- 1International Institute of Tropical Agriculture (IITA), Plant Production and Health, Nampula, Mozambique
- 2Germplasm Resource Collection, United States Department of Agriculture-Agricultural Research Service, Soybean Genomics and Improvement Laboratory, Beltsville, MD, United States
- 3International Institute of Tropical Agriculture (IITA), Plant Production and Health, Lusaka, Zambia
Inoculation of soybean [Glycine max (L.) Merr.] with rhizobia strains is a low-cost investment which can increase yields of smallholder farmers in Mozambique. The performance of four Bradyrhizobium strains was evaluated to identify the best strain to inoculate soybean grown in different agro-ecologies. Field experiments were conducted in three ecological zones in 2018 and 2019 using soybean variety Zamboane inoculated with Bradyrhizobium diazoefficiens strain USDA 110, B. japonicum strains USDA 136, USDA 442 and WB74, and a non-inoculated control in a randomized complete block design with four replications. Indigenous rhizobia populations at the sites ranged from 9.0 x 101 to 2.2 x 103 cells g−1 soil. All four strains increased nodulation, but USDA 110 was superior at two sites with low native rhizobia population, whereas USDA 442 and WB74 were the best at the site with relatively high native rhizobia population. On an average, the strains doubled the number of nodules and increased the dry weight up to 5.8-fold. Inoculation increased shoot dry weight and N content at podding, plant biomass, and number of pods plant−1 across sites but the effects of the strains on seeds per pod, and 100-seed weight were inconsistent. Shoot N content did not differ among inoculant strains and ranged from 15.70 g kg−1 in the control to 38.53 g kg−1 across inoculation. All four strains increased soybean grain yield across sites in 2018 but USDA 110 outperformed the other strains and was also the best at one of the two sites in 2019. Grain yield responses associated with USDA 110 ranged from 552 kg ha−1 (56%) to 1,255 kg ha−1 (76%). Positive correlations between nodule dry weight plant−1 and seed yield, and number of pods plant−1 and grain yield were observed. The gross margin ranged from $343.50–$606.80 ha−1 for the control, but it increased to $688.34–$789.36 when inoculants were applied. On an average, inoculation increased gross margin by $182.57-$395.35 ha−1 over that of non-inoculated control in 2018 but drought stress in 2019 reduced the benefit. The results demonstrate that USDA 110 was the best inoculant strain and has the potential of increasing smallholder productivity and net returns.
Introduction
Soybean is one of the most widely grown crops in the world with USA, Brazil, Argentina, China, and India being the major producers (Chang et al., 2015). The grain contains about 40% protein and 20% oil; hence, it is in high demand for human nutrition, feed for livestock and for use as industrial raw material. In Sub-Saharan Africa (SSA), soybean is also an important component of the smallholder farming systems because of its ability to fix atmospheric nitrogen (Sanginga et al., 2002; Rusinamhodzi et al., 2012). Soybean production in Mozambique is relatively new and it is mainly produced in the medium to high altitude agroecologies with average annual rainfall above 1,200 mm which are in Zambézia, Tete, Nampula, Niassa and Manica provinces. There is a growing interest among smallholder farmers especially in Zambézia and Tete provinces which produce over 70% of soybean in Mozambique because of the steady increase in demand from the domestic poultry industry for chicken feed; thus, there is ready market with attractive farmgate prices. Despite the high demand, soybean producers in Mozambique, largely smallholder farmers (over 95%) are not able to meet the domestic demand because of low yields due to poor agronomic practices and lack of inputs (Matteo et al., 2016). FAO statistics indicate that 55,000 and 75,000 tons of soybean grains were produced in 2019 and 2020, respectively with estimated four-year (2016–2020) average yield of 1.4 ton/ha (FAOSTAT, 2022). The demand gap is met by imported soybean cake from Brazil, Argentina, and India. Several interventions have been proposed to address the low productivity challenges faced by smallholder farmers but the most significant and affordable one is soil fertility improvement using rhizobia inoculants which supply atmospheric N to the crop.
Soybean requires high amount of N for optimum growth and productivity due to the high seed protein content (Hungria and Kaschuk, 2014; Hungria and Mendes, 2015). The N requirement is met by inorganic N uptake from the soil and through symbiotic nitrogen fixation in association with compatible and effective rhizobia strains in the rhizosphere of the plant. The rhizobia infect and colonize the soybean roots inducing nodule formation through complex chemical mediated signals between the rhizobia and the soybean plant involving isoflavones and lipochitooligosaccharide (LCO) molecules (Gage, 2004; Vieira et al., 2010; Smith et al., 2015). N2 fixation occurs in the nodules and exported to other parts of the plant tissues to meet the N demand and protein synthesis. Under favorable conditions, soybean can meet up to 94% of its N needs through N2 fixation (Hungria et al., 2006; Salvagiotti et al., 2008; Gyogluu et al., 2016) and contribute significantly to improve soil N for subsequent crops in rotation (Sanginga et al., 2002; Rusinamhodzi et al., 2012). Hence, it reduces the amount of inorganic nitrogen fertilizer required in smallholder farming systems where the use of inorganic fertilizers is limited. However, N uptake by soybean plant through N2 fixation is often limited by many factors including environmental conditions, absence of adequate population of compatible, efficient and competitive indigenous rhizobia (Thies et al., 1991; Brockwell et al., 1995; Giller, 2001). It is difficult for smallholder farmers to apply the required external inputs such as N and P fertilizers since they are expensive and unaffordable (Kyei-Boahen et al., 2017). Therefore, a low cost-investment such as rhizobia inoculation which costs about $10 ha−1 is becoming increasingly important due to affordability and economic incentives associated with the practice (Kyei-Boahen et al., 2017; Savala et al., 2021, 2022). Maximizing N2 fixation by selecting competitive and effective inoculant strains would improve soil N content, increase crop productivity, and contribute to agricultural sustainability.
Several studies have demonstrated the benefits of using microbial inoculants in SSA (Masso et al., 2016; Kyei-Boahen et al., 2017; van Heerwaarden et al., 2017; Chibeba et al., 2020; Savala et al., 2022). In Mozambique, Gyogluu et al. (2016) reported that rhizobia inoculated soybean fixed up to 200 kg N ha−1 which accounted for 90% of N nutrition leading to 500 kg ha−1 yield advantage over the non-inoculated counterpart. Similar results of better crop growth and development, higher grain yields and profitability using microbial inoculant have been reported in soybean from Mozambique (Chibeba et al., 2020; Savala et al., 2021, 2022). In a Large-scale study in 10 SSA countries including Mozambique, Malawi and Zimbabwe, van Heerwaarden et al. (2017) reported more than 200 kg ha−1 yield increases in soybean due to rhizobial inoculation. A meta-analysis study of various soybean experiments conducted in Ghana showed 60% yield increase in the inoculated plants compared with the non-inoculated plants (Buernor et al., 2022). Masso et al. (2016) and Ronner et al. (2016) reported significant yield response of 447 kg ha−1 and 426 kg ha−1, respectively to rhizobial inoculation in soybean. In most of these studies, the application of rhizobia together with P fertilizer increased soybean grain yields more than applying rhizobia or P alone suggesting that external P input is key for maximizing soybean yield response to inoculation in most soils in SSA.
There is consensus in the literature on the positive impacts of rhizobia inoculant strains on soybean productivity through N2 fixation but the results vary since it depends on many factors including the rhizobia strain. A particular strain may perform well in a specific agro-climatic zone but may fail in another agro-climatic zone due to poor adaptability (Thilakarathna and Raizada, 2017; Mendoza-Suárez et al., 2021). In Nigeria, Okereke et al. (2000, 2001) assessed the competitiveness and performance of several Bradyrhizobium strains and found that USDA 110 and USDA 122 were superior in nodule occupancy, nodule formation and yield gain. They also observed that the variables were influenced by the interaction effects of rhizobia strains, soybean cultivar and location. Ulzen et al. (2016) reported no differences in nodulation and grain yield between inoculants containing strain 532C and USDA 110 in soils with <10 rhizobia cells g−1 soil in Ghana. Limited information is available on the performance of rhizobia strains in tropical soils.
Although rhizobia inoculant production in SSA is a recent development, production in Mozambique is lacking and therefore smallholder farmers depend on products imported from Brazil, South Africa, Malawi and other countries. Most of the soybean inoculants in SSA contain strain USDA 110 but there are other Bradyrhizobium strains in the USDA Collection known to be effective and efficient in establishing symbiotic association with soybean which have not been tested widely in SSA. Therefore, the objective of this study was to evaluate the symbiotic performance of B. japonicum strains USDA 136, USDA 442 and WB74 (USDA 122) with the widely used B. diazoefficiens strain USDA 110 in three agro-ecologies with varying levels of indigenous rhizobia population for possible use as inoculant for soybean in Mozambique.
Materials and methods
Description of experimental site
The experiments were conducted at three locations with sandy clay loam soils in Mozambique in 2 years during the 2017/2018 and 2018/2019 cropping seasons. In the first year, the trial was established at Muriaze (15.2737° S, 39.3133° E; 360 m.a.s.l.) in Nampula province; Namarripe (15.3508° S, 36.7888° E; 776 m.a.s.l.) in Zambezia province and Vila Ulongué (14.7472° S, 34.3683° E; 1211 m.a.s.l.) in Tete province. In the second year, the experiment was conducted at two locations: Namarripe and Vila Ulongué due to logistics reasons. The sites have unimodal rainfall that starts in late November and ends in April averaging 900 mm at Muriaze, 1,500 mm at Namarripe and 1,400 mm at Vila Ulongué. During this period, average maximum temperatures are about 29, 26 and 24°C at Muriaze, Namarripe and Vila Ulongué, respectively, whereas minimum temperatures are 21, 18 and 16°C. Mini Weather Stations (WatchDog 2800 series, Spectrum Technologies, Inc, IL, USA) were installed at each site to collect rainfall data and daily minimum and maximum temperatures during the trial period (Figure 1).
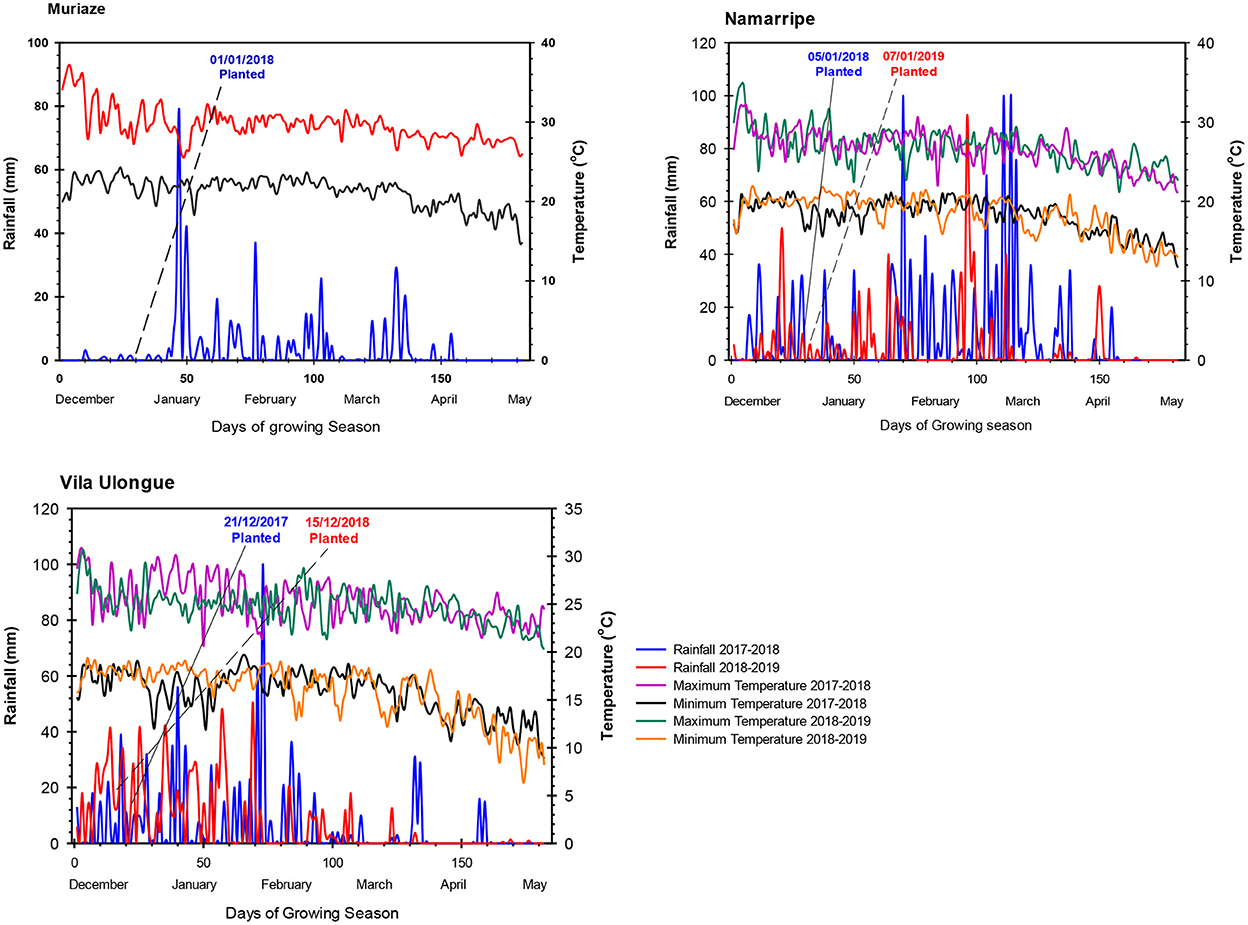
Figure 1. Daily rainfall and temperature at the experimental locations during the 2017/2018 and 2018/2019 cropping seasons at Muriaze, Namarripe, and Vila Ulongué, Mozambique.
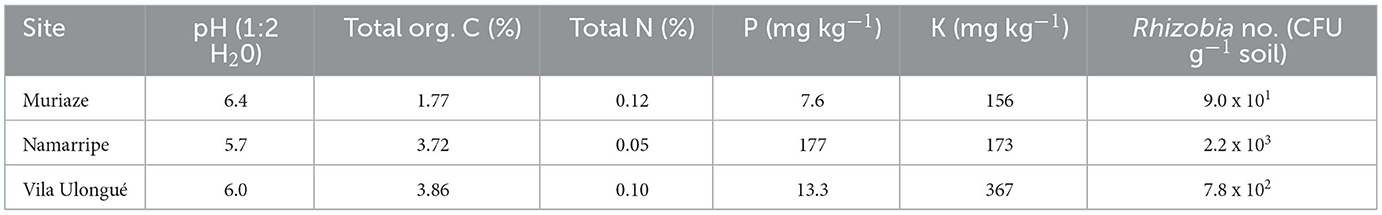
The sites are low input managed fields with maize, fallow and sesame cropping history in the three seasons preceding the current study at Muriaze, maize, fallow, and maize at Namarripe and maize, groundnut, and maize at Vila Ulongué. Based on the Soil Atlas of Africa, the predominant soil type at Muriaze is Haplic Lixisols, and Namarripe and Vila Ulongué are Chromic Luvisols (Jones et al., 2013). Ten soil samples were randomly taken from 0 to 30 cm soil layer using a soil auger from each site 1 week before sowing and combined into a composite sample and four subsamples of the composite from each site were taken to the laboratory for chemical and microbiological analyses (Table 1). The pH was determined using a high impedance voltmeter on 1:2 soil-water suspension (McLean, 1982). Total organic carbon was determined by Walkley-Black Method (Nelson and Sommers, 1996), total N was determined by The Kjeldhal method (Bremner and Mulvaney, 1982), P was determined by Olsen's method (Olsen and Sommers, 1982) and K was determined using ICP-OES after extraction with Mehlich 3 (Mehlich, 1984). Soil subsamples for microbial assay were stored at 4°C until used.
Estimation of soil rhizobia population
Soil samples taken from the three experimental sites were used to estimate the populations of indigenous rhizobia using the most probable number (MPN) technique (Vincent, 1970). Soybean genotype TGx 1987-62F was used as the trap host. The seeds were surfaced sterilized with 95% alcohol for 10 s to remove waxy substances and air bubbles. Another sterilization was conducted for 2 to 5 min in 3% hydrogen peroxide and rinsed with sterile distilled water for 5 to 6 times. The seeds were allowed to fully imbibe sterile distilled water for 4 h (Somasegaran and Hoben, 1994) and pre-germinated in Petri dishes that contained moist sterile tissue and incubated at 28 °C for 48 h. Upon emergence of the radicle, seedlings with straight radicles were selected and transferred aseptically to plastic growth pouches containing 50 ml N-free plant nutrient solution (Broughton and Dilworth, 1970) using forceps. The growth pouches were arranged in a wooden rack and kept in the greenhouse for 1 week prior to inoculation. Ten steps, ten-fold (10−1 to 10−10) serial dilution was employed for the estimation of the total number of rhizobia in the soil samples respectively using saline solution (0.89% NaCl) as the diluent. Each growth pouch was inoculated with 1 ml of the diluent replicated four times changing pipette tips to prevent contamination. The plants were watered with sufficient N—free nutrient solution when required. Nodulation was assessed after 28 days based on the presence or absence of root nodules. The MPN of each bradyrhizobia population at each site was determined using the most probable number enumeration system (MPNES) (Woomer et al., 1990).
Experimental layout and treatment
Soybean variety Zamboane (TGx 1904-6F), a promiscuous and widely grown variety across the three agro-ecologies was used. It is a medium-maturing variety with 102-105 days growth cycle released by the Instituto de Investigação Agrária de Moçambique (IIAM) (National Research Institute of Mozambique) and International Institute of Tropical Agriculture (IITA). Bradyrhizobium diazoefficiens strain USDA 110 and B. japonicum strains USDA 136 and USDA 442 were obtained as single strain inoculants formulated as granular peat containing 2.5 x 109 colony-forming units (CFU) g−1 inoculant from National Rhizobium Germplasm Resource Collection, US Department of Agriculture (USDA-ARS SGIL, Beltsville, MD). These strains were selected based on their competitiveness and effectiveness (Patrick Elia, USDA-ARS SGIL, personal communication; Arachchige et al., 2020). USDA 110 is the most widely used strains in most inoculants in SSA. B. japonicum WB74 (USDA 122) was obtained as commercial inoculant strain from Soygro Pty, South Africa as additional strain for the study. The Soygro inoculant was formulated as peat-based powder containing 1.0 x 109 CFU g−1 inoculant. The experimental design was randomized complete block with four replications. The treatments consisted of non-inoculated control and the four strains placed in the sowing furrows before sowing the seeds. The inoculation application rate was 10 kg ha−1 for the inoculant received from USDA and 20 kg ha−1 for Soygro WB74. The concentration of the rhizobia cells in the Soygro product was half that obtained from USDA; therefore, the application rate for WB74 was doubled to ensure similar number of rhizobial cells of each strain. Basal application of 40 kg P2O5 ha−1 as Triple Superphosphate (TSP) was done at planting. The total experimental area was 18.6 m in length and 18 m wide and each plot consisted of 3 m by 2.5 m with 1.5 m between plots and 2 m between blocks. The row spacing was 0.5 m and intra-row spacing of 0.1 m with two seeds per stand (about 20 plants m−1), resulting in a final plant population of about 340,000 ha−1. Land preparation was accomplished by two passes with a disc harrow. The trials were planted in early January at Muriaze in 2018 and at Namarripe in 2018 and 2019, and in mid-December at Vila Ulongué in 2017 and 2018. To avoid cross-contaminations the non-inoculated control plots were planted first and no person handled more than one strain during planting. In addition to the 1.5 m and 2 m distances that separated plots and blocks respectively, ridges were raised around plots to avoid run-off from one plot to another. Planting was done manually, and manual weed control was conducted two times. The trials were conducted under rainfed conditions (Figure 1). One spraying regime of fungicide formulation consisting of 6.4 ml of Azoxystrobin (250 g active ingredient L−1) and Difenoconazole (125 g active ingredient L−1) in 16 L water (≈ 0.5 L fungicide ha−1) was applied just before flowering to control fungal disease particularly soybean rust.
Data collection
Two weeks after seedling emergence, plant stand count was conducted. At R3 stage, five soybean plants were randomly selected from each plot, the roots were excavated, and the soil was carefully removed to ensure that the roots and the nodules were recovered as much as possible. The roots were carefully washed with water and nodules were removed and counted. The nodules were subsequently placed in envelopes and dried in an oven at 60°C for 48 h to determine nodule dry weight. The plants sampled for nodules assessment were dried in an oven at 60°C for 72 h and shoot dry weight was determined. At maturity, 10 plants were randomly selected and pulled from the ground to determine the number of pods per plant. Forty pods from the 10 plants were randomly selected and seeds in the 40 pods were counted and the average seeds per pod were calculated. A 1 m2 area (two middle rows, 1 m long) of each plot was randomly selected, harvested, and sun-dried for 7 days to determine above-ground biomass. The rest of the plants from each plot were harvested manually and sun-dried for about 3 days. Thereafter, pods from each plot were threshed manually and grain yield was determined. The moisture content of grain samples from each plot was determined using Farmex MT-16 grain moisture Tester (AgraTronix LLC, Streetsboro, OH) and grain yield in kg ha−1 was adjusted to 13% moisture content. Dried shoot samples harvest at R3 stage were ground to pass a 2-mm mesh sieve. Total N content was determined using the Kjeldahl method.
The variable production cost and farmgate prices of soybean grain after harvest were collected to estimate the net returns on investments. The revenue from grain yields of the non-inoculated control plants vs. the average grain yield of the inoculated plants were used to estimate gross margin. The estimated cost included the cost of seed ($33.50 ha−1), land preparation ($30.23 ha−1), planting ($21 ha−1), weeding ($42 ha−1), inoculant ($13.44 ha−1), TSP ($54.60 ha−1), chemical spray ($9.24 ha−1), harvesting and threshing ($42 ha−1), 50 kg storage bags ($0.25 bag−1), transportation of grain to selling points ($0.33 50 kg−1) and other miscellaneous expenses. Average soybean farmgate price of ($0.39 kg−1) for the period between late April to end of July 2018 and 2019 was used since most farmers sell their soybean within this period.
Data analysis
Data collected were analyzed using PROC MIXED of SAS version 9.4 (SAS/STATR, 2018). Combined analysis across sites and cropping seasons was performed. Effects of season and site were dominant and interactions involving season, site and treatment were highly significant for most of the variables. Hence, data for each cropping season were analyzed across site. Season, site, and blocks within sites were considered random effects, whereas treatment (inoculant strains) was considered fixed effect. Significant differences among treatment means were evaluated using LSD at 5% probability. Variables analyzed include number and dry weight of nodules, shoot dry weight at podding, shoot N content, number of pods per plant, number of seed per pod, 100-seed weight, grain yield and plant dry matter at harvest.
Results
Soil properties and weather conditions
The pH of the soils across the three sites ranged from 5.7 to 6.4 and the organic carbon contents at Namarripe and Vila Ulongué were two-fold that at Muriaze (1.77%) (Table 1). Total N at Namarripe was 0.05% indicating low capacity to supply adequate available N, but the Total N contents of the soils at Muriaze (0.12%) and Vila Ulongué (0.10%) could be considered medium in supplying available N. Soil available P level was higher at Namarripe than at Vila Ulongué and Muriaze. However, soil available K levels were very high across the sites. The soils across the three sites contained limited population of indigenous Rhizobium ranging from 9.0 x 101 to 2.2 x 103 cells g−1 soil (Table 1). Namarripe had the highest indigenous rhizobia population, whereas Muriaze had the lowest population.
Total rainfall amount and distribution differed among sites and between cropping seasons (Figure 1). Generally, the frequency of the rains was more favorable for soybean growth in the first season than the second season. During the growing period from December 2017 to April 2018, Muriaze had a total rainfall of 640 mm with most of the rains occurring in January and February, and the distribution was good from January to April 2018 when the crop was in the field. Namarripe received 1,514 mm during the 2017/2018 growing season with almost 70% of the rain occurring in February and March 2018 but the rainfall in 2018/2019 growing season was low (853 mm) with intermittent drought spells especially in February during the flowering stage. The rainfall at Vila Ulongué during the first season was lower (896 mm) than the second season (1,237 mm) but despite the low rainfall in the first year, it was well distributed and available at the time the crop needed water most. Average temperatures at the sites did not vary during the two cropping seasons from December to April (Figure 1). However, the average maximum temperature at Muriaze (30.1° C) was 4.4 o C higher than that at Vila Ulongué (25.7 o C) and 2.8° C higher than that at Namarripe (27.3° C). Similarly, the average minimum temperature at Muriaze was 5.4° C higher compared to that at Vila Ulongué and 2.9° C higher than that at Namarripe (18.9° C). Thus, Muriaze is a dry and hot environment, whereas Vila Ulongué is relatively a cooler environment.
Nodulation and shoot dry matter at R3 growth stage
Inoculation with the four Bradyrhizobium strains increased the number and dry weight of nodules across the experimental sites (P < 0.0001) compared with that of the non-inoculated control plants (Table 2). In the 2017/2018 growing season, USDA 110 treated plants had the highest number and dry weight of nodules at Muriaze and Vila Ulongué, whereas USDA 442 and WB74 (USDA 122) treatments performed significantly better in terms of nodulation at Namarripe. On an average, inoculation almost tripled the number of nodules at Muriaze and Namarripe and increased the dry weight of the nodules by 3.5 times at Muriaze and 5.8 times at Namarripe. At Vila Ulongué, the number of nodules increased by 52%, whereas the nodule dry weight increased 2.5-fold compared to that of the non-inoculated plants. Nodulation at Namarripe in the 2018/2019 cropping season was consistent with the results of the previous season (Table 3). However, nodulation in some of the inoculated plants were not different from that of the control plants at Vila Ulongué. Averaged across the strains, the number and dry weight of nodules doubled when the plants were inoculated at Namarripe and at Vila Ulongué, the number and dry weight of nodules increased by 32 and 50%, respectively.
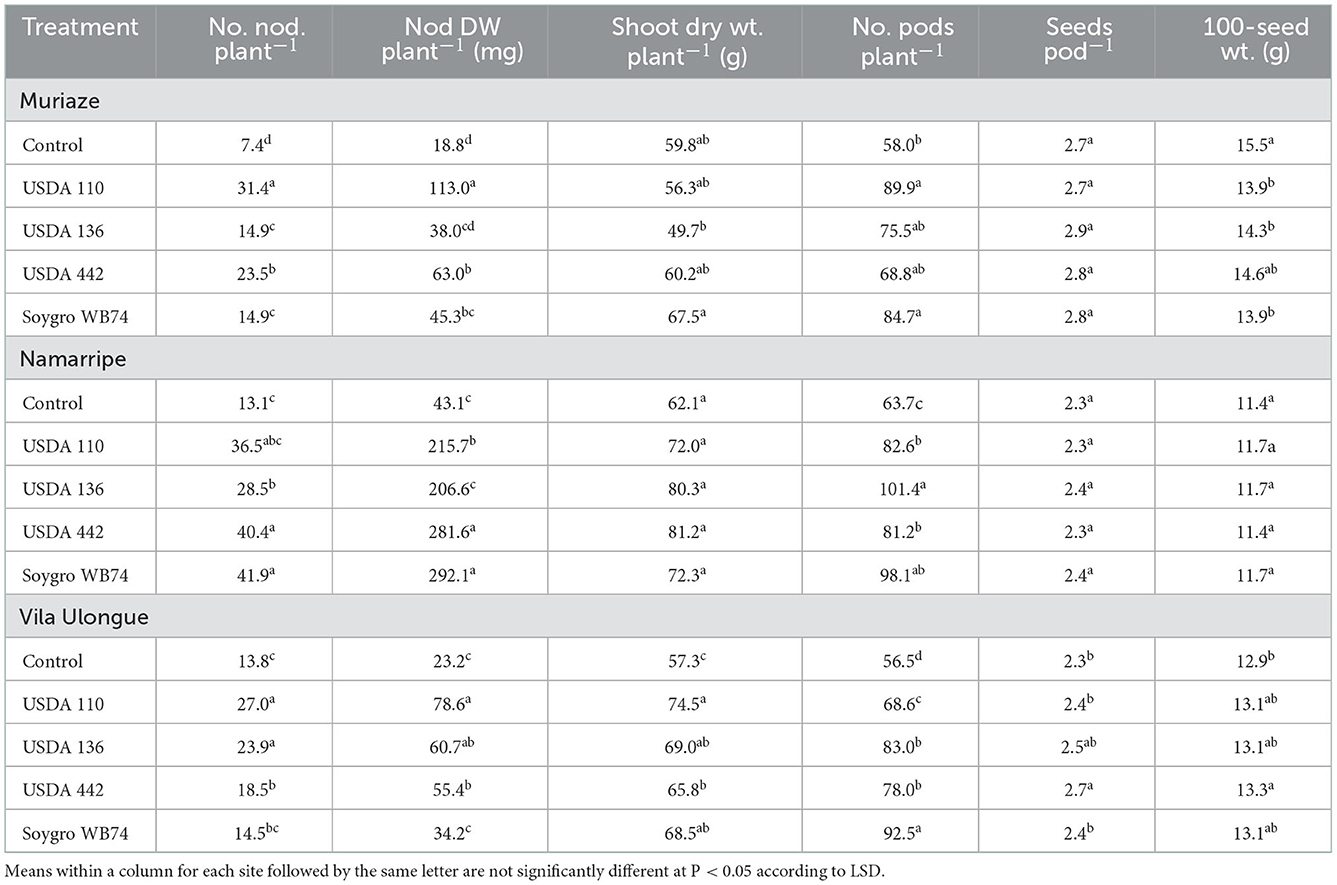
Table 2. Effects of inoculation on number and dry weight of nodules, shoot dry weight at R3 stage, number of pods, seeds per pod and 100-seed weight at Muriaze, Namarripe, and Vila Ulongué in 2017/2018 cropping season.
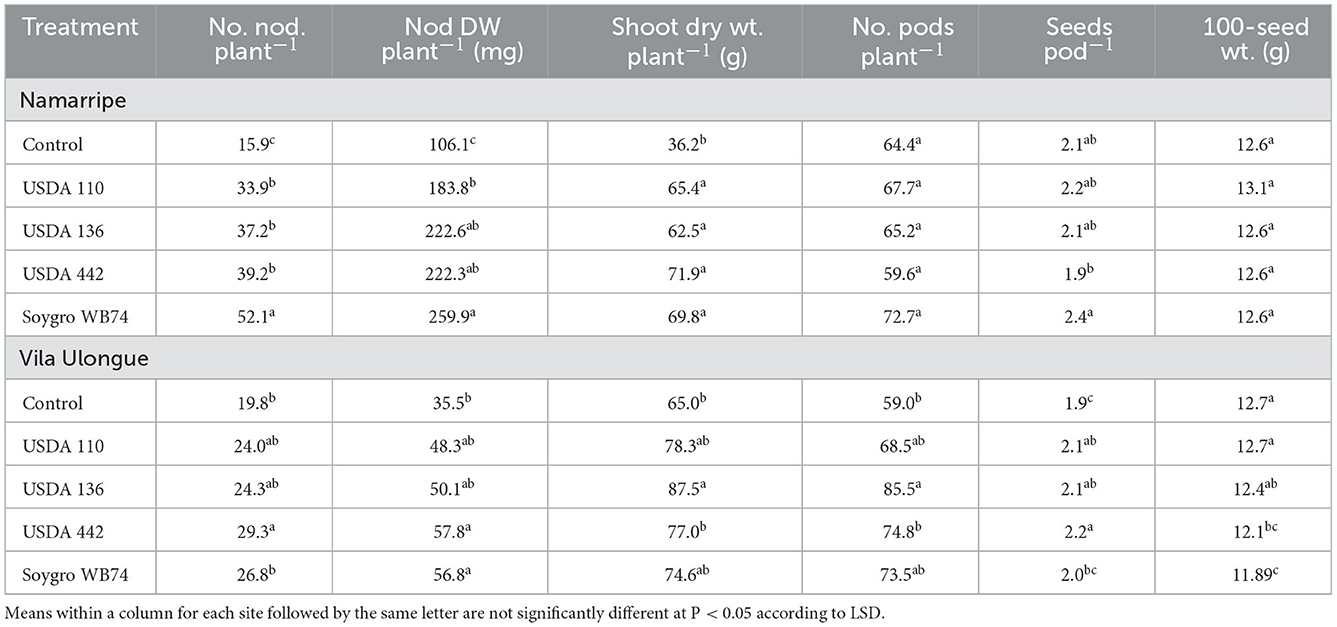
Table 3. Effects of inoculation on number and dry weight of nodules, shoot dry weight at R3 stage, number of pods, seeds per pod and 100-seed weight at Namarripe and Vila Ulongué, in 2018/2019 cropping season.
Plants inoculated with strain WB74 produced the highest shoot dry matter at R3 growth stage at Muriaze, whereas plants associated with USDA 136 had the lowest shoot dry matter in the first season but there were no differences among treatments including the control plants (Table 2). There were no significant differences (P = 0.318) in shoot dry matter among the treatments at Namarripe. All the inoculant treatments at Vila Ulongué increased shoot dry matter compared with that of the non-inoculated plants. In the second season, shoot dry matter at Namarripe did not differ among the strains and was on an average 86% higher than that of the non-inoculated plants (Table 3). In contrast, only the shoot dry matter for USDA 136 was higher than that of the control plants at Vila Ulongué. At this site, the average shoot dry weight of the inoculated plants was 22% more than that of the non-inoculated plants.
Shoot N content at R3 growth stage
Shoot N analysis was conducted in only 2019. Inoculation increased (P = 0.006 at Namarripe and P = 0.0002 at Vila Ulongué) shoot N content at both locations (Figure 2). At Namarripe, shoot N did not differ among the inoculant strains but they were significantly higher than that of the non-inoculated plants. On average, inoculation increased shoot N by 39% (9.91 g kg−1). Similarly, all the inoculated plants at Vila Ulongué had higher shoot N content which averaged 17% (2.69 g kg−1) higher than that of the non-inoculated plants (Figure 2). However, plants treated with strains WB74 had lower shoot N than plants inoculated with USDA 110 and USDA 136. Shoot N accumulation at Namarripe was relatively higher than that at Vila Ulongué.
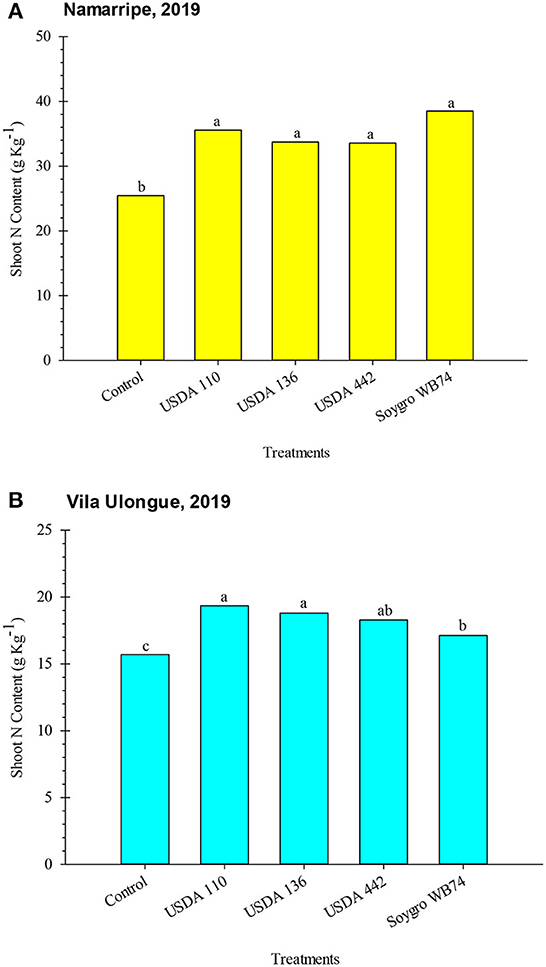
Figure 2. Shoot N contents of soybean inoculated with varoius inoculant strains at Namarripe (A) and Vila Ulongué (B), Mozambique. Means within a location followed by the same letter are not significantly different at P < 0.05 according to LSD.
Yield and dry biomass components
The inoculant strains influenced grain yield and above-ground biomass at harvest across site and cropping season, although the results were more consistent in the first season than the second season (Figures 3, 4). In 2018, grain yield of all the inoculated plants were significantly higher (P = 0.001) than that of the non-inoculated plants but yields among some strains were not different (Figure 3). Generally, USDA 110 outperformed the other strains in grain yield across the three sites (P < 0.0001 at Muriaze and Vila Ulongué and P = 0.037 at Namarripe) in the first season except at Namarripe where USDA 442 performed similar to USDA 110. At Muriaze, USDA 110 increased grain yield by 76% (1,255 kg ha−1) compared with that of the control, and at Vila Ulongué and Namarripe, the yield increased by 80% (1,238 kg ha−1) and 26% (579 kg ha−1), respectively. When averaged over the inoculant strains, soybean grain yield increased by 65% at Muriaze, 61% at Vila Ulongué and 23% at Namarripe compared with the yield of the non-inoculated plants. No significant grain yield differences occurred between the inoculated and non-inoculated plants in 2019 at Namarripe, however yield in USDA 442 was higher than USDA 110 (Figure 3). At Vila Ulongué, only the yield in USDA 110 was higher (56%) than the control in the second season. Furthermore, the yield differences between USDA 110 and two strains (USDA 442 and WB74) were significant.

Figure 3. Grain yield of soybean inoculated with various inoculant strains in 2018 (A) and 2019 (B) in Mozambique. Means within a location followed by the same letter are not significantly different at P < 0.05 according to LSD.
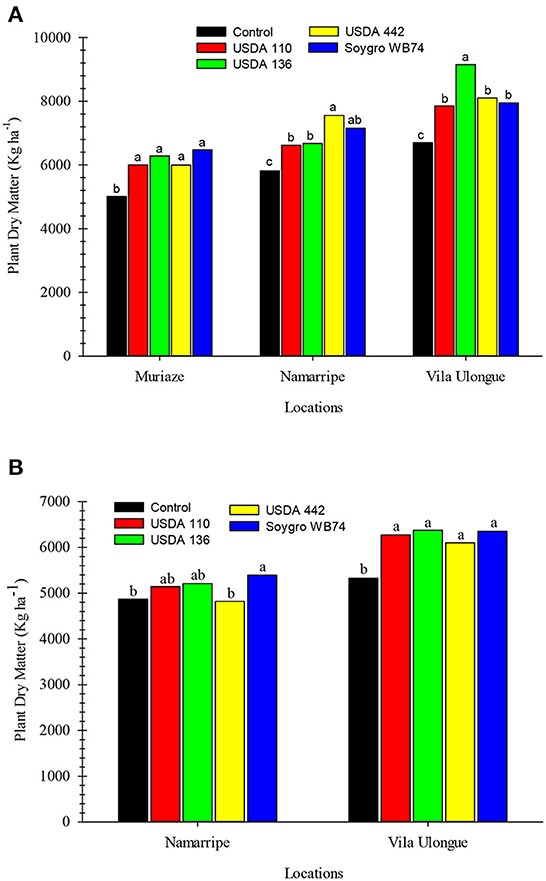
Figure 4. Dry matter yield of soybean inoculated with various inoculant strains in 2018 (A) and 2019 (B) in Mozambique. Means within a location followed by the same letter are not significantly different at P < 0.05 according to LSD.
The above-ground dry matter yield at harvest followed a similar trend as grain yield (Figure 4). In the first season, dry matter yield of the inoculated plants were significantly higher (P = 0.013) than that of the non-inoculated control plants at all sites. However, no differences in dry matter yield occurred among the strains at Muriaze but USDA 442 and USDA 136 treatments at Namarripe and Vila Ulongué, respectively were higher compared to the other strains. Dry matter produced in the first season ranged from 5,008 to 6,475 kg ha−1 at Muriaze, 5,812–7,555 kg ha−1 at Namarripe and 6,700–9,150 kg ha−1 at Vila Ulongué. In the second year, all the inoculated plants produced higher (P = 0.007) dry matter than the control plants at Vila Ulongué but only WB74 increased dry matter yield at Namarripe. As in the previous season, dry matter production was higher at Vila Ulongué than at Namarripe.
Inoculation improved the major yield components such as the number of pods per plants, seeds per pod, and 100-seed weight. However, it was not consistent across sites and cropping season (Tables 2, 3). In the first year, inoculation increased the number of pods across the three sites (P = 0.043 at Muriaze, P = 0.003 at Namarripe, P < 0.0001 at Vila Ulongué), but it had no effect at Namarripe in the second year (P = 0.706) (Table 2). The number of seeds per pod in the first season was not affected by inoculation at Muriaze and Namarripe but at Vila Ulongué, the number for USDA 442 was higher than that of the control plants, USDA 110 and WB74. The differences in the number of pods in the second season did not follow a specific trend since the values for the non-inoculated control were not different from that of the inoculated plants (Table 3). Similarly, the 100-seed weight was not consistent except at Muriaze in the first year where the seed weight of the non-inoculated control plants was higher than that of the inoculated plants which ranged from 13.9 g for WB74 to 15.5 g for the control plants.
Economic benefits of using inoculant
In the first season, variable production cost with no investment in inoculant was $261.77, $268.76, and $260.61 ha−1 for Muriaze, Namarripe and Vila Ulongué, respectively with corresponding revenue of $643.50, $875.55, and $604.11 ha−1 (Table 4). However, the cost increased by 10, 7 and 9%, respectively when inoculants were applied leading to associated revenue of $1,065.09, $1,077.96, and $973.44 ha−1, respectively. Thus, the gross margin ranged from $343.50 to $606.80 ha−1 with no investment in inoculant, but it increased to $688.34 to $789.36 when inoculants were applied. Namarripe had the highest net returns whilst Vila Ulongué had the lowest. In the second year, low productivity due to drought spells negatively affected revenue and drastically reduced the impact of inoculants on net returns (Table 4). Thus, net returns for production with no inoculant was $143.98 for Namarripe to $129.51 for Vila Ulongué which increased by only $4.11 and $95.04, respectively when inoculants were applied.
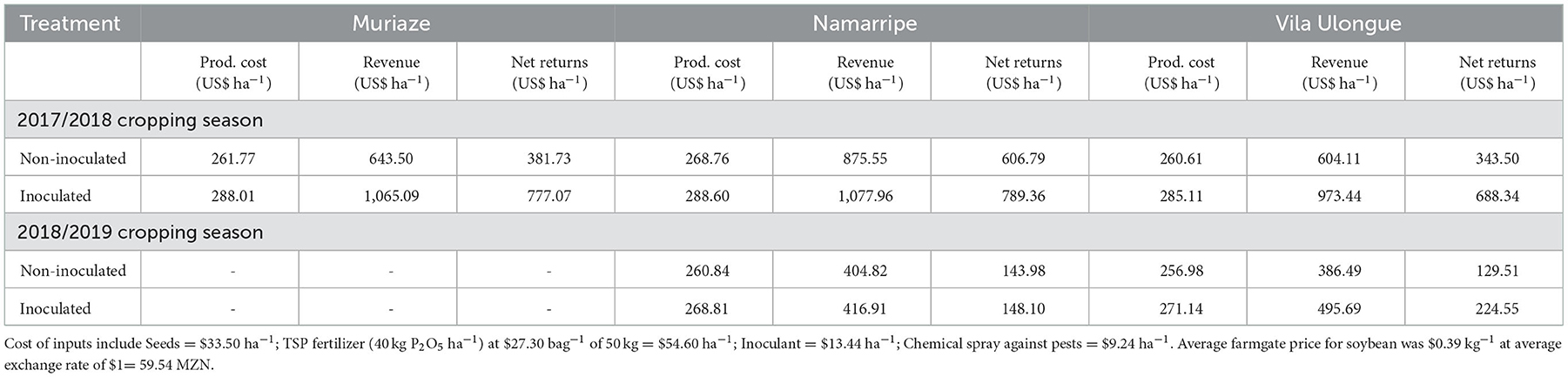
Table 4. Estimated production cost, revenue and net returns for soybean production with and without inoculation in 2017/2018 and 2018/2019 cropping seasons at Muriaze, Namarripe and Vila Ulongué, Mozambique.
Discussion
Performance of different inoculant strains
A promiscuous soybean variety can form nodules freely with indigenous rhizobia (Kueneman et al., 1984). As a result of this varietal trait, varied responses to inoculation have been reported (Okogun and Sanginga, 2003; Osunde et al., 2003; Thuita et al., 2012; Savala et al., 2022). In the present study, soybean variety Zamboane (TGx 1904-6F) demonstrated significant increase in nodulation and yield-related responses to inoculation with all the inoculant strains used. This is consistent with the results of other studies with this variety and others (Gyogluu et al., 2016; Savala et al., 2021, 2022). The success of inoculation depends on many factors including the presence or absence of indigenous rhizobia (Thies et al., 1991; Sanz-Saez et al., 2015), environmental conditions such as soil moisture and nutrient status (Zahran, 1999; Hungria and Vargas, 2000) and the quality of the inoculant applied (Rodríguez-Navarro et al., 2010). Results of soil analysis from the experimental sites indicated slight differences in the soil properties among the sites (Table 1) but were adequate for soybean growth. For example, the pH was 5.7 to 6.4 which was within the range (5–6.4) found to respond well to inoculation in northern Nigeria (Ronner et al., 2016). Soil organic matter, total N and K were sufficient for soybean growth based on soil fertility studies carried out in the same agro-ecological zones of the present study sites (Maria and Yost, 2006). However, soil available P were relatively low at Muriaze and Vila Ulongué compared to Namarripe suggesting that soybean growth response to P input would likely be higher at Muriaze and Vila Ulongué than at Namarripe. Analysis of soil samples from the study sites using MPN technique indicated that the soils contained limited indigenous rhizobia populations (9.0 x 101 to 2.2 x 103 cells g−1 soil) (Table 1). The presence of existing rhizobia led to nodulation of the non-inoculated control plants. However, the inoculant strains effectively improved nodulation, shoot dry matter and N content at R3 growth stage and increased yield components which cumulatively led to increased grain yield and plant biomass production at maturity across sites (Tables 2, 3 and Figures 2–4). The observed nodulation in non-inoculated plants suggested that the existing soil rhizobia formed nodules; however, the population was probably not adequate and could be less effective than the introduced strains.
Inoculation with USDA 110 produced more nodules with corresponding higher nodule dry weight than the other strains in 2018 at Muriaze and Vila Ulongué where the indigenous strain populations were low. However, at Namarripe where the native strain population was relatively high, USDA 442 and WB74 produced more nodules with higher nodule dry weight than the other strains. Furthermore, in 2019, which experienced drought spells, USDA 442 and WB74 performed better in nodulation at both Namarripe and Vila Ulongué. The results suggest that USDA 110 performed well where competition for nodule formation is low, and under relatively favorable soil conditions in 2018, whereas USDA 442 and WB74 were more competitive and adapted to dry conditions. Inoculant strains vary in their ability to successfully colonize rhizosphere and compete against native rhizobia for nodule occupancy (Mendoza-Suárez et al., 2021). Ineffective native rhizobia strains are adapted to the local environment and often compete well against highly efficient introduced strain resulting in low inoculant nodule occupancy (Sanz-Saez et al., 2015; Thilakarathna and Raizada, 2017). Our results agree with the report from Nigeria that used four Bradyrhizobium strains including USDA 110, USDA 122, and USDA 136 (Okereke et al., 2000). In the study by Okereke et al. (2000), inoculation with USDA 110 and USDA 122 (WB74) increased nodule number and nodule dry weight than the other strains including USDA 136 due to the competitive abilities of the two strains for nodule occupancy. They reported that nodule occupancy ranged from 75 to 85% with USDA 110 and 60–65% with USDA 122 compared to 10–15% with USDA 136 at two sites 84 days after planting. In another study, Okereke et al. (2001) reported similar results using several Bradyrhizobium strains where TAL 102 [original designation is USDA 110 (Thies et al., 1991)] was superior to USDA 136 in nodule number and dry weight. Although nodule occupancy was not assessed in our study, Muriaze and Vila Ulongué where USDA 110 performed well had similar populations of resident rhizobia as that of the two study sites in Nigeria which ranged from 1 x 102 to 5 x 102 CFU g−1 soil (Okereke et al., 2000). Our results and those of Okereke et al. (2000, 2001) show that USDA 110 was superior to USDA 122 (WB74) in nodulation in soils with low native rhizobia population, but our data further indicated that WB74 performed better than USDA 110 in soil with relatively high native rhizobia, and under drought conditions. Limited information is available in the literature about the performance of USDA 442 which performed on par with WB74 under similar conditions. In a greenhouse study, Arachchige et al. (2020) observed that plants inoculated with USDA 442 produced more nodules than that of USDA 136 but was not significant in term of seed yield or any biomass related parameters.
In the present study, the performance of the inoculant strains varied across sites possibly due to variability in soil and environmental conditions which led to significant location or season by treatment interactions for most of the variables including number and dry weight of nodules, grain yield and plant biomass. All the inoculant strains had positive effects on soybean growth and yield and demonstrated higher efficiency in N2 fixation than the native rhizobia. The inoculated plants produced higher shoot dry matter at R3 growth stage with high shoot N content and improved yield components compared with the non-inoculated control plants (Tables 2, 3 and Figure 2). The significant improvement in plant growth supported high grain yield and plant dry matter production (Figures 2, 3).
Shoot N concentration was not significant among the inoculant strains as reported by others (Okereke et al., 2000; Arachchige et al., 2020), perhaps the R3 growth stage was too early for the differences to be evident. In the first season, USDA 110 was the best strain across sites in terms of grain yield showing increase of 26, 76, and 80% (579–1,255 kg ha−1) compared to the non-inoculated plants at Namarripe, Muriaze and Vila Ulongué, respectively (Figure 3). In the second season, grain yield was affected by frequent drought spells especially in February and March during flowering and pod-fill (Figure 1); hence, only plants inoculated with USDA 110 at Vila Ulongué increased grain yield (56%). The grain yield of the non-inoculated plants were not different from that of the other strains at both sites. Thus, despite better nodulation observed with USDA 442 and WB74, it did not result in higher grain yield. Ravuri and Hume (1992) reported similar results where no significant differences in grain yield among inoculant strains including USDA 110 occurred due to drought stress, although greater yield responses were observed under favorable growing conditions. In an on-farm study in Ghana, Ulzen et al. (2018) found no response to inoculation at a location where rainfall was low. Soil moisture deficit limits N2 fixation and other growth and developmental processes that lead to biomass accumulation (Sinclair et al., 2007; Muleta et al., 2017; Ulzen et al., 2018) and possibly reduced the potential benefits of inoculation. Strain USDA 442 had the best grain yield at Namarripe in both seasons, but it performed well only at this site which could be associated with the improved nodulation and perhaps competitive ability and adaptation to drought stress. Our results confirm the superior performance of USDA 110 in grain yield as reported by Okereke et al. (2000). However, we found no difference in yield response between USDA 122 (WB74) and USDA 136, whilst the results from Okereke et al. (2000) showed that USDA 122 (WB74) performed better than USDA 136. In Canada, Ravuri and Hume (1992) reported that Bradyrhizobium strain 532C was superior to USDA 110 and had grain yield increase of 11-17% over USDA 110 and CB 1809. They further indicated that the superior performance of strain 532C was not due to greater nodule number per plant but due to higher N2 fixation per milligram nodule weight. In contrast, Bai et al. (2003) found no significant difference between 532C and USDA 110 in grain yield, total plant N, and seed N in Canada. Furthermore, Ulzen et al. (2016) reported no differences in nodulation and grain yield between inoculants 532C and USDA 110 in soils with <10 rhizobia cells g−1 soil in Ghana, emphasizing that response to inoculant strains depends on many factors including soil and environmental conditions and often location specific. The results from Ulzen et al. (2016) showed that USDA 110 increased soybean grain yield by 19% whilst 532C increased yield by 12% relative to the control. Nevertheless, commercial inoculants containing strain 532C are currently available in several African countries and has been found to be superior over locally adapted isolates of acidic soil in Ethiopia (Muleta et al., 2017) and also reported significant grain yield increases in Ghana, Kenya, and Tanzania (Masso et al., 2016; Ulzen et al., 2016).
In the present study, the four inoculant strains on average, increased soybean grain yield by 1,081 kg ha−1 (65%) at Muriaze, 519 kg ha−1 (23%) at Namarripe and 947 kg ha−1 (61%) at Vila Ulongué in 2018. However, the severe drought spells in 2019 drastically reduced the yield advantage for inoculation to 3% at Namarripe and 28% at Vila Ulongué. These results are consistent with previous studies in Mozambique (van Heerwaarden et al., 2017; Chibeba et al., 2020; Savala et al., 2021, 2022), Malawi and Zimbabwe (van Heerwaarden et al., 2017), Nigeria (Ronner et al., 2016) and Ghana (Masso et al., 2016; Ulzen et al., 2016, 2018). Savala et al. (2022) obtained 100, 45 and 28% yield responses to rhizobial inoculation in the same agro-ecologies of the present study in Muriaze, Namarripe and Vila Ulongué, respectively. Data from the present study indicate positive correlations between nodule dry weight per plant and grain yield (r = 0.67–0.91) as well as number of pods per plant and grain yield (r = 0.51–0.85) suggesting that these variables contributed to higher grain yield. However, the correlations between the number of seeds per pod (r = −0.12–0.52) or 100-seed weight (r = −0.91–0.74) and yield parameters were weak and inconsistent. Nodule number and dry weight of the non-inoculated plants at Muriaze were significantly lower than that of the inoculated plants but shoot dry weight at R3 was not different from any of the inoculant treatments. Perhaps the R3 stage was too early for efficient N2 fixation that could lead to differences in shoot dry matter accumulation since grain yield and above-ground biomass at maturity were significantly lower in the control than that of the inoculated plants. However, the explanation why the 100-seed weight of the non-inoculated plants at Muriaze was higher than that of the inoculated plants is not clear. A possible reason could be the low number of pods per plant and number of seeds per pod suggesting less competition for assimilates in the seed leading to large seed size. Ksiezak and Bojarszczuk (2022) reported that plants with higher number of pods per plant and seeds per pod had lower seed weight compared to plants with fewer number of pods per plant and seeds per pod. In the present study and that of Savala et al. (2022), soybean seed weight did not respond consistently to inoculation, whilst Prusinski et al. (2020) found no significant effect of inoculation on seed weight, Although inoculants from different sources were used in the present study and that of Savala et al. (2022), the data on yield responses corroborate, because some of the strains were the same, and perhaps due to the similar agro-climatic conditions and populations of native soil rhizobia. The highest response for grain yield occurred at Muriaze in both studies probably because of the low native rhizobia population at the site compared with the other sites, in addition to the favorable rainfall distribution during the trial period. Nodulation and yield of the non-inoculated plants at Namarripe was relatively high compared to that of the other sites suggesting that the native rhizobia was effective in nodulating roots and were efficient in increasing yield. Thies et al. (1991) reported that at least 66% nodule occupancy by inoculant strains is required to show significant yield response in the presence of high population of local rhizobia. The study at five locations in Hawaii which contained indigenous rhizobia population that ranged from 1.8 x 101 to 3.6 x104 cells g−1 soil, concluded that the response to inoculation and the ability of the inoculant strains to compete successfully is inversely related to the existing population size. They found that as few as 50 rhizobia cells g−1 soil prevented inoculation response. In northern Nigeria, inoculation had no effect on nodulation, yield and plant biomass production in soils which contained 4 x 102 to 2.7 x 104 cells g−1 soil of indigenous rhizobia due to low nodule occupancy by the inoculant strains that ranged from 11 to 36% (Okogun and Sanginga, 2003). In Kenya, Thuita et al. (2012) reported 100% nodule occupancy by commercial inoculant strains which significantly improved nodulation, plant biomass and N2 fixation and attributed this to the low population of native strains (<103 cells g−1 soil) and the competitive abilities of the introduced strains. Our data also show that the inoculant strains supported increased plant dry matter and grain yield production compared with the control across site and season. However, USDA 136 and WB74 were the best among the strains in improving vegetative growth that could have positive implications on soil fertility and for subsequent crops in rotation (Sanginga et al., 2002; Rusinamhodzi et al., 2012).
Economic returns for using inoculant
Smallholder farmers are interested in soybean production because of the high profit margin compared with other crops. However, the magnitude of the profit depends on productivity which creates the incentive for farmers to invest in their production system. Rhizobia inoculation is the most affordable and low-cost investment that can significantly increase soybean productivity and profit. There are great opportunities for soybean producers in Mozambique because of the existing gap between domestic demand, driven by the poultry feed industry and domestic supply. Hence, there is ready market and attractive farmgate prices for soybean. Our data indicated that investment in inoculant which accounted for about 5% ($13.44 ha−1) of the production cost (Table 4) increased grain yield by up to 80% over the yield of the non-inoculated plants (Figure 3). When averaged over the inoculant strains, using inoculant increased profit over that of the non-inoculated control by 104% ($395.35 ha−1) at Muriaze, 100% ($344.83 ha−1) at Vila Ulongué and 30% ($182.57 ha−1) at Namarripe in 2018 (Table 4). The poor rainfall in 2019 decreased the benefits of inoculation at Namarripe. It added only ($4 ha−1) at Namarripe but increased profit over the non-inoculated control at Vila Ulongué by 73% ($95.04 ha−1). Production at Muriaze had the highest gross margin because of the higher yield response to inoculation, whereas investment at Namarripe gave the lowest gross margin because of the relatively low yield response. Under rain-fed smallholder farming systems, frequent drought spells during the growing season is the most important risk but even under adverse weather conditions using inoculant is often better and never worse than without inoculant as demonstrated by the results from Namarripe and Vila Ulongué in 2019. Investment in inoculant is a low-risk management decision because it is not a significant component of the production cost. In this study, the cost of TSP ($54.60 ha−1) and seeds ($33.50 ha−1) were the most expensive inputs which accounted for 19 and 12%, respectively, whereas inoculant accounted for only 5% of the production cost.
Similar results were obtained in a previous study with cowpea where an investment of ($5 ha−1) in inoculant translated to 34% ($152.42 ha−1), 26% ($163.33 ha−1), and 21% ($104.03 ha−1) higher profits relative to the control in the same agro-ecologies where the present study was carried out (Kyei-Boahen et al., 2017). The differences in the gross margins between the two studies are due to the magnitude of the yield responses of soybean and cowpea to inoculation and input costs. The cost of inoculant at the study period was ($5 ha−1) and accounted for only 1.7% of the production cost but the price had almost tripled and accounts for 5% of the production cost in the present study. The cost of P fertilizer was the most significant in both studies. In the previous study, the cost was $187.28 ha−1 and accounted for 39.5% of the production cost and this cost could not be paid by the yield increase due to fertilizer application. However, the cost of P in the present study was 71% lower than that of the previous study making it profitable to use P fertilizer, although it is still the most expensive input. Similar results have been documented in other studies (Ronner et al., 2016; Ulzen et al., 2018).
Conclusion
Soybean grown in soils containing indigenous rhizobia population responded to inoculation irrespective of the inoculant strain. However, some strains performed better than others under variable soil and environmental conditions which resulted in location x inoculant strain interactions for most of the variables evaluated. The yield increase due to inoculation was higher at the site with low indigenous rhizobia than the site where the native rhizobia population was relatively high. This confirms the well-documented knowledge that the size of the native rhizobia population, in addition to other soil factors influence soybean response to inoculation. Strain USDA 110 was superior to the other three inoculant strains evaluated. However, strains USDA 442 and WB74 (USDA 122) performed well at the site with relatively high native rhizobia population and under drought stress. The study demonstrated that investment in inoculant which accounted for only 5% of the production cost could double the gross margin when compared with production without using inoculant. Furthermore, inoculation is profitable even under adverse weather conditions because it is a low-cost and low-risk investment. In situations where the benefit of using inoculant is not directly evident in grain yields, it could be demonstrated in the production of N enriched plant biomass which remains in the field as residues and contribute N and other nutrients to subsequent crops in rotation or companion crops in intercrop systems. Thus, inoculating soybean with effective strain has the potential to contribute to the sustainability of smallholder production system in Mozambique. Since there is no inoculant production facility locally, availability is limited; and thus, these studies provide education and awareness to create demand for inoculants and attract private sector investment in local rhizobia inoculant production. Also production of inoculants within the country would result in increased availability with low price and make it more affordable and improve the economic benefits of smallholder soybean producers.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SK-B, CENS, and DC designed and supervised the experiment. CENS, CPM, CM, and AMC participated in field evaluation and data compilation. SK-B and ANW conducted the data analysis. PE prepared the rhizobia culture and inoculants for the experiments. SK-B oversaw write-up. All authors contributed to the preparation of the manuscript.
Funding
Funding for this work was provided by USAID through Feed the Future Mozambique Improved Seeds for Better Agriculture (SEMEAR) project: Grant #AID-BFS-IO-17-00005.
Acknowledgments
We thank the technical staff of IITA-Mozambique, Irondino Saraiva at Vila Ulongué, Nelito Rosario at Muriaze, Aniceto Mariano, Francisco Domingos, and John Jaquissone at Namarripe for managing the field work and supporting field data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arachchige, P. S. P., Rosso, L. H. M., Hansel, F. D., Ramundo, B., Torres, A. R., Asebedo, R., et al. (2020). Temporal biological nitrogen fixation pattern in soybean inoculated with Bradyrhizobium. Agrosyst. Geosci. Environ. 3, e20079. doi: 10.1002/agg2.20079
Bai, Y., Zhou, X., and Smith, D. L. (2003). Enhanced soybean plant growth resulting from co-inoculation of Bacillus strains with Bradyrhizobium japonicum. Crop Sci. 43, 1774–1781. doi: 10.2135/cropsci2003.1774
Bremner, J. M., and Mulvaney, C. S. (1982). “Nitrogen-Total,” in Methods of Soil Analysis, Part 2, Page, A. L. (ed.). Madison, WI: Am. Soc. Agron. Mon. No. 9. 2nd Edition. p 595–624.
Brockwell, J., Bottomley, P. J., and Thies, J. E. (1995). Manipulation of rhizobia microflora for improving legume productivity and soil fertility: a critical assessment. Plant Soil. 174, 143–180. doi: 10.1007/978-94-011-0055-7_7
Broughton, W. J., and Dilworth, M. J. (1970). “Methods in legume-rhizobium technology: plant nutrient solutions,” in Handbook for Rhizobia, Somasegaran, P., and Hoben, H. J. (eds). Hawaii: NifTAL Project and University of Hawaii. p. 245–249.
Buernor, A. B., Kabiru, M. R., Bechtaoui, N., Jibrin, J. M., Asante, M., Bouraqqadi, A., et al. (2022). Grain legume yield responses to rhizobia inoculants and phosphorus supplementation under Ghana soils: a meta-synthesis. Front. Plant Sci. 13, 877433. doi: 10.3389/fpls.2022.877433
Chang, W.-S., Lee, H.-I., and Hungria, M. (2015). “Soybean production in the Americas,” in Principles of Plant-Microbe Interactions, Lugtenberg, B. (ed.). Switzerland: Springer International Publishing. p. 393–400. doi: 10.1007/978-3-319-08575-3
Chibeba, A. M., Kyei-Boahen, S., Guimarães, M. F., Nogueira, M. A., and Hungria, M. (2020). Towards sustainable yield improvement: field inoculation of soybean with Bradyrhizobium and co-inoculation with Azospirillum in Mozambique. Arch. Microbiol. 202, 2579–2590. doi: 10.1007/s00203-020-01976-y
FAOSTAT (2022). Available online at: https://www.fao.org/faostat/en/#data/QCL
Gage, D. J. (2004). Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev. 68, 280–300. doi: 10.1128/MMBR.68.2.280-300.2004
Giller, K. E. (2001). Nitrogen Fixation in Tropical Cropping Systems, 2nd edition. Wallingford, UK: CAB International. doi: 10.1079/9780851994178.0000
Gyogluu, C., Boahen, S. K., and Dakora, F. D. (2016). Response of promiscuous-nodulating soybean (Glycine max L.) genotypes to Bradyrhizobium inoculation at three field sites in Mozambique. Symbiosis. 69, 81–88. doi: 10.1007/s13199-015-0376-5
Hungria, M., Franchini, J. C., Campo, R. J., Crispino, C. C., Moraes, J. Z., Sibaldelli, R. N. R., et al. (2006). Nitrogen nutrition of soybean in Brazil: contributions of biological N2 fixation and N fertilizer to grain yield. Can. J. Plant Sci. 86, 927–939. doi: 10.4141/P05-098
Hungria, M., and Kaschuk, G. (2014). Regulation of N2 fixation and NO 3–/NH 4+ assimilation in nodulated and N-fertilized Phaseolus vulgaris L. exposed to high temperature stress. Environ. Expt. Bot. 98, 32–39. doi: 10.1016/j.envexpbot.2013.10.010
Hungria, M., and Mendes, I. C. (2015). Nitrogen fixation with soybean: The perfect symbiosis? in Biological Nitrogen Fixation, de Bruijn, F.J. (ed). Hoboken, NJ: Wiley and Sons, Inc. p. 1009–1024. doi: 10.1002/9781119053095.ch99
Hungria, M., and Vargas, M. A. T. (2000). Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crops Res. 65, 151–164. doi: 10.1016/S0378-4290(99)00084-2
Jones, A., Breuning-Madsen, H., Brossard, M., Dampha, A., Deckers, J., Dewitte, O., et al. (2013). Soil Atlas of Africa. Luxembourg: European Commission.
Ksiezak, J., and Bojarszczuk, J. (2022). The effect of mineral N fertilization and Bradyrhizobium japonicum seed inoculation on productivity of soybean (Glycine max (L.) Merrill). Agriculture. 12, 110. doi: 10.3390/agriculture12010110
Kueneman, E. A., Root, W. R., Dashiell, K. E., and Hohenberg, J. (1984). Breeding soybean for the tropics capable of nodulating effectively with indigenous Rhizobium spp. Plant Soil. 82:387–396. doi: 10.1007/BF02184276
Kyei-Boahen, S., Savala, C. E. N., Chikoye, D., and Abaidoo, R. (2017). Growth and yield responses of cowpea to inoculation and phosphorus fertilization in different environments. Front. Plant Sci. 8, 646. doi: 10.3389/fpls.2017.00646
Maria, R. M., and Yost, R. (2006). A survey of soil fertility status of four agroecological zones of Mozambique. Soil Sci. 171, 902–914. doi: 10.1097/01.ss.0000228058.38581.de
Masso, C., Mukhongo, R., Thuita, M., Abaidoo, R., Ulzen, J., Kariuki, G., et al. (2016). “Biological Inoculants for Sustainable Intensification of Agriculture in Sub-Saharan Africa Smallholder Farming Systems,” in Climate Change and Multi-dimensional Sustainability in African Agriculture. New York, NY: Springer. p. 639–658. doi: 10.1007/978-3-319-41238-2_33
Matteo, F., Otsuki, K., and Schoneveld, G. (2016). “Soya bean expansion in mozambique: exploring the inclusiveness and viability of soya business models as an alternative to the land grab,” in The Public Sphere. p. 60–86. Available online at: https://www.cifor.org/publications/pdf_files/articles/ASchoneveld1601.pdf
McLean, E. O. (1982). “Soil pH and lime requirement,” in Methods of soil analysis, part 2, Page, A.L. et al. (ed.). Madison, WI: Agronomy Monogr. 9, 2nd ed. ASA and SSSA. p. 199–223.
Mehlich, A. (1984). Mehlich-3 soil test extractant: a modification of Mehlich-2 extractant. Commun. Soil Sci. Plant Anal. 15, 1409–1416. doi: 10.1080/00103628409367568
Mendoza-Suárez, M., Andersen, S. U., Poole, P. S., and Sánchez-Cañizares, C. (2021). Competition, nodule occupancy, and persistence of inoculant strains: key factors in the Rhizobium-Legume symbioses. Front. Plant Sci. 12:690567. doi: 10.3389/fpls.2021.690567
Muleta, D., Ryder, M. H., and Denton, M. D. (2017). The potential for rhizobial inoculation to increase soybean grain yields on acid soils in Ethiopia. Soil Sci. Plant Nutr. 63, 441–451. doi: 10.1080/00380768.2017.1370961
Nelson, D. W., and Sommers, L. E. (1996). “Total carbon, organic carbon and organic matter,” in Methods of soil analysis: Part 3 Chemical methods. (3rd ed.), Bartels, J. M. et al. (ed.). Madison, WI: ASA and SSSA Book Series 5. p. 961–1010. doi: 10.2136/sssabookser5.3.c34
Okereke, G. U., Onochie, C., Onunkwo, A., and Onyeagba, E. (2001). Effectiveness of foreign bradyrhizobia strains in enhancing nodulation, dry matter and seed yield of soybean (Glycine max L.) cultivars in Nigeria. Biol. Fertil. Soils. 33, 3–9. doi: 10.1007/s003740000264
Okereke, G. U., Onochie, C. C., Onukwo, A. U., Onyeagba, E., and Ekejindu, G. O. (2000). Response of introduced Bradyrhizobium strains infecting a promiscuous soybean cultivar. World J. Microbiol. Biotechnol. 16, 43–48. doi: 10.1023/A:1008927327678
Okogun, J. A., and Sanginga, N. (2003). Can introduced and indigenous rhizobial strains compete for nodule formation by promiscuous soybean in the moist savanna agroecological zone of Nigeria? Biol. Fertil. Soils. 38, 26–31. doi: 10.1007/s00374-003-0611-8
Olsen, S. R., and Sommers, L. E. (1982). “Phosphorus,” in Methods of soil analysis, part 2. Agron. Monogr. 9. 2nd ed, Page, A.L. et al. (eds.). Madison, WI: ASA and SSSA. p. 403–430. doi: 10.2134/agronmonogr9.2.2ed.c24
Osunde, A. O., Gwam, S., Bala, A., Sanginga, N., and Okogun, J. A. (2003). Responses to rhizobial inoculation by two promiscuous soybean cultivars in soils of the Southern Guinea savanna zone of Nigeria. Biol. Fertil. Soils. 37, 274–279. doi: 10.1007/s00374-003-0609-2
Prusinski, J., Baturo-Ciesniewska, A., and Borowska, M. (2020). Response of soybean (Glycine max (L.) Merrill) to mineral nitrogen fertilization and Bradyrhizobium japonicum seed inoculation. Agronomy. 10, 1300. doi: 10.3390/agronomy10091300
Ravuri, V., and Hume, D. J. (1992). Performance of a superior Bradyrhizobium japonicum and a selected Sinorhizobium fredii strain with soybean cultivars. Agron. J. 84, 1051–1056. doi: 10.2134/agronj1992.00021962008400060027x
Rodríguez-Navarro, D. N., Oliver, I. M., Contreras, M. A., and Ruiz-Sainz, J. E. (2010). Soybean interactions with soil microbes, agronomical and molecular aspects. Agron. Sust. Dev. 31, 173–190. doi: 10.1051/agro/2010023
Ronner, E., Franke, A., Vanlauwe, B., Dianda, M., Edeh, E., Ukem, B., et al. (2016). Understanding variability in soybean yield and response to P-fertilizer and rhizobium inoculants on farmers' fields in northern Nigeria. Field Crops Res. 186, 133–145. doi: 10.1016/j.fcr.2015.10.023
Rusinamhodzi, L., Corbeels, M., Nyamangara, J., and Giller, K. E. (2012). Maize–grain legume intercropping is an attractive option for ecological intensification that reduces climatic risk for smallholder farmers in central Mozambique. Field Crops Res. 136, 12–22. doi: 10.1016/j.fcr.2012.07.014
Salvagiotti, F., Cassman, K. G., Specht, J. E., Walters, D. T., Weiss, A., and Dobermann, A. (2008). Nitrogen uptake, fixation and response to fertilizer N in soybeans: a review. Field Crops Res. 108, 1–13. doi: 10.1016/j.fcr.2008.03.001
Sanginga, N., Okogun, J. A., Vanlauwe, B., and Dashiell, K. (2002). The contribution of nitrogen by promiscuous soybeans to maize based cropping systems in moist savanna of Nigeria. Plant Soil. 241, 223–231. doi: 10.1023/A:1016192514568
Sanz-Saez, A., Heath, K. D., Burke, P. V., and Ainsworth, E. A. (2015). Inoculation with an enhanced N2-fixing Bradyrhizobium japonicum strain (USDA110) does not alter soybean (Glycine max Merr.) response to elevated [CO2]. Plant Cell Environ. 1–14. doi: 10.1111/pce.12577
SAS/STATR (2018). 15, 1. User's Guide the GLM Procedure. Available online at: http://support.sas.com/thirdpartylicenses
Savala, C. E. N., Wiredu, A. N., Chikoye, D., and Kyei-Boahen, S. (2022). Prospects and potential of Bradyrhizobium diazoefficiens based bio-inoculants on soybean production in different Agro-ecologies of Mozambique. Front. Sustain. Food Syst. 6, 908231. doi: 10.3389/fsufs.2022.908231
Savala, C. E. N., Wiredu, A. N., Okoth, J. O., and Kyei-Boahen, S. (2021). Inoculant, nitrogen and phosphorus improves photosynthesis and water-use efficiency in soybean production. J. Agri. Sci. 159, 349–362. doi: 10.1017/S0021859621000617
Sinclair, T. R., Purcell, L. C., King, A. C., Sneller, C. H., Chen, P., and Vadez, V. (2007). Drought tolerance and yield increase of soybean resulting from improved symbiotic N2 fixation. Field Crops Res. 101, 68–71. doi: 10.1016/j.fcr.2006.09.010
Smith, S., Habib, A., Kang, Y., Leggett, M., and Diaz-Zorita, M. (2015). “LCO applications provide improved responses with legumes and nonlegumes,” in Biological nitrogen fixation, de Bruijn, F. J. (ed). Hoboken, NJ: John Wiley and Sons. p. 1077–1086. doi: 10.1002/9781119053095.ch107
Somasegaran, P., and Hoben, H. J. (1994). Handbook for Rhizobia: Methods in Legume -Rhizobium technology. Verkag, New York, USA: Springer. P. 366. doi: 10.1007/978-1-4613-8375-8
Thies, J. E., Singleton, P. W., and Bohlool, B. B. (1991). Influence of the size of indigenous rhizobial populations on establishment and symbiotic performance of introduced rhizobia on field grown legumes. Appl. Environ. Microbiol. 57, 19–28. doi: 10.1128/aem.57.1.19-28.1991
Thilakarathna, M. S., and Raizada, M. N. (2017). A meta-analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field conditions. Soil Biol. Biochem. 105, 177-196. doi: 10.1016/j.soilbio.2016.11.022
Thuita, M., Pypers, P., Herrmann, L., Okalebo, R. J., Othieno, C., Muema, E., et al. (2012). Commercial rhizobial inoculants significantly enhance growth and nitrogen fixation of a promiscuous soybean variety in Kenyan soils. Biol. Fertil. Soils. 48, 87–96. doi: 10.1007/s00374-011-0611-z
Ulzen, J., Abaidoo, R. C., Mensah, N. E., and Masso, C. (2018). On-farm evaluation and determination of sources of variability of soybean response to Bradyrhizobium inoculation and phosphorus fertilizer in northern Ghana. Agric. Ecosys. and Environ. 267, 23–32 doi: 10.1016/j.agee.2018.08.007
Ulzen, J., Abaidoo, R. C., Mensah, N. E., Masso, C., and AbdelGadir, A. H. (2016). Bradyrhizobium Inoculants enhance grain yields of soybean and cowpea in northern ghana. Front. Plant Sci. 7, 1770. doi: 10.3389/fpls.2016.01770
van Heerwaarden, J., Baijukya, F., Kyei-Boahen, S., Adjei-Nsiah, S., Ebanyat, P., Kamai, N., et al. (2017). Soyabean response to rhizobium inoculation across sub-Saharan Africa: Patterns of variation and the role of promiscuity. Agric. Ecosyst. Environ. 261, 211–218. doi: 10.1016/j.agee.2017.08.016
Vieira, R. F., Mendes, I. C., Reis-Junior, F. B., and Hungria, M. (2010). “Symbiotic nitrogen fixation in tropical food grain legumes: Current status,” in Microbes for legume improvement, Khan, M. S. et al. (eds). Vienna, Austria: Springer Verlag/Wein. p. 427–472. doi: 10.1007/978-3-211-99753-6_18
Vincent, J. M. (1970). A manual for the practical study of root nodule bacteria. Oxford: Blackwell Scientific. p. 164.
Woomer, P. L., Bennett, J., and Yost, Y. (1990). Overcoming the inflexibility of most - probable number procedures. Agron. J. 82, 349–353. doi: 10.2134/agronj1990.00021962008200020035x
Keywords: soybean, rhizobia strains, inoculation, nodulation, N2 fixation, grain yield, net returns
Citation: Kyei-Boahen S, Savala CEN, Muananamuale CP, Malita C, Wiredu AN, Chibeba AM, Elia P and Chikoye D (2023) Symbiotic effectiveness of Bradyrhizobium strains on soybean growth and productivity in Northern Mozambique. Front. Sustain. Food Syst. 6:1084745. doi: 10.3389/fsufs.2022.1084745
Received: 30 October 2022; Accepted: 21 December 2022;
Published: 18 January 2023.
Edited by:
Mustapha Mohammed, University for Development Studies, GhanaReviewed by:
Ashwin Revanna, Centre for Natural Biological Resources and Community Development (CNBRCD), IndiaRichard Oteng-Frimpong, CSIR-Savanna Agricultural Research Institute, Ghana
Judith Naamala, McGill University, Canada
Copyright © 2023 Kyei-Boahen, Savala, Muananamuale, Malita, Wiredu, Chibeba, Elia and Chikoye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen Kyei-Boahen,  cy5ib2FoZW5AY2dpYXIub3Jn
cy5ib2FoZW5AY2dpYXIub3Jn
†Present addresses: Canon Engoke Norris Savala, The Savalas, The Village Market, Nairobi, Kenya
Alexander Nimo Wiredu, AKDE Solutions Ghana Limited, Accra, Ghana
 Stephen Kyei-Boahen
Stephen Kyei-Boahen Canon Engoke Norris Savala
Canon Engoke Norris Savala Carlos Pedro Muananamuale1
Carlos Pedro Muananamuale1 Carlos Malita
Carlos Malita Alexander Nimo Wiredu
Alexander Nimo Wiredu Amaral Machaculeha Chibeba
Amaral Machaculeha Chibeba Patrick Elia
Patrick Elia David Chikoye
David Chikoye