- 1Animal Sciences Department, Emerging Pathogens Institute, Global Food Systems Institute, University of Florida, Gainesville, FL, United States
- 2Department of Applied Mathematics, Delft University of Technology, Delft, Netherlands
- 3Department of Food Science and Technology, Center for Foodborne Illness Research and Prevention, The Ohio State University, Columbus, OH, United States
- 4Department of Epidemiology and Public Health, Sciensano, Brussels, Belgium
- 5Department of Translational Physiology, Infectiology and Public Health, Ghent University, Merelbeke, Belgium
- 6International Livestock Research Institute, Addis Ababa, Ethiopia
- 7Department of Food Science and Technology, Center for Foodborne Illness Research and Prevention, Translational Data Analytics Institute, The Ohio State University, Columbus, OH, United States
Foodborne disease is a significant global health problem, with low- and middle-income countries disproportionately affected. Given that most fresh animal and vegetable foods in LMICs are bought in informal food systems, much the burden of foodborne disease in LMIC is also linked to informal markets. Developing estimates of the national burden of foodborne disease and attribution to specific food products will inform decision-makers about the size of the problem and motivate action to mitigate risks and prevent illness. This study provides estimates for the burden of foodborne disease caused by selected hazards in two African countries (Burkina Faso and Ethiopia) and attribution to specific foods. Country-specific estimates of the burden of disease in 2010 for Campylobacter spp., enterotoxigenic Escherichia coli (ETEC), Shiga-toxin producing E. coli and non-typhoidal Salmonella enterica were obtained from WHO and updated to 2017 using data from the Global Burden of Disease study. Attribution data obtained from WHO were complemented with a dedicated Structured Expert Judgement study to estimate the burden attributable to specific foods. Monte Carlo simulation methods were used to propagate uncertainty. The burden of foodborne disease in the two countries in 2010 was largely similar to the burden in the region except for higher mortality and disability-adjusted life years (DALYs) due to Salmonella in Burkina Faso. In both countries, Campylobacter caused the largest number of cases, while Salmonella caused the largest number of deaths and DALYs. In Burkina Faso, the burden of Campylobacter and ETEC increased from 2010 to 2017, while the burden of Salmonella decreased. In Ethiopia, the burden of all hazards decreased. Mortality decreased relative to incidence in both countries. In both countries, the burden of poultry meat (in DALYs) was larger than the burden of vegetables. In Ethiopia, the burdens of beef and dairy were similar, and somewhat lower than the burden of vegetables. The burden of foodborne disease by the selected pathogens and foods in both countries was substantial. Uncertainty distributions around the estimates spanned several orders of magnitude. This reflects data limitations, as well as variability in the transmission and burden of foodborne disease associated with the pathogens considered.
Introduction
Food safety is linked, directly or indirectly, to the achievement of many of the Sustainable Development Goals (SDGs), especially those on ending hunger and poverty, and promoting good health and wellbeing. According to the World Health Organization (WHO), 420,000 deaths occurred globally among 600 million cases of foodborne disease (FBD) in 2010 and 230,000 of these deaths were due to foodborne hazards which cause diarrheal disease. In 2010, seven diarrheal disease hazards [Norovirus, Campylobacter spp. (CAMP), enteropathogenic Escherichia coli (EPEC), enterotoxigenic E. coli (ETEC), non-typhoidal Salmonella enterica (NTS), Shigella spp., and Vibrio cholerae] were identified to each cause an estimated global FBD burden of 1–3 million disability-adjusted life years (DALYs), as estimated for 2010 (Havelaar et al., 2015). Africa was estimated to have the highest FBD burden of all regions analyzed (2,460 DALYs per 100,000 population compared to a global average of 477 DALYs per 100,000 population). The World Bank has estimated that the economic cost of FBD in low- and middle-income countries (LMIC) amounts to $95.2 billion per year (Jaffee et al., 2019), with the annual cost of treating FBD being estimated at $15 billion. Due to data limitations, both assessments only capture part of the total burden and cost of FBD in LMIC. For example, there is limited data on the incidence, severity and long-term health outcomes associated with several foodborne pathogens. Costs are often limited to those related to medical treatment and lost productivity. Important public health and economic benefits could be achieved by improving food safety in LMIC.
The objectives of this study are to update estimates of the burden of FBD for the pathogens selected by two projects in Ethiopia and Burkina Faso—TARTARE (TARTARE, 2018) and Pull-Push (International Livestock Research Institute, 2018) and estimate the burden associated with consuming food products selected for intervention in these projects. Further details on the objectives of these projects can be found in Sapp et al. (2022).
Materials and methods
WHO data
Disease burden data were extracted from the 2010 WHO Foodborne Disease Burden Epidemiology Reference Group (FERG) database (Devleesschauwer et al., 2015) for Ethiopia and Burkina Faso. While all calculations were done at the country level, FERG only presented results on a global scale and for subregions. The subregions were based on the official grouping of WHO Member States. FERG further subdivided each region into subregions based on child and adult mortality as described by Ezzati et al. (2002): very low child and adult mortality (stratum A), low child mortality and very low adult mortality (stratum B), low child mortality and high adult mortality (stratum C), high child and adult mortality (stratum D), and high child mortality and very high adult mortality (stratum E). Burkina Faso was assigned to the Africa Region (AFR), stratum D (AFRD) and Ethiopia to AFR, stratum E (AFRE).
Disease burden estimates are expressed as incidence, mortality, and DALY at the population level and per 100,000 population. Monte Carlo samples of the uncertainty distributions of the foodborne disease burden of the pathogens selected by the projects (CAMP, ETEC, STEC, and NTS) were abstracted from the FERG database for the total population, children under 5 years of age, and children over 5 years of age and adults. Details on FERG methods can be found in Devleesschauwer et al. (2015). FERG used 10,000 draws to reach convergence. While FERG reports medians as point estimates for the uncertainty distribution, means are reported here due to their probabilistic properties: in contrast to medians, the sum of means is the same as the mean of sums. We note that at the country level, mean and median disease burdens are very similar for all hazards in both countries (data not shown).
Updating burden estimates to 2017
To provide a clearer picture of the current burden of disease associated with the selected pathogens, estimates were updated from 2010 to 2017. Since the WHO does not provide data to assess trends in diarrheal disease burden by specific pathogens, data from the Global Burden of Disease (GBD) study (Institute of Health Metrics and Evaluation, Seattle, WA, Kyu et al., 2018) were used. Even though there are methodological differences between the FERG and GBD estimates, it was assumed that trends in burden per 100,000 of specific pathogens in GBD could be used to update the FERG estimates. Estimates of mortality, of life lost (YLL), years lived with disability (YLD), and disability-adjusted life years (DALY) for three pathogens (CAMP, ETEC, NTS) were downloaded from the GBD Compare website (https://vizhub.healthdata.org/gbd-compare/, accessed September 17, 2019) for 2010 and 2017, and for the three population groups separately. GBD data are prevalence-based, therefore, it was assumed that the change in incidence was proportional to the change in YLD. GBD does not provide data for the burden of STEC. Furthermore, the currently documented burden of STEC in Africa is very low. Therefore, STEC was excluded from further calculations.
Estimates at the population level were converted to burden per 100,000 by dividing by population numbers in both years (Supplementary material S1). Uncertainty distributions were derived for each pathogen of interest. A request to IHME to provide sample data from the uncertainty distributions was denied. The GBD website provides means and 95% uncertainty intervals for the uncertainty distributions of the variables of interest. Therefore, 10,000 samples from the uncertainty distributions of burden per 100,000 in 2010 and 2017 were reconstructed by fitting Gamma distributions to the available percentiles and means. For all metrics, the GBD burden per 100,000 in 2017 (ρ2017, GBD) divided by the GBD burden per 100,000 in 2010 (ρ2010, GBD) was used as a multiplier to the FERG burden per 100,000 in 2010 (ρ2010, FERG) to calculate the FERG burden per 100,000 in 2017 (ρ2017, FERG):
The burden estimates per 100,000 were applied to 2017 population data (pop2017) to provide absolute burden estimates (β2017, FERG):
2017 estimates for the whole population are presented for all ages and children under 5 years of age. Note that the burden for children under 5 years is correlated with the burden for older children and adults. Given the dependence between the two age groups, the distribution of burden for older children and adults cannot simply be derived by subtraction at the Monte Carlo sample level of the burden for children under 5 from the distribution the burden for all ages. Since we don't have information about this dependence, we refrain from presenting estimates for older children and adults.
Attribution
Estimates of the proportion of FBD incidence associated with CAMP and NTS were attributed to beef, dairy, poultry meat and vegetables based on FERG attribution results for the AFRD and AFRE subregions (Hoffmann et al., 2017) as described by Li et al. (2019). Attribution to food types and food products (see Sapp et al., 2022 for details) were based on a structured expert judgment study organized specifically for this study according to Cooke's Classical Model (Cooke, 1992; Hald et al., 2016). This study also included food group attribution for ETEC as these have not been provided by FERG, who focused their food group attribution on zoonotic pathogens. Experts participating in this study had a diversity of backgrounds with experience in the target countries in one or more of the following domains: diarrheal diseases, zoonoses, microbial food safety, water and sanitation or veterinary public health. We assume, in agreement with FERG, that attribution of mortality and DALY is proportional to the incidence of disease and used the same attribution estimates for all burden metrics.
Monte Carlo simulations (10,000 samples) were used to generate estimates of the uncertainty distributions of the disease burden per food group, type or food product for all hazards individually for three population groups.
All data extraction, manipulation, tables, plots, and statistical testing were done in the R statistical software version 4.1.0 (R Core Team, 2021).
Results
Foodborne disease burden estimates for 2010
Global, sub-regional and country-level data for the standardized FBD burden in 2010 (DALYs per 100,000 population) by the four hazards included in this study are presented in Table 1. The burden per 100,000 of CAMP in AFRD and AFRE was approximately twice as high as the global average, and about the same level in the target countries as in their subregions. The burden per 100,000 of ETEC in AFRD and AFRE was more than three times as high as the global average, and about the same level in the target countries as in their subregions. According to FERG, the burden per 100,000 of STEC at the global level was more than two orders of magnitude lower than the burden of the other three hazards included in this study. It was more than two-fold lower in AFRE than the global average and more than 10 times lower in Ethiopia. Because the currently documented burden of STEC in Africa is very low, this hazard was excluded from further calculations. The burden of NTS in AFRD was more than five times higher than the global average, and more than three times higher in AFRE. It was two times higher in Burkina Faso (more than 10 times higher than the global average) than in AFRD, while it was lower than the AFRE estimate in Ethiopia at approximately twice the global average.
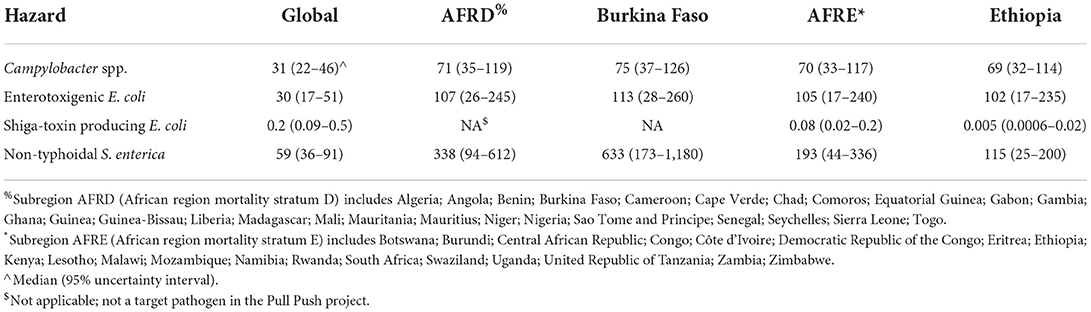
Table 1. Foodborne disability-adjusted life years (DALY) per 100,000 population for four bacterial hazards for the global population, subregions AFRD and AFRE, Burkina Faso and Ethiopia in 2010 according to WHO FERG (Havelaar et al., 2015).
Different metrics for the burden of FBD by the selected hazards in the target countries in 2010 are presented in Table 2. In both countries, the incidence per 100,000 of CAMP was ~3 times higher than that of ETEC and NTS. Mortality per 100,000 of CAMP and ETEC were similar in both countries. In both countries, the mortality per 100,000 of NTS and ETEC was higher than the mortality per 100,000 of CAMP. In Burkina Faso, the mortality per 100,000 of NTS was more than five times higher than that of ETEC, and more than 10 times higher than that of CAMP. While the mortality per 100,000 of CAMP and ETEC were ~10 times higher in children under 5 than in older children and adults, the mortality per 100,000 for NTS was only slightly higher in children under 5 [11.9 (3.24–21.4) per 100,000] than in older children and adults [9.25 (2.41–17.1) per 100,000], see Supplementary materials S2, S4. As noted above, this high mortality per 100,000 of NTS results in a high DALY burden per 100,000 for NTS in Burkina Faso.
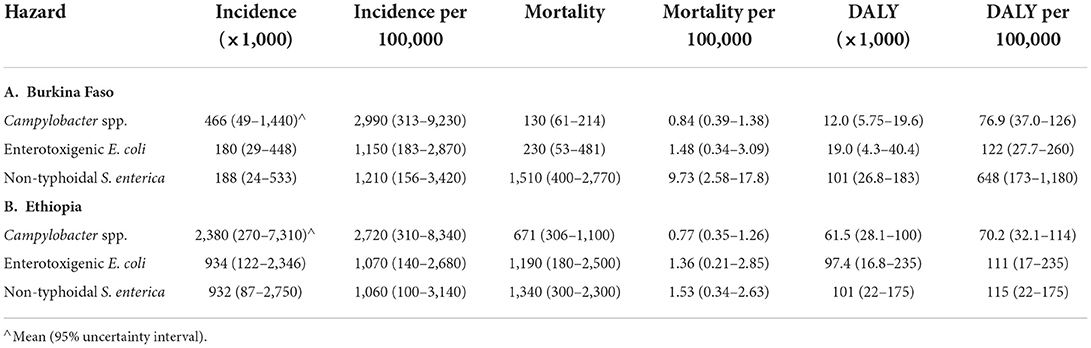
Table 2. Foodborne disease burden for three bacterial hazards in Burkina Faso and Ethiopia in 2010 (all ages) according to WHO FERG (Havelaar et al., 2015).
Updated foodborne disease burden estimates for 2017
The estimated relative changes from 2010 to 2017 in incidence, mortality, and DALY per 100,000 for the three hazards in both countries and per age group based on GBD estimates (Devleesschauwer et al., 2015) are presented in Table 3 by age group. For the total population in Burkina Faso, there was an increase of 4–10% in the burden of CAMP and ETEC for all metrics. At the same time, there was a decrease of 5–20% in the burden of NTS. For children under 5 in Burkina Faso, the increase in the burden of CAMP and ETEC was more substantial (10–20%) than for the total population, while the burden of NTS increased (Supplementary material S2). In Ethiopia, for all metrics and age groups, the FBD burden per 100,000 decreased in 2017 compared to 2010. Mortality and DALY per 100,000 decreased more than incidence per 100,000. The uncertainties in the changes in burden per 100,000 in Ethiopia in the GBD estimates were substantially smaller than in Burkina Faso.
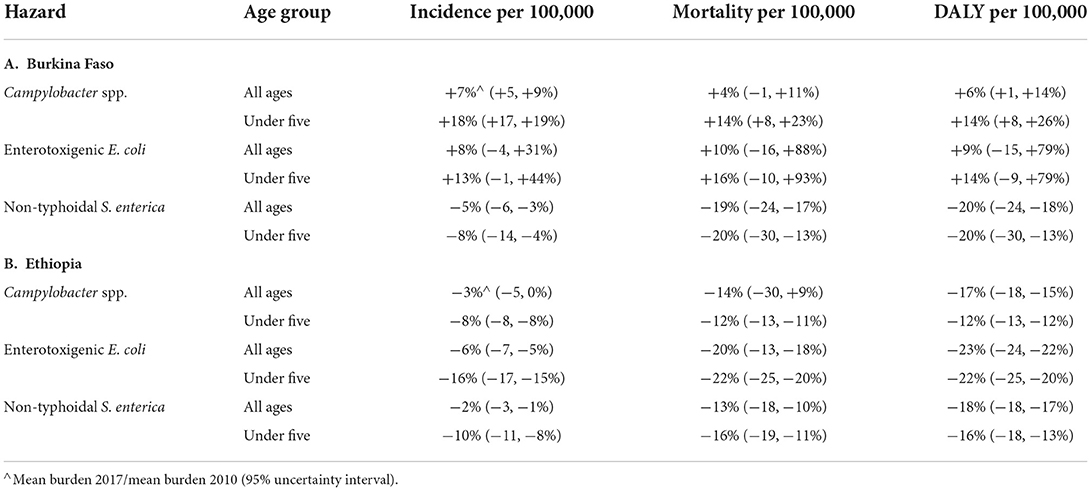
Table 3. Relative change for 2017 compared to 2010 of disease burden per 100,000 for three bacterial hazards in Burkina Faso and Ethiopia according to the Global Burden of Disease study (Kyu et al., 2018).
Updated foodborne disease burden estimates for 2017 were calculated for all hazards, countries and age groups by applying the relative change in GBD burden metrics per 100,000 between 2010 and 2017 to the corresponding FERG estimates. These were then applied to 2017 population data to calculate absolute burden estimates for 2017 (Table 4, detailed results in Supplementary materials S2, S3). Despite decreases in burden per 100,000 for several hazards and age groups, the ordering of hazards and age groups in 2017 was the same as in 2010. All burden estimates were higher in 2017 than in 2010, because the impact of increases in population size outweighed that of a lower burden per 100,000 in both countries. A graphical representation of the uncertainty in the data is shown in Figures 1, 2. Note that the support of the uncertainty distributions spanned several orders of magnitude.
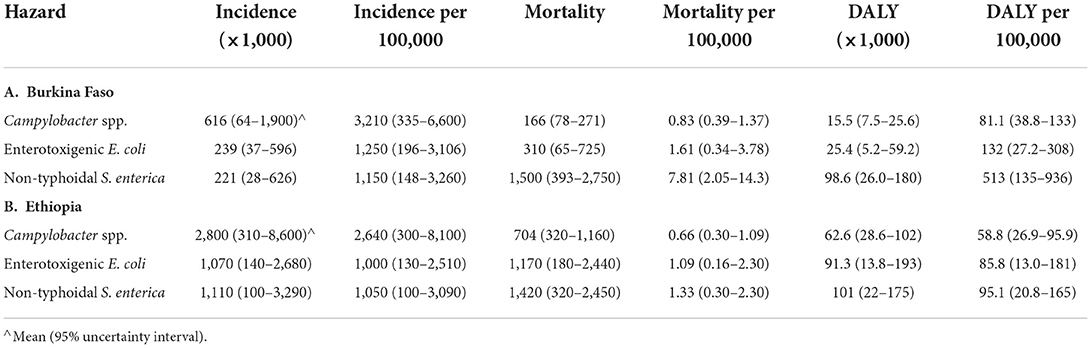
Table 4. Foodborne disease burden for three bacterial hazards in Burkina Faso and Ethiopia in 2017 (all ages) according to WHO FERG (Havelaar et al., 2015), updated according to the Global Burden of Disease study (Kyu et al., 2018).
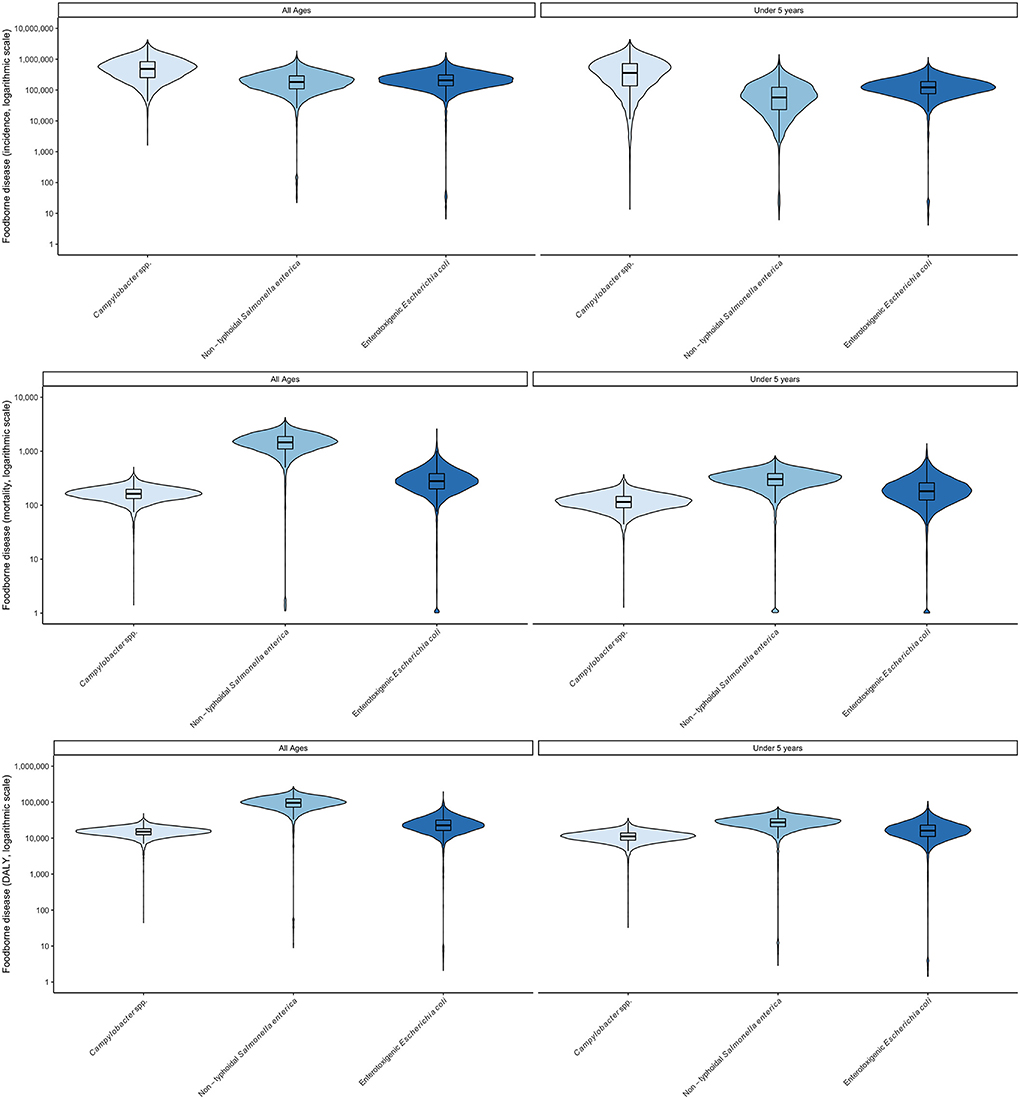
Figure 1. Uncertainty in foodborne disease burden for three bacterial hazards and age groups in Burkina Faso in 2017 (all ages) according to WHO FERG (1), updated according to the Global Burden of Disease study (8). (Top) incidence; (Middle) mortality, (Bottom) DALYs. Violin plots display two mirror images of the probability density function of a distribution, smoothed by a kernel density estimator. Embedded in the violin plot is a boxplot, showing lines for the median, boxes for the interquartile range and whiskers for the 95% uncertainty intervals. Values beyond the 95% UI have been omitted.
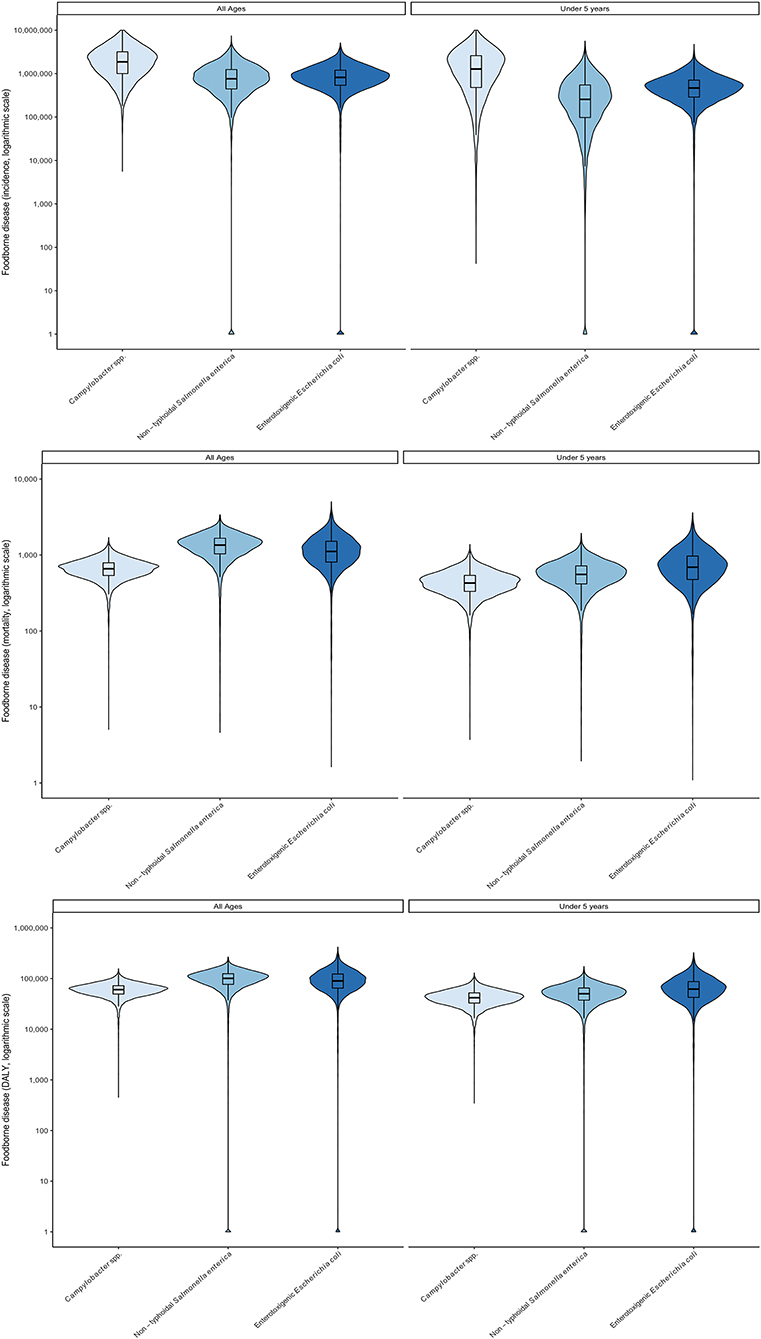
Figure 2. Uncertainty in foodborne disease burden for three bacterial hazards and age groups in Ethiopia in 2017 (all ages) according to WHO FERG (1), updated according to the Global Burden of Disease study (8). (Top) incidence; (Middle) mortality, (Bottom) DALYs. See Figure 1 for explanation of violin plots.
In 2017, children under 5 bore a major share of the total burden of these three pathogens, as measured by DALYs, despite accounting for only 18% of the population of Burkina Faso and 16% in Ethiopia. As shown in Figures 1, 2 the burden for children under 5 per 100,000 was always greater than for the total population. Age distributions differed markedly between pathogens with children under 5 bearing 73% of the burden of CAMP, 73% of ETEC and 30% of NTS in Burkina Faso and 62% of the burden of CAMP, 63% of the burden of ETEC and 49% of the burden of NTS in Ethiopia (see data in Supplementary materials S2–S4).
Attribution of disease burden to specific foods
Attribution estimates are described in detail in Sapp et al. (2022). In this paper, we summarize the results for the four food groups of interest in the TARTARE and Pull Push projects: beef (Ethiopia), dairy (Ethiopia), poultry meat (Burkina Faso and Ethiopia) and vegetables (Burkina Faso and Ethiopia). The burden attributed to these different food groups was further attributed to specific food types and food products as defined in Sapp et al. (2022). We present estimates for the total population only. Full results are available in Supplementary materials S2, S3 as means, medians and 95% uncertainty intervals.
In Burkina Faso, the disease burden associated with poultry meat consumption for CAMP and NTS together was estimated to be 42,600 DALYs in 2017 (Table 5A). Approximately 400,000 persons or 1 out of 50 in the total population fell ill from consuming poultry meat in 2017 by the two hazards and, of these, ~600 or 1 out of 30,000 in the total population died. Incidence of CAMP associated with poultry meat was ~4 times higher than NTS, but mortality and DALY burden of NTS was ~4 times higher than CAMP. The estimated disease burden of NTS and ETEC associated with vegetables was lower than that associated with poultry meat, resulting in ~60,000 cases, 160 deaths and 14,000 DALYs in 2017 (Table 5B). The incidence of ETEC-associated disease was ~3 times higher than NTS, but mortality was two times lower. The DALY burden of the two hazards was comparable.
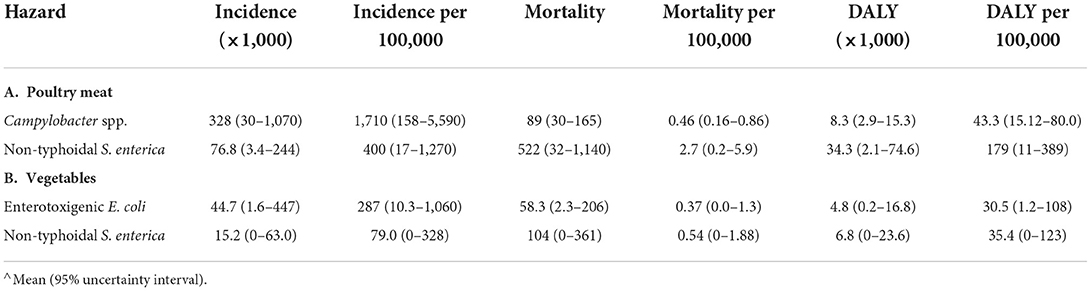
Table 5. Disease burden associated with specific food consumption in Burkina Faso in 2017 (total population).
In Ethiopia, the disease burden associated with CAMP and NTS from beef consumption was ~15,000 DALYs in 2017. An estimated 400,000 persons, or 1 out of 250 of the total population, fell ill; of these, ~190, or 1 out of 2 million, died (Table 6A). The burden of dairy products was slightly higher with 500,000 cases, 200 deaths and 17,000 DALYs than the burden of beef consumption (Table 6B). As in Burkina Faso, the burden of poultry meat was highest among the food groups, with 1.8 million cases, 850 deaths and 65,000 DALYs. Incidence of CAMP associated with poultry meat was ~4 times higher than NTS, but mortality and DALY burden per 100,000 were similar for both pathogens (Table 6C). While the incidence of illness associated with vegetables in Ethiopia was similar to beef and dairy, with ~400,000 cases, there were more deaths (330) and DALYs (24,000), with about 2/3 attributed to ETEC-associated disease (Table 6D).
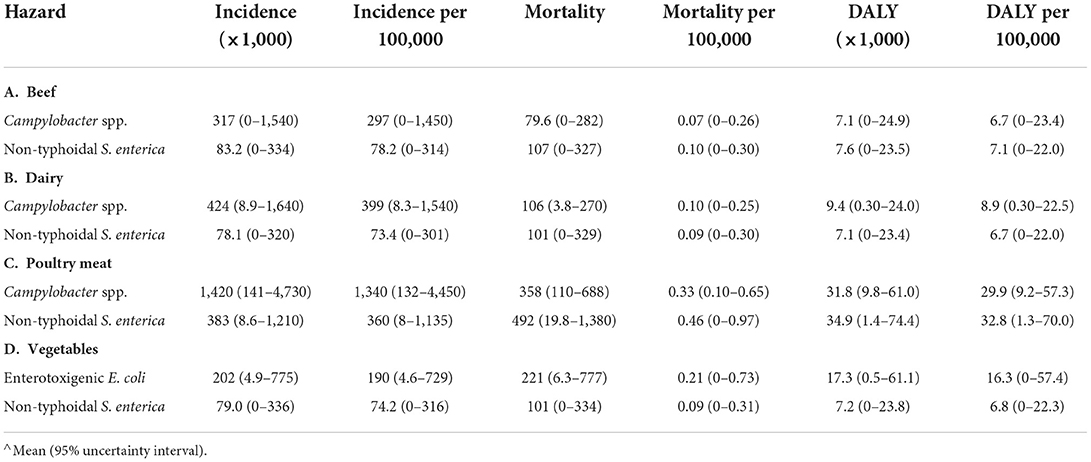
Table 6. Disease burden associated with specific food consumption in Ethiopia in 2017 (total population).
Attribution of disease burden (DALYs per 100,000) to food types and food products is visualized in Figures 3–8. Clearly, uncertainty increases with every step in the attribution process. Note that the burden of poultry meat and chicken meat is the same as chicken is the only poultry species consumed in both countries.
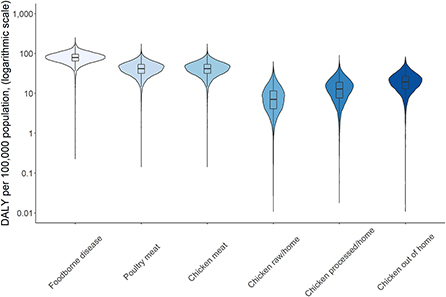
Figure 3. Attribution of disease burden (Disability-Adjusted Life Years per 100,000 population) of Campylobacter spp. to food types and food products in Burkina Faso in 2017. See Figure 1 for explanation of violin plots.
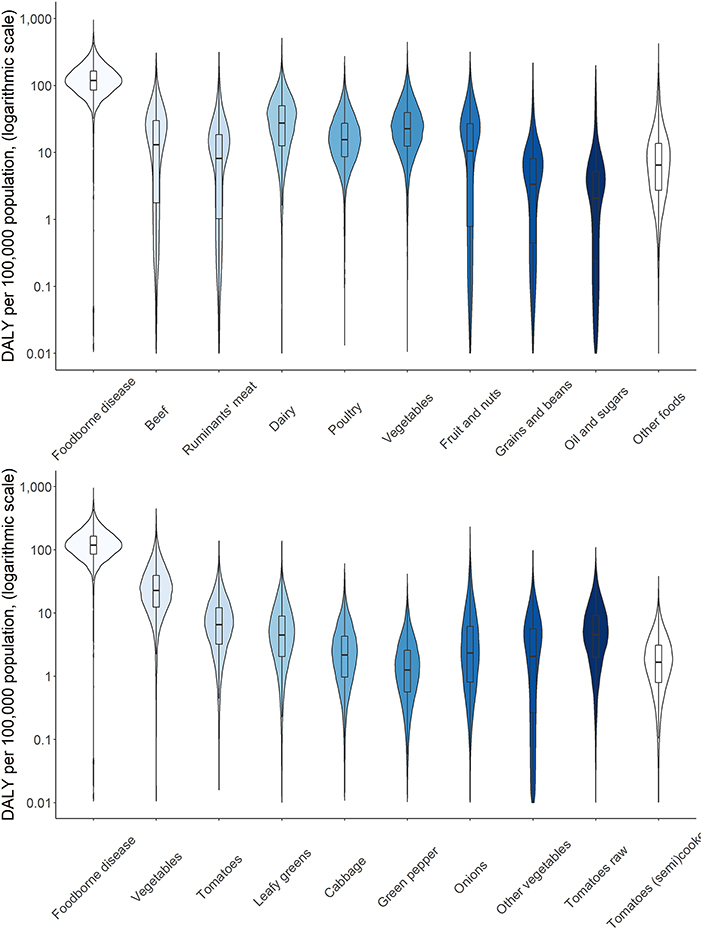
Figure 4. Attribution of disease burden (Disability-Adjusted Life Years per 100,000 population) of enterotoxigenic E. coli to food group, food types and food products in Burkina Faso in 2017. See Figure 1 for explanation of violin plots.
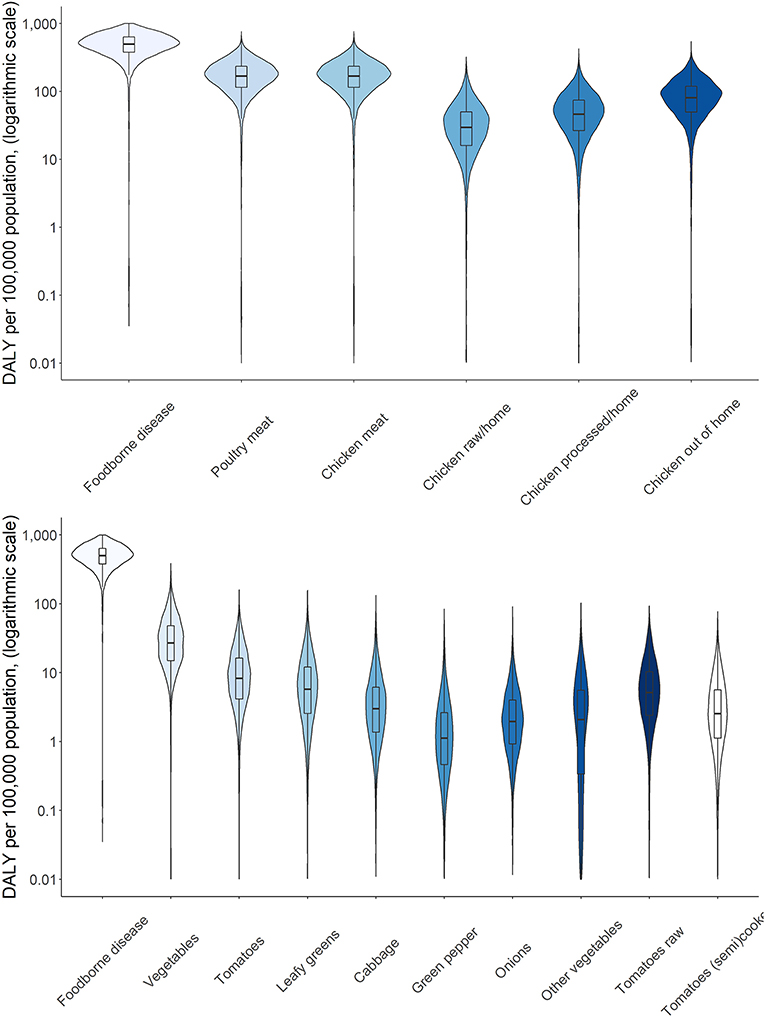
Figure 5. Attribution of disease burden (Disability-Adjusted Life Years per 100,000 population) of non-typhoidal Salmonella enterica to food types and food products in Burkina Faso in 2017. See Figure 1 for explanation of violin plots.
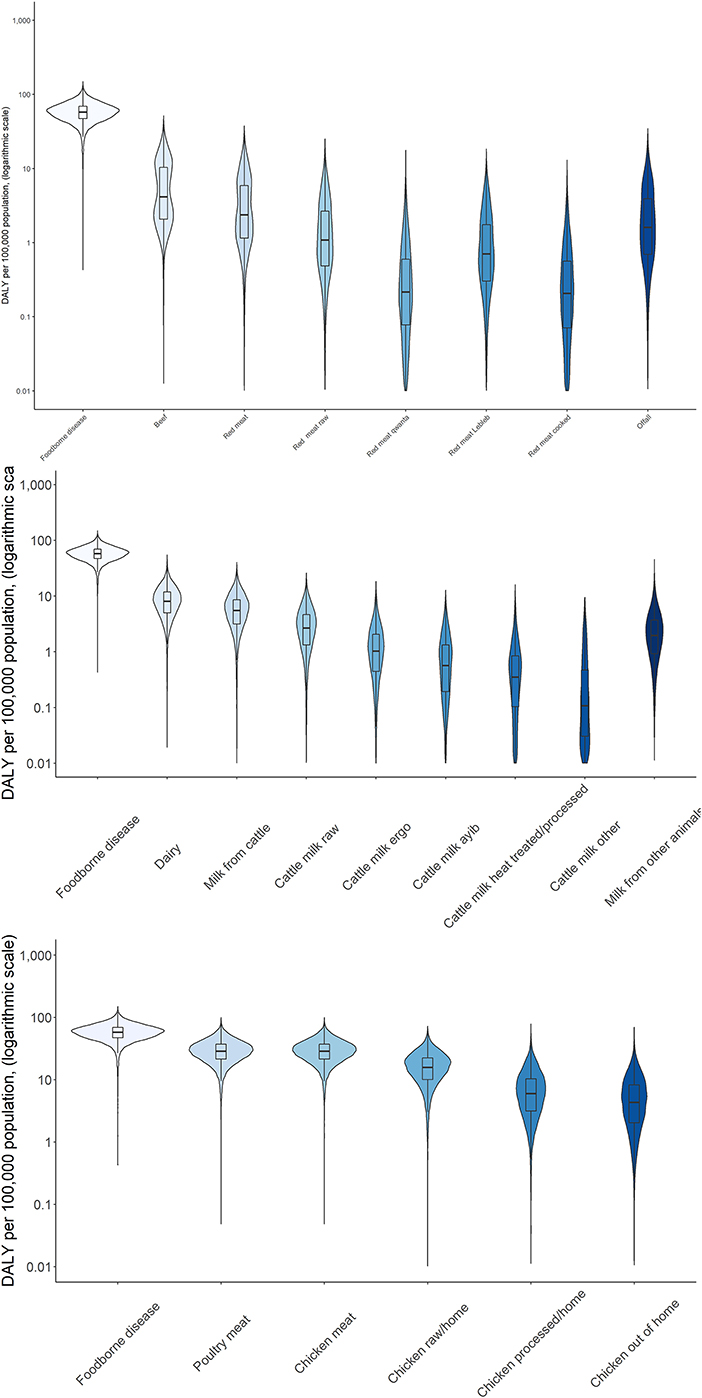
Figure 6. Attribution of disease burden (Disability-Adjusted Life Years per 100,000 population) of Campylobacter spp. to food types and food products in Burkina Faso in 2017. See Figure 1 for explanation of violin plots.
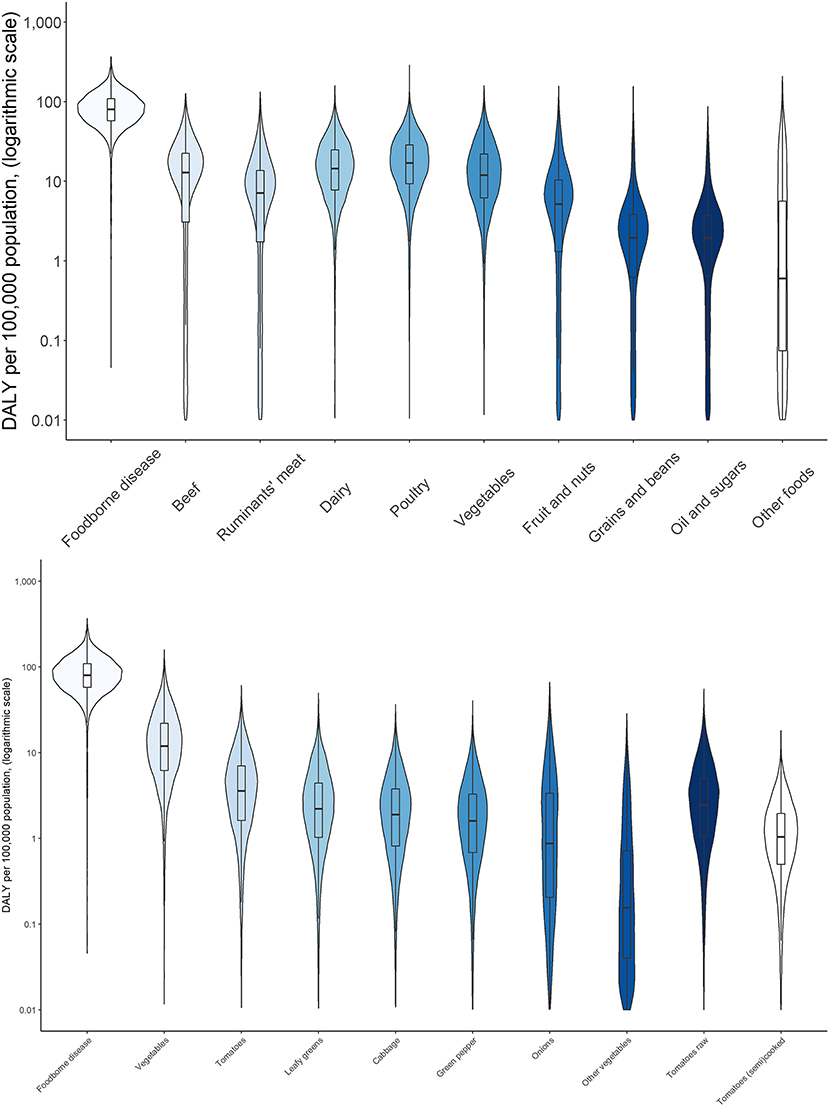
Figure 7. Attribution of disease burden (Disability-Adjusted Life Years per 100,000 population) of enterotoxigenic E. coli to food group, food types and food products in Ethiopia in 2017. See Figure 1 for explanation of violin plots.
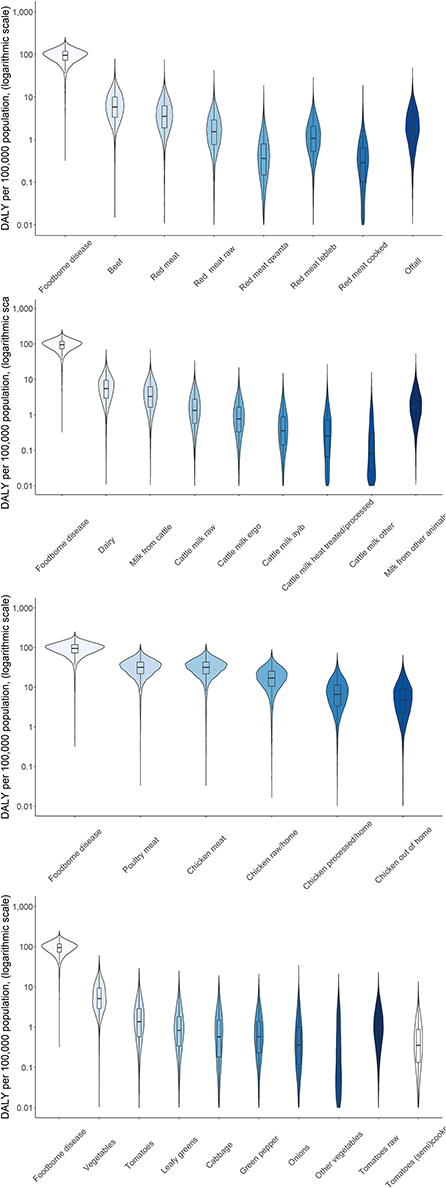
Figure 8. Attribution of disease burden (Disability-Adjusted Life Years per 100,000 population) of non-typhoidal Salmonella enterica to food types and food products in Burkina Faso in 2017. See Figure 1 for explanation of violin plots.
Discussion
While the WHO estimates of the global burden of FBD have increased awareness of food safety as a global health problem, their use for national food safety decision-making is limited because they have only been published on a subregional basis. Furthermore, the reference year for these estimates is 2010, and decision-makers would be better informed by more recent data. Working closely with the governments of two countries in Africa, we obtained permission to extract and publish national estimates from the FERG database. This interaction was formalized in a data request by the University of Florida and a communication plan between the project management of the TARTARE project at the Ohio State University (Columbus, OH), and of the Pull-Push project at the International Livestock Research Institute (Nairobi, Kenya).
The GBD study is the only available data source for annual updates of the burden of three of the four hazards of interest in this study (i.e., CAMP, ETEC, and NTS). Directly using the GBD estimates was not possible because those estimates are prevalence- and outcome-based while the FERG estimates are incidence- and hazard-based. These methods only provide equivalent results under the assumption of a stable epidemiologic situation, which clearly is not the case for foodborne diseases. A detailed discussion of these methods and their differences is provided by Mangen et al. (2013) and Devleesschauwer et al. (2015). Briefly, the incidence- and hazard-based approach attributes the current and future burden of acute disease and sequelae resulting from current exposure to a hazard (e.g., diarrheal disease and Guillain-Barré syndrome due to exposure to Campylobacter spp.) to the year of exposure while the prevalence- and outcome-based approach attributes disease burden to different outcome categories (e.g., diarrheal disease and neurological disease) based on the prevalence of disease in the reference year. The prevalence- and outcome-based method is preferred for the GBD study because it allows straightforward corrections for comorbidities. For chronic diseases or diseases with a long incubation time, the disease burden reflects past exposure. However, in food safety, it is preferable to estimate the impact of infectious disease control programs by using the current burden, resulting in a preference for the incidence- and hazard-based approach. This preference is further supported by the general use of incidence estimates rather than prevalence estimates in infectious disease epidemiology. Notwithstanding these differences in methodology, we suggest that the relative change in the burden of diarrheal disease in GBD estimates can be used to update the FERG estimates because the duration of diarrheal diseases is short, and any changes reflect changes in the current year. The GBD estimates also reflect impacts of non-food related factors such as access to and effectiveness of health care systems, which may explain the larger decrease (or lower increase) of mortality compared to incidence in both countries.
The FERG data, combined with new attribution estimates generated for this study, suggest a considerable burden is associated with CAMP, ETEC and NTS in both countries. In contrast, the burden of STEC in Ethiopia was very small, but these results should be interpreted carefully because the FERG estimates of the burden of STEC for Africa were based on very limited data (Mangen et al., 2013). Importantly, STEC estimates for Africa were derived from data from a single surveillance system in South Africa that had substantial under-reporting biases, including only one case of Hemolytic Uremic Syndrome being reported for the entire continent (Majowicz et al., 2014). Nevertheless, even in countries with better data, such as the US (Hoffmann et al., 2012) and the Netherlands (Havelaar et al., 2012), the population burden of STEC is several orders of magnitude lower than the burden of CAMP and STEC. In contrast, the individual burden of STEC is much higher, largely due to the risk of Hemolytic Uremic Syndrome and End-Stage Renal Disease in children under 5 years of age, which drives much of the concern about these infections.
Due to data limitations, the uncertainty in the estimates of the disease burden is considerable and spans several orders of magnitude. This statistical uncertainty needs to be considered when using these results for further decision-making, e.g., by including the full uncertainty distributions in cost-benefit calculations. There are additional, unquantified sources of uncertainty in our analysis, including those previously documented by FERG. As mentioned earlier, GBD and FERG use different approaches to estimate the burden of disease. We assumed that the relative change in GBD burden metrics is representative of the relative change in FERG estimates. In addition, there are uncertainties introduced from the assumption that attribution results at the subregional level from FERG are applicable to the country level and that mortality and DALY attribution is proportional to incidence attribution. Both assumptions reflect the best current data, and the general problem that data-driven attribution is currently not possible given data gaps and the inability to combine data from different study types (e.g., outbreaks, case-control studies, and microbial typing) in one consistent theoretical framework. These problems occur in high-income countries and low- and middle-income countries alike and expert judgment studies are frequently applied at international and national levels to provide best estimates of the (uncertainty in) foodborne disease attribution (Butler et al., 2015; Beshearse et al., 2021).
Conclusions
Foodborne disease is a global public health problem that disproportionally affects low- and middle-income countries. To support evidence-based food safety decision making, detailed, up-to-date insight on the burden of foodborne disease is required at the national level. The WHO has presented estimates of the global burden of foodborne disease at subregional level for the reference year 2010. We demonstrate how the WHO data can be used in tandem with estimates from the Global Burden of Disease study published by the Institute for Health Metrics and Evaluation to update foodborne disease burden estimates for two countries in Africa (Burkina Faso and Ethiopia) to 2017. Moreover, we demonstrate how the total burden of foodborne disease by specific microbial pathogens can be attributed to specific food using data from structured expert elicitation studies. Our results demonstrate a considerable burden of foodborne disease associated with three pathogenic bacteria (Campylobacter spp., enterotoxigenic E. coli and non-typhoidal Salmonella enterica). The burden of Shiga-toxin producing E. coli was several orders of magnitude lower but should be interpreted with caution since data on the incidence of this pathogen in Africa is very limited. Campylobacter spp. caused the largest number of illnesses in both countries, while non-typhoidal Salmonella enterica caused the largest number of deaths and disability-adjusted life years. Children under 5 years of age bore of the burden of Campylobacter spp. and enterotoxigenic E. coli in Burkina Faso and 2/3 of this burden in Ethiopia. The burden of non-typhoidal Salmonella enterica was more uniformly distributed over age groups. In both countries, the burden (in disability-adjusted life years) of consuming poultry meat was higher than the burden of consuming vegetables. In Ethiopia, estimates were also generated for the burden of consuming beef and dairy, which were similar and somewhat lower than the burden of consuming vegetables.
Data availability statement
The original contributions presented in the study are included in the article and Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AH: conceptualization, formal analysis, funding acquisition, methodology, project administration, resources, supervision, and writing—original. AS: data curation, formal analysis, investigation, software, validation, visualization, and writing—original. MA: data curation, investigation, and writing—review and editing. GN: formal analysis, methodology, software, visualization, and writing—review and editing. KM: conceptualization, supervision, validation, and writing—review and editing. BD: formal analysis, software, validation, and writing—review and editing. DG: funding acquisition, validation, and writing—review and editing. TK-J: project administration, resources, validation, and writing—review and editing. BK: funding acquisition, project administration, validation, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the UK Foreign, Commonwealth & Development Office (FCDO), the Bill & Melinda Gates Foundation and the CGIAR Research Program on Agriculture for Nutrition and Health led by the International Food Policy Research Institute through a project entitled Urban Food Markets in Africa: Incentivizing Food Safety Using a Pull-Push Approach (OPP1195588, The Pull-Push Project) and a project entitled The Assessment and Management of Risk from Non-typhoidal Salmonella, Diarrheagenic Escherichia coli and Campylobacter in Raw Beef and Dairy in Ethiopia (OPP1195643, TARTARE). Under the grant conditions of the Bill & Melinda Gates Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the author accepted manuscript version that might arise from this submission.
Acknowledgments
We acknowledge the inputs from experts in the estimation of the attribution of the disease burden of dairy to specific food products.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2022.1024560/full#supplementary-material
Supplementary material S1. Population size in Burkina Faso and Ethiopia, 2010 and 2017.
Supplementary material S2. Detailed statistics of foodborne disease burden in Burkina Faso, 2010 and 2017.
Supplementary material S3. Detailed statistics of foodborne disease burden in Ethiopia, 2010 and 2017.
Supplementary material S4. Foodborne disease burden for three bacterial hazards in Burkina Faso and Ethiopia in 2010 (children under 5 years of age) according to WHO FERG (1).
References
Beshearse, E., Nane, G. F., and Havelaar, A. H. (2021). “Using the classical model for source attribution of pathogen-caused illnesses,” in Expert Judgement in Risk and Decision Analysis. International Series in Operations Research and Management Science, eds A. M. Hanea, G. F. Nane, T. Bedford, S. French (Cham: Springer International Publishing), 373–385.
Butler, A. J., Thomas, M. K., and Pintar, K. D. M. (2015). Expert elicitation as a means to attribute 28 enteric pathogens to foodborne, waterborne, animal contact, and person-to-person transmission routes in Canada. Foodborne Pathog. Dis. 12, 335–344. doi: 10.1089/fpd.2014.1856
Cooke, R. M. (1992). Experts in Uncertainty: Opinion and Subjective Probability in Science. Oxford, NY: Oxford University Press, 334.
Devleesschauwer, B., Haagsma, J. A., Angulo, F. J., Bellinger, D. C., Cole, D., Döpfer, D., et al. (2015). Methodological framework for world health organization estimates of the global burden of foodborne disease. PLoS ONE 10, e0142498. doi: 10.1371/journal.pone.0142498
Ezzati, M., Lopez, A. D., Rodgers, A., Hoorn, S. V., and Murray, C. J. (2002). Selected major risk factors and global and regional burden of disease. Lancet 360, 1347–1360. doi: 10.1016/S0140-6736(02)11403-6
Hald, T., Aspinall, W., Devleesschauwer, B., Cooke, R., Corrigan, T., Havelaar, A. H., et al. (2016). World health organization estimates of the relative contributions of food to the burden of disease due to selected foodborne hazards: a structured expert elicitation. PLoS ONE 11, e0145839. doi: 10.1371/journal.pone.0145839
Havelaar, A. H., Haagsma, J. A., Mangen, M. J. J., Kemmeren, J. M., Verhoef, L. P. B., et al. (2012). Disease burden of foodborne pathogens in the Netherlands, 2009. Int. J. Food Microbiol. 156, 231–238. doi: 10.1016/j.ijfoodmicro.2012.03.029
Havelaar, A. H., Kirk, M. D., Torgerson, P. R., Gibb, H. J., Hald, T., Lake, R. J., et al. (2015). World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 12, e1001923. doi: 10.1371/journal.pmed.1001923
Hoffmann, S., Batz, M. B., and Morris, J. G. Jr. (2012). Annual Cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J. Food Protect. 75, 1292–1302. doi: 10.4315/0362-028X.JFP-11-417
Hoffmann, S., Devleesschauwer, B., Aspinall, W., Cooke, R., Corrigan, T., Havelaar, A., et al. (2017). Attribution of global foodborne disease to specific foods: findings from a World Health Organization structured expert elicitation. PLoS ONE 12, e0183641. doi: 10.1371/journal.pone.0183641
International Livestock Research Institute (2018). Urban Food Markets in Africa: Incentivizing Food Safety Using a Pull-Push Approach. International Livestock Research Institute. Available online at: https://www.ilri.org/research/projects/urban-food-markets-africa-incentivizing-food-safety-using-pull-push-approach (accessed January 3, 2022).
Jaffee, S., Henson, S., Unnevehr, L., Grace, D., and Cassou, E. (2019). The Safe Food Imperative: Accelerating Progress in Low- and Middle-Income Countries. Washington, DC: World Bank.
Kyu, H. H., Abate, D., Abate, K. H., Abay, S. M., Abbafati, C., Abbasi, N., et al. (2018). Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1859–1922. doi: 10.1016/S0140-6736(18)32335-3
Li, M., Havelaar, A. H., Hoffmann, S., Hald, T., Kirk, M. D., Torgerson, P. R., et al. (2019). Global disease burden of pathogens in animal source foods, 2010. PLoS ONE 14, e0216545. doi: 10.1371/journal.pone.0216545
Majowicz, S. E., Scallan, E., Jones-Bitton, A., Sargeant, J. M., Stapleton, J., Angulo, F. J., et al. (2014). Global incidence of human shiga toxin–producing escherichia coli infections and deaths: a systematic review and knowledge synthesis. Foodborne Pathog. Dis. 11, 447–455. doi: 10.1089/fpd.2013.1704
Mangen, M. J. J., Plass, D., Havelaar, A. H., Gibbons, C. L., Cassini, A., Mühlberger, N., et al. (2013). The pathogen- and incidence-based DALY approach: an appropriated methodology for estimating the burden of infectious diseases. PLoS ONE 8, e79740. doi: 10.1371/annotation/caf33818-3453-4e30-b307-7526427b09b7
R Core Team (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/ (accessed June 2, 2021).
Sapp, A. C., Amaya, M. P., Havelaar, A. H., and Nane, G. F. (2022). Attribution of country level foodborne disease to food group and food types in three African countries: conclusions from a structured expert judgment study. PLoS Neglected Trop. Dis. 16, e0010663. doi: 10.1371/journal.pntd.0010663
TARTARE (2018). TARTARE: The Assessment and Management of Risk from Non-typhoidal Salmonella, Diarrheagenic Escherichia coli and Campylobacter in Raw Beef and Dairy in Ethiopia. CFI. Available online at: https://foodsafety.osu.edu/research/international-research-shifting-food-safety-paradigm/tartare (accessed January 3, 2022).
Keywords: foodborne disease, Africa, Campylobacter, Salmonella, STEC, ETEC
Citation: Havelaar AH, Sapp AC, Amaya MP, Nane GF, Morgan KM, Devleesschauwer B, Grace D, Knight-Jones T and Kowalcyk BB (2022) Burden of foodborne disease due to bacterial hazards associated with beef, dairy, poultry meat, and vegetables in Ethiopia and Burkina Faso, 2017. Front. Sustain. Food Syst. 6:1024560. doi: 10.3389/fsufs.2022.1024560
Received: 21 August 2022; Accepted: 17 October 2022;
Published: 01 November 2022.
Edited by:
John Franklin Leslie, Kansas State University, United StatesReviewed by:
Abhinav Mishra, University of Georgia, United StatesAnne Thebault, Agence Nationale de Sécurité Sanitaire de l'Alimentation, de l'Environnement et du Travail (ANSES), France
Copyright © 2022 Havelaar, Sapp, Amaya, Nane, Morgan, Devleesschauwer, Grace, Knight-Jones and Kowalcyk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arie H. Havelaar, YXJpZWhhdmVsYWFyQHVmbC5lZHU=
†Present address: Amanda C. Sapp, Abrams Public Health Center, Tucson, AZ, United States
‡These authors have contributed equally to this work
 Arie H. Havelaar
Arie H. Havelaar Amanda C. Sapp
Amanda C. Sapp Mirna P. Amaya
Mirna P. Amaya Gabriela F. Nane
Gabriela F. Nane Kara M. Morgan
Kara M. Morgan Brecht Devleesschauwer
Brecht Devleesschauwer Delia Grace
Delia Grace Theo Knight-Jones
Theo Knight-Jones Barbara B. Kowalcyk
Barbara B. Kowalcyk