- Zambia Agriculture Research Institute, Plant Protection Division, Lusaka, Zambia
Africa produces over half of global cassava; however, the continent's average yield is below the potential yields achieved under experimental conditions. Many factors contributing to low yield include lack of quality varieties, poor soils, limited access to capital, competition for labor, as well as pests and diseases. Plant diseases are the major biotic constraints to cassava production and have caused considerable food insecurity in Africa. Although there has been some level of disease management which has contributed to the increase in cassava production, the two viral diseases: cassava mosaic disease (CMD) and cassava brown streak disease (CBSD) still claim between 30–40% and upto 70%, respectively of Africa's cassava harvest. Given the importance of the two diseases in Africa, we review the expansion of CBSD and CMD; impacts of the two diseases on food security and how they can be managed. We provide insights in the spread of the two diseases, management efforts, and future directions.
Introduction
Cassava (Manihot esculenta Crantz), is an important staple crop in many African countries which account for 61.1% the of world production (Nweke, 2005; FAOSTAT, 2020). In sub-Saharan Africa (SSA) there are about 18 major cassava growing countries, each producing from 1 to over 50 million t. The major cassava producing countries include Nigeria (60 million t), Democratic Republic of the Congo (DRC) (41 million t), Ghana (21.8 million t), Angola (8.8 million t), and Tanzania (7.6 million t) (Ritchie et al., 2020) where it is grown mostly by resource-poor farmers, many of them women. Further, it is either grown as a sole crop or intercropped with vegetables, cereals (millet, maize, sorghum) or legumes (beans, cowpea) for food security.
In recent years, utilization and processing of cassava as a raw material has increased especially in the manufacture of many industrial products such as starch, beer, flour and other bio-based products including medicine, feed, cosmetics and biopolymers. For example, in Mozambique and Nigeria, cassava flour has replaced up to 20 and 10% of wheat flour in bread, respectively (Ohimain, 2014; Salvador et al., 2014). Coupled with these developments, its cultivation in many countries is transforming from subsistence to a more commercially-oriented farming enterprise. Because of this, the area under cassava production continues to expand in several African countries. Despite the expansion, productivity remains low and continues to be threatened by abiotic and biotic factors. Among the biotic factors contributing to low productivity are diseases particularly those caused by viruses. According to Patil et al. (2015), cassava is susceptible to over 20 different viruses, of which the most important are viruses causing cassava brown streak virus (CBSD) and CMD.
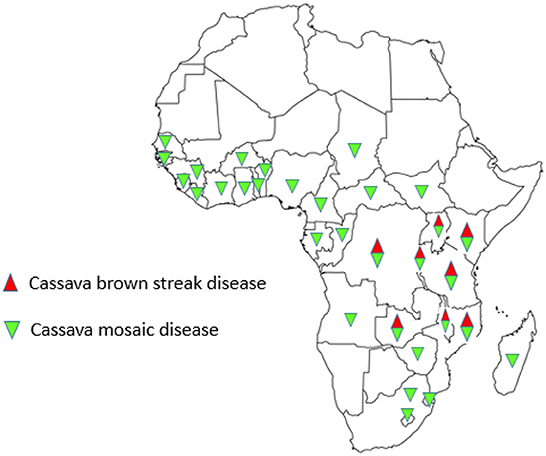
Cassava mosaic geminiviruses (CMGs) causal agent of cassava mosaic disease (CMD) comprise several species of circular single strand DNA (ssDNA) viruses belonging to the genus Begomovirus, family Geminiviridae. Contrastingly, cassava brown streak ipomoviruses (CBSIs) causal of CBSD comprises two positive sense single strand RNA (ss RNA) genomes, belonging to the genus Ipomovirus, family Potyviridae (Winter et al., 2010). The CMGs and CBSIs, are not seed borne but are transmitted by the polyphagous whitefly complex Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) (Maruthi et al., 2005). CMD and CBSD are spread over long distances through planting of infected cuttings originating from diseased plants. However, the presence and abundance of infected whiteflies feeding on the plants quickens the spread from plant to plant within or adjacent fields. Symptoms of CMD and CBSD are characterized by severe mosaic and chlorosis, respectively. During the early years when CBSD was first reported, it was confined to areas <1,000 m above sea level (masl) along the eastern coastal line from Kenya to Mozambique and around the lake shows of lake Malawi. Over the years, the disease has significantly spread covering mid-altitude (1,200–1,500 masl) areas of eastern, central and southern Africa.
Since cassava is vegetatively propagated, the viruses causing the CMD and CBSD are transmitted through the cuttings used as planting materials from one crop cycle to the next. Without intervention, the infection can therefore readily build up from infected plants adjacent to nearby fields particularly where there is a considerable level of vector transmission. Four virus genera are represented among the taxa that have been described as major pests of cassava. Out of the four, two are of economic significance, namely: Ipomovirus (family Potyviridae) where cassava brown streak virus (CBSV) and Uganda cassava brown streak virus (UCBSV) belong: and the Begomovirus (family Geminiviridae) group of cassava infecting geminiviruses. Here we use CBSVs to refer to CBSV and UCBSV. This review discusses the two viral diseases (CMD and CBSD), their causal agents, and their impact on cassava in Africa. Further, the paper addresses the following topics: prevalence of CMD, which occurs in all the main cassava-growing areas, and CBSD expansion, which has been reported to occur mainly in eastern and southern Africa. In addition, the paper examines the impact of the two diseases on cassava and provides insights in the management of the two diseases as well as future directions.
CBSD and the causal viruses
To evaluate the threat of CBSD, it is important to understand the casual viruses, their spread, and the symptoms they cause. CBSD is caused by two viruses, CBSV, and the UCBSV (Mbanzibwa et al., 2009; Winter et al., 2010). Studies have shown that CBSV occurs widely and is the more aggressive virus (Winter et al., 2010; Mbanzibwa et al., 2011; Mohammed et al., 2012), infecting both tolerant and susceptible cultivars as a single or mixed infection with UCBSV. Although there are only two species reported, further speciation is suggested within the UCBSV clade (Ndunguru et al., 2015). The two viruses are transmitted in a semi-persistent manner by the whitefly vector, from plant to plant (Maruthi et al., 2005). Other vectors could transmit CBSVs. Mware et al. (2009) reported transmission of CBSV by Aleurodicus disperses albeit at low rate. Other studies have shown a highly conserved motif of three amino acids Asp-Ala-Gly (DAG) within the CBSV, which is associated with aphid transmission (Ateka et al., 2017). Although mostly associated with cassava, CBSV can also infect other host plants, for example, Nicotiana benthamiana (Munganyinka et al., 2018b). Studies by Amisse et al. (2019) in Mozambique have shown that non-cassava perennial wild plant species: Zanha africana and Trichodesma zeylanicum are alternative hosts to CBSV. The plants are widely distributed in east, central and southern Africa. Manihot carthaginensis subsp. glaziovii, a wild cassava relative native to Brazil is also a host to CBSVs and occurs widely in Mozambique (Amisse et al., 2019).
CBSD affects all the plant parts; leaves, seed capsule, stems, and roots. The CBSVs causes yellowing of the leaves, brown streaks on the stems, and necrosis of the roots, rendering them unpalatable and unsuitable for the market (Figure 1). In addition, they cause concentric necrotic spots on the fruit. Leaf symptoms predominantly appear as leaf chlorosis in feathery patterns along the margins of tertiary veins. In some varieties, chlorosis manifests as pin spots and may later develop into chlorotic blotches. Infected stems show brown lesions or streaks, resulting in stem dieback in severe infections. In younger stems, the streaks appear purplish. Root symptoms are characterized by formation of radial constrictions and necrotic lesions within the root.

Figure 1. Symptoms of cassava brown streak disease. (A) Leaf symptoms of CBSD showing yellow chlorosis, associated with secondary and tertiary veins. (B) Necrotic brown marks on cassava stem. (C) Constrictions in tuberous roots infected with CBSD. (D) Dry brown, corky necrosis in tuberous roots associated with CBSD infection. (E) Circular necrotic lesions on cassava fruit. (F) Necrotic lesions on petiole. (G) Healthy stem.
The symptom expression depends on the virus strain infecting cassava, cassava variety, and environmental factors (rainfall, temperature, soil nutrient). Within the same variety, the CBSD symptoms can be observed either only on the leaves or on the stems or on the roots. In some cases they can appear on all plant parts or only on two plant parts (leaves and stems or on leaves and on the roots).
CMD and causal viruses
Unlike CBSD, CMD is caused by any of the 11 species [commonly referred to as cassava mosaic begomoviruses (CMBs)]. The viruses include African cassava mosaic virus (ACMV), East African cassava mosaic Cameroon virus (EACMCV), Cassava mosaic Madagascar virus (CMMGV), East African cassava mosaic Kenya virus (EACMKV), East African cassava mosaic Malawi virus (EACMMV), East African cassava mosaic virus (EACMV), East African cassava mosaic virus-Uganda (EACMV-UG), East African cassava mosaic Zanzibar virus (EACMZV), South African cassava mosaic virus (SACMV), African cassava mosaic Burkina Faso virus (ACMBFV), and Sri Lankan cassava mosaic virus (SLCMV). EACMV-UG has the most negative impact on cassava yield and contains a recombinant fragment between two distinct begomovirus species (EACMV and ACMV) from the core part of the protein gene (Zhou et al., 1997). All these viruses are transmitted persistently and retained by B. tabaci for much more extended periods than CBSIs, allowing for longer distance spread (Legg et al., 2011; Jeremiah, 2012).
Cassava mosaic disease causes variable leaf symptoms including mosaic, distorted and twisted leaflets, leaf narrowing, stunting, leaf chlorosis (yellow, white or pale spots) and an overall reduction in the size of leaves and plants (Figure 2). Symptom expression depends on the cassava variety, environmental conditions, and virus strains infecting the plants (Alabi et al., 2011). Leaf chlorosis may be pale yellow or nearly white with only a shade of green, or just noticeable paler than usual. Unlike CBSD, CMD does not cause any root necrosis; however, the disease results in reduction of root size. Further, the symptoms are enhanced when plants regenerate after being cut back to stimulate shoot development or when de-topped to provide leaves for home consumption. There is no evidence to show differences between the symptoms caused by the different cassava mosaic geminiviruses (CMGs). However, when two different CMGs are present in a plant, more severe symptoms are observed than either virus alone (Chikoti, 2011).
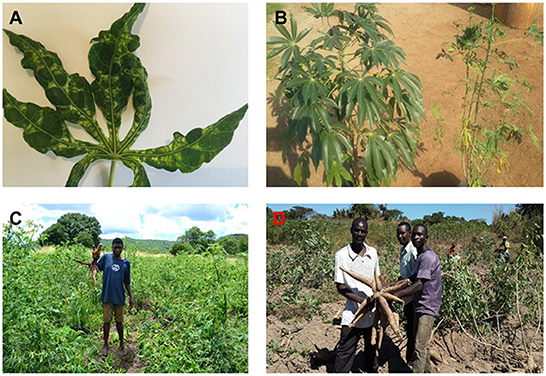
Figure 2. Symptoms of cassava mosaic disease on cassava plants. (A) Severely affected cassava leaf. (B) A resistant variety (right) and a susceptible cassava landrace exposed to infection by CMGs (left). (C) Reduced root size in plants exposed to infection by CMGs (Chikoti et al., 2019). (D) Cassava roots from a resistant CMD variety.
CBSD expansion
Before 2004, CBSD was restricted to coastal East Africa and the shores of Lake Malawi. However, recent studies have shown that CBSD has spread and expanded to previously unaffected areas in many African countries (Figure 3). In Uganda surveys conducted between 2004 and 2017 showed that most areas that were previously disease free are now affected (Alicai et al., 2019). Similarly, in the DRC, geographical expansion of CBSD covering an area of more than 62,000 km2 has been reported in nine previously unaffected territories (Muhindo et al., 2020; Casinga et al., 2021). The expansion of CBSD has also moved southwards reaching northern parts of Zambia (Mulenga et al., 2018). Confirmed in country reports of CBSD expansion from the initial outbreak areas have been made in Rwanda (Munganyinka et al., 2018a; Nyirakanani et al., 2021), Burundi (Bigirimana et al., 2011), and south Sudan (Alicai et al., 2016). The cause of CBSD expansion is difficult to determine. However, it can be speculated that use of infected planting materials transported across long distances could be more probable than through whitefly transmission. This assertion agrees with surveys conducted between November 2015 and August 2018 in DRC close to Lake Tanganyika in Sud-Kivu, Tanganyika and Haut-Kantanga provinces, which have shown the disease, present in Sud-Kivu and Haut-Kantanga provinces (Casinga et al., 2021) and not in Tanganyika, a province that is located between the two provinces.
The first comprehensive survey of CBSD was conducted in Tanzania in 1993/1994 (Legg and Raya, 1998), although this was restricted to an assessment of leaf symptoms and did not take into account stems or roots. Apart from one observation in the western mid-altitude (~1,200 m) region of Tabora, CBSD was confined to the lowland coastal plain. The average incidence for the country was 8.6%, although this rose to 36% for Mtwara region in the southeast, with three other coastal regions having incidences exceeding 19%. A more intensive survey in southern Tanzania broadly confirmed the results of the 1993/1994 survey and highlighted the decrease in incidence from the low altitude coastal zone (29%) to the higher altitude (500–700 m) and Interland (7%) (Hillocks et al., 1999). The considerable variation of CBSD incidence has been reported among cassava varieties (Munganyinka et al., 2018a). For instance, in the most affected areas some varieties had high symptom severity and incidence of infection, others were entirely free of leaf symptoms. In a more recent report in Kenya, foliar incidence ranged from 52.1 to 77.5% and root necrosis incidence from 36.7 to 40% (Masinde et al., 2016). It is not uncommon to find fields with 100% incidence, for example in Tanzania (Hillocks et al., 2002).
Unlike CBSD, CMD is present in all the cassava-growing countries in Africa, from East to West and from south of the Sahara to southern part of the continent (Legg et al., 2006; Ogbe et al., 2008; Alabi et al., 2011; Chikoti et al., 2013) (Figure 3). The earliest distribution of CMGs showed EACMV to occur in the coastal east African areas of Kenya, Tanzania, and Madagascar, as well as Malawi and Zimbabwe (Legg, 2008). In contrast, ACMV occurred throughout Africa. With the discovery of EACMV-Ug, a recombinant CMG variant (Zhou et al., 1997) associated with CMD pandemic (Kumar et al., 2009), the CMD landscape is rapidly changing. The EACMV-Ug has affected much of East and Central Africa, leading to production losses of 47%, equivalent to nearly 14 million tons (Legg, 2008). The CMD continues to expand its range to the west, south and east. Published reports of new occurrences from Angola (Kumar et al., 2009) and Cameroon (Akinbade et al., 2010) corroborate with this assertion. The rapid spread is partly due to super abundant B. tabaci populations as well as movement and exchange of infected planting materials. Recently, Houngue et al. (2020) reported expansion of CMD in Benin following a survey conducted in 2020.
Climate change and abundances of whitefly vectors of viral diseases
Africa has diverse ecosystems: deserts, mountains, savannah (or grassland), forest and coastal environments with savannah being the most common. As the climate changes so does the severity of viral diseases. Climate change-driven temperature rise, affects ecosystems differently. Extreme temperatures and erratic rainfall are likely to exceed the resilience limits of many ecosystems and trigger irreversible effects including that of whiteflies. Although there is no direct link between viruses affecting cassava and climate change, a correlation between whitefly abundance and temperature has been established (Saghafipour et al., 2020). Cassava is not spared as it is impacted directly by the whiteflies through whitefly damage and more so through infection of the viruses. Changes in climatic conditions in different parts of Africa, abundance of whiteflies, and environmental suitability for plant viruses, will likely affect epidemics of CMD and CBSD. In different ecosystems, insects experience stressful temperatures (high and low) and these may affect their distribution and abundances (Cui et al., 2008). Temperature, rainfall and humidity plays an important role in the survival, development and fecundity of whiteflies. Although there is a direct correlation between temperature and whiteflies, at extreme low and high temperatures, survival of the whiteflies is affected. Optimum temperature for B. tabaci (SSA1 SG3) was reported to be in the range of 20–28°C (Aregbesola, 2018). At low (<20°C) or high temperature (>30°C), survivability is reduced (Saghafipour et al., 2020). This suggests that at certain point, high or low temperatures are good as they may reduce whitefly population.
Economic impact of CBSD and CMD
Generally, there is lack of information on the overall estimate of the economic impact of the two diseases. Data for the two diseases from research plots are few from which accurate estimates can be made. What is clear is that the two diseases have devastated the livelihoods of small-scale cassava farmers across the continent. A study by Hillocks et al. (2001) on CBSD in East Africa suggests yield losses of up to 70%. With this yield loss, CBSD represents the greatest threat to millions of cassava farmers on the continent. In monetary value, it is estimated that farmers lose up to US$100 million per year due to CBSD (Mohammed et al., 2012). Though the disease has a limited effect on the growth and appearance of plants, the dry rot in tuberous roots can render entire harvest unpalatable and unmarketable. Two damage categories can be distinguished, quantitative (reduction in yield) and qualitative (changes in chemical content e.g., low starch content or provitamin A). When farmers harvest the roots, they cut out the necrotic lesions of affected tubers and discard tubers that are severely affected hoping to salvage unaffected parts. Consequently, additional labor is required to excise the diseased parts resulting in increased processing costs and food insecurity for the family households. Further, high quality cassava flour cannot be made from affected roots with a severity score of three and above due to decreased starch content and low pH (Hillocks and Maruthi, 2015). CBSD results in rotting of the roots in some varieties but it also reduces the number of marketable roots. In Tanzania, plants expressing foliar symptoms resulted in significant reductions in the number of roots (Ndyetabula et al., 2016). Annual yield losses in Tanzania has been estimated at 860,000 t equivalent to >US$ 51 million (Ndyetabula et al., 2016). In a study conducted in 2013 in Kenya, CBSD resulted in root loss of 24.7% translating to US$1259.50 per hectare (Masinde et al., 2016). Yield losses among small scale farmers in Malawi were estimated at 18–25 % (Gondwe et al., 2003). Effects of CBSD on storage components have been documented. Studies conducted in Kayunga District, Central Uganda, showed 30, 50, and 15% reduction in amylose, amylopectin and starch content, respectively (Nuwamanya et al., 2015). In a similar study in Uganda, 60% of starch content was reduced in susceptible varieties (Nuwamanya et al., 2017). Roots affected in this way fetch lower prices on the market compared to healthy cassava roots. In a similar study 60% of starch content was reduced in susceptible varieties (Nuwamanya et al., 2017). Roots affected in this way fetch lower prices on the market compared to healthy cassava roots. The economic losses due to CBSD could be much higher considering that the disease affects all the plant parts (leaves stems, and roots), which are all utilized either through consumption in households or planting in new fields. What is critical is to have accurate and informative data in countries that are ravaged by the disease.
Similarly, economic losses for CMD are known and few reports are available indicating losses from farmers' fields. The major bottleneck has been in difficulties in quantifying yield losses especially in farmers' fields. Several factors are attributed to this, lack of data and knowledge on how to quantify the losses. In addition, in some countries, farmers plant more than one type of variety in the same field and the varieties bulk at different times. Available information indicates losses ranging between 77.5 and 97.3% (Bisimwa et al., 2015). Losses in monetary terms in East and Central Africa have been estimated at US$ 1.9–2.7 billion dollars per year (Patil and Fauquet, 2009). Fauquet and Fargette (1990) estimated 50% yield loss in areas with CMD infection. In a more recent study, Tembo et al. (2017) estimated annual losses due to CMD at ca. US$ 51.7 million. In cassava plants with single or mixed virus infection, studies on root yield have shown marked differences (Owor, 2003). For example, infection of plants with a mild strain of EACMV-UG2 resulted in minor yield reductions in comparison with healthy controls. In mixed infections, losses of up to 87% were recorded for mixed ACMV and EAMCV-UG2 infections.
Just as much as CBSD affects cassava storage components so does CMD. CMD induces molecular alterations in cassava roots. Effects of the CMBs include reduction of starch and protein content (Terry and Hahn, 1980; Buvaneswari et al., 2018). In Uganda, high starch yield was observed at 25% in healthy plants compared to 21.8% in diseased plants (Nuwamanya et al., 2015). Similarly, in India starch content was reported to be lower (21.5%) in the tubers from infected plants compared to tubers from healthy plants (28%) (Buvaneswari et al., 2018). Furthermore, the protein content was found to be reduced to 0.15% in comparison with tubers collected from healthy plants.
Exploiting efforts to manage CBSD and CMD
Efforts to manage the CBSD and CMD through integrated approaches are being scaled up in many parts of Africa, amidst poor coordination. A few projects covering the breadth and length of Africa such as Cassava Diagnostic Project, West Africa Virus Epidemiology, and Cassava Great Lakes Initiative have attempted to manage the devastating impact of CBSD and CMD. The problem of CBSD and CMD in developing countries is exacerbated by paucity of infrastructure of extension, seed systems, and diagnostic laboratories. The inability to have critical investment in disease management has meant poor performance in the cassava sector resulting in failure to reach achievable yields. Efforts to contain the impacts of the diseases are not well coordinated in Africa, and the existing mitigation programs have little impact in addressing the disease challenges. Consequently, the Global Cassava Partnership for the twenty-first Century (GCP21), a recognized global organization within the cassava community, have been holding conferences with participants from affected countries. Suffice to say that the GCP 21 is a not-for-profit international alliance of 45 organizations whose aims are to fill gaps in cassava research and development in order to unlock the potential of cassava for improving food security and for increasing incomes of poor farmers through work to develop industrial products from cassava (https://gcp21.org/). It provides a platform where scientists share scientific information on cassava. However, most of the participants are not policymakers who can influence their respective governments to institute actions. On the other hand, the International Society for Root and Tuber Crops (ISTRC) with representation from all of the regions of the world where people either produce or consume root and tuber crops, was formed to create enabling environment to improve cassava productivity through scientific research. It has regional branches in Africa, South Pacific, and Asia. Similarly, International Institute of Tropical Agriculture (IITA) with regional hubs across Africa has over the years played a key role in managing cassava viral diseases through developing improved cassava varieties with farmer preferred traits (high yield, disease resistance and nutritional quality). It also produces and shares protocols on disease diagnosis and identification. However, all the key players' efforts need to be strengthened.
Phytosanitation
Although phytosanitation is one technique of controlling CMD and CBSD (Thresh and Otim-Nape, 1994), it has received limited attention. Cassava is propagated using stem cuttings, and both CMD and CBSD are mainly perpetuated and disseminated in this way. As previously stated, in CBSD infected plants, symptoms may appear only on the roots or on the leaves or stems. The appearance of the symptoms makes it difficult in selecting virus-free planting materials as in some varieties the symptoms may not be clear (Ntawuruhunga and Legg, 2007). Monitoring of propagation materials for disease symptoms during the growth of the source plant can help reduce the spread of the disease. There are advantages in farmers selecting propagating material only from uninfected plants at the time the cuttings are being harvested and collected from the field. Unfortunately, most farmers are not familiar with the whole range of CBSD symptoms and tend to collect the cuttings when the source plants are almost leafless. Further farmers in communities, practice phytosanitation in isolation but where farmers cooperate, benefits have been realized. For example, communities in coastal (Mkuranga) and northwestern (Chato) of Tanzania were phytosanitation was practiced, CBSD incidence reduced from >90% to 39.1% after three years (Legg et al., 2017). The approach could also serve as a potential component for integrated cassava virus management programmes, mainly where new cassava plantations are being established in areas severely affected by CBSD.
Seed systems and disease-free planting materials
In many African countries, cassava seed system is still informal serve for a few including Nigeria, Tanzania, and Uganda (Legg et al., 2022). A functional seed system will encompass development of high yielding and disease resistant varieties and investment in infrastructure for early generation of seed. Trading in planting materials that are not certified is common occurrence across Africa. However, what is cardinal is to have the right polices and functional infrastructure. CBSD and CMD can be managed through developing and disseminating resistant or tolerant varieties. However, there have been instances where resistant varieties are not available in some countries. For instance in Zambia where CBSD was recently detected, all the landraces and improved varieties grown by the farmers were susceptible (Mulenga et al., 2018). In Tanzania, where local diseased materials were replaced with disease-free ones, 86% more yield was achieved (Legg et al., 2017). It is thought that the distribution of this clean planting material, often referred to as clean “seed,” reduces disease pressure in communities by ensuring that the majority of crops are, at least initially, relatively disease-free (Kanju et al., 2003).
Recently, CMD was detected in Cambodia, Southeast Asia (Wang et al., 2016). To control the potentially devastating disease, Government representatives, development partners, and cassava research scientists from Cambodia, Thailand, and Vietnam gathered to devise a regional plan. Science and Technology Research Partnership for Sustainable Development (SATREPS) project was launched whose objectives were to develop pest management technologies and a system for the production and cultivation of healthy seedlings (Uke et al., 2022). Prior to first report of CMD outbreak in Cambodia, a study conducted in 2016–2017 on the movement and exchange of cassava planting materials in Cambodia and Vietnam within communes found 82 and 78% of seed provided to others being exchanged between family and acquaintances, respectively (Delaquis et al., 2018). Similarly, in some African countries including Zambia were cassava is mostly grown by small scale farmers, frequent seed exchange occurs between farmers (94%) in the same or nearby communities (Szyniszewska et al., 2021). A similar study in Rwanda indicated that majority (76.9%) of the farmers obtain cuttings for free from either their own fields or from neighbors (Nyirakanani et al., 2021). In Kenya, majority of farmers (83%) recycled planting materials from the previous crop while some 67% obtained the planting material from their neighbors (Kidasi et al., 2021). In the absence of a formal seed system, planting material traded or supplied often suffers from a lack of quality control, farmers are more likely to plant virus-infected cuttings, which can lead to low yield. Without quality control, free movement of infected cuttings efficiently contribute to movement of pathogens including viruses that cause CBSD and CMD. Partnerships being advocated in Southeast Asia involving many countries can be adapted to Africa were CBSD is rapidly spreading and affecting cassava productivity. Cassava seed systems in Africa are largely farmer driven with low seed quality controls. Re-use of the farmer's own seed supply remains the most common source of seed (Kidasi et al., 2021; Szyniszewska et al., 2021).
Host-plant resistance
One of the most effective disease management strategies to combat CMD and CBSD is the use of genetically resistant plants. Genetic resistance is a low cost method of controlling viruses that cause the two diseases. However, many countries lack CBSD resistant/tolerant cassava varieties. Recent studies have shown varying levels of CBSD tolerance. Elite 1,980 full-sib from 106 families from Nigeria evaluated in Uganda exhibited significant susceptibility to CBSD within 2 years of evaluation (Cu et al., 2021). Similarly, farmer preferred cultivars from Ghana and tested for response to mixed infection of CBSVs showed susceptibility to the viruses (Elegba et al., 2020). Despite some of the setbacks, efforts have continued to look elsewhere for resistant genes including South America where ongoing work has shown resistance to CBSD in breeding lines DSC167 and DSC118 (Sheet et al., 2019). In the east and southern Africa, management efforts have focused on identification of superior clones to CBSD (Mukiibi et al., 2019) and introgressing the resistant genes in farmer preferred varieties (Munga, 2008). In Uganda, breeding efforts made for a period of 11 years have resulted in CBSD clones with reasonable resistance/ tolerance (Kawuki et al., 2016). As breeding is a long term activity, clones with resistant genes need to be distributed and evaluated in other countries that are experiencing the CBSD or threatened with the disease. Most of the countries affected by CBSD have reported CBSIs recognized strains, though more unknown strains could be circulating (Ndunguru et al., 2015). Introduced tolerant lines will need to be evaluated in hot spot areas given the ability of CBSV to quickly evolve (Alicai et al., 2016).
From the early 1930s following pandemics that occurred in East Africa, successful CMD control has been through introgression of genes from wild cassava, Manihot glaziovii Muell.-Arg, into cultivated cassava (Fondong, 2017). During the early 1970s, cassava seeds of resistant cassava genotypes were introduced to the International Institute of Tropical Agriculture (IITA) in Nigeria and crosses made with local Nigerian varieties and South American germplasm from the International Center for Tropical Agriculture (CIAT) (Beck, 1982). The resistant varieties from IITA are used as parent material in many cassava breeding programmes in Africa. Unlike the lack of availability of CBSD resistant varieties in many countries, there are several CMD resistant cassava varieties that have been developed (Houngue et al., 2019). The sources of resistance are CMD1 (polygenic recessive resistance), CMD2 (dominant monogenic type), and CMD3 which have been identified and introgressed from wild cassava to cassava cultivars (Akano et al., 2002; Okogbenin et al., 2012; Fondong, 2017). The source of resistance for CMD1 and CMD2 were introgressed from Manihot glaziovii and TME 3 (Tropical Manihot esculenta), respectively (Nichols, 1947). To enhance CMD resistance in plants, CMD1 and CMD2 have been combined using genetic crosses (Fondong, 2017). In Burkina Faso, seven cassava varieties (TMS 91/02312, TMS 92/0067, TMS 92/0325, TMS 92/0427, TMS 4(2)1425, TMS 94/0270, TMS 30572) with origins from IITA and widely used by farmers were reportedly to be highly resistant with molecular markers associated with CMD1, CMD2 and CMD3 genes (Soro et al., 2021). In the DRC CMD incidence was 15% in IITA varieties significantly outperforming the local varieties with 100% incidence in locations assessed (Muengula-Manyi et al., 2012). Although CMD was confined to Africa for centuries, Asia which is affected by the disease is also spearheading breeding efforts. Recently, in Vietnam from the population VNM142, out of 142 clones collected across the country and evaluated in CMD hotspot Tay Ninh, eight clones showed high CMD resistance with CMD severity scores <2.0 (Thuy et al., 2021).
Transgenic cassava option
Improvement of resistance to CBSD and CMD, either through traditional breeding or genetic transformation, is challenging and time-consuming. Needless to say that the growth of transgenic cassava is limited by public opinion across Africa. The varying opinions has contributed to low adoption of potential varieties conferring novel resistance genes to CBSD and CMD. While scientists agree that increasing cassava productivity will require genetically transforming cassava with pest and disease tolerant/ resistant genes. Consumers are wary of long-term effects of genetically modified (GM) cassava on the environment and lack of a regulatory framework to facilitate the adoption of GM cassava (Adenle et al., 2012). Efforts to use transgenic approaches to CMD resistance have increased in recent years to overcome the limitations of conventional breeding (Vanderschuren et al., 2007, 2009). Typical targets for transgenic resistance include the viral non-coding intergenic region and messenger RNAs of rep-associated genes of CMGs, especially ACMV (Zhang et al., 2005; Fondong, 2017). The deployment of virus-resistant transgenic plants has become an important strategy to implement effective and sustainable control measures against major viral diseases. Several transgenic plants with virus resistance have been demonstrated as an effective strategy against virus infections through the expression of coat protein genes, viral replicase genes, or other viral sequences. For example, Zhang et al. (2005) developed transgenic cassava plants with increased ACMV resistance using improved antisense RNA technology by targeting the viral mRNAs of Rep (AC1), TrAP (AC2) and REn (AC3). Similar results have been reported in KU50 transgenic lines expressing dsRNA homologous to the region between the AV2 and AV1 of DNA-A of SLCMV (Ntui et al., 2015) with high levels of resistance displayed to SLCMV compared to the wild-type plants. Transformation of resistant transgenic cassava (TMS60444) to CBSV has been achieved using RNAi technology by targeting a sequence of the CBSV coat protein (Yadav et al., 2011; Vanderschuren et al., 2012) and the gene demonstrated to be integrated within the genome. The mechanism of RNA mediated resistance involves RNA silencing, in which sequence-specific RNA degradation occurs (Kawazu et al., 2009). As the new molecular tools are developed, e.g., Clustered Regularly Interspaced Short Palindromic Repeats (CRISPRs) opportunities to develop CMD and CBSD resistance varieties will be enhanced.
In contrast to CMD as previously stated, limited natural resistance to CBSD has been identified and demonstrated. Because of this development, transgenic approaches are important to reduce the impact of CBSD in Africa. However, host resistance has been identified for a few viruses only, and a limited number of commercial elite crop cultivars and rootstocks exhibit useful resistance. For example, in east Africa, p5001 transgenic plants, 16 of the 25 transgenic lines under field conditions showed foliar resistance to CBSD (Wagaba et al., 2017) compared to the non-transgenic cassava. Researchers in Uganda and Nigeria are conducting a limited number of trials to evaluate how the engineered cassava cultivars will perform in the field. If the materials prove superior over conventionally bred cultivars, farmers can adopt them. In Kenya work spanning several years has resulted in the approval of genetically modified cassava which confers resistance to CBSD for open cultivation (https://geneticliteracyproject.org/2021/06/25/kenya-approves-disease-resistant-gmo-cassava/).
Integrated pest management, surveillance and diagnostics
Integrated pest management (IPM) is a strategy that encompasses all crop protection strategies involving the use of resistant varieties as well as biological, cultural, physical, and chemical control practices. Although researchers agree that overall IPM programmes are required for the whole range of cassava pests and diseases, little progress has been made. This is in spite of likely significant effects on yield arising from CBSD, CMD, cassava bacterial blight, cassava anthracnose, cassava mealybug, and green mite. Recommendations for control of CBSD and CMD include the strict enforcement of quarantine procedures during exchange of cassava planting materials by the farmers. Cultural practices, especially the use of resistant or tolerant cultivars is important and where necessary should be encouraged. In many countries in Africa were cassava seed system is mainly informal, use of virus-free planting material or clean planting material as often referred to should be encouraged.
Priority should be conducting regular surveillance in areas where there are no records of CBSD and CMD. Surveillance should be accompanied by carrying out a diagnosis of the samples collected from the surveys using standardized and harmonized protocols. Further, surveillance must be coordinated between countries and should have a reporting mechanism to ensure effective disease management. Establishing real-time centralized database is cardinal in sharing information on the occurrence of new virus strains and diseases. Surveillance especially for CBSD should also be implemented in cassava-growing regions of Africa not directly connected to the regions of the current distribution of the disease.
Given the difficulties associated with the recognition of symptoms of CMD and CBSD especially in varieties that do not show symptoms fully, laboratory diagnostic methods can play a critical role (Mbanzibwa et al., 2009). Accurate virus diagnosis requires sensitive equipment and standardized procedures. Fortunately, appreciable investment in recent years in laboratory diagnostics has been made ranging from simple to more complex equipment. Polymerase chain reaction (PCR) and Real-time PCR are increasingly being used but the cost is prohibitive for many developing countries. Some low cost rapid test kits are required that can provide quick results in the field and can be valuable to scientists and extension agents. In Asia, an immunochromatographic strip test for detecting SLCMV has been developed and can give a result within 15 mins and does not require laboratory setting (https://www.nstda.or.th/en/news/news-years-2022). CBSD and CMD protocols are available for use by scientists across Africa for disease surveys and for detection (Sseruwagi et al., 2004; Mbanzibwa et al., 2009; Abarshi et al., 2010; Shirima et al., 2017). The ultimate goal will be to document the new and old viruses by establishing a continental system of surveillance and monitoring.
Recently, mobile-based artificial intelligence (AI) tools for cassava pest and disease surveillance have been developed (Ramcharan et al., 2019). The tools perform several functions including real-time diagnosis (<1 min) for presence or absence of pathogens that diseases on cassava (CBSD and CMD). However, affordability and accessibility are the biggest challenges by the end users such as farmers, scientists and extension agents.
Stakeholder networks
Cassava stakeholders in Africa are faced with the gravity of CBSD and CMD and therefore a greatly strengthened and more effectively coordinated network is needed to manage the two diseases. Efforts are in place to tackle CBSD and CMD scourge, however, the efforts are not coordinated by key players such as donors, scientists, extension agents and non-governmental organizations with interest in cassava (Legg et al., 2014). Strengthening the linkages and interaction between Cassava stakeholders is paramount in addressing the impact of CBSD and CMD. The links between the stakeholders involved in cassava technology generation and dissemination, which are end products of cassava research are weak and sometimes inactive.
Although Africa is the world leader in cassava production differences in cassava productivity between Africa, South America and Asia exist. The differences could be attributed to many factors including perception of cassava mostly as a food crop unlike in Asia where it is considered as a cash crop (Costa, 2019). Strong public private partnerships, business environment improvements and R&D technologies are other reasons of high productivity in Asia. For example, Vietnam and Thailand some of the biggest producers in Asia have seen revolution in the supply side triggered by demand from Europe and China (Curran and Cooke, 2008). This has also seen organized plantations unlike in Africa where cassava is still cultivated mainly by smallholder rural families. To unlock industrial potential in Africa there is need to: identify effective seed legislation and policies, understand conducive business environment, identify improved mechanized production methods, and identify effective delivery systems for quality seed.
Conclusions
While cassava continues to be a crop of importance in Africa for enhancing food security especially among rural households, there is need to address the issue of diseases so that the crop can be advocated as a contributor of poverty reduction. The expansion and economic impact of CBSD and CMD on yield requires specific disease management strategies and for continuous revision of these two important viral diseases. To curtail spread and reduce the impact of the two diseases, there is need to have coordinated efforts across the continent and integrate the management strategies. Particular efforts should be placed on quarantine measures since development and deployment of resistant CBSD, and CMD varieties are yet to be fully realized in most of the African countries affected by the two diseases.
Future directions
Going forward, future directions in tackling the two cassava viral diseases are highlighted:
• Conduct annual surveillance surveys including in high risk areas to ascertain extent of viruses causing CBSD and CMD and spread using standardized and harmonized protocols.
• Invest in rapid diagnostic capabilities in national research organizations to respond to threats posed by CBSD and CMD.
• Develop standardized and adapt robust protocols for detection of all species and strains of viruses causing CBSD and CMD.
• Characterize viruses to provide comprehensive data for disease management.
• Establish regional phytosanitary networks in west, east, central and southern African countries.
• Establish and strengthen breeding programmes for developing CBSD and CMD resistant and high yielding cultivars.
• Raise awareness amongst researchers, extension workers, plant protectionists, quarantine officers, farmers, and policymakers of cassava viruses and the threats they pose.
• Establish local and regional early warning systems for CBSD and CMD to facilitate rapid responses in case of new outbreaks.
• Encourage policy makers to participate in national and international conferences or symposiums on CBSD and CMD.
Author contributions
PC conceived the idea, wrote the manuscript, and designed the figures. MT edited the manuscript. Both authors contributed to the article and approved the submitted version.
Funding
This work is an output of the Zambia Skills Development and Entrepreneurship Project supported by the Citizen Economic Empowerment Commission through Project Number P-ZM-IEO-002 with funds provided by Africa Development Bank (AfDB).
Acknowledgments
The authors would like to thank Drs. James Legg and Peter Sseruwagi for insightful advice and comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abarshi, M. M., Mohammed, I. U., Wasswa, P., Hillocks, R. J., Holt., J, Legg, J. P., et al. (2010). Optimization of diagnostic RT-PCR protocols and sampling procedures for the reliable and cost-effective detection of Cassava brown streak virus. J. Virol. Methods 163, 353–359. doi: 10.1016/j.jviromet.2009.10.023
Adenle, A. A., Aworh, O. C., Akromah, R., and Aworh, O. (2012). Developing GM super cassava for improved health and food security: future challenges in Africa. Agric. Food Secur. 1, 11. doi: 10.1186/2048-7010-1-11
Akano, A. O., Dixon, A. G. O., Mba, C., and Barrera, E. (2002). Genetic mapping of dominant gene conferring resistance to cassava mosaic disease. Theor. Appl. Genet. 105, 521–525. doi: 10.1007/s00122-002-0891-7
Akinbade, S. A., Hanna, R., Nguenkam, A., Njukwe, E., Fotso, A., Doumtsop, A., et al. (2010). First report of the East African cassava mosaic virus-Uganda (EACMV-UG) infecting cassava (Manihot esculenta) in Cameroon. New Dis. Rep. 21, 22. doi: 10.5197/j.2044-0588.2010.021.022
Alabi, O. J., Kumar, P. L., and Naidu, R. A. (2011). Cassava Mosaic Disease: A Curse to Food Security in Sub-Saharan Africa. APSnet Features.
Alicai, T., Ndunguru, J., Sseruwagi, P., Tairo, F., Okao-Okuja, G., Nanvubya, R., et al. (2016). Cassava brown streak virus has a rapidly evolving genome: implications for virus speciation, variability, diagnosis and host resistance. Sci. Rep. 6, 36164. doi: 10.1038/srep36164
Alicai, T., Szyniszewska, A. M., Omongo, C. A., Abidrabo, P., Okao-Okuja, G., Baguma, Y., et al. (2019). Expansion of the cassava brown streak pandemic in Uganda revealed by annual field survey data for 2004 to 2017. Sci. Data 6, 327. doi: 10.1038/s41597-019-0334-9
Amisse, J. J. G., Ndunguru, J., Tairo, F., Boykin, L. M., Kehoe, M. A., Cossa, N., et al. (2019). First report of Cassava brown streak viruses on wild plant species in Mozambique. Physiol. Mol. Plant Pathol. 105, 88–95. doi: 10.1016/j.pmpp.2018.10.005
Aregbesola, O. Z. (2018). Understanding the potential impact of climate change on cassava-colonising whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) (Ph.D. Thesis). University of Copenhagen, Copenhagen, Denmark.
Ateka, E., Alicai, T., Ndunguru, J., Tairo, F., Sseruwagi, P., Kiarie, S., et al. (2017). Unusual occurrence of a DAG motif in the Ipomovirus Cassava brown streak virus and implications for its vector transmission. PLoS ONE 12, e0187883. doi: 10.1371/journal.pone.0187883
Beck, B. D. A. (1982). “Historical perspectives of cassava breeding in Africa,” in Proceedings of a Workshop on Root Crops in Eastern Africa, Kigali, Rwanda, eds S. K. Hahn, A. D. R. Ker (Ottawa, ON: IDRC), 13–18.
Bigirimana, S., Barumbanze, P., Ndayihanzamaso, P., Shirima, R., and Legg, J. P. (2011). First report of cassava brown streak disease and associated Ugandan cassava brown streak virus in Burundi. New Dis. Rep. 24, 26. doi: 10.5197/j.2044-0588.2011.024.026
Bisimwa, E., Walangululu, J., and Bragard, C. (2015). Cassava mosaic disease yield loss assessment under various altitude agroecosystemsin the SudKivu Region, Democratic Republic of Congo. Tropicultura 33, 101–110.
Buvaneswari, S., Rageshwari, S., Deivamani, M., Janavi, G., and Rabindran, R. (2018). Impact of Sri Lankan cassava mosaic virus infection on the tuber yield, starch and protein content of cassava tubers of three popular varieties of Tamil Nadu. J. Innov. Agri. 5, 1–4
Casinga, C. M., Shirima, R. R., Mahungu, N. M., Tata-Hangy, W., Bashizi, K. B., Munyerenkana, C. M., et al. (2021). Expansion of the cassava brown streak disease epidemic in Eastern Democratic Republic of Congo. Plant Dis. 105, 2177–2188. doi: 10.1094/PDIS-05-20-1135-RE
Chikoti, P. C. (2011). Development of Cassava (Manihot esculenta Crantz) Cultivars for Resistance to Cassava Mosaic Disease in Zambia. Ph.D Thesis. University of KwaZulu-Natal, Pietermaritzburg, South Africa.
Chikoti, P. C., Mulenga, R. M., Tembo, M., and Sseruwagi, P. (2019). Cassava mosaic disease: a review of a threat to cassava production in Zambia. J. Plant Pathol. 101, 467–477. doi: 10.1007/s42161-019-00255-0
Chikoti, P. C., Ndunguru, J., Melis, R., Tairo, F., Shanahan, P., and Sseruwagi, P. (2013). Cassava mosaic disease and associated viruses in Zambia, occurrence and distribution. Int. J. Pest Manag. 59, 63–72. doi: 10.1080/09670874.2012.752887
Costa, C. (2019). The Cassava Value Chain in Mozambique. Jobs Working Paper. The World Bank's Jobs Umbrella Trust Fund.
Cu, A., Ochwo-Ssemakula, M., Ibanda, A., Ozimati, A., Gibson, P., Onyeka, J., et al. (2021). Cassava brown streak disease response and association with agronomic traits in elite Nigerian cassava cultivars. Front. Plant Sci. 12, 720532. doi: 10.3389/fpls.2021.720532
Cui, X., Wan, F., Xie, M., and Liu, T. (2008). Effects of heat shock on survival and reproduction of two whitefly species, Trialeurodes vaporariorum and Bemisia tabaci biotype B. J. Insect Sci. 8, 24. doi: 10.1673/031.008.2401
Curran, S. R., and Cooke, A. M. (2008). Unexpected outcomes of thai cassava trade: a case of global complexity and local unsustainability. Globalizations. 5, 111–127. doi: 10.1080/14747730802057449
Delaquis, E., Andersen, K. F., Minato, N., Cu, T. T. L., Karssenberg, M. E., Sok, S., et al. (2018). Raising the stakes: cassava seed networks at multiple scales in Cambodia and Vietnam. Front. Sustain. Food Syst. 2, 73. doi: 10.3389/fsufs.2018.00073
Elegba, W., Gruissem, W., and Vanderschuren, H. (2020). Screening for resistance in farmer-preferred cassava cultivars from Ghana to a mixed infection of CBSV and UCBSV. Plants 9, 1026. doi: 10.3390/plants9081026
FAOSTAT (2020). Available online at: http://www.fao.org/faostat/en/#data/QC (accessed January 24, 2020).
Fauquet, C., and Fargette, D. (1990). African cassava mosaic virus: etiology, epidemiology, and control. Plant Dis. 74, 404–411. doi: 10.1094/PD-74-0404
Fondong, V. N. (2017). The search for resistance to cassava mosaic geminiviruses: how much we have accomplished, and what lies ahead. Front. Plant Sci. 8, 408. doi: 10.3389/fpls.2017.00408
Gondwe, F. M. T., Mahungu, N. M., Hillocks, R. J., Raya, M. D., Moyo, C. C., Soko, M. M., et al. (2003). “Economic losses experienced by small-scale farmers in Malawi due to cassava brown streak virus disease,” in Cassava Brown Streak Virus Disease: Past, Present and Future, Proceedings of the International Workshop, Mombasa, Kenya, 27–30 October 2002, eds J. P. Legg and R. J. Hillocks (Aylesford, UK: Natural Resources International Limited).
Hillocks, R., and Maruthi., G. (2015). Post-harvest impact of cassava brown streak disease in four countries in eastern Africa. Food Chain. 5, 116–122. doi: 10.3362/2046-1887.2015.008
Hillocks, R. J., Raya, M. D., Mtunda, K., and Kiozia, H. (2001). Effects of brown streak virus disease on yield and quality of cassava in Tanzania. J. Phytopathol. 149, 389–394. doi: 10.1046/j.1439-0434.2001.00641.x
Hillocks, R. J., Raya, M. D., and Thresh, J. M. (1999). Factors affecting the distribution, spread, and symptom expression of cassava brown streak disease in Tanzania. Afr. J. Root Tuber Crops 3, 57–61.
Hillocks, R. J., Thresh, J. M., Tomas, J., Botao, M., Macia, R., and Zavier, R. (2002). Cassava brown streak disease in northern Mozambique. Int. J. Pest Manag. 48, 178–181. doi: 10.1080/09670870110087376
Houngue, J. A., Houédjissin, S. S., Ahanhanzo, C., Pita, J. S., Houndénoukon, M. S. E., and Zandjanakou-Tachin, M. (2020). Cassava mosaic disease (CMD) in Benin: incidence, severity and its whitefly abundance from field surveys in 2020. Crop Prot. 158, 106007. doi: 10.1016/j.cropro.2022.106007
Houngue, J. A., Zandjanakou-Tachin, M., Ngalle, H. B., Pita, J. S., Cacai, G. H. T., Ngatat, S. E., et al. (2019). Evaluation of resistance to cassava mosaic disease in selected African cassava cultivars using combined molecular and greenhouse grafting tools. Mol. Plant Pathol. 105, 47–53. doi: 10.1016/j.pmpp.2018.07.003
Jeremiah, S. (2012). The role of whitefly (Bemisia tabaci) in the Spread and transmission of cassava brown streak disease in the field (PhD thesis). University of Dar es Salaam, Dares Salaam, Tanzania.
Kanju, E., Mtunda, K. J., Muhanna, M., Raya, M. D., and Mahungu, N. M. (2003). “Management of cassava brown streak virus disease in Tanzania,” in Cassava Brown Streak Disease: Past, Present and Future. Proceedings of an International Workshop, 2002, eds J. P. Legg and R. J. Hillocks (Mombasa, Kenya: Natural Resources International Limited) 66–69.
Kawazu, Y., Fujiyama, R., and Noguchi, Y. (2009). Transgenic resistance to Mirafiori lettuce virus in lettuce carrying inverted repeats of the viral coat protein gene. Transgenic Res. 18, 113–120. doi: 10.1007/s11248-008-9200-9
Kawuki, R. S., Kaweesi, T., Esuma, W., Pariyo, A., Kayondo, I. S., Ozimati, A., et al. (2016). Eleven years of breeding efforts to combat cassava brown streak disease. Breed. Sci. 66, 560–571. doi: 10.1270/jsbbs.16005
Kidasi, P. C., Chao, D. K., Obudho, E. O., and Mwang'ombe, A. W. (2021). Farmers' sources and varieties of cassava planting materials in Coastal Kenya. Front. Sustain. Food Syst. 5, 611089. doi: 10.3389/fsufs.2021.611089
Kumar, P. K., Akinbade, S. A., Dixon, A. G. O., Mahungu, N. M., Mutunda, M. P., Kiala, D., et al. (2009). First report of the occurrence of East African cassava mosaic virus-Uganda (EACMV-UG) in Angola. Plant Pathol. 58, 402. doi: 10.1111/j.1365-3059.2008.02010.x
Legg, J., Ndalahwa, M., Yabeja, J., Ndyetabula, I., Bouwmeester, H., Shirima, R., et al. (2017). Community phytosanitation to manage cassava brown streak disease. Virus Res. 241, 236–253. doi: 10.1016/j.virusres.2017.04.020
Legg, J., Somado, E. A., Barker, I., Beach, L., Ceballos, H., Cuellar, W., et al. (2014). A global alliance declaring war on cassava viruses in Africa. Food Secur. 6, 231–248. doi: 10.1007/s12571-014-0340-x
Legg, J. P. (2008). “African Cassava Mosaic Disease,” in Encyclopedia of Virology (3rd Edition). Elsevier. pp. 30–36. doi: 10.1016/b978-012374410-4.00693-2
Legg, J. P., Diebiru-Ojo, E., Eagle, D., Friedmann, M., Kanju, E., Kapinga, R., et al. (2022). “Commercially sustainable cassava seed systems in Africa,” in Root, Tuber and Banana Food System Innovations, eds G. Thiele, M. Friedmann, H. Campos, V. Polar, J. W. Bentley (Cham: Springer). doi: 10.1007/978-3-030-92022-7_15
Legg, J. P., Jeremiah, S. C., Obiero, H. M., Maruthi, M. N., Ndyetabula, I., Okao-Okuja, G., et al. (2011). Comparing the regional epidemiology of the cassava mosaic and cassava brown streak virus pandemics in Africa. Virus Res. 159, 161–170. doi: 10.1016/j.virusres.2011.04.018
Legg, J. P., Owor, B., Sseruwagi, P., and Ndunguru, J. (2006). Cassava mosaic virus disease in east and central Africa: epidemiology and management of a regional pandemic. Adv. Virus Res. 67, 355–418. doi: 10.1016/S0065-3527(06)67010-3
Legg, J. P., and Raya, D. (1998). Survey of cassava virus diseases in Tanzania. Int. J. Pest Manag. 44, 17–23. doi: 10.1080/096708798228473
Maruthi, M. N., Hillocks, R. J., Mtunda, K., Raya, M. D., Muhanna, M., Kiozia, H., et al. (2005). Transmission of Cassava brown streak virus by Bemisia tabaci (Gennadius). J. Phytopathol. 153, 307–312. doi: 10.1111/j.1439-0434.2005.00974.x
Masinde, E. M., Ogendo, J. O., Maruthi, M. N., Hillocks, R., Mulwa, R. M. S., and Arama, P. F. (2016). Occurrence and estimated losses caused by cassava viruses in Migori County, Kenya. Afr. J. Agric. Res. 11, 2064–2074. doi: 10.5897/AJAR2016.10786
Mbanzibwa, D. R., Tian, Y. P., Tugume, A. K., Mukasa, S. B., Tairo, F., Kyamanywa, S., et al. (2009). Genetically distinct strains of Cassava brown streak virus in the Lake Victoria basin and the Indian Ocean coastal area of East Africa. Arch. Virol., 154, 353–359. doi: 10.1007/s00705-008-0301-9
Mbanzibwa, D. R., Tian, Y. P., Tugume, A. K., Patil, B. L., Yadav, J. S., and Bagewadi, B. (2011). Evolution of cassava brown streak disease associated viruses. J. Gen. Virol. 92, 974–987. doi: 10.1099/vir.0.026922-0
Mohammed, I. U., Abarshi, M. M., Muli, B., Hillocks, R. J., and Maruthi, M. N. (2012). The symptom and genetic diversity of cassava brown streak viruses infecting cassava in East Africa. Adv. Virol. 2012, 1–10. doi: 10.1155/2012/795697
Muengula-Manyi, M., Nkongolo, K. K., Bragard, C., Tshilenge-Djim, P., Winter, S., and Kalonji- Mbuyi, A. (2012). Incidence, severity and gravity of cassava mosaic disease in savannah agroecological region of DR-Congo: analysis of agro-environmental factors. Am. J. Plant Sci. 3, 512–519. doi: 10.4236/ajps.2012.34061
Muhindo, H., Yasenge, S., Casinga, C., Songbo, M., Dhed'a, B., Alicai, T., et al. (2020). Incidence, severity and distribution of Cassava brown streak disease in northeastern Democratic Republic of Congo. Cogent Food Agric. 6, 1789422. doi: 10.1080/23311932.2020.1789422
Mukiibi, D. R., Alicai, T., Kawuki, R., Okao-Okuja, G., Tairo, F., Sseruwagi, P., et al. (2019). Resistance of advanced cassava breeding clones to infection by major viruses in Uganda. Crop. Prot. 115, 104–112. doi: 10.1016/j.cropro.2018.09.015
Mulenga, R. M., Boykin, L. M., Chikoti, P. C., Sichilima, S., Ng'uni, D., and Alabi, O. J. (2018). Cassava brown streak disease and Ugandan cassava brown streak virus reported for the first time in Zambia. Plant Dis. 102, 1410–1418. doi: 10.1094/PDIS-11-17-1707-RE
Munga, T. L. (2008). Breeding for cassava brown streak resistance in coastal Kenya (Ph.D. Thesis). University of KwaZulu-Natal, South Africa.
Munganyinka, E., Ateka, E. M., Kihurani, A. W., Kanyange, M. C., Tairo, F., Sseruwagi, P., et al. (2018a). Cassava brown streak disease in Rwanda, the associated viruses and disease phenotypes. Plant Pathol. 67, 377–387. doi: 10.1111/ppa.12789
Munganyinka, E., Margaria, P., Samar, S., Ateka, E. M., Tairo, F., Ndunguru, J., et al. (2018b). Localization of cassava brown streak virus in Nictotiana and cassava Manihot esculenta (Crantz) using RNAscope in situ hybridization. Virol. J. 15, 128. doi: 10.1186/s12985-018-1038-z
Mware, B. O., Ateka, E. M., Songa, J. M., Narla, R. D., Olubayo, F., and Amata, R. (2009). Transmission and distribution of cassava brown streak virus disease in cassava growing areas of Kenya. J. Appl. Biosci. 16, 864–870.
Ndunguru, J., Sseruwagi, P., Tairo, F., Stomeo, F., Maina, S., Djinkeng, A., et al. (2015). Analyses of twelve new whole genome sequences of cassava brown streak viruses and ugandan cassava brown streak viruses from East Africa: diversity, supercomputing and evidence for further speciation. PLoS ONE 10, e0139321. doi: 10.1371/journal.pone.0139321
Ndyetabula, I. L., Merumba, S. M., Jeremiah, S. C., Kasele, S., Mkamilo, G. S., Kagimbo, F. M., et al. (2016). Analysis of interactions between cassava brown streak disease symptom types facilitates the determination of varietal responses and yield losses. Plant Dis. 100, 1388–1396. doi: 10.1094/PDIS-11-15-1274-RE
Nichols, R. (1947). Breeding cassava for virus resistance. East Afr. Agric. J. 12, 184–194. doi: 10.1080/03670074.1947.11664554
Ntawuruhunga, P., and Legg, J. (2007). New spread of Cassava Brown Streak Virus disease and its implications for the movement of cassava germplasm in the East and Central African region. Crop Crisis Control Project (C3P) Publication, 6. Available online at: http://c3project.iita.org/Doc/A25-CBSDbriefMay6.pdf (accessed September, 2022).
Ntui, V. O., Kong, K., Khan, R. S., Igawa, T., Janavi, G. J., Rabindran, R., et al. (2015). Resistance to Sri Lankan Cassava Mosaic Virus (SLCMV) in genetically engineered Cassava cv. KU50 through RNA Silencing. PLoS ONE 10, e0120551. doi: 10.1371/journal.pone.0120551
Nuwamanya, E., Baguma, Y., Atwijukire, E., Acheng, S., Abidrabo, P., Omongo, C. A., et al. (2017). Cassava brown streak disease effects on leaf metabolites and pigment accumulation. Afri. Crop Sci. J. 25, 33–45. doi: 10.4314/acsj.v25i1.3
>Nuwamanya, E., Baguma, Y., Atwijukire, E., Acheng, S., and Alicai, T. (2015) Effect of cassava brown streak disease (CBSD) on cassava (Manihot esculenta Crantz) root storage components, starch quantities starch quality properties. Int. J. Plant Physiol. Biochem. 7, 12–22. doi: 10.5897/IJPPB2015.0227
Nweke, F. I. (2005). “The cassava transformation in Africa. A review of cassava in Africa with country case studies on Nigeria, Ghana, the United Republic of Tanzania, Uganda and Benin,” in Proceedings of the Validation Forum on the Global Cassava Development Strategy. Vol. 2 (Rome: The Food and Agriculture Organization of the United Nations).
Nyirakanani, C., Bizimana, J. P., Kwibuka, Y., Nduwumuremyi, A., Bigirimana, V. P., Bucagu, C., et al. (2021). Farmer and field survey in cassava-growing districts of rwanda reveals key factors associated with cassava brown streak disease incidence and Cassava productivity. Front. Sustain. Food Syst. 5, 699655. doi: 10.3389/fsufs.2021.699655
Ogbe, F. O., Thottappilly, G., Dixon, A. G. O., Atiri, G. I., and Mignouna, H. D. (2008). Variants of East African cassava mosaic virus and its distribution in double infections with African cassava mosaic virus in Nigeria. Plant Dis. 87, 229–232. doi: 10.1094/PDIS.2003.87.3.229
Ohimain, E. (2014). Review of cassava bread value chain issues for actualization of the 40% cassava bread production in Nigeria. J. Sci. Res. Rep. 3, 1220–1231. doi: 10.9734/JSRR/2014/8825
Okogbenin, E., Egesi, C. N., Olasanmi, B., Ogundapo, O., Kahya, S., Hurtado, P., et al. (2012). Molecular marker analysis and validation of resistance to cassava mosaic disease in elite cassava genotypes in Nigeria. Crop Sci. 52, 2576–2586. doi: 10.2135/cropsci2011.11.0586
Owor, B. (2003). Effect of cassava mosaic geminiviruses (CMGs) on growth and yield of a cassava mosaic disease (CMD) susceptible cultivar in Uganda and cross protection studies. MSc thesis. Makerere University, Kampala, Uganda. doi: 10.1111/j.1744-7348.2004.tb00390.x
Patil, B. L., and Fauquet, C. M. (2009). Cassava mosaic geminiviruses: actual knowledge and perspectives. Mol. Plant Pathol. 10, 685–701. doi: 10.1111/j.1364-3703.2009.00559.x
Patil, B. L., Legg, J., Kanju, E., and Fauquet, C. M. (2015). Cassava brown streak disease: a threat to food security in Africa. J. Gen. Virol. 96:956–968. doi: 10.1099/vir.0.000014
Ramcharan, A., McCloskey, P., Baranowski, K., Mbilinyi, N., Mrisho, L., Ndalahwa, M., et al. (2019). A mobile-based deep learning model for Cassava disease diagnosis. Front. Plant. Sci. 10, 272. doi: 10.3389/fpls.2019.00272
Ritchie, H., Rosado, P., and Roser, M. (2020). Agricultural Production. Available online at: https://ourworldindata.org/agricultural-production (accessed on December 16, 2022).
Saghafipour, A., Zahraei-Ramazani, A., Vatandoost, H., Asadollahi, A., Fouladi-Fard, R., et al. (2020). Relationship between some environmental and climatic factors on outbreak of whiteflies, the human annoying insects. J. Arthropod Borne Dis. 14, 78–87. doi: 10.18502/jad.v14i1.2714
Salvador, E. M., Steenkamp, V., and McCrindle, C. M. E. (2014). Production, consumption and nutritional value of cassava (Manihot esculenta, Crantz) in Mozambique: an overview. J. Agric. Biotech. Sustain. Dev. 6, 29–38. doi: 10.5897/JABSD2014.0224
Sheet, S., Fuerholzner, B., Stein, B., and Winter, S. (2019). Resistance against cassava brown streak viruses from Africa in cassava germplasm from South America. Front. Plant Sci. 10, 567. doi: 10.3389/fpls.2019.00567
Shirima, R. R., Maeda, D. G., Kanju, E., Ceasar, G., Tibazarwa, F. I., and Legg, P. L. (2017). Absolute quantification of cassava brown streak virus mRNA by real-time qPCR. J. Virol. Methods 245, 5–13. doi: 10.1016/j.jviromet.2017.03.003
Soro, M., Som,é, K., Tiendrébéogo, F., Pita, J. S., Romba, R., Néya, B. J., et al. (2021). Evaluation of Ten Cassava Varieties for Resistance to Cassava Mosaic Disease in Burkina Faso. Univers. J. Agric. Res. 9, 266–276. doi: 10.13189/ujar.2021.090605
Sseruwagi, P., Sserubombwe, W. S., Legg, J. P., Ndunguru, J., and Thresh, J. M. (2004). Methods of surveying the incidence and severity of cassava mosaic disease and whitefly vector populations on cassava in Africa: a review. Virus Res. 100, 129–142. doi: 10.1016/j.virusres.2003.12.021
Szyniszewska, A. M., Chikoti, P. C., Tembo, M., Mulenga, R., Gilligan, C. A., Bosch, F., et al. (2021). Smallholder cassava planting material movement and grower behavior in Zambia: implications for the management of cassava virus diseases. Phytopathology 111, 1952–1962. doi: 10.1094/PHYTO-06-20-0215-R
Tembo, M., Mataa, M., Legg, J., Chikoti, P. C., and Ntawuruhunga, P. (2017). Cassava mosaic disease incidence and yield performance of cassava varieties in Zambia. J. Plant Pathol. 99, 681–689.
Terry, E.R., and Hahn, S.K. (1980). The effect of cassava mosaic disease on growth and yield of a local and an improved variety of cassava. Trop. Pest Manag. 26, 34–37. doi: 10.1080/09670878009414280
Thresh, J. M., and Otim-Nape, G. W. (1994). Strategies for controlling African cassava mosaic geminivirus. Adv. Dis Vector Res. 10, 215–236. doi: 10.1007/978-1-4612-2590-4_8
Thuy, C. T. L., Lopez-Lavalle, L. A. B., Vu, N. A., Hy, N. H., Nhan, P. T., Ceballos, H., et al. (2021). Identifying new resistance to cassava mosaic disease and validating markers for the CMD2 locus. Agriculture 11, 829. doi: 10.3390/agriculture11090829
Uke, A., Tokunaga, H., Utsumi, Y., Vu, N. A., Nhan, P. T., Srean, P., et al. (2022). Cassava mosaic disease and its management in Southeast Asia. Plant Mol. Biol. 109, 301–311. doi: 10.1007/s11103-021-01168-2
Vanderschuren, H., Alder, A., Zhang, P., and Gruissem, W. (2009). Dose-dependent RNAi-mediated geminivirus resistance in the tropical root crop cassava. Plant Mol. Biol. 70, 265–272. doi: 10.1007/s11103-009-9472-3
Vanderschuren, H., Moreno, I., Anjanappa, R. B., Zainuddin, I. M., and Gruissem, W. (2012). Exploiting the combination of natural and genetically engineered resistance to cassava mosaic and cassava brown streak viruses impacting cassava production in Africa. PLoS ONE 7, e45277. doi: 10.1371/journal.pone.0045277
Vanderschuren, H., Stupak, M., Fütterer, J., Gruissem, W., and Zhang, P. (2007). Engineering resistance to geminiviruses-review and perspectives. Plant Biotechnol. J. 5, 207–220. doi: 10.1111/j.1467-7652.2006.00217.x
Wagaba, H., Beyene, G., Aleu, J., Odipio, J., Okao-Okuja, G., Chauhan, R. D., et al. (2017). Field level RNAi-mediated resistance to cassava brown streak disease across multiple cropping cycles and diverse EastAfrican agro-ecological locations. Front. Plant Sci. 7, 2060. doi: 10.3389/fpls.2016.02060
Wang, H. L., Cui, X. Y., Wang, X. W., Liu, S. S., Zhang, Z. H., and Zhou, X. P. (2016). First report of Sri Lankan cassava mosaic virus infecting cassava in Cambodia. Plant Dis. 100, 1029–1029. doi: 10.1094/PDIS-10-15-1228-PDN
Winter, S., Koerbler, M., Stein, B., Pietruszka, A., Paape, M., and Butgereitt, A. (2010). Analysis of cassava brown streak viruses reveals the presence of distinct virus species causing cassava brown streak disease in East Africa. J. Gen. Virol. 91, 1365–1372. doi: 10.1099/vir.0.014688-0
Yadav, J. S., Ogwok, E., Wagaba, H., Patil, B. L., Bagewadi, B., Alicai, T., et al. (2011). RNAi-mediated resistance to Cassava brown streak Uganda virus in transgenic cassava. Mol. Plant Pathol. 12, 677–687. doi: 10.1111/j.1364-3703.2010.00700.x
Zhang, P., Vanderschuren, H., Fütterer, J., and Gruissem, W. (2005). Resistance to cassava mosaic disease in transgenic cassava expressing antisense RNAs targeting virus replication genes. Plant Biotechnol. J. 3, 385–397. doi: 10.1111/j.1467-7652.2005.00132.x
Keywords: CBSD, CMD, impact, expansion, Africa
Citation: Chikoti PC and Tembo M (2022) Expansion and impact of cassava brown streak and cassava mosaic diseases in Africa: A review. Front. Sustain. Food Syst. 6:1076364. doi: 10.3389/fsufs.2022.1076364
Received: 21 October 2022; Accepted: 28 November 2022;
Published: 23 December 2022.
Edited by:
Henry Wagaba, National Crops Resources Research Institute (NaCRRI), UgandaReviewed by:
Amadou Sidibé, Agriculture and Agri-Food Canada (AAFC), CanadaAna I. F. Ribeiro-Barros, University of Lisbon, Portugal
Copyright © 2022 Chikoti and Tembo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick Chiza Chikoti, cGNjaGlrb3RpQGdtYWlsLmNvbQ==
 Patrick Chiza Chikoti
Patrick Chiza Chikoti Mathias Tembo
Mathias Tembo