- Department of Agriculture and Animal Health, College of Agriculture and Environmental Sciences, Florida, South Africa
This study investigated the effects of varying soil moisture conditions (through either flooding, drought, or provision of a moderate water supply) on the metabolomic profile of two potato cultivars, namely, Markies and Fianna. Representative tubers of the treated plants were collected 91 days after planting. The samples were freeze-dried, and ground to a fine powder in liquid nitrogen. The fine powder of the tuber samples was analyzed by nuclear magnetic resonance spectroscopy (NMR) to identify their metabolomic profiles. The NMR data was analyzed using principal component analysis and orthogonal partial least square-discriminant analysis to identify any variations between the treatments. In both models, plants exposed to drought clearly separated from the plants that received either excess or moderate water (control). The potato tubers that experienced drought and flood treatments had the highest quantities of aspartic acid, asparagine, and isoleucine. Furthermore, the potatoes exposed to either drought or flood had higher levels of valine and leucine (which are essential for plant defense and resistance against plant pathogens). Potato plants can respond metabolically to varying soil moisture stress.
Introduction
Potato (Solanum tuberosum L.) is a starchy vegetable, and it falls within the 18 major priority crop categories (Leff et al., 2004). Obidiegwu et al. (2015) suggested that potato is the most important priority crop, and ranked in the top 10 most important food crops in the world. The current contribution of potato crops to human nutrition, as well as its ability to respond to climate change challenges, makes potato a highly desirable crop (Manners and Van Etten, 2018). Therefore, potato, as well as many other food crops, may be able to survive climate change and continue to provide nutrition to the ever-growing world population. The survival ability of potato is enhanced by its upregulation of some defense-associated metabolites which have a high nutritional value (Handayani et al., 2019). However, potato plants possess an optimum tolerance level that stressful environmental conditions must not exceed. For example, the optimum temperature for growing potatoes is 22 °C during the day and 14 °C during the night, which correlates with temperate conditions (Trapero-Mozos et al., 2018). When potato plants are exposed to temperature or soil moisture conditions outside the optimum tolerance levels, their growth can be retarded as well as their development, yield and quality. Moreover, either low or high temperatures or water stress can cause physiological changes, including, photoassimilate partitioning, evapotranspiration rate, and photosynthesis (Haverkort et al., 2013). Therefore, for future production, it is essential to understand the molecular responses by potato plants to different environmental stresses for example, the responding metabolites and compounds during the growth and development stages. This knowledge would assist in identifying potato cultivars that can tolerate varying environmental stresses, such as drought, heat, or stress due to flooding (Pereira and Shock, 2006).
Many studies have been undertaken to understand the physiological, biochemical, and genetic basis of drought tolerance in potato crops to improve production under drought conditions (George et al., 2017). The results of these experiments have been used to predict how yields and quality might vary in the field under different climate change scenarios (Beetge and Krüger, 2018). However, knowledge on the simultaneous effects of increasing temperatures and flooding or drought stress (i.e., combined stresses) on potato production is lacking in most African countries. Nonetheless, it is vitally important because the region's struggling economic situation impedes the implementation of additional agricultural infrastructure (e.g., irrigation or cooling systems). This in-depth investigation is important to increase our understanding of how changing environmental conditions by climate change affect potato production to ensure future productivity (Quiroz et al., 2018). Detailed information on stress-dependent changes in metabolites in response to flood or drought treatment is also of vital importance, as these biochemical activities influence potato growth and development. The objective of this study was therefore to investigate the effects of soil moisture stress, such as drought or flooding, on the metabolomic profile of potato crops. This information should assist in identifying drought-or flood-resistant associated potato metabolites that can be used as biomarkers for breeding cultivars that will provide high quality yields despite changing climatic conditions.
Materials and Methods
Plant Material
The experiment was undertaken at the Science Campus of the University of South Africa (26°9'26″S and 27°54'10″E). Four-week-old glasshouse-grown seedlings (in the vegetative growth stage) of the potato cultivars (Markies and Fianna) were selected for study. Three experimental conditions were administered, namely, water flooding, drought and the control (adequate moisture) and three plants were used for each of the experimental treatments in each of the two cultivars. The plants were completely randomized and shifted frequently to minimize the effect of non-experimental factors. The experiment was conducted in two cycles, 2017 and 2018. In total 36 plants were included for the study.
The plants were maintained in pots filled with a mixture of 2:1:1 volume ratio of topsoil, vermiculite and river sand sourced from a local nursery. Throughout the 2017 experiment the glasshouse temperatures were maintained with an average day temperature of 24°C. The 2018 experiment had a slight increase in the average temperature with 24.75°C (day). For both years, the day/night regime was 12 h/12 h and the average night temperature was 8.25°C. This experiment was repeated under the same conditions, to allow for estimation of the variability of the results, and to increase the accuracy of the findings. A greenhouse used had openings at the end of the walls, through which electric fans drew in external air and circulated it throughout the interior, as a means of cooling it. Therefore, a slight day temperature change occurred natural in the greenhouse as it was not controlled.
Experimental Treatments and Data Capture
The experimental treatments (flooding, drought, and control water supply) were administered 28 days after planting (DAP) until 91 DAP. Six (6) Fianna plants received the flooding treatment, six plants were exposed to drought conditions, and six were exposed to the control water supply (Supplementary Table 1). The same experimental format was repeated for Markies. All experimental plants were completely randomized. During the treatment period, flooded plants received 0–10 kPa per day, drought-exposed plants received 60–100 kPa, and plants receiving moderate water received 25–30 kPa. The water volume of each irrigation treatment was determined using a Watermark Soil Moisture Sensor (Irrometer) supplied by Calafrica SA. The range of 0–10 kPa indicates water-saturated soil, 10–30 kPa indicates adequately wet soil, 30–60 kPa indicates irrigation is required, 60–100 kPa indicates the soil is becoming critically dry, and 100–200 kPa indicates severely dry soil conditions. For the control water treatment, plants were only irrigated when the irrometer reading reached 30 kPa. In the drought treatment, plants were irrigated when the irrometer reading reached 100 kPa, and watering ceased when the irrometer reading was 10 kPa. The flooding treated plants were irrigated when the irrometer reading reached 10 kPa.
Plant Tissue Sampling and Storage
The foliage of both the Markies and Fianna cultivars began to senesce at 80 DAP in both 2017 and 2018. Tubers were allowed to set and harden for a further 10 days under the soil by which time senescence was also advancing. Therefore, at 91 DAP in both 2017 and 2018, the Markies and Fianna cultivars plants from all experimental treatments (excluding the guard plants) were harvested. The harvested potato tubers were washed using tap water and individually stored in separate labeled re-sealable freezer bags. All samples were stored in a freezer at −76 °C until required for the metabolomic analysis. Tubers with visible physiological defects were discarded.
Metabolite Measurements
The potato tubers were removed from the −76 °C freezer, one potato tuber per plant was selected for the metabolite analysis resulting in 36 tubers included in the final metabolomic determination. The tubers were lyophilized using a freeze dryer and crushed in liquid nitrogen using a mortar and pestle. For the 1H-NMR analysis, the metabolites were extracted from 50 mg of ground tuber samples with 750 μL of methanol-D4 and 750 μL of buffer (deuterium oxide + potassium dihydrogen phosphate). The mixture was vortexed and sonicated for 20 min before a brief centrifugation at room temperature (Defernez et al., 2004). The samples were centrifuged at 13,000 rpm for an additional 30 min to remove the supernatant from the pellet. The supernatant was dispensed into 5 mm NMR sample tubes for analysis. The 1H-NMR spectroscopic analysis was performed using a 600 MHz Varian NMR spectrometer (Varian Inc., Palo Alto, CA, USA) to obtain the 1D proton spectra of the samples. All NMR analyses were performed at the Council for Scientific and Industrial Research (CSIR), Pretoria (25.7468° S, 28.2789° E).
Visualization and Metabolomics of the Metabolites in Fianna and Markies Potato Cultivars After Different Irrigation Treatments
Mestrenova version 10.1 was used for the phase and baseline correction analysis, for all of the NMR spectra (Figures 2A,B). Initially we eliminated the region of methanol (3.28–3.33 ppm) and water (4.75–5.1 ppm). The ASCII files generated from the spectra and the NMR intensity data were divided into 0.04 ppm bins. The results were imported into Microsoft Excel for analysis with SIMCA.
Principal Component Analysis (PCA)
The principal component analysis (PCA) was performed using SIMCA, and scatter plots were generated. The analysis was an exploratory unsupervised pattern recognition model. The PCA algorithm reduced the dimensionality of the data while retaining most of the variation in the dataset. The PCA identified the variables that cluster together to observe the trends, clusters and outlying data. The data was further analyzed using supervised orthogonal partial least-squares discriminant analysis (OPLS-DA). The OPLS-DA is a systematic variation technique wherein classes that are not correlated are removed, although the level of the variations may not improve, but the interpretation becomes clearer. It incorporated an orthogonal signal correction filter into a PLS model to ensure an accurate interpretation of the data (Akhtar et al., 2021).
Results
The effect of treatment was evident in yield quality and quantity of potatoes in this research. Both Markies and Fianna CVs that were exposed to drought and flooding treatments had reduced yield after all tubers with visible defects were removed–that is, in terms of tuber weight, diameter and height. Therefore, it can be construed that the increase in the number of tubers with visible defects found in potato plants that were subjected to flooding, and those that received drought irrigation treatment, were the reason for the reduced yield quantities obtained from these two treatment groups. Under flooding conditions, oxygen is reduced, and as a result, root respiration and growth are affected, causing gases such as CO2 and ethylene to build up. This condition is responsible for the potato seed cells deteriorating, causing them to be weak, preventing them from establishing a healthy canopy, and eventually impacting negatively on the yield quality and quantity. Hence, in the current study, the plants that were exposed to the flooding treatment were less productive. The effects of drought conditions were equally detrimental to potato plant growth, due to the root system of these plants being shallow and therefore extracting limited quantities of water from the soil, which, in turn, may reduce its capacity to recover after a water stress period. Therefore, the results of this research further emphasized the importance of maintaining an optimal soil moisture status if improved quality and quantity of potato yield is to be achieved.
In this research, it was further observed that the combination of two or more stresses had even worse impacts; for example, the smallest yield was identified in the 2018 experimentation year where flooding treatment was accompanied with a 0.75°C temperature increase, which was more detrimental to the performance of the crop. This suggests that flooding stress, combined with elevated atmospheric temperature, provided an undesirable environment for potato growth.
To ascertain if the metabolites were altered by the different water treatment applications in 2017 and 2018, the samples were analyzed using 1H-NMR spectroscopy followed by PCA of the NMR data. The results as observed in the PCA showed a clear separation between Markies and Fianna potato cultivars after different irrigation treatments (control, flooding or drought). To further investigate the differences in the responses of Markies and Fianna potato cultivars to the treatments, we analyzed the data using a supervisedOPLS-DA model (Figure 1A) and obtained the same results. The OPLS-DA multivariate statistical analysis (Figure 1A) revealed a clear discrimination between the cultivars with only two outliers (Fianna cultivar: samples 1 and 6; Markies cultivar: samples 29 and 30). The PCA analyses, therefore, provided an adequate initial metabolic conclusion of the Markies and Fianna potato cultivar datasets. The OPLS-DA verified the PCA results and provided confirmation of the results. The model showed a good fit and predictability in accordance with R2X(cum) = 0.84, R2Y = 0.99 (cum), and Q2 = 0.99, as provided in Figure 1A and Supplementary Table 2, respectively.
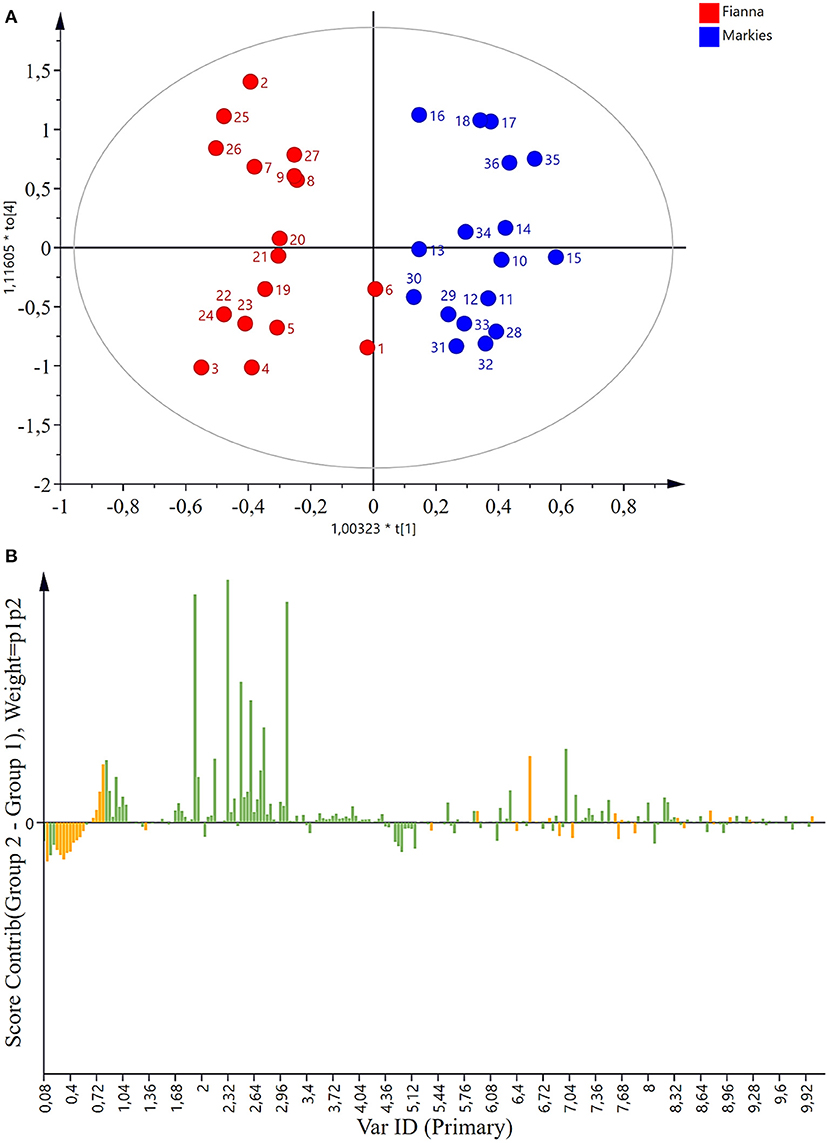
Figure 1. (A) OPLSA-DA statistical plot showing the separation between Markies and Fianna potato cultivars. (B) Loadings of each variable in the contribution plot of OPLS-DA illustrating chemical shifts from the Fianna and Markies potato cultivars that received the control, flooding or drought irrigation treatments.
The loadings (Figure 1B) from the OPLS-DA contribution plot were used to explain the rows and columns, and further summarized the influence of the different variables in the model. The resulting NMR contribution plot (Figure 1B) demonstrated the variations caused by the different water regimes through the score clusters. The loadings of each variable in the contribution plot (Figure 1B) illustrates chemical shifts from the metabolomic databases for metabolite identification. The bins of the chemical shifts in this contribution plot were linked with compounds using existing literature and the NMR spectra (Figures 2A,B). The contribution plot showed clear separation between the cultivars and with the water treatments, suggesting high metabolite variations between samples. Therefore, the variables in each position on the plot contributed heavily to the observed compounds whose scores were positioned similarly on the score plot. Figure 1B presents a spectra contribution plot, consisting of metabolites with varying quantities. The loading pattern revealed the chemical shifts responsible for these variations. The specific regions of Solanum tuberosum L. are represented by buckets (positive bars). The contribution plots for both the Markies and Fianna potato cultivars revealed the sugars were responsible for the observed differences (Figure 1B). The other peaks of the chemical shifts demonstrated variations which may be due to changes in the metabolite pattern that were influenced by the different water treatments.

Figure 2. (A) Annotated NMR spectra of the compounds used for metabolite identification in samples of Fianna and Markies potato cultivars that received control or flooding or drought irrigation treatments. (B) Annotated NMR spectra of the compounds used for the metabolite identification in samples of Fianna and Markies potato cultivars that received control, flooding or drought irrigation treatments.
The high abundance of compounds in the 1H NMR spectra (Figures 2A,B) corresponded with amino acids, such as valine, isoleucine and leucine metabolites. In addition, there were many significant loadings consistent with the transitions of the metabolites, including arginine, cystine, dimethylglycine, glutamine, glycine, histidine, homocysteine, lysine, methionine, phenylalanine, serine, threonine and tryptophan.
The 1H NMR spectra from which the 20 metabolites of interest were annotated after pre-processing and aligning are provided in Figures 2A,B. Whereas, Table 1 shows the characteristic chemical shifts of the compounds in comparison to literature values. The major metabolic changes, such as in the sugars (i.e., maltose, sucrose, α-glucose, and β-glucose) due to the different water regimes are shown, except for the potato samples that received the drought treatment. The major metabolic changes in the Markies cultivars after drought included a reduced concentration of linolenic acid. Overall, an increase in metabolic concentration (Figures 2A,B) occurred after the treatment was applied, except for Markies potatoes in drought conditions. Major concentration changes occurred in the amino acids, sugars, amines, and aromatics, particularly during drought and flood stress. This result suggests the plants developed a defense against moisture stress. The contribution plots showed the branched chain amino acids were among the most abundant compounds, whereas the precursor aspartate decreased.
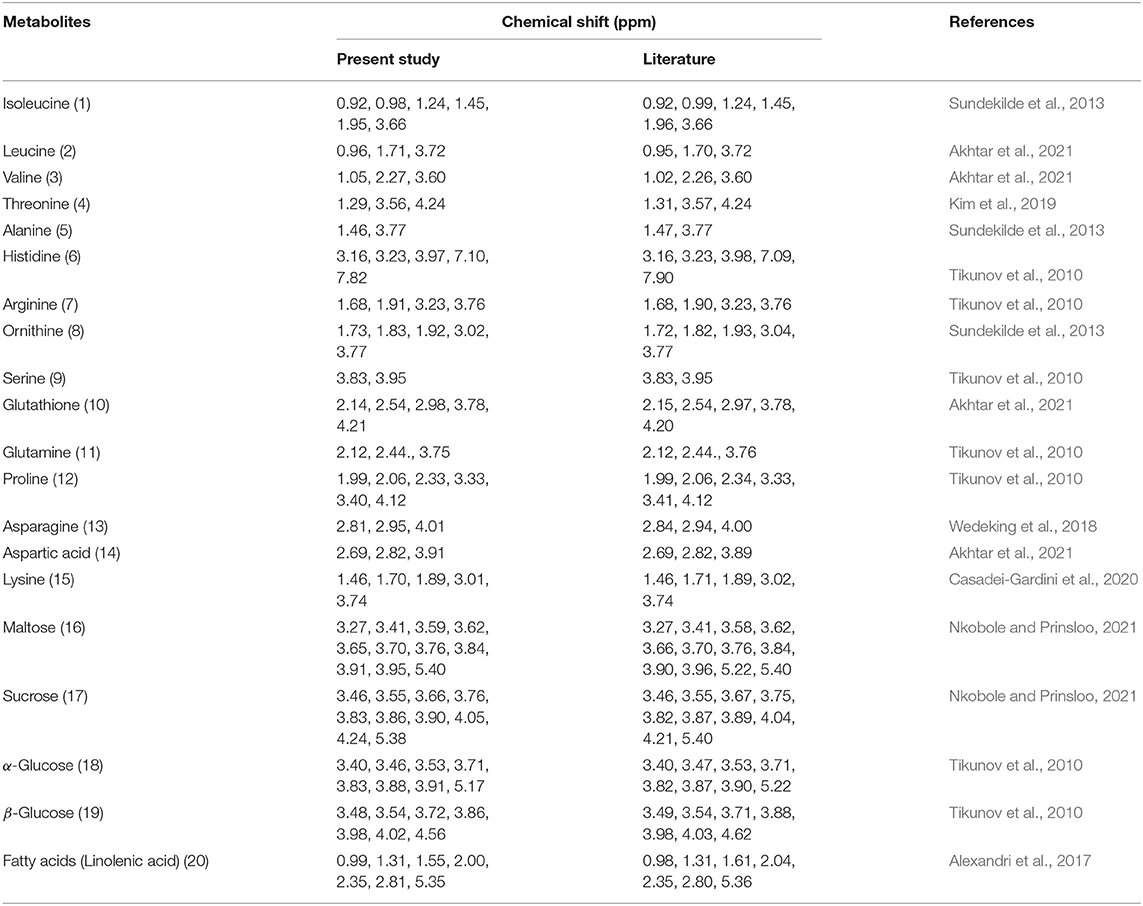
Table 1. Representative of the compounds used for metabolite identification in samples of Fianna and Markies potato as presented in the annotated NMR spectra which caused sample separation between the treatments (i.e., control, flooding and drought).
Figures 3A,B show a clear separation between the treatments. The scores for either drought, flood, or control were in similar positions for both the Fianna and Markies potato cultivars. The OPLS-DA model successfully separated the data between cultivars and in relation to the treatments applied (Figure 3B). The separation was constantly associated with the treatment applied to both the Markies and Fianna potato cultivars.
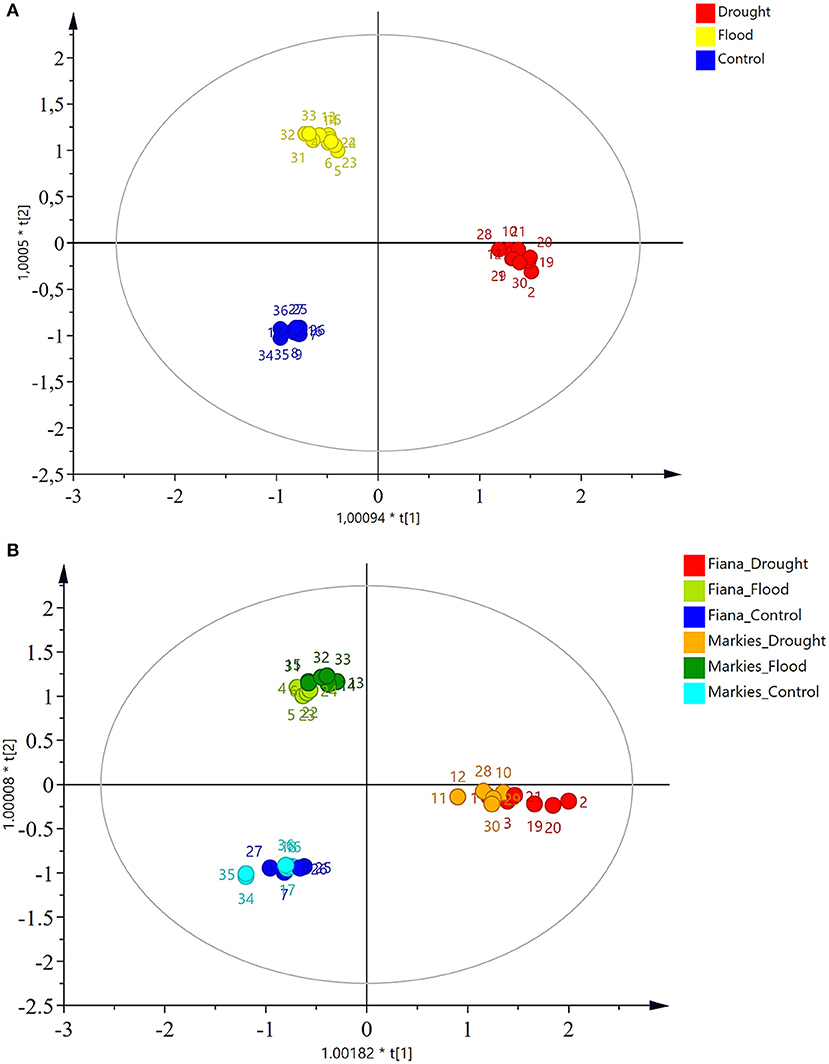
Figure 3. (A) Score plot of OPLS-DA of Fianna and Markies potato cultivars and treated with control, flooding, or drought irrigation. (B) Score plot of OPLS-DA of Fianna CV showed a clear separation between the treatments of control, flooding, or drought irrigation, this analysis was repeated using Markies CV.
Discussion
In the present study, the negative impacts of flooding and drought conditions were observed in potato growth and development and caused significant shifts in the metabolome. However, cultivar differences were observed in the performance of Fianna and Markies even though the water regime treatments were the same. These two cultivars were selected because they are among the predominant potato varieties that are used by the farmers. There are other widely-grown cultivars such as Mondial and Valor but we selected only two cultivars for a manageable and credible experiment. However, the approach was also that two cultivars would be selected initially and the number of cultivars would be incremented until differing metabolomic responses were discovered. Fortunately, the first two cultivars yielded differing responses. For sustainability, planting more than one potato cultivar is essential under the ever-changing climatic conditions that are associated with a wide range of less predictable weather patterns. The growing of two or more cultivars could be considered as one of the climate change adaptation strategies which could assist in mitigating risks associated with potato production in the current era of looming climate change threats. Therefore, understanding the variation between potato cultivars in relation to the effect of water stress on potato growth, development, and yield is essential in managing the effects of climate change (Hijmans, 2003). Extreme flooding or water logging events are sufficient to affect plant growth, such as leaf water potential, roots and tuber development (Morris and Brewin, 2014). This research is one of the starting points, which means more data is still required on the potato cultivars' genotypic variations to allow farmers to find appropriate cultivars for cultivation under climate change conditions. More in depth research is still required on developing a database of potato cultivars and their metabolic responses to combined environmental stress.
The 1H-NMR combined with PLS-DA analysis successfully classified the cultivars according to the irrigation treatments based on the chemical composition of the tubers. Since a clear discrimination between both the treatments and cultivars was observed, the discrimination was further identified using a PLS-DA model. The 1H-NMR spectroscopic analysis revealed the metabolic components produced by Markies and Fianna potato cultivars after exposure to varying soil moisture stress. Although the changes in the metabolite peaks as a result of environmental stresses induced were controlled, the results revealed that underlying metabolic variations existed between the two cultivars, as they clearly separated into two clusters (i.e., Markies and Fianna cultivar). Changes in the soil moisture altered the metabolism in the potato tuber, affecting both development and yield (Obidiegwu et al., 2015), the potato plants subjected to the drought and flooding treatments resulted in decreased yield quantity and quality when compared to plants that received control irrigation treatment. In addition, there was clear separation between the water regime treatments, although there was a clear overlap between Fianna and Markies samples. Both the Markies and Fianna potato plants were more drought-resistant than flood resistant, as there were more pronounced effects in the PLS-DA results of the flood treated potato tubers (Figures 3A,B).
Other identified metabolites (Table 1) included an aromatic amine (dopamine), a metabolite of the amino acid (isoleucine), and an organic nitrogenous compound (histamine). There was increased production of many metabolites, some of which are associated with methylation reactions, signal transduction, the recycling of nitrogen and the modification of oxidative stress. The results revealed how the Markies and Fianna cultivars reacted to water stress, to enable a good quality yield. The amino acids identified were classified as aspartate and glutamate. The availability of more pronounced amino acids has previously been reported in various plant tissues under control and stressful conditions, as they often accumulate to maintain nutrient uptake and intracellular ionic balance to sustain plants during stress (Sprenger et al., 2018).
The data analysis used in this study aimed to ascertain whether there were any metabolic variations in the potato plants as a result of different water regimes. The stress response of both Markies and Fianna potato cultivars contained a metabolic sensing component which centered around various amino acids and defense metabolites such as arginine, cystathionine, cysteine, cystine, dimethylglycine, dopa, glutamine, glycine, histidine, homocysteine, isoleucine, leucine, lysine, methionine, phenylalanine, serine, threonine, tryptophan and valine. These metabolites were diverse, although the quantities of the chemical compounds varied depending on the type of moisture stress. Biswas and Kalra (2018) suggest these compounds are often involved in protein protection, membrane and osmotic adjustment of the reactive oxygen species (ROS). They may have also undertake critical functions, including defense against drought or flood stress to which these potato cultivars were exposed. The use of two cultivars, (Markies and Fianna) added a layer of complexity to this research and revealed variations related to the individual cultivar.
The PCA and OPLS-DA analyses provided essential techniques for the interpretation of information-rich spectral data which assist in inferring scientific conclusions and improve our understanding of potato metabolic systems. Therefore, if the potatoes were exposed to a combination of stresses, either simultaneously or consecutively, this research revealed these cultivars will respond differently. Specifically, the Fianna cultivar responded to water stress better than the Markies cultivar. Differences in the potato cultivars are generally associated with their intrinsic ability to adjust to varying environmental conditions (Sprenger et al., 2018). This study assessed the relationships between different water irrigation treatments to Markies and Fianna potato cultivars and assessed the resulting quantities of different metabolites. The supervised model (i.e., OPLS-DA) indicated clear separation in the quantities of different metabolites, and the Markies and Fianna potato cultivars. Contribution plots were used to determine the compounds that were affected by the water treatment applied.
The annotation of important metabolites identified in these samples are presented in the NMR spectra (Figures 2A,B). The intense peaks within the range 3.5–4.5 ppm, were attributed to carbohydrates which usually account for more than 80% of the potato tuber dry weight (Barnaby et al., 2019). These peaks were more pronounced under the control and flood treatments (Markies cultivar), as well as in control and drought treatments (Fianna cultivar). Signals for sugars (maltose, sucrose, α-glucose and β-glucose) within the range of 4.2–5.4 ppm were clearly distinguished in the spectra and were likely to belong to the branched polysaccharides, amylopectin and amylose. Signals belonging to fatty acids (linolenic acid) were clearly pronounced in the Fianna cultivar and less intense in the Markies cultivar. Low intensity peaks were identified between 7.44 and 7.48 ppm and could be assigned to the high-molecular-weight aromatics, possibly polyphenols. These results confirm the findings of Barnaby et al. (2019), who stated that the plant's biochemical compounds, such as fructose, glucose, phosphorus, potassium and myo-inositol activity in the leaves, might be reduced in plants subjected to drought, due to the inhibition of photosynthesis caused by stomatal closure.
Moreover, the regions of chained amino acids increased, whereas defense metabolite levels varied with the cultivar (Figures 1A,B, 2A,B) and treatment application (Figures 3A,B). Therefore, this research suggests a positive relationship between plant stress (drought and flooding treatment) and amino acids (such as valine, arginine, ornithine and alanine) in the region of 1–3.5 ppm. Low concentrations of phosphates and antioxidants (such as glutathione) were associated with the flooding treatment in both Markies and Fianna cultivars. Some amino acids (such as asparagine, aspartic acid and isoleucine) showed low concentrations in the drought treatment, although the quantities varied remarkably among the cultivars. The amino acids; valine, isoleucine, and leucine, are essential in the production of plant defense metabolites and resistance against plant pathogens were also identified (Moroz et al., 2017). The non-protein amino acid (2-aminobutyric acid) was also found in both the drought and flood treated potato plants. This metabolite is known to induce resistance in plants against biotic and abiotic stresses (Sós-Hegedus et al., 2014). Similar levels of proline existed in all three treatment samples. Proline is believed to be of benefit in potato plants under stress, as discussed in Kishor et al. (2015).
The clearly distinct clustering indicated the water regime treatment effects and cultivar variations. The metabolite variations indicated the possibility of breeding potato cultivars that can withstand drought or flooding conditions. The level of metabolic production reflects the cellular state of the potatoes under abiotic stress (control, drought, or flood conditions). The results provide data on the metabolites that are responsible for the biochemical phenotype of the cell, tissue or entire living organism (Tiago et al., 2016). However, more in-depth research is required to develop a database of potato cultivars and their metabolic responses to combined environmental stresses. This database will provide insight into suitable potato cultivars that could yield marketable and nutritious potatoes under changing climatic conditions.
Conclusion
This study demonstrated the 1H-NMR-based metabolomic approach could be used to examine metabolic changes in potato plants exposed to soil moisture stress. The annotation of the 1H-NMR spectra allowed us to determine the compounds that altered with moisture stress and included metabolism processes associated with sugars, carbohydrates, aromatics, amino acids and organic acids. Clear metabolic separations were observed between the cultivars and between water treatments. Potato plants exposed to drought treatments exhibited a certain degree of resistance to moisture stress. Understanding the combination of these metabolic alterations may assist in the identification of potato cultivars that can withstand drought or flood stress associated with climate change. This study has implications for biomarker discovery and breeding. It has been demonstrated that potato cultivars have differing responses to water regimes. A wider study which should include more potato varieties and a rigorous search for biomarkers is possible given the findings of this study. Biomarkers can be utilized as markers for selection during breeding for potato cultivars which have tolerance to water stress.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
SM and DM initiated the project. DM also supervised this research. KN served as project advisor, assisted during planting, data analysis, and provided critical reading of the manuscript. MR did NMR laboratory analysis and provided inputs during the drafting of the manuscript. OB assisted with NMR analysis, its figures, and provided feedback during the drafting of the manuscript. SM further prepared the manuscript. All authors have read and approved the final submitted version.
Funding
This research was funded by University of South Africa (Doctoral Bursary).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Highly appreciated Mr. G. Magaseng as well as other staff members for assistance at the greenhouse. SM would also like to thank Editage for editing this paper. SM would like to thank her husband Mr. Z. M. Mdlalose, her two sons Mr. T. L. M. Mdlalose and Mr. T. Z. K. Mdlalose for their support, assistance, always being there for me, and their endless love throughout this research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2022.801504/full#supplementary-material
References
Akhtar, M. T., Samar, M., Shami, A. A., Mumtaz, M. W., Mukhtar, H., Tahir, A., et al. (2021). 1H-NMR-based metabolomics: an integrated approach for the detection of the adulteration in chicken, chevon, beef and donkey meat. Molecules. 26, 4643. doi: 10.3390/molecules26154643
Alexandri, E., Ahmed, R., Siddiqui, H., Choudhary, M. I., Tsiafoulis, C. G., and Gerothanassis, I. P. (2017). High resolution NMR spectroscopy as a structural and analytical tool for unsaturated lipids in solution. Molecules. 22, 1663. doi: 10.3390/molecules22101663
Barnaby, J. Y., Fleisher, D. H., Singh, S. K., Sicher, R. C., and Reddy, V. R. (2019). Combined Effects of drought and CO2 enrichment on foliar metabolites of potato (Solanum tuberosum L.) cultivars. J. Plant Interact. 14, 110–118.
Beetge, L., and Krüger, K. (2018). Drought and heat waves associated with climate change affect performance of the potato aphid macrosiphum euphorbiae. Scientific Rep. South Africa. 9, 1–9. doi: 10.1038/s41598-018-37493-8
Biswas, J. C., and Kalra, N. (2018). Effect of waterlogging and submergence on crop physiology and growth of different crops and its remedies: Bangladesh perspectives. Saudi J. Eng. Technol. 3, 315–329. doi: 10.21276/sjeat.2018.3.6.1
Casadei-Gardini, A., Del Coco, L., Marisi, G., Conti, F., Rovesti, G., Ulivi, P., et al. (2020). 1H-NMR based serum metabolomics highlights different specific biomarkers between early and advanced hepatocellular carcinoma stages. Cancers. 12, 241. doi: 10.3390/cancers12010241
Defernez, M., Gunning, Y. M., Parr, A. J., Shepherd, L. V. T., Davies, H. V., and Colquhoun, I. J. (2004). NMR and HPLC-UV profiling of potatoes with genetic modifications to metabolic pathways. J Agri Food Chem. 52, 6075–6085. doi: 10.1021/jf049522e
George, T. S., Taylor, M. A., Dodd, I. C., and White, P. J. (2017). Climate change and consequences for potato production: a review of tolerance to emerging abiotic stress. Potato Res. Netherlands. 60, 239–268. doi: 10.1007/s11540-018-9366-3
Handayani, T., Gilani, S. A., and Watanabe, K. N. (2019). Climatic changes and potatoes: how can we cope with the abiotic stresses? Breeding Science, Japan. 69, 545–563. doi: 10.1270/jsbbs.19070
Haverkort, A. J., Franke, A. C., Engelbrecht, F. A., and Steyn, J. M. (2013). Climate change and potato production in contrasting South African agro-ecosystems. 1. effects on land and water use efficiencies. Potato Res. USA. 56, 1–20. doi: 10.1007/s11540-013-9230-4
Hijmans, R. J (2003). The Effect of Climate Change on Global Potato Production. American Journal of Potato Research 80, 271–280. doi: 10.1007/BF02855363
Kim, E. R., Kwon, H. N., Nam, H., Kim, J. J., Park, S., and Kim, Y. H. (2019). Urine-NMR metabolomics for screening of advanced colorectal adenoma and early stage colorectal cancer. Scientific Rep. 9, 1–10. doi: 10.1038/s41598-019-41216-y
Kishor, P. B. K., Kumari, P. H., Sunita, M. S. L., and Sreenivasulu, N. (2015). Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front. Plant Sci. 6, 1–17. doi: 10.3389/fpls.2015.00544
Leff, B., Ramankutty, N., and Foley, J. A. (2004). Geographic distribution of major crops across the World. Global Biogeochemical Cycles. 18, 1–33. doi: 10.1029/2003GB002108
Manners, R., and Van Etten, J. (2018). Are agricultural researchers working on the right crops to enable food and nutrition security under future climates? Glob Environ Change. 53, 182–194. doi: 10.1016/j.gloenvcha.2018.09.010
Moroz, N., Fritch, K. R., Marcec, M. J., Tripathi, D., Smertenko, A., and Tanaka, K. (2017). Extracellular alkalinization as a defense response in potato cells. Front. Plant Sci. 8, 1–11. doi: 10.3389/fpls.2017.00032
Morris, J., and Brewin, P. (2014). The impact of seasonal flooding on agriculture: the spring 2012 floods in somerset, england. J. Flood Risk Manag. 7, 128–140. doi: 10.1111/jfr3.12041
Nkobole, N., and Prinsloo, G. (2021). 1H-NMR and LC-MS based metabolomics analysis of wild and cultivated amaranthus spp. Molecules. 26, 795. doi: 10.3390/molecules26040795
Obidiegwu, J. E., Bryan, G. J., Jones, H. G., and Prashar, A. (2015). Coping with Drought: Stress and Adaptive Responses in Potato and Perspectives for Improvement. Front. Plant Sci. 6, 1–26. doi: 10.3389/fpls.2015.00542
Pereira, A. B., and Shock, C. C. (2006). Development of irrigation best management practices for potato from a research perspective in the United States. Am J Potato Res. USA. 84, 1–20. www.sakia.org/ep_2006_01_01
Quiroz, R., Ramírez, D. A., Kroschel, J., Andrade-Piedra, J., Barreda, C., Condori, B., et al. (2018). Impact of climate change on the potato crop and biodiversity in its center of origin. Open Agri. South America. 3, 273–283. doi: 10.1515/opag-2018-0029
Sós-Hegedus, A., Juhász, Z., Poór, P., Kondrák, M., and Antal, F. (2014). Soil drench treatment with ß-aminobutyric acid increases drought tolerance of potato. PLoS ONE. 9, 1–15. doi: 10.1371/journal.pone.0114297
Sprenger, H., Erban, A., Seddig, S., Rudack, K., Thalhammer, A., Le, M. Q., et al. (2018). Metabolite and transcript markers for the prediction of potato drought tolerance. Plant Biotech J. 16, 939–950. doi: 10.1111/pbi.12840
Sundekilde, U. K., Larsen, L. B., and Bertram, H. C. (2013). NMR-based milk metabolomics. Metabolites. 3, 204–222. doi: 10.3390/metabo3020204
Tiago, F. G., Ana, T. M., and Carla, A. (2016). Mass spectrometry as a quantitative tool in plant metabolomics. Philosophical Transact. A. 374, 1–26. doi: 10.1098/rsta.2015.0370
Tikunov, A. P., Johnson, C. B., Lee, H., Stoskopf, M. K., and Macdonald, J. M. (2010). Metabolomic investigations of American Oysters using 1H-NMR spectroscopy. Marine Drugs. 8, 2578–2596. doi: 10.3390/md8102578
Trapero-Mozos, A., Wayne, L., Morris, W. L., Ducreux, L. J. M., McLean, K., Stephens, J., et al. (2018). Engineering heat tolerance in potato by temperature-dependent expression of a Specific allele of heat-shock cognate 70. Plant Biotech. J. 16, 197–207. doi: 10.1111/pbi.12760
Keywords: potato tuber (Solanum tuberosum), nuclear magnetic resonance, principal component analysis, water stress, climate change
Citation: Mdlalose SP, Raletsena M, Ntushelo K, Bodede O and Modise DM (2022) 1H-NMR-Based Metabolomic Study of Potato Cultivars, Markies and Fianna, Exposed to Different Water Regimes. Front. Sustain. Food Syst. 6:801504. doi: 10.3389/fsufs.2022.801504
Received: 25 October 2021; Accepted: 04 March 2022;
Published: 04 April 2022.
Edited by:
Amos P. K. Tai, The Chinese University of Hong Kong, ChinaReviewed by:
Benoit Bizimungu, Agriculture and Agri-Food Canada, CanadaTahira Fatima, Purdue University, United States
Copyright © 2022 Mdlalose, Raletsena, Ntushelo, Bodede and Modise. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samukelisiwe P. Mdlalose, NTc2Njc2MDhAbXlsaWZlLnVuaXNhLmFjLnph
†Present address: David M. Modise, Faculty of Natural and Agricultural Sciences, Food Security, and Safety, North-West University, Mahikeng, South Africa
 Samukelisiwe P. Mdlalose
Samukelisiwe P. Mdlalose Maropeng Raletsena
Maropeng Raletsena Khayalethu Ntushelo
Khayalethu Ntushelo Olusola Bodede
Olusola Bodede David M. Modise
David M. Modise