- Department of Soil Science and Agricultural Chemistry, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi, India
A majority of agricultural activities are conducted under fragile lands or set-up. The growth and development of crops are negatively affected due to several biotic and abiotic stresses. In the current situation, research efforts have been diverted toward the short-term approaches that can improve crop performance under changing environments. Seed treatment or priming technology is in a transition phase of its popularity among resource-poor farmers. Suitable policy intervention can boost low-cost techniques to implement them on a larger scale in developing countries and to harness the maximum benefits of sustainable food production systems. Primed seeds have high vigor and germination rate that help in seedling growth and successful crop stand establishment under stress conditions. This review is attempted to assess different seed priming techniques in terms of resource use efficiency, crop productivity, cost–benefit balance, and environmental impacts. Moreover, a comprehensive study of the mechanisms (physiological and biochemical) of seed priming is also elaborated. A detailed examination of the applications of priming technology under diverse agroecosystems can improve our understanding of the adaptive management of natural resources.
Introduction
Nearly 60% of the Indian population relies upon agriculture and its associated sectors for their livelihood. Enormous improvement in food grain production was achieved through the Green Revolution with the adoption of modern approaches such as high-yielding varieties, agrochemicals and intensified farming (Akhilesh and Kavitha, 2020). Although high-yielding varieties are being promoted, the bulk of the tropical land fails to provide suitable growth conditions for these high-yielding crops; and consequently, low yield is obtained even after the application of necessary agrochemicals. Indiscriminate use of these chemicals causes environmental pollution, soil degradation and economical burden to farmers. Different factors such as rapid growth in population rate, cultivating lands that are ill-suited for agriculture, inappropriate farming practices and eradication of trees for fuel and shelter can render tropical soils to be fragile. Further, the regional agricultural hubs under tropical conditions are highly affected by extreme events of climate change, e.g., heavy rainfall, floods and droughts (González-Orozco et al., 2020; Sarkar et al., 2020a).
Dealing with fragile ecosystems is challenging for agricultural researchers to provide restorative solutions. In this context, to curtail the effects of climate change and continue cultivation practices under fragile ecosystems, the adoption of eco-friendly and economical techniques such as seed priming, low-input sustainable agriculture, conservation agriculture, etc., are imperative (Rakshit and Singh, 2018; Sarkar et al., 2020a). Pre-sowing techniques like seed priming grabbed the attention of researchers and grew into the subject of extensive investigation and interest when it addressed the problems of slow and non-uniform germination, low seed vigor, poor crop stand, biotic and abiotic stresses, poor product quality, etc. (Paparella et al., 2015; Chatterjee et al., 2018; Zulfiqar, 2021). Seed priming eases germination even under adverse conditions, lifts crop performance and enhances yield potential (Ajouri et al., 2004; Ibrahim, 2016; Marthandan et al., 2020). To alleviate the effects of modern agriculture, this technique emerged as a viable strategy that protects plants against both biotic and abiotic stresses (Sarkar et al., 2018). The absolute performance of seed priming is more prominent under adverse conditions (Parera and Cantliffe, 1994) such as fragile ecosystems than favorable conditions.
Fragile Ecosystems
Fragile lands comprise tropics, semi-arid areas, deserts, wetlands, islands, coastal regions and mountains (ENVIS Resource Partner on Biodiversity, 2020). It is projected that over 33% of land resources in the world are degraded owing to intensive agricultural practices, deforestation, desertification, salinization, pollution, industrialization, urbanization, unscientific land-use practices, climate change, etc. (Abhilash, 2021). Globally, drylands are distributed around 6.45 billion hectares, and 70% of these lands are used for cultivation purposes (Karim and Rahman, 2015). Peculiar characteristics of desert soils are drought and aridity circumstances where crop experiences water-deficit conditions. In desert farming, crop strives with water-scarce situations as a result of inadequate rainfall, and rapid land surface evaporation leads to crop failure or reduction in yield. Annual rainfall in semi-arid regions falls in between desert ecosystem and potential evapotranspiration. The major hazards in semi-arid regions are low rainfall, soil erosion and salinity that lead to low soil organic carbon content, available nitrogen and poor soil structure (Garcia-Franco et al., 2018). According to Chivasa et al. (2001), the poor stand establishment of tropical crops in semi-arid regions of Zimbabwe is primarily due to low-quality seeds, poor sowing techniques and low soil moisture. Salinity has affected above 800 million hectares of the global area (Gopalakrishnan and Kumar, 2020). The expansion rate of soil salinization indicates that more than 50% of global arable land will be affected by 2050 (Tisarum et al., 2020). The germination percentage, as well as germination time, decreases with an increase in salt concentration (Ibrahim, 2016).
In India, mountains are sustaining 6% of the population and 18% of the geographical area (Wani, 2011) where the potential of agriculture is extensive. The major constraint in hilly lands is undulating topography, which causes soil erosion and a reduction in soil fertility. Further, low temperature or chilling stress generates reactive oxygen species (ROS), which hampers seed germination (Farooq et al., 2017). Wetlands contribute 4.7% of India's geographical area (Bassi et al., 2014), which are saturated with water throughout the year or differing durations. The main limitations in wetland agriculture are excessive flood and drought damage and nutrient run-off. In small islands, the challenge is to meet people's demands with limited resources. Similar to other categories of fragile lands, small island soils too struggle with complications such as irregular topography, ecosystem instability, soil erosion and loss of fertility. Coastal areas occupy more than 10% of land on the Earth's surface (Ministry of Environment Forest, 2012). Agriculture in coastal areas faces considerable threats such as seawater inundation, soil salinity, and erosion.
Integration of Resources is Key
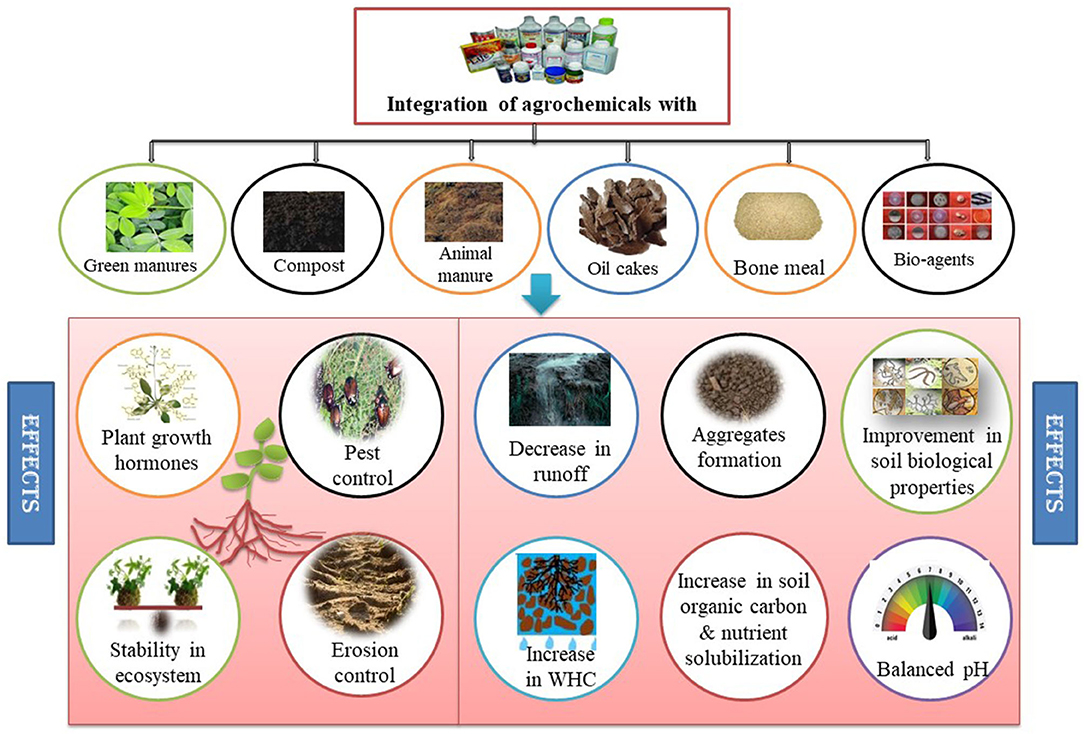
Fragile ecosystems are extremely vulnerable to climate change that induces adverse effects on crop production and food security. For example, diffusion and mass flow of nutrients (especially water-soluble ones) decrease due to drought stress (Vurukonda et al., 2016). To meet the nutrient demand of crops and for the finest yields, chemical fertilizers play a key role. Despite several advantages, chemical fertilizers and pesticides are unfriendly to the environment, causing damage to soil, plant, animal, and human health and also placing an economic burden on farmers. Indiscriminate application of synthetic fertilizers interrupts sustainability and environmental quality, while the reduction of organic matter application causes adverse impacts on soil health (Sarkar et al., 2017a; Ferdous et al., 2020). Loss of soil fertility is accompanied by decreased soil aggregation, water-holding capacity (WHC), biodiversity and crop stress tolerance (Sergaki et al., 2018). To minimize these impaired effects of climate change and agrochemicals on soils, agriculture and environment, switching to the integration of fertilizers and naturally available resources that sustain environment and restore nutrients naturally through supporting internal cycling and assuring harmony of ecosystem is an appropriate practice to restore the quality of degraded systems (Singh and Reddy, 2011; Dubey et al., 2021a,b). These are basically practiced with a customized approach. Integration of agrochemicals with organic or natural resources such as farmyard manure (FYM), compost and bio-fertilizers replaces some quantity of fertilizers, supplements a part of energy, improves soil properties and maintains sustainability (Figure 1). In a 5-years field experiment on wheat, integrating 50 and 75% of recommended dose of NPK fertilizers with FYM and bio-fertilizer enhanced grain yield significantly in all seasons and also reduced the quantity of chemical fertilizer requirement (Cisse et al., 2019). Further, integration of manure and bio-agent with 50% recommended dose of fertilizers greatly enhanced yield in the long run. Single and combined application of organics, viz. press mud, FYM and vermicompost, in bread wheat revealed the improvement in grain yield by 30–68%, and the highest B:C ratio was recorded in FYM treatments (Ali et al., 2020).
Seed Priming—A Viable Answer
Modern agricultural practices with an intensive approach (e.g., heavy tillage, synthetic agrochemicals, hybrid varieties, and monocropping) may further deteriorate fragile lands by accelerating the loss of organic matter, degradation of soil and environmental damage (e.g., deterioration of water and air quality, loss of biodiversity, and biomagnification); thus, several attempts have been carried out to achieve ecosystem stability. Vital aspects of crop improvement through maintaining sustainability can be accomplished by modulating the metabolism of seed that would be achieved by seed priming technique. Seed priming is a pre-sowing seed treatment that allows controlled hydration of seeds to imbibe water and go through the first stage of germination but does not allow radical protrusion through the seed coat (McDonald, 2000).
Seed priming hastens the germination process and enhances the rate of seedling emergence even under extreme climatic conditions and in problem soils. Seed priming is categorized into different types, viz. hydropriming, osmopriming, halopriming, hormonal priming, and biopriming, and provides extensive crop benefits. Seed priming techniques can deal with detrimental conditions in fragile lands such as drought, heat stress, salinity, nutrient stress and several environmental stresses. Seed soaking in distilled water and osmotic solutions is considered hydropriming and osmopriming, respectively. Hydropriming in paddy crop enhanced resistance against CO2 stress and oxidative damage (Nedunchezhiyan et al., 2020), while osmopriming with CaCl2 in wheat provided resistance against drought stress (Hussain et al., 2018). Seed imbibition using phytohormones to activate seed metabolism is known as hormonal priming. Seed priming with cytokinins (plant growth substance) imparted salt stress in wheat (Iqbal et al., 2006) and drought tolerance in soybean (Mangena, 2020). Beneficial microorganisms or plant growth-promoting microorganisms used for seed treatment are crucial for biopriming. Groundnut seeds treated with Pseudomonas fluorescens mitigated salt stress under field conditions (Saravanakumar and Samiyappan, 2007). Enhanced plant stress tolerance against drought was also recorded due to bacterial priming (Bacillus thuringiensis) of wheat seeds (Timmusk et al., 2014). Chemical priming is another type of priming where seed treatment is carried using natural substances (organic acids, plant extracts, chitosan, polyamines, mannose, trehalose, etc.) or synthetic compounds (sodium nitroprusside, sodium hypochlorite, etc.) (Jisha et al., 2013; Paparella et al., 2015; Lutts et al., 2016). However, combined application of natural and synthetic substances is also a common practice in seed priming. For example, Bajwa et al. (2018) found that priming wheat seeds with sorghum extracts and benzyl aminopurine improved crop performance under saline soil conditions. Choosing an appropriate priming approach in accordance with the limitations of a fragile land would be pertinent to combat stress.
Mechanisms of Seed Priming
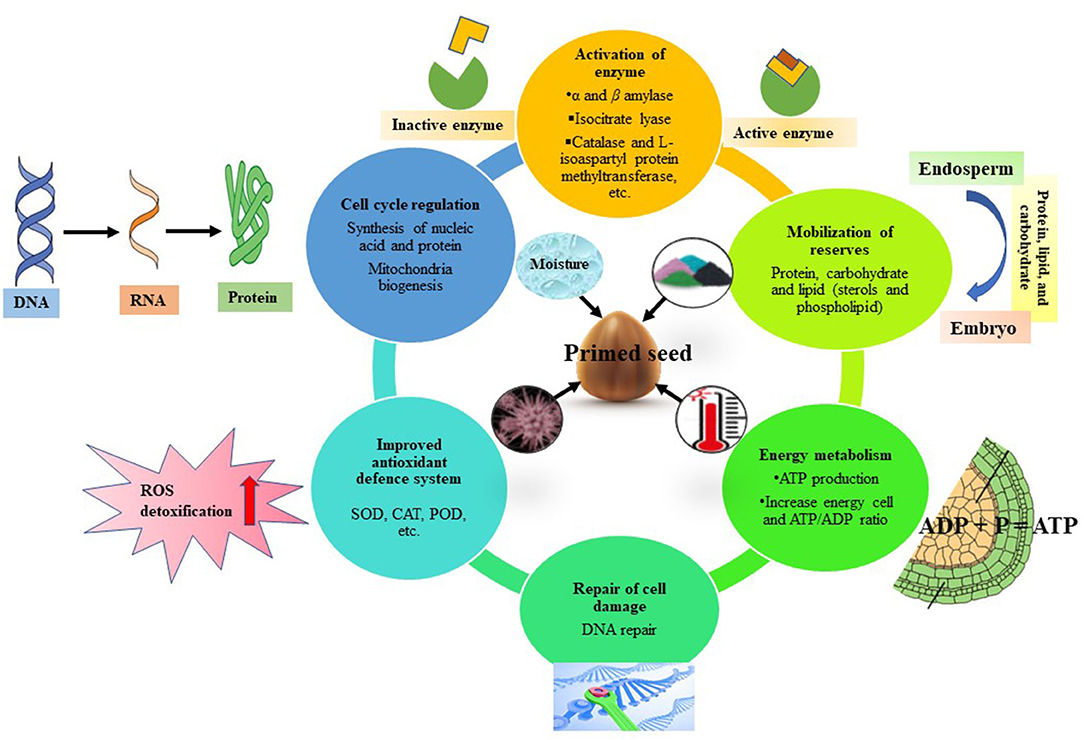
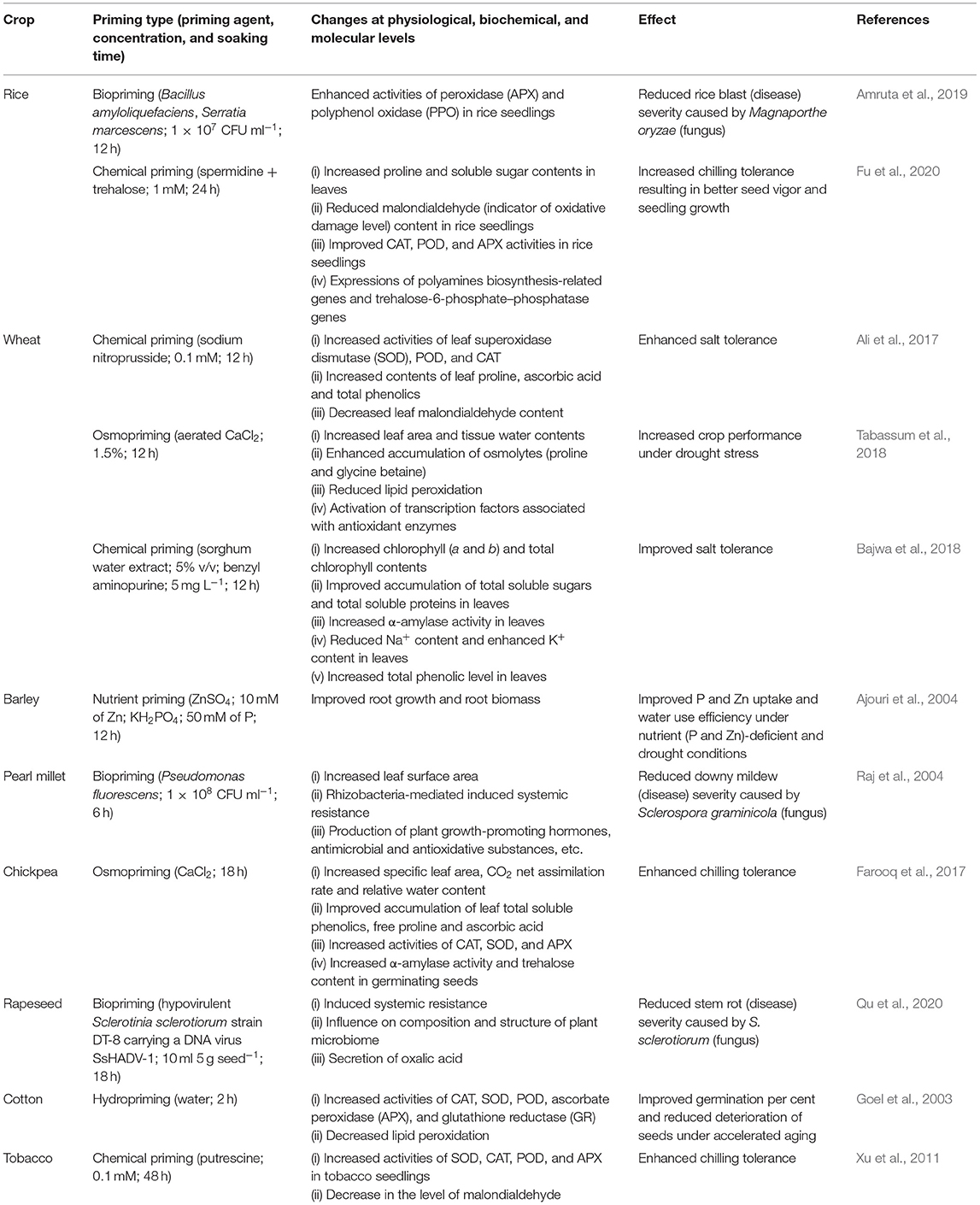
Priming exposes seed to stimuli, in response to which a set of interlinked biochemical changes occurs such as activation of enzymes, synthesis of growth-promoting substances, metabolism of germination inhibitors and repair of cell damage (Farooq et al., 2010; Chatterjee et al., 2018). The process of seed priming is accomplished in three stages (Ibrahim, 2016; Lutts et al., 2016; Marthandan et al., 2020). Seed imbibition is the first (I) stage, where the seed uptakes water rapidly as the water potential of the seed is low. Stage II is known as the activation phase. This stage is characterized by a series of metabolic and repairing events at the cellular level (Figure 2). The moisture content decreases, and the major changes documented during this stage include synthesis of protein, formation of new mitochondria, activation of enzymes and antioxidant system, and DNA repair. Seed imbibition is stopped after this stage. Rehydration during seed priming induce changes at the cellular level such as cell division, synthesis of nucleic acids, protein, ATP production, increase in cell energy, ATP/ADP ratio for energy requirement, accumulation of essential lipids, production of antioxidants and activation of DNA repair mechanism (Varier et al., 2010; Paparella et al., 2015; Lutts et al., 2016). In the cell damage repair system, DNA repair is most crucial because, in case of defective repair, oxidative injury can lead to cell death during germination (Paparella et al., 2015). Studies revealed that proteins, carbohydrates and lipid-mobilizing enzymes are activated during seed priming (Varier et al., 2010; Di Girolamo and Barbanti, 2012; Hameed et al., 2013). For example, α-amylase activity along with total soluble sugars increased in wheat when primed with benzyl aminopurine and sorghum water extract (Bajwa et al., 2018). α-Amylase is known to hydrolyse the starch (polysaccharides) reserves into sugars (simple forms). Priming enhances protein synthesis by increasing the synthesis of rRNA and improving the integrity of ribosomes. Production of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) and maintaining a balance between generation and destruction of ROS such as hydrogen peroxide, superoxide and hydroxyl radicals under stress conditions are important effects of seed priming (Wojtyla et al., 2016; Farooq et al., 2017). In stage III, the uptake of water is rapid, and protrusion of the radicle indicates that the germination process has entered into the growth and cell elongation phase (Marthandan et al., 2020). Metabolic changes of seeds induced by priming are detailed with some research findings in Table 1.
Practices and Performances of Seed Priming
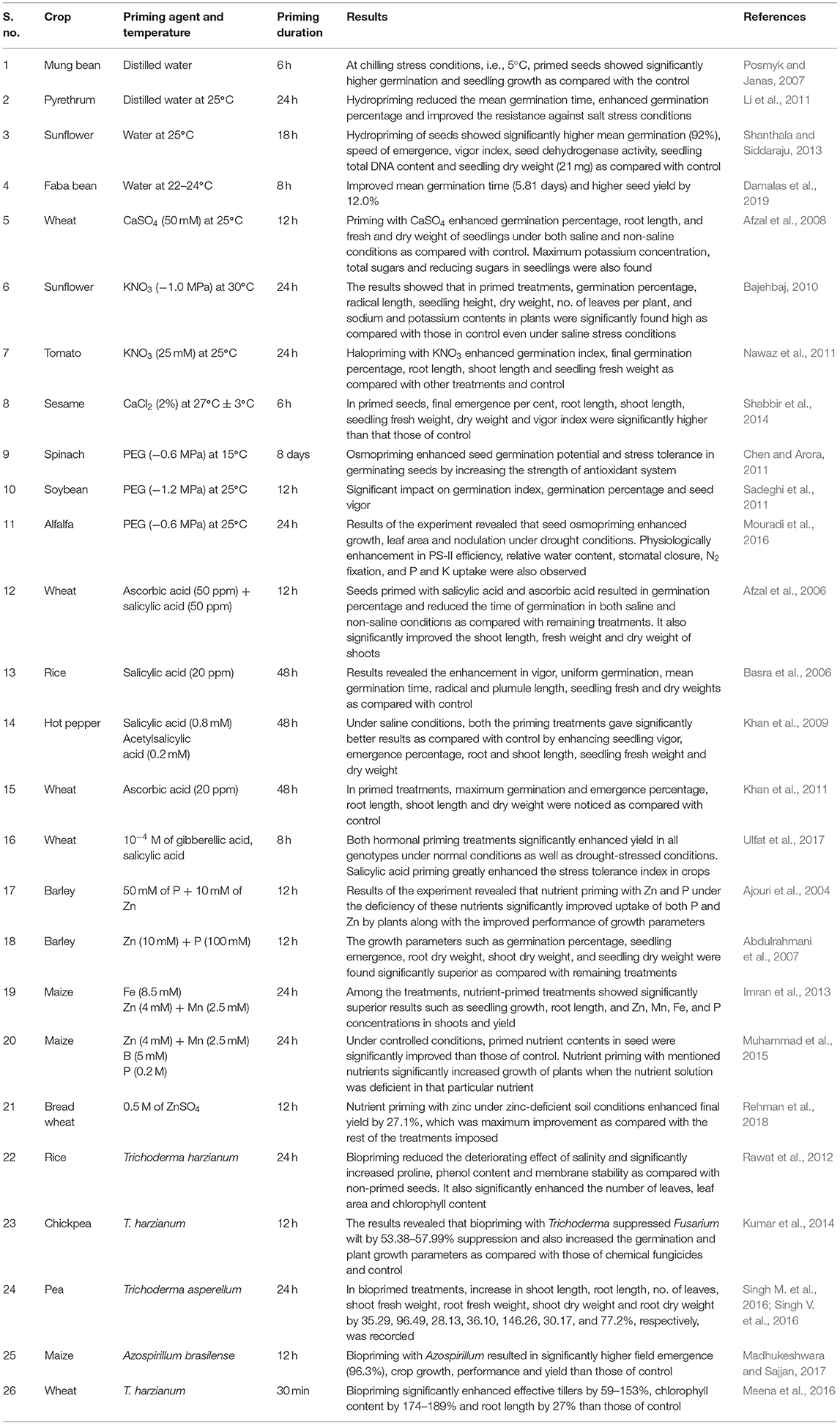
The objectives of seed priming related to improved crop performance are achieved by different methods as described in the earlier section. Each priming technique involves restricted hydration to allow imbibition and pre-germinative metabolic changes (Figure 2). The efficiency of priming is affected by numerous factors and straightaway depends on treated crop species, particular cultivar and selected priming method. Physical, chemical and biological factors such as nature of solute and water content, primers, time, temperature, existence or non-existence of luminosity, aeration and seed condition also control priming success and decide germination rate and time, seedling vigor and finally plant growth and development. In hydropriming, the soaking of seeds in water for a specific period of time before sowing is ensured, which will finally be dried to a certain moisture content (Singh et al., 2015) and is an effortless, economical and environment-friendly technique. It is a potent approach to combat abiotic stresses such as heat and drought stress to crop. Different chemical solutions are also used as priming agents. Soaking of seeds in the solution with inorganic salts, viz. NaCl, KNO3, CaCl2, and CaSO4, is known as halopriming (Nawaz et al., 2013). Halopriming stimulates the crop to raise robust even under soil salinity. This is a common observation in hydropriming that due to the high water potential of pure water entry to seed is very fast, which allows abrupt seed imbibition, which may not be congenial for germination metabolic activation and cell elongation under diverse agro-ecologies. To overcome this hurdle, seeds are soaked in an osmotic solution such as sugar, polyethylene glycol (PEG), glycerol, sorbitol and mannitol, followed by air drying. Osmosis controls the excess water entry into the seed during imbibition, hence reducing the ROS accumulation and thus protecting the cell from oxidative injury. Osmopriming improves crop performance in both saline and non-saline conditions. Because of its direct impact on seed metabolism, plant growth regulators in minuscule quantities are also used in pre-sowing seed treatment with different organic compounds, viz. IAA, salicylic acid, ascorbate and kinetin (Nawaz et al., 2013), and not only promotes growth and development but also alleviates the impacts of several environmental stresses. In diverse stressed agro-ecologies, it is a common practice where seeds are soaked in a nutrient solution with certain concentration for a specific time period before being sowed with a resultant positive effect on growth, yield and nutrient uptake (Shivay et al., 2016). Researchers have documented that seed priming with macro- or micronutrients can increase water uptake efficiency, enhance nutrient substances and hasten the seed germination rate and seedling development (Bhowmick et al., 2013; Rakshit et al., 2013, 2014).
The application of beneficial microorganisms (Trichoderma harzianum, Azospirillum lipoferum, Pseudomonas fluorescens, Rhizobium spp., Bacillus spp., etc.) improves soil properties, enhances plant growth and increases the activity of symbiotic microbes (Javaid, 2010; Sarkar et al., 2017b). Further, they improve plant performance by releasing plant growth hormones, mobilizing nutrients and suppressing pests and diseases. Among all methods of microbial applications, the advanced method is seed biopriming. It is the seed pre-soaking (seed hydration) along with inoculation of beneficial microorganisms. Biopriming promotes microbial colonization at the root zone of the plant and thus enhances plant growth (Raj et al., 2004; Sarkar et al., 2020b).
The capability of seed priming to enhance nutrient use efficiency can be an answer for limited mineral reserves of essential nutrients as it is being depleted day by day. Under the current agricultural scenario, ~50% of applied fertilizers are indeed utilized by crop (Parihar et al., 2019), and the rest is subjected to losses through leaching, soil fixation, low mobility, denitrification, etc., posing threat to the environment and economy, which could be minimized by seed priming that enhances uptake nutrients and minimize losses (Sarkar et al., 2021). Biopriming of wheat seed with T. harzianum improved nitrogen use efficiency (agronomic) up to 3.36% when applied with 1/4th N and recommended dose of PK (Meena et al., 2016). Nutrient priming of barley seed with P and Zn individually or in combination enhanced the nutrient uptake and water use efficiency under nutrient (P and Zn)-deficient and drought conditions (Ajouri et al., 2004). In a P-deficient tropical soil, priming of mung bean seeds with 0.01 and 0.02% P enhanced the P uptake (Shah et al., 2012). Kumar et al. (2020) reported maximum P solubilizing activity in a consortium of Burkholderia gladioli, Pseudomonas sp., and Bacillus subtilis, and the application of this consortium through seed biopriming improved the soil available P by 54%.
Seeds priming improves water use efficiency and, hence, suitable in drought-prone areas as well. Seed priming is one of the lifelong practices with new age interventions that proved to be an efficient technology for plants under water stress conditions. Under mild-to-severe drought conditions, priming of sesame seeds with different species of mycorrhizal fungi (Funneliformis mosseae and Rhizoglomus intraradices) enhanced water use efficiency by 6% to 10% by improving root length and overall root system (Askari et al., 2019). Priming of rice seed with moringa leaf extract increased the water productivity to the highest level (compared with CaCl2 and KCl) when managed under alternate wetting and drying conditions (Rehman et al., 2015). Osmopriming with different molecules (gibberellic acid and ammonium molybdate) improved water use efficiency in summer cowpea seeds sown under limited water availability conditions (Arun et al., 2017).
The economical profit of a crop via any technology can be determined through a benefit–cost (B:C) ratio analysis that can be described as an evaluation of expenditure through comparing economic benefit with the economic cost of an activity (Shively and Galopin, 2013). Seed priming techniques reduce chemical input requirements of the crop by increasing efficiency (Rakshit et al., 2014), which helps in enhancing the B:C ratio. In a 3-years experiment on wheat, the economic returns and B:C ratio were higher in osmopriming compared with hydropriming (Farooq et al., 2020). Enhancement in economic yield per cent by 4–39% was reviewed in wheat, rice, maize, and linola crops through the adoption of different seed priming techniques (Farooq et al., 2019). The seed biopriming technique regulates defense systems of crops to overcome climatic conditions under varied agro-ecologies and showed a higher B:C ratio than that of the recommended dose of fertilizer (Devika and Rakshit, 2019). While comparing the effects of hydropriming, chemical priming and hormonal priming on developing salt tolerance in Indian mustard, Srivastava et al. (2010) concluded that hydropriming is a simple and cost-effective approach to achieve the target.
Seed priming is an economical and trending technique that can resist environmental stresses as well as biotic stresses (pests and pathogens). The benefits of seed priming with the help of research evidence are shown in Table 2.
Conclusions
Seed priming is a viable option to boost the performance of crops under fragile ecosystems. An enhanced understanding of the metabolic events taking place throughout the priming intervention and the subsequent germination should facilitate to use this simple and inexpensive technology for maximizing seed performance in a more resourceful way under varied fragile ecologies. However, many popular technologies failed to reach the farmers' field due to a lack of awareness. Suitable policy intervention to strengthen the extension services moves the targets to the next level. It is desirable to highlight the advantages of seed priming in terms of ecosystem restoration and harness sustainable biomass production from degraded lands.
Author Contributions
OSD, SS, PB, and JS: writing. AR: writing, editing, and supervision. DS: writing and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdulrahmani, B., Ghassemi-Golezani, K., Valizadeh, M., and Asl, V. F. (2007). Seed priming and seedling establishment of barley (Hordeum vulgare L.). J. Food Agricult. Environ. 5:179.
Abhilash, P. C. (2021). Restoring the unrestored: strategies for restoring global land during the UN decade on ecosystem restoration (UN-DER). Land 10:201. doi: 10.3390/land10020201
Afzal, I., Basra, S. M., Farooq, M., and Nawaz, A. (2006). Alleviation of salinity stress in spring wheat by hormonal priming with ABA, salicylic acid and ascorbic acid. Int. J. Agric. Biol. 8, 23–28.
Afzal, I., Rauf, S., Basra, S. M. A., and Murtaza, G. (2008). Halopriming improves vigor, metabolism of reserves and ionic contents in wheat seedlings under salt stress. Plant Soil Environ. 54, 382–388. doi: 10.17221/408-PSE
Ajouri, A., Asgedom, H., and Becker, M. (2004). Seed priming enhances germination and seedling growth of barley under conditions of P and Zn deficiency. J. Plant Nutr. Soil Sci. 167, 630–636. doi: 10.1002/jpln.200420425
Akhilesh, K. B., and Kavitha, S. (2020). “A study on impact of technology intervention in the field of agriculture in India,” in Smart Technologies, eds K. B. Akhilesh and D. Möller (Singapore: Springer), 373–385. doi: 10.1007/978-981-13-7139-4_28
Ali, N., Khan, M. N., Ashraf, M. S., Ijaz, S., Saeed-ur-Rehman, H., Abdullah, M., et al. (2020). Influence of different organic manures and their combinations on productivity and quality of bread wheat. J. Soil Sci. Plant Nutr. 20, 1949–1960. doi: 10.1007/s42729-020-00266-2
Ali, Q., Daud, M. K., Haider, M. Z., Ali, S., Rizwan, M., Aslam, N., et al. (2017). Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol. Biochem. 119, 50–58. doi: 10.1016/j.plaphy.2017.08.010
Amruta, N., Kumar, M. P., Kandikattu, H. K., Sarika, G., Puneeth, M. E., Ranjitha, H. P., et al. (2019). Bio-priming of rice seeds with novel bacterial strains, for management of seedborne Magnaporthe oryzae L. Plant Physiol. Rep. 24, 507–520. doi: 10.1007/s40502-019-00492-6
Arun, M. N., Hebbar, S. S., Bhanuprakas, K., and Senthivel, T. (2017). Seed priming improves irrigation water use efficiency, yield and yield components of summer cowpea under limited water conditions. Legume Res. 40, 864–871. doi: 10.18805/LR-3785
Askari, A., Ardakani, M. R., Paknejad, F., and Hosseini, Y. (2019). Effects of mycorrhizal symbiosis and seed priming on yield and water use efficiency of sesame under drought stress condition. Sci. Hortic. 257, 108749. doi: 10.1016/j.scienta.2019.108749
Bajehbaj, A. A. (2010). The effects of NaCl priming on salt tolerance in sunflower germination and seedling grown under salinity conditions. Afr. J. Biotechnol. 9, 1764–1770. doi: 10.5897/AJB10.1019
Bajwa, A. A., Farooq, M., and Nawaz, A. (2018). Seed priming with sorghum extracts and benzyl aminopurine improves the tolerance against salt stress in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 24, 239–249. doi: 10.1007/s12298-018-0512-9
Basra, S. M. A., Farooq, M., Wahid, A., and Khan, M. B. (2006). Rice seed invigoration by hormonal and vitamin priming. Seed Sci. Techn. 34, 753–758. doi: 10.15258/sst.2006.34.3.23
Bassi, N., Kumar, M. D., Sharma, A., and Pardha-Saradhi, P. (2014). Status of wetlands in India: A review of extent, ecosystem benefits, threats and management strategies. J. Hydrol. Reg. Studies 2, 1–19. doi: 10.1016/j.ejrh.2014.07.001
Bhowmick, M. K., Duary, B., Biswas, P. K., Rakshit, A., and Adhikari, B. (2013). Seed priming, row spacing and foliar nutrition in relation to growth and yield of chickpea under rainfed condition. SATSA Mukhapatra-Annu. Techn. Issue 17, 114–119.
Chatterjee, N., Sarkar, D., Sankar, A., Sumita, P. A. L., Singh, H. B., Singh, R. K., et al. (2018). On-farm seed priming interventions in agronomic crops. Acta Agric. Slov. 111, 715–735. doi: 10.14720/aas.2018.111.3.19
Chen, K., and Arora, R. (2011). Dynamics of the antioxidant system during seed osmopriming, post-priming germination, and seedling establishment in spinach (Spinacia oleracea). Plant Sci. 180, 212–220. doi: 10.1016/j.plantsci.2010.08.007
Chivasa, W., Harris, D., and Nyamudeza, P. (2001). On-farm seed priming: a key technology to improve crop establishment and yield in semi-arid tropics. Int. Sorgh. Mill. Newsletter. 42, 112–113.
Cisse, A., Arshad, A., Wang, X., Yattara, F., and Hu, Y. (2019). Contrasting impacts of long-term application of biofertilizers and organic manure on grain yield of winter wheat in North China Plain. Agronomy 9:312. doi: 10.3390/agronomy9060312
Damalas, C. A., Koutroubas, S. D., and Fotiadis, S. (2019). Hydro-priming effects on seed germination and field performance of faba bean in spring sowing. Agriculture 9:201. doi: 10.3390/agriculture9090201
Devika, O. S., and Rakshit, A. (2019). Economic appraisal of bio-priming mediated stress moderation in crop plants. Econ. Affairs 64:295570. doi: 10.30954/0424-2513.3.2019.12
Di Girolamo, G., and Barbanti, L. (2012). Treatment conditions and biochemical processes influencing seed priming effectiveness. Ital. J. Agron. 7:e25. doi: 10.4081/ija.2012.e25
Dubey, P. K., Singh, A., Raghubanshi, A., and Abhilash, P. C. (2021a). Steering the restoration of degraded agroecosystems during the United Nations Decade on Ecosystem Restoration. J. Environ. Manage. 280, 111798. doi: 10.1016/j.jenvman.2020.111798
Dubey, R. K., Dubey, P. K., Chaurasia, R., Rao, C. S., and Abhilash, P. C. (2021b). Impact of integrated agronomic practices on soil fertility and respiration on the indo-gangetic plain of North India. Agronomy 11, 402. doi: 10.3390/agronomy11020402
ENVIS Resource Partner on Biodiversity. (2020). Fragile Ecosystems of India. Botanical Survey of India, Kolkata, West Bengal. Available online at: http://www.bsienvis.nic.in/Database/FragileEcosystems_23603.aspx
Farooq, M., Basra, S. M., Wahid, A., and Ahmad, N. (2010). Changes in nutrient-homeostasis and reserves metabolism during rice seed priming: consequences for seedling emergence and growth. Agricult. Sci. China 9, 191–198. doi: 10.1016/S1671-2927(09)60083-3
Farooq, M., Hussain, M., Habib, M. M., Khan, M. S., Ahmad, I., Farooq, S., et al. (2020). Influence of seed priming techniques on grain yield and economic returns of bread wheat planted at different spacings. Crop Past. Sci. 71, 725–738. doi: 10.1071/CP20065
Farooq, M., Hussain, M., Nawaz, A., Lee, D. J., Alghamdi, S. S., and Siddique, K. H. (2017). Seed priming improves chilling tolerance in chickpea by modulating germination metabolism, trehalose accumulation and carbon assimilation. Plant Physiol. Biochem. 111, 274–283. doi: 10.1016/j.plaphy.2016.12.012
Farooq, M., Usman, M., Nadeem, F., Rehman, H., Wahid, A., Basra, S. M., et al. (2019). Seed priming in field crops: potential benefits, adoption and challenges. Crop Past. Sci. 70, 731–771. doi: 10.1071/CP18604
Ferdous, Z., Ullah, H., Datta, A., Attia, A., Rakshit, A., and Molla, S. H. (2020). Application of biogas slurry in combination with chemical fertilizer enhances grain yield and profitability of maize (Zea mays L.). Commun. Soil Sci. Plant Anal. 51, 2501–2510. doi: 10.1080/00103624.2020.1844728
Fu, Y., Zhang, Z., Liu, J., Chen, M., Pan, R., Hu, W., et al. (2020). Seed priming with spermidine and trehalose enhances chilling tolerance of rice via different mechanisms. J. Plant Growth Regul. 39, 669–679. doi: 10.1007/s00344-019-10009-y
Garcia-Franco, N., Hobley, E., Hübner, R., and Wiesmeier, M. (2018). “Climate-smart soil management in semiarid regions,” in Soil Management and Climate Change. eds M. Á. Muñoz and R. Zornoza (Academic Press), 349–368. doi: 10.1016/B978-0-12-812128-3.00023-9
Goel, A., Goel, A. K., and Singh, S. I. (2003). Changes in oxidative stress enzymes during artificial ageing in cotton (Gossypium hirsutum L.) seeds. J. Plant Physiol. 160, 1093–1100. doi: 10.1078/0176-1617-00881
González-Orozco, C. E., Porcel, M., Velásquez, D. F. A., and Orduz-Rodríguez, J. O. (2020). Extreme climate variability weakens a major tropical agricultural hub. Ecol. Indic. 111:106015. doi: 10.1016/j.ecolind.2019.106015
Gopalakrishnan, T., and Kumar, L. (2020). Modeling and mapping of soil salinity and its impact on paddy lands in jaffna peninsula, Sri Lanka. Sustainability 12:8317. doi: 10.3390/su12208317
Hameed, A., Sheikh, M. A., Hameed, A., Farooq, T., Basra, S. M. A., and Jamil, A. (2013). Chitosan priming enhances the seed germination, antioxidants, hydrolytic enzymes, soluble proteins and sugars in wheat seeds. Agrochimica 57, 97–110.
Hussain, M., Farooq, M., Sattar, A., Ijaz, M., Sher, A., and Ul-Allah, S. (2018). Mitigating the adverse effects of drought stress through seed priming and seed quality on wheat (Triticum aestivum L.) productivity. Pakistan J. Agricult. Sci. 55. 313–319. doi: 10.21162/PAKJAS/18.5833
Ibrahim, E. A. (2016). Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 192, 38–46. doi: 10.1016/j.jplph.2015.12.011
Imran, M., Mahmood, A., Römheld, V., and Neumann, G. (2013). Nutrient seed priming improves seedling development of maize exposed to low root zone temperatures during early growth. Europ. J. Agronomy 49, 141–148. doi: 10.1016/j.eja.2013.04.001
Iqbal, M., Ashraf, M., and Jamil, A. (2006). Seed enhancement with cytokinins: changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regul. 50, 29–39. doi: 10.1007/s10725-006-9123-5
Javaid, A. (2010). “Beneficial microorganisms for sustainable agriculture,” in Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming (Dordrecht: Springer), 347–369. doi: 10.1007/978-90-481-8741-6_12
Jisha, K. C., Vijayakumari, K., and Puthur, J. T. (2013). Seed priming for abiotic stress tolerance: an overview. Acta Physiol. Plant. 35, 1381–1396. doi: 10.1007/s11738-012-1186-5
Karim, M. R., and Rahman, M. A. (2015). Drought risk management for increased cereal production in Asian least developed countries. Weather Clim. Extremes. 7, 24–35. doi: 10.1016/j.wace.2014.10.004
Khan, H. A., Pervez, M. A., Ayub, C. M., Ziaf, K., Balal, R. M., Shahid, M. A., et al. (2009). Hormonal priming alleviates salt stress in hot pepper (Capsicum annuum L.). Soil Environ. 28, 130–135.
Khan, M. B., Gurchani, M. A., Hussain, M., Freed, S., and Mahmood, K. (2011). Wheat seed enhancement by vitamin and hormonal priming. Pak. J. Bot. 43, 1495–1499.
Kumar, P., Aeron, A., Shaw, N., Singh, A., Bajpai, V. K., Pant, S., et al. (2020). Seed bio-priming with tri-species consortia of phosphate solubilizing rhizobacteria (PSR) and its effect on plant growth promotion. Heliyon 6:e05701. doi: 10.1016/j.heliyon.2020.e05701
Kumar, V., Shahid, M., Singh, A., Srivastava, M., Mishra, A., Srivastava, Y. K., et al. (2014). Effect of biopriming with biocontrol agents Trichoderma harzianum (Th. Azad) and Trichoderma viride (01pp) on chickpea Genotype (Radhey). J. Plant Pathol Microb. 5:2.
Li, J., Yin, L. Y., Jongsma, M. A., and Wang, C. Y. (2011). Effects of light, hydropriming and abiotic stress on seed germination, and shoot and root growth of pyrethrum (Tanacetum cinerariifolium). Ind. Crops Prod. 34, 1543–1549. doi: 10.1016/j.indcrop.2011.05.012
Lutts, S., Benincasa, P., Wojtyla, L., Kubala, S., Pace, R., Lechowska, K., et al. (2016). “Seed priming: new comprehensive approaches for an old empirical technique,” in New Challenges in Seed Biology – Basic and Translational Research Driving Seed Technology, eds S. Araújo and A. Balestrazzi (Rijeka, Croatia: InTechOpen), 1–46. doi: 10.5772/64420
Madhukeshwara, B. P., and Sajjan, A. S. (2017). Influence of bio-priming on field performance and yield in maize hybrid. ACTA Sci. Agricult. 1, 16–19.
Mangena, P. (2020). Effect of hormonal seed priming on germination, growth, yield and biomass allocation in soybean grown under induced drought stress. Indian J. Agricult. Res. 54:441. doi: 10.18805/IJARe.A-441
Marthandan, V., Geetha, R., Kumutha, K., Renganathan, V. G., Karthikeyan, A., and Ramalingam, J. (2020). Seed priming: a feasible strategy to enhance drought tolerance in crop plants. Int. J. Mol. Sci. 21:8258. doi: 10.3390/ijms21218258
McDonald, M. B. (2000). Seed Priming. Seed Technology and Its Biological Basis. Sheffield: Sheffield Academic Press, 287–325.
Meena, S. K., Rakshit, A., and Meena, V. S. (2016). Effect of seed bio-priming and N doses under varied soil type on nitrogen use efficiency (NUE) of wheat (Triticum aestivum L.) under greenhouse conditions. Biocatal. Agric. Biotechnol. 6, 68–75. doi: 10.1016/j.bcab.2016.02.010
Ministry of Environment and Forest govt. of India. (2012). Coastal Zones of India. Ahmedabad: Space Applications Centre.
Mouradi, M., Bouizgaren, A., Farissi, M., Latrach, L., Qaddoury, A., and Ghoulam, C. (2016). Seed osmopriming improves plant growth, nodulation, chlorophyll fluorescence and nutrient uptake in alfalfa (Medicago sativa L.)–rhizobia symbiosis under drought stress. Sci. Hortic. 213, 232–242. doi: 10.1016/j.scienta.2016.11.002
Muhammad, I., Kolla, M., Volker, R., and Günter, N. (2015). Impact of nutrient seed priming on germination, seedling development, nutritional status and grain yield of maize. J. Plant Nutr. 38, 1803–1821. doi: 10.1080/01904167.2014.990094
Nawaz, A., Amjad, M., Pervez, M. A., and Afzal, I. (2011). Effect of halopriming on germination and seedling vigor of tomato. Afr. J. Agric. Res. 6, 3551–3559.
Nawaz, J., Hussain, M., Jabbar, A., Nadeem, G. A., Sajid, M., Subtain, M. U., et al. (2013). Seed priming a technique. Int. J. Agricult. Crop Sci. 6:1373.
Nedunchezhiyan, V., Velusamy, M., and Subburamu, K. (2020). Seed priming to mitigate the impact of elevated carbon dioxide associated temperature stress on germination in rice (Oryza sativa L.). Arch. Agron. Soil Sci. 66, 83–95. doi: 10.1080/03650340.2019.1599864
Paparella, S., Araújo, S. S., Rossi, G., Wijayasinghe, M., Carbonera, D., and Balestrazzi, A. (2015). Seed priming: state of the art and new perspectives. Plant Cell Rep. 34, 1281–1293. doi: 10.1007/s00299-015-1784-y
Parera, C. A., and Cantliffe, D. J. (1994). Presowing seed priming. Hortic. Rev. 16:109–114 doi: 10.1002/9780470650561.ch4
Parihar, M., Meena, V. S., Mishra, P. K., Rakshit, A., Choudhary, M., Yadav, R. P., et al. (2019). Arbuscular mycorrhiza: a viable strategy for soil nutrient loss reduction. Arch. Microbiol. 201, 723–735. doi: 10.1007/s00203-019-01653-9
Posmyk, M. M., and Janas, K. M. (2007). Effects of seed hydropriming in presence of exogenous proline on chilling injury limitation in Vigna radiata L. seedlings. Acta Physiologiae Plantarum. 29, 509–517. doi: 10.1007/s11738-007-0061-2
Qu, Z., Zhao, H., Zhang, H., Wang, Q., Yao, Y., Cheng, J., et al. (2020). Bio-priming with a hypovirulent phytopathogenic fungus enhances the connection and strength of microbial interaction network in rapeseed. NPJ Biofilms Microb. 6:45. doi: 10.1038/s41522-020-00157-5
Raj, S. N., Shetty, N. P., and Shetty, H. S. (2004). Seed bio-priming with Pseudomonas fluorescens isolates enhances growth of pearl millet plants and induces resistance against downy mildew. Int. J. Pest Manag. 50, 41–48. doi: 10.1080/09670870310001626365
Rakshit, A., Pal, S., Meena, S., Manjhee, B., Rai, S., Rai, A., et al. (2014). Seed bio-priming: a potential tool in integrated resource management. SATSA Mukhaptra Annu, Techn. Issue. 18, 94–103.
Rakshit, A., Pal, S., Rai, S., Rai, A., Bhowmick, M. K., and Singh, H. B. (2013). Micronutrient seed priming: a potential tool in integrated nutrient management. SATSA Mukhaptra Ann. Techn. Issue. 17, 77–89.
Rakshit, A., and Singh, H. B. (Eds.). (2018). Advances in Seed Priming. Singapore: Springer. doi: 10.1007/978-981-13-0032-5
Rawat, L., Singh, Y., Shukla, N., and Kumar, J. (2012). Seed biopriming with salinity tolerant isolates of Trichoderma harzianum alleviates salt stress in rice: growth, physiological and biochemical characteristics. J. Plant Path. 353–365.
Rehman, A., Farooq, M., Naveed, M., Nawaz, A., and Shahzad, B. (2018). Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Europ. J. Agronomy 94, 98–107. doi: 10.1016/j.eja.2018.01.017
Rehman, H., Kamran, M., Basra, S. M. A., Afzal, I., and Farooq, M. (2015). Influence of seed priming on performance and water productivity of direct seeded rice in alternating wetting and drying. Rice Sci. 22, 189–196. doi: 10.1016/j.rsci.2015.03.001
Sadeghi, H., Khazaei, F., Yari, L., and Sheidaei, S. (2011). Effect of seed osmopriming on seed germination behavior and vigor of soybean (Glycine max L.). ARPN J. Agricult. Biol. Sci. 6, 39–43.
Saravanakumar, D., and Samiyappan, R. (2007). ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogaea) plants. J. Appl. Microbiol. 102, 1283–1292. doi: 10.1111/j.1365-2672.2006.03179.x
Sarkar, D., Chattopadhyay, A., Singh, S., Devika, O. S., Pal, S., Parihar, M., et al. (2020b). “Modulation of microbiome through seed bio-priming,” in Trichoderma: Agricultural Applications and Beyond. Soil Biology, Vol. 61., eds C. Manoharachary, H. B. Singh, and A. Varma (Cham: Springer), 209–218. doi: 10.1007/978-3-030-54758-5_10
Sarkar, D., Kar, S. K., Chattopadhyay, A., Rakshit, A., Tripathi, V. K., Dubey, P. K., et al. (2020a). Low input sustainable agriculture: a viable climate-smart option for boosting food production in a warming world. Ecol. Indic. 115:106412. doi: 10.1016/j.ecolind.2020.106412
Sarkar, D., Pal, S., Mehjabeen, M., Singh, V., Singh, S., Pul, S., et al. (2018). “Addressing stresses in agriculture through bio-priming intervention,” in Advances in Seed Priming, eds A. Rakshit and H. B. Singh (Singapore: Springer), 107–113. doi: 10.1007/978-981-13-0032-5_7
Sarkar, D., Pal, S., Singh, H. B., Yadav, R. S., and Rakshit, A. (2017b). “Harnessing bio-priming for integrated resource management under changing climate,” in Advances in PGPR Research, eds H. B. Singh, B. K. Sarma, and C. Keswani (London, UK: CAB International), 349–363.
Sarkar, D., Rakshit, A., Al-Turki, A. I., Sayyed, R. Z., and Datta, R. (2021). Connecting bio-priming approach with integrated nutrient management for improved nutrient use efficiency in crop species. Agriculture 11:372. doi: 10.3390/agriculture11040372
Sarkar, D., Shikha, R. S., Ganguly, S., and Rakshit, A. (2017a). Management of increasing soil pollution in the ecosystem. Adv. Res. 12, 1–9. doi: 10.9734/AIR/2017/36622
Sergaki, C., Lagunas, B., Lidbury, I., Gifford, M. L., and Schäfer, P. (2018). Challenges and approaches in microbiome research: from fundamental to applied. Front. Plant Sci. 9:1205. doi: 10.3389/fpls.2018.01205
Shabbir, I., Ayub, M., Tahir, M., Bilal, M., Tanveer, A., Hussain, M., et al. (2014). Impact of priming techniques on emergence and seedling growth of sesame (Sesamum indicum L.) genotypes. Scientia 1, 92–96. doi: 10.15192/PSCP.SA.2014.1.3.9296
Shah, H., Jalwat, T., Arif, M., and Miraj, G. (2012). Seed priming improves early seedling growth and nutrient uptake in mungbean. J. Plant Nutr. 35, 805–816. doi: 10.1080/01904167.2012.663436
Shanthala, J., and Siddaraju, R. (2013). Effect of hydro priming on biochemical properties sunflower hybrid and its parental line during seeds storage. Int. J. Plant Sci. 8, 221–229.
Shivay, Y. S., Singh, U., Prasad, R., and Kaur, R. (2016). Agronomic interventions for micronutrient biofortification of pulses. Indian J. Agron 61, 161–172.
Shively, G., and Galopin, M. (2013). An overview of benefit-cost analysis. Available online at: http://www2.econ.iastate.edu/classes/crp274/swenson/URP290/Readings/Purdue_An%20Overview%20of%20Benefit.pdf
Singh, H., Jassal, R. K., Kang, J. S., Sandhu, S. S., Kang, H., and Grewal, K. (2015). Seed priming techniques in field crops-a review. Agric. Rev. 36, 251–264. doi: 10.18805/ag.v36i4.6662
Singh, H., and Reddy, M. S. (2011). Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils. Eur. J. Soil Biol. 47, 30–34. doi: 10.1016/j.ejsobi.2010.10.005
Singh, M., Dotaniya, M. L., Mishra, A., Dotaniya, C. K., Regar, K. L., and Lata, M. (2016). “Role of biofertilizers in conservation agriculture,” in Conservation Agriculture (Singapore: Springer), 13–134. doi: 10.1007/978-981-10-2558-7_4
Singh, V., Upadhyay, R. S., Sarma, B. K., and Singh, H. B. (2016). Seed bio-priming with Trichoderma asperellum effectively modulate plant growth promotion in pea. Int. J. Agricult. Environ. Biotechnol. 9, 361–365. doi: 10.5958/2230-732X.2016.00047.4
Srivastava, A. K., Lokhande, V. H., Patade, V. Y., Suprasanna, P., Sjahril, R., and D'Souza, S. F. (2010). Comparative evaluation of hydro-, chemo-, and hormonal-priming methods for imparting salt and PEG stress tolerance in Indian mustard (Brassica juncea L.). Acta Physiol. Plant. 32, 1135–1144. doi: 10.1007/s11738-010-0505-y
Tabassum, T., Farooq, M., Ahmad, R., Zohaib, A., Wahid, A., and Shahid, M. (2018). Terminal drought and seed priming improves drought tolerance in wheat. Physiol. Mol. Biol. Plants 24, 845–856. doi: 10.1007/s12298-018-0547-y
Timmusk, S., Abd El-Daim, I. A., Copolovici, L., Tanilas, T., Kännaste, A., Behers, L., et al. (2014). Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE. 9:e96086. doi: 10.1371/journal.pone.0096086
Tisarum, R., Theerawitaya, C., Samphumphuang, T., Polispitak, K., Thongpoem, P., Singh, H. P., et al. (2020). Alleviation of salt stress in upland rice (Oryza sativa L. ssp. indica cv. Leum Pua) using arbuscular mycorrhizal fungi inoculation. Front. Plant Sci. 11:348. doi: 10.3389/fpls.2020.00348
Ulfat, A. N. E. E. L. A., Majid, S. A., and Hameed, A. (2017). Hormonal seed priming improves wheat (Triticum aestivum l.) field performance under drought and non-stress conditions. Pak. J. Bot. 49, 1239–1253.
Varier, A., Vari, A. K., and Dadlani, M. (2010). The subcellular basis of seed priming. Curr. Sci. 450–456.
Vurukonda, S. S. K. P., Vardharajula, S., Shrivastava, M., and Sk,Z, A. (2016). Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 184, 13–24. doi: 10.1016/j.micres.2015.12.003
Wani, M. H. (2011). Hill agriculture in India: problems and prospects of mountain agriculture. Ind. J. Agricult. Econ. 66, 64–66. doi: 10.22004/ag.econ.204732
Wojtyla, Ł., Lechowska, K., Kubala, S., and Garnczarska, M. (2016). Molecular processes induced in primed seeds—increasing the potential to stabilize crop yields under drought conditions. J. Plant Physiol. 203, 116–126. doi: 10.1016/j.jplph.2016.04.008
Xu, S., Hu, J., Li, Y., Ma, W., Zheng, Y., and Zhu, S. (2011). Chilling tolerance in Nicotiana tabacum induced by seed priming with putrescine. Plant Growth Regul. 63, 279–290. doi: 10.1007/s10725-010-9528-z
Keywords: seed treatment, biotic and abiotic stresses, hydropriming, mitigation strategies, crop performance
Citation: Devika OS, Singh S, Sarkar D, Barnwal P, Suman J and Rakshit A (2021) Seed Priming: A Potential Supplement in Integrated Resource Management Under Fragile Intensive Ecosystems. Front. Sustain. Food Syst. 5:654001. doi: 10.3389/fsufs.2021.654001
Received: 15 January 2021; Accepted: 25 May 2021;
Published: 07 July 2021.
Edited by:
Urs Feller, University of Bern, SwitzerlandReviewed by:
Mohamed Sheteiwy, Mansoura Universiy, EgyptMohammad Ehsan Dulloo, Alliance Bioversity International and CIAT, France
Copyright © 2021 Devika, Singh, Sarkar, Barnwal, Suman and Rakshit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deepranjan Sarkar, ZGVlcC5nb2dyZWVuQGdtYWlsLmNvbQ==; Amitava Rakshit, YW1pdGF2YXJAYmh1LmFjLmlu
 O. Siva Devika
O. Siva Devika Sonam Singh
Sonam Singh Deepranjan Sarkar
Deepranjan Sarkar Prabhakar Barnwal
Prabhakar Barnwal Jarupula Suman
Jarupula Suman Amitava Rakshit
Amitava Rakshit