
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 14 May 2021
Sec. Crop Biology and Sustainability
Volume 5 - 2021 | https://doi.org/10.3389/fsufs.2021.606379
This article is part of the Research Topic Plant Growth-Promoting Microorganisms for Sustainable Agricultural Production View all 50 articles
The study aim was to evaluate the potential nitrogen fixation and denitrification in the rhizosphere soil of potato plants, crop yield and output quality in response to the different fertilization systems and the inoculation with Azospirillum brasilense 410. Field stationary experiment was conducted between 2016 and 2019 with potato in a crop rotation system on leached chernozem soil. Farmyard manure, 40 t/ha, applied prior to potatoes planting promotes nitrogen fixation (0.8–2.0 times compared to control). However, it has also affected denitrification (in 1.4–2.2 times higher compared to control). The lowest rate of mineral fertilizers used in the experiment, N40P40K40, was shown as most environmentally feasible. Under its use the increase of soil nitrogenase activity and low denitrification levels were observed. Same trends were also noted for the medium fertilizer rate, N80P80K80. The highest doses of mineral fertilizers, N120P120K120, substantially affected the denitrification process and reduced the nitrogen fixation activity (in 1.9–2.2 times). The combination of manure with the medium fertilizers rate has also resulted in high denitrification levels, while the soil nitrogen fixation activity has restored only at flowering stage. Crop inoculation with A. brasilense combined with the manure application, has not affected studied processes. However, crop inoculation after the green manure intercropping has shown the growth of nitrogenase activity. Used on the mineral fertilizers background inoculation has activated nitrogen fixation and has ensured the decrease of denitrification levels, subject to the fertilization background. High fertilizer rates have hampered the inoculation efficiency. Inoculation has promoted crop yields on unfertilized and mineral backgrounds or following green manure. Crop inoculation following organic and the organo-mineral backgrounds had no significant effect, probably due to the competition for A. brasilense from microorganisms that have created a competitive environment for A. brasilense. Despite its environmental expediency, inoculation combined with the low fertilizer doses underperforms the action of inoculation combined with the medium fertilizer rates showing the latter as the compromise between the environmental requirements and crop productivity. The use of inoculation has promoted the accumulation of starch and ascorbic acid and has contributed to the reduction of nitrate contents in the tubers of inoculated plants.
Traditional crop fertilization systems do not take into account rising environmental requirements and concerns. The major attention is brought to the determination of the mineral fertilizer rates based on the nutrient removal values and planned harvest indicators while leaving aside the variability of the utilization rates of active ingredients from fertilizers. However, the later, according to various scientific data, should always be considered as the degree of assimilation of the active substance from the fertilizers remains low for crop macroelements, with the nitrogen uptake being within 35–50%, phosphorus – not exceeding 20%, and potassium staying withing 25–60% range, subject to the soil type, fertilizer origin, and used crop production system (Korenkov, 1990; Tilman, 1998). Applied mineral fertilizers cannot be assimilated by crops in full, resulting in the application of higher rates to achieve the planned productivity. At the same time, despite the availability of different practices for coherent crop fertilization systems (Juchenko, 1990; Johnston and Bruulsema, 2014; Sposari and Flis, 2017), they are rarely followed in practice. That results in significant environmental pollution issues related to fertilizer residuals, including mineral nitrogen among the largest contaminant of croplands (Luis et al., 2014; Khan et al., 2018), as more than 50% of the total amount of applied nitrogen can be lost from agricultural systems (Vitousek et al., 1997; Tilman, 1998). In addition to the nitrate pollution, nitrogen fertilizers are the main source of N2O emissions from agriculture, accounting for 60–80% of emissions globally (Dalal et al., 2003; Signor et al., 2013; Mazzetto et al., 2016; Millar et al., 2018).
Nitrogen fertilizers, in theory, should only be used within the range of their physiological and ecological feasibility to minimize the risk of environmental pollution with nitrogen compounds. However, most fertilization systems account only for the agrochemical and economic indicators for the calculation of nitrogen fertilization doses. The ability to include environmental indices in the design of crop fertilization systems appeared with the development of biological testing methods, in particular using indicators of functional activity of nitrogen-fixing bacteria associated with the roots of cultivated plants (Umarov et al., 1985; Ladha et al., 1986; Volkogon, 2013).
Nitrogen fixing microorganisms fix nitrogen from the atmosphere only in the absence of the excess amounts of mineral nitrogen compounds in the environment (including the soil) (Shah et al., 1972; Lvov, 1989). Knowing the dynamics of nitrogenase activity in the root zone of plants grown on different fertilization backgrounds (from deficit to excess nitrogen in the soil) the physiologically justified from crop standpoint doses of mineral nitrogen that will not reduce the nitrogen fixation activity can be determined (Ladha et al., 1986).
Under the excess nitrogen fertilizers conditions diazotrophs cease atmospheric nitrogen fixation and switch to available mineral nitrogen compounds, as they are more energy-efficient for the bacterial cell. For this reason, the assimilation of mineral nitrogen used for constructive metabolism in bacteria is accompanied with the denitrification in soil (Eskew et al., 1977; Bothe et al., 1981). Consequently, the increase of the denitrification activity in the rhizosphere under the influence of mineral nitrogen fertilizers will also indicates on an excess of nitrogen compounds for the crops. Understanding the activity of N2O emission in the rhizosphere soils of croplands in response to different doses of mineral nitrogen will allow the selection of the most appropriate amount of mineral nitrogen, accounting for the minimal losses of gaseous nitrogen compounds from the soil compared to the unfertilized control. Based on the comparative analysis of two processes – nitrogen fixation and denitrification in croplands, the physiologically appropriate doses of mineral nitrogen can be selected (Volkogon, 2013).
Cavigelli and Robertson (2000) have stated that due to the high importance of soil microbiota in the nitrogen cycle, changes in its composition and number can change the rate of nitrogen transformation in the soil. Therefore, changes in groups of soil microorganisms caused by chemical compounds or seeds inoculation with specific microorganisms can potentially alter nitrogen transformation processes in the soil. Such microorganisms capable to stimulate the processes of transformation of nitrogen compounds in croplands are called Plant Growth Promoting Bacteria (PGPB) (Kloepper and Schroth, 1978; Bashan and Holguin, 1998). Among these bacteria, representatives of the Azospirillum genus have been studied in great detail. The stimulating effect of Azospirillum sp. on plant growth and development is explained by several mechanisms, including the synthesis of plant hormones and other biologically active substances, biological nitrogen fixation, and enhancement of mineral compounds absorption by plants (Bashan and Levanony, 1990; Okon and Itzigsohn, 1995; Kennedy et al., 1997; Ruppel and Merbach, 1997; Saubidet and Barneix, 1998; Mirza et al., 2000, Rodrigues et al., 2008; Bashan et al., 2014; O'Callaghan, 2016; Zeffa et al., 2019).
It is rational to assume that Azospirillum sp., like other PGPBs promote the assimilation of nitrogen compounds by inoculated plants and establish conditions for the reduction of the chemical fertilizer rates and N2O emissions from the soil through the more efficient use and application of lower rates of mineral fertilizers. That was confirmed by various studies showing a significant increase in crop production efficiency in response to inoculation (Freitas and Germida, 1990; Okon and Itzigsohn, 1995; Bashan and Holguin, 1998; Shaharooma et al., 2008; Martins et al., 2017).
The present study focuses on the characteristics of nitrogen fixation and biological denitrification (as the specific biological testing criteria) in crops rhizosphere under the use of different fertilization systems and microbial inoculation, as well as on the influence of the studied processes on the potato yield and output quality.
The research was conducted during 2016–2019 in a field stationary experiment of the Institute of Agricultural Microbiology and Agroindustrial Production of the National Academy of Agrarian Sciences of Ukraine (established in 2009) on leached chernozem soil [pH salt – 5.2; soil organic matter – 3.01%; easily hydrolyzed nitrogen – 109 mg/kg; labile forms of phosphates (as P2O5) – 168 mg/kg; exchangeable potassium (as K2O) – 58 mg/kg of soil].
The potatoes of the Bellarosa variety were grown in crop rotation: potatoes – spring barley – peas – winter wheat under the seven different fertilization systems: farmyard manure, three different doses of complex mineral fertilizer, combination of farmyard manure and the mineral fertilizers, green manure and unfertilized control (see Table 1 for detailed test variants description), each studied into blocks with and without crop inoculation.
The experimental design was the same across the years of studies, with the only shifting factor of plots' distribution following the crop rotation scheme (potatoes always planted after winter wheat). The test plots (7.2 × 12.0 m each) were randomly spread across the experimental field in 4-fold repetition following the described crop rotation, accounting total of 56 test plots.
Crop Inoculation was performed with the microbial preparation Biogran (Technical Specifications of Ukraine 24.1-00497360-006:2009, State registration certificate A 05575), created based on the active nitrogen fixing bacterium Azospirillum brasilense 410 [deposited in the collection of microorganisms of the All-Russian Institute of Agricultural Microbiology (ARIAM) under the number VNIISHM B-36, and in the Depository of the Institute of Microbiology and Virology of the National Academy of Sciences of Ukraine, under the number IMV B-7222] (Volkogon and Dimova, 2004). Potato tubers were manually treated prior to planting with the aqueous suspension of the microbial preparation at the rate of 2 L per 4 tons of potatoes (1.5 × 109 of bacterial cells in 1 ml of suspension).
Rhizosphere soil samples for analysis were taken from the crop roots within 0.3 cm range around the roots of sampled plants after shuddering. The samples obtained from all plant roots in a specific test plot were mixed and used for preparation of average sample.
To study the differences in the potential nitrogenase activity in the rhizosphere soil of potato plants in response to the action of fertilizers and inoculation the acetylene method developed by Hardy et al. (1968) in the modification of Umarov (1976) was used. For this purpose, the average sample of the rhizosphere soil without crop residues was taken and used for subsequent analyses. Soil samples weighing 10 g were placed in 40 cm3 glass vessels. 1 cm3 of 20% D-glucose solution was added to the soil samples. The soil moisture level was determined separately and adjusted with sterile distilled water to achieve 70% of the full soil moisture-holding capacity. All samples were mixed thoroughly, closed with cotton plugs and kept for 72 h in a dark room with controlled temperature regulated with a thermostat at 26°C. After the incubation the cotton plugs were replaced with rubber ones. Acetylene was injected into each vessel in the amount of 10% from the gaseous phase volume (3 cm3). After 1-h exposure of the samples with acetylene, gas samples were taken with a syringe and analyzed on a gas chromatograph with a flame ionization detector [3 m steel column filled with Parapak Q 60-80 mesh sorbent (Waters Corporation, USA), thermostat temperature 40°C, gas flow: hydrogen – 15 cm3/min, nitrogen – 100 cm3/min, air – 500 cm3/min]. All measurements were performed in 5-fold repetition.
Potential nitrogenase activity (PNA) in rhizosphere soil, expressed in moles of C2H4 per one gram of soil per hour was calculated using the next formula:
E, amount of ethylene in analyzed gaseous sample; V1, volume of gaseous phase in vessel, cm3; K, soil moisture coefficient; V2, analyzed sample volume injected in chromatograph, cm3; m, soil sample weight, g; t, acetylene exposure time, hours.
Taking into account that biological denitrification with the formation of N2 as the final product is observed only in a limited number of microorganisms, so-called true denitrifiers, and naturally does not always end with the formation of N2 (Conrad, 1996) but is often suspended at the stage of N2O formation the acetylene blockage method developed by Zviagincev (1991) was used to determine the potential denitrification activity in rhizosphere soil of potato plants in response to the action of fertilizers and inoculation. This method is based on the ability of acetylene to inhibit the nitric oxide reductase and suspend the process of and dissimilation at the stage of nitric oxide reduction, thus allowing the determination of the N2O emissions from denitrification (N2OD) and assess the activity of all denitrifying microorganisms in soils, the ones that reduce nitrogen compounds to N2O and those that perform complete denitrification – to N2. To determine the potential denitrification activity, samples of rhizosphere soil, weighing 10.0 g, were prepared and placed in 40 cm3 vessels. 1 cm3 of 20% D-glucose solution and 1 cm3 of 2% KNO3 solution were added to the samples. Soil moisture was adjusted with sterile water to 70% of the full soil moisture-holding capacity. To create anaerobic conditions vessels were closed with rubber stoppers and filled under the pressure with helium to displace the air. The rubber stopper was punctured with two injection needles, one of which was attached to the helium source, while the other was used to release gases and balance the pressure in vessels. Vessels were filled with helium for 30 s, after which the needles were simultaneously removed. 3 cm3 of the gas mixture was taken from each vessel followed by the injection of 3 cm3 of the acetylene (to maintain the normal partial pressure of gases in the vessels). Prepared soil samples in vessels were kept in a dark room with controlled temperature regulated with a thermostat at 26°C for 24 h. At the end of the exposure period, gas samples were taken with an injection syringe and analyzed on a gas chromatograph with an electron capture detector [columns temperature – 40°C, evaporator temperature – 120°C, detector temperature – 330°C. Carrier gas (argon with methane 95/5) consumption rate – 35 cm3/min, 3 m steel column filled with Parapak Q 60-80 mesh sorbent (Waters Corporation, USA)]. All measurements were performed in 5-fold repetition.
Potential denitrification activity (N2OD) in rhizosphere soil, expressed in nmoles N2O per gram of soil per day was calculated using the next formula:
E, amount of N2O in analyzed gaseous sample; V1, volume of gaseous phase in vessel, cm3; K, soil moisture coefficient; V2, analyzed sample volume injected in chromatograph, cm3; m, soil sample weight, g; t, acetylene exposure time, days.
To estimate the number of ammonifiers in the rhizosphere soil of potato plants the dilution culture of soil suspensions on meat-peptone agar plates was used (Gerhardt, 1981). The number of nitrogen fixation microorganisms was determined using a semi-liquid medium with malate (Dobereiner and Baldani, 1979) and acetylene test (Villemin et al., 1974). The number of denitrifiers was determined on Giltay liquid medium with Gris reagent (nitrite test) (Zviagincev, 1991). The final numbers of nitrogen fixing bacteria and denitrifiers were calculated using the McCrady table based on the growth of the microorganisms in the extreme dilutions (Gerhardt, 1981).
Taking into the account that the concentration of mineral nitrogen compounds in the soil is subject to change during the growing season influencing, respectively, the interrelation of the studied processes all experiments were conducted in dynamics. For this all soil samples and the analysis of the potential nitrogenase activity, the denitrification activity and microorganisms' counts were performed during three main crop growth stages: budding (BBCH 51), flowering (BBCH 61) and crop senescence (BBCH 91).
Potato yield was determined by weighing all tubers harvested from the single test plot for the experimental seasons of 2016–2019.
The starch content in tubers was determined using the Evers method (Ermakov, 1972), the ascorbic acid – using the method based on the reduction properties of the vitamin C (Ermakov, 1972), the nitrates content – using the potentiometric assay (Gorodniy, 2005).
The differences between the variants in the potential activity of nitrogen fixation (potential nitrogenase activity), the potential denitrification activity, product quality indices were analyzed using the arithmetic mean and standard deviation values calculated with MS Excel build-in Analysis ToolPak. The dynamics of nitrogen fixing microorganisms and denitrifying bacteria in the rhizosphere soil of potato plants were statistically processed using the McCrady tables. Crop yields were analyzed using the two-way ANOVA algorithm with two probability levels (<5 and <1%) using Statistica 6.0 software (StatSoft Inc., USA). The least significant difference was calculated for (a) the whole experiment, (b) only for different fertilizer type), (c) only for inoculation with A. brasilense) and their interaction.
Taking into the account that the course of the nitrogenase and denitrification activity in the rhizosphere of potato plants was uniform throughout the research years (2016–2019) the data discussed further represents the results obtained in 2018.
The dynamics of the potential activity of nitrogen fixation in the rhizosphere soil of potato plants indicates its stable increase in response to farmyard manure application. High levels of nitrogenase activity were also observed in the variants with green manure, while the use of organo-mineral fertilizer system has reduced the potential nitrogen fixation activity for a prolonged time, with the slight performance increase starting from the flowering stage (BBCH 61) and beyond (Figure 1).
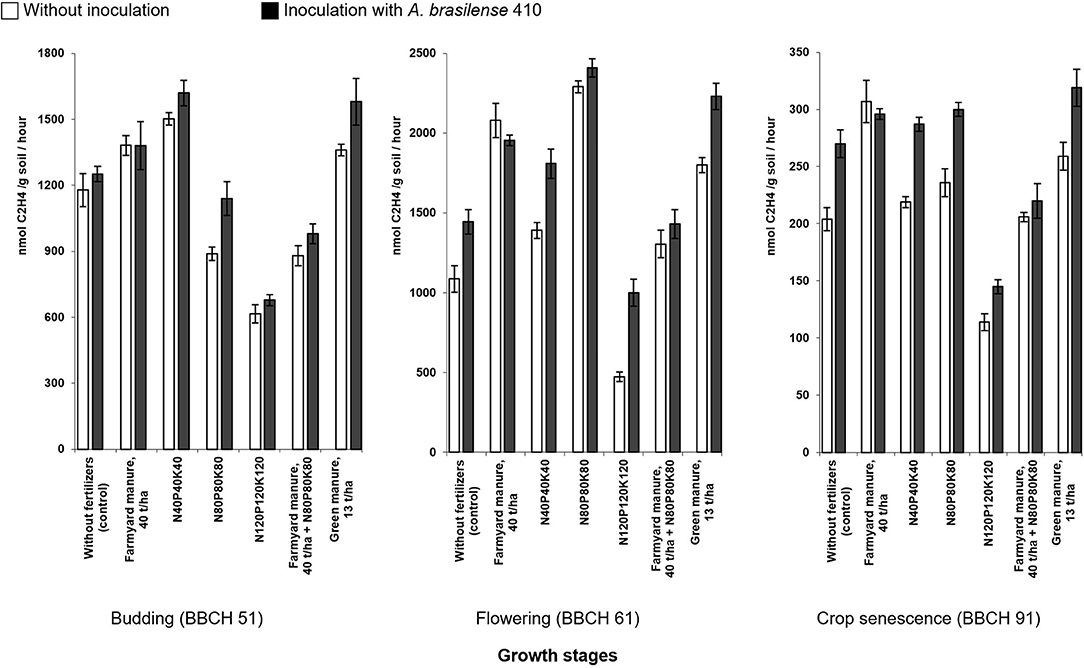
Figure 1. The dynamics of the potential nitrogenase activity (ethylene production) in the rhizosphere soil of potato plants under the action of fertilizers and microbial inoculation with A. brasilense 410 (2018) (bars indicate standard deviation).
The lowest studied doses of mineral fertilizers, N40P40K40, have stimulated nitrogen fixation activity, especially at the beginning of the growing season. The intensification of the mineral nutrition doses, N80P80K80, has reduced the potential nitrogen fixation at initial growth stages but gained higher levels later – at the flowering stage (BBCH 61) and the beginning of crop senescence (BBCH 91). The use of the highest rates of mineral fertilizers, N120P120K120, leads to the reduction of nitrogen fixation throughout the growing season (Figure 1).
Crops inoculation has stimulated the nitrogenase activity in the rhizosphere soil in the variants with low and medium rates of mineral fertilizers. The combination of inoculation with the highest studied rate of mineral fertilizers, N120P120K120, has also stimulated the nitrogen fixation activity, although the numbers have remained below the control values.
Inoculation of potato tubers had no effect on the potential nitrogen fixation activity in the variants with the farmyard manure, unlike in the variants with green manure where inoculation with A. brasilense has significantly stimulated the nitrogenase activity in the rhizosphere soil of potato plants. A slight increase in nitrogenase activity was observed at the flowering stage (BBCH 61) and the beginning of crop senescence (BBCH 91) in response to crop inoculation on the organo-mineral fertilization background. However, the obtained values were below the numbers observed in the test plots with low and medium fertilizer rates.
All types and doses of fertilizers used in the given research led to the intensification of the biological denitrification in the rhizosphere of potato plants (Figure 2). Test plots with the farmyard manure were characterized by significant N2OD losses throughout the growing season. The combination of farmyard manure with the mineral fertilizers has induced these losses. In the case of mineral fertilizers, the potential denitrification activity was proportional to the applied rates of fertilizers. Even crop cultivation on the background of green manure has a slight increase in the N2OD emissions activity.
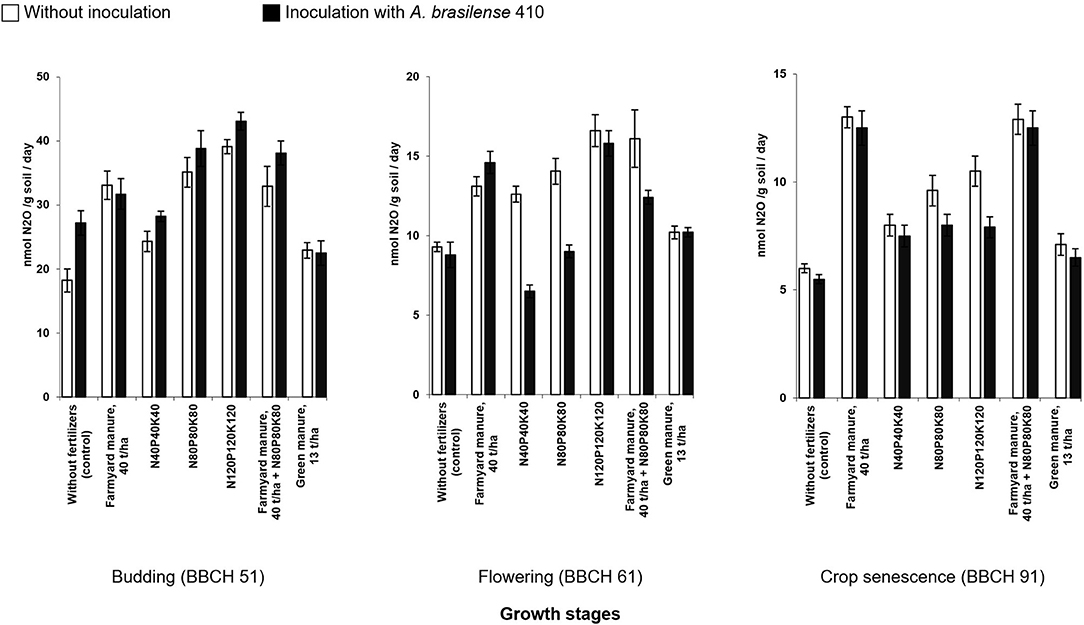
Figure 2. Potential activity of N2OD emissions in the rhizosphere soil of potato plants under the action of fertilizers and microbial inoculation with A. brasilense 410 (2018) (bars indicate standard deviation).
Inoculation with A. brasilense has induced a significant reduction in N2OD emissions in variants with low and medium rates of mineral fertilizers (Figure 2) starting from the flowering stage (BBCH 61). At the end of the growing season (crops senescence, BBCH 91), the denitrification intensity under the influence of the inoculation has decreased even in the variant with the highest studied dose of fertilizers, N120P120K120.
The use of farmyard manure has significantly increased the number of ammonifiers, nitrogen fixers and denitrifiers whereas inoculation practically had no impact on the number of microorganisms in the rhizosphere soil of plants grown on the farmyard manure background (Table 2). At the same time, the inoculation of potatoes grown after green manure has stimulated the development of both ammonifiers and diazotrophs while the number of denitrifying microorganisms has remained stable.
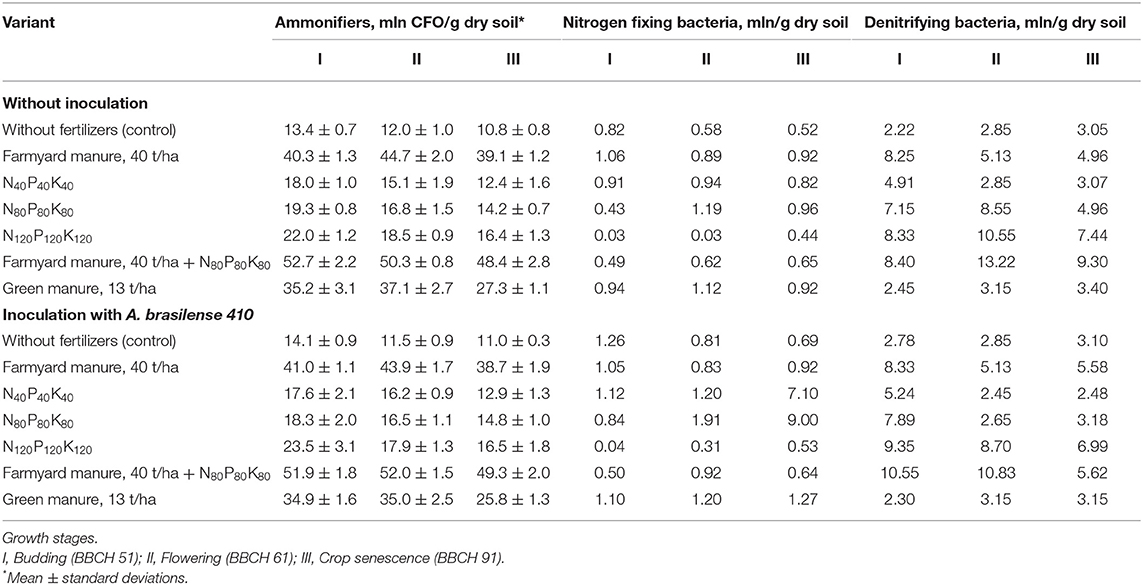
Table 2. The quantitative dynamics of ammonifiers, nitrogen fixing microorganisms and denitrifying bacteria in the rhizosphere soil of potato plants under the action of fertilizers and inoculation (2018).
Inoculation of plants grown on low and medium mineral backgrounds, N40P40K40 and N80P80K80, respectively, has significantly contributed to the increase of the diazotrophs number and decrease of the number of denitrifying microorganisms in the rhizosphere soil. The highest used rate of mineral fertilizers, N120P120K120, has reduced the effect of inoculation as the number of diazotrophs in the rhizosphere of plants remained unchanged. At the same time, crop inoculation with A. brasilense has promoted the growth of denitrifying bacteria at the early stages of crop development with a gradual decrease in their number starting from the flowering stage (Table 2).
Potato yield records indicate the highest increase in crop productivity with the use of farmyard manure, 40 t/ha, organo-mineral fertilizers and intense mineral fertilization backgrounds. The application of low and medium fertilizer rates has stimulated crop productivity by 24.6 and 93.2%, respectively. At the same time, the combination of the inoculation with mineral fertilizers has the highest impact on output results (Table 3). In particular, the introduction of A. brasilense has increased the crop yield by 42.4% on the N40P40K40 background, and by 116.9% on the N80P80K80 background, compared to the control. The combination of the highest mineral fertilizer rate, N120P120K120, with inoculation has shown that with the increasing agrochemical load, the efficiency of inoculation decreases.
Crop inoculation has practically no effect on its productivity in variants with farmyard manure, 40 t/ha, application. No significant difference was observed in the variants with combined inoculation and organo-mineral background.
Analysis of output quality parameters has shown that the inoculation of potato plants has substantially influenced the quality of the obtained products (Table 4). Thus, the application of microbial preparation has stimulated the accumulation of starch in potato tubers while reducing the nitrate contents.
The study of ascorbic acid content has shown a significant increase in the variants with mineral fertilizers, green manure and organo-mineral background (up to 14%). The increase was even more significant in response to inoculation (Table 4).
The dependence of associative nitrogen fixation activity on nitrogen fertilizer doses was first shown by Balandreau and Villemin (1973). Umarov et al. (1985) formulated the concepts of optimal doses of mineral nitrogen for the process of associative nitrogen fixation as “the ones that do not exceed the physiological needs of plants in nitrogen.” The same conclusion was reached by Ladha et al. (1986). In the development of these studies, we proposed (Volkogon, 2013) to determine not only the optimal doses of mineral nitrogen (the ones that promote the highest rates of nitrogen fixation), but also the physiologically, or environmentally, acceptable doses [the ones that do not reduce the nitrogen fixation activity below control (without fertilizers) indicators]. In our opinion, such biological approach can be a significant addition to the practice of agrochemical justification of crop fertilization systems.
Since the introduction of the acetylene reduction method by Hardy et al. (1968) for the determination of the nitrogenase activity of diazotrophs, a number of its modifications have been developed. Particularly, in 1976 Umarov proposed a modification of Hardy's method for determining the potential nitrogenase activity in the soil. Notwithstanding that the method cannot be used for the evaluation of the activity and productivity of nitrogen fixation, its application allows locating differences in the functional activity of rhizosphere diazotrophs in response to various agricultural techniques, including fertilizers application and inoculation.
The changes of the ecologically acceptable doses of mineral nitrogen can be achieved upon the introduction of certain PGPBs into the crop lands, as inoculated plants uptake larger amounts of nitrogen to ensure a constructive metabolism. At the same time, crop inoculation expands the range of environmentally acceptable doses of fertilizers (Volkogon, 2013; Volkogon et al., 2014). So, higher nitrogenase activity in rhizosphere soil of experimental variants compared to unfertilized control is indicating the ecological feasibility of the selected level of nitrogen nutrition while the reduction of nitrogenase activity, on the contrary, indicates the inhibition of the nitrogen fixation process and possible environmental risks.
Thus, the analysis of the nitrogenase activity dynamics in the rhizosphere soil of potato plants demonstrates that mineral fertilizers in the doses that are not exceeding N80P80K80 can be considered physiologically and ecologically expedient. Intensification of mineral nutrition, as well as the combination of farmyard manure with the mineral fertilizers, were shown to be environmentally unfavorable, as the nitrogenase activity indices in these variants were dramatically lower compared to the control numbers. Apparently, the high concentration of nitrogen compounds in the soil reduces the synthesis of the nitrogenase nitrogen-fixing complex of diazotrophic bacteria. To collect more evidence on the joint use of mineral nitrogen with the farmyard manure the application of lower doses of mineral nitrogen fertilizers should be tested for the organo-mineral fertilization system.
It is known that the denitrification process does not always end with the dinitrogen formation. Under natural conditions, this process can result in incomplete reduction of nitrates to nitrite (), nitric oxide (NO), or nitrous oxide (N2O) (Conrad, 1996). Complete denitrification with the formation of dinitrogen is observed only for a limited number of microorganisms, so-called true denitrifiers. To reveal the activity of all denitrifying microorganisms in the rhizosphere of potato plants we used acetylene, as a specific inhibitor of nitrous oxide reductase, to block the reduction of N2O to N2.
At the same time, it should be emphasized that the determination of the potential denitrification activity in the rhizosphere soil does not characterize the total loss of N2O from a certain area, but only reflects the reaction of rhizosphere microorganisms to a certain number of mineral nitrogen compounds, and thus corresponds to the crop response to the shortage or excessive amount of mineral nitrogen.
With N2OD emission values exceeding control in all studied combinations throughout the growing season, farmyard manure, 40 t/ha, used stand-alone and in combination with mineral fertilizer have demonstrated the highest increase. The losses of gaseous nitrogen in variants with mineral fertilizers have corresponded to the applied rate as they have increased with the fertilization intensity. This data confirms the previously reported findings of Barton et al. (1999) and Shcherbak et al. (2014) on denitrification rates and N2O emissions from croplands.
The use of an inoculant based on A. brasilense 410 in potato growing technology significantly influences the processes of nitrogenase activity and biological denitrification in the rhizosphere of plants, but the effect of inoculation depends on the fertilization background. High levels of nitrogenase activity at the beginning of the growing season were observed in variants with N40P40K40 and, starting from the flowering phase, for N80P80K80. Under the use of the highest rate of mineral fertilizer, N120P120K120, inoculation, even though it stimulates the nitrogenase activity, has not reached the control values. One of the highest indices of the nitrogenase activity in the rhizosphere of potato plants was observed in rhizosphere soil under the combined action of inoculation with green manure.
At the beginning of the growing season, inoculation has contributed to the growth of N2OD emissions in all variants except for the farmyard manure. Microorganisms introduced into the crop soils can also contribute to the reduction of the excess amount of nitrogen in the soil as even a small amount of mineral nitrogen in the soil can be considered excessive for crop seedlings with the Azospirillum sp. involved in both denitrification and nitrogen fixation processes (Bashan and Levanony, 1990). After the utilization of a certain amount of applied mineral nitrogen by potato plants, initiated with the inoculation, bacteria reflect the changes in the soil environment and reveal its nitrogen-fixing function. This, in turn, significantly reduces the denitrification activity in the rhizosphere of inoculated plants. Thus, in the flowering stage, the N2OD emissions were even below the control levels in the variants with the lowest rate of mineral fertilizers, N40P40K40, and crop inoculation. The numbers observed in the variants with the medium fertilizer rate, N80P80K80, were similar to the control.
The reduction of N2O emissions in response to crop inoculation with PGPB-based preparations used under mineral nitrogen fertilizer background was reported earlier by Calvo et al. (2013) based on the results obtained in a greenhouse experiment. These findings were confirmed in the following experiment, which showed that the use of inoculants in corn production reduced the emissions of N2O from 15 to 49%, subject to the type of nitrogen fertilizer (except for urea) and microorganisms. In general, they demonstrated that PGPBs enhance mineral nitrogen uptake following the fertilizer application while reducing N2O emissions (Calvo et al., 2016). Our findings have confirmed these findings. However, the introduction of the biological preparation in the cultivation of potatoes on the background of high rates of mineral fertilizer has increased the emission of N2OD. It can be related to the ability of Azospirillum sp. to perform different functions depending on the availability of the mineral nitrogen in the soil. Since bacteria of Azospirillum genus are capable of denitrification, it can be assumed that at high nitrogen background and presence of introduced or present bacteria in the soil, they are involved in the natural regulation process of the nitrogen cycle, accounting for N2OD emissions aimed to reduce the excess of mineral nitrogen forms in soil. This can be avoided through the introduction of a sufficient amount of fresh organic matter into the soil with a broad C/N ratio or reducing nitrogen rate application. It would promote the active transformation of mineral nitrogen compounds into organic forms and reduce nitrogen losses.
So, under inoculation, plants utilize mineral nitrogen compounds to a much greater extent, which is extremely important from the ecological point of view. In fact, inoculants can serve as an inhibitor of the denitrification process since their introduction to the “plant-soil” system increases nitrogen utilization coefficients from mineral fertilizer.
The effect of A. brasilense 410 on the studied processes was leveled under potatoes growing on the background of farmyard manure, 40 t/ha. Both the nitrogenase activity and the emission of N2OD in the rhizosphere of inoculated plants remained unchanged on a farmyard manure background. That is related to the fact that a significant number of microorganisms are introduced into the soil with manure, creating a prevailing competitive environment for nitrogen fixing bacteria from biological products. Under these circumstances, the positive effect of inoculation is offset. These assumptions are indirectly confirmed by the counts of microorganisms of individual ecological and trophic groups in the rhizosphere soil of potato plants (Table 2). Thus, the introduction of farmyard manure into the soil has significantly increased the number of ammonifiers, nitrogen fixation microorganisms and denitrifying bacteria. Inoculation practically has not significantly influenced the number of microorganisms in the rhizosphere soil. In this case, farmyard manure can ensure non-specific bacterization of the soil, which prevents the successful introduction of A. brasilense.
Scientists have already paid attention to the abundant number of microorganisms in the farmyard manure. Thus, one of the founders of scientific soil science –Dokuchaev (1948), wrote: “Along with manure, a vast number of microorganisms are introduced into the soil, the role of which is no less important than fertilizers.” Our results, to some extent, confirm these findings and expand the knowledge about the effectiveness of inoculation upon the cultivation of the crops on the farmyard manure background.
Unlike with the farmyard manure, potato inoculation following the green manure incorporation has promoted the growth of ammonifiers and diazotrophs in the rhizosphere soil of plants. The number of denitrifying microorganisms has remained unchanged. Therefore, the incorporation of green manure, lupine, in particular, creates optimal conditions for A. brasilense efficiency effect.
The biological preparation applied on low mineral fertilizer backgrounds has considerably increased the number of diazotrophs, which confirms the creation of favorable soil conditions for the development of the introduced microorganism.
Regardless of the fertilization system, an increase in crop productivity was observed for all studied variants. Therewith, the inoculation ensured the highest gains in combination with low and medium fertilizer rates with a 14.3% yield increase on the N40P40K40 background and 12.3% on the N80P80K80 background. The highest studied rate of mineral fertilizer, N120P120K120, had also promoted the yield increase, but to a much lesser extent, indicating the disadvantage of the chosen fertilizer rate for the efficiency effect of A. brasilense inoculation.
Analyzing the yield levels of potatoes grown on mineral backgrounds without inoculation and in combination with inoculation, it was noted that the effect of A. brasilense on the crop productivity was equivalent to the action of a certain number of mineral fertilizers. Thus, the difference in yield means between variants with the highest applied dose (N120P120K120) and N80P80K80 without inoculation was 4.2 t/ha. At the same the yield difference between N120P120K120 and N80P80K80 backgrounds combined with the action of crop inoculation was only 1.4 t/ha. In some years, the effect of inoculation on crop yields was equivalent to the action of mineral fertilizers at the lowest studied dose (N40P40K40). Overall these findings indicate the possibility of reducing the amount of mineral fertilizers in crop production when combine with the inoculation to achieve the planned result.
Higher efficiency of inoculation used in the cultivation of different crops grown on low rates of nitrogen fertilizers has been reported in numerous publications. According to Freitas and Germida (1990), PGPBs are more effective with the reduced amount of nutrients in the soil. Shaharooma et al. (2008) reported that nitrogen utilization efficiency in response to Pseudomonas fluorescens inoculation has increased in response to all levels of wheat fertilization. Depending on the mineral fertilizer rates (25, 50, 75, and 100% to the recommended rate of nitrogen, phosphorus, and potassium), it ensured 115, 52, 26, and 27% yield increase compared to the uninoculated wheat plants, respectively.
Our results have also indicated the optimality of low and medium fertilizer rates for the manifestation of inoculation efficiency. Thus, in particular, potato inoculation on the medium fertilizer background, N80P80K80, averaged 25.6 t/ha over the 4 years of research, which is close to the figures obtained in the variant with the highest used fertilizer rate, N120P120K120 – 27.0 t/ha. The inoculation effect, in this case, was equivalent to the action of a certain amount of mineral fertilizers. That can be used in estimations of mineral fertilizers as inoculation can reduce the rates without affecting crop yields.
The inoculation efficiency effect was noticeable upon the crop cultivation following the green lupine manure. The increase of the nitrogenase activity in the rhizosphere of potato plants and the crop yield was observed upon the A. brasilense introduction. This data has confirmed the results of other researchers. Thus, the maximum diameter of cobs, their raw and dry weight was recorded for lettuce inoculated with Azospirillum bacteria (GM1M1Az3) on the background of green manure and mulching (Borthakur et al., 2012). It has also been reported that the interaction between green manure and seed inoculation with Herbaspirillum seropedicae has had a positive effect on corn yield, contributing to the higher number of kernels and their weight (Avila et al., 2020).
In contrast to the effect on green manure, background inoculation did not affect the yield of potatoes in the variants where the farmyard manure was applied. The latest research findings on this topic were reviewed by Wani (1990) indicating the high efficiency of the combination of non-symbiotic nitrogen-fixing bacteria with manure. However, freely existing diazotrophs cannot be compared with associative bacteria, given the mechanisms of their interaction with plants. At the same time, there is evidence of the high efficacy of PGPB used following a pig slurry background. Thus, a study by Lai et al. (2008) showed that lettuce growth in soil fertilized with pig slurry and inoculated with Azospirillum rugosum IMMIB AFH-6 was significantly lower compared to the one of the inoculated plants grown on the mineral background. However, the addition of only half of the recommended rate of mineral fertilizer to the pig slurry background had ensured the highest yield gains and an increase of other studied parameters of inoculated plants (Lai et al., 2008).
Studies conducted by Yildirim et al. (2011) have shown that broccoli inoculation with Bacillus cereus, Rhizobium rubi, and Brevibacillus reuszeri following the farmyard manure had contributed to the increase of crop productivity (up to 24.3%), chlorophyll content (up to 14.7) and nutrients uptake compared to control (manure only) values. Combination of farmyard manure with crop inoculation with Azotobacter has increased the biological yield of wheat (Esmailpour et al., 2013) and corn (Dutta et al., 2014).
According to Hadi et al. (2015), the maximum productivity of black cumin seeds was recorded for the crops inoculated with Azotobacter and Azospirillum on farmyard manure, 5 t/ha, background. Used separately, both bacteria and manure applications have a positive effect on crop yields. However, their combination ensured a better outcome. In their review on the importance of PGPB for the absorption of nutrients by inoculated plants from fertilizers, Adesemoye and Kloepper (2009), do not differentiate manure and chemical fertilizers as substrates containing chemical elements and consider promising the use of microbial preparations on the manure background. The result obtained in our experiment, which is quite the opposite of the findings mentions above, may be explained by the conditions of the experiment (crop choice, characteristics of inoculant, manure dose, soil type, etc.). On other hand, it designates the study prospects of the combination of PGPB with farmyard manure. The data also indicates a certain limitation of the use of biological preparations in the organic production systems, as their efficiency can be compromised. At the same time, the effectiveness of biologicals may even advance with their introduction into the soil not with manure, but through vermicomposting, as noted by Song et al. (2015).
The inoculation had ensured the reduction of the nitrates content in potato tubers, especially, when used on mineral fertilizer background. Besides its effect on the output product quality, crop inoculation ensured higher content of starch and ascorbic acid in potato tubers. The increase in vitamin C content can be beneficial for its possible positive effects. Potatoes are an important source of vitamin C, not only because of their relatively high content but also because they can be stored for a prolonged period. Therefore, the improvement in the ascorbic acid content in potatoes will have a beneficial effect on human nutrition (Love and Pavek, 2008). It is also known, that ascorbic acid can neutralize to a great extent the harmfulness of nitrates for warm-blooded organisms (Hirneth and Classen, 1984; Shehata, 2005). Thus, the increase in its content following the reduction of nitrates content in the variants with A. brasilense inoculation promotes the value of the product.
Even so, the lowest dose of mineral fertilizers was shown to be the most optimal in terms of environmental sustainability of production, higher doses (N80P80K80) are a more reasonable compromise between environmental feasibility and crop productivity. Green manure use despite its vital role in regulating processes of biological transformation of nitrogen was practically disadvantageous, while the application of farmyard manure is advisable with some reservations. Crop inoculation with A. brasilense was proved to be beneficial influencing the optimization of the ecological conditions of croplands, the increase of potatoes productivity and the improvement of the output quality of potato crops.
The study of nitrogenase activity and N2OD emissions in the rhizosphere of potato plants in dynamics has shown that the application of low (N40P40K40) and medium (N80P80K80) doses of mineral fertilizers are physiologically and ecologically optimal for cultivation on leached chernozem, especially in combination with inoculation. Crops inoculation with A. brasilense on these mineral fertilizer backgrounds has ensured higher nitrogen fixation activity, a significant reduction of denitrification levels, and the highest crop productivity compared to the other fertilizer options. The inoculation effect on the course of nitrogen fixation and denitrification crop cultivation on the background of 40 t/ha of manure was significantly leveled, while the highest manifestation of the efficiency of biological preparation was observed under the green manure background. Taken as a whole, the use of A. brasilense in potato growing technologies has a positive effect on the accumulation of starch and ascorbic acid and helps to reduce the content of nitrates in the output products.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
VV performed the experimental data analysis and worked on the manuscript discussion session. SD and KV carried out the field experimental data acquisition, quantification of basic physiological groups of microorganisms, and data analysis. KV and VS performed the study of potential nitrogenase activity and potential denitrification activity in rhizosphere soil and data analysis. MV helped with the data interpretation and manuscript preparation. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are thankful to the reviewers of given manuscript as their critical comments, suggestions and feedback have improved the clarity and quality of this paper.
Adesemoye, A. O., and Kloepper, J. W. (2009). Plant–microbes interactions in enhanced fertilizer-use efficiency. Appl. Microbiol. Biotechnol. 85, 1–12. doi: 10.1007/s00253-009-2196-0
Avila, J. S., Ferreira, J. S., Santos, J. S., Rocha, P. A., and Baldani, V. L. D. (2020). Green manure, seed inoculation with Herbaspirillum seropedicae and nitrogen fertilization on maize yield. Revista Brasileira de Engenharia Agrícola e Ambiental 24, 590–595. doi: 10.1590/1807-1929/agriambi.v24n9p590-595
Balandreau, J., and Villemin, G. (1973). Fixation biologique de l'azote moleculairee en Savane de Lamto. Rev. Ecol. Biol. Sol. 10, 25–33.
Barton, L., McLay, C. D. A., Schipper, L. A., and Smith, C. T. (1999). Annual denitrification rates in agricultural and forest soils: a review. Aust. J. Soil Res. 37, 1073–1093. doi: 10.1071/SR99009
Bashan, Y., de Bashan, L. E., Prabhu, S. R., and Hernandez, J.-P. (2014). Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998-2013). Plant Soil. 378, 1–33. doi: 10.1007/s11104-013-1956-x
Bashan, Y., and Holguin, G. (1998). Proposal for the division of plant growth promoting rhizobacteria into two classifications: biocontrol-PGPB (plant growth promoting bacteria) and PGPB. Soil Biol. Biochem. 30, 1225–1228. doi: 10.1016/S0038-0717(97)00187-9
Bashan, Y., and Levanony, H. (1990). Current status of Azospirillum inoculation technology: Azospirillum as a challenge for agriculture. Can. J. Microbiol. 36, 591–608. doi: 10.1139/m90-105
Borthakur, P. K., Tivelli, S. W. P., and Urquerio, L. F. V. (2012). Effect of green manuring, mulching, compost and microorganism inoculation on size and yield of lettuce. Acta Hortic. 933, 165–171. doi: 10.17660/ActaHortic.2012.933.19
Bothe, H., Klein, B., Stephan, M. P., and Dobereiner, J. (1981). Transformation of inorganic nitrogen by Azospirillum spp. Arch. Microbiol. 130, 96–100. doi: 10.1007/BF00411058
Calvo, P., Watts, D. B., Ames, R. N., Kloepper, J. W., and Torbert, H. A. (2013). Microbial-based inoculants impact nitrous oxide emissions from an incubated soil medium containing urea fertilizers. J. Environ. Qual. 42, 704–712. doi: 10.2134/jeq2012.0300
Calvo, P., Watts, D. W., Kloepper, J. W., and Torbert, H. A. (2016). The influence of microbial-based inoculants on N2O emissions from soil planted with corn (Zea mays L.) under greenhouse conditions with different nitrogen fertilizer regimens. Can. J. Microbiol. 62, 1041–1056. doi: 10.1139/cjm-2016-0122
Cavigelli, M. A., and Robertson, G. P. (2000). The functional significance of denitrifier community composition in a terrestrial ecosystem. Ecology 81, 1402–1414. doi: 10.1890/0012-9658(2000)081[1402:TFSODC]2.0.CO;2
Conrad, R. (1996). Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O and NO). Microbiol. Rev. 60, 609–640. doi: 10.1128/MR.60.4.609-640.1996
Dalal, R. C., Wang, W., Robertson, P. G., and Parton, W. J. (2003). Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Aust. J. Soil Res. 41, 165–195. doi: 10.1071/SR02064
Dobereiner, J., and Baldani, V. L. D. (1979). Selective infection of maize roots by streptomycin-resistant Azospirillum lipoferum and other bacteria. Can. J. Microbiol. 25, 1264–1269. doi: 10.1139/m79-199
Dokuchaev, V. V. (1948). On the Question of Opening the Departments of Soil Science at Russian Universities and the Doctrine of Microorganisms. Selected Works, Vol. 2. Moscow: State Publishing House for Agricultural Literature, 290–318.
Dutta, D., Kumar, V., and Singh, S. (2014). Effect of Nitrogen, Farmyard manure, Azotobacter interaction on rhizospheric population of Azotobacter and yield of maize in mollisol of Uttarakhand. Biosci. Bioeng. Biotechnol. 1, 11–15.
Ermakov, A. I. (1972). Biochemical Research Methods of Plants. Leningrad: Kolos Publishing House [in Russian].
Eskew, D. L., Focht, D. D., and Ting, I. P. (1977). Nitrogen fixation, denitrification, and pleomorphic growth in a highly pigment Spirillum lipoferum. Appl. Env. Microbiol. 34, 582–586. doi: 10.1128/AEM.34.5.582-585.1977
Esmailpour, A., Hassanzadehdelouei, M., and Madani, A. (2013). Impact of livestock manure, nitrogen and biofertilizer (azotobacter) on yield and yield components wheat (Triticum aestivum L.). Cercetări Agronomice în Moldova 2, 5–14. doi: 10.2478/v10298-012-0079-5
Freitas, J. R., and Germida, J. J. (1990). Plant growth-promoting rhizobacteria for winter wheat. Can. J. Microbiol. 36, 265–272 doi: 10.1139/m90-046
Gerhardt, P. (1981). Manual of Methods for General Bacteriology. Washington, DC: American Society for Microbiology.
Hadi, M. R. H. S., Ghanepasand, F., and Darzi, M. T. (2015). Evaluation of biofertilizer and manure effects on quantitative yield of Nigella sativa L. Int. J. Biol. Biomol. Agricul. Food Biotechnol. Eng. 9, 853–855.
Hardy, R. W., Holsten, R. D., Jacson, E. K., and Burns, R. C. (1968). The acetylene-ethylene assay for N2-fixation: laboratory and field evaluation. Plant. Physiol. 43, 1185–1207. doi: 10.1104/pp.43.8.1185
Hirneth, H., and Classen, H. G. (1984). Inhibition of nitrate-induced increase of plasma nitrite and methemoglobinemia in rats by simultaneous feeding of ascorbic acidor tocopherol. Arzneimittelforschung 34, 988–991.
Johnston, A. M., and Bruulsema, T. (2014). 4R nutrient stewardship for improved nutrient use efficiency. Proc. Eng. 83, 365–370. doi: 10.1016/j.proeng.2014.09.029
Juchenko, A. A. (1990). Adaptive Crop Production (Eco-Genetic Foundations). Kishinev: Publishing house Shtiinca [in Russian].
Kennedy, I. R., Pereg-Gerk, L. L., Wood, C., Deaker, R., Gilchrist, K., and Katupitiya, S. (1997). Biological nitrogen fixation in nonleguminous field crops: facilitating the evolution between Azospirillum and wheat. Plant Soil 194, 65–79. doi: 10.1023/A:1004260222528
Khan, M. N., Mobin, M., Abbas, Z. K., and Alamri, S. A. (2018). “Fertilizers and their contaminants in soils, surface and groundwater,” in The Encyclopedia of the Anthropocene, Vol. 5, eds D. A. DellaSala and M. I. Goldstein (Oxford: Elsevier), 225–240.
Kloepper, J. W., and Schroth, M. N. (1978). “Plant growth promoting rhizobacteria on radishes,” in: Proceedings of the 4th International Conference on Plant Pathogenic Bacteria, ed. Station de Pathologic Vegetal et Phytobacteriologic. Vol. 2. Angers, France, 879–882.
Korenkov, D. A. (1990). Issues of nitrogen agrochemistry and ecology. Agrochemistry 11, 28–37 [in Russian].
Ladha, J. K., Tiror, A. C., Caldo, G., and Watanabe, I. (1986). Rice-plant-accociated N2-fixation as affected by genotype, inorganic N fertilizer and organic manure. In transaction of XIII congr. Int. Soc. Soil Sci. Hamburg. 2, 598–599.
Lai, W. A., Rekha, P. D., Arun, A. B., and Young, C. C. (2008). Effect of mineral fertilizer, pig manure, and Azospirillum rugosum on growth and nutrient contents of Lactuca sativa L. Biol. Fertil. Soils. 45, 155–164. doi: 10.1007/s00374-008-0313-3
Love, S. L., and Pavek, J. J. (2008). Positioning the potato as a primary food source of vitamin C. Am. J. Pot Res. 8, 277–285. doi: 10.1007/s12230-008-9030-6
Luis, L., Gilles, B., Bruna, G., Anglade, J., and Josette, G. (2014). 50 year trends in nitrogen use efficiency of world cropping systems: the relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 9:105011. doi: 10.1088/1748-9326/9/10/105011
Lvov, N. P. (1989). Molybdenum in Nitrogen Assimilation in Plants and Microorganisms. Moskow: Publishing house “Science” [in Russian].
Martins, M. R., Jantalia, C. P., Reis, V. M., Alves, B. J. R., Boddey, R. M., and Urquiaga, S. (2017). Impact of plant growth-promoting bacteria on grain yield, protein content, and urea-15 N recovery by maize in a Cerrado Oxisol. Plant Soil. 422, 239–250. doi: 10.1007/s11104-017-3193-1
Mazzetto, A. M., Barneze, A. S., Feigl, B. J., Cerri, C. E. P., and Cerri, C. C. (2016). Nitrogen fertilizer effects on nitrous oxide emission from Southwest Brazilian Amazon pastures. J. Fertil. Pestic. 7:167. doi: 10.4172/2471-2728.1000167
Millar, N., Urreac, A., Kahmarka, K., Shcherbak, I., Robertson, G. P., and Ortiz-Monasterio, I. (2018). Nitrous oxide (N2O) flux responds exponentially to nitrogen fertilizer in irrigated wheat in the Yaqui Valley, Mexico. Agricul. Ecosyst. Environ. 261, 125–132. doi: 10.1016/j.agee.2018.04.003
Mirza, M. S., Rasul, G., Mehnaz, S., Ladha, J. K., So, R. B., Ali, S., et al. (2000). “Beneficial effects of nitrogen-fixing bacteria on rice,” in: The Quest for Nitrogen Fixation in Rice, eds J. K. Ladha, P. M. Reddy. Los Baños, Philippines: IRRI, 191–204.
O'Callaghan, M. (2016). Microbial inoculation of seed for improved crop performance: issues and opportunities. Appl. Microbiol. Biotechnol. 100, 5729–5746. doi: 10.1007/s00253-016-7590-9
Okon, Y., and Itzigsohn, R. (1995). The development of Azospirillum as a commercial inoculant for improving crop yields. Biotechnol. Adv. 13, 365–374. doi: 10.1016/0734-9750(95)02004-M
Rodrigues, E. P., Rodrigues, L. S., de Oliveira, A. L. M., Baldani, V. L. D., Teixeira, K. R. D., Urquiaga, S., et al. (2008). Azospirillum amazonense inoculation: effects on growth, yield and N2 fixation of rice (Oryza sativa L.). Plant Soil 302, 249–261. doi: 10.1007/s11104-007-9476-1
Ruppel, S., and Merbach, W. (1997). Effect of ammonium and nitrate on 15N2-fixation of Azospirillum spp., and Pantoea agglomerans in association with wheat plants. Microbiol. Res. 152, 377–383. doi: 10.1016/S0944-5013(97)80055-9
Saubidet, M. I., and Barneix, A. J. (1998). Growth stimulation and nitrogen supply to wheat plants inoculated with Azospirillum brasilense. J. Plant Nutrition. 21, 2565–2577. doi: 10.1080/01904169809365588
Shah, V. K., Davis, L. C., and Brill, W. J. (1972). Nitrogenase. I. Repression and derepression of the iron-molybdenum and iron proteins of nitrogenase in Azotobacter vinelandii. Biochim. Biophys. Acta 256, 498–511. doi: 10.1016/0005-2728(72)90078-3
Shaharooma, B., Naveed, M., Arshad, M., and Zahir, Z. A. (2008). Fertilizer-dependent efficiency of Pseudomonads for improving growth, yield, and nutrient use efficiency of wheat (Triticum aestivum L.). Appl. Microbiol. Biotechnol. 79, 147–155. doi: 10.1007/s00253-008-1419-0
Shcherbak, I., Millar, N., and Robertson, G. P. (2014). Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. U.S.A. 111, 9199–9204. doi: 10.1073/pnas.1322434111
Shehata, S. A. (2005). Nitrate detoxification of drinking water by ascorbic acid in growing rabbits. World Rabbit Sci. 13, 93–106. doi: 10.4995/wrs.2005.526
Signor, D., Cerri, C. E. P., and Conant, R. (2013). N2O emissions due to nitrogen fertilizer applications in two regions of sugarcane cultivation in Brazil. Environ. Res. Lett. 8:015013. doi: 10.1088/1748-9326/8/1/015013
Song, X., Liu, M., Wu, D., Griffiths, B. S., Jiao, J., Li, H., et al. (2015). Interaction matters: synergy between vermicompost and PGPR agents improves soil quality, crop quality and crop yieldin the field. Appl. Soil Ecol. 89, 25–34 doi: 10.1016/j.apsoil.2015.01.005
Sposari, M., and Flis, S. (2017). 4R framework implementation: precision and adoption by farmers and dealers. Crops Soil 5, 24–26. doi: 10.2134/cs2017.50.0507
Umarov, M., Shabaev, V., Smolin, V., and Aseeva, J. (1985). “Incorporation of biological nitrogen by nonlegumenous plants during associative N2-Fixation,” in: IX Int. Symp. Soil Biol. and Conservation of the Biosphere. Pap. Sorpon, 65.
Umarov, M. M. (1976). Acetylene method of studying nitrogen fixation in soil-microbiological studies. Pochvovedenie 11, 119–123 [in russian].
Villemin, G., Balandreau, J., and Dommergues, Y. (1974). Utilization du test de reduction de'acetylene pour la numeration des bacteries libres fixatuces d'azote. Ann. Microbiol. End. Enzimol. 24, 87–94.
Vitousek, P. M., Aber, J. D., Howarth, R. W., Likens, G. E., Matson, P. A., Schindler, D. W., et al. (1997). Technical report: human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 7, 737–750. doi: 10.2307/2269431
Volkogon, V., Berdnikov, O., Dimova, S., and Volkogon, M. (2014). Orientation of nitrogen transformation processes in the soil with corn growing under the different fertilization practices. Agricul. Sci. Pract. 1, 26–31. doi: 10.15407/agrisp1.03.026
Volkogon, V. V. (2013). Biological Nitrogen Transformation. The Direction of Processes at Different Levels of Crop Fertilizer. Saarbrucken: Palmarium Academic Publishing [in russian].
Volkogon, V. V., and Dimova, S. B. (2004). Biological preparations of complex action in potato cultivation. Bull. Agrar. Sci. 10, 29–33.
Wani, S. P. (1990). Inoculation with associative nitrogen-fixing bacteria: role in cereal grain production improvement. Indian J. Microbiol. 30, 363–393.
Yildirim, E., Karlidag, H., Turan, M., Dursun, A., and Goktepe, F. (2011). Growth, nutrient uptake, and yield promotion of broccoli by plant growth promoting rhizobacteria with manure. Hortscience 46, 932–936. doi: 10.21273/HORTSCI.46.6.932
Zeffa, D. M., Perini, L. J., Silva, M. B., Sousa, N. V., Scapim, C. A., Oliveira, A. L. M., et al. (2019). Azospirillum brasilense promotes increases in growth and nitrogen use efficiency of maize genotypes. PLoS ONE 14:e0215332. doi: 10.1371/journal.pone.0215332
Keywords: nitrogen fixation, denitrification, inoculation, fertilizers, potato, starch, nitrates, ascorbic acid
Citation: Volkogon VV, Dimova SB, Volkogon KI, Sidorenko VP and Volkogon MV (2021) Biological Nitrogen Fixation and Denitrification in Rhizosphere of Potato Plants in Response to the Fertilization and Inoculation. Front. Sustain. Food Syst. 5:606379. doi: 10.3389/fsufs.2021.606379
Received: 14 September 2020; Accepted: 13 April 2021;
Published: 14 May 2021.
Edited by:
Everlon Cid Rigobelo, São Paulo State University, BrazilReviewed by:
Noemi Carla Baron Cozentino, São Paulo State University, BrazilCopyright © 2021 Volkogon, Dimova, Volkogon, Sidorenko and Volkogon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mykola V. Volkogon, bXZvbGtvZ29uQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.