- 1Department of Soil Science, School of Agriculture, College of Basic and Applied Sciences, University of Ghana, Accra, Ghana
- 2Institute for Environment and Sanitation Studies, College of Basic and Applied Sciences, University of Ghana, Accra, Ghana
- 3The Plant Protection and Regulatory Services Directorate, Ministry and Food and Agriculture, Accra, Ghana
Introduction: Soybean is an important legume whose nitrogen-fixing ability may be exploited to improve the fertility status of soils. In Ghana, where most of the soils are poor in fertility, cultivation of soybean presents an inexpensive way for resource-poor farmers to earn appreciable income and improve the fertility of arable land at the same time. However, the yield and N-fixing response of soybean to inoculation in most soils with poor fertility in Ghana are not well-researched.
Method: A screen house study on the efficacy of Histick Soy (an inoculum manufactured by a German chemical company) on improving the nodulation of soybean in P-deficient soils comprising two Plinthustalfs, Ny1 and Ny2, with a history and no history, respectively, of soybean cultivation and a Kandiustalf with no history of soybean cultivation was evaluated in Ghana. Sterile riverbed sand was included as a check. Soybean seeds were inoculated with Histick Soy at three different rates, namely, zero, half, and recommended rate, and grown in a screen house to ascertain the efficacy of the inoculant in nodulating soybean. Nitrogen was applied at 0 and 10 kg/ha, K was applied at 60 kg/ha, and P was applied at 0, 30, and 60 kg/ha. These treatments were completely randomized with four replicates at a moisture content equivalent to 80% field capacity and grown till flowering. At flowering, the number of nodules per plant was counted. A parallel experiment was carried out to physiological maturity where 100-seed weight per pot was determined.
Results and discussion: Results obtained revealed that plants from the uninoculated seeds in the riverbed sand and the Kandiustalf did not nodulate. In the case of Ny2, the number of nodules at harvesting was statistically similar for half and full recommended application rate of the inoculant. The uninoculated Ny2 with 4.4 average nodules per pot did not increase at half recommended application rate. At the recommended rate, nodule numbers increased 2.3-fold to 10.3. The Ny1 showed no response to inoculation. Treatments, which received the application of 60 and 30 kg P2O5/ha triggered higher responses to inoculation in low and high Bradyrhizobia populations, respectively, in the Plinthustalfs.
Introduction
Soybean [Glycine max (L.) Merr.] is one of the most valuable foods and feed crops, with high levels of protein and oil (Tikde et al., 2015; Vollmann, 2016; Sandhu et al., 2018). It is an important agronomic crop for smallholder farmers in Ghana, as they take advantage of the nitrogen-fixing potential of the crop to improve soil quality while addressing the protein deficiency of their diets. In some parts of Ghana, soybean is gradually assuming the status of a cash crop for small-scale resource-poor farmers. The high cost of chemical fertilizer and the need to reduce nitrogen losses to minimize eutrophication of water bodies during crop production serve as an added incentive to resource-poor farmers to grow soybean. Cultivation of legumes to exploit the beneficial effects of “Biological Nitrogen Fixation” (BNF) is viewed as an environmentally friendly approach to increase crop yields and maintain or improve soil health at the same time (Vollmann, 2016). The cultivation of soybean also has the advantage of controlling the spread of Striga hemonthica, a parasitic weed in northern Ghana that has the potential to cause yield losses in crop production. The plant exudes chemicals that stimulate the germination of Striga seeds, which subsequently die off, as their roots are not able to attach to the host (soybean) for nutrient absorption (MoFA and CSIR, 2005; IITA, 2009).
Most Ghanaian soils are poor and have low levels of nitrogen (Awuni et al., 2020). As part of measures to improve the fertility status of soils in Ghana, most resource-poor farmers include legumes in cropping systems to take advantage of the fixation of N. Under optimum conditions, including adequate P availability and optimum pH levels, soybean fixes atmospheric nitrogen in the soil through a symbiotic relationship (Iannetta et al., 2016; Thilakarathna and Raizada, 2017), thereby decreasing the quantity of inorganic N application on arable land and increasing crop productivity in an environmentally sustainable production system (Zander et al., 2016). The symbiotic relationship between rhizobia and host legumes leads to the formation of nodules on the roots of the host plant where the microbes inhabit and proliferate by extracting nutrients from the host plant. In return, the rhizobia convert atmospheric nitrogen into plant-usable forms and make it available for utilization by the host plant (Beyan et al., 2018). This beneficial process accounts for about 60% of the total biological nitrogen fixation (Beyan et al., 2018). In soils that are not inhabited by the right rhizobia strains, nodulation of soybean is limited and this results in poor yields. Nodule formation on the roots of soybean and its habitation with efficient rhizobia strain is therefore known to improve nodulation and increase the yield of the legume crop while maintaining soil health. The main challenge besetting atmospheric N-fixation in the soil during soybean production is that the crop is not a promiscuous legume (Beyan et al., 2018), and thus does not nodulate freely, especially in Ghanaian soils. Most of the native rhizobia strains that nodulate legumes in Ghanaian soils are ineffective (Fening and Danso, 2002; Ulzen et al., 2016). Therefore, soybean nodulation, and subsequent N fixation thereof, has been a major problem associated with the production of the crop in most Ghanaian soils.
To improve the nodulation of soybean and increase its yield, there have been conscious efforts to introduce the right strain of rhizobia that ensures proficient symbiotic interactions from external sources (Ulzen et al., 2016). Presently, only a few Ghanaian soils with a history of soybean cultivation are inhabited with the strain of bacteria that can promote nodulation of the legume (Boakye et al., 2016; Ulzen et al., 2016). Inoculation of seeds with Bradyrhizobium japonicum is consequently an important practice in soybean cultivation (Martyniuk et al., 2013; Jarecki et al., 2016). To optimize legume–rhizobium interactions for the effective production of soybean, especially in soils with no history of the crop's cultivation, inoculation of soybean presents a promising option for increasing the efficiency of the N symbiotic relationship for enhanced crop productivity (Tewari et al., 2003).
Most soils, particularly those in the northern parts of Ghana where large tracts of land are cultivated to soybean are deficient in P due to a myriad of reasons, chief of which are their high concretionary nature, acidic pH, and kaolinitic nature (Nartey et al., 1997). Ironically, P is a very important nutritional element in the effective nodulation of soybean and affects its growth and N fixation (Míguez-Montero et al., 2020). Some studies have suggested that even low levels of P stress resulting from the deficiency of the element in soils can inhibit nitrogen-fixing enzyme activity in legume nodules due to reduced nodule ATP energy (Ulzen et al., 2016). Thus, the application of effective rhizobia strains in the presence of optimum levels of P in the soil can stimulate root nodule growth and nitrogenase activity (Fening and Danso, 2002; Ulzen et al., 2016). Additionally, the availability of adequate amounts of P can enhance the N-fixing capacity of root nodules nitrogen to improve N fixation (Ulzen et al., 2016). The use of inoculants has therefore proved to be effective in improving biological nitrogen fixation in soils (Calvo et al., 2014; Ronner et al., 2016). In most developed countries where the use of inoculants in legume production has advanced, some of these inoculants have been described to improve nodulation and yield even under some conditions of environmental stress (Calvo et al., 2014; Ronner et al., 2016). In Ghana, however, the use of rhizobia inoculants in soybean production is not well-advanced due to considerable technological challenges in effective strain selection and the lack of appropriate inoculants for use by resource-poor farmers (Boakye et al., 2016).
There is, however, some evidence to suggest that introduced inoculants could be subjected to environmental stress conditions that may not favor the activities of these rhizobia relative to indigenous strains (Cernay et al., 2015; Goyal et al., 2021). This may have implications on nodulation, and subsequently crop yield, and can serve as one of the major drivers of yield variability in soybean production (Ronner et al., 2016; Gunnabo et al., 2019). It is therefore important to assess the efficacy of introduced inoculants to determine their suitability under the unpredictable environmental conditions that most Ghanaian farmers operate.
Histick Soy is a commercial inoculant of Bradyrhizobium japonicum of 4 × 109 viable cells/g produced by BASF of Germany. It has been introduced into Ghana with a view of improving the productivity of soybean cultivation. There is, however, very little information on the efficacy of the inoculant in low-fertility soils, especially under P-deficient soil conditions. Since a lot of local farmers grow legumes, especially soybean in soils with poor fertility that are mostly deficient in P, they may do so with the expectation that the application of an inoculant will lead to an improvement in nodulation of the crop irrespective of the P status of the soil. It has therefore become necessary to ascertain the soil P conditions under which the effectiveness of this inoculant will be optimum. It is in light of this that a screen house pot experiment was conducted using three soils from the Savannah zone of Ghana and sterile riverbed sand to ascertain the efficacy of Histick Soy on soybean nodulation. The objectives of the study were, therefore, as follows:
I. Ascertain the effectiveness of Histick Soy in nodulating soybean plants in some P-deficient Ghanaian soils.
II. Compare the effectiveness of Histick Soy on nodulation of soybean in soils with indigenous Rhizobium.
III. Determine the effect of varying P rates on the effectiveness of Histick Soy on soybean nodulation.
Materials and methods
Three soils, two of which were sampled from the interior Savannah zone of Ghana and the third was sampled from the Coastal Savannah zone, were used for the study. The soils from the interior Savanna zone, which were both Nyankpala series (Plinthustalfs), had one with a history of soybean production (Nyankpala 1) and the other with no history of soybean production (Nyankpala 2). The choice of Nyankpala 1 was to see how the Bradyrhizobium japonicum of the Histick Soy would perform in the presence of the indigenous rhizobia. The soil, which was sampled from the Coastal Savannah, is a Toje series (Rhodic Kandiustalf) with no history of soybean production. Sterile riverbed sand collected from the Volta Lake at Asutsuare in the Eastern Region of Ghana, to ascertain the effect of Histick Soy on soybean nodulation, was included. The bulk density of the riverbed sand was determined after which sampling was done. The sand was first washed with 6 M HCl to disinfect and sterilize it, and thereafter, washed several times with sterile distilled water amidst testing with silver nitrate to get rid of the HCl.
The bulk densities of the undisturbed Savannah soils were taken after which the plow layer (0–20 cm) was sampled and processed for characterization of physicochemical properties. Concretions, which were present in the Nyankpala soils (Plinthustalfs), were determined by hand-picking and wet-sieving and washed thoroughly with water to remove soil materials adhering to the surfaces (Mitsuchi, 2012). The particle size distribution of the fine earth fraction was determined using the Bouyoucos hydrometer method (Day, 1965). Subsequently, the texture of the soil samples was determined, according to the USDA system of classification. The bulk density of the samples was determined using the core method (Blake and Hartge, 1986). The moisture content of the soil at maximum water holding capacity (WHC) was determined by covering the water-saturated sample of the undisturbed soils with polythene sheets and determining the moisture content after allowing gravitational water to drain for 48 h.
The pH of the fine earth was determined electrometrically in water using a 1:1 soil-water ratio (w/v) on an Oakton PC 2,700 pH meter. The organic carbon content of the fine earth was determined by a Leco TruMac CNS analyzer after the destruction of carbonates in the soil samples with 6 M HCl. The total N in the fine earth fraction was analyzed using the Leco TruMac CNS analyzer. Available P in the fine earth fraction was extracted according to the method of Bray and Kurtz (1945), and the P concentration in the extracts was determined by a UV spectrophotometer after color development by the method of Murphy and Riley (1962). The CEC of the soil was determined by the ammonium acetate method.
The populations of indigenous Bradyrhizobium in each of the soils capable of nodulating the test legume were estimated by the most probable number (MPN) plant infection assay (Vincent, 1970) using growth pouches. The growth pouches were half-filled with N-free nutrient solution (Somasegaran and Hoben, 1994) and autoclaved. The soybean seeds were surface sterilized with 70% ethanol for 3 min and rinsed thoroughly in several changes of sterile distilled water (Somasegaran and Hoben, 1994). The seeds were further sterilized with mercuric chloride (HgCl2) and pre-germinated on moist filter paper in Petri dishes until the radicles were about 2 cm long. A pair of sterilized forceps was used to pick the sterilized seeds, which were planted at two seedlings per growth pouch, with the radicle facing downwards. The pouches were randomly arranged on a rack in the screen house. Ten-fold serial dilutions up to level 6 (10−1, 10−2, 10−3, 10−4, 10−5, and 10−6) were prepared for each of the soils using sterilized distilled water as a diluent, which was then used as inoculum. One milliliter (1 mL) of the inoculum was used to inoculate the seedlings for every dilution level. The plants were assessed for the presence of nodules 6 weeks after planting by scoring plus (+) for the presence of nodules and minus (–) for the absence of nodules. The most probable number of rhizobia cells per gram of soil was calculated (Vincent, 1970) using the formula in equation 1,
where m is the most likely number from the MPN table (Beck et al., 1993), d is the lowest dilution in the series, and v is the aliquot used for inoculation (Somasegaran and Hoben, 1994).
The bulk soils of the plow layer of the two Nyankpala soils and Toje series as well as the sterile riverbed sand were packed in plastic bags to attain their respective field bulk densities. Soybean seeds with about 90–100% germination capacity were inoculated with the Histick Soy at rates of zero (no inoculant), half the manufacturer's application rate (0.5) (2 g Histick Soy/kg seed), and the full recommended application rate (1) (4 kg Histick Soy/kg seed). These seeds were sown at four seeds per pot and later thinned to two. Nitrogen from ammonium sulfate was applied at two rates of 0 and 10 kg/ha to the treatments. The 10 kg N/ha application rate was to evaluate the effect of a low dose of N on the inoculant's ability to nodulate soybean. Potassium oxide (K2O) was applied at 60 kg/ha to all the treatments. Phosphorus pentoxide (P2O5), which is essential for nodulation was applied at three different rates, namely, 0, 30, and 60 kg P2O5 /ha. All the treatments were kept at 80% field capacity to avoid leaching. The treatments were replicated four times and completely randomized in a screen house. Thus, with four soil types, three inoculum treatments, three P fertilizer rates, and four replications in a completely randomized design, the total treatments were 144.
Six weeks after planting (which coincided with flowering), the experiment was terminated. The soils were saturated for easy removal of the whole plant, washed off, and the number of nodules per plant was determined. The nodules removed were incised and the presence of leghemoglobin was confirmed or otherwise by visual appraisal. A second experiment, which run parallel to the first one with the same treatments, was also carried out but the crops were grown to physiological maturity. At maturity, the seeds per pot were extracted and 100 seed weight per treatment was determined. All measured agronomic parameters for the experiments terminated at 6 weeks and physiological maturity was subjected to analysis of variance using GenStat (2009) to establish if there were any significant treatment effects at p <0.05. Mean separations were done using Tukey's Lsd 0.05.
Results
Soil characterization
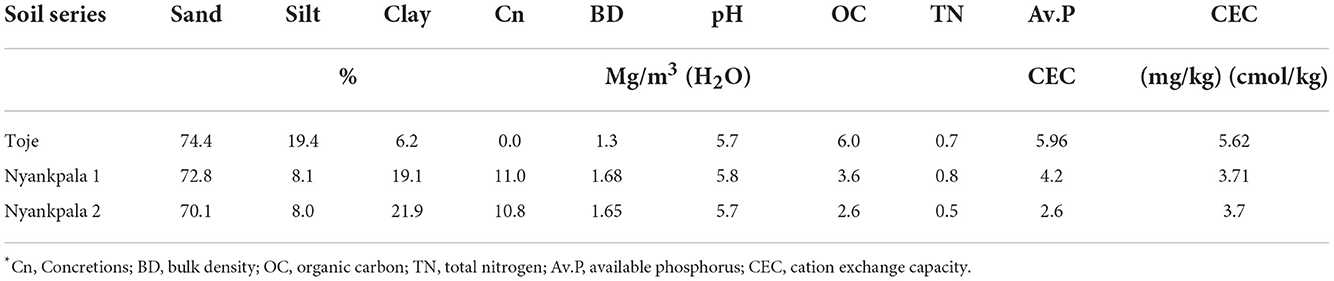
Some of the physical and chemical properties of the two Nyankpala soils (Plinthustalfs) and the Toje series (Kandiustalf) used for the study are presented in Table 1. It is evident from Table 1 that all three agricultural soils have high sand contents between 70.1 and 74.4%. With a clay content of 21.9% and silt content of 8.0%, 70.1% sand content gave Ny2 a sandy clay loam texture. Although Ny1 has a clay content of 19.1%, its sand content is 72.8%, which was not very different from the 74.4% sand content ascribed to the Toje series with 6.2% clay content that gave the two soils a sandy loam texture, according to the USDA system of classification.
The pH in the water of the three agricultural soils is 5.7, which makes them slightly acidic. The soils have a low organic carbon content of < 10 g/kg. Consequently, the total N, available P, an CEC of the soils are very low as presented in Table 1.
The population of indigenous rhizobia in the soils capable of nodulating soybean is presented in Table 2. As evidenced from the table, the Nyankpala 1 soil with a history of soybean cultivation had the highest population of 150,000 bacteria cells/g soil. The Nyankpala 2 and Toje soils with no history of soybean cultivation had marginal values of 5 and 2 cells/g soil, respectively. The riverbed sand, as expected, did not have any bacterial cells, as it had been disinfected with HCl.
Effect of Histick Soy on mean nodule numbers
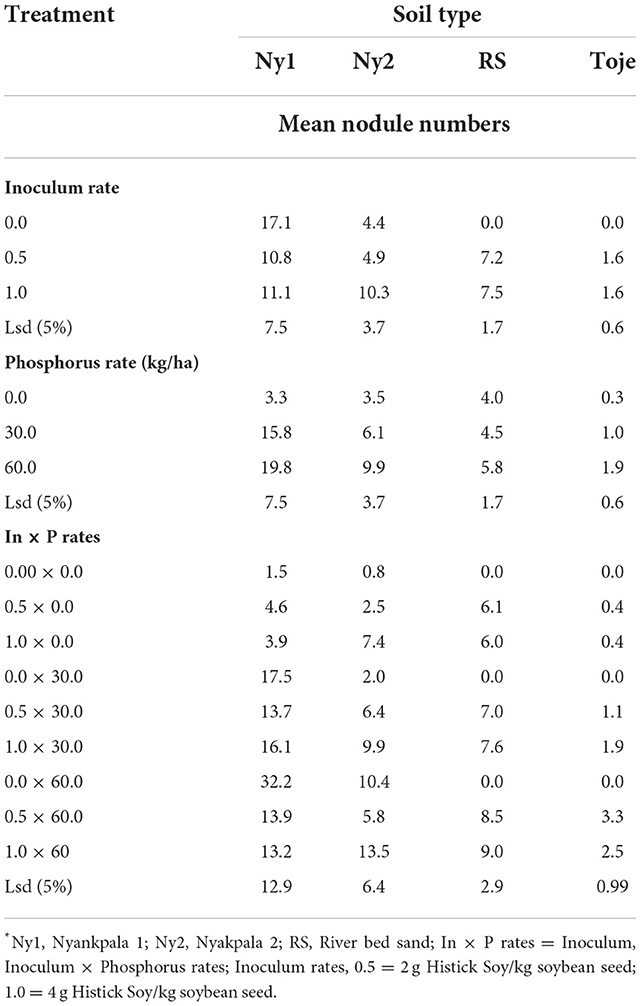
Effects of varying rates of Histick Soy on nodulation of soybean grown in the four soil types are presented in Table 3. The nodule numbers at the two Histick Soy rates in the Nyankpala soil, with a history of soybean production (Ny1), were not significantly different. The mean number of nodules counted on plants that were grown on the uninoculated Ny1 was 17.1. On inoculating at half the recommended rate of Histick Soy, nodulation decreased, although insignificantly at a 5% probability level, to 10.8. At the full recommended rates of the Histick Soy inoculant, the number of nodules, which was 11.1, was not significantly different from that found on plants grown in the uninoculated Ny1.
Inoculating the Nyankpala series with no history of soybean production (Ny2), the Toje series and the sterilized riverbed sand with Histick Soy had a positive effect on the nodulation of soybean. The uninoculated Nyankpala series (Ny2), which had five indigenous rhizobia cells/g soil had a mean number of nodules per plant being 4.4. This increased insignificantly to 4.9 when the soil was inoculated with half the recommended rate of Histick Soy. On application of Histick Soy at the manufacturer's recommended rate, however, there was a significant (p < 0.05) 2.3-fold increase in the number of nodules to 10.3 from the uninoculated. The uninoculated riverbed sand, as expected, had no nodules.
When the inoculant was applied at half the recommended rate of Histick Soy, the number of nodules was 7.5, which was not significantly different from the numbers obtained when the same medium was inoculated at the recommended rate.
There were only two indigenous rhizobia cells/g in the uninoculated Toje series. Consequently, nodulation was zero on soybean grown in the uninoculated soil. On inoculation, however, the mean number of nodules increased significantly to 1.6 per plant at the two rates of inoculation.
Effect of phosphorus on mean nodule numbers
Roots of soybean plants with nodules upon incision were pink in color implying the presence of leghemoglobin and connoting effectiveness. Increasing the P rate from 0 to 30 kg/ha in Ny1 led to 4.8-fold increase (p < 0.05) in nodule numbers from 3.3 to 15.8 (Table 3). However, a further increase in P rate to 60 kg/ha did not show any significant increase in nodule numbers from that at 30 kg P2O5/ha.
When the P rate was increased from 0 to 30 kg/ha in Ny2, an insignificant increase in nodule numbers on soybean was observed. An additional 30 kg/ha P increase to 60 kg/ha, however, gave a significant increase (p < 0.05) in nodule numbers from 3.5 at no amendment to 9.9. Thus, it appears that the response of soils with relatively low indigenous Bradyrhizobia populations such as in Ny2 to added P, when inoculated with Histick Soy, is high at 60 kg P2O5/ha, while for the same soil with high populations of indigenous rhizobia, the response to nodulation is rather high at 30 kg P2O5/ha when inoculated with the product. This assertion is corroborated by the highest nodule numbers observed in Ny1 (16.1) at 1.0 × 30 and in Ny2 (13.5) at 1.0 × 60 interactive rates of inoculum and P (Table 3).
The pattern of P response to nodulation in the riverbed sand media was similar to that of Ny2 as significant increases (p < 0.05) in nodule numbers on P application manifested only at the highest P rate of 60 kg/ha. There were no indigenous rhizobia in the riverbed sand and, with no other nutrients apart from what was supplied from an external source, it was a matter of consequence that the highest response to nodulation upon inoculation with Histick Soy was at the highest P rate.
With the rhizobia in an entirely new environment, it stands to reason that they will need a high dose of P to nodulate.
The Toje series had a similar very low indigenous rhizobia population (Table 2) and inherent chemical properties as the Ny2 (Table 1). However, this soil had a different pattern of response to P fertilization on inoculation, as it had significant increase in nodule numbers with an increasing P rate from 0 through 30–60 kg/ha.
Effect of soil type on nodulation
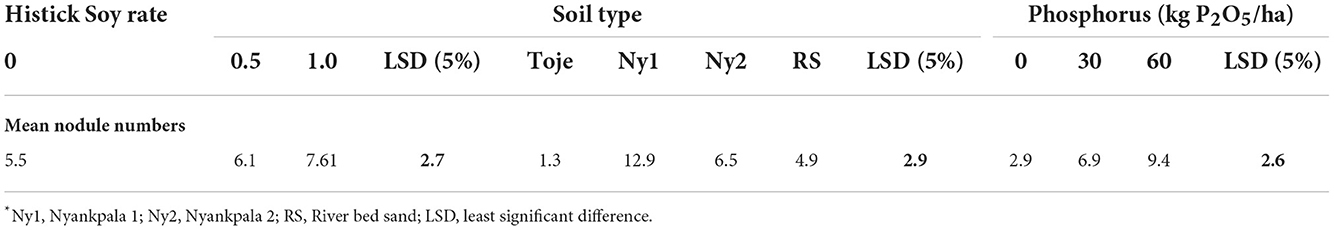
To ascertain the effect of soil type on nodulation, the data were pooled for statistical analysis and are presented in Table 4. There appears to be a significant soil-type effect on nodulation. From the table, it is clear that effectiveness in nodulation is in the order of Ny1 > Ny2 = RS > Toje. The Ny1 had the highest indigenous Bradyrhizobium population of 15 × 104 cells/g soil. It is, therefore, not surprising that it is the most effective among the four growing media used in nodulating soybean. The riverbed sand had no indigenous Rhizobium while the Ny2 and Toje series had, albeit very low numbers. The riverbed sand is, however, not significantly different from Ny2 in terms of nodulating soybean but significantly superior to the Toje series in nodulating the crop.
Effect of N rates on nodule numbers
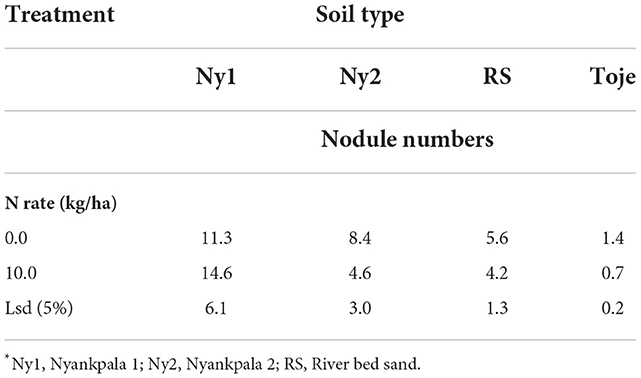
Apart from Ny1, which did not show any significant difference in nodule numbers on plants fertilized at the two rates of N, namely, 0 and 10 kg/ha, all the other treatments had higher nodule numbers in plants that were unfertilized than those which were fertilized at 10 kg N/ha (Table 5). The higher nodule numbers in soybean grown in the Ny2, RS, and Toje series at 0 N rate show the inhibition effect of applied N to nodulation.
Yield (100-seed weight)
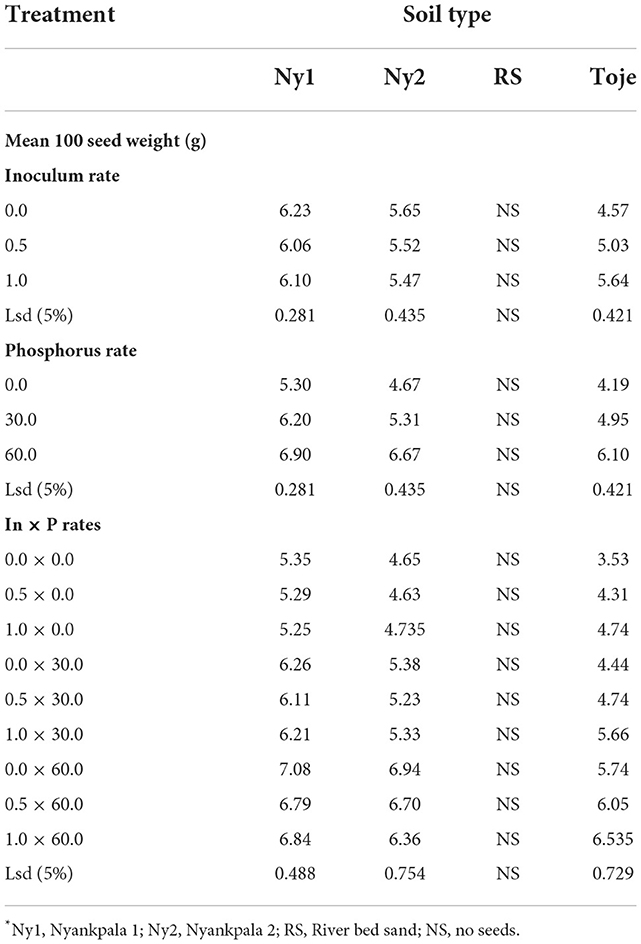
The effect of Histick Soy and P rates on 100-seed weight of the crop at maturity is presented in Table 6. It is clear from the table that inoculating the two Nyankpala soils with Histick Soy at no P application had no effect on seed weight as all the inoculum rates had statistically similar seed weights at harvest. The increasing rate of inoculation of Histick Soy on the Toje series increased seed weight significantly. In the uninoculated Toje series, seed weight was 4.57 g, which increased significantly to 5.0 g and then to 5.6 g at the half and full rates of Histick Soy applications, respectively. There were no seeds obtained in the RS as most of the plants could not survive to maturity.
Increasing P rates in the two Nyankpala soils increased seed weights significantly (Table 6). At no P amendment, Ny1 produced 100 seeds that weighed 5.3 g. This increased (p < 0.05) to 6.2 at 30 kg/ha and further increased significantly to 6.9 at 60 kg/ha. The uninoculated Ny2 had 100 seeds weighing 4.7 g, which increased (p < 0.05) to 5.3 and 6.7, at 30 and 60 kg P2O5/ha, respectively. From Table 6, it is evident that combining inoculation of Histick Soy on soybean seeds and P fertilization gave higher seed weights in all the agricultural soils used than sole inoculation with Histick Soy.
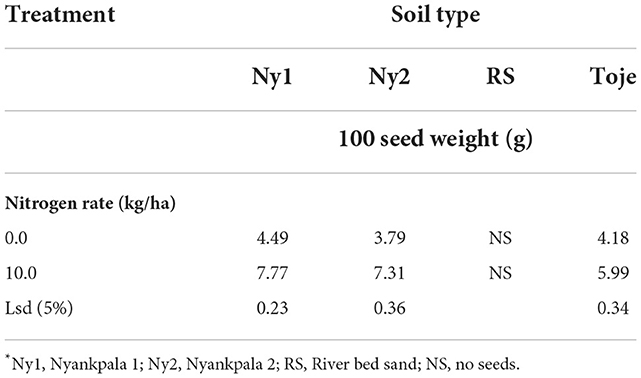
Table 7 shows the effect of N on 100 seed weights. In general, seed weight increased significantly when N was applied to the three agricultural soils, such as. Ny1, Ny2, and Toje series at 10 kg/ha from that of the unamended soil. The Ny2 soil had the lowest total N content of 0.5 g/kg. It, therefore, stands to reason that it had the highest response to N application as it increased (p < 0.05) in seed weight 1.93 times from the 3.79 g at no N application to 7.31 g at 10 kg N/ha.
Discussion
Soil characteristics
The almost 11% concretions of the Plinthustalfs (Nyankpala series) would decrease the effective soil volume for root growth and also limit P availability to the crop. The lower soil volume in the Nyankpala soils would culminate in a higher bulk density as reflected in Table 1. The high sand content of 74.4% coupled with the low clay content of the Kandiustalf (Toje series) would make the soils prone to leaching should water above field capacity be applied. The clay content of the three soils, mostly dominated by the low activity clays such as kaolinite (Eze, 2008; Ziblim et al., 2012) may account for the low CEC of the soils. Additionally, the low organic carbon and total N content of the soils give the indication that the soils are low in fertility and with their available P levels particularly very low (<6 mg/kg) response to P fertilization, and hence, nodulation should be high, thus, justifying their choice as soil media for the study.
The Nyankpala 1 has a history of soybean cultivation. It is, therefore, not surprising that the soil has higher TN than its Nyankpala 2 counterpart, as the crop may have fixed N into the soil. Additionally, the crop residue left on the Nyankpala 1 soil after harvests could have contributed to the higher organic carbon content with a concomitant higher TN and available P. The slightly higher available P in the Nyankpala 1 soil (4.2 mg/kg) compared with the Nyankpala 2 (2.6 mg/kg) could also be due to the residual P from P fertilizer that may have been applied during the cultivation of soybean. The relatively high population of indigenous rhizobia in the Nyankpala 1 soils underscores its inclusion in the study to evaluate the effectiveness of Histick Soy in Ghanaian soils. The two Nyankpala soils offer the platform to compare the effectiveness of the introduced Bradyrhizobium species against the indigenous ones.
Effect of inoculant on nodulation
As indicated in Table 2, the Ny1 had the highest number of indigenous Bradyrhizobia of 15 × 104 cells/g soil, but inoculation with the Histick Soy did not have any significant effect on nodule numbers relative to that of the uninoculated Ny1. The presence of indigenous rhizobia in the soil can mask or suppress the effect of the introduced rhizobia from the inoculant (Van Kessel and Hartley, 2000). The indigenous rhizobia had adapted to the soil's environment and may have out-competed the introduced rhizobia from the inoculant, resulting in lower nodule occupancy by the latter (Sanz-Sáez et al., 2015) at both half and recommended manufacturer's rate, thus, accounting for the apparent non-significant effect of Histick Soy in nodulating soybean in Ny1. With time and continuous application of the inoculant, the introduced rhizobia would get adapted to the Ny1 soil conditions, which may culminate in a significant increase in nodulation of soybean (Yang et al., 2018).
Response to rhizobia inoculation was more pronounced in the Nyankpala series with no history of soybean production (Ny2), the Toje series, and the sterilized riverbed sand. Studies have demonstrated that the response of legumes to inoculation is generally affected by the nature of the existing soil population of rhizobia (Yang et al., 2018; Mathenge et al., 2019). The respective 2.3- and 1.6-fold significant increases in nodule numbers in the Ny2 and Toje soils following inoculation at the recommended manufacturer's rate relative to their uninoculated counterparts give credence to the fact that the Bradyrhizobia from the inoculant did not have to compete with any indigenous strains for nodulation. The fact that nodulation occurred on soybean plants grown in both Ny2 and Toje series, which had a very low population of indigenous rhizobia, and especially the sterile riverbed sand also proves the efficacy of Histick Soy as an effective inoculant for soybean production.
Effect of phosphorus on nodulation
Phosphorus is the most essential element in ATP formation, which in turn is involved in energy transport, a key factor in nodulation. Additionally, P enhances root development and proliferation, thereby increasing the sites for rhizobia infection and initiation of nodule formation (Dabesa and Tana, 2021). With the relatively high population of indigenous rhizobia in the Ny1 coupled with its low inherent P concentration, a sharp response to any added P is not unexpected. It is, therefore, not surprising that the 30 kg P2O5/ha application manifested in a significant increase in nodule numbers as reported in similar studies by Ao et al. (2014) and Adjei-Nsiah et al. (2022). With subsequent increases in P rates, the increase in nodule numbers would be at a decreasing rate as evident in the insignificant increases in P application between the 30 and the 60 kg/ha. In the Ny2 and the riverbed sand media, however, a slightly different trend was observed with significantly higher nodule numbers at the P application rate of 60 kg/ha.
Although the Toje series had a very low indigenous rhizobia population and inherent chemical properties similar to the Ny2, there was a marked increase in nodule numbers with an increase in P application rates. The fact that the pattern of response to P fertilization in the Toje series was different from that of Ny2 is an indication that the inherent physical properties of the soils may have influenced their respective responses to P fertilization when inoculated with Histick Soy. The Toje series, a Kandiustalf, is not plinthic, and thus, has no concretions, while the Plinthustalf has about 10.8% concretion content (Table 1). Furthermore, the Plinthustalf has a higher clay content. Clay and concretion contents have been found to control P availability in soils in northern Ghana (Nartey et al., 1997). These differences in clay and concretion contents may, in part, be responsible for the different patterns of responses to P fertilization. The P contents in all the soils were very low (<6 mg/kg). Consequently, any small increase in P fertilization in the soils should lead to a sharp response. It is, therefore, not surprising that when the data were pooled (Table 4), there was a significant increase in nodulation with an increase in P fertilization at 30 kg/ha while any increase in P rate thereafter was not significant. This gives credence to the fact that the highest response of nodulation to P amendment in this study was at 30 kg/ha.
Effect of soil type on nodulation and 100-seed weight of soybean
The fact that the sterilized or disinfected riverbed sand with no indigenous Bradyrhizobia is at par with Ny2 and better than the Toje series in nodulation suggests that the indigenous rhizobia in the uninoculated Nyankpala and the Toje series were either not effective or did not have the optimum number of bacterial cells to nodulate soybean. This may, in part, explain the response of the Ny2 and Toje series to the application of Histick Soy. Just as was observed in the nodule numbers, increasing P rates increased seed weight in the Toje series. The response of all the soils to increased P fertilization underscores the importance of nutrients in soybean production in Ghana. It is, therefore, important to determine the P application rate that would provide the optimum seed production in different soils.
Conclusion
The use of inoculants in cropping systems presents an eco-friendly means of improving food production by most resource-poor farmers in developing countries. Application of the right rhizobia strain to improve nodulation in legumes and enhance biological nitrogen fixation in low-fertility soils is an environmentally friendly option for improving soil fertility and increasing crop yields at the same time. The results obtained in this research have demonstrated that Histick Soy is effective in nodulating soybean if applied at the manufacturer's recommended rate of 4 g/kg seeds. Additionally, this research has shown that although the application of P at 30 kg P2O5/ha is appropriate for enhancing nodulation, 60 kg P2O5/ha would enhance both nodulation and seed production in soils with low indigenous rhizobia.
This study also showed that the effectiveness of Histick Soy in nodulating soybean in Ghana would be most pronounced in soils with no history of soybean production.
Author's note
This research underscores the relevance of inoculation with the right rhizobia strain in the formation of nodules and the fixation of nitrogen and how this can inform farmers of the need for the application of relevant inoculants in soybean production.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Acknowledgments
The authors are most grateful to the School of Agriculture of the College of Basic and Applied Sciences, University of Ghana for the support in conducting this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adjei-Nsiah, S., Martei, D., Yakubu, A., and Ulzen, J. (2022). Soybean (Glycine max L. Merrill) response to phosphorus application and rhizobium inoculation on acrisols of the semi-deciduous forest agro-ecological zone of Ghana. PeerJ 10, e12671. doi: 10.7717/peerj.12671
Ao, X., Guo, X. H., Zhu, Q., Zhang, H. J., Wang, H. Y., Ma, Z. H., et al. (2014). Effect of phosphorus fertilization to P uptake and dry matter accumulation in soybean with different P efficiencies. J. Integr. Agric. 13, 326–334. doi: 10.1016/S2095-3119(13)60390-1
Awuni, G. A., Reynolds, D. B., Goldsmith, P. D., Tamimie, C. A., and Denwar, N. N. (2020). Agronomic and economic assessment of input bundle of soybean in moderately acidic savanna soils of Ghana. Agrosyst. Geosci. Environ. 3, 2–15. doi: 10.1002/agg2.20085
Beck, D. P., Materon, L. A., and Afandi, F. (1993). Practical Rhizobium Legume technology manuel, Technical Manual No: 19. Aleppo: International Centre for Agricultural Research in the Dry Areas (ICARDA).
Beyan, S. M., Wolde-meskel, E., and Dakora, F. D. (2018). An assessment of plant growth and N2 fixation in soybean genotypes grown in uninoculated soils collected from different locations in Ethiopia. Symbiosis 75, 189–203. doi: 10.1007/s13199-018-0540-9
Blake, G. R., and Hartge, K. H. (1986). “Bulk density,” in Methods of Soil Analysis, Part 1—Physical and Mineralogical Methods, 2nd Edn, ed A. Klute (Madison, WI: American Society of Agronomy), 363–382.
Boakye, E. Y., Lawson, I. Y. D., Danso, S. K. A., and Offei, S. K. (2016). Characterization and diversity of rhizobia nodulating selected tree legumes in Ghana. Symbiosis 69, 89–99. doi: 10.1007/s13199-016-0383-1
Bray, R. H., and Kurtz, L. T. (1945). Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 59, 39–46. doi: 10.1097/00010694-194501000-00006
Calvo, P., Nelson, L., and Kloepper, J. W. (2014). Agricultural uses of plant biostimulants. Plant Soil 383, 3–41. doi: 10.1007/s11104-014-2131-8
Cernay, C., Ben-Ari, T., Pelzer, E., Meynard, J. M., and Makowski, D. (2015). Estimating variability in grain legume yields across Europe and the Americas. Sci. Rep. 5, 11171. doi: 10.1038/srep11171
Dabesa, A., and Tana, T. (2021). Response of soybean [(Glycine max L. (Merrill)] to bradyrhizobium inoculation, lime, and phosphorus applications at Bako, Western Ethiopia. Int. J. Agron. 2021, 6686957. doi: 10.1155/2021/6686957
Day, P. R. (1965). “Particle fractionation and particle-size analysis,” in Methods of Soil Analysis, Part 1—Physical and Mineralogical Methods, ed A. Klute (Madison,WI: American Society of Agronomy), 545–567. doi: 10.2134/agronmonogr9.1.c43
Eze, P. N. (2008). Characterization, Classification and Pedogenesis of Soils on a Legon catena in the Accra Plains, Ghana. (M.Phil. Thesis), University of Ghana, Legon (Accra).
Fening, J. O., and Danso, S. K. A. (2002). Variation in symbiotic effectiveness of cowpea bradyrhizobia indigenous to Ghanaian soils. Appl. Soil Ecol. 21, 23–29. doi: 10.1016/S0929-1393(02)00042-2
Goyal, R. K., Mattoo, A. K., and Schmidt, M. A. (2021). Rhizobial–Host interactions and symbiotic nitrogen fixation in legume crops toward agriculture sustainability. Front. Microbiol. 12, 669404. doi: 10.3389/fmicb.2021.669404
Gunnabo, A. H., Geurts, R., Wolde-meskel, E., Degefu, T., Giller, K. E., and van Heerwaarden, J. (2019). Genetic interaction studies reveal superior performance of Rhizobium tropici CIAT899 on a range of diverse East African common bean (Phaseolus vulgaris L.) genotypes. Appl. Environ. Microbiol. 85, e01763–e01719. doi: 10.1128/AEM.01763-19
Iannetta, P. P., Young, M., Bachinger, J., Bergkvist, G., Doltra, J., Lopez-Bellido, R. J., et al. (2016). A comparative nitrogen balance and productivity analysis of legume and non-legume supported cropping systems: the potential role of biological nitrogen fixation. Front. Plant Sci. 7, 1700. doi: 10.3389/fpls.2016.01700
Jarecki, W., Buczek, J., and Bobrecka-Jamro, D. (2016). Response of soybean [Glycine max (L.) Merr.] to bacterial soil inoculants and foliar fertilization. Plant Soil Environ. 62, 422–427. doi: 10.17221/292/2016-PSE
Martyniuk, S., Kozieł, M., and Stalega, J. (2013). Effect of various strains of symbiotic bacteria on yields and nodulation of lupine and soybean. J. Res. Appl. Agric. Eng. 58, 67–70.
Mathenge, C., Thuita, M., Masso, C., Gweyi-Onyango, J., and Vanlauwe, B. (2019). Variability of soybean response to rhizobia inoculant, vermicompost, and a legume-specific fertilizer blend in Siaya County of Kenya. Soil Tillage Res. 194, 104290. doi: 10.1016/j.still.2019.06.007
Míguez-Montero, M. A., Valentine, A., and Pérez-Fernández, M. A. (2020). Regulatory effect of phosphorus and nitrogen on nodulation and plant performance of leguminous shrubs. AoB Plants 12, 1–11. doi: 10.1093/aobpla/plz047
Mitsuchi, M. (2012). Characteristics and genesis of nodules and concretions occurring in soils of the R. Chinit area, Kompong Thom Province, Cambodia. Soil Sci. Plant Nutr. 22, 409–421. doi: 10.1080/00380768.1976.10433003
MoFA and CSIR. (2005). Soybean Production Guide. Food Crops Development Project. Accra: Ghana's Ministry of Food and Agriculture.
Murphy, J., and Riley, J. P. (1962). A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 27, 31–36. doi: 10.1016/S0003-2670(00)88444-5
Nartey, E., Dowuona, G. N., Ahenkorah, Y., Mermut, A. R., and Tiessen, H. (1997). Amounts and distributions of some forms of phosphorus in ferruginous soils of the interior savanna zone of Ghana. Ghana J. Agric. Sci. 30, 135–143. doi: 10.4314/gjas.v30i2.1965
Ronner, E., Franke, A. C., Vanlauwe, B., Dianda, M., Edeh, E., Ukem, B., et al. (2016). Understanding variability in soybean yield and response to P-fertilizer and rhizobium inoculants on farmers' fields in northern Nigeria. Field Crop. Res. 186, 133–145. doi: 10.1016/j.fcr.2015.10.023
Sandhu, D., Coleman, Z., Atkinson, T., Rai Krishan, M., and Mendu, V. (2018). Genetics and physiology of the nuclearly inherited yellow foliar mutants in soybean. Front. Plant Sci. 9, 471. doi: 10.3389/fpls.2018.00471
Sanz-Sáez, Á., Heath, K. D., Burke, P. V., and Ainsworth, E. A. (2015). Inoculation with an enhanced N2-fixing Bradyrhizobium japonicum strain (USDA110) does not alter soybean (Glycine max Merr.) response to elevated CO2. Plant Cell Environ. 38, 1–14. doi: 10.1111/pce.12577
Somasegaran, P., and Hoben, H. J. (1994). Hand Book for Rhizobia. New York, NY: Springer–verlag, 79–158. doi: 10.1007/978-1-4613-8375-8_18
Tewari, K., Minagawa, R., Suganuma, T., Fujikake, H., Ohtake, N., Sueyoshi, K., et al. (2003). Effect of deep placement of slow-release nitrogen fertilizers and inoculation of Bradyrhizobia on the first cropping of soybean in the field dressed with mountain soil. Soil Sci. Plant Nutr. 74, 183–189.
Thilakarathna, M. S., and Raizada, M. N. (2017). A meta-analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field conditions. Soil Biol. Biochem. 105, 177–196. doi: 10.1016/j.soilbio.2016.11.022
Tikde, S. A., Ramakrishna, D., Kiran, S., Kosturkova, G., and Ravishankar, G. A. (2015). Nutraceutical potential of soybean: review. Asian J. Clin. Nutr. 7, 22–32. doi: 10.3923/ajcn.2015.22.32
Ulzen, J., Abaidoo, R. C., Mensah, N. E., Masso, C., and AbdelGadir, A. H. (2016). Bradyrhizobium inoculants enhance grain yields of soybean and cowpea in Northern Ghana. Front Plant Sci. 7, 1770. doi: 10.3389/fpls.2016.01770
Van Kessel, C., and Hartley, C. (2000). Agricultural management of grain legumes: has it led to an increase in nitrogen fixation? Field Crops Res. 65, 165–181. doi: 10.1016/S0378-4290(99)00085-4
Vincent, J. M. (1970). A Manual for the Practical Study of Root-Nodule Bacteria. London: Blackwell Scientific Publications.
Vollmann, J. (2016). Soybean versus other food grain legumes: a critical appraisal of the United Nations International year of pulse 2016. J. Land Manage. Food Environ. 67, 17–24. doi: 10.1515/boku-2016-0002
Yang, S., Chen, W., Wang, E., Chen, W., Yan, J., Han, X., et al. (2018). Rhizobial biogeography and inoculation application to soybean in four regions across China. J. Appl. Microbiol. 125, 853–866. doi: 10.1111/jam.13897
Zander, P., Amjath-Babu, T. S., Preissel, S., Reckling, M., Bues, A., Schläfke, N., et al. (2016). Grain legume decline and potential recovery in European agriculture: a review. Agron. Sustain. Dev. 36, 26. doi: 10.1007/s13593-016-0365-y
Keywords: Bradyrhizobia, efficacy, nitrogen fixation, nodulation, inoculant
Citation: Nartey EK, Darko DA, Sulemana N and Assibey EO (2022) Efficacy of Histick Soy in soybean nodulation in two Alfisols of Ghana. Front. Sustain. Food Syst. 6:1004090. doi: 10.3389/fsufs.2022.1004090
Received: 26 July 2022; Accepted: 21 November 2022;
Published: 14 December 2022.
Edited by:
Benedicta Essel Ayamba, Council for Scientific and Industrial Research (CSIR), GhanaReviewed by:
Bharat Prakash Meena, Indian Institute of Soil Science (ICAR), IndiaJaime H. Mejías, Instituto de Investigaciones Agropecuarias, Chile
Copyright © 2022 Nartey, Darko, Sulemana and Assibey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel A. Darko, ZGFkYXJrb0B1Zy5lZHUuZ2g=
 Eric K. Nartey
Eric K. Nartey Daniel A. Darko
Daniel A. Darko Nasirudeen Sulemana
Nasirudeen Sulemana Ernest Osei Assibey3
Ernest Osei Assibey3