
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 10 November 2022
Sec. Sustainable Food Processing
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.1006467
This article is part of the Research TopicSustainable Food Systems in Ibero-AmericaView all 5 articles
Vegetable oil extraction generates high amounts of by-products, which are designated as oil cakes. Since the current strategies employed for oil cakes' reuse are linked with some drawbacks, identification of alternative approaches to decrease the environmental impact and promote a circular economy is of vital importance. In general, these materials are characterized by high fiber content, making them suitable to be employed in solid state fermentation (SSF). Filamentous fungi have been the microorganisms mostly applied in SSF and yeasts were applied in less extent. In the present work, three by-products from the extraction of olive, sunflower, and rapeseed oils were used as solid substrates in SSF for lipase and protease production by Yarrowia lipolytica W29. Oil cakes mixtures composition was optimized for the production of each enzyme using a simplex-centroid design of experiments. A 50% (w/w) mixture of olive cake (OC) and sunflower cake (SC) led to the highest lipase production, while a combination of the three oil cakes was most suitable for maximum protease production. Both enzymes were produced at maximum levels in a short period of 48 h. This work demonstrated that enzyme production by Y. lipolytica W29 in SSF can be modulated by the different combinations of oil cakes in the substrate mixture. Additionally, the potential of using by-products from vegetable oil industries in SSF processes was also demonstrated, showing alternative strategies for their valorization.
Oleaginous crops are highly cultivated worldwide and their production reached 500 million tons between 2018 and 2020. Moreover, an increase in the production is expected, reaching 600 million tons by 2030 (OECD-FAO, 2021). These crops are mainly used for oil extraction, which can be used for human consumption and biodiesel production (Waseem et al., 2017). The oil extraction generates large amounts of solid by-products, named oil cakes, and the great demand for vegetable oil production in the next decade will certainly result in increased production of these by-products (OECD-FAO, 2019; Singh et al., 2022).
Oil cakes obtained after rapeseed and sunflower oils extraction, two of the most oilseed crops produced, can be employed as animal feed since these by-products are rich in protein (Lomascolo et al., 2012). However, the presence of antinutritional factors, such as tannins, phytic acid, and glucosinolates, may have a negative impact in animal health and growth performance. In contrast, olive oil extraction results in the production of olive cake (OC) with low protein content (Leite et al., 2016). These materials are mainly used for energy production by combustion owing to their high calorific value and are often submitted to a drying process and solvent extraction processes that have a negative environmental impact (Christoforou and Fokaides, 2016). Therefore, more eco-friendly options should be explored. Olive cakes are a source of value-added compounds and several green techniques have been used for their extraction (Gullón et al., 2020). Though OC is not usually utilized as animal feedstuff due to its low protein and high fiber content, there are some studies attempting their incorporation in animal feed formulations (Joven et al., 2014; Abid et al., 2020; Neofytou et al., 2020). Regardless of the oil cakes applications, these by-products are still unexploited and other approaches for their up-grading must be evaluated. Due to their composition, oil cakes can act as a substrate in solid state fermentation (SSF) processes for their biotransformation and the production of bioactive compounds, such as enzymes. However, single oil cakes may not have the suitable nutritional composition to allow optimal microbial growth and target compounds production. Thus, mixing oil cakes from different sources may lead to optimal composition for SSF.
Lipases, which are enzymes that can be obtained by SSF of oil cakes, are involved in several reactions, such as esterification, transesterification, and aminolysis of lipids, hydrolysis of triacylglycerols, and synthesis of esters. Their versatility in terms of applications, such as in food industry, wastewater treatment, pharmaceuticals, cosmetics, leather processing, and biodiesel production, increased the interest in the production of these enzymes (Salihu et al., 2012; Navvabi et al., 2018). Another type of enzyme with industrial relevance is protease, which is responsible for the hydrolysis of peptide bonds from proteins and polypeptides. This enzyme is mostly used in the detergent, pharmaceutical, and food industries (Razzaq et al., 2019).
Yarrowia lipolytica is a non-conventional yeast naturally found in environments rich in fats and/or proteins, such as dairy products (Gonçalves et al., 2014). This oleaginous yeast is broadly used in biotechnological processes to produce enzymes, surfactants, aromas, erythritol, and lipids (Lopes et al., 2022). Most of these studies were performed in submerged fermentation, however, it the recent years, a growing interest arose in the application of Y. lipolytica in SSF processes. The ability of Y. lipolytica to grow under mycelium form constitutes an advantage for SSF since the formation of hyphae can increase substrate colonization and nutrients assimilation, resulting in higher microbial growth and production rates. Lipase has been successfully produced by this yeast using, as solid substrates, palm kernel (Imandi et al., 2010), mustard oil (Imandi et al., 2013), cottonseed (Farias et al., 2014) and canola (Souza et al., 2017) cakes, and soybean meal (Souza et al., 2017). In most cases, medium supplementation was needed for lipase production. Though two studies reported the production of lipase using by-products from olive oil extraction (Moftah et al., 2013; Lopes et al., 2016), to our knowledge, rapeseed cake (RC) and sunflower cake (SC) have not yet been explored as solid substrates for the production of this enzyme by Y. lipolytica. Despite some reports of protease production by Y. lipolytica in submerged fermentation and its application in milk proteins hydrolysis (Dabrowska et al., 2020) and biopeptide synthesis (Pokora et al., 2017), its production in SSF is still underexplored.
In this work, the potential of using OC, SC, and RC as solid substrates for lipase and protease production by Y. lipolytica was evaluated. A simplex centroid mixture design was used to obtain the optimum oil cake mixture for the production of these enzymes. Additionally, characterization of the remaining solid substrate after enzyme extraction was performed to evaluate its potential use in other industries, promoting a circular economy.
Yarrowia lipolytica W29 (ATCC 20460) were stored at −80°C in 30% glycerol solution and revived in YPD agar medium (glucose 20 g/L, peptone 20 g/L, yeast extract 10 g/L, agar 20 g/L). For inoculum preparation, yeast cells from an agar plate were cultivated overnight in 100 mL of YPD (glucose 20 g/L, peptone 20 g/L, yeast extract 10 g/L) medium in 500-mL Erlenmeyer flasks in a rotary shaker at 200 rpm and 27°C.
Rapeseed cake and sunflower cake, acquired in dry conditions from a Portuguese vegetable oil production industry (Iberol SA), were milled and stored at room temperature. Olive cake, collected in wet conditions after olive oil extraction in a two-phase olive mill of the Portuguese northern region (Achsula SA), was stored at −18°C owing to its high moisture content.
Moisture content and ashes were determined by drying the oil cakes at 105°C for 24 h and 550°C for 2 h, respectively, according to the standard methods from AOAC (2005). The quantification of lipids was performed gravimetrically after extraction with chloroform and methanol (2:1, v/v) as described by Ferreira et al. (2020). Total nitrogen was measured by the Kjeldahl method, which was converted to crude protein content by using a factor of 6.25. Organic carbon was quantified using a Thermo Finnigan Flash Element Analyzer 1112 (San Jose, CA USA). Quantification of cellulose, hemicellulose, and lignin was performed as described by Leite et al. (2016). Acid detergent fiber (ADF) was calculated using the percentages of hemicellulose and lignin. The values of hemicellulose, lignin, and cellulose were used to determine neutral detergent fiber (NDF).
A simplex centroid mixture design was used to select the best mixture of oil cakes for lipase and protease production by Y. lipolytica W29. The components of the mixture, OC, RC, and SC, were studied in four levels: 0%, 33.3%, 50%, and 100% (w/w) and nine runs were performed with two replicates of the central point. The proportions of oil cakes in each run are presented in Table 2.
The following equation is a representation of the model that best fitted the experimental results:
Where Y represents the predicted response, β is the regression coefficients of the model, and x is the independent variables.
Fermentations were carried in 500-mL Erlenmeyer flasks containing 10 g of dry solid substrate. Distilled water was added to moist the substrates prior to sterilization at 121°C for 15 min. The solid substrate was inoculated with 2 mL (3.8 mg of cells per g of solid substrate) of inoculum suspension leading to a moisture content of 75% (wet basis) and incubated at 27°C for 2 days.
Using the best substrate composition, fermentations were performed to evaluate the enzymes production over time by sampling all fermented material in one flask each day. Autoclaved solid substrate without inoculation was used as control and was extracted as described for SSF experiments.
For enzymes extraction, a solution of 10 g/L NaCl and 5 g/L Triton X-100 was added to the fermented solid substrate in a solid/liquid ratio of 1:5 (g:mL). After 30 min of agitation in a rotary shaker at 200 rpm and room temperature, a liquid extract was obtained by pressing and a sample of the liquid extract was collected for cell counting. This step was followed by centrifugation at 8,000 rpm and 4°C for 10 min. The supernatant was stored at −18°C. The remaining solid was dried and stored at room temperature for further analysis.
The aqueous extracts were characterized in terms of cellular density, lipase and protease activity, reducing sugars, and soluble proteins concentration.
Cellular density was determined by cell counting in an Olympus BX51 microscope. The cell number was converted to dry cell mass per gram of dry substrate using the correspondent conversion factor.
Lipase activity measurement was based on a spectrophotometric assay using 4-nitrophenyl butyrate as substrate. Briefly, the enzymatic extract was incubated with 4-nitrophenyl butyrate in sodium acetate buffer 50 mM (pH 5.6) during 15 min at 37°C. Afterwards, the reaction was stopped by the addition of acetone and 4-nitrophenol release was measured at 405 nm. One unit of lipase activity (U) was defined as the amount of enzyme that produced 1 μmol of 4-nitrophenol per minute. Lipase activity was expressed in units per gram of dry substrate (U/g).
Protease activity was determined with a spectrophotometric method based on the reaction of the enzymatic extract with 5 g/L azocasein (Sigma) in sodium acetate buffer 50 mM (pH 5). After the incubation for 40 min at 37°C, 100 g/L trichloroacetic acid was added followed by centrifugation (3,000 rpm, 15 min). Potassium hydroxide 5 M was added to the supernatant and the absorbance was measured at 428 nm. One unit of protease activity was defined as the amount of enzyme that produced an increase of 0.01 of absorbance relatively to the blank per minute. Protease activity was expressed in units per gram of dry substrate (U/g). Total phenols were quantified using the Folin-Ciocalteau method as described by (Sousa et al., 2022) and gallic acid was used as a standard. Total phenols were expressed in mg of phenols per gram of dry substrate (mg/g).
Reducing sugars were determined using the dinitrosalicylic (DNS) acid reagent method (Miller, 1959) and glucose was used as a standard. Reducing sugars were expressed in mg of reducing sugar per gram of dry substrate (mg/g).
Soluble protein was determined using the Bradford method (Bradford, 1976) and Bovine serum albumin (BSA) was used as a standard. Protein content was expressed in mg of protein per gram of dry substrate (mg/g). The ratio between lipase activity and soluble protein was used to calculate lipase specific activity, which was expressed in units of lipase activity per mg of soluble protein (U/mg).
The experimental design analysis was performed using the Statgraphics Centurion XVI software. The experimental data were statistically evaluated using GraphPad Prism. Data were subjected to one-way analysis of variance (ANOVA) and Tukey's test for multiple comparison. Analysis was performed with a confidence interval of 95%.
The selection of a solid substrate for SSF, must take into account the target compound that will be produced since the substrate composition can affect their production. In the present work, oil cakes, that have residual lipids in their composition and that are generated from olive, rapeseed, and sunflower oils extraction were used alone or in combination, leading to different substrate composition. The characterization of each oil cake is presented in Table 1. The OC used in this work was obtained in a two-phase extraction system, being mainly composed of olive pulp, skins, and water. It also presents a high moisture content, which is related to the use of a two-phase process for oil extraction (Dermeche et al., 2013; Gullón et al., 2020). Additionally, this by-product has the highest lipid percentage among the three oil cakes. The fact that rapeseed and sunflower oils are extracted with solvents and olive oil extraction is performed only from mechanical processes may explain these differences in the residual oil content. While a similar carbon content was detected in OC and RC, the carbon percentage in SC is slightly lower. Moreover, OC has a lower nitrogen percentage than the others, resulting in the highest C/N ratio for this oil cake (84 for OC and 7 for SC and RC). Crude protein content in OC is 10-fold lower than the values for SC and RC. Some studies reported that a low C/N ratio is needed for biomass production in Y. lipolytica (Hapeta et al., 2020) and for lipase production in filamentous fungi (Lima et al., 2003; Rigo et al., 2009). By contrast, synthesis and accumulation of some biocompounds (e.g., microbial lipids by Y. lipolytica) are improved by high C/N ratio (Morin et al., 2011). Thus, C/N ratio is an important parameter to control for the production of target bioactive compounds. This can be achieved by medium supplementation, which can often lead to higher production costs, or by the mixture of agro-industrial by-products with different chemical composition. As reported in the literature (Molina-Alcaide and Yáñez-Ruiz, 2008), OC has high fiber content, having the highest percentage among the three oil cakes used in this work.
The differences regarding oil cakes' chemical composition described so far may be associated with the fruit or seed that originated the oil cake and the method used for oil extraction (Ramachandran et al., 2007). Moreover, with the mixture of oil cakes at different proportions, it is possible to modulate substrate composition, which can affect microbial growth and biocompounds production.
The utilization of a simplex centroid design allowed the identification of the optimum substrate mixture to obtain the maximum lipase and protease activities after 2 days of SSF (Table 2).
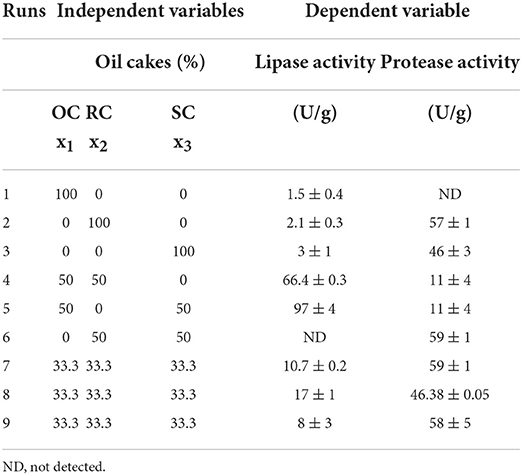
Table 2. Substrate composition for each run of SSF experiments and results of the dependent variables studied in simplex centroid design.
Besides the quantification of enzymatic activity, cellular density in the extracts obtained in each experiment was also measured to estimate the influence of oil cakes in yeast growth. Figure 1 shows the cellular density obtained in the experiments as a function of the percentage of OC in the substrate mixture. The lowest cellular density was obtained when OC was used as the single component of the solid substrate. Studies using Y. lipolytica (Lopes et al., 2016) and filamentous fungi (Salgado et al., 2014) in SSF showed similar results when using this oil cake as the single solid substrate. In spite of the higher content in phenolic compounds of OC in comparison with the other oil cakes, this difference may not explain the lowest cell growth, which is most related with the low nitrogen percentage in the oil cake (Table 1). Thereby, lowering the percentage of OC in the substrate mixture led to an increase in cellular density. This result demonstrates that mixture of oil cakes is a suitable strategy for the revalorization of by-products from olive oil production. The highest cellular density was obtained when SC and RC were used alone or in binary mixtures, showing that these by-products are more suitable for Y. lipolytica growth than OC.
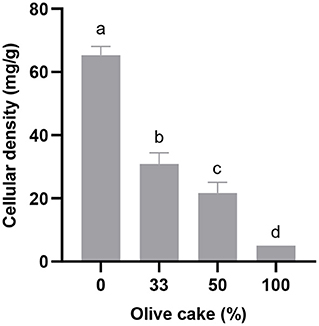
Figure 1. Cellular density obtained after 2 days of SSF as a function of the percentage of olive cake in the substrate mixture. Bars with the same letter are not significantly different (p > 0.05).
It has been reported that the presence of lipids in the culture medium induces lipase production (Gonçalves et al., 2014). However, a reduced lipase activity was detected when OC was used as the single component of the solid substrate since yeast growth was low in these conditions (Figure 1). The mixture of OC with the other oil cakes improved lipase activity, especially in binary mixtures. As previously mentioned, this combination improved cell proliferation and the residual oil present in OC led to an increase in lipase activity. Similarly, Farias et al. (2014) observed that residual oil of cottonseed cake was essential to attain high values of lipase production by Y. lipolytica IMUFRJ 50682. Despite the high cellular density attained when SC and RC were used alone or in binary mixtures, low lipase activity was observed. This result could be related with the low residual oil in these substrates as well as the release of proteases to the medium that may lead to other enzymes hydrolysis (Ogrydziak, 2013).
In fact, Y. lipolytica also presents proteolytic activity, being able to synthesize extracellular proteases (Ogrydziak, 2013). The presence of OC alone or in binary mixtures resulted in low protease production. In contrast, high protease activity was detected in the presence of RC and SC in the substrate, either alone, in the binary mixture with these two oil cakes and in ternary mixtures. It has been reported that protease synthesis is induced in protein-rich media (Ogrydziak, 2013; de Castro et al., 2015) and Matkawala et al. (2008) observed a positive correlation between protease production by Aspergillus niger and the protein content in the SSF substrates. Similarly, in this study, protease production by Y. lipolytica W29 was improved by the increase of protein content in the solid substrate, that is the case of RC and SC addition in the mixture.
The Equations (2) and (3) represent the mathematical models obtained in the simplex centroid mixture design for each enzyme.
The regression coefficients values are directly proportional to the impact that the independent variables have on enzyme activity. Likewise, the behavior of these variables individually or in combination can be examined through these equations. Oil cakes alone had a reduced impact on lipase activity and the use of binary mixtures with OC had a significant positive effect on the production of this enzyme, particularly for the mixture of OC and SC. In contrast, the negative values in the binary mixture with RC and SC and in the ternary mixture revealed an antagonist effect on lipase activity, being the latter term significant at a 5 % level. Regarding the second equation, linear terms had a positive effect, except OC, which had no influence on protease activity. Binary mixture containing OC had an antagonist effect while the binary mixture with SC and RC as well as the ternary mixtures contributed positively for protease activity.
Through variance analysis, a coefficient of determination (R2) of 0.99 was obtained for lipase activity, which suggests a good fit of the model. Additionally, the p-value obtained is lower than 0.05 (p = 0.0341), indicating that there is a statistically significant relation, at a 5 % level, between lipase activity and the components of the mixture. Concerning protease activity, a high R2 was also obtained (0.98) and the p-value was 0.0635, which revealed that this model is significant at 10% level. Likewise, these results showed that these models can be used for predictive purposes.
The mixture contour plots (Figure 2) show the estimated response of lipase and protease activities predicted by the model with the different mixtures of oil cakes. The corners of the triangle represent the components of the mixture and every point inside the triangle corresponds to the enzyme's activities predicted with different proportions of oil cakes in the substrate mixture. The regions colored in red represent the substrate composition that led to the maximum enzyme production. According to the plot in Figure 2A, highest lipase activity can be achieved when SC and OC are the main components of the mixture. Moreover, the model predicted that lipase activity reaches its maximum, 97 U/g, with a mixture of OC and SC (1:1, w/w). On the other hand, it appears that highest protease activity is reached with a combination of the three by-products (Figure 2B). In particular, a mixture of OC (12%), SC (41%), and RC (47%) led to the optimum protease activity, 64 U/g. Likewise, combination of different agro-industrial by-products with OC appears to be a suitable approach for microbial growth and bioactive compounds production, allowing its revalorization by SSF. Lopes et al. (2016) observed an improvement of lipase activity with the mixture of OC with wheat bran. Similarly, a combination of OC and winery by-products led to higher production of lipase (Salgado et al., 2014) and lignocellulosic enzymes (Filipe et al., 2020). The presence of OC in binary mixtures is essential to attain high lipase activity, while an antagonist effect is observed in these conditions for protease production. Likewise, it appears that the production of lipase and protease can be modulated by the percentage of OC in the solid substrate. The results obtained in the present work demonstrate that different substrate combinations are required for the optimum production of lipase or protease by Y. lipolytica. Thus, changing the substrate composition, allows to increase the production of one enzyme over another, facilitating the downstream purification processes. Furthermore, is possible, at any time, to change the substrate composition to attain the maximum lipase or protease production, depending on by-products availability or market demands, which is in line with the biorefinery concept.
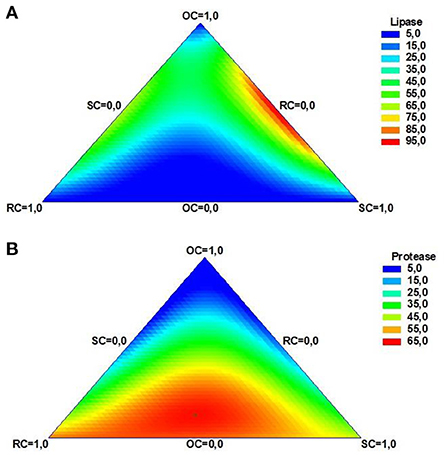
Figure 2. Contour plots for the dependent variables obtained in the simplex centroid mixture design. (A) Lipase and (B) protease.
Incubation time is an important parameter to optimize when establishing a SSF process for enzymes production. Hence, after the selection of the best substrate mixture using as a standard operation time of 2 days, SSF of the mixture of 50% (w/w) OC and SC was performed for 4 days to evaluate the profile of lipase and protease production throughout the time. A five-fold increase in cellular density was attained after 1 day of cultivation, reaching 26 mg of cellular dry mass per gram of dry substrate in the end of the experiments (Figure 3A). The increase of cellular density was accompanied by a decrease of reducing sugars in the first 2 days and a residual value was maintained until the end of the SSF process (Figure 3A). The total sugars depletion was not observed since Y. lipolytica is unable to assimilate some monosaccharides, such as xylose, which is included in the hemicellulose fraction of oil cakes. Lipase activity increased in the first day, reaching a maximum of (102 ± 17) U/g in the second day (Figure 3B), which validates the selection of 2 days of SSF in the experimental design. Moreover, a specific activity of 3.9 U/mg of total soluble protein was obtained at this point. Lipase production in oily substrates is essential to convert triacylglycerols into free fatty acids that can be incorporated by the cells (Lopes et al., 2022). After being bounded to the yeast cell wall, lipase is gradually released into the medium, increasing the contact between the enzyme and substrate and nutrients assimilation. Lipase secretion begins during the transition to the stationary growth phase when the carbon source is reaching limiting concentrations (Pereira-Meirelles et al., 2000). In this work, maximum lipase activity was observed at the second day of SSF where the cellular growth was already stabilized (Figure 3A). Similar lipase activity values were obtained using soybean meal (Souza et al., 2017), a mixture of OC and wheat bran (Lopes et al., 2016), and canola cake (Souza et al., 2017) as solid substrates after 10, 24, and 28 h, respectively, in SSF processes using Y. lipolytica IMUFRJ 50682. Moreover, Farias et al. (2014) obtained (46 ± 1) U/g after 14 h of fermentation with Y. lipolytica IMUFRJ 50682 using soybean cake supplemented with soybean sludge. In the same work, the potential of cottonseed cake as a SSF substrate was also assessed and, after 28 h, a maximum lipase activity of (50 ± 1) U/g was attained. In contrast, other authors reported longer incubation times to reach maximum enzyme production by Y. lipolytica. For instance, 4 days was the optimum incubation time for lipase production by Y. lipolytica NCIM 3589, reaching 18.58 U/g with palm kernel cake as solid substrate (Imandi et al., 2010). The same incubation time was selected in SSF with Y. lipolytica NCIM 3589 using mustard oil cake supplemented with glucose and urea (Imandi et al., 2013) and with Y. lipolytica NRRL Y-1095 using OC submitted to an alkaline pre-treatment (Moftah et al., 2013), leading to a lipase activity of 57.89 and 40 U/g, respectively. In the present work, high values of lipase activity were obtained in a short period of time and without any medium supplementation, which improves enzyme productivity and reduces the production costs of the process. Additionally, these results show the potential of using by-products from olive oil production in combination with SC for lipase production.
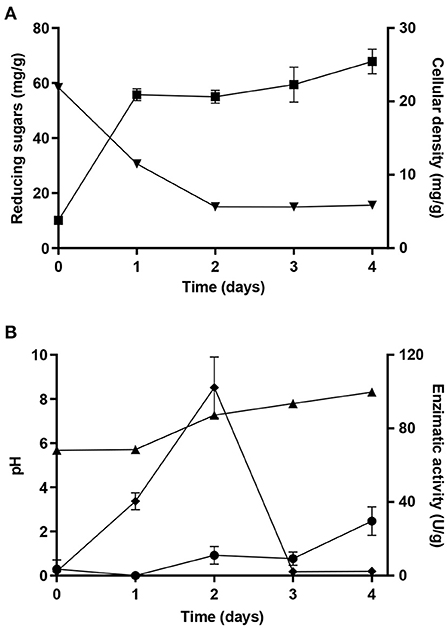
Figure 3. (A) Cellular density (■), reducing sugars concentration (▾), (B) enzymatic activity of lipase (♦) and protease (•), and pH (▴) obtained during SSF with Y. lipolytica W29 for 4 days with the optimum substrate mixture for lipase production.
After the maximum value of lipase activity was reached, it abruptly decreased until the end of the fermentation. Similar results were obtained during lipase production by Y. lipolytica NRRL Y-1095 under SSF using OC (Moftah et al., 2013) and a mixture of OC and wheat bran (Lopes et al., 2016) as solid substrates with Y. lipolytica IMUFRJ 50682. To understand the reduction on lipase activity after the second day, protease activity and pH values were also examined. As can be observed in Figure 3B, when lipase activity reached its maximum value, low protease activity was attained. However, in the fourth day of cultivation, when lipase production was very low, an increase in protease activity was observed. Braga et al. (2012) observed an abrupt reduction on lipase activity, which was followed by an increase of protease production. Moreover, a similar enzymatic activity profile was obtained during SSF using canola cake as the solid substrate (Souza et al., 2017). The increase in pH values and the release of alkaline proteases to the fermentation medium may explain the decrease in lipase production since lipases can be degraded by proteolysis.
The kinetics of protease activity by SSF was also evaluated, with the mixture of the three oil cakes that led to the highest protease production. The yeast cellular density reached (73 ± 8) mg per gram of dry substrate at the end of the experiments (Figure 4A). Despite the lower reducing sugars concentration in this substrate mixture, cellular density was approximately three-fold higher than that observed in the mixture used at optimal conditions for lipase production. The lower percentage of OC in the optimal substrate mixture for protease production could explain the improved microbial growth, since this mixture is characterized by higher nitrogen percentage. Regardless of the initial concentration, the final sugar concentration was similar in the two SSF processes. Concerning protease activity, it increased in the first day and (63 ± 3) U/g was obtained after 2 days of cultivation with the optimized substrate composition (Figure 4B), demonstrating that the experimental design executed to predict the protease optimal mixture was correctly performed at 2 days of SSF. Values of pH also play an important role in protease induction, since Y. lipolytica is able to synthesize acid and alkaline proteases, being the latter the most secreted enzyme. As expected, the production of this extracellular protease is induced in a medium with neutral and alkaline pH (Ogrydziak, 2013). Thus, the high protein content in the substrate and the increase of pH values (Figure 4B) improved protease activity and the levels of the proteolytic enzyme were maintained until the end of the experiments. The alkaline protease is produced by Y. lipolytica during the exponential growth phase and production is very reduced or absent in the stationary phase (Ogrydziak, 1993). Unlike lipase, a peak was not observed during protease production possibly because this enzyme was not degraded during the SSF process. To our knowledge, the optimization of SSF processes aiming at protease production by Y. lipolytica has not been reported yet. However, as previously mentioned, some reports on lipase production also quantified the proteolytic activity of Y. lipolytica. In particular, protease production by Y. lipolytica was reported in SSF using canola cake (Souza et al., 2017), soybean by-products (Farias et al., 2014; Souza et al., 2017), and cottonseed cake (Farias et al., 2014) as solid substrates. These results, together with the ones found in the present study, demonstrate the potential of using Y. lipolytica as a cell factory for protease production while reusing by-products from vegetable oil industries. Despite the maintenance of protease activity until the fourth day, since shorter incubation times enhances the process productivity and reduces the production costs, a SSF process of 2 days can also be selected for protease production by Y. lipolytica W29 in these conditions.
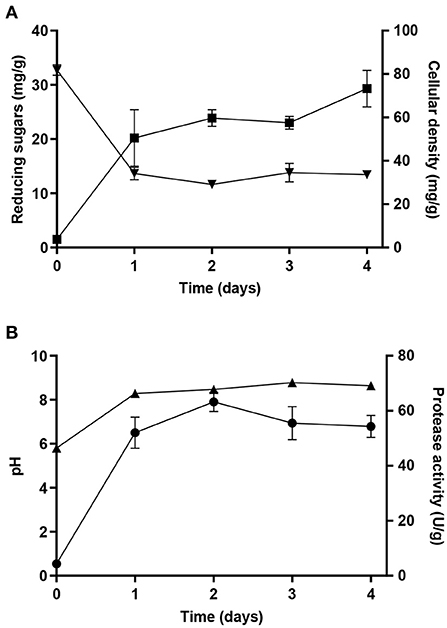
Figure 4. (A) Cellular density (■), reducing sugars concentration (▾), (B) protease activity (•), and pH (▴) obtained during SSF with Y. lipolytica W29 for 4 days with the optimum substrate mixture for protease production.
Besides the target biocompounds production, Y. lipolytica demonstrated promising results in the biotransformation of agro-industrial by-products, in particular okara (Vong et al., 2016, 2018), which is a by-product from soybean processing. Moreover, the high lipolytic and proteolytic activities attained in this work could result in changes in substrate composition. For this reason, after selection of the optimum substrate mixture and incubation time for enzyme production, a characterization of the remaining solid after enzyme extraction was performed to evaluate its potential application in other industries or bioprocesses.
Solid state fermentation carried out in the optimal conditions for the production of lipase and protease led to a significant increase in the ash content of the final substrate (Table 3). Microbial growth can lead to the loss of dry organic matter such as carbohydrates, lipids, organic acids, and proteins. As a result, an apparent increase in the mineral content in the substrate was observed. Yarrowia lipolytica growth did not change fiber content, which was an expected outcome since this microorganism is unable to produce lignocellulosic enzymes responsible for the degradation of these materials. In both cases, a slight reduction in crude protein content was observed, which could indicate protein consumption or increased protein solubilization that was removed in the extract. However, these differences were not statistically significant. Finally, a decrease in lipid percentage was observed, however, these differences were only statistically significant in the optimum substrate mixture for lipase production. This is not surprising since lipase is responsible for the hydrolysis of triacylglycerols and free fatty acids, which are then assimilated by the yeast cells and used as a carbon source (Morin et al., 2011). In the present study, the high lipolytic activity observed with the mixture of OC and SC (1:1) could explain the decrease in lipids observed after 2 days of SSF. Similarly, Yano et al. (2008) reported a reduction in the lipid content of fish mince after SSF with Y. lipolytica NBRC-10073. Several studies performed to access the safety of Y. lipolytica showed that this microorganism can be employed as a production host for food or feed (Groenewald et al., 2014) and Y. lipolytica biomass has been successfully incorporated in feed formulations (Czech et al., 2016). Thus, SSF of the mixture of OC with the other oil cakes enhanced yeast biomass production, which is also a source of protein and can be kept in the solid after SSF if no extraction is performed. The enzymes (lipases and proteases) produced, if also kept in the final fermented mixtures, may have an important role in enhancing nutritional properties of the mixtures by improving lipids and proteins digestibility by the animals (Ojha et al., 2019).
Olive cake, an underexplored oil cake with very limited use as animal feed, was successfully incorporated in SSF substrate mixtures, opening new opportunities for its valorization. The combination of SC and RC with OC improved the composition of the latter, in particular reduced the C/N ratio, enhancing yeast cellular growth and lipase and protease production in comparison with the utilization of single OC. A simplex centroid mixture design was applied to identify the optimum substrate mixture of OC, RC, and SC for maximum lipase and protease production. The content of residual oil in OC was an important factor for lipase production, where 50% (w/w) of OC and SC lead to the highest lipase production by SSF. In contrast, high protein content in the SSF substrate, owing to RC and SC, improved protease production. Likewise, the combination of different oil cakes modulated enzyme secretion, resulting in a targeted production. This work showed the promising application of by-products from vegetable oils production in SSF by Y. lipolytica, allowing the production of enzymes of interest for many industries.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AC: experiments, data analysis, and manuscript writing. IB and JS: conceptualization of the study, manuscript writing, and reviewing. ML: manuscript writing and reviewing. All authors contributed to the article and agree with the final version of the manuscript.
This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UID/BIO/04469/2020, by LABBELS, Associate Laboratory in Biotechnology, Bioengineering and Microelectromechanical Systems, LA/P/0029/2020 and by the project BIOECONORTE (NORTE-01-0145-FEDER-000070). AC was supported by Ph.D. grant SFRH/BD/139098/2018 funded by FCT.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abid, K., Jabri, J., Ammar, H., Ben Said, S., Yaich, H., Malek, A., et al. (2020). Effect of treating olive cake with fibrolytic enzymes on feed intake, digestibility and performance in growing lambs. Anim. Feed Sci. Technol. 261, 114405. doi: 10.1016/j.anifeedsci.2020.114405
AOAC (2005). Official Methods of Analysis of the Association of Official Analytical Chemists. 18th Edn. Washington DC: AOAC.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 7, 248–254.
Braga, A., Gomes, N., and Belo, I. (2012). Lipase induction in Yarrowia lipolytica for castor oil hydrolysis and its effect on γ-decalactone production. J. Amer. Oil Chem. Soc. 89, 1041–1047. doi: 10.1007/s11746-011-1987-5
Christoforou, E., and Fokaides, P. A. (2016). A review of olive mill solid wastes to energy utilization techniques. Waste Manage. 49, 346–363. doi: 10.1016/j.wasman.2016.01.012
Czech, A., Smolczyk, A., Ognik, K., and Kiesz, M. (2016). Nutritional value of Yarrowia lipolytica yeast and its effect on growth performance indicators n piglets. Ann. Anim. Sci. 16, 1091–1100. doi: 10.1515/aoas-2016-0034
Dabrowska, A., Bajzert, J., Babij, K., Szołtysik, M., Stefaniak, T., Willak-Janc, E., et al. (2020). Reduced IgE and IgG antigenic response to milk proteins hydrolysates obtained with the use of non-commercial serine protease from Yarrowia lipolytica. Food Chem. 302:125350. doi: 10.1016/j.foodchem.2019.125350
de Castro, R. J. S., Ohara, A., Nishide, T. G., Albernaz, J. R. M., Soares, M. H., and Sato, H. H. (2015). A new approach for proteases production by Aspergillus niger based on the kinetic and thermodynamic parameters of the enzymes obtained. Biocatalysis Agri. Biotechnol. 4, 199–207. doi: 10.1016/j.bcab.2014.12.001
Dermeche, S., Nadour, M., Larroche, C., Moulti-mati, F., and Michaud, P. (2013). Olive mill wastes : biochemical characterizations and valorization strategies. Process Biochem. 48, 1532–1552. doi: 10.1016/j.procbio.2013.07.010
Farias, M. A., Valoni, E. A., Castro, A. M., and Coelho, M. A. Z. (2014). Lipase production by Yarrowia lipolytica in solid state fermentation using different agro industrial residues. Chem. Eng. Trans. 38, 301–306. doi: 10.3303/CET1438051
Ferreira, M., Fernandes, H., Peres, H., Oliva-Teles, A., Belo, I., and Salgado, J. M. (2020). Bio-enrichment of oilseed cakes by Mortierella alpina under solid-state fermentation. LWT Food Sci. Technol. 134, 109981. doi: 10.1016/j.lwt.2020.109981
Filipe, D., Fernandes, H., Castro, C., Peres, H., Oliva-Teles, A., Belo, I., et al. (2020). Improved lignocellulolytic enzyme production and antioxidant extraction using solid-state fermentation of olive pomace mixed with winery waste. Biofuels Bioprod. Bioref. 14, 78–91. doi: 10.1002/bbb.2073
Gonçalves, F. A. G., Colen, G., and Takahashi, J. A. (2014). Yarrowia lipolytica and its multiple applications in the biotechnological industry. Sci. World J. 2014:476207. doi: 10.1155/2014/476207
Groenewald, M., Boekhout, T., Neuvéglise, C., Gaillardin, C., Van Dijck, P. W. M., and Wyss, M. (2014). Yarrowia lipolytica: safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 40, 187–206. doi: 10.3109/1040841X.2013.770386
Gullón, P., Gullón, B., Astray, G., Carpena, M., Fraga-Corral, M., Prieto, M. A., et al. (2020). Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 137, 109683. doi: 10.1016/j.foodres.2020.109683
Hapeta, P., Kerkhoven, E. J., and Lazar, Z. (2020). Nitrogen as the major factor influencing gene expression in Yarrowia lipolytica. Biotechnol. Rep. 27, e00521. doi: 10.1016/j.btre.2020.e00521
Imandi, S. B., Karanam, S. K., and Garapati, H. R. (2010). Optimization of media constituents for the production of lipase in solid state fermentation by Yarrowia lipolytica from palm Kernal cake (Elaeis guineensis). Adv. Biosci. Biotechnol. 1, 115–121. doi: 10.4236/abb.2010.12016
Imandi, S. B., Karanam, S. K., and Garapati, H. R. (2013). Use of Plackett-Burman design for rapid screening of nitrogen and carbon sources for the production of lipase in solid state fermentation by Yarrowia lipolytica from mustard oil cake (Brassica napus). Brazil. J. Microbiol. 44, 915–921. doi: 10.1590/S1517-83822013005000068
Joven, M., Pintos, E., Latorre, M. A., Suárez-Belloch, J., Guada, J. A., and Fondevila, M. (2014). Effect of replacing barley by increasing levels of olive cake in the diet of finishing pigs: growth performances, digestibility, carcass, meat and fat quality. Anim. Feed Sci. Technol. 197, 185–193. doi: 10.1016/j.anifeedsci.2014.08.007
Leite, P., Salgado, J. M., Venâncio, A., Domínguez, J. M., and Belo, I. (2016). Ultrasounds pretreatment of olive pomace to improve xylanase and cellulase production by solid-state fermentation. Bioresour. Technol. 214, 737–746. doi: 10.1016/j.biortech.2016.05.028
Lima, V. M. G., Krieger, N., Sarquis, M. I. M., Mitchell, D. A., Ramos, L. P., and Fontana, J. D. (2003). Effect of nitrogen and carbon sources on lipase production by Penicillium aurantiogriseum. Food Technol. Biotechnol. 41, 105–110.
Lomascolo, A., Uzan-Boukhris, E., Sigoillot, J. C., and Fine, F. (2012). Rapeseed and sunflower meal: a review on biotechnology status and challenges. Appl. Microbiol. Biotechnol. 95, 1105–1114. doi: 10.1007/s00253-012-4250-6
Lopes, M., Miranda, S. M., Costa, A. R., Pereira, A. S., and Belo, I. (2022). Yarrowia lipolytica as a biorefinery platform for effluents and solid wastes valorization–challenges and opportunities. Crit. Rev. Biotechnol. 42, 163–183. doi: 10.1080/07388551.2021.1931016
Lopes, V. R. O., Farias, M. A., Belo, I. M. P., and Coelho, M. A. Z. (2016). Nitrogen sources on TPOMW valorization through solid state fermentation performed by Yarrowia lipolytica. Braz. J. Chem. Eng. 33, 261–270. doi: 10.1590/0104-6632.20160332s20150146
Matkawala, F., Nighojkar, S., Kumar, A., and Nighojkar, A. (2008). Enhanced production of alkaline protease by Neocosmospora sp. N1 using custard apple seed powder as inducer and its application for stain removal and dehairing. Biocatalysis Agri. Biotechnol. 21, 101310. doi: 10.1016/j.bcab.2019.101310
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428.
Moftah, O. A. S., Grbavcic, S. Z., Moftah, W. A. S., Lukovic, N. D., Prodanovic, O. L., Jakovetic, S. M., et al. (2013). Lipase production by Yarrowia lipolytica using olive oil processing wastes as substrates. J. Serb. Chem. Soc. 78, 781–794. doi: 10.2298/JSC120905005M
Molina-Alcaide, E., and Yáñez-Ruiz, D. R. (2008). Potential use of olive by-products in ruminant feeding: a review. Anim. Feed Sci. Technol. 147, 247–264. doi: 10.1016/j.anifeedsci.2007.09.021
Morin, N., Cescut, J., Beopoulos, A., Lelandais, G., Le Berre, V., Uribelarrea, J. L., et al. (2011). Transcriptomic analyses during the transition from biomass production to lipid accumulation in the oleaginous yeast Yarrowia lipolytica. PLoS ONE 6:e27966. doi: 10.1371/journal.pone.0027966
Navvabi, A., Razzaghi, M., Fernandes, P., Karami, L., and Homaei, A. (2018). Novel lipases discovery specifically from marine organisms for industrial production and practical applications. Process Biochem. 70, 61–70. doi: 10.1016/j.procbio.2018.04.018
Neofytou, M. C., Miltiadou, D., Sfakianaki, E., Constantinou, C., Symeou, S., Sparaggis, D., et al. (2020). The use of ensiled olive cake in the diets of Friesian cows increases beneficial fatty acids in milk and Halloumi cheese and alters the expression of SREBF1 in adipose tissue. J. Dairy Sci. 103, 8998–9011. doi: 10.3168/jds.2020-18235
OECD-FAO (2019). “Oilseeds and oilseed products,” in Agricultural Outlook 2019–2028 (OECD-FAO), 142–153. doi: 10.1787/5f037977-en
OECD-FAO (2021). OECD-FAO Agricultural Outlook OECD Agriculture Statistics (Database). doi: 10.1787/agr-outl-data-en
Ogrydziak, D. (2013). “Acid and alkaline extracellular proteases of Yarrowia lipolytica,” in Yarrowia lipolytica. Microbiology Monographs, ed G. Barth (Berlin; Heidelberg: Springer), 77–97. doi: 10.1007/978-3-642-38583-4
Ogrydziak, D. M. (1993). Yeast extracellular proteases. Crit. Rev. Biotechnol. 13, 1–55. doi: 10.3109/07388559309069197
Ojha, B. K., Singh, P. K., and Shrivastava, N. (2019). “Enzymes in the animal feed industry,” in Enzymes in Food Biotechnology: Production, Applications, and Future Prospects, ed M. Kuddus (London: Elsevier), 93–109. doi: 10.1016/B978-0-12-813280-7.00007-4
Pereira-Meirelles, F. V., Rocha-Leão, M. H. M., and Sant'Anna, G. L. (2000). Lipase location in Yarrowia lipolytica cells. Biotechnol. Lett. 22, 71–75. doi: 10.1023/A:1005672731818
Pokora, M., Zambrowicz, A., Zabłocka, A., Dabrowska, A., Szołtysik, M., Babij, K., et al. (2017). The use of serine protease from Yarrowia lipolytica yeast in the production of biopeptides from denatured egg white proteins. Acta Biochim. Pol. 64, 245–253. doi: 10.18388/ABP.2016_1316
Ramachandran, S., Singh, S. K., Larroche, C., Soccol, C. R., and Pandey, A. (2007). Oil cakes and their biotechnological applications - a review. Bioresour. Technol. 98, 2000–2009. doi: 10.1016/j.biortech.2006.08.002
Razzaq, A., Shamsi, S., Ali, A., Ali, Q., Sajjad, M., Malik, A., et al. (2019). Microbial proteases applications. Front. Bioeng. Biotechnol. 7:110. doi: 10.3389/fbioe.2019.00110
Rigo, E., Ninow, J. L., Polloni, A. E., Remonatto, D., Arbter, F., Vardanega, R., et al. (2009). Improved lipase biosynthesis by a newly isolated Penicillium sp. Indus. Biotechnol. 5, 119–126. doi: 10.1089/ind.2009.5.119
Salgado, J. M., Abrunhosa, L., Venâncio, A., Domínguez, J. M., and Belo, I. (2014). Integrated use of residues from olive mill and winery for lipase production by solid state fermentation with Aspergillus sp. Appl. Biochem. Biotechnol. 172, 1832–1845. doi: 10.1007/s12010-013-0613-4
Salihu, A., Alam, M. Z., AbdulKarim, M. I., and Salleh, H. M. (2012). Lipase production: an insight in the utilization of renewable agricultural residues. Resour. Conserv. Recycling 58, 36–44. doi: 10.1016/j.resconrec.2011.10.007
Singh, R., Langyan, S., Sangwan, S., Rohtagi, B., Khandelwal, A., and Shrivastava, M. (2022). Protein for human consumption from oilseed cakes: a review. Front. Sustain. Food Syst. 6:856401. doi: 10.3389/fsufs.2022.856401
Sousa, D., Salgado, J. M., Cambra-López, M., Dias, A. C. P., and Belo, I. (2022). Degradation of lignocellulosic matrix of oilseed cakes by solid-state fermentation: fungi screening for enzymes production and antioxidants release. J. Sci. Food Agric. 102, 1550–1560. doi: 10.1002/jsfa.11490
Souza, C., Farias, M. A., Ribeiro, B. D., and Coelho, M. A. Z. (2017). Adding value to agro-industrial co-products from canola and soybean oil extraction through lipase production using Yarrowia lipolytica in solid-state fermentation. Waste Biomass Valor. 8, 1163–1176. doi: 10.1007/s12649-016-9690-2
Vong, W. C., Au Yang, K. L. C., and Liu, S. Q. (2016). Okara (soybean residue) biotransformation by yeast Yarrowia lipolytica. Int. J. Food Microbiol. 235, 1–9. doi: 10.1016/j.ijfoodmicro.2016.06.039
Vong, W. C., Hua, X. Y., and Liu, S. Q. (2018). Solid-state fermentation with Rhizopus oligosporus and Yarrowia lipolytica improved nutritional and flavour properties of okara. LWT Food Sci. Technol. 90, 316–322. doi: 10.1016/j.lwt.2017.12.050
Waseem, S., Imadi, S. R., Gul, A., and Ahmad, P. (2017). “Oilseed crops: present scenario and future prospects,” in Oilseed Crops: Yield and Adaptations Under Environmental Stress, ed P. Ahmad (John Wiley and Sons Ltd.), 1–306.
Keywords: Yarrowia lipolytica, lipase, protease, solid state fermentation, oil cakes
Citation: Costa AR, Salgado JM, Lopes M and Belo I (2022) Valorization of by-products from vegetable oil industries: Enzymes production by Yarrowia lipolytica through solid state fermentation. Front. Sustain. Food Syst. 6:1006467. doi: 10.3389/fsufs.2022.1006467
Received: 29 July 2022; Accepted: 27 October 2022;
Published: 10 November 2022.
Edited by:
Lorenzo Pastrana, International Iberian Nanotechnology Laboratory (INL), PortugalReviewed by:
Sapna Langyan, National Bureau of Plant Genetic Resources (ICAR), IndiaCopyright © 2022 Costa, Salgado, Lopes and Belo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabel Belo, aWJlbG9AZGViLnVtaW5oby5wdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.