
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 24 November 2022
Sec. Waste Management in Agroecosystems
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.1010890
This article is part of the Research Topic Circular Economy and Sustainability in the Agro-Food System View all 12 articles
 Claudio Cacace1†
Claudio Cacace1† Claudio Cocozza1†
Claudio Cocozza1† Andreina Traversa1
Andreina Traversa1 Rossana Coda2,3
Rossana Coda2,3 Carlo Giuseppe Rizzello4
Carlo Giuseppe Rizzello4 Erica Pontonio1
Erica Pontonio1 Francesco De Mastro1
Francesco De Mastro1 Gennaro Brunetti1
Gennaro Brunetti1 Michela Verni1*
Michela Verni1*Introduction: The use of novel soil amendments and the exploitation of plant growth-promoting microorganisms are considered promising tools for developing a more sustainable agriculture in times when ensuring high-yield productions with limited resources is essential.
Methods: In this study, the potential of brewers' spent grain (BSG), the major by-product of the brewing industry, as organic soil amendment, was investigated. Bioprocessed BSG, obtained by an enzymatic treatment coupled with fermentation, together with native BSG, were used as amendments in a pot-trial. An integrated analytical approach aimed at assessing the modification of the physicochemical properties of a typical Mediterranean alkaline agricultural soil, and the plant growth-promoting effect on escarole (Cichorium endivia var. Cuartana), was carried out.
Results: The use of biomasses led to soil organic content and total nitrogen content up to 72 and 42% higher, compared to the unamended soils. Moreover, the lower pH and the higher organic acids content doubled phosphorus availability. Although the number of leaves per plant in escaroles from pots amended with native and bioprocessed BSG did not show any difference compared to plants cultivated on unamended pots, the average fresh weight per escarole head, was higher in pots amended with bioprocessed BSG.
Discussion: Hence, the results collected so far encourage BSG application for agricultural purpose, while solving the problem of disposing of such abundant side stream.
Beer is one of the most consumed beverages across the world and its major byproduct is represented by brewers' spent grain (BSG). BSG is generated during the malting mashing processes of brewing and comprises the outer layers of barley grains and, of the other cereals potentially used. Every 100 L of beer produced, 20 kg of BSG is generated (Yoo et al., 2021), leading to production volumes of 39 million tons of BSG every year worldwide, 3.4 million of which, just in Europe (Bianco et al., 2020). A small quantity of BSG is used as low-value animal feed, while the remaining part is discarded (Ravindran and Jaiswal, 2016). Such residual biomass represents, on one hand, a huge cost and risk for the environment, on the other, a source of organic carbon and nutrients that could be valorised as soil amendment.
BSG contains up to 20% of proteins, composed mainly by essential amino acids, it is also rich in cellulose (17%) and arabinoxylans and shows an acidic pH due to the presence of organic acids (Mussatto et al., 2006). Although the amount of available fermentable sugar is low, BSG can be used as substrate for fermentation with lactic acid bacteria (LAB) whose activity further lowers the pH and enhances the release of phenolic compounds, flavonoids, and protein derivatives (Verni et al., 2019, 2020). It was also showed that fermentation of BSG by Lactiplantibacillus plantarum or Leuconostoc pseudomesenteroides induces an overexpression of genes involved in the metabolism of arabinose and xylose, the most abundant sugars composing BSG arabinoxylans (Acin-Albiac et al., 2022).
Nowadays ensuring high production yields with limited resources is of utmost importance to guarantee the sustainability of the global food system. Amendments with residual biomasses are highly recommended because they restore fertility of degraded soils (Abdelrahman et al., 2020), improve the quality of growing substrates in pot cultivation (Mininni et al., 2015), positively influence both chemical and microbiological soil properties (Ozores-Hampton et al., 2011), and allow carbon sequestration (Zhang et al., 2012). For example, for alkaline soils, which are characterized by high pH values leading to the precipitation of iron as iron oxyhydroxides and to the adsorption of phosphate by soil minerals (Sposito, 2016), application of organic matter is essential to increase yields; and BSG, native and bioprocessed, used as soil amendments, besides providing organic matter could provide several benefits. Organic acids could be involved in the competition for phosphate sorption sites, helping the desorption of P and increasing its availability (Brunetti et al., 2019), as well as in the solubilization of iron by lowering the pH (Cocozza and Ercolani, 1997). In addition, LAB are plant growth promoting microorganisms (PGPM) that can act as biocontrol agents, help plants to withstand biotic and abiotic stress and produce compounds that directly stimulate plant growth (Lamont et al., 2017). From an environmental perspective, bacterial species belonging to the former genus Lactobacillus are among the microorganisms able to bioaccumulate metals. Ameen et al. (2020) showed that the former Lactobacillus plantarum (recently reclassified as Lactiplantibacillus plantarum) absorbed on the surface and inside the cells a great amount of Ni2+ and Cr2+ from industrial wastewaters. The superficial adsorption is possibly due to the electrostatic interaction of metals with the functional groups of the bacterial cell wall (Kargar and Shirazi, 2020).
Based on the above consideration, we hypothesized that the integration of BSG into Mediterranean alkaline agricultural soils could positively affect their physicochemical properties and promote plant growth. In this framework, this study aimed at investigating the potential of BSG as organic soil amendment, referring to a proof-of-concept escarole cultivation model (Figure 1). In particular, brewers' spent grain, native or processed (bBSG) according to a biotechnological protocol previously optimized for the release of phenolic compounds and bioactive peptides through enzymatic treatment and LAB fermentation (Verni et al., 2020), were used in a pot trial. An integrated approach for the characterization of native and processed BSG amended soils and plants was applied.
BSG was kindly provided by Peroni brewery (Bari, Italy) and had the following proximal composition: moisture 80%; protein 21% of dry matter (d.m.); fat 10.9% of d.m.; cellulose 22.5% of d.m.; hemicellulose, 25% of d.m, lignin, 15.3% of d.m; ashes, 5.1% of d.m.
Lactiplantibacillus plantarum PU1, belonging to the Culture Collection of the Department of Soil, Plant and Food Sciences (University of Bari, Italy), selected as starter based on the kinetics of growth and acidification and the ability to increase antioxidant activity of BSG (Verni et al., 2020), was used in this study. The strain was routinely propagated on De Man, Rogosa and Sharpe (MRS) medium (Oxoid, Basingstoke, Hampshire, UK) at 30°C. Before inoculation it was cultivated until the late exponential phase of growth (ca. 10 h), harvested by centrifugation at 10,000 x g for 10 min at 4°C, washed twice in 50 mM sterile phosphate buffer (4°C, pH 7.0), resuspended in sterile distilled water, and used to inoculate BSG.
The commercial hydrolytic enzyme, Depol™ 761P (Biocatalysts, Chicago, IL), a preparation derived from Bacillus subtilis having xylanase activity (14,670 nkat g−1), was used for the BSG treatment before fermentation.
BSG bioprocessing was carried out as described by Verni et al. (2020). Briefly, BSG homogenized with water at a 60:40 ratio was added of Depol™ 761P (100 nkat g−1) and incubated at 50°C for 5 h. After the enzymatic treatment, L. plantarum PU1, cultivated as above described, was inoculated (initial cell density ca. 7.5 log cfu g−1) and the mixture incubated at 30°C for 24 h.
Fermentation was monitored by measuring, before and after incubation, pH and enumerating presumptive LAB using MRS (Oxoid, Basingstoke, Hampshire, United Kingdom) agar medium, supplemented with cycloheximide (0.1 g L−1). Plates were incubated in anaerobiosis condition (AnaeroGen and AnaeroJar, Oxoid) at 30°C for 48 h. The amendments were also characterized for the presence of yeasts, molds, and Enterobacteriaceae. Yeasts and molds were cultivated on Yeast Peptone Dextrose Agar medium (Sigma-Merck, Darmstadt, Germany), supplemented with chloramphenicol (0.1 g L−1), through pour and spread plate enumeration, respectively, and incubated at 25°C whereas Enterobacteriaceae were determined on Violet Red Bile Glucose Agar (Oxoid) at 37°C for 24 h. pH values were determined by a pH meter (Model 507, Crison, Milan, Italy) with a food penetration probe. The AACC method 02–31.01 (AACC, 2010) was used for the determination of total titratable acidity (TTA) of samples and expressed as the amount (mL) of 0.1M NaOH necessary to reach pH of 8.4. Native and bioprocessed spent grain were also characterized for electrical conductivity (EC), moisture, total nitrogen (TN), total phosphorous (TP) and organic carbon (OC) contents, following the methods by Trinchera et al. (2006). The moisture, expressed as percentage of the initial weight, was determined by drying samples at 105°C overnight. The EC was measured on sample/water extracts (1:10 w/v) after shaking for 30 min using a Hanna Edge® EC instrument. The TN content was determined by the Kjeldahl method, while, according to Ciavatta et al. (1989), the OC content was determined by dichromate oxidation and subsequent titration with ferrous sulfate. The total P content was measured spectrophotometrically at 650 nm, after incinerating biomass samples at 550°C, suspending ashes in 10% hydrochloric acid solution, and developing the blue color in the filtered solution in accordance with the Olsen (1954).
Water/salt-soluble extracts (WSE) of the biomasses were prepared according to the method originally described by Osborne and modified by Weiss et al. (1993) using 50 mM Tris–HCl (pH 8.8). After centrifugation, the supernatants were used to determine sugars, organic acids, peptides, and total free amino acids (TFAA) concentration.
Glucose was measured using the D-Fructose D-Glucose Assay Kit K-FRUGL (Megazyme International Ireland Limited, Bray, Ireland), following the manufacturer's instructions, whereas organic acids were quantified by High Performance Liquid Chromatography (HPLC), using an ÄKTA Purifier system (GE Healthcare, Buckinghamshire, UK) equipped with an Aminex HPX-87H column (ion exclusion, Biorad, Richmond, CA), as described by Rizzello et al. (2010).
For the analysis of peptides, WSE were treated with trifluoroacetic acid (0.05% wt/vol), centrifuged (10,000 x g for 10 min), and subject to dialysis (cut-off 500 Da) to remove proteins and free amino acids, respectively. Then, peptides concentration was determined by the o-phtaldialdehyde method as described by Church et al. (1983), and dialysates analyzed through Reversed-Phase Fast Performance Liquid Chromatography (RP-FPLC), using an ÄKTA FPLC equipped with a Resource RPC column, with the UV detector operating at 214 nm (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) as described by Rizzello et al. (2010). TFAA were analyzed by a Biochrom 30+ series Automatic Amino Acid Analyzer (Biochrom Ltd., Cambridge Science Park, United Kingdom), equipped with a Li-cation-exchange column (4.6 x 200 mm internal diameter) (De Pasquale et al., 2021).
An alkaline and tilled soil classified Haplic and Petric Calcisol, according to IUSS Working Group WRB (2015), was collected in Southern Italy, air dried and used for the pot experiments. Treatments were: (i) not amended soil, without plant (CTA); (ii) soil amended with BSG, without plant (BSGA); (iii) soil amended with bBSG, without plant (bBSGA); (iv) not amended soil, with plant (CTP); (v) soil amended with BSG, with plant (BSGP); (vi) soil amended with bBSG and with plant (bBSGP). Pots were distributed in a completely randomized design with three replications for each treatment, for a total of 18 experimental pots, and the trial was performed in a greenhouse at the University of Bari, Italy. The amended pots received BSG or bBSG at a dose of about 25,000 kg ha−1 according to the good local agricultural practices (Abdeldaym et al., 2018). Thirty-days-old seedlings of Cichorium endivia var. Cuartana, a variety of escarole, were transplanted at the end of the first period of February 2020 and the trial was stopped at the beginning of April. The first irrigation was performed immediately after the transplanting for the rooting and establishment of the plants. The subsequent irrigations were carried out when water lost by evapotranspiration (ET) reached about 40% of the available water depletion in the soil. The ET was calculated utilizing values of a class A pan evaporation and following the FAO procedure (Allen et al., 1998). During the trial, the temperature ranged from 5°C in the night to 23°C at mid-day, and all pots did not receive any further kind of fertilization. The escarole was intended for the fresh consumption, as salads, or cooked, as a side dish.
The soil was characterized at the beginning of the trial (T0) for pH, EC, TN, available phosphorous (Pava), and OC content, according to the conventional analytical methods described by Sparks et al. (1996). Briefly, the pH was measured in deionized water (pHH2O) and in 1 M KCl (pHKCl) suspensions at 1:2.5 soil to liquid ratio, whereas the electrical conductivity (EC) was measured in filtrates from a 1:2 soil to water ratio. The TN content was determined by the Kjeldahl method. The OC was measured by dichromate oxidation and ferrous sulfate titration according to the Walkley-Black method (De Vos et al., 2007). The Pava was extracted with a 0.5 M NaHCO3 solution and determined spectrophotometrically at 650 nm, according to the Olsen (1954). Diethylenetriaminepentaacetic acid (DTPA)-extractable fractions of Mn, Fe, and Cu were obtained from a 1:2 soil to DTPA solution. DTPA extracts were filtered by gravity through Whatman No. 42 filter paper, and the solutions were then analyzed using an inductively coupled plasma iCAP 6000 Series ICP-OES Spectrometer (Thermo Electron Corporation, Walthman (MA), USA). Particle size analysis was determined by the pipette method.
At the end of the experiment, all soils were analyzed again to investigate the effects of native and bioprocessed BSG in the presence and in absence of plants on soil parameters with respect to T0.
To verify the effects of BSG and bBSG on plant during the trial, indirect measurements of the chlorophyll content were carried out using SPAD-502 (Konica Minolta, Japan). At the end of the test (50 days from transplanting) the number of plant leaves was recorded, as well as their fresh and dry weight to determine production yield of each treatment. Moreover, leaf samples were analyzed for their P, Mn, Fe, and Cu content, aiming at verifying the effects of each treatment on leaf composition. The total P was obtained according to Trinchera et al. (2006). The total Mn, Fe, and Cu content were determined using the microwave-assisted acid digestion method, adding a Suprapur® HNO3:H2O2:HCl mixture (6:1:1, v:v:v) to each sample. At the end of the digestion, samples were cooled, filtered through Whatman No. 42 filter paper, diluted with distilled Milli-Q Reagent grade water and, finally, analyzed by means of an inductively coupled plasma iCAP 6000 Series ICP-OES Spectrometer (Thermo Electron Corporation).
Experimental data were tested against the normal distribution of variables (Shapiro—Wilk test) and the homogeneity of variance (Bartlett test) using R studio. The variables normally distributed with homogeneity of variances verified were subjected to an ANOVA and HSD test.
In bBSG, the initial cell density of presumptive LAB corresponded to the targeted inoculum and, after fermentation, increased of ca. 1 log cycle, reaching 8.32 ± 0.12 log cfu g−1. The presence of potentially spoiling microorganisms was also assessed. Yeasts and molds in BSG were 4.7 ± 0.3 and 1.2 ± 0.2 log cfu g−1, respectively, whereas Enterobacteriaceae were 3.3 ± 0.1 log cfu g−1. After bioprocessing, compared to BSG, a significant decrease of yeasts and molds was observed, remaining below 2.5 log cfu g−1, whereas Enterobacteriaceae were not detected.
Fermentation led to a relevant acidification. The pH decreased from 4.49 ± 0.15 of BSG to 3.75 ± 0.11 of bBSG, with a production of roughly 68 and 13 mmol kg−1 d.m. of lactic and acetic acid, respectively, which were detected in traces in native BSG. As consequence, TTA value was significantly higher in bBSG (11.72 ± 0.62 mL) compared to BSG (3.59 ± 0.21 mL). Glucose was not detected in both biomasses.
Bioprocessing of the biomass led to an increase in peptides concentration of ca. 20%, reaching 75 mg g−1 d.m. in bBSG. This trend was confirmed by the FPLC chromatograms, where although the number of total peaks detected was lower in bBSG, compared to BSG, a higher total area was found after bioprocessing (1,472 ± 80 against 854 ± 42 mAU*mL, respectively). Moreover, the treatment led to a shift toward less hydrophilic peptides. Indeed, almost 60% of all peptides detected in bBSG eluted in the range 46–100% of acetonitrile, 20% more than BSG. On the contrary, TFAA significantly (P < 0.05) decreased after bioprocessing reaching 980 mg kg−1 d.m. against the 3.7 g kg−1 d.m. found in native BSG.
As shown in Table 1, bioprocessing increased the EC content of the biomass, while BSG showed significantly higher total phosphorous content compared to bBSG. The content of OC, TN, and the C/N ratio did not significantly differ between biomasses.
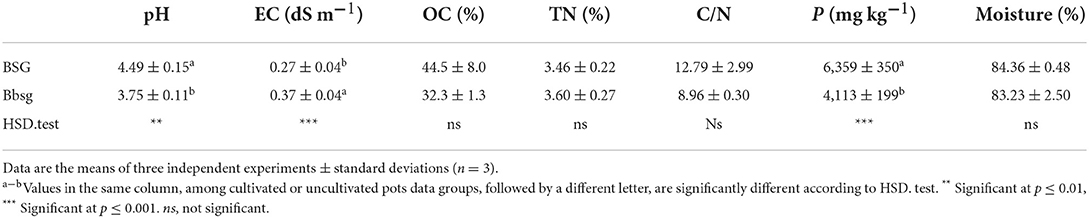
Table 1. Chemical and physicochemical characteristics of native (BSG) and bioprocessed brewers' spent grains (bBSG).
Table 2 reports soils physicochemical properties at the beginning (T0) and at the end of the trial. The pHH2O of T0, CTP and CTA was alkaline and ranged from 8.37 ± 0.12 to 8.71 ± 0.04, while that of soils with bBSG and BSG supplementation was significantly lower, even if they did not show significant differences among each other. In contrast, the pHKCl of all soils did not show any significant difference. Treated soils, with or without plant, had significantly higher OC and TN than T0 and corresponding control pots. The availability of P increased significantly with the addition of the biomasses and with plants, while the Pava content raised only in treated pots without plants.
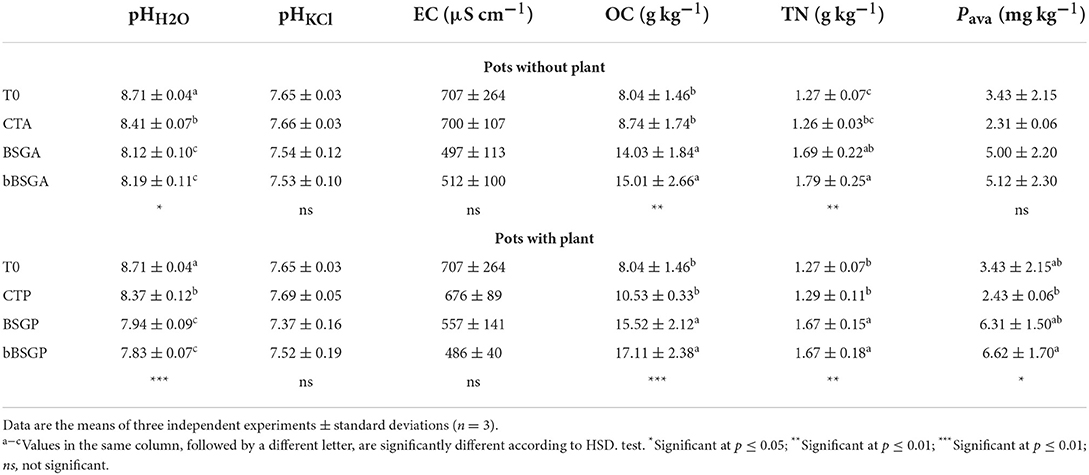
Table 2. Chemical and physicochemical properties of soils unamended (CT) and amended with native (BSG) or bioprocessed brewers' spent grain (bBSG), uncultivated (A) or cultivated with escarole plants (P).
No significant differences were observed in the content of available iron, manganese, and copper in soils, with or without plants, and treated with bioprocessed or native BSG (data not shown).
Table 3 reports the mean biometric features of escaroles at the end of the experiment. The plants from BSG and bBSG amended pots had number of leaves 1.21- and 1.14-times higher, respectively, than plants cultivated on control pots, although all treatments did not have any significant difference among each other. Regarding the yield, bBSG amended pots showed the highest fresh weight and their yield was 1.26-fold higher than control pots, followed by BSG amended pots, whose yield was 1.18-time higher than CTP. The application of biomasses also significantly increased the dry weight of plants with respect to CTP. In fact, BSG and bBSG treated escaroles showed a dry weight 1.19- and 1.24-fold higher than CTP, respectively.
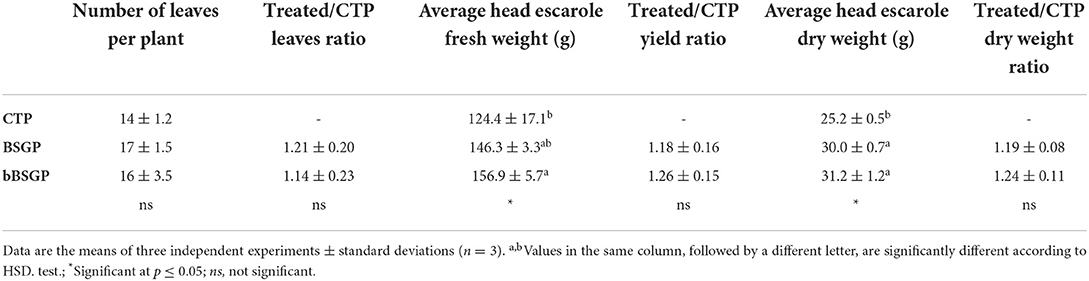
Table 3. Biometric features of escarole plants, grown in soil unamended (CTP) and amended with native (BSG) or bioprocessed brewers' spent grain (bBSG), at the end of the trial.
No difference in the content of micronutrients and phosphorus were observed among plants grown in control soil or soils treated with BSG or bBSG, as reported in Table 4. Whereas, chlorophyll content of escarole leaves, initially unaffected by the treatments, from the 27th day after transplantation, was significantly higher in leaves from plants grown in treated soils, with respect to the control pots (Figure 2). However, no significant difference was observed between the bioprocessed and native BSG tested.
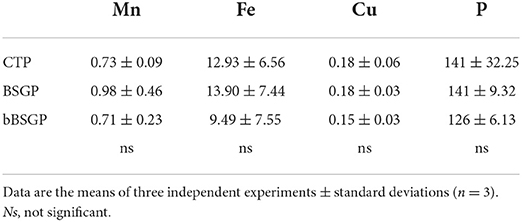
Table 4. Micronutrients and phosphorous content expressed as mg kg−1 of escarole leaves grown in soil unamended (CTP) and amended with native (BSG) or bioprocessed brewers' spent grain (bBSG).
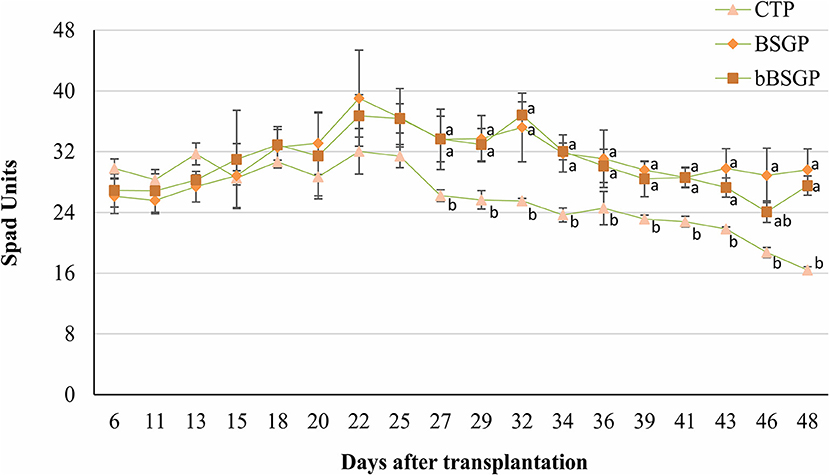
Figure 2. Effect of the biomasses on chlorophyll content of escaroles grown in control soil (CTP), soil amended with native (BSGP) or bioprocessed brewers' spent grain (bBSGP). a, bDifferent letters indicate significant differences among the data according to HSD test. Vertical bars represent standard deviation.
Most of the BSG generated is used as feed or disposed of as landfill, whereas a little part of it is used for biogas/bioethanol production (Mussatto et al., 2006), nevertheless, up-cycling strategies that include its complete reutilization without further generation of by-products should be favored. Due to the potential health benefits deriving from its components, the inclusion of BSG in bakery products has been proposed by several authors (for a review see Lynch et al., 2016) and examples of such products can also be found at retail level. Since the incorporation of untreated BSG often entails negative repercussion on the structure of such products, BSG valorization as functional food ingredient, through bioprocessing technology, has also been recently proposed (Verni et al., 2020; Schettino et al., 2021; Koirala et al., 2022), while its the use as soil amendment is uncommon and it has been only partially investigated. The use of BSG as organic amendment in soils cultivated with maize was first proposed by Mbagwu and Ekwealor (1990). They found that the highest BSG dose (10% w/w) determined the better soil conditions due to its capacity to improve aggregate stability and water retention capacity, whereas the lowest dose (2.5% w/w) determined the highest crop production yield. Aboukila et al. (2018) tested BSG in comparison to compost in calcareous soils cultivated with squash. Authors concluded that the application of BSG was more economical than compost and determined the best results in terms of soil pH reduction, soil water holding capacity and squash yield. No further studies have been conducted with the use of BSG as soil amendment, while in a recent review, Chetrariu and Dabija (2020) reported the use of BSG for obtaining a biochar to employ as nutrient supplier for plant growth and soil improver.
On the other hand, LAB can regulate the fate of phosphate in soil (Levering et al., 2012) or fix atmospheric nitrogen (Giassi et al., 2016), can act as biocontrol agent and increase the shelf life of the amendment (Cacace et al., 2022), therefore their use in agriculture is desirable.
Bioprocessing of the biomass is crucial to enable higher microbial stability of the spent grain while leading to the synthesis/release of compounds of interest. Indeed, the significant lower pH of bBSG compared to that of BSG, ascribed to the higher content of lactic and acetic acids and, to a lesser extent, to hydroxycinnamic acids released by the enzymatic treatment (Verni et al., 2020), prevented the proliferation of other microorganisms, either bacteria or molds, potentially spoiling the biomass. The great impact of the bioprocessing on the biomass shelf-life is an aspect particularly appealing in view of the potential large-scale application of brewers' spent grain as soil amendment. In addition, as previously reported, the sequential enzymatic/fermentative treatment also enabled the release of peptides having higher hydrophobic ratio than those in BSG, a feature that enhances their solubility in lipids, thus facilitating access to hydrophobic radical species and to hydrophobic polyunsaturated fatty acids (Sarmadi and Ismail, 2010; Verni et al., 2020). On the downside, bioprocessing determined a 70% decrease of FAA most likely metabolized by the LAB used as starter.
The partial mineralization of BSG, operated by microorganisms during the bioprocessing, could have determined the release of salts causing a slight but significant increase of the EC of bBSG compared to BSG. Further, the lower content of total P in bBSG compared to BSG was probably due to the interaction of phosphates with the surface and the stirrer of the bioreactor during the bioprocessing, leading to a certain removal of that nutrient from the biomass.
The biomasses slightly but significantly decreased the soil pH. In addition, soil in cultivated pots showed lower pH values than uncultivated soils, thus highlighting the plant rhizosphere contribution to the soil pH level. As expected, biomasses addition to the soil led to significantly higher content of OC and TN in treated soils compared to the unamended ones, regardless of the plant presence. The Pava content did not differ among pots without plants, even if the treated but uncultivated pots (BSGA and bBSGA) showed slightly (but not significantly) higher values of such parameter than the corresponding control (CTA). bBSG supplied less total P than BSG, but the Pava content of the corresponding amended soils was similar. This is possibly due to the abundant monocarboxylic acids production of the L. plantarum strains used as starter for bioprocessing, that could promote the solubilization of P. It is indeed generally accepted that P solubilization is also associated with the release of low molecular weight organic acids which, through their hydroxyl and carboxyl groups, chelate the cations bound to phosphate, thus converting it into soluble forms (Tabatabai, 1994). In contrast, the rhizosphere effect of the escarole roots induced a significantly higher availability of phosphate in cultivated pots (BSGP and bBSGP), probably due to the release of more suitable di- and tri-carboxylic organic acids and phosphatases (Brunetti et al., 2019). In any case, all treatments shared a very low Pava content (lower than 6.6 mg kg−1) that could have represented a limiting factor for the crop.
The first 25 days after transplantation were possibly needed for the rooting and establishment of the plants while, after this period, both BSG and bBSG supported similarly the crop due to their almost equal N contribution to the plant nutrition. Although the number of leaves did not differ among treatments, their fresh and dry weight was different, leading to the highest final yield when bBSG amendment was applied. The dry weight of escaroles was influenced by the application of biomasses since all treatments received the same amount of water during irrigations, but the amended pots retained more water precisely because of the organic amendments. The higher availability of water provided a better photosynthesis, as confirmed also by the SPAD readings, thus a greater accumulation of photosynthates. Nevertheless, other cultivars or crops could respond differently to the same treatments due to their different genotypes, hence more studies should be performed. It can be hypothesized that the bioprocessing played an important promotion of the decomposition and mineralization processes of BSG and/or stimulating PGPM of the rhizosphere microbial community. A great deal of this stimulation might be due the organic acids produced, during the bioprocessing, by the carbohydrates metabolism of L. plantarum PU1. Indeed, it was recently showed that lactic, oxalic, and citric acids are used as source of carbon and energy by soil microorganisms, confirmed by the increase in dehydrogenase and phosphatase activity (Macias-Benitez et al., 2020), bioindicator of soil fertility and phosphate bioavailability (Karaca et al., 2010; Navnage et al., 2018). Treating soils with organic acids, especially lactic acid, not only affects soil physicochemical performances but also induces changes in the soil microbiota composition favoring the proliferation of microorganisms (Bacillus spp. and Micrococcaceae) involved in soil degradation and fertility (Macias-Benitez et al., 2020). The authors observed that, once the lactic acid was degraded, although the biodiversity tended to return to phyla similar to those found before the treatment, an induction pattern of PGPM was left (Macias-Benitez et al., 2020).
The similar P content found in leaves from all treatments is probably due to the low availability of such nutrient in the soils, while the similar content of Cu, Fe and Mn in leaves can be ascribed to their nature as micronutrients (even their level in control soil satisfied the plant nutrition). Although similar biometric parameters of escarole plants were found between amended and unamended soils, from the 27th day of treatment onward, BSG and bBSG prompted to a better chlorophyll content compared to unamended soils. Such effect, most likely caused by the higher TN content of amended soils, was similar to that previously found in escarole cultivated in soil amended with wasted bread, used as such or bioprocessed with amylolytic enzymes and lactic acid bacteria (Cacace et al., 2022).
Generally, the application of plant- or animal-based organic amendment residues is known to increase soil enzymatic activities with a crucial role in C (β-glucosidase and β-galactosidase), N (urease), P (phosphatases), and S (sulphatase) cycles and are also used as quality indicators for pollution, ecosystem perturbations, and agricultural practice (Karaca et al., 2010). Still, the contribution of microbial enzymes brought about by bBSG cannot be excluded as a factor influencing soil biochemical and microbial properties. As a matter of fact, during BSG bioprocessing, the environmental pressure exerted by the low availability of energy sources shifts L. plantarum phenotype toward the metabolism of arabinose and xylose and increases the expression of genes encoding for cellobiose metabolism (Acin-Albiac et al., 2022), all of which could be of great importance in the degradation of fibrous material in soil as well as the ability of the strain to adapt to soil conditions and keep exerting beneficial functions long after amendment practice. Moreover, the intense metabolic activity of β-glucosidases, whose genes are present in high redundance in LAB genomes, has been negatively correlated with soil heavy metals (Karaca et al., 2010) suggesting that these enzymes, involved in the degradation of carbohydrates as well as in the release of a wide range of phenolic compounds in BSG (Acin-Albiac et al., 2022), might be a key element to fight soil pollutants.
In conclusion, the use of brewers' spent grains as soil amendment determined higher yield of escarole compared to the unamended soil, especially when the biomass was previously subjected to bioprocessing. Overall, brewers' spent grains supplied organic matter and total nitrogen to soils, improving their fertility. The acidic nature of this biomass, especially when subjected to lactic acid fermentation, can improve alkaline soils increasing the solubility of nutrients. In contrast, the use of these biomasses is not recommended in acid soils, because can determine an excessive availability of potentially toxic elements and an excessive presence of Al deriving from mineral weathering. Although further investigation on the agronomical responses of other cultivars or plants, as well as LAB survival in amended soil, their ability to modulate soil microbiota, or their potential in chelating heavy metals or other soil pollutant are needed, the results collected in this preliminary study encourage its application for agricultural purpose, also solving the problem of disposing of such residues widely produced all over the world.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
CGR, CCo, and GB contributed to the conception and design of the study. CCa and MV performed the laboratory analyses. AT and FD conducted the samplings and participated in the analysis. CCa wrote the first draft of the manuscript and CGR, CCo, MV, RC, and EP revised it. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AACC (2010). Approved methods of the American Association of Cereal Chemistry, 11th edn. St. Paul, MN: AACC.
Abdeldaym, E. A., Traversa, A., Cocozza, C., and Brunetti, G. (2018). Effects of a 2-year application of different residual biomasses on soil properties and potato yield. Clean 46, 1800261. doi: 10.1002/clen.201800261
Abdelrahman, H., Cocozza, C., Olk, D. C., Ventrella, D., Montemurro, F., and Miano, T. (2020). Changes in labile fractions of soil organic matter during the conversion to organic farming. J. Soil Sci. Plant Nutr. 20, 1019–1028. doi: 10.1007/s42729-020-00189-y
Aboukila, E. F., Nassar, I. N., Rashad, M., Hafez, M., and Norton, J. B. (2018). Reclamation of calcareous soil and improvement of squash growth using brewers' spent grain and compost. J. Saudi Soc. Agric. Sci. 17, 390–397. doi: 10.1016/j.jssas.2016.09.005
Acin-Albiac, M., Filannino, P., Coda, R., Rizzello, C. G., Gobbetti, M., and Di Cagno, R. (2022). How water-soluble saccharides drive the metabolism of lactic acid bacteria during fermentation of brewers' spent grain. Microb. Biotechnol. 15, 915–930. doi: 10.1111/1751-7915.13846
Allen, R. G., Pereira, L. S., Raes, D., and Smith, M. (1998). Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements—FAO Irrigation and Drainage Paper 56. Rome: FAO.
Ameen, F. A., Hamdan, A. M., and Moustafa, Y. E. (2020). Assessment of the heavy metal bioremediation efficiency of the novel marine lactic acid bacterium, Lactobacillus plantarum MF042018. Sci. Rep. 10, 314. doi: 10.1038/s41598-019-57210-3
Bianco, A., Budroni, M., Zara, S., Mannazzu, I., Fancello, F., and Zara, G. (2020). The role of microorganisms on biotransformation of brewers' spent grain. Appl. Microbiol. Biotechnol. 104, 8661–8678. doi: 10.1007/s00253-020-10843-1
Brunetti, A., Traversa, A., De Mastro, F., and Cocozza, C. (2019). Short term effects of synergistic inorganic and organic fertilization on soil properties and yield and quality of plum tomato. Sci. Hortic. 252, 342–347. doi: 10.1016/j.scienta.2019.04.002
Cacace, C., Rizzello, C. G., Brunetti, G., Verni, M., and Cocozza, C. (2022). Reuse of wasted bread as soil amendment: bioprocessing, effects on alkaline soil and escarole (Cichorium endivia) production. Foods 11, 189. doi: 10.3390/foods11020189
Chetrariu, A., and Dabija, A. (2020). Brewer's spent grains: possibilities of valorization, a review. Appl. Sci. 10, 5619. doi: 10.3390/app10165619
Church, F. C., Swaisgood, H. E., Porter, D. H., and Catignani, G. L. (1983). Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 66, 1219–1227. doi: 10.3168/jds.S0022-0302(83)81926-2
Ciavatta, C., Antisari, L. V., and Sequi, P. (1989). Determination of organic carbon in soils and fertilizers. Commun. Soil Sci. Plant Anal. 20, 759–773. doi: 10.1080/00103628909368115
Cocozza, C., and Ercolani, G. L. (1997). Siderophore production and associated characteristics in rhizosphere and non-rhizosphere flourescent pseudomonads. Ann. Microbiol. 47, 17–28.
De Pasquale, I., Verni, M., Verardo, V., Gómez-Caravaca, A. M., and Rizzello, C. G. (2021). Nutritional and functional advantages of the use of fermented black chickpea flour for semolina-pasta fortification. Foods 10, 182. doi: 10.3390/foods10010182
De Vos, B., Lettens, S., Muys, B., and Deckers, J. A. (2007). Walkley–Black analysis of forest soil organic carbon: recovery, limitations and uncertainty. Soil Use Manag. 23, 221–229. doi: 10.1111/j.1475-2743.2007.00084.x
Giassi, V., Kiritani, C., and Kupper, K. C. (2016). Bacteria as growth – promoting agents for citrus rootstocks. Microbiol. Res. 190, 46–54. doi: 10.1016/j.micres.2015.12.006
IUSS Working Group WRB (2015). “World Reference Base for Soil Resources,” in International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports No. 106 (Rome: FAO).
Karaca, A., Cetin, S. C., Turgay, O. C., and Kizilkaya, R. (2010). “Soil enzymes as indication of soil quality,” in Soil enzymology ed. G. Shukla, A. Varma (Berlin: Springer), pp. 119–148.
Kargar, S. H. M., and Shirazi, N. H. (2020). Lactobacillus fermentum and Lactobacillus plantarum bioremediation ability assessment for copper and zinc. Arch. Microbiol. 202, 1957–1963. doi: 10.1007/s00203-020-01916-w
Koirala, P., Costantini, A., Maina, H. N., Rizzello, C. G., Verni, M., Beni, V. D., et al. (2022). Fermented brewers' spent grain containing dextran and oligosaccharides as ingredient for composite wheat bread and its impact on gut metabolome in vitro. Fermentation 8, 87. doi: 10.3390/fermentation,8100487
Lamont, J. R., Wilkins, O., Bywater-Ekegärd, M., and Smith, D. L. (2017). From yogurt to yield: Potential applications of lactic acid bacteria in plant production. Soil Biol. Biochem. 111, 1–9. doi: 10.1016/j.soilbio.2017.03.015
Levering, J., Musters, M. W. J. M., Bekker, M., Bellomo, D., Fiedler, T., de Vos, W. M., et al. (2012). Role of phosphate in the central metabolism of two lactic acid bacteria—a comparative systems biology approach. FEBS J. 279, 1274–1290. doi: 10.1111/j.1742-4658.2012.08523.x
Lynch, K. M., Steffen, E. J., and Arendt, E. K. (2016). Brewers' spent grain: a review with an emphasis on food and health. J. Inst. Brewing 122, 553–568. doi: 10.1002/jib.363
Macias-Benitez, S., Garcia-Martinez, A. M., Caballero Jimenez, P., Gonzalez, J. M., Tejada Moral, M., and Parrado Rubio, J. (2020). Rhizospheric organic acids as biostimulants: monitoring feedbacks on soil microorganisms and biochemical properties. Front. Plant Sci. 11, 633. doi: 10.3389/fpls.2020.00633
Mbagwu, J. S. C., and Ekwealor, G. C. (1990). Agronomic potential of brewers' spent grains. Biol. Wastes 34, 335–347. doi: 10.1016/0269-7483(90)90034-P
Mininni, C., Grassi, F., Traversa, A., Cocozza, C., Parente, A., Miano, T., et al. (2015). Posidonia oceanica (L.) based compost as substrate for potted basil production. J. Sci. Food Agric. 95, 2041–2046. doi: 10.1002/jsfa.6917 https://doi.org/10.1002/jsfa.6917
Mussatto, S. I., Dragone, G., and Roberto, I. C. (2006). Brewers' spent grain: generation, characteristics and potential applications. J. Cereal Sci. 43, 1–14. doi: 10.1016/j.jcs.2005.06.001
Navnage, N. P., Patle, P. N., and Ramteke, P. R. (2018). Dehydrogenase activity (DHA): measure of total microbial activity and as indicator of soil quality. Int. J. Chem. Stud 6, 456–458.
Olsen, S. R. (1954). Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate (No. 939). Washington, DC: US Department of Agriculture.
Ozores-Hampton, M., Stansly, P. A., and Salame, T. P. (2011). Soil chemical, physical, and biological properties of a sandy soil subjected to long-term organic amendments. J. Sustain. Agric. 35, 243–259 doi: 10.1080/10440046.2011.554289
Ravindran, R., and Jaiswal, A. K. (2016). Exploitation of food industry waste for high-value products. Trends Biotechnol. 34, 58–69. doi: 10.1016/j.tibtech.2015.10.008
Rizzello, C. G., Nionelli, L., Coda, R., De Angelis, M., and Gobbetti, M. (2010). Effect of sourdough fermentation on stabilisation, and chemical and nutritional characteristics of wheat germ. Food Chem. 119, 1079–1089. doi: 10.1016/j.foodchem.2009.08.016
Sarmadi, B. H., and Ismail, A. (2010). Antioxidative peptides from food proteins: a review. Peptides 31, 1949–1956. doi: 10.1016/j.peptides.2010.06.020
Schettino, R., Verni, M., Acin-Albiac, M., Vincentini, O., Krona, A., Knaapila, A., et al. (2021). Bioprocessed brewers' spent grain improves nutritional and antioxidant properties of pasta. Antioxidants 10, 742. doi: 10.3390/antiox10050742
Sparks, D. L., Page, A. L., Helmke, P. A., Loeppert, R. H., Soltanpour, P. N., Tabatabai, M. A., et al. (1996). “Methods of soil analysis,” in Chemical Methods. (Madison, WI: SSSA Book).
Tabatabai, K. A. K. (1994). Effect of organic acids on release of phosphorus from phosphate rocks. Soil Sci. 158, 442–453.
Trinchera, L., Leita, P., and Sequi, P. (2006). Metodi di Analisi per I Fertilizzanti. Roma : Ministero delle Politiche Agricole Alimentari e Forestali.
Verni, M., Pontonio, E., Krona, A., Jacob, S., Pinto, D., Rinaldi, F., et al. (2020). Bioprocessing of brewers' spent grain enhances its antioxidant activity: characterization of phenolic compounds and bioactive peptides. Front. Microbiol. 11, 1831. doi: 10.3389/fmicb.2020.01831
Verni, M., Rizzello, C. G., and Coda, R. (2019). Fermentation biotechnology applied to cereal industry by-products: nutritional and functional insights. Front. Nutr. 6, 42. doi: 10.3389/fnut.2019.00042
Weiss, W., Vogelmeier, C., and Görg, A. (1993). Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers' asthma. Electrophoresis 14, 805–816. doi: 10.1002/elps.11501401126
Yoo, J. H., Luyima, D., Lee, J. H., Park, S. Y., Yang, J. W., An, J. Y., et al. (2021). Effects of brewer's spent grain biochar on the growth and quality of leaf lettuce (Lactuca sativa L. var. crispa.). Appl. Biol. Chem. 64, 10. doi: 10.1186/s13765-020-00577-z
Keywords: organic waste, sustainable agriculture, brewers' spent grain, lactic acid bacteria, soil amendment
Citation: Cacace C, Cocozza C, Traversa A, Coda R, Rizzello CG, Pontonio E, De Mastro F, Brunetti G and Verni M (2022) Potential of native and bioprocessed brewers' spent grains as organic soil amendments. Front. Sustain. Food Syst. 6:1010890. doi: 10.3389/fsufs.2022.1010890
Received: 03 August 2022; Accepted: 04 November 2022;
Published: 24 November 2022.
Edited by:
Claudio Bellia, University of Catania, ItalyReviewed by:
Zina Flagella, Università degli Studi di Foggia, ItalyCopyright © 2022 Cacace, Cocozza, Traversa, Coda, Rizzello, Pontonio, De Mastro, Brunetti and Verni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michela Verni, bWljaGVsYS52ZXJuaUB1bmliYS5pdA==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.