
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Sustain. Food Syst. , 21 March 2022
Sec. Crop Biology and Sustainability
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.796113
This article is part of the Research Topic Microbial Services for Sustainable Agriculture View all 8 articles
Currently, the tropics harbor a wide variety of crops to feed the global population. Rapid population expansion and the consequent major demand for food and agriculture-based products generate initiatives for tropical forest deforestation, which contributes to land degradation and the loss of macro and micronative biodiversity of ecosystems. Likewise, the entire dependence on fertilizers and pesticides also contributes to negative impacts on environmental and human health. To guarantee current and future food safety, as well as natural resource preservation, systems for sustainable crops in the tropics have attracted substantial attention worldwide. Therefore, the use of beneficial plant-associated microorganisms is a promising sustainable way to solve issues concerning modern agriculture and the environment. Efficient strains of bacteria and fungi are a rich source of natural products that might improve crop yield in numerous biological ways, such as nitrogen fixation, hormone production, mobilization of insoluble nutrients, and mechanisms related to plant biotic and abiotic stress alleviation. Additionally, these microorganisms also exhibit great potential for the biocontrol of phytopathogens and pest insects. This review addresses research regarding endophytic and rhizospheric microorganisms associated with tropical plants as a sustainable alternative to control diseases and enhance food production to minimize ecological damage in tropical ecosystems.
The ever-growing demand for agriculture-based products and food security does not meet the rapid increase in world population (Pawlak and Kołodziejczak, 2020; Fasusi et al., 2021). To overcome this global challenge, in the past few decades, agricultural systems have been adapted to this reality. Within a limited time, the use of chemical fertilizers and agrochemicals is capable of increasing food production and improving crop yield, quality, and shelf life. However, these practices are costly in terms of ecosystem degradation, climate change, soil erosion, and biodiversity loss (Fasusi et al., 2021; Mitter et al., 2021). Reports have reiterated the gradual decrease in soil health in the long term as an effect of the indiscriminate use of agrochemicals during the past few decades (El-Ghamry et al., 2018; Guha et al., 2020). Furthermore, Berkelmann et al. (2020) showed that the functional potential of soil microbial communities in rainforests is drastically affected by their conversion into managed land-use systems.
Regarding conventional fertilizers (calcium ammonium nitrate (CAN), diammonium phosphate (DAP), monoammonium phosphate (MAP), nitrogen, phosphorus and potassium (NPK), single superphosphate (SSP), triple superphosphate (TSP), and urea, although widely used, it has been reported that in subhumid zones, low nutrient use efficiency is a consequence of using large amounts of these agronutrients (Rop et al., 2018; Lawrencia et al., 2021). According to the International Fertilizer Industry Association (2021) database (https://www.ifastat.org/databases/plant nutrition), between 2016 and 2018, Brazil exported 529.2 thousand tons and consumed on average 15,000,000 tons of N, P2O5, and K2O derivatives. Additionally, diseases caused by phytopathogenic fungi represent a severe threat to the production of several crops worldwide (Brauer et al., 2019; Cullen et al., 2019; Omomowo and Babalola, 2019).
Plant-associated microbes are a well-known source of natural secondary metabolites, as well as a vast and unexplored source of unique chemical structures of phytochemical compounds, biofertilizers, and growth promoters, which can be considered natural and sustainable approaches to minimize the use of agrochemicals. The involvement of these microbiological tools in the development of agriculture and biotechnological processes is evident since issues concerning sustainable agriculture vs. practices entirely dependent on agrodefensives and synthetic fertilizers might cause negative impacts on the environment and human health (Brauer et al., 2019; Cullen et al., 2019; Omomowo and Babalola, 2019).
Endophytes can establish a mutualistic interaction with the host plant by exchanging nutrients and protection; they produce antibiotics and other substances that can protect the plant against stress conditions such as attacks by herbivores and pests and plant pathogens. These microorganisms live in intimate interactions with the host plant without causing any apparent disease symptoms (Azevedo et al., 2000; Pacifico et al., 2019). In contrast, rhizospheric space is a narrow soil zone that surrounds the roots and possesses a dynamic and highly diversified microbial community (bacteria, fungi, arbuscular mycorrhizae, oomycetes, viruses, and archeas) that is attracted by plant exudates and nutrients and capable of directly and indirectly modulating plant growth, nutrition, and health (Figure 1) (Philippot et al., 2013).
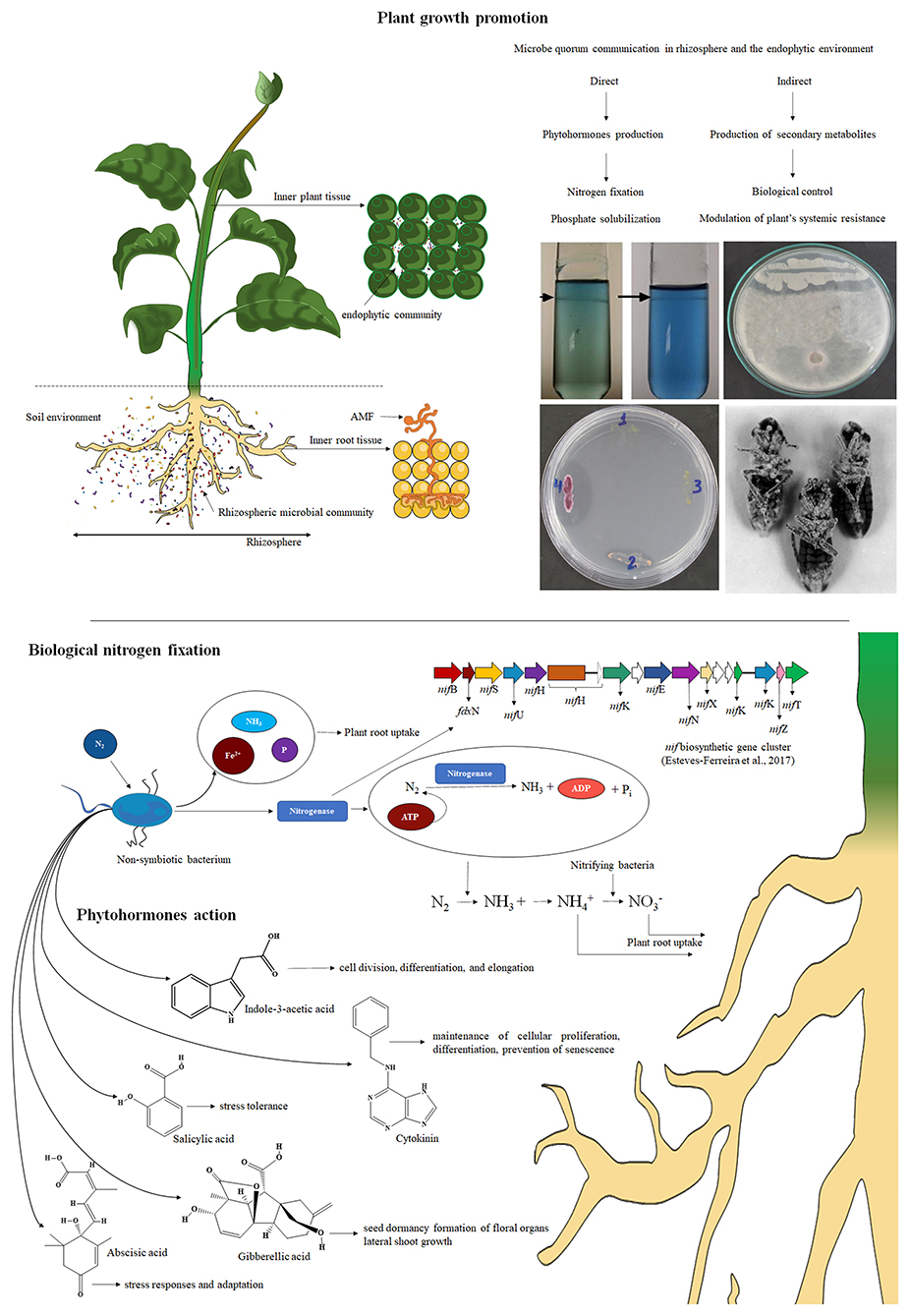
Figure 1. The rhizosphere is a dynamic and complex habitat of innumerable microbes and invertebrates that are attracted by nutrients, exudates, border cells, and mucilage released by plant roots. Thus, symbiotic PGPR indirectly/directly regulates the composition and biomass of the plant microcommunity in natural ecosystems. Indirect mechanisms might occur during competition for nutrients, where PGPR suppresses the effects of pathogens by the production of antibiotics, siderophores, and lytic enzymes, whereas nitrogen fixation, phosphate biodisponibilization, and phytohormone production directly affect plant growth.
Tropical ecosystems are major reservoirs of biological diversity on our planet, and with the recent availability of rigorous methods for characterizing microbial communities, the tremendous diversity of microorganisms in tropical environments is now also evident. Microorganisms compose a large proportion of biological diversity on Earth and have fundamental roles in the structure and function of ecosystems around the globe (Azevedo and Quecine, 2017). In this scenario, this review provides insights into the roles of microorganisms in a range of tropical environments, including agricultural fields, mangroves, savannahs, and Atlantic forests, and provides a glimpse into the microbial communities present in a range of tropical environments to explore the many potential applications of tropical microorganisms to agricultural productivity.
Endophytes were originally described in 1866 by de Bary, who observed and outlined possible distinctions between plant pathogens (Gouda et al., 2016). Such microorganisms might be found from roots to aerial plant structures, living a part of or all their life cycle within their host plants without causing apparent damage or disease (Liu et al., 2017). Endophytic microorganisms typically colonize internal plant tissues through the vascular system, apoplasts, and outer cell layers, where the pathways of penetration might be wounds or emergency areas of lateral roots and rootlets in germination (Liu et al., 2017).
Furthermore, endophytes constitute an important source of a plethora of natural compounds (alkaloids, phenolic acids, quinones, steroids, saponins, tannins, and terpenoids), which play a critical role in plant resistance to phytopathogenic infections (Fadiji and Babalola, 2020; Morelli et al., 2020). However, bacterial secondary metabolism is also mediated by quorum sensing (QS), which allows the production of multiple substances, where cell density creates a complex signaling system that transcribes specific genes for their interaction with their hosts (Krzyzek, 2019). Moreover, plants have been reported to possess quorum quenching (QQ) molecules, which can directly influence quorum sensing and respond to QS molecules, promoting direct plant growth (Kandel et al., 2017).
Likewise, the biodisponibilization of phytonutrients in the rhizosphere is regulated by quorum communication of the soil microbial community (Philippot et al., 2013). In summary, soil harbors a competitive and diversified microbiome (Onaka, 2017). The crucial influence of nutrients, exudates, border cells, and mucilage released by the plant in its root environment can significantly affect its rhizospheric microbial colonization (Hassan et al., 2019). Therefore, the role of rhizomicrobes in plant health is crucial (Omotayo and Babalola, 2020).
Plant growth-promoting rhizobacteria and endophytes have diverse beneficial effects on host plants through different mechanisms; these microorganisms are generally referred to as plant growth-promoting microbes (PGPMs). In return, the roots of the plant release exudates containing nutrients and compounds that can be used by PGPM for their development (Lopes et al., 2021). The inoculation of PGPM into crops is considered an environmentally friendly alternative to chemical fertilization (Lopes et al., 2021).
Innumerable interactions between plants and the soil microcommunity, such as PGPM, occur by indirect and direct mechanisms (Hassan et al., 2019). Indirectly, competition for nutrients in the rhizosphere might suppress potential pathogens (Khatoon et al., 2020). However, direct mechanisms include the ability to biosynthesize phytohormones that promote root and shoot growth (e.g., indole-3-acetic acid and cytokinin), solubilization of phosphate, zinc, potassium, and fixation of atmospheric nitrogen (Batista et al., 2018; Khatoon et al., 2020). Figure 1 Plant and scheme summarize the influence of endophytes and rhizosymbiotic microbes on the plant.
Many plant species currently cultivated and used in agriculture originate or were domesticated in the tropics (Gepts, 2008). Geographic localization, mean annual temperature, and mean precipitation favor high levels of biodiversity in tropical ecosystems (Andresen et al., 2018). Plants residing in tropical regions constitute a promising source of a great diversity of bacteria and fungi with beneficial potential to be explored as biological controllers of pathogens and plant growth promoters in a more sustainable and ecofriendly way (Harman et al., 2020; Umokoro, 2020).
According to Azevedo et al. (2000), the first reports on the isolation of microorganisms associated with tropical hosts in South America date back to the 1980's (Petrini and Dreifuss, 1981; Dreyfuss and Petrini, 1984). Since then, the number of studies focused on the isolation and exploration of endophytic and rhizospheric microorganisms inhabiting plants from different tropical ecoregions has increased worldwide (Azevedo and Quecine, 2017). Some studies related to this theme are discussed in further detail below.
Paullinia cupana var. sorbilis (Mart.) Ducke (Sapindaceae), or guarana, is a typical tropical plant originally from the Amazon rainforest, whose genetic basis is present mainly in the Maués state of Amazonas (Henman, 1982). Brazil is a unique country as a commercial-scale producer of guarana, and soft drinks and other products commercialized as syrup, powder, extract, dietary supplements, cosmetics, and pharmaceuticals serve both domestic and foreign markets (Kuri, 2008; Hamerski et al., 2013).
In recent decades, anthracnose has been limited to the production and expansion of guarana in the Amazon State (Atroch and Nascimento Filho, 2018), and currently, Bahia State leads production (Meneghetti et al., 2021). Since guarana has great social and economic importance for Brazil and methods to control anthracnose are not effective (Tavares et al., 2005), research groups have joined efforts to find the microbiota associated with P. cupana var. sorbilis as a solution for reducing the incidence of the disease and increasing production.
In a study carried out by Bogas et al. (2015), the authors verified differences in the structure of the endophytic bacterial communities associated with P. cupana leaves collected from Santa Helena farm, Maués. Plants with symptoms of anthracnose showed significantly higher diversity, density, and number of operational taxonomic units than asymptomatic plants. The Firmicutes phylum, represented mainly by the Bacillus genus, was significantly more isolated from asymptomatic plants. In 16S rRNA gene clone library analysis, Proteobacteria was the phylum significantly more present in asymptomatic plants. The Pseudomonas genus was observed in both treatments but at the highest frequency in asymptomatic plants. Based on other studies, the authors suggested that the presence of Colletotrichum sp. seems to be important to cause shifts in the P. cupana microbial communities, and the Bacillus and Pseudomonas genera could confer protection to asymptomatic plants against infection by phytopathogens.
In a similar study, Bonatelli et al. (2019) evaluated endophytic bacterial communities of leaves of P. cupana collected from Santa Helena farm, Maués. In general, the culture-dependent approach revealed a predominance of Acinetobacter, Enterobacter, and Serratia genera in symptomatic leaves and Erwinia, Pantoea, Pseudomonas, and Stenotrophomonas genera in asymptomatic leaves. The Bacillus genus was accessed similarly in both treatments. Using pyrosequencing, Proteobacteria was the most abundant phylum. Acinetobacter, Pseudomonas, and Klebsiella were the most abundant genera in symptomatic leaves. In vitro assays showed that especially Bacillus sp. EpD2-5 isolated from asymptomatic leaves inhibited the growth of Colletotrichum sp., which was attributed to lytic enzymes and siderophores produced by the bacteria evaluated.
Liotti et al. (2018) isolated endophytic bacteria from seed, leaf, and root tissues collected from two P. cupana genotypes in Embrapa (Brazilian Agricultural Research Company) experimental farms located in Manaus and Maués, Brazil. The colonization rate, richness, diversity, and species composition varied across the plant organs and geographic location. The main phyla identified were Proteobacteria, Actinobacteria, and Firmicutes. Some strains induced Sorghum bicolor growth and antagonistic traits against Colletotrichum guaranicola and Fusarium decencellulare.
Batista et al. (2018) evaluated the diversity and plant growth-promoting potential of bacteria isolated from rhizosphere samples collected from asymptomatic and symptomatic guarana plants from Santa Helena farm, Maués. Proteobacteria and Firmicutes were the dominant phyla, and Burkholderia and Bacillus were the most abundant genera found in the rhizosphere samples evaluated. The multitrait PGP strains Bacillus sp. RZ2MS9 and Burkholderia ambifaria RZ2MS16 expressively promoted corn and soybean growth under greenhouse conditions. The strains RZ2MS9 and RZ2MS16 increased the root dry weight in corn and significantly increased the dry weights in both corn and soybean. The data suggested that the RZ2MS9 and RZ2MS16 strains have the potential to be explored as inoculants within a broad range of hosts, which could be related to biological nitrogen fixation and auxin, phosphate, and siderophore production. The agronomic potential of Bacillus sp. the RZ2MS9 strain (Batista et al., 2018) was evaluated in other studies. Andrade et al. (2020) verified an increase in the seed germination and dry mass of seedlings of DKB390 and 30A37PW® maize hybrids treated with rhizobacteria, as well as an increase in the seed speed of the 30A37PW® hybrid treated with bacteria-free filtrate. Almeida et al. (2021) showed a synergistic effect on maize root and shoot dry weight coinoculated with Bacillus sp. RZ2MS9 and the commercial inoculant Azospirillum brasilense Ab-V5.
Additionally, studies have demonstrated the importance of the fungal community associated with guarana plants. Silva et al. (2018) isolated and identified endophytic fungi from roots and seeds of P. cupana susceptible and tolerant to anthracnose cultivated in Embrapa experimental farms in the cities of Manaus and Maués. The plant geographic location, clonal type, and organ influenced the structure of the endophytic fungal community of P. cupana, which harbors species that synthesize lytic enzymes (i.e., Fusarium oxysporum, Mycoleptodiscus terrestres, Trichoderma harzianum) and indole-3 acid acetic acid (i.e., F. oxysporum, Fusarium solani, T. harzianum, Trichoderma asperellum) in vitro, opening possibilities to propose their applications to plant growth and protection.
In a more recent study, Casas et al. (2021) demonstrated the reduction of lesions caused by Colletotrichum fructicola in guarana seedlings (cultivar BRS-Maués) after treatment with a conidial suspension of the endophyte Colletotrichum siamense isolated from healthy leaves of guarana plants at the Santa Helena farm. The endophyte promoted total protein and pathogenesis-related protein (PRP) synthesis at different times after C. fructicola inoculation. This study seems to be the first about resistance induction in guarana plants for the control of phytopathogens.
Jatropha curcas L. (Euphorbiaceae), is native to Central America and distributed worldwide in tropical regions. Genetically close to the castor plant (Ricinus communis L), J. curcas is a rapidly growing plant that is capable of adapting to a wide range of environmental stress conditions, including nutrient-limited soils and low to high rainfall seasons, and might take advantage of anthropic/unproductive areas (Kumar et al., 2016). Moreover, J. curcas is recommended for cultivation for soil health improvement, climate change mitigation, carbon sequestration, and socioeconomic development, and in the past few decades, J. curcas has been shown to be a biodiesel source (Islam et al., 2014). In Brazil, J. curcas is also reported as an alternative supply of vegetable oil from its seeds (Laviola et al., 2015).
Studies concerning the biotechnological and agricultural potential of bacterial microbiota associated with J. curcas represent an alternative that might contribute to research aiming to increase productivity and establish this crop. Mohanty et al. (2017) hypothesize that its ability to adapt to environmental stresses could be due to its endophytes (Madhaiyan et al., 2013a).
Sousa and Silva (2012) discuss the differential growth promotion in J. curcas by different endophytic strains of Bacillus. In vitro studies were performed to test the phosphate solubilization and indole-3-acetic acid (IAA) production of these strains. The best-performing endophytic strain was identified as B. megaterium (BP4D), which exhibited the highest phosphate solubilization level and IAA production level. In vivo and under greenhouse conditions, the capacity to promote plant growth was observed in certain vegetative parameters, such as mass and the length of the roots, by the BP4D strain.
Jha and Saraf (2012) described a novel Enterobacter cancerogenus strain, MSA2, as a plant growth-promoting (PGP) bacterium isolated from the J. curcas rhizosphere. MSA2 was then used as an inoculant in J. curcas vegetatives under greenhouse conditions. J. curcas culture showed an increase in almost all vegetative characteristics of the plant in comparison with the uninoculated control, as well as increased root length and root dry weight.
Madhaiyan et al. (2013b) reported a novel N-fixing endophyte, Enterobacter sp. R4-368, which was able to colonize root and stem tissues and significantly promoted early plant growth and seed productivity of J. curcas in sterilized and non-sterilized soils. In addition, Madhaiyan et al. (2013c) announced the complete genome sequence of R4-368. Draft genome sequence analysis showed the discovery of a complete set of genes for nitrogen fixation and synthesis and efflux of volatile compounds, auxins, and siderophores. Some authors consider Enterobacter strains to be elite plant growth-promoting bacteria (Ramesh et al., 2014; Ikeda et al., 2020).
Madhaiyan et al. (2015) presented a detailed study regarding the role of a novel leaf-colonizing diazotroph, Methylobacterium radiotolerans L2-4, on J. curcas biomass and seed production. Foliar spray of L2-4 improved plant height, leaf number, chlorophyll content, and stem volume. At the same time, Madhaiyan et al. (2014) reported the draft genome sequence of L2-4, which exhibits several genes involved in metabolic pathways involved in PGP.
Mohanty et al. (2017) characterized the endophytic bacteria of J. curcas and their PGP effects on maize (Zea mays L.). The main genera found were Brevibacillus, Paenibacillus, Rhizobium, and Sphingomonas. All isolates were positive for phosphate and IAA production. Inoculation of the endophytic strains on maize seeds significantly increased the shoot and root length of seedlings compared with the shoot and root length of non-inoculated seedlings. Similarly, Machado (2019) described inoculation in Z. mays seeds of the bacterial strains EPM-41A, EPM-54, and EPM-92 - Bacillus sp.; EPM-63B - Citrobacter sp., EPM-34 Curtobacterium sp.; EPM-4 and EPM-63 Klebsiella sp.; and EPM-2 Serratia sp., where the treatment with EPM2- Serratia sp. exhibited significantly higher values for all growth parameters.
There is a great incentive for the commercial cultivation of J. curcas in Brazil as a renewable raw material for biodiesel production. However, the great expansion of cultivated areas has been accompanied by the occurrence of new pathogens and the appearance of diseases called descending dryness and stem base rot, caused by species of the Lasiodiplodia fungus, causing losses in productivity and mortality of 80% of physic nut plants (Machado et al., 2014). Thus, chemical control alone does not provide protection or curative treatment. The adoption of a sequence of prophylactic care has been recommended in the past few decades (Silva et al., 2015).
Recently, Machado et al. (2020) identified bacterial strains belonging to the genera Arthrobacter, Bacillus, Citrobacter, Curtobacterium, Enterococcus, Klebsiella, Leucobacter, Lysinibacillus, Microbacterium, Rhodococcus, and Serratia endophytically associated with J. curcas. Additionally, a total of 37 strains were screened in vitro for PGP, where 56.75% of evaluated bacteria could fix nitrogen, 62% of the isolates were able to synthesize the phytohormone IAA in the presence of the tryptophan precursor, and 32% of the evaluated isolates had the capacity to solubilize insoluble inorganic phosphate. Additionally, these strains were evaluated in vitro against the phytopathogenic fungi Lasidioplodia subglobosa, L. euphorbicola, and L. pseudotheobromae, where 35% exhibited antagonism to the three tested phytopathogens, whereas the Bacillus genus showed higher antagonistic activity than the other strains.
The Citrus genus (Rutaceae family) comprises one of the fruit crops of major economic, social and cultural importance worldwide. Originating from southeastern Asia, where Citrus domestication probably started, the crop is well-adapted to tropical and subtropical climate zones (Talón et al., 2020). Brazil, followed by China, India, Mexico, and the United States, is among the largest citrus producers in the world (FAOSTAT, 2019). Global production of citrus fruits was estimated to increase by 4 percent to 98 million tons in 2020/2021 (United States Department of Agriculture, 2021). However, diseases caused by pests and pathogens limit citrus production, leading to considerable damage to orchards and economic losses in the sector.
Among the most important citrus diseases caused by bacteria are huanglongbing (HLB), citrus variegated chlorosis (CVC), and citrus canker (CCK) (Mendonça et al., 2017). Although integrated management maintains citrus disease incidence at lower levels, there is no effective cure or treatment. While CVC incidence has remained lower in 2020 than in 2019, the CCK incidence increased by 15% (Fundecitrus, 2020).
Citrus plants treated with beneficial microorganisms can have important metabolic responses to infection by Candidatus Liberibacter spp. (Munir et al., 2021). This phloem-limited bacterium is transmitted to citrus trees by Diaphorina citri (Hemiptera: Liviidae) (Ammar et al., 2011) and causes HLB, a disease currently considered the most destructive affecting the citrus industry worldwide (Wang et al., 2017).
Bacillus species have been isolated from healthy/asymptomatic citrus plants at high frequency, which suggests their role in HLB resistance (Munir et al., 2020a). Munir et al. (2020b) showed that primary and secondary metabolites were activated in HLB-affected plants in response to inoculation with the endophyte Bacillus subtilis L1-21. Tang et al. (2018) demonstrated the potential of Bacillus amyloliquefaciens GJ1 isolated from healthy leaves of Citrus sinensis (L.) Osbeck as a novel biocontrol agent in Candidatus Liberibacter asiaticus-infected plants. Different genes and proteins involved in detoxification mechanisms were upregulated by endophytic bacteria, suggesting a use for HLB biocontrol to improve plant health. Recently, Nan et al. (2021) demonstrated that B. amyloliquefaciens GJ1 improved the immunity of C. Liberibacter asiaticus-infected citrus by increasing photosynthesis and enhancing the expression of defense-related genes.
Another strategy for HLB management is the discovery of microorganisms to control D. citri. Dorta et al. (2018) showed that Bacillus thuringiensis (Bt) strains translocated from roots to shoots in citrus seedlings and caused mortality of D. citri nymphals. The mortality was 42–77% and 66–90% at 2 and 5 days, respectively, after inoculation in citrus plants of the recombinant Bt strains harboring a single cry or cty gene.
The endophytic microbial community associated with citrus plants can be used as a biocontrol agent of the xylem-limited bacteria Xylella fastidiosa, the causal agent of CVC, one of the diseases that caused the most damage to the Brazilian citrus industry in the 2000's (Azevedo et al., 2016).
Lacava et al. (2004) showed that X. fastidiosa growth was reduced by endophytic Methylobacterium mesophylicum SR1.6/6 in vitro. Later, in experiments of co-inoculation using Catharanthus roseus as a model plant, Lacava et al. (2006) showed that the population of M. mesophylicum SR1.6/6 was lower in the presence of X. fastidiosa, suggesting that these bacteria interact inside xylem vessels competing for space and nutrients. Since M. mesophilicum SR 1.6/6 was isolated from CVC-free citrus plants at high frequency (Araújo et al., 2002), this endophyte could contribute to preventing the disease in the host plants (Lacava et al., 2006; Azevedo et al., 2016). Plant exudates and N-acyl-homoserine lactones regulate specific gene expression in M. mesophilicum SR 1.6/6 (Dourado et al., 2013), and co-cultivation of this endophyte and X. fastidiosa leads to specific adaptive responses of the phytopathogen (Dourado et al., 2015).
X. fastidiosa synthesizes exopolysaccharides (EPSs) or fastidian gum, which are essential for biofilm formation, and occlusion of the sap flow in the xylem, which leads to CVC symptoms (Simpson et al., 2000). Based on the premise of symbiotic control (Miller, 2007), Ferreira Filho et al. (2012) modified Methylobacterium extorques AR1.6/2 isolated from citrus (Araújo et al., 2002) to express endoglucanase A (EglA), which was cloned from a citrus endophytic Bacillus pumilus able to degrade xanthan gum (Lima et al., 2005). Using scanning electron microscopy, the bacterial strain (AREglA) and X. fastidiosa were observed to possibly coinhabit the xylem vessels of C. roseus, and AREglA may decrease biofilm formation. AREglA also stimulated the production of the resistance protein catalase in the inoculated plants. The results suggested the potential use of AR1.6/2 and other bacteria isolated from citrus for the symbiotic control of X. fastidiosa in its niche.
Bogas et al. (2016) demonstrated for the first time the potential of Methylobacterium sp. to promote the growth of Citrus limonia and Citrus sunki seedlings under commercial nursery conditions. M. mesophylicum SR 1.6/6, M extorquens AR 1.6/2 and M. extorquens AR 1.6/11 isolated from citrus (Araújo et al., 2002) significantly increased the height and/or biomass of roots and shoots at the end of rootstock production. The pathway of indole-e-acetic acid (IAA) biosynthesis identified in the M. mesophylicum SR 1.6/6 genome suggested that its mechanism was involved in citrus growth promotion. These effects seem to be dependent on specific interactions between Methylobacterium and citrus rootstock varieties and on the inoculation method. In general, Methylobacterium sp. can accelerate citrus growth, contributing to reducing the costs and the time that plants remain in the nursery.
Endophytes are also promising for the biocontrol of Xanthomonas citri subsp. citri (Xcc), the causal agent of CCK (Brunings and Gabriel, 2003). This disease affects all commercially important citrus species and varieties in many tropical and subtropical citrus-producing areas worldwide (Sharma and Sharma, 2009).
Liu et al. (2021) showed that the differences in bacterial endophytic communities and their abundance in citrus cultivars may be related to host CCK resistance or susceptibility. Firmicutes were significantly more abundant in resistant Satsuma mandarin leaves than in susceptible Newhall Navel Orange leaves. However, the abundance of this phylum was lower in all summer samples, with this number being much lower in resistant Satsuma mandarin, suggesting that this season would be the period with the highest citrus canker disease risk. At the genus level, Bacillus subtilis with antagonistic potential against Xcc was the main species present in all samples but was more abundant in the susceptible Newhall navel oranges. Pseudomonas, which also has antagonistic potential, was present in higher abundance in summer samples, particularly in resistant Satsuma mandarin samples.
In a study carried out by Daungful et al. (2019), the endophytes Bacillus amyloliquefaciens LE109 and B. subtilis LE24, both isolated from healthy Citrus aurantifolia, and Bacillus tequilensis PO80 isolated from healthy Citrus maxima, significantly inhibited the growth of Xcc in vitro. Lime plants did not display symptoms of CCK after inoculation with cell suspensions of Xcc and LE109 or LE24 or with crude bioactive compounds obtained from these endophytes. The study suggested that endophytes could compete with the pathogen for nutrients and produce metabolites that inhibited the growth of Xcc on lime plants.
Mangroves are typical tropical ecosystems situated between the land and the sea. These biological communities are frequently found in tropical and subtropical areas and occupy ~18.1 million hectares of the planet. These ecosystems demand high nutrient availability at the start of the trophic chain, which confers high importance on the activities of microorganisms, such as bacteria and fungi, that are responsible for the processes of degradation and formation of essential compounds and most of the carbon flow in the sediments of mangrove forests (Holguin et al., 2001). The adaptation of microbial species to mangrove conditions indicates a potential source of biotechnological resources to be explored, including the discovery of new microorganisms that produce compounds that can be used for agriculture (Dias et al., 2009). In this scenario, endophytes are of agronomic interest because these organisms can enhance plant growth and improve plant nutrition through diverse mechanisms, such as phosphorus solubilization and nitrogen fixation (Castro et al., 2014; Sebastianes et al., 2017).
In this context, Gayathri and Muralikrishnan (2013) collected root, stem, and leaf samples from mangrove plants at Chidambaram, Tamil Nadu, India. The authors obtained 24 isolates. All isolates were evaluated for plant growth-promoting activity using a PGPR activity test to detect the phosphate solubilization and biological nitrogen fixation activities of endophytic bacteria.
Deivanai et al. (2014) examined the compatible association of bacterial endophytes from Rhizophora apiculata of the Merbok brackish river in Semeling, Kedah, Malaysia, in colonizing rice tissues and discussed the likelihood of utilizing the symbiotic association of these endophyte isolates to increase the fitness of rice seedlings. The inoculation of rice seeds with endophytic strains of Bacillus cereus, B. amyloliquefaciens, and Pantoea ananatis significantly increased the root and shoot length, suggesting that endophytic bacteria from mangrove trees can increase the fitness of rice seedlings under controlled conditions.
Brazilian mangroves are primarily made up of three tree species, Rhizophora mangle, Laguncularia racemosa, and Avicennia sp. (Dias et al., 2009), from which several diverse endophytic bacteria have been isolated (Castro et al., 2014). In this scenario, Castro et al. (2014) performed a diversity analysis on endophytic bacteria obtained from the branches of Brazilian mangrove forests. Bacillus was the most frequently isolated genus, comprising 42% of the species isolated. However, other common endophytic genera, such as Curtobacterium, Enterobacter, and Pantoea, were also identified. Additionally, in Brazil, Castro et al. (2018) evaluated a large number of endophytic bacterial strains from three different plant species, namely, R. mangle, L. racemosa, and Avicennia sp., to examine phosphate solubilization. All 115 strains examined produced a halo during the phosphate solubilization test in vitro. The endophytic strain MCR1.48 (Enterobacter sp.), which has a high P solubilization index, was selected for in vivo assays in Acacia polyphylla. These authors selected the commonly used reforestation tree A. polyphylla, which has few published studies involving inoculation by bacteria of agronomic interest and used for the reforestation of degraded areas in Brazil (Rao et al., 2007). Inoculation with Enterobacter sp. strain MCR1.48 increased the dry mass of A. polyphylla shoots and roots, suggesting that the presence of the endophyte generates important benefits that promote the growth and fitness of this plant.
The biological control method using endophytic fungi capable of inhibiting phytopathogens seems to be a more ecofriendly and sustainable tool in agricultural management than using agrochemicals that negatively impact the environment, humans, and other organisms (Tewari et al., 2019). In this regard, Moreira et al. (2020) evaluated the antagonistic potential of Diaphorte sp. FS-94(4) strain isolated from Avicennia nitida, Brazilian mangrove forests (Sebastianes et al., 2013) against the phytopathogens Colletotrichum sp., Fusarium oxysporum, Phytophthora sojae, and Rhizopus microspores. The antagonism index (AI) ranged from 20 to 62%. The endophyte produced in vitro cellulase, suggesting that this enzyme is involved in suppressing phytopathogens by breaking down the cell wall.
The Brazilian tropical savannah, known as the Cerrado, has ~6,000 vascular plant species, featuring the highest floral biodiversity among the world's savannahs. The Cerrado also has a high diversity of microorganisms (Lacava and de Sousa, 2016; de Sousa et al., 2017; Lisboa et al., 2021). Much of the biodiversity of this microbiome is not yet known, which makes it one of the 25 most important terrestrial biodiversity hotspots on the planet (Oliveira-Filho and Ratter, 2002; Bogas et al., 2020). Among the diverse trees in the Brazilian savannah, the research groups have considered two species: Stryphnodendron adstringens (Mart.) Coville (Fabaceae) (Figure 2A) and Solanum lycocarpum Saint-Hill (Solanaceae) (Figure 2B). S. adstringens trees have the potential for medicinal applications. Its bark and fruit contain anti-inflammatory (Lima et al., 1998), antiviral (Felipe et al., 2006), and antimicrobial properties (Almeida et al., 2017). S. lycocarpum produces secondary metabolites that exhibit antioxidant, antibacterial, cytotoxic, and anti-inflammatory properties (da Costa et al., 2015).
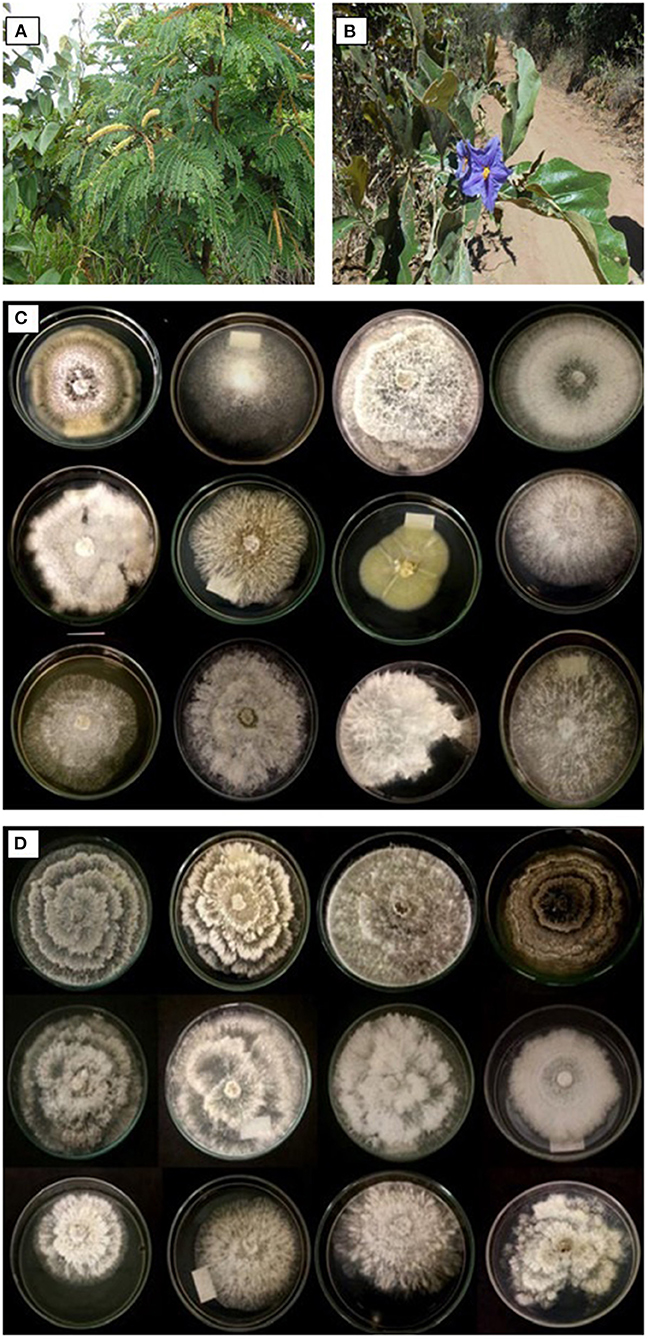
Figure 2. Examples of endemic Brazilian tropical savannah trees (22°01′03″S, 47° 53′27″W). (A) Stryphnodendron adstringens (Mart.) Coville (Fabaceae); (B) Solanum lycocarpum Saint-Hill (Solanaceae). Fungal morphological groups retrieved in the culture media isolation procedure. Endophytic fungi (morphological groups) isolated from leaves of S. adstringens (C) and S. lycocarpum (D).
Recently, Lisboa et al. (2021) reported that bacterial strains were isolated from rhizosphere samples of S. lycocarpum and were tested in vitro for direct mechanisms of plant growth promotion, such as phosphate solubilization. Among the 131 strains evaluated, the calcium phosphate solubilizing capacity trials showed that 45.8% of the strains exhibited a positive reaction. Some bacterial strains identified as Bacillus sp., Burkholderia sp., and Microbacterium sp. showed high solubilization potential. In this same study, the rhizobacteria strains were evaluated in visual agar plate assays against phytopathogenic fungi. A total of 50% of the straisn showed antagonist activity against Sclerotinia sclerotiorium, Moniliophthora perniciosa, Fusarium sp., Sphaceloma sp., Ceratocystis paradoxa, Alternaria alternata, Colletotrichum sp., Phytophthora sojae and Rhizopus microsporus. According to the authors, this research is the first report on the characterization, identification, and isolation of rhizospheric bacteria from S. lycocarpum in Brazil.
Torres (2018) reported the isolation and identification of 66 endophytic fungi from leaves of S. adstringens and S. lycocarpum collected in the savannah area of São Paulo State, Brazil (Figures 2C,D). The endophytic strains were evaluated in vitro for their antagonistic potential against the phytopathogens Colletotrichum sp., Fusarium oxysporum, and Lasiodiplodia subglobosa. The results showed that endophytic strains exhibited antagonistic activity against the phytopathogens tested. Additionally, Torres (2018) reported the ability of an endophytic fungal strain isolated from Brazilian savannah species (Bogas et al., 2020) to solubilize inorganic phosphate. The author observed the potential to solubilize inorganic phosphate with a mean phosphate solubilization index ranging from 1.46 to 1.93 and 1.19 to 2.61 by endophytic strains.
In a similar study, Sharma and Roy (2015) reported that fungal endophytes were isolated from the root, stem, and leaves of the plant Amaranthus spinosus, another species occurring in the savannah biome, and stated that endophytic fungal isolates of the plant A. spiunosus showed a positive test for phosphate solubilization. The phosphate solubilization efficiency was found to be highest for the fungal genus Aspergillus isolated from the stem of the plant.
Finally, Noriler et al. (2018) assessed the fungal community associated with Stryphnodendron adstringens leaves and petioles collected in the savannah area of Mato Grosso do Sul State, Brazil. The authors verified the antimicrobial activity of crude extracts produced by the endophytes and suggested that Diaporthe cf. heveae LGMF 1631 strain may represent an alternative to be used in the biocontrol of the phytopathogens Phyllosticta citricarpa and Colletotrichum abscissum.
Archaea constitute a group of microorganisms initially found only in environments with extremes conditions of pH, temperature salinity, pressure among other factors (Rampelotto, 2013). In the last few decades, archaea also are considered a substancial component of complex microbiomes, including plant-associated microbiomes in the above and belowground (Moissl-Eichinger et al., 2017).
Although the ecological role of archaea and its interaction with their host plants still remain unclear, the metagenomic data set have been essential to reveal archaeal groups living in the rhizosphere and endosphere that could be used to plant improvement. Data obtained by Taffner et al. (2018) provided evidences of the importance of archaea to promote plant growth through carbon and nitrogen cycling and auxin biosynthesis. In addition, the results suggested the potential role of these microorganism in plant protection against abiotic stress. Other metagenomic studies have revealed the importance of archaea in the ammonia oxidation in the soils (Bartossek et al., 2012; Clark et al., 2021).
Some studies have explored metagenomic data set for investigate archaea diversity and community structure in tropical agroecosystems. Fadiji et al. (2020) verified for the first time that organic fertilizer boosts the abundance and diversity of endophytic archaea in roots of maize as compared with chemical fertilizers. The phyla Crenarchaeota, Euryarchaeota, and Thaumarchaeota were identified in the samples with higher abundance collected from the organic fertilizer site. The results suggested a better understanding of the endophytic archaea roles in the maize to reduce the dependence on chemical fertilizers and improve the sustainable practices. Xu et al. (2018) performed the metagenomic sequencing of the rhizosphere and bulk soil samples to define citrus microbiome, and verified a low relative abundance of archaea phyla, such as Crenarchaeota and Euryarchaeota. Despite, the results provided relevant information to explore the potential of the citrus microbiome for sustainable production and improve health plant. Dubey (2016) used metagenomic to analyzed the rhizosphere of bioenergy crop J. curcas adapted to grow under salt stress and high temperature conditions. The results showed high abundances of uncultured archaea Crenarchaeota and Euryarchaeota and suggested their role in the adaptation of J. curcas to stress conditions.
Despite the archaeal potential to improve plant's adaptation and health, only few studies have demonstrated its direct effects during symbiosis with their hosts. Recently, Song et al. (2019) showed that soil archaea Nitrosocosmicus oleophilus MY3 promote growth of model plant Arabidopsis thaliana by colonizing its roots and releases volatile compounds that elicit systemic resistance (ISR) against the necrotrophic bacterium Pectobacterium carotovorum subsp. carotovorum SCC1 and biotrophic bacterium Pseudomonas syringae pv. tomato DC3000 via the salicylic acid-independent signaling pathway. However, the literature about symbiotic relationship among tropical crops and archaea is scarce.
The knowledge about the dynamics of archaeal communities, and they physiological and molecular mechanisms to mediate plant growth and stress tolerance are one of challenges to overcome in archaea application in sustainable agriculture (Naitam and Kaushik, 2021).
Burkholderia spp. is a gram-negative β-proteobacterium that might be found in several habitats, ranging from human pathogens to plants, as endophytes (Esmaeel et al., 2018; Furlan et al., 2019; Kunakom and Eustáquio, 2019; Mullins et al., 2019). Burkholderia consists of an emergent genus in terms of biotechnological applications and the diversity of natural products with potential therapeutic relevance and plant growth-promoting properties.
The metabolic arsenal of Burkholderia species is evident. Their large genomes encode numerous natural products. Several compounds have been reported from Burkholderiales, such as siderophores and lipopeptides, non-ribosomal peptides, and polyketides. The specialized holometabolite pyrrolnitrin plays an important role in plant protection against a wide range of soil- and seed-borne phytopathogens. Pyrrolnitrin was originally isolated from Pseudomonas pyrrocinia and produced by several strains of B. cepacia (Pawar et al., 2019). In B. cepacia strains, the biosynthesis of pyrrolnitrin involves mechanisms of quorum-sensing (QS) signaling molecules known as N-acyl-homoserine-lactones (Jenul et al., 2018). According to Keum et al. (2009), nutrients, such as glucose, are capable of decreasing pyrrolnitrin production to the detriment of increasing cell density. However, the full potential of the Burkholderia genus has gained notoriety with recent advances in genomic tools (Kunakom and Eustáquio, 2019).
According to the systematic review of Kunakom and Eustáquio (2019), free-living Burkholderia species are skilled producers of hydrolytic enzymes that can degrade natural and synthetic pollutants. Moreover, several studies have been reported the isolation of Burkholderiales from tropical regions which showed promising results on biological control of economic important phytopathogens and in PGP.
Tropical crops such as Banana (Musa spp.) holds a worldwide production of 116.8 million tons in 2019 (FAOSTAT, 2021) but is severely affected by fungal diseases mainly caused by Mycosphaerella fijiensis (black leaf streak disease) and Fusarium oxysporum f.sp. cubense (Fusarium wilt), which are responsible for significant losses in annual production (Nakkeeran et al., 2021; Soares et al., 2021). Xu et al. (2020) isolated a rhizosphere-borne Burkholderia sp. HQB-1 from healthy plants collected in a pathogen-infected banana orchard is capable of potently inhibiting Botrytis cinerea, Colletotrichum gloeosporioides, Curvularia fallax, and F. oxysporum through phenazine-1-carboxylic acid.
A Burkholderia fungorum strain UFLA 04-226, isolated from root nodules of Macroptilium atropurpureum (da Silva et al., 2012), showed common bean plant (Phaseolus vulgaris) growth promotion properties in the presence of nitrogen fertilizer. Additionally, another Burkholderia strain UFLA 04-155 was capable of increasing the dry weight of the nodules and shoots, increasing P, Mg, Cu, Fe, K, Ca, S, and Zn in leaves, and inducing nodule formation by the indigenous rhizobial community (de Oliveira-Longatti et al., 2015).
Ganoderma boninense (basal stem rot disease) is a serious threat to the oil palm (Elaeis guineensis) cultivars. G. boninense might cause the death of the palm by the destruction of the basal tissues, causing significant economic losses (Tan et al., 2021). Palm oil is a valuable raw material used in the manufacturing of several products (e.g., biodiesel, cosmetics, detergents, food, and plastics) and therefore constitutes an important economic activity in Indonesia and Malaysia (Siddiqui et al., 2021). In Nadhrah et al. (2016), an endophyte-oil palm-associated Burkholderia GanoEB2, when associated with a bioorganic powder (real strong bioorganic fertilizer) composed of N2, P, K, Mg, B, Fe, Zn, and trace elements combined with plant-based organic matter, resulted in a reduction of 18.2% of disease incidence, 26.6% of the severity of foliar symptoms, and 27.7% of disease severity foliar index.
Several studies report the successful association between beneficial Burkholderia spp. and sugarcane (Saccharum spp.) crops. According to Luvizotto et al. (2010), Burkholderiales are often found in association with this cultivar, which confers a wide range of physiological activities. In this same publication, a study regarding the bacterial diversity harbored by this plant was also reported. In addition, phylogenetic analysis based on the sequencing of 16S rRNA and the housekeeping gene gyrB revealed that most of the isolates belong to the Burkholderia cepacia complex (Bcc). Sugarcane is native to regions ranging from warm temperate to tropical climates of south and southeast Asia, where Brazil holds the largest global production, followed by India (Bordonal et al., 2018; El Chami et al., 2020). However, fungal diseases, such as those affected by red rot (Colletotrichum falcatum), seedling rot (Pythium spp.), sett rot (Ceratocystis paradoxa), sugarcane smut (Sporisorium scitamineum), and wilt (Fusarium sacchari) are responsible for substantial losses in production (Viswanathan and Malathi, 2019). Cui et al. (2020) reported that the isolation of a toxoflavin produced by B. gladioli strain CGB10 endophytically isolated from sugarcane leaves showed strong inhibitory activity against S. scitamineum. Studies have shown the antifungal potential of such molecules. Li et al. (2019) also reported the antifungal activity of such a compound. Burkholderia gladioli HDXY-02 isolated from the medicinal plant Lycoris aurea exhibited broad-spectrum activity against plant (Fusarium graminearum, Magnaporthe oryzae, and Rhizoctonia solani) and human (Aspergillus fumigatus, A. nidulans, Candida albicans, and Cryptococcus neoformans) fungal pathogens.
Polygala paniculata is a folk medicinal plant in Brazilian coast which is known for the abundant presence of methyl salicylate in its roots. Moreover, Polygalaceae have reported several pharmacological effects, including anti-inflammatory, anxiolytic, trypanocidal, antinociceptive, and antimicrobial effects, as well as other specialized molecules, such as alkaloids, flavonoids, terpenes, lignins, and coumarins. In Brazil, these plants are widely used for the treatment of lesions and sprains (Lapa et al., 2009). As promising and still unexplored niche-harboring microbes, the literature reports a novel Burkholderia cepacia strain endophytically associated with these plants. Showing novel alleles of gyrB, lepA, and phaC, B. cepacia ST 1870 (strain COPS) revealed broad-spectrum and potent activity toward the phytopathogenic fungi Moniliophthora perniciosa and Sclerotinia sclerotirium (Figure 3) (Cruz et al., 2021).
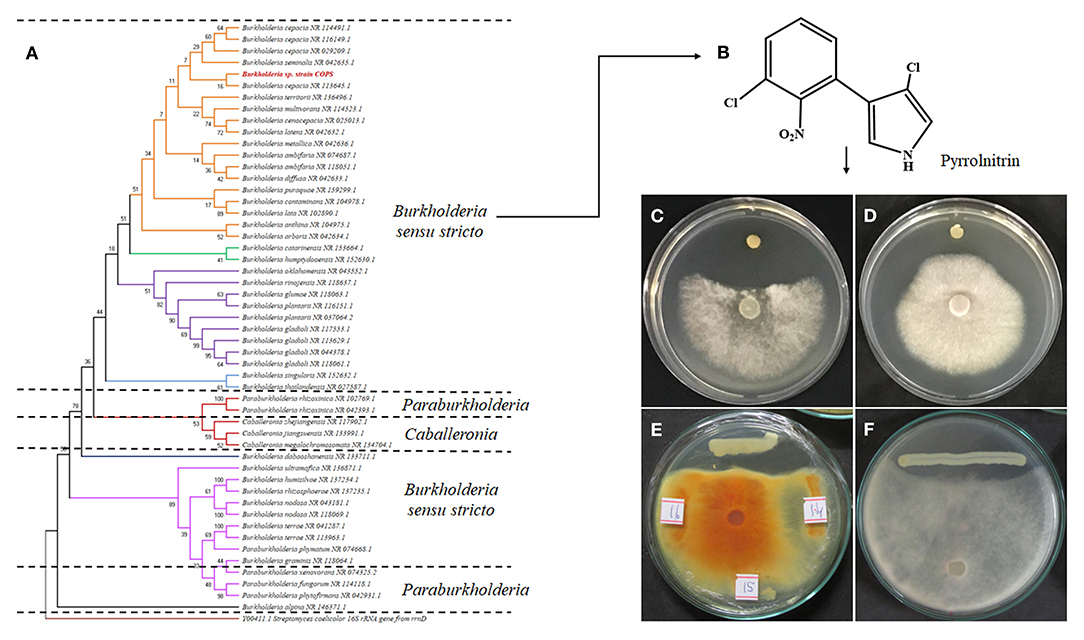
Figure 3. Burkholderiales comprise a diverse taxonomic group (Burkholderia, Caballeronia, Mycetohabitans, Paraburkholderia, Pararobbisais, Robbsia, and Trinickia) (A) that is capable of adapting to various ecological niches and is considered an emerging source of natural products for therapeutic and biotechnological applications. In terms of agricultural practices, antifungal pyrrolnitrin (B) is known for its broad spectrum of activity against several phytopathogenic fungi. Both the dual culture assay and the crude extract of the novel B. cepacia strain COPS showed potent antifungal effects against Ceratocystis paradoxa (C,F), Fusarium proliferatum (D), and Alternaria alternata (E).
Both endophytic and rhizospheric microorganisms have attracted attention based on their potential use in sustainable agriculture, since they represent a key strategy for integrated managment practices to improve plant growth and control pests and diseases (Le Cocq et al., 2016; Kumar et al., 2018). Adopt sustainable farming practices is of great importance to enhance crop production while concurrently addressing food security needs for the growing global population (Arora, 2018).
As mentioned before, chemical compounds or methods offer disadvantages to the environment and human health (Brauer et al., 2019; Cullen et al., 2019; Omomowo and Babalola, 2019). Nanoparticles (NPs) are biotechnologically attractive for their biological effects on pathogenic microbes and plant growth promotion (Ibrahim et al., 2019). In this way, the plant-associated microbes are attaing the new horizons and have been employed in the green synthesis of NPs for use in the agriculture sector (Mishra et al., 2017; Ali et al., 2020). A recent example is the synthesis of silver nanoparticles (Ag-NPs) by Bacillus siamensis endophytically isolated from the medicinal health plant Coriandrum sativum. Ag-NPs strongly inhibited bacterial growth, biofilm formation, and swimming motility of the rice phytopathogen Xanthomonas oryzae pv. oryzae and Acidovorax oryzae and promoted a significant increase in root length, shoot length, fresh weight, and dry weight of rice seedlings (Ibrahim et al., 2019). Furthermore, green synthesized Ag-NPs by the garlic (Allium sativum) endophyte Pseudomonas poae showed strong inhibition of the wheat pathogen Fusarium graminearum in terms of mycelium growth, spore germination, length of the germ tubes, and mycotoxin production (Ibrahim et al., 2020).
Despite the promising potential of endophytic and rhizospheric microorganisms for the synthesis of NPs, some challenges still need to be overcome for its efficient application in the plant breeding. The physicochemical properties (e.g., size, shape) and synthesis parameters (e.g., biomass quantity, temperature, pH) should be optimized for green NPs formulations development in large-scale. In addition, the physicochemical properties (e.g., size, shape) and synthesis parameters (e.g., biomass quantity, temperature, pH) should be optimized for green NPs formulations development in large-scale. In addition, is essential know the specificity and toxicity of NPs for target and no-target organisms and environment (Raj et al., 2021).
Therefore, basic and applied researches using both endophytic and rhizospheric microorganisms, comminated or not with new technologies, have essential role in ensuring development, productivity and protection of tropical crops and food security in the imminent future.
The use of microbes and their products is a reality on drug development and agricultural processes which is constantly increasing since the past few decades. The search for interesting natural biological activity has been the basis for the development of various biotechnological and agricultural applications. Therefore, plant-symbiont microbes constitute a rich and unexplored source of natural secondary metabolites. The microbial world and endophytes and rhizobacteria, in particular, exhibit vast genetic and metabolic biodiversity that has not yet been thoroughly explored. According to Azevedo and Quecine (2017), most studies on endophytes have been carried out using hosts from temperate countries, while data from tropical regions remain scarce. Consequently, the exploitation of the tropical microbiome is critically important since there is ever increasing demand for eco-friendly practices in agriculture which can effectively improve production and neutralize imminent biological threats. Herein, we highlighted the role of these microbes considering fundamental properties of application of biotechnology in strategic areas of agri-business. The available literature has revealed that tropical plants harbor a great diversity of microorganisms which remain unknown.
PL designed the project. PL, AB, and FC wrote the manuscript and edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the São Paulo Research Foundation, FAPESP (Proc. Nos. 2015/10974-8 and 2020/11315-6).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ali, A., Ahmed, T., Wu, W., Hossain, A., Hafeez, R., Masum, M. I., et al. (2020). Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials 10:1146. doi: 10.3390/nano10061146
Almeida, A. C., Andrade, V. A., Fonseca, F. S. A., Macêdo, A. A., Santos, R. L., Colen, K. G. F., et al. (2017). Acute and chronic toxicity and antimicrobial activity of the extract of Stryphnodendron adstringens (Mart.) Coville. Pesq. Vet. Bras. 37, 840–846. doi: 10.1590/s0100-736x2017000800010
Almeida, R. J., Bonatelli, M. L., Batista, B. D., Teixeira-Silva, N. S., Mondin, M., Santos, R. C., et al. (2021). Bacillus sp. RZ2MS9, a tropical PGPR, colonizes maize endophytically and alters the plant's production of volatile organic compounds both independently and when co-inoculated with Azospirillum brasilense Ab-V5. Environ. Microbiol. Rep. 27:427998. doi: 10.1101/2021.01.04.425352
Ammar, E., Shatters, R. G., and Hall, D. G. (2011). Localization of Candidatus Liberibacter asiaticus, associated with citrus Huanglongbing, in its psyllid vector using fluorescence in situ hybridization. J. Phytopathol. 159, 726–734. doi: 10.1111/j.1439-0434.2011.01836.x
Andrade, P. A. M., Pimenta, L. S., Cardilho, B. E. S., Marcon, J., Silva, J. A. S., Azevedo, J. L., et al. (2020). Bacillus sp. RZ2MS9 and the bacteria-free filtrate in the seed germination and growth of maize seedlings. Braz. J. Agricul. 95, 95–105. doi: 10.37856/bja.v95i2.4232
Andresen, E., Arroyo-Rodríguez, V., and Escobar, F. (2018). “Tropical biodiversity: the importance of biotic interactions for its origin, maintenance, function, and conservation,” in Ecological Networks in the Tropics, eds W. Dáttilo and V. Rico-Gray (Cham: Springer). doi: 10.1007/978-3-319-68228-0_1
Araújo, W. L., Marcon, J., Maccheroni, W., Van Elsas, J. D., Van Vuurde, J. W. L., and Azevedo, J. L. (2002). Diversity of endophytic bacterial population and interaction with Xylella fastidiosa in citrus plants. Appl. Environ. Microbiol. 68, 4906–4914. doi: 10.1128/AEM.68.10.4906-4914.2002
Arora, N. K. (2018). Agricultural sustainability and food security. Environ. Sustain. 1, 217–219. doi: 10.1007/s42398-018-00032-2
Atroch, A. L., and Nascimento Filho, F. J. (2018). “Guarana - Paullinia cupana Kunth var. sorbilis (Mart.) Ducke,” in Exotic Fruits, eds S. Rodrigues, E. O. Silva, E. S. Brito, S. Rodrigues, E. O. Silva, and E. S. Brito (Cambridge, MA: Press). doi: 10.1016/B978-0-12-803138-4.00029-0
Azevedo, J. L., Araújo, W. L., and Lacava, P. T. (2016). The diversity of citrus endophytic bacteria and their interactions with Xylella fastidiosa and host plants. Genet. Mol. Biol. 39, 476–491. doi: 10.1590/1678-4685-gmb-2016-0056
Azevedo, J. L., Maccheroni, W., Pereira, J. O., and Araújo, W. L. (2000). Endophytic microorganisms: a review on insect control and recent advances on tropical plants. EJB Electron. J. Biotechnol. 3:1. doi: 10.2225/vol3-issue1-fulltext-4
Azevedo, J. L., and Quecine, M. C. (2017). Diversity and Benefits of Microorganisms From the Tropics. Switzerland: Springer Nature. doi: 10.1007/978-3-319-55804-2
Bartossek, R., Spang, A., Weidler, G., Lanzen, A., and Schleper, C. (2012). Metagenomic analysis of ammonia-oxidizing archaea affiliated with the soil group. Front. Microbiol. 3, 208. doi: 10.3389/fmicb.2012.00208
Batista, B. D., Lacava, P. T., Ferrari, A., Silva, N. S. T., Bonatelli, M. L., Tsui, S., et al. (2018). Screening of tropically derived, multi-trait plant growth-promoting rhizobacteria and evaluation of corn and soybean colonization ability. Microbiol. Res. 206, 33–42. doi: 10.1016/j.micres.2017.09.007
Berkelmann, D., Schneider, D., Meryandini, A., and Daniel, R. (2020). Unravelling the effects of tropical land use conversion on the soil microbiome. Environ. Microbiome 15:5. doi: 10.1186/s40793-020-0353-3
Bogas, A. C, Torres, F. L., Sousa, C. P., and Lacava, P. T. (2020). Bioactivity of endophytes from the Brazilian tropical savannah. Acta Sci. Microbiol. 3, 15–22. doi: 10.31080/ASMI.2020.03.0670
Bogas, A. C., Aguilar-Vildoso, C. I., Camargo-Neves, A. A., and Araújo, W. L. (2016). Effects of growth-promoting endophytic Methylobacterium on development of Citrus rootstocks. Afr. J. Microbiol. Res. 10, 646–653. doi: 10.5897/AJMR2016.7926
Bogas, A. C., Ferreira, A. J., Araújo, W. L., Astolfi-Filho, S., Kitajima, E. W., Lacava, P. T., et al. (2015). Endophytic bacterial diversity in the phyllosphere of Amazon Paullinia cupana associated with asymptomatic and symptomatic anthracnose. SpringerPlus 4:258. doi: 10.1186/s40064-015-1037-0
Bonatelli, M. L., Tsui, S., Batista, B. D., Dourado, M. N., Kitajima, E. W., Andreote, F. D., et al. (2019). Bacterial communities associated with anthracnose symptomatic and asymptomatic leaves of guarana, an endogenous tropical crop, and their pathogen antagonistic effects. Arch. Microbiol. 201, 1061–1073. doi: 10.1007/s00203-019-01677-1
Bordonal, R. O., Carvalho, J. L. N., Lal, R., Figueiredo, E. B., Oliveira, B. G., and La Scala, N. (2018). Sustainability of sugarcane production in Brazil. A review. Agron. Sustain. Dev. 38:13. doi: 10.1007/s13593-018-0490-x
Brauer, V. S., Rezende, C. P., Pessoni, A. M., De Paula, R. G., Rangappa, K. S., Nayaka, S. C., et al. (2019). Antifungal agents in agriculture: Friends and foes of public health. Biomolecules. 9:521. doi: 10.3390/biom9100521
Brunings, A. M., and Gabriel, D. W. (2003). Xanthomonas citri: breaking the surface. Mol. Plant Pathol. 4, 141–157. doi: 10.1046/j.1364-3703.2003.00163.x
Casas, L. L., Pereira, J. O., Costa-Neto, P. Q., Silva, J. F., Almeida, L. N., Bianco, R. A., et al. (2021). Endophytic Colletotrichum siamense for biocontrol and resistance induction in guarana seedlings. Int. J. Microbiol. 2021:1925226. doi: 10.1155/2021/1925226
Castro, R. A., Dourado, M. N., de Almeida, J. R., Lacava, P. T., Nave, A., de Melo, I. S., et al. (2018). Mangrove endophyte promotes reforestation tree (Acacia polyphylla) growth. Braz. J. Microbiol. 49, 59–66. doi: 10.1016/j.bjm.2017.04.002
Castro, R. C, Quecine, M. C, Lacava, P. T., Batista, B. D., Luvizotto, D. M., Marcon, J., Ferreira, A., et al. (2014). Isolation and enzyme bioprospection of endophytic bacteria associated with plants of Brazilian mangrove ecosystem. SpringerPlus 3:382. doi: 10.1186/2193-1801-3-382
Clark, I. M., Hughes, D. J., Fu, Q., Abadie, M., and Hirsch, P.R. (2021). Metagenomic approaches reveal differences in genetic diversity and relative abundance of nitrifying bacteria and archaea in contrasting soils. Sci. Rep. 11:15905. doi: 10.1038/s41598-021-95100-9
Cruz, F. P. N., de Paula, A. F., Nogueira, C. T., Andrade, P. H. M., Borges, L. M., Lacava, P. T., et al. (2021). Discovery of a novel lineage Burkholderia cepacia ST 1870 endophytically isolated from medicinal Polygala paniculata which shows potent in vitro antileishmanial and antimicrobial effects. Int. J. Microbiol. 2021:6618559. doi: 10.1155/2021/6618559
Cui, G., Yin, K., Lin, N., Liang, M., Huang, C., Chang, C., et al. (2020). Burkholderia gladioli CGB10: A novel strain biocontrolling the sugarcane smut disease. Microorganisms 8:1943. doi: 10.3390/microorganisms8121943
Cullen, M. G., Thompson, L. J., Carolan, J. C., Stout, J. C., and Stanley, D. A. (2019). Fungicides, herbicides and bees: A systematic review of existing research and methods. PLoS ONE 14, e0225743. doi: 10.1371/journal.pone.0225743
da Costa, G. A., Morais, M. G., Saldanha, A. A., Assis Silva, I. C., Aleixo, Á. A., Ferreira, J. M., et al. (2015). Antioxidant, antibacterial, cytotoxic, and anti-inflammatory potential of the leaves of Solanum lycocarpum A. St. Hil. (Solanaceae). Evidence-based complementary and alternative medicine: eCAM. 2015:315987. doi: 10.1155/2015/315987
da Silva, K., Cassetari Ade, S., Lima, A. S., De Brandt, E., Pinnock, E., Vandamme, P., et al. (2012). Diazotrophic Burkholderia species isolated from the Amazon region exhibit phenotypical, functional and genetic diversity. Syst. Appl. Microbiol. 35, 253–262. doi: 10.1016/j.syapm.2012.04.001
Daungful, O., Youpensuk, S., and Lumyong, S. (2019). Endophytic bacteria isolated from citrus plants for biological control of Citrus Canker in Lime plants. Trop. Life Sci. Res. 30, 73–88. doi: 10.21315/tlsr2019.30.1.5
de Oliveira-Longatti, S. M., Martins de Sousa, P., Marciano Marra, L., Avelar Ferreira, P. A., and de Souza Moreira, F. M. (2015). Burkholderia fungorum promotes common bean growth in a dystrophic oxisol. Ann. Microbiol. 65, 1825–1832. doi: 10.1007/s13213-014-1020-y
de Sousa, C. P., Serrano, N. F. G., and Lacava, P. T. (2017). “Endophytic microorganisms of the Tropical Savannah: A promising source of bioactive molecules,” in Diversity and Benefits of Microorganisms From the Tropics, eds J. de Azevedo and M. Quecine M. (Cham: Springer). doi: 10.1007/978-3-319-55804-2_4
Deivanai, S, Bindusara, A. S., Prabhakaran, G., and Bhore, S. J. (2014). Culturable bacterial endophytes isolated from mangrove tree (Rhizophora apiculata Blume) enhance seedling growth in Rice. J. Nat. Sci. Biol. Med. 5, 437–444. doi: 10.4103/0976-9668.136233
Dias, A. C. F., Andreote, F. D., Dini-Andreote, F., Lacava, P. T., Sá, A. L. B., Melo, I. S., et al. (2009). Diversity and biotechnological potential of culturable bacteria from Brazilian mangrove sediment. World J. Microbiol. Biotechnol. 25, 1305–1311. doi: 10.1007/s11274-009-0013-7
Dorta, S. O., Balbinotte, J., Monnerat, R., Lopes, J. R. S., da Cunha, T., Zanardi, O. Z., et al. (2018). Selection of Bacillus thuringiensis strains in citrus and their pathogenicity to Diaphorina citri (Hemiptera: Liviidae) nymphs. Insect Sci. 27, 519–530. doi: 10.1111/1744-7917.12654
Dourado, M. N., Bogas, A. C., Pomini, A. M., Andreote, F. D., Quecine, M. C., Marsaioli, A. J., et al. (2013). Methylobacterium-plant interaction genes regulated by plant exudate and quorum sensing molecules. Braz. J. Microbiol. 44, 1331–1339. doi: 10.1590/S1517-83822013000400044
Dourado, M. N., Santos, D. S., Nunes, R. L., Costa de Oliveira, R. L. B., Oliveira, M. V., and Araújo, W. L. (2015). Differential gene expression. in Xylella fastidiosa 9a5c during co-cultivation with the endophytic bacterium Methylobacterium mesophilicum SR1.6/6. J. Basic Microbiol. 55, 1357–1366. doi: 10.1002/jobm.201400916
Dreyfuss, M., and Petrini, O. (1984). Further investigations on the occurrence and distribution of endophytic fungi in tropical plants. Bot. Helv. 94, 33–40.
Dubey, G., Kollah, B., Gour, V. K., Shukla, A. K., and Mohanty, S. R. (2016). Diversity of bacteria and archaea in the rhizosphere of bioenergy crop Jatropha curcas. 3 Biotech. 6, 257. doi: 10.1007/s13205-016-0546-z
El Chami, D., Daccache, A., and El Moujabber, M. (2020). What are the impacts of sugarcane production on ecosystem services and human well-being? A review. Ann. Agric. Sci. 65, 188–199. doi: 10.1016/j.aoas.2020.10.001
El-Ghamry, A., Mosa, A., Alshaal, T., and El-Ramady, H. (2018). Nanofertilizers vs. biofertilizers: New insights. EBSS 2, 51–72. doi: 10.21608/jenvbs.2018.3880.1029
Esmaeel, Q., Pupin, M., Jacques, P., and Leclère, V. (2018). Nonribosomal peptides and polyketides of Burkholderia: new compounds potentially implicated in biocontrol and pharmaceuticals. Environ. Sci. Pollut. Res. Int. 25, 29794–29807. doi: 10.1007/s11356-017-9166-3
Fadiji, A.E., Ayangbenro, A.S., and Babalola, O.O. (2020). Organic farming enhances the diversity and community structure of endophytic archaea and fungi in maize plant: a shotgun approach. J. Soil Sci. Plant Nutr. 20:9. doi: 10.1007/s42729-020-00324-9
Fadiji, A. E., and Babalola, O. O. (2020). Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 15, 467. doi: 10.3389/fbioe.2020.00467
FAOSTAT (2019). FAOSTAT Online Database. Available online at: http://www.fao.org/faostat/en/#data/QCL (accessed August 29, 2021).
FAOSTAT (2021). FAOSTAT Online Database. Available online at: http://faostat.fao.org/ (accessed June 10, 2021).
Fasusi, O. A., Cruz, C., and Babalola, O. O. (2021). Agricultural sustainability: Microbial biofertilizers in rhizosphere management. Agriculture 11:163. doi: 10.3390/agriculture11020163
Felipe, A. M., Rincão, V. P., Benati, F. J., Linhares, R. E., Galina, K. J., de Toledo, C. E., et al. (2006). Antiviral effect of Guazuma ulmifolia and Stryphnodendron adstringens on poliovirus and bovine herpesvirus. Biol. Pharm. Bull. 29, 1092–1095. doi: 10.1248/bpb.29.1092
Ferreira Filho, A. S., Quecine, M. C., Bogas, A. C., Rossetto, P. B., Lima, A. O. S., Lacava, P. T., et al. (2012). Endophytic Methylobacterium extorquens expresses a heterologous ß-1,4-endoglucanase A (EglA) in Catharanthus roseus seedlings, a model host plant for Xylella fastidiosa. World J. Microbiol. Biotechnol. 28, 1475–1481. doi: 10.1007/s11274-011-0949-2
Fundecitrus (2020). Doenças e Pragas. Available online at: https://www.fundecitrus.com.br/ (accessed September 3, 2021).
Furlan, J. P. R., Pitondo-Silva, A., Braz, V. S., Gallo, I. F. L., and Stehling, E. G. (2019). Evaluation of different molecular and phenotypic methods for identification of environmental Burkholderia cepacia complex. World J. Microbiol. Biotechnol. 35:39. doi: 10.1007/s11274-019-2614-0
Gayathri, P., and Muralikrishnan, V. (2013). Isolation of endophytic bacteria from mangrove, bananas and sugarcane for their biological activities. Asian J Res. Biol. Pharm. Sci. 1, 19–27.
Gepts, P. (2008). “Tropical environments, biodiversity, and the origin of crops, in genomics of tropical crop plants,” in Plant Genetics and Genomics: Crops and Models, eds P. H. Moore and R. Ming (New York, NY: Springer). doi: 10.1007/978-0-387-71219-2_1
Gouda, S., Das, G., Sen, S. K., Shin, H. S., and Patra, J. K. (2016). Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 7, 1538. doi: 10.3389/fmicb.2016.01538
Guha, T., Gopal, G., Kundu, R., and Mukherjee, A. (2020). Nanocomposites for delivering agrochemicals: A comprehensive review. J. Agric. Food. Chem. 68, 3691–3702. doi: 10.1021/acs.jafc.9b06982
Hamerski, L., Somner, G. V., and Tamaio, N. (2013). Paullinia cupana Kunth (Sapindaceae): A review of its ethnopharmacology, phytochemistry and pharmacology. J. Med. Plants. Res. 7, 2221–2229. doi: 10.5897/JMPR2013.5067
Harman, G., Khadka, R., Doni, F., and Uphoff, N. (2020). Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 11, 610065. doi: 10.3389/fpls.2020.610065
Hassan, M. K., McInroy, J. A., and Kloepper, J. W. (2019). The interactions of rhizodeposits with plant growth-promoting rhizobacteria in the rhizosphere: A review. Agriculture 9:142. doi: 10.3390/agriculture9070142
Henman, A. R. (1982). Guaraná (Paullinia cupana var. sorbilis): Ecological and social perspectives on an economic plant of the central amazon basin. J. Ethnopharmacol. 6, 311–338. doi: 10.1016/0378-8741(82)90054-X
Holguin, G., Vazquez, P., and Bashan, Y. (2001). The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biol. Fert. Soils 33, 265–278. doi: 10.1007/s003740000319
Ibrahim, E., Fouad, H., Zhang, M., Zhang, Y., Qiu, W., Yan, C., et al. (2019). Biosynthesis of silver nanoparticles using endophytic bacteria and their role in inhibition of rice pathogenic bacteria and plant growth promotion. RSC Adv. 9, 29293–29299. doi: 10.1039/C9RA04246F
Ibrahim, E., Zhang, M., Zhang, Y., Hossain, A., Qiu, W., Chen, Y., et al. (2020). Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat fusarium head blight pathogen Fusarium graminearum. Nanomaterials 10:219. doi: 10.3390/nano10020219
Ikeda, A. C., Savi, D. C., Hungria, M., Kava, V., Glienke, C., and Galli-Terasawa, L. V. (2020). Bioprospecting of elite plant growth-promoting bacteria for the maize crop. Acta Sci. Agron. 42:e44364. doi: 10.4025/actasciagron.v42i1.44364
International Fertilizer Industry Association (2021). Consumption. Available online at: https://www.ifastat.org/databases/plant-nutrition (accessed June 10, 2021).
Islam, A. K. M. A., Yaakob, Z., Ghani, J. A., and Anuar, N. (2014). Jatropha curcas L.: A future energy crop with enormous potential. Biomass Bioenergy 2014, 31–61. doi: 10.1007/978-3-319-07578-5_2
Jenul, C., Sieber, S., Daeppen, C., Mathew, A., Lardi, M., Pessi, G., et al. (2018). Biosynthesis of fragin is controlled by a novel quorum sensing signal. Nat. Commun. 9:1297. doi: 10.1038/s41467-018-03690-2
Jha, C. K., and Saraf, M. (2012). Evaluation of multispecies plant-growth-promoting consortia for the growth promotion of Jatropha curcas L. J. Plant. Growth Regul. 31, 588–598. doi: 10.1007/s00344-012-9269-5
Kandel, S. L., Joubert, P. M., and Doty, S. L. (2017). Bacterial endophyte colonization and distribution within plants. Microorganisms 5:77. doi: 10.3390/microorganisms5040077
Keum, Y. S., Lee, Y. J., Lee, Y. H., and Kim, J. H. (2009). Effects of nutrients on quorum signals and secondary metabolite productions of Burkholderia sp. O33. J. Microbiol. Biotechnol. 19, 1142–1149. doi: 10.4014/jmb.0901.465
Khatoon, Z., Huang, S., Rafique, M., Fakhar, A., Kamran, M. A., and Santoyo, G. (2020). Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manage. 273:111118. doi: 10.1016/j.jenvman.2020.111118
Krzyzek, P. (2019). Challenges and limitations of anti-quorum sensing therapies. Front. Microbiol. 10, 2473. doi: 10.3389/fmicb.2019.02473
Kumar, A., Patel, J. S., and Meena, V. S. (2018). “Rhizospheric microbes for sustainable agriculture: an overview,” in Role of Rhizospheric Microbes in Soil, ed V. Meena (Singapore: Springer). doi: 10.1007/978-981-10-8402-7_1
Kumar, P., Srivastava, V. C., and Jha, M. K. (2016). Jatropha curcas phytotomy and applications: Development as a potential biofuel plant through biotechnological advancements. Renew. Sust. Energ. Rev. 59, 818–858. doi: 10.1016/j.rser.2015.12.358
Kunakom, S., and Eustáquio, A. S. (2019). Burkholderia as a source of natural products. J. Nat. Prod. 82, 2018–2037. doi: 10.1021/acs.jnatprod.8b01068
Kuri, C. M. B. (2008). The Guarana industry in Brazil. IBER J. 7, 87–98. doi: 10.19030/iber.v7i5.3258
Lacava, P. T., Araújo, W. L., Marcon, J., Maccheroni, W, and Azevedo, J. L. (2004). Interaction between endophytic bacteria from citrus plants and the phytopathogenic bacterium Xylella fastidiosa, causal agent of citrus variegated chlorosis. Lett. Appl. Microbiol. 39, 55–59. doi: 10.1111/j.1472-765X.2004.01543.x
Lacava, P. T., and de Sousa, C. P. (2016). “Role of endophytic actinomycetes in crop protection: plant growth promotion and biological control,” in Plant Growth Promoting Actinobacteria, eds G. Subramaniam, S. Arumugam, and V. Rajendran (Singapore: Springer). doi: 10.1007/978-981-10-0707-1_9
Lacava, P. T., Li, W. B., Araújo, W. L., Azevedo, J. L., and Hartung, J. S. (2006). Rapid, specific and quantitative assays for the detection of the endophytic bacterium Methylobacterium mesophilicum in plants. J. Microbiol. Methods 65, 535–541. doi: 10.1016/j.mimet.2005.09.015
Lapa, F. R., Gadotti, V. M., Missau, F. C., Pizzolatti, M. G., Marques, M. C., Dafré, A. L., et al. (2009). Antinociceptive properties of the hydroalcoholic extract and the flavonoid rutin obtained from Polygala paniculata L. in mice. Basic Clin. Pharmacol. Toxicol. 104, 306–315. doi: 10.1111/j.1742-7843.2008.00365.x
Laviola, B. G, Alves, A. A., Kobayashi, A. K., and Formighieri, E. F. (2015). Situação atual do pinhão-manso no Brasil e no mundo. Brasília, DF: Embrapa Agroenergia.
Lawrencia, D., Wong, S.K., Low, D.Y.S., Goh, B.H., Goh, J.K., Ruktanonchai, U.R., et al. (2021). Controlled release fertilizers: A review on coating materials and mechanism of release. Plants 10:238. doi: 10.3390/plants10020238
Le Cocq, K., Gurr, S. J., Hirsch, P. R., and Mauchline, T. H. (2016). Exploitation of Endophytes for sustainable agricultural intensification. Mol. Plant Pathol. 18, 469–473. doi: 10.1111/mpp.12483
Li, X., Li, Y., Wang, R., Wang, Q., and Lu, L. (2019). Toxoflavin produced by Burkholderia gladioli from Lycoris aurea is a new broad-spectrum fungicide. Appl. Environ. Microbiol. 85, e00106–e00119. doi: 10.1128/AEM.00106-19
Lima, A. O. S., Quecine, M. C., Fungaro, M. H. P., Andreote, F., Araújo, W. L., Silva-Filho, M. C., et al. (2005). Molecular characterization of a novel beta-1, 4 endoglucanase from endophytic Bacillus pumilus strain. Appl. Microbiol. Biotechnol. 68, 57–65. doi: 10.1007/s00253-004-1740-1
Lima, J. C. S., Lima, J. C. D. S., Martins, D. T. O., and de Souza, P. T. (1998). Experimental evaluation of stem bark of Stryphnodendron adstringens (Mart.) Coville for anti-inflammatory activity. Phytother. Res. 12, 218–220. doi: 10.1002/(SICI)1099-1573(199805)12:3&<218::AID-PTR220&>3.0.CO;2-4
Liotti, R. Z., Figueiredo, M. I. S., da Silva, G. F., de Mendonça, E. A. F., and Soares, M. A. (2018). Diversity of cultivable bacterial endophytes in Paullinia cupana and their potential for plant growth promotion and phytopathogen control. Microbiol. Res. 207, 8–18. doi: 10.1016/j.micres.2017.10.011
Lisboa, P. H. G., de Andrade, P. H. M., Machado, P. C., de Sousa, C. P., and Lacava, P. T. (2021). Isolation and in vitro screening of plant growth-promoting rhizobacteria from Solanum lycocarpum St. Hil., an endemic plant of the Brazilian tropical savannah. Afr. J. Microbiol. Res. 15, 253–261. doi: 10.5897/AJMR2021.9524
Liu, B., Lai, J., Wu, S., Jiang, J., and Kuang, W. (2021). Endophytic Community diversity in two citrus cultivares with different citrus cancker disease resistance. Arch. Microbiol. 203, 5453–5462. doi: 10.1007/s00203-021-02530-0
Liu, H., Carvalhais, L. C., Crawford, M., Singh, E., Dennis, P. G., Pieterse, C. M. J., et al. (2017). Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 8, 2552. doi: 10.3389/fmicb.2017.02552
Lopes, M. J. S., Dias-Filho, M. B., and Gurgel, E. S. C. (2021). Successful plant growth-promoting microbes: inoculation methods and abiotic factors. Front. Sustain. Food Syst. 5, 606454. doi: 10.3389/fsufs.2021.606454
Luvizotto, D. M., Marcon, J., Andreote, F. D., Dini-Andreote, F., Neves, A. A. C., Araújo, W. L., et al. (2010). Genetic diversity and plant-growth related features of Burkholderia spp. from sugarcane roots. World J. Microbiol. Biotechnol. 26, 1829–1836. doi: 10.1007/s11274-010-0364-0
Machado, A. R., Pinho, D. B., and Pereira, O. L. (2014). Phylogeny, identification and pathogenicity of the Botryosphaeriaceae associated with collar and root rot of the biofuel plant Jatropha curcas in Brasil, with a description of new species of Lasiodiplodia. Fungal Divers. 67, 231–247. doi: 10.1007/s13225-013-0274-1
Machado, P. C. (2019). Diversidade e potencial biotecnológico da comunidade bacteriana associada ao pinhão-manso (Jatropha curcas L.) [Master's thesis]. Universidade Federal de São Carlos, Sao Carlos, Brazil. Available online at: https://repositorio.ufscar.br/bitstream/handle/ufscar/12097/TESE%20DE%20DOUTORADO_VF_%20.pdf?sequence=1&isAllowed=y
Machado, P. C., Andrade, P. H. M., Sousa, C. P., Souza, C. W. O., and Lacava, P. T. (2020). In vitro characterization of endophytic bacteria associated with physic nut (Jatropha curcas L.) and their potential for plant-growth promotion and biocontrol. Braz. J. Dev. 6, 88572–88589. doi: 10.34117/bjdv6n11-326
Madhaiyan, M., Alex, T. H. H., Ngoh, S. T., Prithiviraj, B., and Ji, L. (2015). Leaf-residing Methylobacterium species fix nitrogen and promote biomass and seed production in Jatropha curcas. Biotechnol. Biofuels. 8:222. doi: 10.1186/s13068-015-0404-y
Madhaiyan, M., Chan, K. L., and Ji, L. (2014). Draft genome sequence of Methylobacterium sp. strain L2-4, a leaf-associated endophytic N-fixing bacterium isolated from Jatropha curcas L. Genome Announc. 2:e01306–14. doi: 10.1128/genomeA.01306-14
Madhaiyan, M., Jin, T. Y., Roy, J. J., Kim, S. J., Weon, H. Y., Kwon, S. W., et al. (2013b). Pleomorphomonas diazotrophica sp. nov., an endophytic N-fixing bacterium isolated from root tissue of Jatropha curcas L. Int. J. Syst. Evol. Microbiol. 63, 2477–2483. doi: 10.1099/ijs.0.044461-0
Madhaiyan, M., Peng, N., and Ji, L. (2013c). Complete genome sequence of Enterobacter sp. strain R4-368, an endophytic N-fixing gammaproteobacterium isolated from surface-sterilized roots of Jatropha curcas L. Genome Announc. 1:e00544–13. doi: 10.1128/genomeA.00544-13
Madhaiyan, M., Peng, N., Te, N. S., Hsin, I C., Lin, C., Lin, F., et al. (2013a). Improvement of plant growth and seed yield in Jatropha curcas by a novel nitrogen-fixing root associated Enterobacter species. Biotechnol. Biofuels. 6:140. doi: 10.1186/1754-6834-6-140
Mendonça, L. B. P., Zambolim, L., and Badel, J. L. (2017). Bacterial citrus diseases: major threats and recent progress. J. Bacteriol. Mycol. Open Access. 25, 340–350. doi: 10.15406/jbmoa.2017.05.00143
Meneghetti, G. A., Silva, L. J. S., Ferreira, M. A. C., and Santos, A. C. (2021). Elements for reflection and analysis of conditions of guarana production in Amazonas. BJAER. 4, 427–442. doi: 10.34188/bjaerv4n1-037
Mishra, S., Singh, B., Naqvi, A. H., and Singh, H. B. (2017). Potential of biosynthesized silver nanoparticles using Stenotrophomonas sp. BHU-S7 (MTCC 5978) for management of soil-borne and foliar phytopathogens. Sci. Rep. 7:45154. doi: 10.1038/srep45154
Mitter, E. K., Tosi, M., Obregón, D., Dunfield, K. E., and Germida, J. J. (2021). Rethinking crop nutrition in times of modern microbiology: Innovative biofertilizer technologies. Front. Sustain. Food Syst. 5, 29. doi: 10.3389/fsufs.2021.606815
Mohanty, S. R., Dubey, G., and Kollah, B. (2017). Endophytes of Jatropha curcas promote growth of maize. Rhizosphere 3, 20–28. doi: 10.1016/j.rhisph.2016.11.001
Moissl-Eichinger, C., Pausan, M., Taffner, J., Berg, G., Bang, C., and Schmitz, R. A. (2017). Archaea are interactive components of complex microbiomes. Trends Microbiol. 26, 70–85. doi: 10.1016/j.tim.2017.07.004
Moreira, C. C., Luna, G. L. F., Soriano, B., Cavicchioli, R., Bogas, A. C., de Sousa, C. P., et al. (2020). Leishmanicidal, cytotoxic, antimicrobial and enzymatic activities of Diaporthe species, a mangrove-isolated endophytic fungus. Afr. J. Microbiol. Res. 14, 516–524. doi: 10.5897/AJMR2020.9397
Morelli, M., Bahar, O., Papadopoulou, K. K., Hopkins, D. L., and Obradović, A. (2020). Editorial: Role of endophytes in plant health and defense against pathogens. Front. Plant Sci. 11, 1312. doi: 10.3389/fpls.2020.01312
Mullins, A. J., Murray, J. A. H., Bull, M. J., Jenner, M., Jones, C., Webster, G., et al. (2019). Genome mining identifies cepacin as a plant-protective metabolite of the biopesticidal bacterium Burkholderia ambifaria. Nat. Microbiol. 6, 996–1005. doi: 10.1038/s41564-019-0383-z
Munir, S., Ahmed, A., Li, Y., He, P., Singh, B. K., He, P., et al. (2021). The hidden treasures of citrus: finding Huanglongbing cure where it was lost. Crit. Rev. Biotecnol. 30, 1–16. doi: 10.1080/07388551.2021.1942780
Munir, S., Li, Y., He, P., He, P., Ahmed, A., Wu, Y., et al. (2020b). Unraveling the metabolite signature of citrus showing defense response towards Candidatus Liberibacter asiaticus after application of endophyte Bacillus subtilis L1-21. Microbiol. Res. 31:126425. doi: 10.1016/j.micres.2020.126425
Munir, S., Li, Y., He, P., Huang, M., He, P., He, P., et al. (2020a). Core endophyte communities of different citrus varieties from citrus growing regions in China. Sci. Rep. 10:3648. doi: 10.1038/s41598-020-60350-6
Nadhrah, N.I., Nulit, R., Nurrashyeda, R., and Idris, A.S. (2016). Effect of formulated bioorganic containing Burkholderia GanoEB2 in suppressing Ganoderma disease in oil palm seedlings. Plant Protect. Sci. 51, 80–87. doi: 10.17221/26/2014-PPS
Naitam, M. G., and Kaushik, R. (2021). Archaea: An agro-ecological perspective. Curr. Microbiol. 78, 2510–2521. doi: 10.1007/s00284-021-02537-2
Nakkeeran, S., Rajamanickam, S., Saravanan, R., Vanthana, M., and Soorianathasundaram, K. (2021). Bacterial endophytome-mediated resistance in banana for the management of Fusarium wilt. 3 Biotech. 11:267. doi: 10.1007/s13205-021-02833-5
Nan, J., Shaoran, Z., and Ling, J. (2021). Antibacterial Potential of Bacillus amyloliquefaciens GJ1 against Citrus Huanglongbing. Plants 10:261. doi: 10.3390/plants10020261
Noriler, S.A., Savi, D.C., Aluizio, R., Palácio-Cortes, A.M., Possiede, Y.M., and Glienke, C. (2018). Bioprospecting and structure of fungal endophyte communities found in the Brazilian Biomes, Pantanal, and Cerrado. Front. Microbiol. 9, 1526. doi: 10.3389/fmicb.2018.01526
Oliveira-Filho, A.T., and Ratter, J.A. (2002). “Vegetation physiognomies and woody flora of the Cerrado biome,” in The Cerrados of Brazil: Ecology and Natural History of a Neotropical Savanna, eds P. S. Oliveira and R. J. Marquis (Columbia: Columbia University Press). doi: 10.7312/oliv12042-007
Omomowo, O.I., and Babalola, O.O. (2019). Bacterial and fungal endophytes: tiny giants with immense beneficial potential for plant growth and sustainable agricultural productivity. Microorganisms 7:481. doi: 10.3390/microorganisms7110481
Omotayo, O. P., and Babalola, O. O. (2020). Resident rhizosphere microbiome's ecological dynamics and conservation: Towards achieving the envisioned sustainable development goals, a review. ISWCR 9, 127–142. doi: 10.1016/j.iswcr.2020.08.002
Onaka, H. (2017). Novel antibiotic screening methods to awaken silent or cryptic secondary metabolic pathways in actinomycetes. J. Antibiot. 70, 865–870. doi: 10.1038/ja.2017.51
Pacifico, D., Squartini, A., Crucitti, D., Barizza, E., Lo Schiavo, F., Muresu, R., et al. (2019). The role of the endophytic microbiome in the grapevine response to environmental triggers. Front. Plant Sci. 10, 1256. doi: 10.3389/fpls.2019.01256
Pawar, S., Chaudhari, A., Prabha, R., Shukla, R., and Singh, D.P. (2019). Microbial pyrrolnitrin: Natural metabolite with immense practical utility. Biomolecules. 9:443. doi: 10.3390/biom9090443
Pawlak, K., and Kołodziejczak, M. (2020). The role of agriculture in ensuring food security in developing countries: Considerations in the context of the problem of sustainable food production. Sustainability 13:5488. doi: 10.3390/su12135488
Petrini, O., and Dreifuss, M. (1981). Endophytische pilze in epiphytischen Aracea, Bromeliaceae und Orchidaceae. Sydowia. 34, 135–148.
Philippot, L., Raaijmakers, J.M., Lemanceau, P., and van der Putten, W.H. (2013). Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 11, 789–799. doi: 10.1038/nrmicro3109
Raj, N. B., Swamy, M. K., Purushotham, B., and Sukrutha, S. K. (2021). “Applications of microbe-based nanoparticles in agriculture: present state and future challenges,” in Microbial Nanobiotechnology: Principles and Applications, eds A. Lateef, B. Gueguim-Kana, N. Dasgupta, and S. Ranjan (Singapore: Springer). doi: 10.1007/978-981-33-4777-9_12
Ramesh, A., Sharma, S., Sharma, M., Yadav, N., and Joshi, O. (2014). Plant growth-promoting traits in Enterobacter cloacae subsp. dissolvens MDSR9 isolated from soybean rhizosphere and its impact on growth and nutrition of soybean and wheat upon inoculation. Agric. Res. 3, 53–66. doi: 10.1007/s40003-014-0100-3
Rampelotto, P. H. (2013). Extremophiles and extreme environments. Life 3, 482–485. doi: 10.3390/life3030482
Rao, K. P. C., Verchot, L. V., and Joshi, L. M. (2007). Adaptation to climate change through sustainable management and development of agroforestry systems. J SAT Agric Res. 4, 1–30.
Rop, K., Karuku, G.N., Mbui, D., Michira, I., and Njomo, N. (2018). Formulation of slow release NPK fertilizer (cellulose-graft-poly (acrylamide)/nano-hydroxyapatite/soluble fertilizer) composite and evaluating its N mineralization potential. Ann. Agri. Sci. 63, 163–172. doi: 10.1016/j.aoas.2018.11.001
Sebastianes, F. L. S., de Azevedo, J. L., and Lacava, P. T. (2017). “Diversity and biotechnological potential of endophytic microorganisms associated with tropical mangrove forests,” in Diversity and Benefits of Microorganisms From the Tropics, eds J. Azevedo and M. Quecine (Cham: Springer). doi: 10.1007/978-3-319-55804-2_3
Sebastianes, F. L. S., Romão-Dumaresq, A. S., Lacava, P. T., Harakava, R., Azevedo, J. L., de Melo, I. S., et al. (2013). Species diversity of culturable endophytic fungi from Brazilian mangrove forests. Curr. Gen. 59, 153–166. doi: 10.1007/s00294-013-0396-8
Sharma, S., and Roy, S. (2015). Isolation and identification of a novel endophyte from a plant Amaranthus spinosus. Int. J. Curr. Microbiol. App. Sci. 4, 785–798. Available online at: https://www.ijcmas.com/vol-4-2/ShilpaSharmaandShikhaRoy.pdf
Siddiqui, Y., Surendran, A., Paterson, R. R. M., Ali, A., and Ahmad, K. (2021). Current strategies and perspectives in detection and control of basal stem rot of oil palm. Saudi. J. Biol. Sci. 5, 2840–2849. doi: 10.1016/j.sjbs.2021.02.016
Silva, F. A., Liotti, R. G., Boleti, A. P. A., Reis, E. M., Passos, M. B. S., Santos, E. L., et al. (2018). Diversity of cultivable fungal endophytes in Paullinia cupana (Mart.) Ducke and bioactivity of their secondary metabolites. PLoS ONE 13, e0195874. doi: 10.1371/journal.pone.0195874
Silva, J.A., Matos, D.L., David, G.Q., Ramalho, A.B., and Peres, W.M. (2015). Biocontrole in vitro de Lasiodiplodia theobromae por isolados de Trichoderma spp. Cáceres 2, 444–449.
Simpson, A. J., Reinach, F. C., Arruda, P., Abreu, F. A., Acencio, M., Alvarenga, R., et al. (2000). The genome sequence of the plant pathogen Xylella fastidiosa. The Xylella fastidiosa consortium of the organization for nucleotide sequencing and analysis. Nature 406, 151–159. doi: 10.1038/35018003
Soares, J. M. S., Rocha, A. J., Nascimento, F. S., Santos, A. S., Miller, R. N. G., Ferreira, C. F., et al. (2021). Genetic improvement for resistance to black sigatoka in bananas: A systematic review. Front. Plant Sci. 12, 657916. doi: 10.3389/fpls.2021.657916
Song, G. C., Im, H., Jung, J., Lee, S., Jung, M. Y., Rhee, S. K., et al. (2019). Plant growth-promoting archaea trigger induced systemic resistance in Arabidopsis thaliana against Pectobacterium carotovorum and Pseudomonas syringae. Environ. Microbiol. 21, 940–948. doi: 10.1111/1462-2920.14486
Sousa, P.L., and Silva, M.J. (2012). Differential growth of Jatropha curcas L. by different strains of endophytic Bacillus sp. Int. Res. J. Agric. Sci. Soil Sci. 2, 306−311. Available online at: https://www.interesjournals.org/articles/differential-growth-of-jatropha-curcas-l-by-differentstrains-of-endophytic-bacillus-sp.pdf
Taffner, J., Erlacher, A., Bragina, A., Berg, C., Moissl-Eichinger, C., Berg, G, and Tamaki, H. (2018). What is the role of archaea in plants? New insights from the vegetation of alpine bogs. Sphere 3, e00122–e00118. doi: 10.1128/mSphere.00122-18
Talón, M., Wu, G. A., Gmitter, Jr, F. G., and Rokhsar, D. S. (2020). The origin of citrus. In: The Genus Citrus. Elsevier Inc. doi: 10.1016/B978-0-12-812163-4.00002-4
Tan, M. I. S. M. H., Jamlos, M. F., Omar, A. F., Dzaharudin, F., Chalermwisutkul, S., and Akkaraekthalin, P. (2021). Ganoderma boninense disease detection by near-infrared spectroscopy classification: A review. Sensors 21:3052. doi: 10.3390/s21093052
Tang, J., Ding, Y., Nan, J., Yang, X., Sun, L., Zhao, X., et al. (2018). Transcriptome sequencing and ITRAQ reveal the detoxification mechanism of Bacillus GJ1, a potential biocontrol agent for Huanglongbing. PLoS ONE 13, e0200427. doi: 10.1371/journal.pone.0200427
Tavares, A.M., Atroch, A.L., Nascimento Filho, F.J., Pereira, J.C.R., Araújo, J.C.A., Moraes, L.A.C., et al. (2005). Cultura do guaranazeiro no Amazonas. Embrapa Amazônia Ocidental, Manaus.
Tewari, S., Shrivas, V. L., Hariprasad, P., and Sharma, S. (2019). Harnessing endophytes as biocontrol agents. In: Plant Health Under Biotic Stress. Singapore: Springer. doi: 10.1007/978-981-13-6040-4_10
Torres, F.L. (2018). Isolamento, caracterização e potencial biotecnológico de fungos endofíticos associados às plantas de Cerrado. [dissertation/master's thesis]. São Carlos (SP) Universidade Federal de São Carlos, Brazil.
United States Department of Agriculture (2021). U.S. Production and Exports Forecast Down Despite Global Gains. Available online at: https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed August 29, 2021).
Viswanathan, R., and Malathi, P. (2019). Biocontrol strategies to manage fungal diseases in sugarcane. Sugar Tech. 21, 202–212. doi: 10.1007/s12355-018-0690-3
Wang, N., Pierson, E.A., Setubal, J.C., Xu, J., Levy, J.G., Zhang, Y.Z., et al. (2017). The Candidatus Liberibacter-host interface: insights into pathogenesis mechanisms and disease control. Ann. Rev. Phytopathol. 107, 451–482.
Xu, J., Zhang, Y., Zhang, P., Trivedi, P., Riera, N., Wang, Y., et al. (2018). The structure and function of the global citrus rhizosphere microbiome. Nat. Commun. 9:4894. doi: 10.1038/s41467-018-07343-2
Keywords: biological control, endophytes, plant-microbial symbiosis, rhizobacteria, sustainable food production, tropical crops
Citation: Lacava PT, Bogas AC and Cruz FdPN (2022) Plant Growth Promotion and Biocontrol by Endophytic and Rhizospheric Microorganisms From the Tropics: A Review and Perspectives. Front. Sustain. Food Syst. 6:796113. doi: 10.3389/fsufs.2022.796113
Received: 15 October 2021; Accepted: 21 February 2022;
Published: 21 March 2022.
Edited by:
Diego Cunha Zied, São Paulo State University, BrazilReviewed by:
Ana I. F. Ribeiro-Barros, University of Lisbon, PortugalCopyright © 2022 Lacava, Bogas and Cruz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paulo Teixeira Lacava, cHRsYWNhdmFAdWZzY2FyLmJy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.