- Food Security and Safety Focus Area, Faculty of Natural and Agricultural Sciences, North-West University, Mmabatho, South Africa
To meet the global demand for food, several factors have been deployed by agriculturists to supply plants with nitrogen. These factors have been observed to influence the soil nitrification process. Understanding the aftermath effect on the environment and health would provoke efficient management. We review literature on these factors, their aftermath effect on the environment and suggest strategies for better management. Synthetic fertilizers and chemical nitrification inhibitors are the most emphasized factors that influence the nitrification process. The process ceases when pH is <5.0. The range of temperature suitable for the proliferation of ammonia oxidizing archaea is within 30 to 37oC while that of ammonia oxidizing bacteria is within 16 to 23oC. Some of the influencing factors excessively speed up the rate of the nitrification process. This leads to excess production of nitrate, accumulation of nitrite as a result of decoupling between nitritation process and nitratation process. The inhibition mechanism of chemical nitrification inhibitors either causes a reduction in the nitrifying micro-organisms or impedes the amoA gene's function. The effects on the environment are soil acidification, global warming, and eutrophication. Some of the health effects attributed to the influence are methemoglobinemia, neurotoxicity, phytotoxicity and cancer. Biomagnification of the chemicals along the food chain is also a major concern. The use of well-researched and scientifically formulated organic fertilizers consisting of microbial inoculum, well-treated organic manure and good soil conditioner are eco-friendly. They are encouraged to be used to efficiently manage the process. Urban agriculture could promote food production, but environmental sustainability should be ensured.
Introduction
Nitrification process (NP) is an oxidation reaction that usually occurs under aerobic conditions. The process serves as an intermediate of oxidized and reduced forms of nitrogen in its cycling. The nitrate produced serves as a substrate for denitrification and a nutrient for plant growth. This has made it important to environmental sustainability and agricultural intensification. Compounds such as ammonium (NH4), ammonia (NH3), hydroxylamine (NH2OH), nitrous oxide (NO), nitrite (NO2-), and nitrate (NO3-) are the major forms of nitrogen associated with the process. The soil nitrification process is divided into two major phases which are nitritation and nitratation, and the order of microbial oxidation of ammonia via nitrite to nitrate is sequential (Amoo and Babalola, 2017). Nitritation accomplishes the oxidation of ammonia to nitrite, while nitratation phase oxidizes nitrite to nitrate. This process is majorly engineered by some group of nitrifying bacteria and archaea in a complex chemical transformation, and they are affected by several factors. The factors include synthetic fertilizers, chemical nitrification inhibitors and other agrochemicals. The effects are evaluated with total soil nitrogen, mean annual temperature, pH and microbial biomass nitrogen (Li et al., 2020).
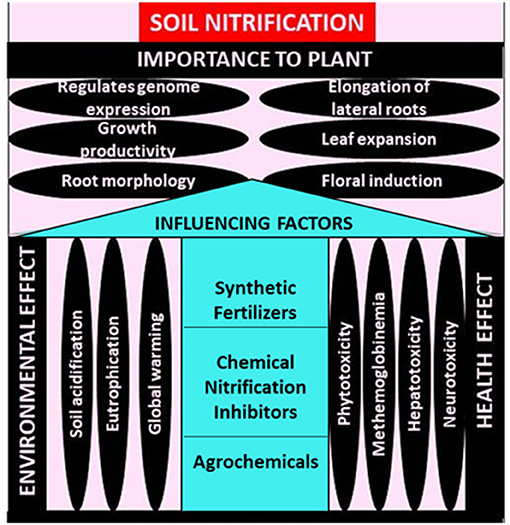
The universal cycling of nitrogen is being massively distressed due to the activities of man on the lithosphere. Manipulation of the soil nitrification process for agricultural benefit has been one of such activity. This has led to and would continue to lead to negative effects which many researchers do not foresee. Reviewing this will enlighten scientists on the importance of the soil nitrification process, its influencing factors and the effect on environment and biotic health (Figure 1). This would provoke better management and cause amendments to be made. The influence is measured by the rate at which associating chemicals are produced or by the dynamics of the soil organism, especially those directly associated with the process ‘the nitrifying bacteria and archaea'.
Importance of Nitrification
The modern nitrification process has led to a 50% loss of nitrogen and has reduced the availability of nitrogen for the use of plants (Beeckman et al., 2018). Despite the present situation, the importance of the nitrification process cannot be overemphasized. Its most important goal is to provide nitrate for plant use. Although there are other available nitrogen forms in the soil, nitrate seems preferable to most plants and other soil organisms and leads to better functioning of the ecosystem if produced in the right proportion.
Crop nitrogen demand is unpredictable. The time of greatest demand is normally during the stem elongation phase, except for crops targeted for high protein grain whose highest demand is during the flowering phase (Angus, 2001). However, the presence of external induces the expression of the transporter gene, causing elongation of lateral roots (Mantelin and Touraine, 2004). Also, high-affinity transport system (HATS) becomes active if concentration of NO3 in soil is low (<250 micrometers) and low-affinity transport system (LATS) becomes activated if the concentration of NO3 is high (>250 micrometers) (Plett et al., 2018). Subsequently, an excess supply of nitrate reduces the demand for nitrate (Mantelin and Touraine, 2004); therefore, it is needed in a gradual release and at the right time.
In addition to being a nutrient, nitrate is a local and systemic signal that regulates genome-wide gene expression, root morphology, leaf expansion, seed dormancy and floral induction (Hachiya and Sakakibara, 2016). It helps in the production of embryos during the early stages of reproduction and carries out anthesis (Yoneyama et al., 2016). Several responses to nitrate are mediated via calcium and phytohormone signaling pathways including auxin, cytokines and abscises acid (Hachiya and Sakakibara, 2016). A decrease in nitrate assimilation causes a decline in protein concentration in cereals. This leads to retardant growth, and the subsequent effect on animal and human nutrition can be detrimental (Dier et al., 2018).
An additional benefit of nitrification is the oxidation of ammonia. Ammonia has a negative effect on plant, biotic and abiotic components of the environment. Excess ammonia affects the uptake of nutrients, disturbs hormonal balance, decreases soluble carbohydrates of plants, and distorts photosynthesis and metabolic pathways (Wang et al., 2016). Directly or indirectly, ammonia plays a crucial role in environmental damage (Lehtovirta-Morley, 2018). This could be the result of its higher acid level when compared to the oxidized nitrogen forms. Ammonia in agricultural runoff negatively affects water bodies as it reduces dissolved oxygen resulting in aquatic biota toxicity (Wang et al., 2016). Plant tolerance of ammonia varies within plant species (Byrnes et al., 2017), and few plants can conveniently use ammonia.
The availability of nitrates is one of the main factors that determine the productivity and growth of plants. Unfortunately, they are scarce in natural soil due to soil physical and chemical properties, microorganism activities and drainage (Kiba and Krapp, 2016). Of all the nitrogen forms, nitrate is the most susceptible to leaching, thus making it often unavailable for plant use at the moment needed. The anthropogenic input of nitrogen has done more harm than good to the agricultural system. Although done purposely to improve crop yield, the excessive and repeated input of anthropogenic nitrogen has increased nitrate leaching (Nevison et al., 2016) and reactive nitrous oxide gas production. This is alarming as agriculturists believing they have made available sufficient nitrates for plant growth have indirectly affected productivity.
Mechanism of Nitrification Process
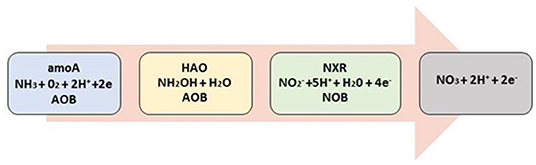
The mechanism of nitrification is a complex one, being a mixture of biological and chemical processes (Figure 2). The biochemical reaction takes place on the membrane site of the associating microorganisms. Primarily, ammonia (NH3) is used as the major substrate. It is transformed by ammonia monooxygenase enzyme (amoA) into hydroxylamine (NH2OH), while hydroxylamine with the aid of the enzyme hydroxylamine oxidoreductase (HAO) reacts with water to produce nitrite (NO2) (Amoo and Babalola, 2017). Nitrite oxidoreductase (NXR) found in nitrite-oxidizing bacteria transforms nitrite into nitrate (NO3) (Fu et al., 2020). The reaction requires the use of oxygen and hydrogen; electrons are usually released from the membrane. In an unperturbed environment, the nitrification process is usually stable, however, when disturbed by anthropogenic activities it varies. The variation is dependent on factors that affect the availability of ammonia as well as the abundance and function of nitrifying bacteria. At suitable conditions such as sufficient amount of substrate and pH that is balanced, the rate of nitrification as reported by Tarre and Green (2004) is 0.55 g of N.g of biomass−1, day−1.
Factors Influencing the Nitrification Process
Categorically, the factors that affect the nitrification process can be chemical or physical. These factors were adopted to intensify crop production and meet global food demand. The chemical factors include synthetic fertilizer, chemical nitrification inhibitors and pesticides. Some of the notable physical factors are temperature, pH, and oxygen (Schaefer and Hollibaugh, 2017). Li et al. (2020), evaluated the global soil nitrification rate across terrestrial ecosystems. It was observed that the total soil nitrogen contributed mostly to the nitrification with a coefficient of 0.29, next was the mean annual temperature (0.25), followed by the pH (0.24), and microbial biomass nitrogen (0.19).
Synthetic Fertilizer
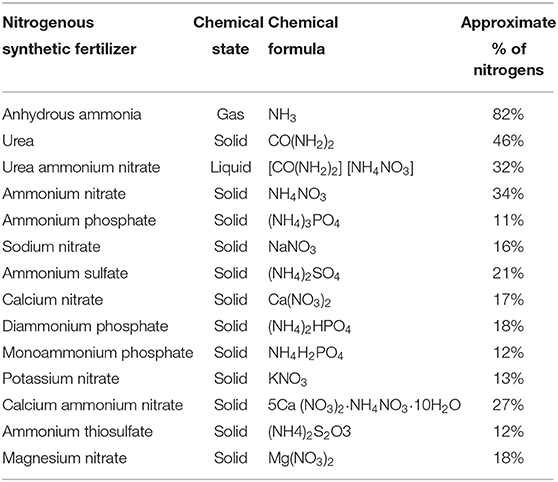
Synthetic fertilizers come in various types, brands and formulae (Table 1), and they could be in solid, liquid or gaseous state. The different kinds of fertilizer majorly are made of phosphorus, potassium, nitrogen, and a combination of either two or the three elements (Cai et al., 2019). Koli et al. (2019) classified them as straight (supply only one nutrient), complex (containing two or three nutrients), and mixed fertilizer (has more than three nutrients). Majority are nitrogen-based as a result of high requirement of the element by the plants. Farmers rely on fertilizers made of nitrogen to have an exponential increase in crops produced. However, the efficiency of its use is low (30–50%) when comparing it to the amount of crop produced (Liang et al., 2019).
In time past, the rotation of crops was carried out in farming to exploit endophyte nitrogen-fixing rhizobia inhabiting legumes and microorganisms in organic waste to produce beneficial nutrients, ammonia and nitrate for plant use. The practice was safe but could not continually be relied on because it does not provide enough for the plant usage. This resulted in the use of synthetic fertilizer which provides an immediate replacement to naturally produced nutrients. Unfortunately, it negatively affects the rate of nitrification in the long run (Verma et al., 2018). Those with ammonia speed up the rate of nitrification excessively since they provide an immediate substrate for ammonia oxidizers to act on. Also, synthetic fertilizers with phosphate elevate the process of nitrification 12 times by raising soil pH to favor the process (DeForest and Otuya, 2020). This often leads to an oversaturation of nutrients beyond what the biota in the environment can assimilate. Generally, where there is an increase in soil nitrifying microorganisms as a result of synthetic fertilizer application, it is only temporal (Quemada et al., 2019).
Chemical Nitrification Inhibitors
Nitrogen is lost from the soil through leaching, volatilization of NH3 and other nitrogenous gases associated with the microbial reaction in the denitrification and nitrification processes (Coskun et al., 2017). Due to the detrimental effects of the gases on health and the environment, inhibitors have been recently used to restrict the rate of nitrification. This causes the transformation of ammonium () to nitrate () to be delayed in the soil. The actions of the inhibitors are noticed by restraining the action of the genes associated with process (Liu et al., 2020). Also, growth of the acting bacteria and archaea be inhibited (Elrys et al., 2020). However, their use and mechanism of inhibition are yet to be fully understood.
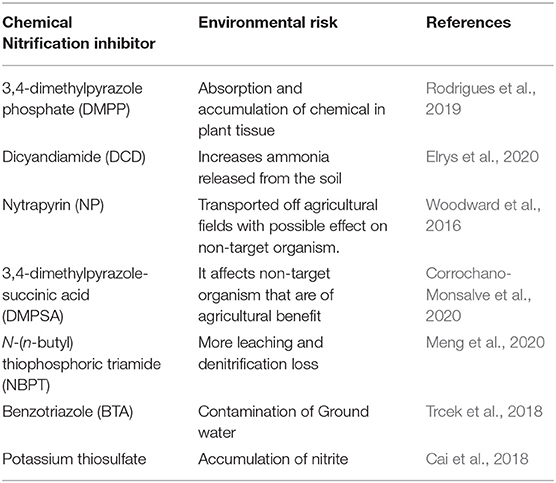
Nitrapyrin (NP), Dicyandiamide (DCD) and 3,4-dimethylpyrazole phosphate (DMPP) are well-known synthetic nitrification inhibitors (Lu et al., 2019). They are usually used along with synthetic nitrogen fertilizers or organic waste. Infusing organic waste with DMPP can prolong the nitrification time (Kong et al., 2018). This is achieved by chelating chemicals like Cu which inhibit the first enzymatic step of nitrification (Wu D. et al., 2018). Moreover, the mechanisms of inhibition vary within the different nitrification inhibition (Rodrigues et al., 2018). Application of DCD with urea decreased the rate of loss (1.8 mg N kg−1 soil day−1) which could have been a result of an inhibitory effect on ammonia-oxidizing microbial communities (Duncan et al., 2016).
DMPP is considered less toxic than DCD because its recommended application rate is one-tenth of DCD (Rodrigues et al., 2018). However, according to Yang et al. (2016), an increase in yield by the application of DMPP was noticed only in alkaline soil. The shortcomings of chemical nitrification inhibition as reported by Lu et al. (2019) include difficulties in application, high cost, environmental pollution and food safety risks (Table 2). Other than these few mentioned shortcomings, there are likely to be more. Knowing the specific species that are targeted by this organism would be of great advantage to agricultural and environmental management.
Other Agrochemicals and Substance
Aside from the use of fertilizer and nitrification inhibitors, there are some other agrochemicals and substances used in farms that influence the nitrification process. They are frequently used to promote plant productivity. Pesticides are one of them and they are of various categories, such as fungicides, insecticides, and rodenticides. Iprodione a fungicide has an antagonistic effect on amoA genes, it decreases their abundance and reduces the rate of nitrification (Zhang et al., 2018a). Another is herbicides which can be synthetic or organic. Atrazine and glyphosphate are synthetic herbicides observed to grossly reduce the rate of nitrification in the soil by inhibiting the microbial functional genes responsible for the process (Zhang et al., 2018b).
Clinoptilolites are synthetic substances with high cation exchange properties with the potential to retain ammonium ions (Jakkula and Wani, 2018). Hydrogel, polyvinyl alcohol, and anionic polyacrylamide are soil conditioners reported by Seddik et al. (2019) noticed to increase the total nitrogen content of the soil. Although, according to Youssef et al. (2019), polyvinyl alcohol had no significant effect on the nitrification process. Also, quartz sand used to control soil nutrient leaching in agricultural soil affects nitrogen transformation dynamics. It was observed to grossly inhibit the autotrophic nitrification process and stimulate the immobilization of and thus should be used cautiously (Wang et al., 2017). This must have been a result of altering the agricultural soil's physical and chemical properties.
Flue gas desulphurization gypsum (FGDG) has also been used as a soil amendment and noticed to influence the nitrification process by inhibiting and delaying the occurrence of amoA genes (Li et al., 2016). Industrial waste from dairy factories escalates the availability of ammonium, this rapidly increases nitrification process. Other forms of human activities that have brought excess influx of nitrogen include, combustion of fossil fuel, biomass burning, and biological activities in the natural soil. The terrestrial anthropogenic activities have been increasing tremendously over the past years and would continue to increase. Researchers need to find a way to create a pseudo-balanced ecosystem continuously.
Climate Change
Agriculture practices such as bush burning, tree cutting have affected climate change. One of the observed effects of climate change is an unusual increase in atmospheric temperature. Increased temperature increases the volatilization and emission of nutrients. The nitrification process driven by AOA and AOB is strongly affected by elevation and fundamental differences in temperature. Taylor et al. (2017) evaluated AOA and AOB across a gradient of (4–42oC), it was observed that the maximum nitrification potential rates of AOA are within the range 30 to 37oC while that of AOB is within the range 16 to 23oC. Hu et al. (2016) reported an increase in AOA and a gradual decrease in AOB under elevated temperatures. Akram et al. (2018) observed a correlation between change in climate, nitrogen fertilizer application and emission of N2O. According to Sahrawat (2008), plotting the response of temperature to climate change gives a bell-shape with an optimum temperature of 30–35oC.
Physical Factors
Anthropogenic activities often affect physical factors of soil environment. These in turn affect the soil nitrification process. Notable physical factors that affect the process are temperature, pH, moisture, oxygen, and aeration. The two most important physical factors are temperature and pH. The response of the process to temperature is similar to that observed in climate change. Le et al. (2019) reported that ammonia oxidation is inhibited at pH 5 while nitrite oxidation is inhibited at pH 8.5, optimum activity of AOB and NOB are 7.5 and 7.0. The optimum pH varies but there is an agreement of the process ceasing at 5.0 since oxidation of ammonia is the first. Also, Soil moisture closes up pore spaces, this affects aeration and reduces the oxygen level. Nitrification is a biochemical oxidation process, low oxygen levels in the soil would negatively affect the process.
AOB diversity differs among soil types; the presence of clay in soil affects the nitrification process. Waterlogging which could arise as a result of frequent irrigation reduces the soil oxygen level decreasing the nitrification potential rate and the abundance of ammonia oxidizing microorganisms (Nguyen et al., 2018). Tillage is an age-long agricultural practice done to increase productivity by removing weeds and increasing soil aeration. However, it has a subsequent disadvantage of reducing soil biomass, which negatively affects soil structure and quality (Vazquez et al., 2019). The mechanism of the influencing physical factors is not fully understood as a result of the complex interaction among the various factors.
Effect of the Influencing Factors on the Environment
In the past, scientists managing the nitrification process have focused on agricultural intensification, paying little or no attention to environmental degradation. The addition of fertilizer initially brought an enormous boom in agricultural productivity with little or no side effects. However, it is presently clear that the use of nitrogen fertilizer is causing serious environmental issues. Excessive levels of in the soil can be imputed to the increasing use of fertilizer made of synthetic nitrogen in agroecosystems (Zhai et al., 2017). Significant changes were observed in soil bacteria community structure, and soil organic matter mineralization tends to be negatively affected by the use of DMPP (Zhang et al., 2017).
The efficiency of nitrogen use in crops is low. Fifty percent of the synthetic nitrogen applied to agricultural systems is not mopped up, instead, it is distributed to the surroundings as oxides of nitrogen (NOx) and ammonia (Coskun et al., 2017). The excess nitrogenous compounds are lost to surface water, groundwater and the atmosphere as a result of over saturation in the soil, propelling detrimental effects to the environment. The increased ammonia leads to soil acidification and eutrophication of surface water bodies (Ni et al., 2018).
Soil Acidification
Fertilizers with ammonia, especially urea, reduce the pH of the soil; this increases its acidity (Goulding, 2016). An acidic soil affects the normal functioning of the ecosystem, especially the biotic component. Also, high acid levels of soil negatively affect the biodiversity dwelling in it, this is detrimental to soil quality (Li et al., 2017). Farmers resolve the challenge by manipulating the soil with various chemicals and substances, thus the land eventually becomes degraded and undesirable for planting in the long run.
Nitrification inhibitors can also decrease the rate of nitrification by disrupting the activities of the bacteria leading to low soil pH (Alonso-Ayuso et al., 2016). Soil with low pH affects the uptake of nutrients such as calcium (Ca), magnesium (Mg), potassium (K), phosphorus (P) and molybdenum in plants (Shi et al., 2019). Inhibiting nitrification is believed to reduce agricultural production costs, pollution and climate change (Coskun et al., 2017). However, the detrimental effects of nitrification inhibition in increasing the volatilization of NH3 outweigh its benefits.
Eutrophication
Eutrophication is a global challenge that impairs the quality of marine and inland waters (Le Moal et al., 2019). Ammonia, nitrite, and nitrate are widely spread in natural waters, and they increase the occurrence of eutrophication (Wu S.-H. et al., 2018). The leached nitrogenous substances result in eutrophication and they affect surface and groundwater, causing algal blooms and loss of biodiversity (Beeckman et al., 2018). The occurrence of eutrophication often results in the production of cyanobacteria in rivers and waterways (Le Moal et al., 2019). This has led to the threatening of aquatic resources (Paerl, 2018).
The management and mitigation of the global expansion of toxic cyanobacterial harmful algal blooms (CyanoHABs) is a major challenge facing researchers and water resource managers (Paerl et al., 2019). In June 2016, St Lucie River in Florida had high concentrations of cyanotoxins that greatly exceeded WHO guidelines for consumable and recreational water (Metcalf et al., 2018). The degradation of the environment and abuse of agrochemicals has prompted researchers into searching for environmentally friendly ways of improving crop yields (Enagbonma and Babalola, 2019). Replacing synthetic fertilizers with a more environmentally friendly biofertilizer could limit the occurrence of algal blooms.
Global Warming
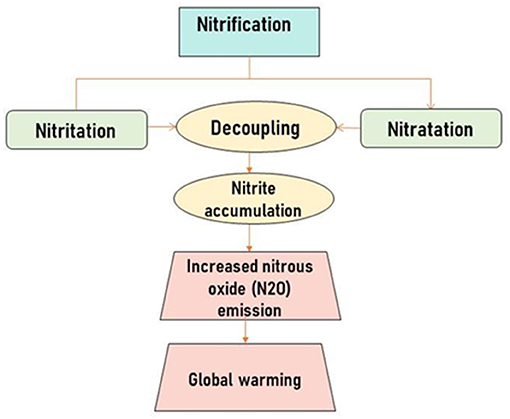
According to NOAA (2021), in 2020 the average temperature globally was 0.98oC warmer than in previous years. Modernized agriculture areas would contribute to global warming as they depend on fertilizer and other agrochemicals to maximize plant growth. The inputting of synthetic and organic nitrogenous materials in the soil by agroecosystems has contributed largely to anthropogenic N2O emissions (Charles et al., 2017). In a study carried out by Feng et al. (2019) in North Eastern China, chemical fertilizer was observed to increase nitrous oxide emission by increasing nitrifying and denitrifying microorganisms. Decoupling is usually observed in the two stages of nitrification (Heiss and Fulweiler, 2016). Accelerating soil nitrification with rapid microbial activity could cause decoupling (Figure 3) as a result of nitrite accumulation and a reduction in nitrogen use efficiency (NUE) of plants. This often leads to the escape of excess nitrite and other reactive nitrogen into the environment (nitrogen cascade). Nitrogen dioxides are greenhouse gases with 300 times greater global warming effect than carbon dioxide (Beeckman et al., 2018). NO is chemically reactive, the gas is involved in photochemical processes in the troposphere and acts as the major pioneer of ozone (O3) formation at ground level (Recio et al., 2019).
Nitrification and denitrification are closely related, and the types of gas used up and produced by the different processes pose a challenge to scientific researchers. Denitrification produces higher amounts of N2O when compared to nitrification, as nitrification simply produces the substrate on which denitrifying bacteria act (Siljanen et al., 2019). If this is so, then it would be more appropriate to inhibit denitrification process and not nitrification process for reducing global warming which is the goal of chemical nitrification inhibitors.
Effect of the Influencing Factors on Health
The influencing factors on the nitrification process have directly and indirectly affected biotic health. Biomass, crops and animals' health is affected at low pH (Zou et al., 2018). Acidic soil increases the bioavailability of heavy metals making the soil toxic for organisms (Ayangbenro et al., 2018). Low pH accumulates and increases the toxicity of aluminum (Al) and manganese (Shi et al., 2019). The metals accumulate in plants and biomagnify along the food chain, disrupting the physiology of animals. Furthermore, bacterial wilt disease develops more quickly and severely in acidic conditions, causing mechanical blockage of the water transport system in the plant (Li et al., 2017). Also, retarding the nitrification process by using nitrification inhibitors might effectively decrease the emission of N2O. However, more NH3 would be retained in the soil and its volatilization to the atmosphere would be increased (Fan et al., 2018; Ni et al., 2018). The emission of ammonia negatively affects the health of humans and vegetation (Ni et al., 2018).
Plants
The continual application of nitrification inhibitors in a farm can negatively affect the growth and development of plants. According to Rodrigues et al. (2018), plants can take up N-(n-butyl) thiophosphoric triamide (NBPT) urease inhibitor, which can affect their metabolism by influencing their endogenous urease. NBPT reduces the possibility of urea reaching the nickel atom. This causes transient yellowing of leaf tips as a result of urea toxicity soon after application (Cantarella et al., 2018). Nitrapyrin used with liquid fertilizers shows symptoms of phytotoxicity (Rodrigues et al., 2018). Bioaccumulation of DMPP in plant leaves showed signs of phytotoxicity and affects plant metabolism and hormone signaling (Rodrigues et al., 2019). Soil factors, management factors and crop types often determine the efficiency of nitrification inhibitors (Yang et al., 2016). Also, the hindrance in NO formation as a result of inhibiting the nitrification process could negatively affect the resistance of plants to disease (Yun et al., 2016). Plants produced are weak, disease-prone with less fruiting, accumulate salt and burn plant roots at high concentrations. Although NO can have a positive effect on plants; however, at high concentrations it poses potential damage to cellular structures under conditions of redox imbalance (Farnese et al., 2016). An excessive increase in the rate of nitrification which would produce high concentration of NO should also be checked.
Soil Organism
High concentration of nitrite is caused by varying factors which include heavy use of synthetic fertilizer and treatment of soil with biocidal chemicals (Siontorou and Georgopoulos, 2016). It can also accumulate in soil when oxidation of ammonia proceeds faster than the consumption of nitrate and when nitrate consumption is slower than its reduction (Heil et al., 2016). Nitrite at high concentrations is toxic to soil organisms. Nitrification inhibitors might have an undesirable effect on non-target soil organisms (Rodrigues et al., 2018).
Animals and Humans
Contamination of water bodies has been on the increase in emerging urban cities of developing countries (Fashae and Obateru, 2021). Fashae and Obateru (2021) observed a river located at Ibadan, Nigeria was polluted. This was partly attributed to agricultural activities. Also, groundwater with nitrate, the by-product of the soil nitrification process, is a global challenge, particularly in agrarian countries. The influence on the nitrification process has made it readily available in the environment. Nitrate dissolves easily in water, diffusing quickly toward the groundwater especially in sandy soil. Consumption of groundwater contaminated with nitrate can cause adverse health challenges. The health hazard of nitrate contamination varies for individuals in a population, and often it is in decreasing order from infants, children, adult females and adult males (Zhai et al., 2017). Infants and children are seen to be most susceptible to the contaminant.
Methemoglobinemia is a common physiological disorder in infants as a result of ingesting high levels of nitrate either through formula or water. The nitrate binds with methemoglobin and this affects the ability of the blood to react with oxygen, it often leads to death (Ward et al., 2018). Besides methemoglobinemia, other health effects associated with nitrate consumption include cancer of the colon, disease of the thyroid, neural tube defects, and adverse pregnancy outcomes (Ward et al., 2018). Nitrate can transform into N-nitroso compounds which have the potential to cause cancer, especially cancer of the colon (Schullehner et al., 2018).
Ammonia volatilization would increase with increasing urea-based fertilizer application. Ammonia has been associated with irritation of the eyes and respiratory system, and it also intensifies the production of particulate matter which damages the respiratory tissue (Naseem and King, 2018). Excessive amount of both ammonia and nitrate in the soil increases the occurrence of eutrophication. The toxins produced by cyanobacteria associated with eutrophication are known to be hepatotoxic, neurotoxic, irritating to the gastro intestine and cause contact dermatitis (Metcalf et al., 2018).
Nitrification inhibitors have been detected in open water environments and their effects on aquatic ecosystems and human health are still unclear (Qin and Lin, 2019). DCD has been discovered in milk products obtained from animals fed on plants cultivated with DCD (Rodrigues et al., 2018), and consumption of contaminated products is a potential health risk in humans (Ning et al., 2018). The health of people living in the region where nitrification inhibitors are continuously applied can be negatively affected (Yang et al., 2016).
Managing Nitrification Process
Recent agroecosystem practice depends heavily on chemicals, machinery, and other forms of management that dilapidate soil structuring and quality (Rillig and Lehmann, 2019). Additional expenditure on fertilizer is still increasing and encouraged in many areas even when the nitrogen fertilizer efficiency is not profitable. Management of nitrous oxide is best done locally and regionally since no best solution is permanent. Continual feedback from the agricultural system is necessary and immediate mitigation should be proffered where necessary (Coyne and Ren, 2017). An efficient nitrification program can be established by the stakeholders. They are to determine if and when nitrification is a challenge, which parameters are associated with the challenge and proffer solutions.
The rate of nitrification is observed to be positively correlated to the abundance of AOB (Tao et al., 2017). Monitoring it and factors that tend to overtly influence their growth would initiate a good procedure for management. Afterward, some organisms known to counteract the adverse effect of the nitrification and denitrification process could be used. Inoculating microbes into soil has been considered an environmentally sustainable means to increase production (Alori et al., 2017). Enebe and Babalola (2018), suggested integration of microorganisms with other mediums as biofertilizers. Modern biotechnologies can be used to decrease the contamination of food associated with organic and microbial biofertilizers.
Verma et al. (2018) suggested that agrochemicals produced should be incorporated with organic manures or biofertilizer, a system referred to as integrated plant nutrient management. However, Pathak et al. (2016) recommend a management system that eradicates chemicals by using microbial bioinoculants and organic manure. Organic fertilizer could be made from living organism, dead organism or their waste. They could directly or indirectly increase the supply of nitrogen in the soil naturally and in a stable way. According to Wang et al. (2018), Trichoderma viride inoculated into the topsoil increases the abundance of AOA and AOB. Phanerochaete chrysosporium and Bacillus thuringiensis can promote nitrate and ammonia supply in soil (Shang et al., 2017). Organic manure has been produced using the combination of microbial bioinoculants and vermicomposting (Arumugam et al., 2017).
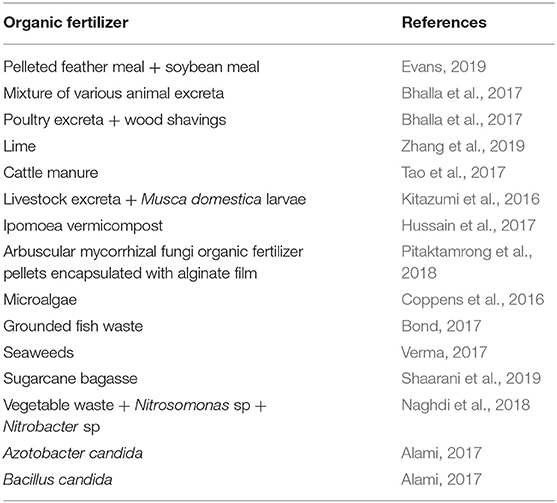
Biochar made from the burning of organic waste is a carbon-rich product used also as soil amendment. According to He et al. (2016), rice straw biochar causes an increase in nitrifiers activities and enhances the nitrification process. Zeolites are naturally occurring mineral compounds used in agriculture as soil conditioners. It is known to have a nutrient holding capacity, retain nitrogenous substances and gradually release them in a controlled manner (Jakkula and Wani, 2018). Zeolites have the potential to efficiently stabilize the nitrification process. Scientifically formulated organic fertilizers have been produced by researchers (Table 3). The acceptance of organic fertilizer for agricultural intensification should promote crop yields by improving nutrient storage, physical and chemical parameters of the soil (Cai et al., 2019). Applying the right amount of manure to plants when needed is also very crucial. This would require the agriculturist to know the growth stage when individual species of plants need nitrate the most and the quantity needed.
Biological nitrification inhibitors are produced by certain plants which include Brachiaria humidicola cv. (Byrnes et al., 2017), rice (Oryza sativa), sorghum (Sorghum bicolor), pearl millet (Pennisetum glaucum), wheat relative (Leymus racemosus), Neem (Azadirachta indica) (Cantarella et al., 2018) and peanut (O'Sullivan et al., 2016). Brachiaria humidicola is known to produce brachialactone (a powerful nitrification inhibitor) in its rooting systems and has the highest biological nitrifying inhibiting capacity established so far (Subbarao et al., 2017). 1,9-decanediol, a biological nitrification inhibitor in rice root exudates, was recently identified and proved to inhibit nitrification in bioassays using Nitrosomonas (Lu et al., 2019). The use of these biological nitrification inhibitors is better options if nitrification inhibition must be used. Also, since inhibition aims to retain nitrate and reduce nitrous oxide emission, then denitrification should be focused on to reduce the emission of greenhouse gas. Biological denitrification inhibition would be a better strategy to make nitrate more available in the soil for the use of plants (William et al., 2019).
Urban Agriculture
Cultivation of food in the cities, termed “urban agriculture,” is becoming popular and of paramount importance globally. If well managed in a sustainable way, it would be a good strategy for combating food security. McDougall et al. (2019), evaluated the stress of urban agriculture on the ecosystem in Sydney, Australia, it was observed that the environmental loading ratio was on the increase (5.82) with 14.66% renewable input. They concluded that the system was inefficient. However, with a better management strategy, there was a drastic improvement, with an environmental loading ratio of 1.32. The use of synthetic fertilizers and other agrochemicals should be discouraged. Alternatively, organic waste and self-composting that promote plant growth should be encouraged, and bioinoculants proven to be safe could be incorporated for efficiency. Considering proximity to human settlement, urban agriculturists should be trained, certified, and continuously monitored before and during agricultural practice. Failure to do this could result in the indiscriminate use of synthetic fertilizers and agrochemicals, thereby increasing the exposure of many to their environmental and health risks.
Limitations and Prospect
Intensification of agriculture has proved to be a threat to the security of food at the present and in the future (El Mujtar et al., 2019). Techniques in the agronomic management of soil should be improved. Research on soil nitrification process still has gaps to be covered and should be continuous. Considering the urgent need to manage the process as a result of its environmental and health effect, some of the prospects and suggestions for further research include:
1. Extensive environmental toxicological studies of the influencing factors should be carried out on agrochemicals and weighed with their intended benefit before approval for usage. Also, bioaccumulation and biomagnification along the food chain should be evaluated. Already, the use of agrochemicals (herbicide, fungicide, insecticide and synthetic fertilizer) is discouraged because of their negative effects in the long run. However, nitrification inhibitors are being encouraged and the usage is gradually increasing. Many of them are still under long term toxicological studies, they appear alright at first usage, but with time the negative effect is noticed. There should be a thorough investigation of its effects on the environment and health.
2. Production of scientifically formulated and modified organic fertilizer that can serve as an alternative to nitrogen-based fertilizer. Plants express inert proteins that could promote or suppress growth in plants when they are in contact with factors externally (Olanrewaju et al., 2019). Also, the fertility of soil needs to be considered when increasing crop production (Omomowo and Babalola, 2019). Fertilizers made from neem oil or cake can stabilize the nitrification process and increase nitrogen use efficiency (Sarwar et al., 2019). Using biotechnologically improved organic substances to immobilize nitrate for later gradual release into the soil environment would be beneficial.
3. Identifying and classifying nitrifying bacteria and archaea associated with specific crop plants species using new generation sequencing (NGS). The divergent thoughts of researchers on nitrification processes result from incomplete knowledge of the full range of its microbial network. During the 4th International Conference on nitrification, early career investigators were encouraged to manage nitrogen concentrations for the benefit of soil biodiversity (Klotz, 2016). Nitrifying bacteria and archaea can be biotechnologically worked on and their proliferation in soil environment can be optimized for the management of nitrification process.
4. Influenced nitrification and denitrification processes' contribution to global warming, and the use of micro bioinoculants as a management strategy. Without the influencing factors, the nitrification process's contribution to global warming would likely be minimal. However, yield may be low except with the use of bioinoculants, which would provide a gradual release of nutrient.
Conclusion
The process of nitrification affects global cycling of nitrogen and its derivatives, nitrogen use efficiency, ecosystem health and services. In unperturbed natural agroecosystems, only small amounts of nitrogen and its derivatives are lost. However, the present agroecosystem has highly increased the rate of nitrification beyond what the biotic system can absorb. They depend on synthetic fertilizers, nitrification inhibition and other agro-substance which influences the soil nitrification process. The effects of their influence are observed negatively on the environment and biotic health in general. Proper management and biotechnology need to be put in place to reduce and remediate their effect. Managing nitrification can be achieved by having an in-depth understanding of the process, initiating a well-planned monitoring strategy, using eco-friendly and sustainable materials to improve the availability of nitrogen in soils, deploying several strategies wholly and specifically for the various chemicals and organisms distributed within its system. Urban agriculture can be used to boost food production, but it must be managed properly to ensure environmental sustainability.
Author Contributions
OEA reviewed and wrote the first draft of the article. OOB conceptualized the work, secured funding, provided academic input, expertise to co-author, and commented on the manuscript at all stages and thoroughly critiqued the article. Both authors contributed to the article and approved the submitted version.
Funding
The study was funded by the National Research Foundation of South Africa through OOB from the grants (UID 123634, UID 132595).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
OOB appreciates the National Research Foundation of South Africa for the grant (UID123634, UID132595) that has supported research in her lab. OEA would like to thank the North-West University for its doctoral bursary.
References
Akram, R., Turan, V., Wahid, A., Ijaz, M., Shahid, M. A., Kaleem, S., et al. (2018). “Paddy land pollutants and their role in climate change,” in Environmental Pollution of Paddy Soils, eds M. Hashmi and A. Varma (Cham: Springer), 113–124. doi: 10.1007/978-3-319-93671-0_7
Alami, N. H.. (2017). Effect of yeast based biofertilizer combined with bacteria on mustard plant growth. Int. J. Appl. Biol. 1, 46–57. doi: 10.20956/ijab.v1i2.3093
Alonso-Ayuso, M., Gabriel, J. L., and Quemada, M. (2016). Nitrogen use efficiency and residual effect of fertilizers with nitrification inhibitors. Eur. J. Agron. 80, 1–8. doi: 10.1016/j.eja.2016.06.008
Alori, E. T., Glick, B. R., and Babalola, O. O. (2017). Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 8, 971. doi: 10.3389/fmicb.2017.00971
Amoo, A. E., and Babalola, O. O. (2017). Ammonia-oxidizing microorganisms: key players in the promotion of plant growth. J. Soil Sci. Plant Nutr. 17, 935–947. doi: 10.4067/S0718-95162017000400008
Angus, J.. (2001). Nitrogen supply and demand in Australian agriculture. Aust. J. Exp. Agric. 41, 277–288. doi: 10.1071/EA00141
Arumugam, K., Renganathan, S., Renganathan, K., Sharma, N. K., and Babalola, O. O. (2017). Enhancing the post consumer waste management through vermicomposting along with bioinoculumn. Int. J. Eng. Trends Technol. 44, 1–4. doi: 10.14445/22315381/IJETT-V44P235
Ayangbenro, A. S., Olanrewaju, O. S., and Babalola, O. O. (2018). Sulfate-reducing bacteria as an effective tool for sustainable acid mine bioremediation. Front. Microbiol. 9, 1986. doi: 10.3389/fmicb.2018.01986
Beeckman, F., Motte, H., and Beeckman, T. (2018). Nitrification in agricultural soils: impact, actors and mitigation. Curr. Opin. Biotechnol. 50, 166–173. doi: 10.1016/j.copbio.2018.01.014
Bhalla, S. K., Dicosola, G. J., Hooper, D. K., Randhava, S. S., and Laughlin, M. A. (2017). Process for Manufacturing Liquid and Solid Organic Fertilizer From Animal Waste. Philadelphia: Google Patents. Available online at: https://patents.google.com/patent/US9688584B2/en (accessed June 4, 2021).
Bond, Z. S.. (2017). Effects of Organic Fertilizer and Spatial Analysis of Phosphate at Babe+ Sage Farm Soils. Available online at: https://kb.gcsu.edu/src/2017/friday/24/ (accessed June 13, 2021).
Byrnes, R. C., Nùñez, J., Arenas, L., Rao, I., Trujillo, C., Alvarez, C., et al. (2017). Biological nitrification inhibition by Brachiaria grasses mitigates soil nitrous oxide emissions from bovine urine patches. Soil Biol. Biochem. 107, 156–163. doi: 10.1016/j.soilbio.2016.12.029
Cai, A., Xu, M., Wang, B., Zhang, W., Liang, G., Hou, E., et al. (2019). Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil Tillage Res. 189, 168–175. doi: 10.1016/j.still.2018.12.022
Cai, Z., Gao, S., Xu, M., and Hanson, B. D. (2018). Evaluation of potassium thiosulfate as a nitrification inhibitor to reduce nitrous oxide emissions. Sci. Total Environ. 618, 243–249. doi: 10.1016/j.scitotenv.2017.10.274
Cantarella, H., Otto, R., Soares, J. R., and De Brito Silva, A. G. (2018). Agronomic efficiency of NBPT as a urease inhibitor: a review. J. Adv. Res. 13, 19–27. doi: 10.1016/j.jare.2018.05.008
Charles, A., Rochette, P., Whalen, J. K., Angers, D. A., Chantigny, M. H., and Bertrand, N. (2017). Global nitrous oxide emission factors from agricultural soils after addition of organic amendments: a meta-analysis. Agric. Ecosys. Environ. 236, 88–98. doi: 10.1016/j.agee.2016.11.021
Coppens, J., Grunert, O., Van Den Hende, S., Vanhoutte, I., Boon, N., Haesaert, G., et al. (2016). The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 28, 2367–2377. doi: 10.1007/s10811-015-0775-2
Corrochano-Monsalve, M., González-Murua, C., Estavillo, J.-M., Estonba, A., and Zarraonaindia, I. (2020). Unraveling DMPSA nitrification inhibitor impact on soil bacterial consortia under different tillage systems. Agric. Ecosyst. Environ. 301, 107029. doi: 10.1016/j.agee.2020.107029
Coskun, D., Britto, D. T., Shi, W., and Kronzucker, H. J. (2017). Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 3, 1–10. doi: 10.1038/nplants.2017.74
Coyne, M. S., and Ren, W. (2017). Managing nitrous oxide emissions in agricultural fields. Plant Soil Sci. 6, 1–7. doi: 10.13023/PSSRR.2017.1
DeForest, J. L., and Otuya, R. K. (2020). Soil nitrification increases with elevated phosphorus or soil pH in an acidic mixed mesophytic deciduous forest. Soil Biol. Biochem. 142, 107716. doi: 10.1016/j.soilbio.2020.107716
Dier, M., Meinen, R., Erbs, M., Kollhorst, L., Baillie, C. K., Kaufholdt, D., et al. (2018). Effects of free air carbon dioxide enrichment (FACE) on nitrogen assimilation and growth of winter wheat under nitrate and ammonium fertilization. Global Change Biol. 24, 40–54. doi: 10.1111/gcb.13819
Duncan, E. G., O'sullivan, C. A., Simonsen, A. K., Roper, M. M., Treble, K., and Whisson, K. (2016). A composite guanyl thiourea (GTU), dicyandiamide (DCD) inhibitor improves the efficacy of nitrification inhibition in soil. Chemosphere 163, 1–5. doi: 10.1016/j.chemosphere.2016.07.103
El Mujtar, V., Muñoz, N., Mc Cormick, B. P., Pulleman, M., and Tittonell, P. (2019). Role and management of soil biodiversity for food security and nutrition; where do we stand? Global Food Secur. 20, 132–144. doi: 10.1016/j.gfs.2019.01.007
Elrys, A. S., Raza, S., Elnahal, A. S., Na, M., Ahmed, M., Zhou, J., et al. (2020). Do soil property variations affect dicyandiamide efficiency in inhibiting nitrification and minimizing carbon dioxide emissions? Ecotoxicol. Environ. Saf. 202, 110875. doi: 10.1016/j.ecoenv.2020.110875
Enagbonma, B. J., and Babalola, O. O. (2019). Environmental sustainability: a review of termite mound soil material and its bacteria. Sustainability 11, 1–10. doi: 10.3390/su11143847
Enebe, M. C., and Babalola, O. O. (2018). The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: a survival strategy. Appl. Microbiol. Biotechnol. 102, 7821–7835. doi: 10.1007/s00253-018-9214-z
Evans, J. M.. (2019). Pelleted feather Meal and Soybean Meal Based Organic Fertilizer. California: Google Patents. Available online at: https://patents.google.com/patent/US10167239B2/en (accessed June 4, 2021).
Fan, C., Li, B., and Xiong, Z. (2018). Nitrification inhibitors mitigated reactive gaseous nitrogen intensity in intensive vegetable soils from China. Sci. Total Environ. 612, 480–489. doi: 10.1016/j.scitotenv.2017.08.159
Farnese, F. S., Menezes-Silva, P. E., Gusman, G. S., and Oliveira, J. A. (2016). When bad guys become good ones: the key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front. Plant Sci. 7, 471–486. doi: 10.3389/fpls.2016.00471
Fashae, O. A., and Obateru, R. O. (2021). “Geospatial assessment of surface water pollution and industrial activities in Ibadan, Nigeria,” in Spatial Modeling and Assessment of Environmental Contaminants, eds P. K. Shit, P. P. Adhikary, and D. Sengupta (Cham: Springer), 189–211. doi: 10.1007/978-3-030-63422-3_12
Feng, X., Gao, H., Lal, R., Zhu, P., Peng, C., Deng, A., et al. (2019). Nitrous oxide emission, global warming potential, and denitrifier abundances as affected by long-term fertilization on Mollisols of Northeastern China. Arch. Agron. Soil Sci. 65, 1831–1844. doi: 10.1080/03650340.2019.1578959
Fu, Q., Abadie, M., Blaud, A., Carswell, A., Misselbrook, T. H., Clark, I. M., et al. (2020). Effects of urease and nitrification inhibitors on soil N, nitrifier abundance and activity in a sandy loam soil. Biol. Fertil. Soils 56, 185–194. doi: 10.1007/s00374-019-01411-5
Goulding, K.. (2016). Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 32, 390–399. doi: 10.1111/sum.12270
Hachiya, T., and Sakakibara, H. (2016). Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 68, 2501–2512. doi: 10.1093/jxb/erw449
He, L., Zhao, X., Wang, S., and Xing, G. (2016). The effects of rice-straw biochar addition on nitrification activity and nitrous oxide emissions in two Oxisols. Soil Tillage Res. 164, 52–62. doi: 10.1016/j.still.2016.05.006
Heil, J., Vereecken, H., and Brüggemann, N. (2016). A review of chemical reactions of nitrification intermediates and their role in nitrogen cycling and nitrogen trace gas formation in soil. Eur. J. Soil Sci. 67, 23–39. doi: 10.1111/ejss.12306
Heiss, E. M., and Fulweiler, R. W. (2016). Coastal water column ammonium and nitrite oxidation are decoupled in summer. Estuarine Coast. Shelf Sci. 178, 110–119. doi: 10.1016/j.ecss.2016.06.002
Hu, H.-W., Macdonald, C. A., Trivedi, P., Anderson, I. C., Zheng, Y., Holmes, B., et al. (2016). Effects of climate warming and elevated CO2 on autotrophic nitrification and nitrifiers in dryland ecosystems. Soil Biol. Biochem. 92, 1–15. doi: 10.1016/j.soilbio.2015.09.008
Hussain, N., Abbasi, T., and Abbasi, S. (2017). Toxic and allelopathic ipomoea yields plant-friendly organic fertilizer. J. Cleaner Prod. 148, 826–835. doi: 10.1016/j.jclepro.2017.01.176
Jakkula, V., and Wani, S. (2018). Zeolites: potential soil amendments for improving nutrient and water use efficiency and agriculture productivity. Sci. Rev. Chem. Commun. 8, 1–15.
Kiba, T., and Krapp, A. (2016). Plant nitrogen acquisition under low availability: regulation of uptake and root architecture. Plant Cell Physiol. 57, 707–714. doi: 10.1093/pcp/pcw052
Kitazumi, K., Nakano, Y., Polutova, Y., Nagae, K., Sekiya, R., and Yamawaki, H. (2016). Organic Fertilizer Production System. Tokyo: Google Patents.
Klotz, M. G.. (2016). Proposal to Support the 4th International Conference on Nitrification and Related Processes (ICoN4). Charlotte, NC: Univ. of North Carolina. doi: 10.2172/1330971
Koli, P., Bhardwaj, N. R., and Mahawer, S. K. (2019). “Agrochemicals: Harmful and Beneficial Effects of Climate Changing Scenarios,” in Climate Change and Agricultural Ecosystems, eds K. K. Choudhary, A. Kumar, and A. K. Singh (Sawston: Elsevier), 65–94. doi: 10.1016/B978-0-12-816483-9.00004-9
Kong, X., Eriksen, J., and Petersen, S. O. (2018). Evaluation of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) for mitigating soil N2O emissions after grassland cultivation. Agric. Ecosyst. Environ. 259, 174–183. doi: 10.1016/j.agee.2018.02.029
Le Moal, M., Gascuel-Odoux, C., Ménesguen, A., Souchon, Y., Étrillard, C., Levain, A., et al. (2019). Eutrophication: a new wine in an old bottle? Sci. Total Environ. 651, 1–11. doi: 10.1016/j.scitotenv.2018.09.139
Le, T. T. H., Fettig, J., and Meon, G. (2019). Kinetics and simulation of nitrification at various pH values of a polluted river in the tropics. Ecohydrol. Hydrobiol. 19, 54–65. doi: 10.1016/j.ecohyd.2018.06.006
Lehtovirta-Morley, L. E.. (2018). Ammonia oxidation: ecology, physiology, biochemistry and why they must all come together. Fed. Eur. Microbiol. Soc. Microbiol. Lett. 365, 1–9. doi: 10.1093/femsle/fny058
Li, H., Peng, T., Wang, Q., Wu, Y., Chang, J., Zhang, M., et al. (2017). Development of incompletely fused carpels in maize ovary revealed by miRNA, target gene and phytohormone analysis. Front. Plant Sci. 8, 463. doi: 10.3389/fpls.2017.00463
Li, Q., Guo, X., Lu, Y., Shan, G., and Huang, J. (2016). Impacts of adding FGDG on the abundance of nitrification and denitrification functional genes during dairy manure and sugarcane pressmud co-composting. Waste Manag. 56, 63–70. doi: 10.1016/j.wasman.2016.07.007
Li, Z., Zeng, Z., Tian, D., Wang, J., Fu, Z., Zhang, F., et al. (2020). Global patterns and controlling factors of soil nitrification rate. Glob. Change Biol. 26, 4147–4157. doi: 10.1111/gcb.15119
Liang, D., Zhang, Q., Zhang, W., Liu, L., Liang, H., Quirino, R. L., et al. (2019). Tunable thermo-physical performance of castor oil-based polyurethanes with tailored release of coated fertilizers. J. Cleaner Prod. 210, 1207–1215. doi: 10.1016/j.jclepro.2018.11.047
Liu, C., Liu, H., Liu, X., Zhang, Y., Wang, L., Guan, D., et al. (2020). Nitrification inhibitor 3, 4-dimethylpyrazole phosphate (DMPP) reduces N2O emissions by altering the soil microbial community in a wheat–maize rotation on the North China Plain. Eur. J. Soil Sci. 72, 1270–1291. doi: 10.1111/ejss.13017
Lu, Y., Zhang, X., Jiang, J., Kronzucker, H. J., Shen, W., and Shi, W. (2019). Effects of the biological nitrification inhibitor 1,9-decanediol on nitrification and ammonia oxidizers in three agricultural soils. Soil Biol. Biochem. 129, 48–59. doi: 10.1016/j.soilbio.2018.11.008
Mantelin, S., and Touraine, B. (2004). Plant growth-promoting bacteria and nitrate availability: impacts on root development and nitrate uptake. J. Exp. Bot. 55, 27–34. doi: 10.1093/jxb/erh010
McDougall, R., Kristiansen, P., and Rader, R. (2019). Small-scale urban agriculture results in high yields but requires judicious management of inputs to achieve sustainability. Proc. Natl. Acad. Sci. 116, 129–134 doi: 10.1073/pnas.1809707115
Meng, X., Li, Y., Yao, H., Wang, J., Dai, F., Wu, Y., et al. (2020). Nitrification and urease inhibitors improve rice nitrogen uptake and prevent denitrification in alkaline paddy soil. App. Soil Ecol. 154, 103665. doi: 10.1016/j.apsoil.2020.103665
Metcalf, J. S., Banack, S. A., Powell, J. T., Tymm, F. J., Murch, S. J., Brand, L. E., et al. (2018). Public health responses to toxic cyanobacterial blooms: perspectives from the 2016 Florida event. Water Policy 20, 919–932. doi: 10.2166/wp.2018.012
Naghdi, M., Cledon, M., Brar, S. K., and Ramirez, A. A. (2018). Nitrification of vegetable waste using nitrifying bacteria. Ecol. Eng. 121, 83–88. doi: 10.1016/j.ecoleng.2017.07.003
Naseem, S., and King, A. J. (2018). Ammonia production in poultry houses can affect health of humans, birds, and the environment—techniques for its reduction during poultry production. Environ. Sci. Pollut. Res. 25, 15269–15293. doi: 10.1007/s11356-018-2018-y
Nevison, C., Hess, P., Riddick, S., and Ward, D. (2016). Denitrification, leaching, and river nitrogen export in the community earth system model. J. Adv. Model. Earth Syst. 8, 272–291. doi: 10.1002/2015MS000573
Nguyen, L. T., Osanai, Y., Anderson, I. C., Bange, M. P., Braunack, M., Tissue, D. T., et al. (2018). Impacts of waterlogging on soil nitrification and ammonia-oxidizing communities in farming system. Plant Cell Physiol. 426, 299–311. doi: 10.1007/s11104-018-3584-y
Ni, K., Kage, H., and Pacholski, A. (2018). Effects of novel nitrification and urease inhibitors (DCD/TZ and 2-NPT) on N2O emissions from surface applied urea: an incubation study. Atmos. Environ. 175, 75–82. doi: 10.1016/j.atmosenv.2017.12.002
Ning, J., Ai, S., and Cui, L. (2018). Dicyandiamide has more inhibitory activities on nitrification than thiosulfate. PLoS ONE 13, e200598. doi: 10.1371/journal.pone.0200598
NOAA (2021). Global Climate Report. Available online at: https://www.ncdc.noaa.gov/sotc/global/2020 (accessed February 10, 2022).
Olanrewaju, O. S., Ayangbenro, A. S., Glick, B. R., and Babalola, O. O. (2019). Plant health: feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 103, 1155–1166 doi: 10.1007/s00253-018-9556-6
Omomowo, O. I., and Babalola, O. O. (2019). Bacterial and fungal endophytes: tiny giants with immense beneficial potential for plant growth and sustainable agricultural productivity. Microorganisms 7, 481. doi: 10.3390/microorganisms7110481
O'Sullivan, C. A., Fillery, I. R., Roper, M. M., and Richards, R. A. (2016). Identification of several wheat landraces with biological nitrification inhibition capacity. Plant Soil 404, 61–74. doi: 10.1007/s11104-016-2822-4
Paerl, H. W.. (2018). Mitigating toxic planktonic cyanobacterial blooms in aquatic ecosystems facing increasing anthropogenic and climatic pressures. Toxins 10, 76. doi: 10.3390/toxins10020076
Paerl, H. W., Havens, K. E., Hall, N. S., Otten, T. G., Zhu, M., Xu, H., et al. (2019). Mitigating a global expansion of toxic cyanobacterial blooms: confounding effects and challenges posed by climate change. Mar. Freshw. Res. 7, 579. doi: 10.1071/MF18392
Pathak, H., Jain, N., Bhatia, A., Kumar, A., and Chatterjee, D. (2016). Improved nitrogen management: a key to climate change adaptation and mitigation. Indian J. Fertil. 12, 151–162.
Pitaktamrong, P., Kingkaew, J., Yooyongwech, S., Cha-Um, S., and Phisalaphong, M. (2018). Development of arbuscular mycorrhizal fungi-organic fertilizer pellets encapsulated with alginate film. Eng. J. 22, 65–79. doi: 10.4186/ej.2018.22.6.65
Plett, D. C., Holtham, L. R., Okamoto, M., and Garnett, T. P. (2018). Nitrate uptake and its regulation in relation to improving nitrogen use efficiency in cereals. Semin. Cell Dev. Biol. 74, 97–104. doi: 10.1016/j.semcdb.2017.08.027
Qin, J., and Lin, C. (2019). Effects of micro-molar H2O2 on inhibiting soil nitrification. Geoderma 333, 145–148. doi: 10.1016/j.geoderma.2018.07.028
Quemada, M., Alonso-Ayuso, M., Castellano-Hinojosa, A., Bedmar, E. J., Gabriel, J. L., García González, I., et al. (2019). Residual effect of synthetic nitrogen fertilizers and impact on Soil Nitrifiers. Eur. J. Agron. 109, 125917. doi: 10.1016/j.eja.2019.125917
Recio, J., Alvarez, J. M., Rodriguez-Quijano, M., and Vallejo, A. (2019). Nitrification inhibitor DMPSA mitigated N2O emission and promoted NO sink in rainfed wheat. Environ. Pollut. 245, 199–207. doi: 10.1016/j.envpol.2018.10.135
Rillig, M. C., and Lehmann, A. (2019). Exploring the agricultural parameter space for crop yield and sustainability. New Phytol. 223, 517–519. doi: 10.1111/nph.15744
Rodrigues, J. M., Lasa, B., Aparicio-Tejo, P. M., González-Murua, C., and Marino, D. (2018). 3,4-Dimethylpyrazole phosphate and 2-(N-3,4-dimethyl-1H-pyrazol-1-yl) succinic acid isomeric mixture nitrification inhibitors: Quantification in plant tissues and toxicity assays. Sci. Total Environ. 624, 1180–1186. doi: 10.1016/j.scitotenv.2017.12.241
Rodrigues, J. M., Lasa, B., Betti, M., Fernández-Irigoyen, J., Santamaría, E., González-Murua, C., et al. (2019). Multi-omic and physiologic approach to understand Lotus japonicus response upon exposure to 3, 4 dimethylpyrazole phosphate nitrification inhibitor. Sci. Total Environ. 660, 1201–1209. doi: 10.1016/j.scitotenv.2019.01.047
Sahrawat, K.. (2008). Factors affecting nitrification in soils. Commun. Soil Sci. Plant Anal. 39, 1436–1446. doi: 10.1080/00103620802004235
Sarwar, N., Wasaya, A., Saliq, S., Reham, A., Farooq, O., Mubeen, K., et al. (2019). Use of natural nitrogen stabilizers to improve nitrogen use efficiency and wheat crop yield. Cercetari Agronomice in Moldova 52, 107–115. doi: 10.2478/cerce-2019-0011
Schaefer, S. C., and Hollibaugh, J. T. (2017). Temperature decouples ammonium and nitrite oxidation in coastal waters. Environ. Sci. Technol. 51, 3157–3164. doi: 10.1021/acs.est.6b03483
Schullehner, J., Hansen, B., Thygesen, M., Pedersen, C. B., and Sigsgaard, T. (2018). Nitrate in drinking water and colorectal cancer risk: A nationwide population-based cohort study. Int. J. Cancer 143, 73–79. doi: 10.1002/ijc.31306
Seddik, W., Osman, M. A., and Kenawy, M. H. (2019). Physico-chemical behavior of natural minerals along with synthetic soil conditioners on nutritional status and yield productivity. J. Soil Sci. Agric. Eng. 10, 397–403. doi: 10.21608/jssae.2019.53685
Shaarani, S. M., Mokhtar, N. J., Arshad, Z. I. M., Man, R. C., Mudalip, S. K. A., and Sulaiman, S. Z. (2019). “Co-composting landfill leachate with sugarcane bagasse for biofertilizer production,” in AIP Conference Proceedings. Pahang. p. 1–9. doi: 10.1063/1.5117092
Shang, C., Chen, A., Chen, G., Li, H., Guan, S., and He, J. (2017). Microbial biofertilizer decreases nicotine content by improving soil nitrogen supply. Appl. Biochem. Biotechnol. Genetic Eng. Rev. 181, 1–14. doi: 10.1007/s12010-016-2195-4
Shi, R.-Y., Ni, N., Nkoh, J. N., Li, J.-Y., Xu, R.-K., and Qian, W. (2019). Beneficial dual role of biochars in inhibiting soil acidification resulting from nitrification. Chemosphere 234, 43–51. doi: 10.1016/j.chemosphere.2019.06.030
Siljanen, H. M. P., Alves, R. J. E., Ronkainen, J. G., Lamprecht, R. E., Bhattarai, H. R., Bagnoud, A., et al. (2019). Archaeal nitrification is a key driver of high nitrous oxide emissions from arctic peatlands. Soil Biol. Biochem. 137, 1–10. doi: 10.1016/j.soilbio.2019.107539
Siontorou, C. G., and Georgopoulos, K. N. (2016). A biosensor platform for soil management: the case of nitrites. J. Cleaner Prod. 111, 133–142. doi: 10.1016/j.jclepro.2015.07.038
Subbarao, G., Arango, J., Masahiro, K., Hooper, A., Yoshihashi, T., Ando, Y., et al. (2017). Genetic mitigation strategies to tackle agricultural GHG emissions: the case for biological nitrification inhibition technology. Plant Sci. 262, 165–168. doi: 10.1016/j.plantsci.2017.05.004
Tao, R., Wakelin, S. A., Liang, Y., and Chu, G. (2017). Response of ammonia-oxidizing archaea and bacteria in calcareous soil to mineral and organic fertilizer application and their relative contribution to nitrification. Soil Biol. Biochem. 114, 20–30. doi: 10.1016/j.soilbio.2017.06.027
Tarre, S., and Green, M. (2004). High-rate nitrification at low pH in suspended-and attached-biomass reactors. Appl. Environ. Microbiol. 70, 6481–6487. doi: 10.1128/AEM.70.11.6481-6487.2004
Taylor, A. E., Giguere, A. T., Zoebelein, C. M., Myrold, D. D., and Bottomley, P. (2017). Modeling of soil nitrification responses to temperature reveals thermodynamic differences between ammonia-oxidizing activity of archaea and bacteria. ISME J. 11, 896–908. doi: 10.1038/ismej.2016.179
Trcek, B., Žigon, D., Zidar, V., and Auersperger, P. (2018). The fate of benzotriazole pollutants in an urban oxic intergranular aquifer. Water Res. 131, 264–273. doi: 10.1016/j.watres.2017.12.036
Vazquez, E., Benito, M., Espejo, R., and Teutscherova, N. (2019). Effects of no-tillage and liming amendment combination on soil carbon and nitrogen mineralization. Eur. J. Soil Biol. 93, 103090. doi: 10.1016/j.ejsobi.2019.103090
Verma, N.. (2017). Effect of Organic and Inorganic Sources of Nitrogen With Biofertilizer on Growth, Yield and Quality of Forage Sorghum Sorghum bicolor (L.) Moench. Raipur: Indira Gandhi Krishi Vishwavidhyalaya. Available online at: http://krishikosh.egranth.ac.in/handle/1/5810032110 (accessed June 14, 2021).
Verma, N., Chaudhary, S., and Goyal, S. (2018). Long term effects of inorganic fertilizers and organic amendments on ammonification and nitrification activity of soils under cotton-wheat cropping system. Int. J. Curr. Microbiol. App. Sci 7, 718–724. doi: 10.20546/ijcmas.2018.704.080
Wang, X., Xu, S., Wu, S., Feng, S., Bai, Z., Zhuang, G., et al. (2018). Effect of Trichoderma viride biofertilizer on ammonia volatilization from an alkaline soil in Northern China. J. Environ. Sci. 66, 199–207. doi: 10.1016/j.jes.2017.05.016
Wang, Y., Wang, J., Zhao, X., Song, X., and Gong, J. (2016). The inhibition and adaptability of four wetland plant species to high concentration of ammonia wastewater and nitrogen removal efficiency in constructed wetlands. Bioresour. Technol. 202, 198–205. doi: 10.1016/j.biortech.2015.11.049
Wang, Z., Liu, L., Chen, Q., Wen, X., Liu, Y., Han, J., et al. (2017). Conservation tillage enhances the stability of the rhizosphere bacterial community responding to plant growth. Agron. Sustain. Dev. 37, 38–44. doi: 10.1007/s13593-017-0454-6
Ward, M. H., Jones, R. R., Brender, J. D., De Kok, T. M., Weyer, P. J., Nolan, B. T., et al. (2018). Drinking water nitrate and human health: an updated review. Int. J. Environ. Res. Public Health 15, 1–31. doi: 10.3390/ijerph15071557
William, G., Piola, F., Burlet, A., Mathieu, C., Nardy, M., Poussineau, S., et al. (2019). Biological denitrification inhibition (BDI) in the field: a strategy to improve plant nutrition and growth. Soil Biol. Biochem. 136, 1–9. doi: 10.1016/j.soilbio.2019.06.009
Woodward, E. E., Hladik, M. L., and Kolpin, D. W. (2016). Nitrapyrin in streams: the first study documenting off-field transport of a nitrogen stabilizer compound. Environ. Sci. Technol. Lett. 3, 387–392. doi: 10.1021/acs.estlett.6b00348
Wu, D., Zhao, Z., Han, X., Meng, F., Wu, W., Zhou, M., et al. (2018). Potential dual effect of nitrification inhibitor 3,4-dimethylpyrazole phosphate on nitrifier denitrification in the mitigation of peak N2O emission events in North China Plain cropping systems. Soil Biol. Biochem. 121, 147–153. doi: 10.1016/j.soilbio.2018.03.010
Wu, S.-H., Huang, B.-H., Huang, C.-L., Li, G., and Liao, P.-C. (2018). The Aboveground vegetation type and underground soil property mediate the divergence of soil microbiomes and the biological interactions. Microb. Ecol. 75, 434–446 doi: 10.1007/s00248-017-1050-7
Yang, M., Fang, Y., Sun, D., and Shi, Y. (2016). Efficiency of two nitrification inhibitors (dicyandiamide and 3, 4-dimethypyrazole phosphate) on soil nitrogen transformations and plant productivity: a meta-analysis. Sci. Rep. 6, 1–10. doi: 10.1038/srep22075
Yoneyama, T., Tanno, F., Tatsumi, J., and Mae, T. (2016). Whole-plant dynamic system of nitrogen use for vegetative growth and grain filling in rice plants (Oryza sativa L.) as revealed through the production of 350 grains from a germinated seed over 150 days: a review and synthesis. Front. Plant Sci. 7, 1–13. doi: 10.3389/fpls.2016.01151
Youssef, G., El-Etr, W., Zein El-Abdeen, H., and El-Farghal, W. (2019). Evaluation of some synthetic soil conditioners and nitrogen rates on nitrogen use efficiency by maize-wheat crops system in calcareous soil. J. Soil Sci. Agric. Eng. 10, 1–11. doi: 10.21608/jssae.2019.36660
Yun, B. W., Skelly, M. J., Yin, M., Yu, M., Mun, B. G., Lee, S. U., et al. (2016). Nitric oxide and S-nitrosoglutathione function additively during plant immunity. New Phytol. 211, 516–526. doi: 10.1111/nph.13903
Zhai, Y., Zhao, X., Teng, Y., Li, X., Zhang, J., Wu, J., et al. (2017). Groundwater nitrate pollution and human health risk assessment by using HHRA model in an agricultural area, NE China. Ecotoxicol. Environ. Saf. 137, 130–142. doi: 10.1016/j.ecoenv.2016.11.010
Zhang, J., Bei, S., Li, B., Zhang, J., Christie, P., and Li, X. (2019). Organic fertilizer, but not heavy liming, enhances banana biomass, increases soil organic carbon and modifies soil microbiota. Appl. Soil Ecol. 136, 67–79. doi: 10.1016/j.apsoil.2018.12.017
Zhang, M., Wang, W., Bai, S. H., Zhou, X., Teng, Y., and Xu, Z. (2018a). Antagonistic effects of nitrification inhibitor 3,4-dimethylpyrazole phosphate and fungicide iprodione on net nitrification in an agricultural soil. Soil Biol. Biochem. 116, 167–170. doi: 10.1016/j.soilbio.2017.10.014
Zhang, M., Wang, W., Tang, L., Heenan, M., and Xu, Z. (2018b). Effects of nitrification inhibitor and herbicides on nitrification, nitrite and nitrate consumptions and nitrous oxide emission in an Australian sugarcane soil. Biol. Fertility Soils 54, 697–706. doi: 10.1007/s00374-018-1293-6
Zhang, M., Wang, W., Zhang, Y., Teng, Y., and Xu, Z. (2017). Effects of fungicide iprodione and nitrification inhibitor 3, 4-dimethylpyrazole phosphate on soil enzyme and bacterial properties. Sci. Total Environ. 599, 254–263. doi: 10.1016/j.scitotenv.2017.05.011
Keywords: agricultural intensification, agroecosystems, environmental challenge, nitrification inhibitor, nitrifying microorganism, synthetic fertilizer
Citation: Ayiti OE and Babalola OO (2022) Factors Influencing Soil Nitrification Process and the Effect on Environment and Health. Front. Sustain. Food Syst. 6:821994. doi: 10.3389/fsufs.2022.821994
Received: 25 November 2021; Accepted: 25 February 2022;
Published: 24 March 2022.
Edited by:
Adeyemi Oludapo Olusola, University of Ibadan, NigeriaReviewed by:
Israel Ropo Orimoloye, University of Fort Hare, South AfricaRotimi Obateru, Adekunle Ajasin University, Nigeria
Copyright © 2022 Ayiti and Babalola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olubukola Oluranti Babalola, b2x1YnVrb2xhLmJhYmFsb2xhQG53dS5hYy56YQ==
†ORCID: Oluwatobi Esther Ayiti orcid.org/0000-0002-6164-8965
Olubukola Oluranti Babalola orcid.org/0000-0003-4344-1909
 Oluwatobi Esther Ayiti†
Oluwatobi Esther Ayiti† Olubukola Oluranti Babalola
Olubukola Oluranti Babalola