Turkish. J. Fish. Aquat. Sci. 11, 491–497. doi: 10.4194/1303-271-v11_3_33
Szucs, I., Tikász, I. E., Fehér, M., and Stundl, L. (2018). Testing for consumer preferences of smoked Asian seabass (Barramundi) filet products in Hungary. Cogent Bus. Manag. 5, 1432158. doi: 10.1080/23311975.2018.1432158
Tocher, D. R., Mourente, G., Van De Eeken, A., Evjemo, J. O., Diaz, E., Wille, M., et al. (2003). Comparative study of antioxidant defence mechanisms in marine fish fed variable levels of oxidised oil and vitamin E. Aquac. Int. 11, 195–216. doi: 10.1023/A:1024127003997
Tu, W. C., Mühlhäusler, B. S., James, M. J., Stone, D. A. J., and Gibson, R. A. (2012). An alternative n-3 fatty acid elongation pathway utilizing 18:3n-3 in barramundi (Lates calcarifer). Biochem. Biophys. Res. Commun. 423, 176–182. doi: 10.1016/j.bbrc,.2012.05.110
U.S. Department of Agriculture (2021). Palm Oil Explorer. U.S. Department of Agriculture. Available online at: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=4243000&sel year=2021&rankby=Production (accessed January 10, 2022).
Walford, J., and Lam, T. J. (1993). Development of the digestive tract and proteolytic enzyme activity in seabass (Lates calcarifer) larvae and juveniles. Aquaculture 109, 187–205. doi: 10.1016/0044-8486(93)90215-K
Wan Ahmad, W. A., David, A., Stone, J., and Schuller, K. A. (2013). Dietary fish oil replacement with palm or poultry oil increases fillet oxidative stability and decreases liver glutathione peroxidase activity in barramundi (Lates calcarifer). Fish Physiol. Biochem. 39, 1631–1640. doi: 10.1007/s10695-013-9815-5
Williams, K., and Barlow, C. G. (1999). Dietary requirement and optimal feeding practices for Barramundi (Lates calcarifer). Canberra, ACT: Project 92/63, Final report to Fisheries R&D Corporation
Yong, A. S. K., Mubarak, N. S. S., and Shapawi, R. (2019). Effects of partial replacement of fish oil with different vegetable oils on growth, feed utilization and fatty acid profile of hybrid grouper juvenile (Epinephelus fuscoguttatus × Epinephelus lanceolatus). J. Oil Palm Res. 31, 110–121. doi: 10.21894/jopr.2019.0003
Keywords: co-feeding, weaning, palm oil, fish oil replacement, Asian seabass
Citation: Safiin NSZ, Ching FF and Shapawi R (2022) Successful Co-Feeding of Asian Seabass, Lates calcarifer Larvae With Palm Oil-Based Microdiets and Live Feeds. Front. Sustain. Food Syst. 6:836275. doi: 10.3389/fsufs.2022.836275
Received: 15 December 2021; Accepted: 07 March 2022;
Published: 15 April 2022.
Edited by:
Keitaro Kato, Kindai University, JapanReviewed by:
Kwasi Adu Adu Obirikorang, Kwame Nkrumah University of Science and Technology, GhanaCopyright © 2022 Safiin, Ching and Shapawi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rossita Shapawi, cm9zc2l0YUB1bXMuZWR1Lm15
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.