- 1Department of Botany, Rampurhat College, Birbhum, India
- 2Department of Botany and Forestry, Vidyasagar University, Midnapore, India
The yield of many crops benefits from pollinating insects; thus, recently reported declines in pollinator abundance and diversity are concerning for global food production. The pollinator dependency and amount of yield enhancement may vary according to crop species and geographical location. Fennel (Foeniculum vulgare) is an important spice crop cultivated in Indian states. However, comprehensive knowledge about pollination demand and yield enhancement potential of managed bees is still unknown. Here, we conducted a replicated study in two successive years (2020 and 2021) in West Bengal by combining pollinator surveys, pollinator-exclusion experiments, and field manipulation on fennel, which quantifies the impacts of supplemental stingless bees (Tetragonula iridipennis) pollination on native pollinators and crop yield. The crop species attracted many insect species belonging to diverse groups. Among those, important native pollinators (based on “approximate pollination value”) were Apis cerana, Apis dorsata, Apis florea, and Oxybelus furculatus in open condition (i.e., without field manipulation and in the absence of managed bees). We derived the coefficient of pollination deficit (D) from the fruit set percentages in open and manual cross-pollination treatments. The obtained value (D = 0.18) implies that the crop species have pollen transfer limitations, resulting in retardation of crop yield. From field manipulation with managed stingless bee colonies, the abundance of visitors (especially stingless bee foragers) on fennel increased (without altering the foraging activity of other native pollinators), thereby fruit set and crop yield increased by about 14.89 and 19.31%, respectively. Native managed stingless bees had no negative impacts on other native unmanaged species and can be promoted as complementary and short-term means of boosting yields and improving agricultural sustainability.
Introduction
Insect pollination of flowering plants is crucial in terrestrial environments, and it provides emergent ecosystem services for human beings (Garibaldi et al., 2011; Schulp et al., 2014), especially on crop production. About 75% of agricultural crop species depend on animal pollination, and cross-pollination through insects ensures more significant benefit via developing higher fruit quantity and/or quality (Klein et al., 2007; Classen et al., 2014; Wietzke et al., 2018). Bees are the most important pollinators recognized worldwide (Greenleaf and Kremen, 2006; Winfree et al., 2007; Tucker and Rehan, 2018; Patel et al., 2021). However, non-bee insects also act as important pollinators of several crop plants (Rader et al., 2015; Cusser et al., 2021). During recent decades, land-use change, habitat fragmentation, and use of agrochemicals are important anthropogenic factors responsible for the decline of insect pollinators (Klein et al., 2007; Ollerton et al., 2014). One potential consequence of declining populations of pollinators is a decline in the rate of pollination, which will lead to lower seed or fruit set, lower plant regeneration rates which exhibit knock-on effects to the animals that rely on plants and their products for food.
Fennel (Foeniculum vulgare Mill.) is an important umbelliferous spice crop grown widely in Indian states. Inflorescence of the crop species is a compound-umbel comprising many umbellets. Each umbellet carries several hermaphrodite flowers and exhibits the protandrous type of dichogamy (Koul et al., 1996). Protandry is strong at the level of flower and umbel, leading to the separation of male and female phases at those levels. The phases overlap in each individual and thereby facilitate both geitonogamy and xenogamy. The crop depends on insect pollinators; and in the absence of a biotic pollinator (i.e., within the bagged umbels), seed set is extremely low—about 5.49% (Koul et al., 1996). Fennel flowers are visited by several insect groups like honeybees (Mishra, 1995; Layek and Karmakar, 2016, 2018b; Layek et al., 2020a), stingless bees (Layek and Karmakar, 2018a), and syrphid flies (Sagar, 1981; Baswana, 1984), and these visitors may provide pollination services for the crop species. For enhancing the yield of entomophilous (insect-mediated pollination) crops, using wild and managed bee species and improving crop field environment (through habitat management and pesticide stewardship) are effective strategies (Isaacs et al., 2017). The utilization of western honeybees (Apis mellifera L.) in crop pollination is well-established globally (Carreck et al., 1997; Kumar and Singh, 2017; Rollin and Garibaldi, 2019). The dependence on a single pollinator species, especially one that has suffered from disease and colony collapse (Lee et al., 2015; Van Engelsdorp et al., 2017), is precarious. Furthermore, the western honeybee is non-native to several tropical countries, including India. Non-native species can reach high abundance and dominate in native environments. The dominance of the western honeybees has negative consequences for the abundance and species richness of native pollinators (Garibaldi et al., 2021). They can also alter the foraging behavior of native pollinators (Layek et al., 2021b). Therefore, using alternative managed pollinators makes agriculture more resilient. In this sense, we can utilize stingless bees for supplemental pollination services on crops that receive stingless bee visits (Layek and Karmakar, 2018a; Bisui et al., 2021). Unfortunately, the installation of stingless bee colonies for crop pollination is limited, maybe due to the unavailability of managed colonies and insufficient data about their foraging behavior. Again, installing alien stingless bees may risk biological introduction and invasion events (Dos Santos et al., 2021). The Indian stingless bee (Tetragonula iridipennis Smith) is the most common native bee in almost all the Indian states, including West Bengal. Their nesting biology (Danaraddi et al., 2009; Layek and Karmakar, 2018a), floral resources (Layek and Karmakar, 2018a; Bisui et al., 2019), and foraging activities (Devanesan et al., 2002; Danaraddi et al., 2011; Layek and Karmakar, 2018a) were also determined. The bee species shows polylectic foraging habits with a high floral constancy (Layek and Karmakar, 2018a). Therefore, we have an immense opportunity to utilize this stingless bee for crop pollination to increase the fruit quality and/or quantity.
To assess the yield enhancement potential of Indian stingless bees on fennel through supplementary pollination services, we carried out a novel combination of the studies on floral visitors' richness, native pollinators, and impacts of stingless bee colonies on native pollinators and the yield of fennel. Here, we determine (1) the native floral visitors and their importance as a pollinator on fennel; (2) fruit set in different pollination treatments and pollination deficit of the crop species within the study area; (3) the impacts of the application of managed stingless bee colonies on native pollinators, if any; and (4) the yield enhancement potential of Indian stingless bee on fennel.
Materials and Methods
Experimental Site
The experiment was conducted in 2 successive years (i.e., 2020–2021) on agricultural fields of farmers in the Jenadihi village (latitude: 23.4468° N, longitude: 87.0449° E) in the Bankura district of West Bengal. We selected fennel fields in two study sites (I and II) separated by about 1 km, beyond the flight distance of the chosen stingless bee species (Layek et al., 2021a). In that sense, when we use supplemental stingless bee pollination in one study site, bees of this site cannot affect the other study site. Five fields (I-1, I-2, I-3, I-4, and I-5) were selected from study site I and three (II-1, II-2, and II-3) in study site II for each year. The selected fields are small (average area of 0.0095 ± 0.0023 hectares; length: 10.68 ± 1.36 m, breadth: 8.84 ± 1.06 m) and almost rectangular. In each site, fields are closely situated side by side or partitioned by one to three fields of uncultivated or cultivated by other crops like giant pumpkin, lentil, onion, etc. All the selected fields are characterized by lateritic soil and similar climatic conditions. The cultivating time of the fennel is February to April. The temperature and relative humidity (RH) gradually increase from February (average day temperature: 24°C, RH = 55%) to April (average day temperature: 28°C, RH = 62%).
In study site II, we installed three stingless bee (Tetragonula iridipennis) colonies, namely colony A, B, and C on the field edge of II-1, II-2, and II-3, respectively. The colonies were installed at nearly the middle point of the shorter edges of the three fields before the flowering period of the crop species (oldest flowers buds are close to mature) for each year. The height of the hives was almost at flower level (about 4 ft height from the ground level), and the orientation of the nest entrance was to the fennel fields. The distances among the installed colonies were about 9.29 m (between A and B), 9.18 m (B and C), and 18.47 m (A and C).
Data Collection About Native Floral Visitors
Three fennel fields (I-3, I-4, and I-5) of site I (excluding I-1 and I-2, the fields are utilized for the pollinator-exclusion experiment) were utilized to record the native floral visitors. We observed the visitors at different timeslots (6.00–8.00 h, 8.00–10.00 h, 10.00–12.00 h, 12.00–14.00 h, 14.00–16.00 h, and 16.00–18.00 h) during peak flowering time of fennel (i.e., mid-February–mid-March). We carried out field surveys on the sunny days at 3 days intervals in general. Floral visitors were identified in the field. A few insect species (those are not identified by us) were captured with the help of an insect net from field I-5. We put the entrapped insects into glass vials containing a piece of cotton (soaked with ethyl acetate). Later, we sent them to entomologists (at the ZSI, Kolkata) for identification.
The data about foraging behavior (including abundance, type of visits, collection of floral rewards, visitation rate, etc.) were collected on a separate sampling day from the surveys in which we caught the visitors for identification (as during catching, abundance, and foraging behavior of visitors may change). The data were taken separately on the two study sites, as we can compare the collected data between site I (i.e., without the managed bee) and site II (i.e., with managed bees) to evaluate the impacts of the managed stingless bee species.
To measure the abundance of the floral visitors, we recorded the number of encountered individuals of different insect species in 1 m2 field area (i.e., plot) per 5 min over the six time slots and repeated this 10 times each year (N = 6 × 10 × 2, for each study site). The plots were selected at different regions (near the boundary and toward the center) of the crop fields (I-3, I-4, and I-5 of the site I and II-1, II-2, and II-3 of the site II). Then, we calculated relative abundance (RA) of each insect species as follows:
For each flower-visitor species, the type of visit—legitimate (visitor touches the reproductive parts of the flower during visitation) or illegitimate (does not touch the reproductive parts of the flower)—and their collected floral reward (nectar, pollen, or floral tissue) were recorded. To estimate the flower visitation rate of the visitors, we considered the number of umbellets visited per minute rather than individual flowers. Because fennel flowers are tiny and clustered in compound-umbel, most visitors touch more than one flower in a single visit. We targeted an individual of an insect species visiting the fennel flowers and watched for 1 min. When the targeted individual just started contact with an umbellet (to collect rewards from the fennel flowers), we began to record the time with a stopwatch. We counted the number of umbellets visited in 1 min. We repeated the process 30 times to each dominant insect species. We also recorded the foraging time (i.e., the amount of time spent per visit on an umbellet) for each dominant flower-visitor species in open condition (n = 30 for each species).
To count the number of pollen grains attached to the visitor's body surface, we randomly entrapped the visitors during peak activity time (i.e., 10.00–12.00 h) with an insect net. We utilized field I-5 of site I (for open pollination) and field II-3 of site II (for managed bee pollination) for this purpose. We captured the insects (n ≥ 10 for each dominant species; we did not consider the insect species for which sample size was <10) gently by forceps, and hind legs were amputated to separately consider the body surface pollens and the pollens on corbiculae or scopae (because we think that the amount of stored pollen loads on corbiculae or scopae does not have a proportionate role in pollination like other parts of the insect's body surface). Each specimen (insect without hind legs) was put into a glass vial. The pollen loads on corbiculae (in the case of honeybees, stingless bees) were separated from the hind legs and put into a glass vial (as paired—collected from an individual). Then, we added 1.0–6.0 mL (depending on the body size of the captured visitor) of 0.4 M sucrose solution to the vials containing the insect body and 1.0 mL to the vials containing corbicular pollen loads. After vigorously shaking the vials containing the insect's body, 10 μL of solution from the vial was taken by micropipette on a clear glass slide and the number of pollen grains suspended in the solution were counted with the help of a light microscope. We repeated this procedure five times for each sample and estimated the average number of pollen grains in 10 μL and then the total number of pollen grains (on the body surface) in accordance with the initial volume of the solution (Nikkeshi et al., 2019). The number of pollen grains per paired corbicular loads was estimated using a hemocytometer. After shaking the vial containing pollen loads, 1 μL of each sample was charged into the counting chamber of a hemocytometer. Then, we counted the number of pollen grains (present within the large square for WBC) with the help of a light microscope at 10 × 15 magnification. The total number of pollen grains per sample (i.e., per paired corbicular pollen loads) was calculated as follows:
Some visitors carried pollen grains of other crop species cultivated in neighboring fields (Figure 1). We considered only fennel pollen, and ignored others pollen types, if present.

Figure 1. Halictus acrocephalus visiting fennel flowers with other type (Cucurbita maxima) of pollen grains on the body surface.
As most visitors touch more than one flower on a single visit (due to the smaller size of flowers and arranged in a compound-umbel), it is not easy to measure the exact impact (pollination efficiency) of a single visit performed by the visitors. Here, we postulated a combined parameter, namely “approximate pollination value (APV)” to determine the important native pollinators of fennel. The APV was based on three parameters— relative abundance, flower visitation rate, and amount of pollen carried by a visitor; and it was calculated for a flower-visiting species as follows:
Here, we applied the number of umbellets visited per minute as flower visitation rate. The “pollen carrying value (PCV)” of floral visitors derived from the summation of two components— (i) PCV 1 (i.e., body surface pollen content excluding pollen loads on corbiculae or scopae, ranged from 0 to 5) and (ii) PCV 2 (i.e., corbicular or scopal pollen content, ranged from 0 to 3) (Table 1).
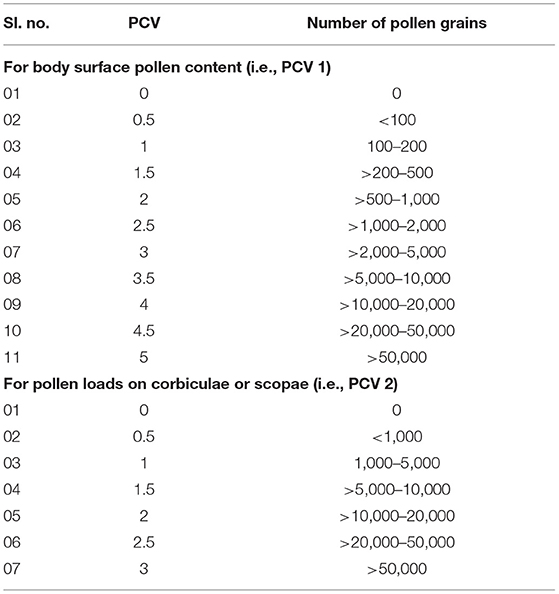
Table 1. Pollen carrying value (PCV) is based on the number of pollen grains on visitor's body surface (excluding pollen loads on corbiculae or scopae) and pollen loads on corbiculae or scopae.
Fruit Set and Pollination Deficiency
We estimated the fruit set in three different pollination treatments, namely: (1) pollinator-exclusion treatment, (2) open-pollination (without field manipulation or without managed bees—allowed unrestricted visitation by native floral visitors), and (3) manually cross-pollination treatment. In site I, fennel field I-1 and I-2 are used for pollinator-exclusion treatment, I-3 and I-4 for open-pollination, and I-5 used for manual cross-pollination treatments. For each of these pollination treatments, we randomly selected 10 flowers (from the above-mentioned fennel fields) on a sampling day, and repeated 10 times each year of 2020 and 2021 (N = 10 × 10 × 2, for each pollination treatment). The selected flowers were marked by being given black dots of ink on the pedicel. Fruit set percentages were recorded for these treatments.
For pollinator-exclusion treatment, just before the flowering period (~7 days remained to start the opening of flowers), we sprayed plants with endosulfan 35 EC (0.07%) to remove the insects. After half an hour of spraying, plants were caged with nylon nets for several days up to maturation of fruits. At regular intervals of 3 days, we mildly sprayed the insecticide on the net to repel flower visitors. For manual cross-pollination experiments, we surveyed the fields in the early morning (at 4:00–5:00 a.m.), and randomly selected 10 flowers (in the incomplete opened state, before anther dehiscence), and removed the stamens of the chosen flowers. Then, we bagged the emasculated flowers containing compound-umbels with a fine nylon net. Again, the net was removed at about 10:00 h, and emasculated flowers were manually pollinated with the pollens of the dehisced anthers of different individuals. For pollen transfer, we have chosen a few stamens with dehisced anther with many pollen grains (by closed observation with 10 × lens). We took a stamen with small forceps by holding the filament. The anther touched gently to the stigmatic surface, as sufficient pollens can transfer from the anther without damaging the stigmatic part. Then, we re-enclosed the selected umbels immediately to prevent visitation by floral visitors until the specified flowers lose stigmatic receptivity (apparently determined by observing the senescence of petals and stamens; and dried out of the stigmatic surface).
After obtaining the data of fruit set in open-pollination and manual cross-pollination treatments, we measured a coefficient of pollination deficit (D) by using the method of Layek et al. (2020b, 2021b) as follows:
The value of the coefficient of pollination deficit (D) ranges between 0 and 1. The higher value of D implies more pollination deficiency. According to the value of D, we categorized the crop species under one of the following classes: (1) high pollination deficit (D > 0.5), (2) medium pollination deficit (D = 0.3–0.5), (3) low pollination deficit (0.3 > D ≥ 0.1), and (4) negligible pollination deficit (D < 0.1).
The number of fruits (nearly to mature) per compound-umbel was counted for the pollinator-exclusion and open-pollination treatments. After maturation of the fruits, weights of dried fruits were also taken. For weighing, 100 fruits were taken together (constituting a group or lot), as fennel fruits are small and difficult to weigh individually. In this manner, 10 lots were taken for each system to weigh. Additionally, we recorded yield in terms of the fruit weight per plant (n = 30 in each system) and per hectare field area for these treatments. For estimation of yield per hectare, we selected an area of 5 m × 5 m (n = 3 in each treatment per year) for harvesting the ripened fruits, and the obtained values were multiplied by 400 to convert the yield for hectare.
Impact of Application of Stingless Bees on Native Pollinators
During the peak flowering period of the fennel, the species richness of floral visitors in study site II (i.e., with managed stingless bees and other native pollinators) was recorded. Additionally, the abundance, visitation rate, foraging time, and the number of pollen grains carried by the floral visitors were recorded similarly to that of open-pollination treatment.
Impact of Application of Stingless Bees on Crop Yield
We repeated the same experimental setup for the 2 years, 2020 and 2021. During the peak flowering period, we randomly selected 10 flowers from the two fennel fields (II-1, II-2) of study site II, marked by small black dots of ink on their petiole and repeated 10 times each year. Fruit set (%) was recorded. Later, the number of fruits (nearly to mature) per compound-umbel (n = 30), weights of ripened dried fruits (10 lots; 100 fruits per lot) were recorded. The crop yield was estimated similarly to that of pollinator-exclusion and open-pollination treatments, by estimating the fruit weight per plant and per hectare field area.
Statistical Analysis
Statistical analyses of the data were conducted using SPSS (v. 16.0) and Microsoft Excel software packages. We performed “Shapiro–Wilk” and “Kolmogorov-Smirnov” tests to check whether the data are normally distributed. In the case of normally distributed data, we followed parametric tests. To examine whether the number of pollen grains on the visitor's body surfaces differed among the insect species and different groups, we used a generalized linear model (GLM). We used linear mixed-effects models (LMM) to analyze the number of fruits per compound-umbel in the three pollination treatments. Pollination treatments are regarded as fixed effects. One-way ANOVA was also used to analyze the data about fruit set, fruit weight, and yield for three pollination treatments. If a significant difference was detected, then Tukey-b post-hoc test was applied (at a significance level of 0.05) to recognize the differences among the means.
Results
Floral Visitors
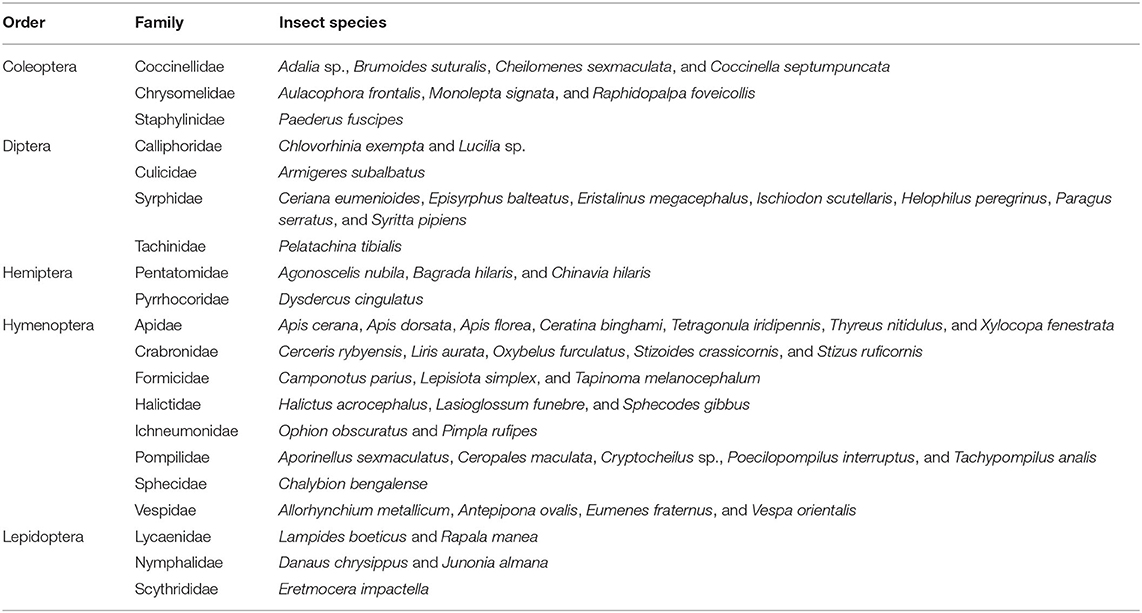
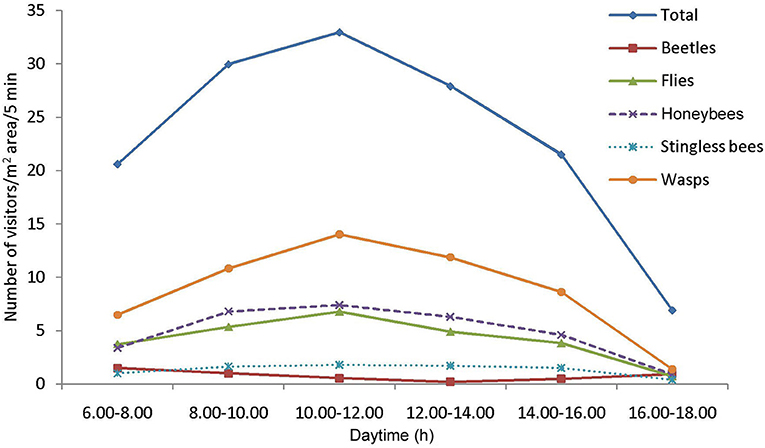
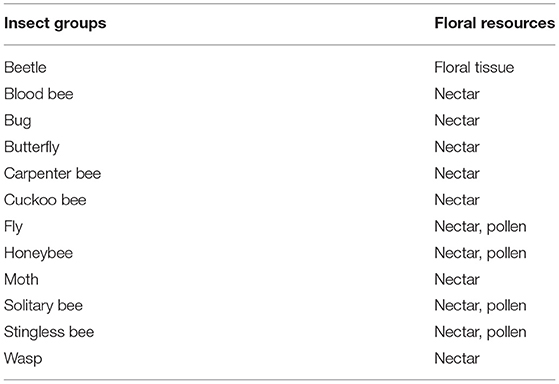
Fifty-eight insect species belonging to 20 families of 5 orders were recorded as floral visitors of fennel in West Bengal, India (Table 2; Figures 2, 3). The highest represented insect order is Hymenoptera (8 families and 30 species), followed by Diptera (4 families and 11 species), Coleoptera (3 families and 8 species), Lepidoptera (3 families and 5 species), and Hemiptera (2 families and 4 species). Among Hymenoptera, dominated insect families were Apidae (with 7 species), Crabronidae (with 5 species), and Pompilidae (with 5 species). In open-condition (i.e., without field manipulation), abundant insect species were Oxybelus furculatus (2.17 ± 2.36 wasps/m2/5 min; 9.30% of total visitors, i.e., relative abundance), Apis florea (2.09 ± 1.94 bees/m2/5 min; 8.98% of total visitors), Apis cerana (1.82 ± 1.97 bees/m2/5 min; 7.83% of total visitors), Episyrphus balteatus (1.45 ± 1.32 flies/m2/5 min; 6.22% of total visitors), and Tetragonula iridipennis (1.34 ± 1.33 bees/m2/5 min; 5.76% of total visitors). Considering different insect groups, most abundant was wasps (8.89 ± 5.28 wasps/m2/5 min; 38.16% of total visitors), followed by honeybees (4.89 ± 3.44 individuals/m2/5 min; 20.99% of total visitors), flies (4.23 ± 2.60 flies/m2/5 min; 18.17% of total visitors), stingless bees (1.34 ± 1.33 bees/m2/5 min; 5.76% of total visitors), and beetles (0.78 ± 0.78 beetles/m2/5 min; 3.36% of total visitors). The abundance of flower-visiting insects was varied at different daytimes [F(5, 114) = 74.66, P < 0.001; Supplementary Table 1]. The total number of encountered visitors was low (20.60 ± 5.13 individuals/m2/5 min) in early morning at 6:00–8:00 a.m. Then, visitor's abundance increased and reached the highest during 10:00 a.m.−12:00 noon (32.95 ± 4.66 individuals/m2/5 min). After that, abundance gradually decreased with time elapsed. In our experimental timeslots, the lowest abundance of 6.90 ± 2.81 individuals/m2/5 min was recorded during 2:00–6:00 p.m. (Figure 4). Flies, honeybees, and wasps were dominant from 10:00 a.m. to 12:00 noon. A higher abundance of stingless bees was observed during 10:00 a.m.−2:00 p.m. However, beetles were more frequent during the early morning and late afternoon (Figure 4). All the visitors touched reproductive parts of the fennel flowers and were regarded as legitimate visitors. Flies, honeybees, solitary bees, and stingless bees foraged on fennel flowers to collect nectar and pollen grains (Table 3). Other insect groups (beetles, blood bees, bugs, butterflies, carpenter bees, cuckoo bees, moths, and wasps) visited the flowers for nectar, whereas beetles were also fed floral tissues. During the morning, floral visitors especially Tetragonula iridipennis collected pollen grains and visited mostly freshly opened flowers. In the afternoon (2:00–6:00 p.m.), they act mainly as nectar foragers and visit both the current-day flowers and one-day old flowers (with senesced petals and stamens). The flower visitation rate (number of umbellets visits per minute) was extremely high for Apis cerana (13.13 ± 3.45 umbellets/min), Apis dorsata (11.20 ± 3.00 umbellets/min), Oxybelus furculatus (8.90 ± 2.17 umbellets/min), and Liris aurata (8.03 ± 2.99 umbellets/min). Among the dominant Hymenopteran members, stingless bees (Tetragonula iridipennis) had a low rate of umbellet visitation (2.27 ± 1.08 umbellets/min). The amount of time spent per umbellet was higher for stingless bees (31.89 ± 7.45 s) and flies (Episyrphus balteatus: 25.44 ± 6.90 s, Ischiodon scutellaris: 21.72 ± 7.11 s, Syrttia pipiens: 19.44 ± 6.44 s) compared to honeybees, solitary bees, and wasps (Table 4). Body surface pollen content of actively flower-visiting insects varied from species to species (GLM, type III: Wald χ2 = 24,972.56, d.f. = 1, P < 0.001; Intercept: Wald χ2 = 15.59E6, d.f. = 1, P < 0.001) as well as among different insect groups (GLM, type III: Wald χ2 = 19,574.84, d.f. = 1, P < 0.001; Intercept: Wald χ2 = 11.96E6, d.f. = 1, P < 0.001). Among the different insect groups (whose body surface pollen counted), honeybees have greater pollen content on their body surface (Apis cerana: 15,264 ± 2,386.94 pollen grains, Apis dorsata: 22,672 ± 2,191.36 pollen grains, Apis florea: 10,432 ± 2,102.60 pollen grains), followed by wasps, stingless bees, and flies (Table 4). In addition, honeybees and stingless bees carried corbicular pollen loads. The load size was higher for giant honeybees and lower for stingless bees. In open pollination, the “approximate pollination value (APV)” was the highest in Apis cerana (APV = 668.25), followed by Apis florea (APV = 412.68), Apis dorsata (APV = 351.12), and Oxybelus furculatus (APV = 206.92) (Table 5).
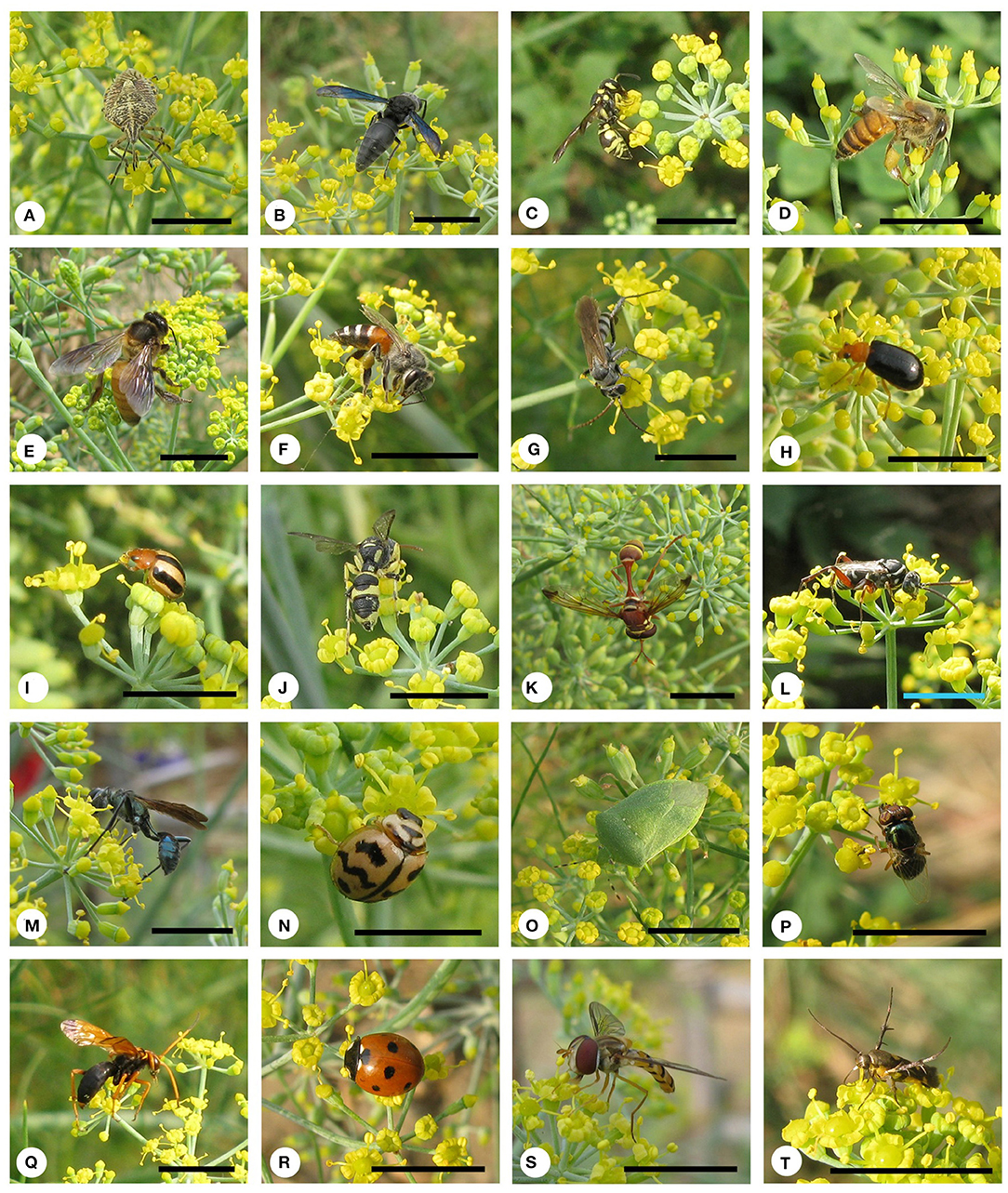
Figure 2. Floral visitors of fennel in West Bengal. (A) Agonoscelis nubila, (B) Allorhynchium metallicum, (C) Antepipona ovalis, (D) Apis cerana, (E) Apis dorsata, (F) Apis florea, (G) Aporinellus sexmaculatus, (H) Aulacophora frontalis, (I) Brumoides suturalis, (J) Cerceris rybyensis, (K) Ceriana eumenioides, (L) Ceropales maculata, (M) Chalybion bengalense, (N) Cheilomenes sexmaculata, (O) Chinavia hilaris, (P) Chlovorhinia exempta, (Q) Cryptocheilus sp., (R) Coccinella septumpunctata, (S) Episyrphus balteatus, and (T) Eretmocera impactella. Scale bar = 10 mm.
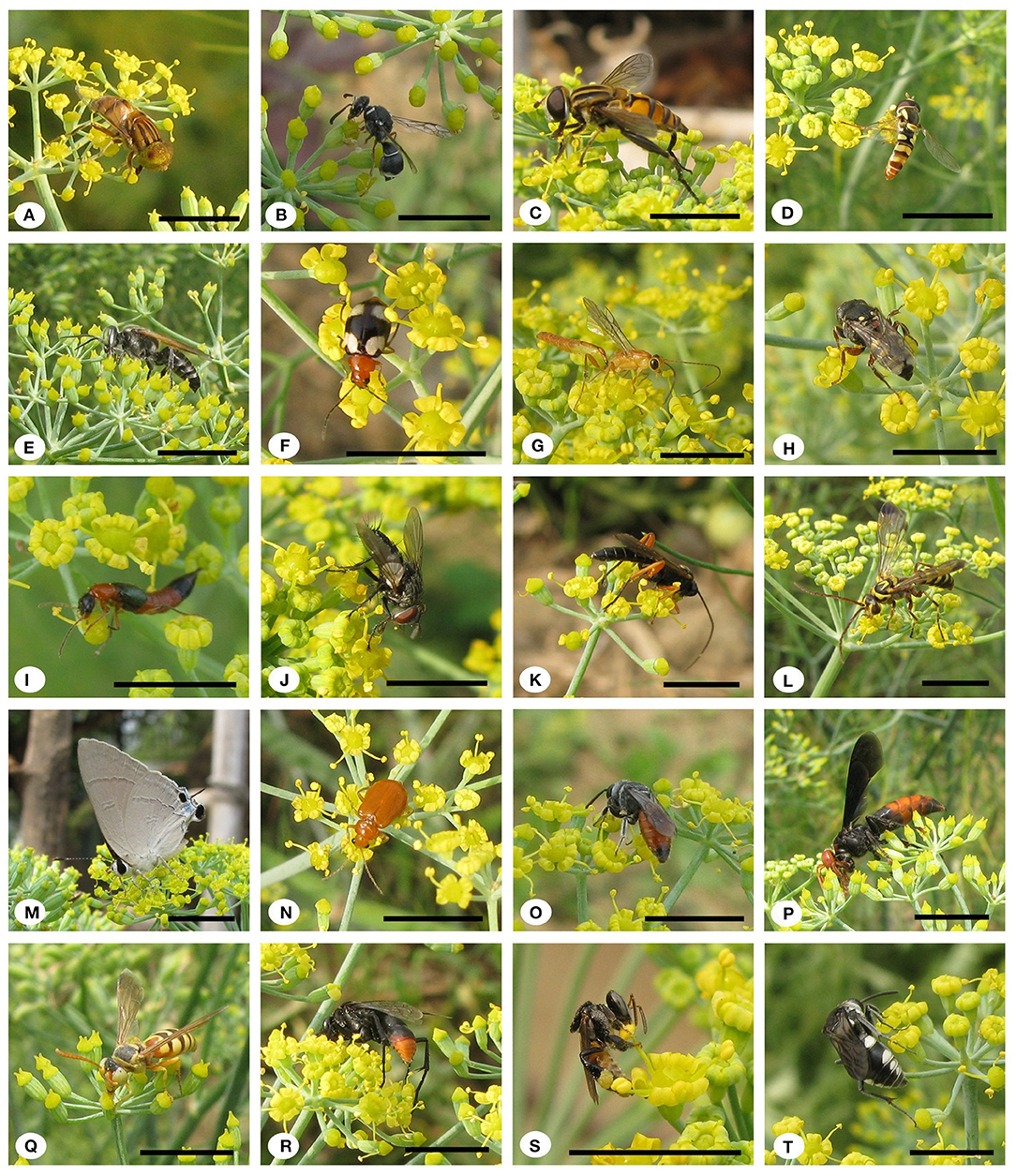
Figure 3. Floral visitors of fennel in West Bengal. (A) Eristalinus megacephalus, (B) Eumenes fraternus, (C) Helophilus peregrinus, (D) Ischiodon scutellaris, (E) Liris aurata, (F) Monolepta signata, (G) Ophion obscuratus, (H) Oxybelus furculatus, (I) Paederus fuscipes, (J) Pelatachina tibialis, (K) Pimpla rufipes, (L) Poecilopompilus interruptus, (M) Rapala manea, (N) Raphidopalpa foveicollis, (O) Sphecodes gibbus, (P) Stizoides crassicornis, (Q) Stizus ruficornis, (R) Tachypompilus analis, (S) Tetragonula iridipennis, and (T) Thyreus nitidulus. Scale bar = 10 mm.
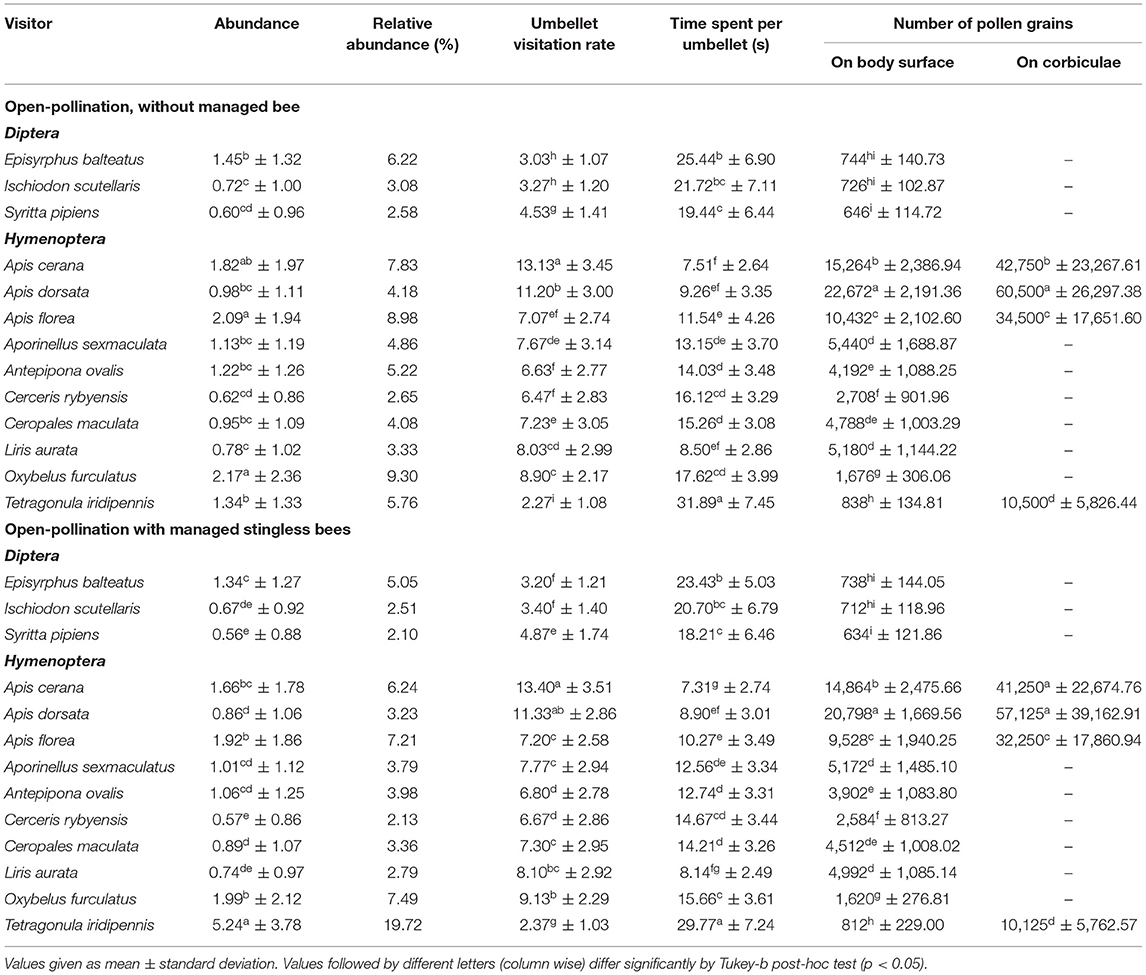
Table 4. Abundance (number/m2 area/5 min), relative abundance, umbellet visitation rate, amount of time spent per umbellet, and number of pollen grains carried by the dominant floral visitors.
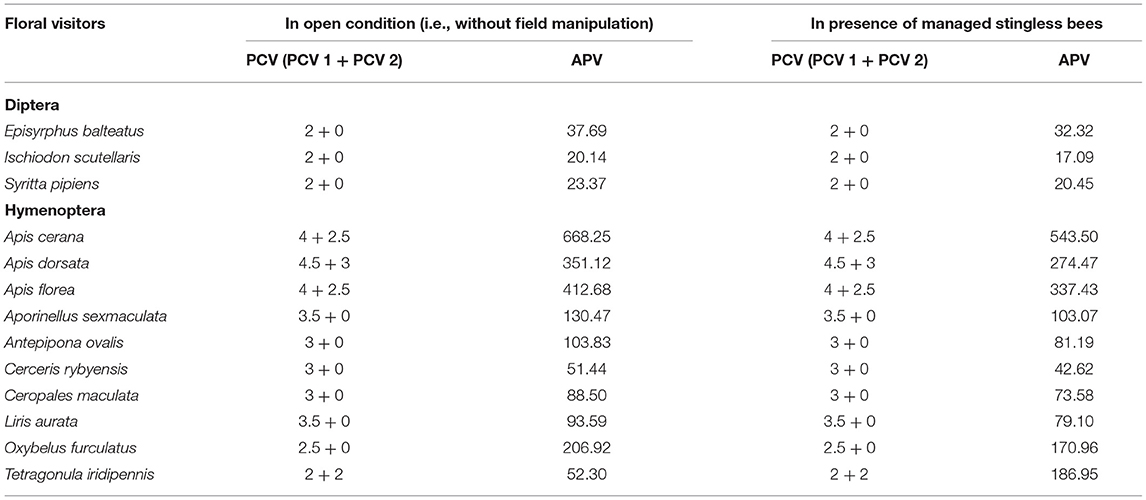
Table 5. Pollen carrying value (PCV) and approximate pollination value (APV) of dominant floral visitors.
Fruit Set and Pollination Deficiency
The percentage of fruit set in fennel differed significantly among the three pollination treatments, namely, pollinator-exclusion, open-pollination, and manual cross-pollination treatments [year 2020: F(2,27) = 24.13, P < 0.001; year 2021: F(2,27) = 22.66, P < 0.001]. The highest percentage of fruit set obtained in manual cross-pollinated treatment (year 2020: 87 ± 9.49; year 2021: 85 ± 10.08), followed by open-pollination (year 2020: 72 ± 12.29; year 2021: 69 ± 11.97), and pollinator-exclusion treatment (year 2020: 48 ± 15.49; year 2021: 46 ± 15.78) (Figure 5). This result corresponded to a considerable amount of pollination deficit (year 2020: D = 0.17; year 2020: D = 0.19; average: D = 0.18).
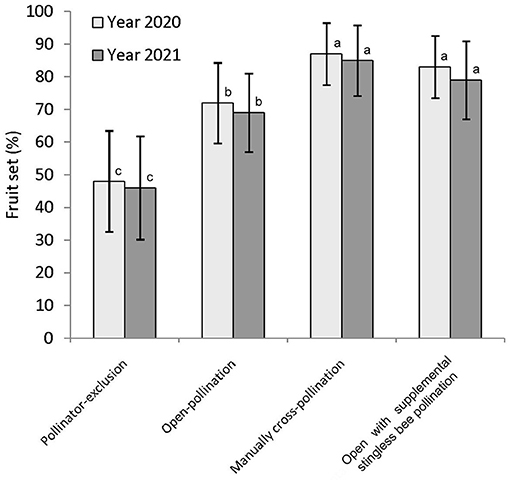
Figure 5. Fruit set of fennel in different pollination treatments. Values are given by mean ± standard deviation. Different letters indicate significant differences by Tukey-b post-hoc test (p < 0.05).
Impact of Managed Stingless Bees on Native Pollinators
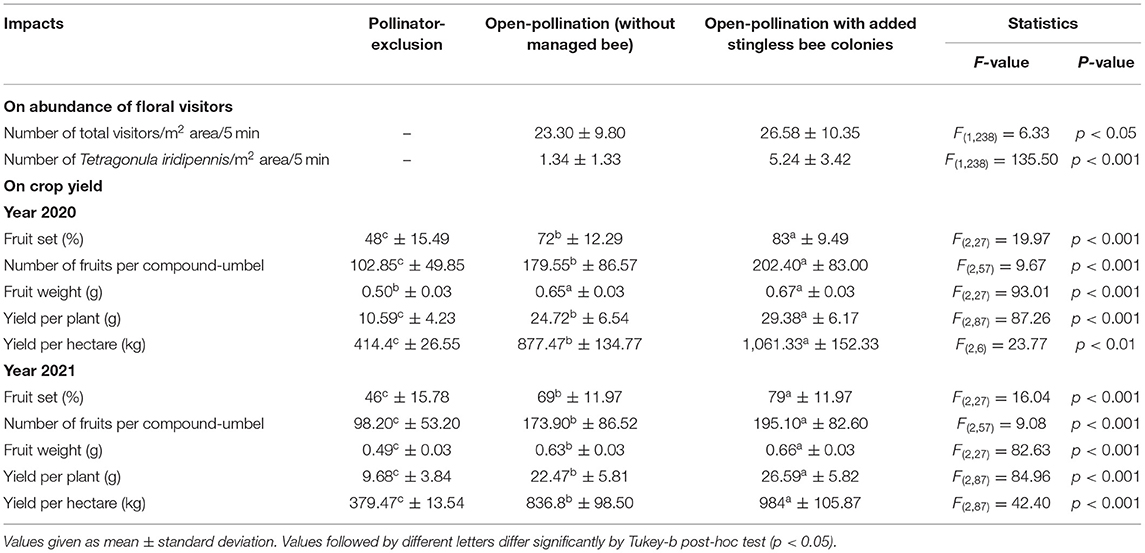
The species richness of floral visitors did not alter in the presence of the managed Indian stingless bee colonies. The abundance of stingless bees (5.24 ± 3.42 bees/m2 area/5 min), as well as total visitors (26.58 ± 10.35 visitors/m2 area/5 min) was increased significantly compared to the open condition (Tables 4, 6). Abundances of other flower-visiting species did not differ significantly. The umbellet visitation rate, amount of time spent per visit on an umbellet, and body surface pollen content of dominant native pollinators did not differ significantly (Table 4).
Impact of Managed Stingless Bees on Yield of Fennel
When we applied stingless bee colonies in addition to the native pollinators, year-wise obtained fruit set percentages were 83 ± 9.49 (in 2020) and 79 ± 11.97 (in 2021). On average, of these two years of study, the value (81%) was about 14.89 and 72.34% higher than the open-pollination (i.e., without managed bees) and pollinator-exclusion treatments, respectively. The counted number of fruits per compound-umbel were varied among the three pollination treatments (year 2020: LMM, type III— Treatments: F = 9.67, d.f. = 57, P < 0.001; Intercept: F = 278.64, d.f. = 57, P < 0.001; year 2021: LMM, type III— Treatments: F = 9.08, d.f. = 57, P < 0.001; Intercept: F = 254.71, d.f. = 57, P < 0.001). The number of matured fruits per compound-umbel (year 2020: 202.40 ± 83.00 fruits/compound-umbel; year 2021: 195.10 ± 82.60 fruits/compound-umbel) was also significantly higher in open-pollination with the addition of managed stingless bee colonies compared to open-pollination and pollinator-exclusion treatments (Table 6). Fruit weight of fennel also depends on the degree of pollination services perception, with the lowest fruit weight (year 2020: 0.50 ± 0.03 g/100 fruits; year 2021: 0.49 ± 0.03 g/100 fruits) in the pollinator-exclusion treatment and the highest fruit weight (year 2020: 0.67 ± 0.03 g/100 fruits; year 2021: 0.66 ± 0.03 g/100 fruits) achieved in supplementary pollination through managed stingless bee colonies in addition to native pollinators. The crop yield (in terms of fruit weight per plant and per hectare field area) was significantly different among the three pollination treatments (Table 6). Fennel yield was higher in open-pollination with managed stingless bee colonies (year 2020: 29.38 ± 6.17 g/plant, 1,061.33 ± 152.33 kg/ha; year 2021: 26.59 ± 5.82 g/plant, 984 ± 105.87 kg/ha; on average: 27.99 ± 6.11 g/plant, 1,022.67 ± 124.74 kg/ha) compared to the open-pollination and pollinator-exclusion systems (Table 6). Therefore, with the addition of managed stingless bee colonies within the fennel fields, yield increased about 19.31% (estimated from the yield per hectare area) compared to the open-pollinated system.
Discussion
We recorded a vast amount of species richness for floral visitors in fennel grown in West Bengal. The crop species may be regarded as magnet plants (i.e., they attract many insect species) in restoration projects (Zych et al., 2007). Among the recorded visitors, some are common to the studies of other regions (Chaudhary, 2006; Bharati et al., 2015), a few insect species (namely, Antepipona ovalis, Cerceris rybyensis, Liris aurata, Oxybelus furculatus, Stizoides crassicornis, Stizus ruficornis, etc.) were newly documented as floral visitors of fennel from West Bengal. The richness and abundance of floral visitors may vary from location to location because floral visitors of a plant species depend not only on floral characteristics, but also on geographical location, surrounding vegetation, and climatic conditions. Higher represented insect orders were Hymenoptera, Diptera, and Coleoptera. The dominance of Hymenoptera within the visitor's spectrum of fennel was well-established in other Indian states (Bharati et al., 2015) and outside the country (Skaldina, 2020). However, our study revealed that the insect orders Coleoptera and Diptera were also dominant in addition to Hymenoptera. The most abundant flower visitors were native honeybees (Apis florea, Apis cerana), crabonidae wasps (Oxybelus furculatus), syrphid flies (Episyrphus balteatus, Ischiodon scutellaris), and stingless bees (Tetragonula iridipennis). In other Indian states, the prominence of the western honeybee (Apis mellifera) was also recognized (Chaudhary, 2006). The numbers of body surface pollen grains (excluding pollen loads on corbiculae or scopae) were higher in honeybees, moderate in wasps, and lower in flies and stingless bees. Honeybees and stingless bees actively collected pollens and temporarily stored them as pollen loads on their corbiculae to carry them into their hive. Pollen attachment depends on visitors' body size, surface architecture (hairy or non-hairy), and foraging strategies. However, the amount of body surface pollen grains does not proportionately influence pollination efficiency of an insect species because it also depends on population size (number of foraging individuals), visitation rate, foraging strategies (legitimate or illegitimate), and timing of forage regarding stigmatic-receptivity. As the flowers are very small and bloomed in a cluster, we cannot determine single-visit pollination efficiency of the legitimate flower visitors. As an alternative, we calculated “approximate pollination value (APV)” based on relative abundance, visitation rate, and pollen carrying value of the flower visitors. According to the APV, important native pollinators are Apis cerana, Apis dorsata, Apis florea, and Oxybelus furculatus in the open condition.
Besides a pollinator's richness and abundance, the crop species showed a significant pollination deficit (D = 0.18). That means there is an opportunity to optimize the fennel yield through supplementary pollination services. Sufficient bee pollination stabilizes the yield and maintains genetic variability of the crop species, counteracts with inbreeding depression, and facilitates system resilience (Stein et al., 2017). Some workers (Kumar and Singh, 2017) utilized western honeybee colonies to fulfill the demand. However, Layek et al. (2021b) showed that the installation of western honeybee colonies in a watermelon field has significant negative impacts on native pollinator species and may render sustainable agricultural practices. Again, exotic stingless bees may interact and compete for resources with local pollinator populations. Therefore, stingless beehive displacement to non-native places should be impeded in negotiations to safeguard their welfare and the sustainability of local insect populations in the long term (Dos Santos et al., 2021). In the present experiment, we selected a native stingless bee species (Tetragonula iridipennis) to pollinate the fennel. To our knowledge, it is the first attempt to utilize stingless bee colonies in fennel pollination. After establishing stingless bee colonies, floral visitors' abundance (especially the number of stingless bees) was significantly increased. The greater abundance of stingless bee workers does not substantially alter the native visitor's richness and foraging behavior (abundance, flower visitation rate, and amount of time spent per umbellet) on fennel— it may be due to smaller body size, non-aggressive behavior of the stingless bee workers, and is native to the study areas. Using stingless bee colonies, the fruit set (%), fruit weight, and yield of fennel were significantly increased compared to the open-pollination treatment. Though stingless bees have a lower visitation rate, they spend more time on the flowers of umbellets than the other visitors. This suggests that the bee's coverage in the field and potential as a pollinator are strongly influenced by two factors—(i) the number of opened flowers in umbellets and (ii) the number of bees in fields. The higher fruit set and increased fruit weight are desirable features for crop species from both the commercial and ecological points of view, as they indicate plant reproductive success. Thus, our study clearly reveals the value of stingless bee pollination to fennel production.
Conclusion
While many insect species from different groups visited the fennel flowers, we recognized significant pollen transfer limitations in open-conditions. This may hinder the optimum yield of the crop species. To overcome the pollination deficiency, utilization of managed stingless bee colonies remains effective and significantly increases crop yield quantity and quality. The managed stingless bees do not trigger any negative impact on the foraging activities of native pollinators. Therefore, supplemental stingless bee pollination appears to be economically and ecologically important to support the sustainable agricultural practices.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
UL: conceptualization, investigation, data collection, statistical analysis, and writing—original draft. AD: data collection, writing—review, and editing. PK: supervision, writing—review, and editing. All authors approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the farmers who allowed us to conduct the field experiment on their fennel fields. We also gratefully acknowledged the entomologists in ZSI, Kolkata for identification of the floral visitors.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2022.820264/full#supplementary-material
References
Baswana, K. S. (1984). Role of insect pollinator on seed production in coriander and fennel. South Indian Hort. 32, 117–118.
Bharati, V., Singh-Ahlawat, D., Sharma, S. K., Singh, N. V., Jitender, J., and Singh, N. (2015). Diversity, abundance and pollination efficiency of insect pollinators of fennel (Foeniculum vulgare Miller) and effect of abiotic factors on insect pollinator activity. J. Appl. Nat. Sci. 7, 786–793. doi: 10.31018/jans.v7i2.684
Bisui, S., Layek, U., and Karmakar, P. (2019). Comparing the pollen forage pattern of stingless bee (Trigona iridipennis Smith) between rural and semi-urban areas of West Bengal, India. J. Asia-Pac. Entomol. 22, 714–722. doi: 10.1016/j.aspen.2019.05.008
Bisui, S., Layek, U., and Karmakar, P. (2021). Determination of nectar resources through body surface pollen analysis: a study with the stingless bee Tetragonula iridipennis Smith (Apidae: Meliponini) in West Bengal, India. Sociobiology 68, e6173. doi: 10.13102/sociobiology.v68i3.6173
Carreck, N. L., Williams, I. H., and Little, D. J. (1997). The movement of honey bee colonies for crop pollination and honey production by beekeepers in great Britain. Bee World 78, 67–77. doi: 10.1080/0005772X.1997.11099337
Chaudhary, O. P. (2006). Diversity, foraging behaviour of floral visitors and pollination ecology of fennel (Foeniculum vulgare Mill.). J. Spices Aromat. Crops 15, 34–41.
Classen, A., Peter, M. K., Ferger, S. W., Helbig-Bonitz, M., Schmack, J. M., Maassen, G., et al. (2014). Complementary ecosystem services provided by pest predators and pollinators increase quantity and quality of coffee yields. Proc. R. Soc. B. 281, 20133148. doi: 10.1098/rspb.2013.3148
Cusser, S., Haddad, N. M., and Jha, S. (2021). Unexpected functional complementarity from non-bee pollinators enhances cotton yield. Agric. Ecosyst. Environ. 314, 107415. doi: 10.1016/j.agee.2021.107415
Danaraddi, C. S., Hakkalappanavar, S., Biradar, S. B., Tattimani, M., and Vinod, S. K. (2011). Studies on foraging behaviour of stingless bee, Trigona iridipennis Smith at Dharwad, Karnataka. Int. J. For. Crop Improv. 2, 163–169.
Danaraddi, C. S., Viraktamath, S., Basavanagoud, K., and Bhat, A. R. S. (2009). Nesting habits and nest structure of stingless bee, Trigona iridipennis Smith at Dharwad, Karnataka. Karnataka J. Agric. Sci. 22, 310–313.
Devanesan, S., Nisha, M. M., Bennet, R., and Shailaja, K. K. (2002). Foraging behaviour of stingless bees, Trigona iridipennis Smith. Insect Environ. 8, 131–133.
Dos Santos, C. F., Acosta, A. L., Halinski, R., De Souza dos Santos, P. D., Borges, R. C., Gianinni, T. C., et al. (2021). The widespread trade in stingless beehives may introduce them into novel places and could threaten species. J. Appl. Ecol. doi: 10.1111/1365-2664.14108
Garibaldi, L. A., Aizen, M. A., Klein, A. M., Cunningham, S. A., and Harder, I. D. (2011). Global growth and stability of agricultural yield decrease with pollinator dependence. Proc. Natl. Acad. Sci. U. S. A. 108, 5909–5914. doi: 10.1073/pnas.1012431108
Garibaldi, L. A., Pérez-Méndez, N., Cordeiro, G. D., Hughes, A., Orr, M., Alves-dos-Santos, I., et al. (2021). Negative impacts of dominance on bee communities: does the influence of invasive honey bees differ from native bees?. Ecology 102, e03526. doi: 10.1002/ecy.3526
Greenleaf, S. S., and Kremen, C. (2006). Wild bees enhance honey bees pollination of hybrid sunflower. Proc. Natl. Acad. Sci. U. S. A. 103, 13890–13895. doi: 10.1073/pnas.0600929103
Isaacs, R., Williams, N., Ellis, J., Pitts-Singer, T. L., Bommarco, R., and Vaughan, M. (2017). Integrated crop pollination: combining strategies to ensure stable and sustainable yields of pollination-dependent crops. Basic Appl. Ecol. 22, 44–60. doi: 10.1016/j.baae.2017.07.003
Klein, A. M., Vaissière, B. E., Cane, J. H., Steffan-Dewenter, I., Cunninghum, S. A., Kremen, C., et al. (2007). Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B. 274, 303–313. doi: 10.1098/rspb.2006.3721
Koul, P., Sharma, N., and Koul, A. K. (1996). Reproductive biology of wild and cultivated fennel (Foeniculum vulgare Mill.). Proc. Indian Natn. Sci. Acad. 62, 125–135.
Kumar, M., and Singh, R. (2017). Role of honeybees in the pollination of fennel (Foeniculum vulgare L.). J. Pharmacogn. Phytochem. 6, 214–218.
Layek, U., Bisui, S., and Karmakar, P. (2021a). Flight range and resource loading-unloading behavior of stingless bee Tetragonula iridipennis (Smith). J. Apic. Res. doi: 10.1080/00218839.2021.1994259
Layek, U., and Karmakar, P. (2016). Bee plants used as nectar sources by Apis florea Fabricius in Bankura and Paschim Medinipur districts, West Bengal. Geophytology 46, 1–14.
Layek, U., and Karmakar, P. (2018a). Nesting characteristics, floral resources, and foraging activity of Trigona iridipennis Smith in Bankura district of West Bengal, India. Insect. Soc. 65, 117–132. doi: 10.1007/s00040-017-0593-4
Layek, U., and Karmakar, P. (2018b). Pollen analysis of Apis dorsata Fabricius honeys in Bankura and Paschim Medinipur districts, West Bengal. Grana 57, 298–310. doi: 10.1080/00173134.2017.1390604
Layek, U., Kundu, A., Bisui, S., and Karmakar, P. (2021b). Impact of managed stingless bee and western honey bee colonies on native pollinators and yield of watermelon: a comparative study. Ann. Agric. Sci. 66, 38–45. doi: 10.1016/j.aoas.2021.02.004
Layek, U., Kundu, A., and Karmakar, P. (2020b). Floral ecology, floral visitors and breeding system of Gandharaj lemon (Citrus × limon L. Osbeck). Bot. Pac. 9, 113–119. doi: 10.17581/bp.2020.09208
Layek, U., Manna, S. S., and Karmakar, P. (2020a). Pollen foraging behaviour of honey bee (Apis mellifera L.) in southern West Bengal, India. Palynology 44, 114–126. doi: 10.1080/01916122.2018.1533898
Lee, K. V., Steinhauer, N., Rennich, K., Wilson, M. E., Tarpy, D. R., Caron, D. M., et al. (2015). A national survey of managed honey bee 2013-2014 annual colony losses in the USA. Apidologie 46, 292–305. doi: 10.1007/s13592-015-0356-z
Nikkeshi, A., Inoue, H., Arai, T., Kishi, S., and Kamo, T. (2019). The bumblebee Bombus ardens ardens (Hymenoptera: Apidae) is the most important pollinator of Oriental persimmon, Diospyros kaki (Ericales: Ebenaceae), in Hiroshima, Japan. Appl. Entomol. Zool. 54, 409–419. doi: 10.1007/s13355-019-00637-x
Ollerton, J., Erenler, H., Edwards, M., and Crockett, R. (2014). Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 346, 1360–1362. doi: 10.1126/science.1257259
Patel, V., Pauli, N., Biggs, E., Barbour, L., and Boruff, B. (2021). Why bees are critical for achieving sustainable development. Ambio 50, 49–59. doi: 10.1007/s13280-020-01333-9
Rader, R., Bartomeus, I., Garibaldi, L. A., Garratt, M. P. D., Howlett, B. G., Winfree, R., et al. (2015). Non-bee insects are important contributors to global crop pollination. Proc. Natl. Acad. Sci. U. S. A. 113, 146–151. doi: 10.1073/pnas.1517092112
Rollin, O., and Garibaldi, L. A. (2019). Impacts of honeybee density on crop yield: a meta-analysis. J. Appl. Ecol. 56, 1152–1163. doi: 10.1111/1365-2664.13355
Sagar, P. (1981). Role of insects in crop pollination of fennel crop at Ludhiana. Agric. Res. J. 18, 388–392.
Schulp, C. J. E., Lautenbach, S., and Verburg, P. H. (2014). Quantifying and mapping ecosystem services: demand and supply of pollination in the European Union. Ecol. Indic. 36, 131–141. doi: 10.1016/j.ecolind.2013.07.014
Skaldina, O. (2020). Insect associated with sweet fennel: beneficial visitors attracted by a generalist plant. Arthropod-Plant Interact. 14, 399–407. doi: 10.1007/s11829-020-09752-x
Stein, K., Coulibaly, D., Stenchly, K., Goetze, D., Porembski, S., Lindner, A., et al. (2017). Bee pollination increases yield quantity and quality of cash crops in Burkina Faso, West Africa. Sci. Rep. 7, 17691. doi: 10.1038/s41598-017-17970-2
Tucker, E. M., and Rehan, S. M. (2018). Farming for bees: annual variation in pollinator populations across agricultural landscapes. Agri. For. Entomol. 20, 541–548. doi: 10.1111/afe.12287
Van Engelsdorp, D., Traynor, K. S., Andree, M., Lichtenberg, E. M., Chen, Y., Saegerman, C., et al. (2017). Colony collapse disorder (CCD) and bee age impact honey bee pathophysiology. PLoS One 12, e0179535. doi: 10.1371/journal.pone.0179535
Wietzke, A., Westphal, C., Gras, P., Kraft, M., Pfohl, K., Karlovsky, P., et al. (2018). Insect pollination as a key factor for strawberry physiology and marketable fruit quality. Agric. Ecosyst. Environ. 258, 197–204. doi: 10.1016/j.agee.2018.01.036
Winfree, R., Williams, N. M., Dushoff, J., and Kremen, C. (2007). Native bees provide insurance against ongoing honeybee losses. Ecol. Lett. 10, 1105–1113. doi: 10.1111/j.1461-0248.2007.01110.x
Keywords: approximate pollination value, field manipulation, foraging activity, managed bee, pollination deficiency
Citation: Layek U, Das A and Karmakar P (2022) Supplemental Stingless Bee Pollination in Fennel (Foeniculum vulgare Mill.): An Assessment of Impacts on Native Pollinators and Crop Yield. Front. Sustain. Food Syst. 6:820264. doi: 10.3389/fsufs.2022.820264
Received: 22 November 2021; Accepted: 18 January 2022;
Published: 11 March 2022.
Edited by:
Matteo Dainese, Eurac Research, ItalyReviewed by:
Alistair John Campbell, Embrapa Amazônia Oriental, BrazilPalatty Sinu, Central University of Kerala, India
Copyright © 2022 Layek, Das and Karmakar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prakash Karmakar, cHJha2FzaGJvdDE5NzNAZ21haWwuY29t
 Ujjwal Layek
Ujjwal Layek Alokesh Das
Alokesh Das Prakash Karmakar
Prakash Karmakar