- 1Infectious Diseases Division, Venezuelan Science Incubator and Emerging Pathogens Regional Collaborative Network, Barquisimeto, Venezuela
- 2Global WASH, Houston, TX, United States
- 3Instituto de Investigaciones Biomédicas, Barquisimeto, Venezuela
- 4Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 5Programa de Medicina, Universidad Centroccidental Lisandro Alvarado, Barquisimeto, Venezuela
- 6Academia Nacional de Medicina, Caracas, Venezuela
- 7Sociedad Venezolana de Puericultura y Pediatría, Caracas, Venezuela
- 8Infectious Diseases Department, Baylor College of Medicine, Houston, TX, United States
Venezuela is in the midst of a humanitarian crisis with a dangerous cocktail of hyperinflation, violence, minimal local food production, and policies that impact the nutrition for millions of Venezuelans. Independent data suggests that most Venezuelans are food insecure, with alarming rates of acute and chronic malnutrition, especially among children. A re-emergence of poverty-related intestinal parasitoses and anemia has aggravated their health. With little to no response from public authorities, Venezuela is now the lowest-ranked country in the world in deworming coverage. Modest independent and private epidemiological studies suggest prevalence rates as high as 60% in some regions. This article reviews public health policies regarding malnutrition and intestinal parasitoses and aims to provide a rational approach based on international recommendations for countries in crisis.
Introduction
Venezuela's humanitarian emergency demands a multi-sector approach aimed at reducing parasite-induced malnutrition. Over the last decade, Venezuela has transitioned from being one of the richest oil-producing countries in the world to becoming a crisis-hit nation amid a deep socioeconomic collapse. This complex situation primarily reflects three phenomena: (a) inadequate economic measures that have led to hyperinflation and increased poverty; (b) a spike in violence, sociopolitical turmoil, and massive migration; (c) and the decay of the public health care system.
The Venezuelan crisis has caused multidimensional impoverishment of most of its population, with a steady decay in education, quality of life, and employment rates that affect nearly 65% of Venezuelans (Encuesta Nacional de Condiciones de Vida, 2020). Hyperinflation has impacted food security and access to utilities (water, electricity, gas) for at least three-quarters of the population; in actuality, this rough estimate may be much higher given the political restrictions imposed by the national government on research concerning these topics (World Food Program, 2019).
Currently, Venezuelan public healthcare is practically non-existent as most facilities have critical shortages of medicines, equipment, supplies, and qualified medical staff. Furthermore, the majority of epidemiological surveillance and control programs have been abandoned, resulting in an unprecedented spike in vaccine-preventable and vector-borne diseases that, fueled by massive migration, have created the largest disease exodus of modern times (Grillet et al., 2019; Paniz-Mondolfi et al., 2019a). Intestinal parasitoses have also reached epidemic proportions among Venezuelans since these agents have an infectious life cycle that is propelled by poor water, sanitation, and hygiene (WASH) status. The arrival of SARS-CoV-2 has aggravated the situation as the remaining, already-limited resources have been diverted (or redistributed or rerouted) to try to lower the impact of Coronavirus Disease 2019 (COVID-19).
We aim to summarize the effects that poverty, malnutrition, and intestinal parasitoses exert on the most vulnerable populations in Venezuela: children and indigenous peoples. We also examine current policies and recommend measures to address these issues.
Policy Options and Implications
Malnutrition
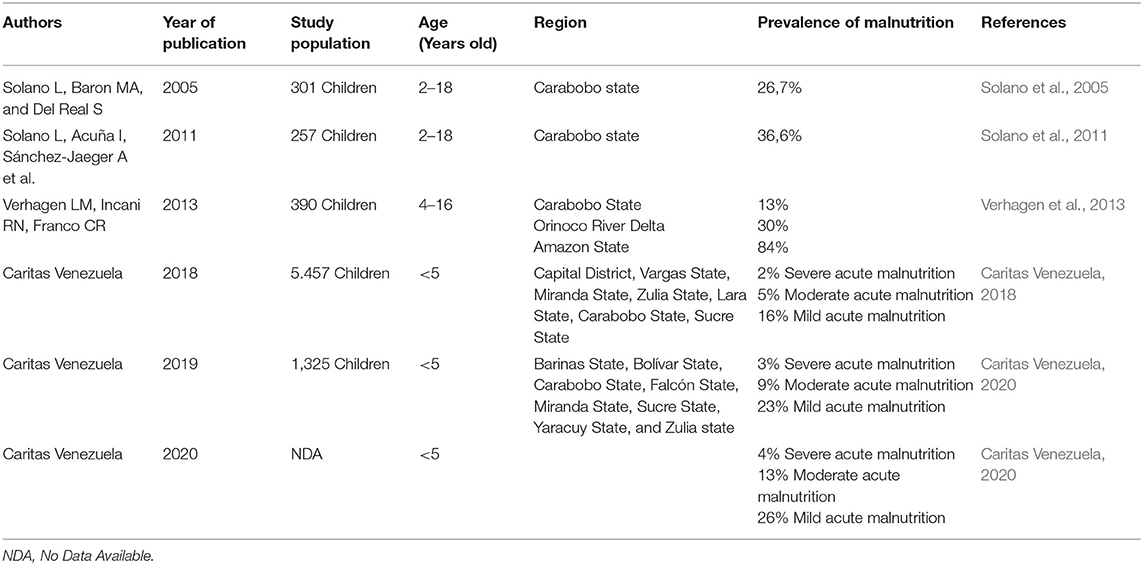
In 2019, The World Food Program (WFP) reported that 2.3 million (7.9%) Venezuelans were severely food insecure, mainly in Zulia, Falcon, Delta Amacuro, and Amazonas states (World Food Program, 2019). Between December 2019 and March 2020, child malnutrition rates increased to 26% (Caritas Venezuela, 2020). General undernutrition rates skyrocketed to 21.2% by 2018, while the prevalence of stunted growth among children reached 13.4% in 2012 (FAO et al., 2019).
The prevalence of severe acute and chronic malnutrition exceeds critical thresholds in many states with a heavy presence of Amerindian peoples, including the states of Zulia, Amazonas, and Delta Amacuro (Table 1). In the last decades, indigenous peoples have disproportionally suffered as a consequence of the ongoing national crisis, particularly in isolated areas where access to healthcare facilities is most challenging (Paniz-Mondolfi et al., 2019b). In these areas, the already low healthcare coverage reaches critical rates (Gómez et al., 2019). Previous studies report that 45.5% of health centers that treat Indigenous Peoples lack adequate medical personnel and 22.7% have non-medical personnel providing medical care (Gómez et al., 2019).
The arrival of the SARS-CoV-2 pandemic deeply worsened the Venezuelan food crisis by increasing prices and reducing food availability for the general population. In border regions, the situation has been complicated by containment measures resulting in the closure of food centers managed by international agencies that supported Venezuelans in extreme poverty (González, 2020). Furthermore, the humanitarian corridor through the Colombian-Venezuelan border became bottlenecked. Illegal transit coupled with insufficient food and medical care are common in this region, which has witnessed an increase in violence as armed groups are constantly fighting to take control over the zone (Collins, 2020).
There is a similar scenario in the south, where poverty and marginalization have forced a massive exodus of Warao Amerindians in precarious nutritional status to Brazil. In an attempt to address cultural and linguistic barriers that hinder the social adaptation of Indigenous Venezuelan migrants, public health authorities in Brazil have created humanitarian shelters just for this group (Prengaman, 2018).
Hunger, malnutrition, and obesity, are just some of the challenges Venezuela faces. Linear growth in children is a good marker to identify inequalities in human development and a sensitive indicator for the health and well-being of populations (Orellana et al., 2019). Early nutritional disturbances result in adverse effects for individuals in their adult lives and can also be transmitted to future generations. Studies carried out in Yanomami communities confirm the intergenerational transmission of low maternal height as a consequence of the most severe nutritional deficits (Orellana et al., 2019).
Intestinal Parasitoses
Public authorities in Venezuela have severely curtailed epidemiological surveillance activities, and the lack of equipment and reagents have hampered efficient diagnosis and reporting of nutritional status and infectious diseases. Due to the abandonment of most epidemiological programs, current data for the prevalence of intestinal parasitoses and the attributable morbidity is scarce and may be underestimated. Cross-sectional studies that used combined microscopy and PCR methods have reported prevalence rates of over 65% in rural communities (Incani et al., 2017). Others have pointed at Blastocystis spp, Giardia intestinalis and Entamoeba coli, Trichuris trichiura, and Ascaris lumbricoides as the most common agents (Table 2) (Hagel et al., 2001; Nastasi-Miranda, 2015; Orozco et al., 2019). Intensity of infection (measured as eggs/larvae per gram of feces) has also been reported in some Venezuelan communities with a tendency toward light-to-moderate parasite loads (Incani et al., 2020). Prevalence and intensity of infection are not necessarily related as they may share some—but not all—risk factors in an agent-specific fashion (Incani et al., 2020).
Intestinal parasitoses have become a persistent public health issue in Venezuela, remaining at high or unacceptable levels for the last 30 years (Chacín de Bonilla, 1990). Neighboring countries such as Colombia and Brazil have reported prevalence rates of 25.5 and 33.4% respectively, while Venezuela displayed a larger prevalence of 39.4% (Chammartin et al., 2013). Reports from indigenous populations have also shown elevated occurrence for several intestinal protozoa and helminths (Devera et al., 2005; Gastiaburu, 2019), with predominance toward polyparasitism in children from certain communities (Acurero-Yamarte et al., 2016; Gastiaburu, 2019).
The long-term effects of intestinal parasitoses on the general population—and especially on children—are considerable: anthropometric alterations, malnutrition, physical growth and mental impairment, anemia, and reduced community productivity (Crompton and Nesheim, 2002; Nastasi-Miranda, 2015). In poor urban and rural communities, intestinal parasitoses are related to overcrowding, inadequate hygiene, and precarious housing materials. Additional spatial clustering of “wormy houses” with individuals with high prevalence and intensity of helminth infection represent local hotspots for community transmission that should be heavily targeted in mass deworming strategies (Incani et al., 2020).
Chronic parasitoses may also elicit immunomodulatory effects on human hosts, which have been described in Amerindian peoples. Reports from Warao communities show Th2-skewed cytokine profiles that may facilitate Mycobacterium tuberculosis infection in patients with Ascaris lumbricoides (Verhagen et al., 2012). Other studies suggest that this cytokine shift could provide protection against other gastrointestinal agents such as Helicobacter pylori (Fuenmayor-Boscán et al., 2016) and modulate immunological responses in allergic or inflammatory processes (Gazzinelli-Guimaraes and Nutman, 2018).
Recently, the Pan-American Health Organization declared Venezuela the country with the lowest mass deworming coverage (Ault et al., 2011). Mass deworming is a technique recommended by WHO to improve children's health (World Health Organization, 2017) by drastically reducing the prevalence of parasites and slowly improving nutrition in low-income communities.
The cost of mass deworming is mitigated in part by the donation of classic deworming drugs such as albendazole/mebendazole by their pharmaceutical manufacturers including Merck, Johnson & Johnson, and GlaxoSmithKline. Emerging strategies, such as the massive use of oral ivermectin could potentially help tackle intestinal parasites, as well as other hyper-prevalent neglected tropical diseases (NTDs) such as river blindness, malaria, Chagas disease, and the Leishmaniases (Perez-Garcia et al., 2020).
Integrated WASH and NTD Strategy
The provision of safe water, sanitation, and hygiene (WASH) is one of the five key intervention methods within WHO's global roadmap for the elimination of NTDs (World Health Organization, 2020). Yet, the WASH component of this strategy and its potential role in reducing NTDs has received little to no attention globally. In Venezuela, this strategy is non-existent.
First, the collaboration helps to develop a large-scale needs assessment that can collect data needed for investment opportunities from the humanitarian sector. Water pollution and the interruption of water services across Venezuela have intensified in the past decade, and there is little documentation of its severity and impact, particularly toward the most vulnerable indigenous groups. Reports from local NGOs indicate that at least 82% of Venezuelans have very sporadic water access and the quality is far below WHO standards (CEPAZ, 2018). The risks of parasitic infections posed by poor water quality can be more threatening to a population already weakened by shortages of food and health services. Open defecation is also prevalent in many regions across the country, and about 70% of the wastewater volume produced in the country is not collected or treated (CEPAZ, 2018).
Second, the collaboration promotes joint planning and implementation of activities in a country where multisector action is needed to simultaneously control several NTDs (Perez-Garcia et al., 2020). Rapid reinfection after massive deworming can occur when poor hygiene practices exist and the environment is still contaminated with parasites at infective stages. Having access to safe water, sanitation facilities, and improved hygiene can enhance the effectiveness of deworming campaigns. The WASH component of the strategy can plan and implement adequate access to WASH.
Third, it serves to track progress across multisectoral actions beyond the health sector. The multisectoral effort can use target indicators not only in vector control but also in transmission interruption through adequate access to WASH. For example, measuring access to safe collection and disposal of feces, access to potable water, and access to handwashing facilities.
Finally, the combined intervention can guide humanitarian response plans in education. Mass deworming together with educational WASH campaigns could effectively reduce the burden of intestinal parasitoses, by interrupting the reinfection cycle that involves contaminated soil, water, or food.
Actionable Recommendations
Combining WASH and NTD efforts is critical to target investments from the humanitarian sector needed for large-scale strategies to reduce parasite-induced malnutrition in Venezuela. This approach serves to create a robust collaboration platform among researchers and NGOs working to reduce the burden of parasitic infections in the country. Although international aid to Venezuela discretely increased in 2018, there is no coordinated strategy or agreement upon priorities to use these funds. An integrated WASH-NTD approach is key to create a strategy for long-term impact on communities' health and nutrition.
Achieving long-term impact requires proper diagnostics. More investment is needed to support research institutions working with nucleic acid-based methods for the detection and identification of parasitic infections. These methods provide higher sensitivity and specificity with simpler standardization of diagnostic procedures. Another advantage is that DNA samples can be stored and used for genetic characterization, posing as a valuable tool for surveys and surveillance studies that provide the basis for WASH-NTD interventions aimed at reducing parasite-induced malnutrition.
Finally, international humanitarian assistance should focus on coordinating strategies between stakeholders of multiple sectors to increase capacity in the WASH and NTD sectors. Capacity building is critical in Venezuelan research institutions, NGOs, and the private sector to search for international and technical support with scientific evidence-based proposals.
Conclusions
The humanitarian crisis in Venezuela requires a multi-sector approach aimed at reducing parasite-induced malnutrition. An integrated WASH-NTD strategy is essential to facilitate the planning, implementation, and evaluation of large-scale programs that target a reduction of the parasite burden in the most vulnerable populations of Venezuela.
Author Contributions
IM-C, AP-M, LD-N, EM-R, and LP-G contributed conception and design of the study. LD-N, EM-R, and LP-G organized the database. AP-M, LD-N, EM-R, and LP-G wrote the first draft of the manuscript. AP-M, LD-N, EM-R, IM-C, HU-M, LV-P, JH, ES, and LP-G wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study received funding from Global Grant No. GG2015574 through the Rotary Foundation. The authors are grateful to the Rotary e-Club of Houston, the Rotary Club of Barquisimeto-Nueva Segovia, the Rotary Club of Humble Intercontinental, the Rotary Club of Corsicana, Rotary D7305, and Rotary D5890.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge the Rotary Club of Somerset and the Rotary eClub of Houston for their initial efforts toward funding the Deworming Venezuela campaign.
References
Acurero-Yamarte, E., Suarez, O. D., Rivero-Rodríguez, Z., Mora, Á. B., La Corte, M. C., Terán, R., et al. (2016). Enteroparásitos en niños de una comunidad indígena del municipio Machiques de Perijá, estado Zulia Venezuela. Kasmera 44, 26–34. Available online at: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0075-52222016000100005&lng=es&nrm=iso
Al Rumhein, F., Sánchez, J., Requena, I., Blanco, Y., and Devera, R. (2005). Parasitosis intestinales en escolares: relación entre su prevalencia en heces y en el lecho subungueal. Rev Biomed. 16, 227–237. doi: 10.32776/revbiomed.v16i4.423
Ault, S., Nicholls, R., Saboya, M., and Gyorkos, T. (2011). Workshop on integrating deworming intervention into preschool child packages in the Americas. Pan American Health Organization, 1–70. Available online at: https://www.paho.org/en/documents/workshop-integrating-deworming-intervention-preschool-child-packages-americas-2011 (accessed November 28, 2020).
Caritas Venezuela (2018). Monitoreo de la Situación Nutricional en Niños menores de 5 años. Octubre-Diciembre 2018. Available online at: http://caritasvenezuela.org/mapas-y-boletines-de-nuestra-accion/ (accessed January 27, 2021).
Caritas Venezuela (2020). Monitoreo centinela de la desnutrición aguda y la seguridad alimentaria familiar. Available online at: http://caritasvenezuela.org/mapas-y-boletines-de-nuestra-accion/ (accessed October 1, 2020).
CEPAZ (2018). Emergencia Humanitaria Compleja en Venezuela: Derecho al Agua. Available online at: https://cepaz.org/documentos_informes/emergencia-humanitaria-compleja-en-venezuela-derecho-al-agua/ (accessed November 27, 2020).
Chacín de Bonilla, L. (1990). El problema de las parasitosis intestinales en Venezuela. Invest. Clin. 31, 1–2.
Chammartin, F., Scholte, R. G., Guimarães, L. H., Tanner, M., Utzinger, J., and Vounatsou, P. (2013). Soil-transmitted helminth infection in South America: a systematic review and geostatistical meta-analysis. Lancet Infect. Dis. 13, 507–518. doi: 10.1016/S1473-3099(13)70071-9
Collins, J. (2020). COVID-19 and the crisis for migrants and Indigenous people on the Venezuela-Colombia border. The New Humanitarian. Available online at: https://www.thenewhumanitarian.org/news-feature/2020/09/23/Venezuela-Colombia-border-coronavirus-migration-Indigenous (accessed November 27, 2020).
Crompton, D. W. T., and Nesheim, M. C. (2002). Nutritional impact of intestinal helminthiasis during the human life cycle. Annu. Rev. Nutr. 22, 35–59. doi: 10.1146/annurev.nutr.22.120501.134539
Devera, R., Blanco, Y., and Cabello, E. (2005). Elevada prevalencia de Cyclospora cayetanensis en indígenas del estado Bolívar, Venezuela. Cad. Saude Publica 21, 1778–1784. doi: 10.1590/S0102-311X2005000600025
Devera, R., Cordero, A., Uzcategui, Y., Blanco, Y., Amaya, I., and Requena, I. (2016). Blastocistosis en niños y adolescentes de una comunidad Indígena del estado Bolívar, Venezuela. Saber, Universidad de Oriente 28, 73–82. Available online at: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S1315-01622016000100007&lng=es&nrm=iso
Díaz, I., Rivero, Z., Bracho, A., Castellanos, M., Acurero, E., Calchi, M., et al. (2006). Prevalencia de enteroparásitos en niños de la etnia Yukpa de Toromo, estado Zulia, Venezuela. Rev. Méd. Chile 134, 72–78. doi: 10.4067/S.0034-98872006000100010
Encuesta Nacional de Condiciones de Vida (2020). Informe de resultados 2019/20. Available online at: https://www.proyectoencovi.com/informe-interactivo-2019 (accessed November 15, 2020).
FAO UNICEF, FIDA, OMS, PMA (2019). El estado de la seguridad alimentaria y la nutrición en el mundo 2019. Protegerse frente a la desaceleración y el debilitamiento de la economía. Available online at: http://www.fao.org/3/ca5162es/ca5162es.pdf (accessed November 27, 2020).
Fuenmayor-Boscán, A. D., Hernández, I. M., Valero, K. J., Paz, A. M., Sandrea, L. B., and Rivero, Z. (2016). Association between Helicobacter pylori and intestinal parasites in an Añu indigenous community of Venezuela. Indian J. Gastroenterol. 35, 106–112. doi: 10.1007/s12664-016-0641-4
Gastiaburu, P. K. (2019). Prevalencia de parasitosis intestinales en niños indígenas Warao y criollos de Barrancas del Orinoco. Venezuela. Cienc. e Investig. Med. Estud. Latinoam. 24:1110. doi: 10.23961/cimel.v24i1.1110
Gazzinelli-Guimaraes, P. H., and Nutman, T. B. (2018). Helminth parasites and immune regulation [version 1; peer review: 2 approved]. F1000Research 7:F1000 Faculty Rev-1685. doi: 10.12688/f1000research.15596.1
Gómez, H., Montiel, H., Pizarro, I., Naveda, J., and Ávila M., Rojas, S. (2019). Informe de salud y enfermedades endémicas en comunidades indígenas. Kapé Kapé. Available online at: https://kape-kape.org/informes/ (accessed November 14, 2020).
González, J. D. (2020). Venezuelan Guajira: between malnutrition and disease. Hearts On Venezuela. Available online at: http://www.heartsonvenezuela.com/venezuelan-guajira-between-malnutrition-and-disease/ (accessed November 27, 2020).
Grillet, M. E., Hernández-Villena, J. V., Llewellyn, M. S., Paniz-Mondolfi, A. E., Tami, A., Vincenti-Gonzalez, M. F., et al. (2019). Venezuela's humanitarian crisis, resurgence of vector-borne diseases, and implications for spillover in the region. Lancet Infect. Dis. 19, e149–e161. doi: 10.1016/S1473-3099(18)30757-6
Hagel, I., Salgado, A., Rodríguez, O., Ortiz, D., Hurtado, M., Puccio, F., et al. (2001). Factores que influyen en la prevalencia e intensidad de las parasitosis intestinal en Venezuela. Gac. méd. Caracas. 109, 82–90. Available online at: https://www.researchgate.net/publication/338825954_Factores_que_influyen_en_la_prevalencia_e_intensidad_de_las_parasitosis_intestinales_en_Venezuela
Incani, R. N., Ferrer, E., Hoek, D., Ramak, R., Roelfsema, J., Mughini-Gras, L., et al. (2017). Diagnosis of intestinal parasites in a rural community of Venezuela: Advantages and disadvantages of using microscopy or RT-PCR. Acta Trop. 167, 64–70. doi: 10.1016/j.actatropica.2016.12.014
Incani, R. N., Grillet, M. E., and Mughini-Gras, L. (2020). Hotspots and correlates of soil-transmitted helminth infections in a Venezuelan rural community: which are the “wormy” houses? J. Infect. 82, 143–149. doi: 10.1016/j.jinf.2020.10.037
Nastasi-Miranda, J. A. (2015). Prevalencia de parasitosis intestinales en unidades educativas de Ciudad Bolívar, Venezuela. Revista CUIDARTE 6:181. doi: 10.15649/cuidarte.v6i2.181
Orellana, J. D. Y., Marrero, L., Alves, C. L. M., Ruiz, C. M. V., Hacon, S. S., Oliveira, M. W., et al. (2019). Association of severe stunting in indigenous Yanomami children with maternal short stature: clues about the intergerational transmission. Cien Saude Colet 24, 1875–1883. doi: 10.1590/1413-81232018245.17062017
Orozco, M., Marchán, E., and Rondón, R. (2019). Enteroparasites, epidemiological indicators and nutritional status in preschoolers of “Coropo”, Aragua State, Venezuela. Rev Vzlana Salud Pub. 6, 9–16. Available online at: https://www.scipedia.com/public/Mata_et_al_2018h
Paniz-Mondolfi, A. E., Grillet, M. E., Tami, A., Oliveira-Miranda, M. A., Delgado Noguera, L. A., Hotez, P., et al. (2019a). Venezuela's upheaval threatens Yanomami. Science. 365, 766–767. doi: 10.1126/science.aay6003
Paniz-Mondolfi, A. E., Tami, A., Grillet, M. E., Márquez, M., Hernández-Villena, J., Escalona-Rodríguez, M. A., et al. (2019b). Resurgence of vaccine-preventable diseases in venezuela as a regional public health threat in the Americas. Emerging Infect. Dis. 25, 625–632. doi: 10.3201/eid2504.181305
Perez-Garcia, L. A., Mejias-Carpio, I. E., Delgado-Noguera, L. A., Manzanarez-Motezuma, J. P., Escalona-Rodriguez, M. A., Sordillo, E. M., et al. (2020). Ivermectin: repurposing a multipurpose drug for Venezuela's humanitarian crisis. Int. J. Antimicrob. Agents 56:106037. doi: 10.1016/j.ijantimicag.2020.106037
Prengaman, P. (2018). Brazil struggles to care for Venezuela's indigenous Warao. Available online at: https://apnews.com/article/d19b805f7a384e2481429e4db236d676 (accessed November 27, 2020).
Rivero-Rodríguez, Z., Hernández, A., Bracho, A., Salazar, S., and Villalobos, R. (2013). Prevalencia de microsporidios intestinales y otros enteroparásitos en pacientes con VIH positivo de Maracaibo, Venezuela. Biomédica. 33, 538–545. doi: 10.7705/biomedica.v33i4.1468
Solano, L., Acuña, I., Sánchez-Jaeger, A., Barón, M. A., and Morón, A. (2011). Pobreza estructural y déficit nutricional en niños preescolares, escolares y adolescentes del Sur de Valencia Estado Carabobo-Venezuela. Salus 15. Available online at: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S1316-71382011000100005&lng=es&nrm=iso
Solano, L., Baron, M. A., and Del Real, S. (2005). Situación nutricional de preescolares, escolares, y adolecentes de Valencia, Carabobo, Venezuela. An Venez Nutr. 18. Available online at: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0798-07522005000100014&lng=es&nrm=iso
Verhagen, L. M., Hermans, P. W. M., Warris, A., De Groot, R., Maes, M., Villalba, J. A., et al. (2012). Helminths and skewed cytokine profiles increase tuberculin skin test positivity in Warao Amerindians. Tuberculosis 92, 505–512. doi: 10.1016/j.tube.2012.07.004
Verhagen, L. M., Incani, R. N., Franco, C. R., Ugarte, A., Cadenas, Y., Sierra Ruiz, C. I., et al. (2013). High malnutrition rate in Venezuelan Yanomami compared to Warao Amerindians and Creoles: significant associations with intestinal parasites and anemia. PLoS ONE 8:e77581. doi: 10.1371/journal.pone.0077581
World Food Program (2019). Venezuela Food Security Assessment Main Findings Data Collected between July and September 2019. WFP. Available online at: https://reliefweb.int/sites/reliefweb.int/files/resources/Main%20Findings%20WFP%20Food%20Security%20Assessment%20in%20Venezuela_January%202020-2.pdf (accessed November 28, 2020).
World Health Organization (2017). WHO recommends large-scale deworming to improve children's health and nutrition. Available online at: https://www.who.int/news/item/29-09-2017-who-recommends-large-scale-deworming-to-improve-children-s-health-and-nutrition (accessed November 19, 2020).
World Health Organization (2020). Ending the Neglect to attain the Sustainable Development Goals. A road map for neglected tropical diseases 2021–2030. WHO. Available online at: https://www.who.int/neglected_diseases/Ending-the-neglect-to-attain-the-SDGs–NTD-Roadmap.pdf (accessed November 27, 2020).
Keywords: Venezuela, malnutrition, policy, crisis, parasitoses, wash, water, sanitation
Citation: Mejias-Carpio IE, Paniz-Mondolfi AE, Mogollon-Rodriguez EA, Delgado-Noguera LA, Sordillo EM, Urbina-Medina HA, Hayon J, Vetencourt-Pineda LA and Perez-Garcia LA (2021) Assessment of Malnutrition and Intestinal Parasitoses in the Context of Crisis-Hit Venezuela: A Policy Case Study. Front. Sustain. Food Syst. 5:634801. doi: 10.3389/fsufs.2021.634801
Received: 28 November 2020; Accepted: 12 February 2021;
Published: 05 March 2021.
Edited by:
Gioconda San-Blas, Academia de Ciencias Físicas, Matemáticas y Naturales de Venezuela (ACFIMAN)/Instituto Venezolano de Investigaciones Científicas, VenezuelaReviewed by:
Isabel Hagel, Central University of Venezuela, VenezuelaMatthew D. Moore, University of Massachusetts Amherst, United States
Copyright © 2021 Mejias-Carpio, Paniz-Mondolfi, Mogollon-Rodriguez, Delgado-Noguera, Sordillo, Urbina-Medina, Hayon, Vetencourt-Pineda and Perez-Garcia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto E. Paniz-Mondolfi, YWxiZXJ0by5wYW5pei1tb25kb2xmaUBtb3VudHNpbmFpLm9yZw==
 Isis E. Mejias-Carpio
Isis E. Mejias-Carpio Alberto E. Paniz-Mondolfi
Alberto E. Paniz-Mondolfi Euler A. Mogollon-Rodriguez1,3,5
Euler A. Mogollon-Rodriguez1,3,5 Emilia M. Sordillo
Emilia M. Sordillo Huniades A. Urbina-Medina
Huniades A. Urbina-Medina Jesica Hayon
Jesica Hayon Luis A. Perez-Garcia
Luis A. Perez-Garcia