
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Sustain. Food Syst., 27 June 2019
Sec. Agro-Food Safety
Volume 3 - 2019 | https://doi.org/10.3389/fsufs.2019.00047
Rice is one of the most economically important foods in the world today. The FAO has reported that managing rice processing and the resulting byproducts into more sustainable applications would be beneficial for a variety of reasons. Rice processing involves several milling stages to produce edible final products. The milling process is the most important step in rice production because it determines the nutritional, cooking, and sensory qualities of crude rice. As crude rice goes through the milling process, byproducts are generated, such as bran that have been shown to exhibit beneficial impacts on human and animal nutrition. While several rice byproducts have applications in agriculture, rice bran has probably received the most attention for its functional properties. Rice bran is a mixture of protein, fat, ash, and crude fiber. However, rice bran's composition is largely dependent on the type of rice and the efficiency of the milling system. Based on studies with mice, rice bran has been shown to elicit prebiotic-like properties by preventing colonization of Salmonella in the gastrointestinal tract. More recently, in vitro incubation studies with chicken cecal contents have demonstrated that certain rice varieties are more inhibitory to Salmonella than others. Moreover, the byproducts of the rice milling process can also provide an economic boost for rice producing nations. In this review, the byproducts of the milling process, how they are utilized, and potential application for rice milling byproducts are discussed.
Rice is a critical source of food for more than 3 billion people annually (FAOSTAT, 2017). Approximately 680 million tons of rice is grown yearly, only second to wheat for the most food produced around the world (Foo and Hameed, 2009; Friedman, 2013). Rice, along with wheat and corn, belong to the genus Oryza, which are considered optimal sources of vitamins, minerals, and nutrition (FAOSTAT, 2017). Two species of rice are cultivated and twenty-two identified species are wild. The cultivated species are Oryza sativa and Oryza glaberrima. Oryza sativa is grown all over the world while Oryza glaberrima has been cultivated in West Africa for the last 3,500 years (Foo and Hameed, 2009; Friedman, 2013).
Several projects and studies have been directed toward promoting rice sustainability (Savant et al., 1996; Choudhury and Kennedy, 2004; Klotzbücher et al., 2015). Key factors toward long term sustainable rice cultivation include nitrogen fixation and the availability of silicon in the soil (Savant et al., 1996; Choudhury and Kennedy, 2004; Klotzbücher et al., 2015). However, in cultivating the rice, several byproducts are also generated (Dhankhar, 2014). Depending on the strain of rice and technique utilized, up to 40% of the yield is lost in the milling process due to the discarding of the byproducts (Linscombe, 2006). The byproducts of rice are broken rice, the husk, and the bran layer (Figure 1). The milling process is important because it improves the nutritional, cook time, and sensory characteristics of rice (Dhankhar, 2014). Being able to utilize these byproducts in alternative industries would, therefore, improve the yield and sustainability of rice production (Sanchez et al., 2018).
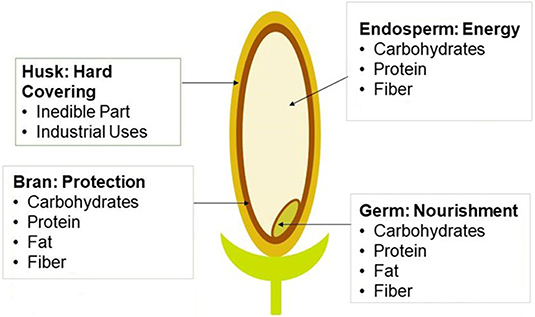
Figure 1. Diagram of a rice kernel. Shows the four different parts of a rice kernel, the rice husk, rice bran, rice germ, and the rice endosperm. Also shows the nutritional content inside different parts of the kernel (Smith, 2019).
The byproducts of rice have a 2-fold contribution to food sustainability. Firstly, bioactive compounds and nutrients contained in byproducts, such as rice bran and husk can be extracted, providing use in food products, as well as generate indirect income (Iriondo-DeHond et al., 2018). According to Esa et al. (2013), the demand for rice is expected to remain strong over the next few decades, causing the production of rice byproducts to remain high. With the increase in demand for healthier food products, rice byproduct could possibly increase as a food additive. In 2015, global rice bran oil (RBO) market size was estimated at over 1.2 million tons in 2015 (Rice Bran Oil Market, 2016). Production is mostly concentrated in countries, such as India, China, Japan, Thailand, and Vietnam. In 2013, the International Council of Rice Bran Oil (ICRBO) was formed to establish a scientific standard and focus on value-added products (Rice Bran Oil Market, 2016). The RBO market is anticipated to increase at a significant compound annual growth rate (CAGR) of 5.14% during the years 2017 to 2021 (Rice Bran Oil Market, 2016). The health benefits, a high smoke point meaning less impurities, and the balance of fatty acids make RBO a desirable choice for consumers (Nayik et al., 2015).
Byproducts of rice milling have been investigated within animal production systems, particularly poultry (Ugwu et al., 2008; Ebling et al., 2015; Rubinelli et al., 2017). Impacts on growth rate, pathogens, and the gastrointestinal microbial community have been observed (Ugwu et al., 2008; Ebling et al., 2015; Rubinelli et al., 2017). One pathogen of interest to target is Salmonella as it caused 23,662 hospitalizations in the US from 2009 to 2015 and has been linked to the consumption of poultry products (Dewey-Mattia et al., 2018). These byproducts may be beneficial to alternative animal production systems as they can be grown and certified as organic (Faccin et al., 2009; United States Department of Agriculture, 2017). They also have beneficial uses in cosmetics, construction materials, and as food additives. The objective of this review is to describe the characteristics of byproducts resulting from the rice milling process and discuss potential avenues for utilization, such as in alternative poultry production systems.
There are three milling systems used; one-step, two-step and multi-stage milling (Odior and Oyawale, 2011). The objective of the rice milling systems is to remove the husk and the bran layer of the rice kernel, which makes the rice edible and free from impurities. Depending on the requirements of the customer, the rice should have the minimum number of broken kernels possible (typically 12–15%) (Siebenmorgen et al., 2011; Dhankhar, 2014).
In a one-step milling system, bran and husk layers are removed in a single pass, which results in white rice directly from the paddy (Prabhakaran et al., 2017). The single pass, also known as the iron huller, is an adaptation of the Engelberg coffee huller. It is known to break the head rice (International Rice Research Institute (IRRI), 2016), which is why single pass use is discouraged. However, the single pass is highly regarded in several of the developing rice-growing countries and is widely used for custom milling (Atungulu and Pan, 2014). In a two-step milling process, the husk and bran layers are removed in separate steps, either in the same machine or isolated machines. The two-stage mill has better milling yield compared to the single-pass milling system (International Rice Research Institute (IRRI), 2016), and is widely used for custom milling and in more agrarian regions of rice production.
Rice milling at the commercial level is generally multi-stage. This method involves a more intricate system to minimize grain breakage. This is done by thermal buildup and reducing stress (International Rice Research Institute (IRRI), 2016). The eight steps in the multistage process are listed in Table 1. Rice kernels are composed of ~20% rice husk, 11% rice bran, and 69% starchy endosperm (milled rice) (Dhankhar, 2014). The endosperm portion is typically packaged and sold for either direct consumption or further processing. The other 31% of the rice kernel becomes waste byproducts. The byproducts of the milling process are fine broken rice, rice germ, husks and bran (Kennedy and Burlingame, 2003). In the past, these products were discarded as waste (Sharif et al., 2014); however, rice bran, rice husk, and broken rice have industrial and bioactive applications for humans and animals (Rohman et al., 2014). In the following section, the primary byproducts associated with the milling process are discussed.
Rice husk, also widely known as rice hull, is an available agricultural waste in numerous rice-producing countries (Kumar et al., 2013). Approximately 120 million tons of rice husk is available each year after it has been removed from the whole rice paddy. Rice husk is composed of 15% carbon, 18% ash, and 67% volatile matter (Lim et al., 2012). It is formed from two polyphenolic compounds, silica and lignin, and these compounds are structural materials used to protect the seed during the germination. The high content of lignin and silica enables the husk to exhibit an antioxidative defense system that helps prevent the seed from oxidative stress (Kim et al., 2012). The silica gives the husk its protective nature and also contributes to allowing rice husk to burn slowly. The husk decomposes slowly while resisting fungal decomposition. Rice husk is burned to recover its ash content. Different forms of silica can be present in ash formation, which is controlled by different temperatures when burning (Singh et al., 2008). The two forms are amorphous silica and crystalline silica, which possess different properties and can be used for different applications (Singh et al., 2008; Chao-Lung et al., 2011). The amorphous forms of silica are composed of silica tetrahedrals arranged in a random three-dimensional network (Prasara and Gheewala, 2017). The tetrahedral three-dimensional network helps to increase the reactivity since a large surface area is available for the reaction to take place. The structure of crystalline silica is built by repetition of a basic unit of the silicon tetrahedron in a three-dimensional oriented framework (Soltani et al., 2015).
When rice husk is burned, it generates ~17–26% of rice husk ash (RHA) (Asavapisit and Ruengrit, 2005). Rice husk contains roughly 97% silica, making it highly porous and lightweight with a high external surface area (Kumar et al., 2013). Rice husk burnt at 700°C produces amorphous ash. At temperatures above 850°C, the husk produces crystalline ash (Singh et al., 2008; Chao-Lung et al., 2011). Rice husk ash has a high melting point at ~1,440°C, the temperature at which silica melts. Rice husk ash possesses numerous advantageous properties, which upon further research will benefit society by providing a cost-effective alternative for utilization in a variety of applications as discussed in the next section (Kumar et al., 2013).
The conversion of rice husk into fuel is one of the most important uses of rice husk (Hossain et al., 2018). Rice husk, once removed from the kernel, can be a source of energy because of the organic compounds present (International Rice Research Institute (IRRI), 2016). The husk is a biomass fuel that must undergo thermal processing, such as combustion, pyrolysis, or gasification (Sikarwar et al., 2016). In countries, such as India where large rice production occurs annually, industries process the husk to produce fuel. Energy produced from thermal processing includes heat, electric, syngas, and biofuels (Pradhan et al., 2013). Using these methods, rice husk can provide power to communities in countries with high rice production, such as China and India (Pradhan et al., 2013). The husk's ability to produce electricity from biomass is based on gasification for small scale power generation. The gasification process involves heating the rice husks to high temperatures, which causes the materials to decompose into a mixture of combustible gases. The gases are subsequently burned to produce heat or steam that activates a gas turbine and produces electricity (Kumar et al., 2013). In the rice mills, the husk is used as fuel to generate steam for the parboiling process of rice (Kapur et al., 1998; Roy et al., 2006).
Soil fertilization is an emerging trend for rice husk agricultural application. Rice husk can be used to fertilize soil due to the high lignin content (Kumar et al., 2013). Husk, with its rich reserves of potassium and silicon, helps to amend the soil, enhance its properties by decreasing soil bulk density, and improve its fertility with the air pockets created underground (Badar and Qureshi, 2014). Using a composting technique to degrade the rice husk is very slow, taking up to ~4 months before it is converted to fertilizer. However, plants and flowers are shown to increase in growth and stability with rice husk as a fertilizer source (Badar and Qureshi, 2014).
Rice husk has also been implemented in the purification of bacteriocins (Janes et al., 1998). Bacteriocins are small molecular weight proteins produced by microorganisms with demonstrated ability to inhibit specific foodborne pathogens and are often incorporated in the preparation and processing of food products (Cleveland et al., 2001; Joerger, 2003; Micciche et al., 2018a). One significant drawback with utilizing bacteriocins is the high cost associated with the purification of the product from the microbial cultures (Bradshaw, 2003; Micciche et al., 2018a). Rice husk ash (RHA) has potential application within purification processes as it is primarily silica which proteins can bind to (Kalapathy et al., 2000). Janes et al. (1998) utilized RHA, comprised of 97% silica, to purify nisin, lactocin GI3, pediocin RS2, leucocin BC2, and enterocin CS1. The optimal recovery rates for RHA were nisin, 63% at pH 7.0; lactocin GI3, 92% at pH 6.0; pediocin RS2, 97% at pH 8.0 to 9.0; leucocin BC2, 88% at pH 9.0; and enterocin CS1, 92% at pH 5.0. These rates were comparable to silicic acid with the exception of nisin (97%) and were higher for the extractions of pediocin RS2 (82 vs. 97%) leucocin BC2 (33 vs. 88%). This indicates that RHA may be a suitable cost-effective alternative to silicic acid.
Rice husk has also been proposed as an alternative to sawdust and wood shavings for litter and bedding in poultry houses. The idea was initially suggested to the poultry industry in Arkansas by the transportation company J. B. Hunt in 1961 (Schwartz, 1992; Strausberg, 1995). By transporting broiler chickens from Northwest Arkansas to the rice-rich areas of Missouri and Southeastern Arkansas, J. B. Hunt and company recognized they could transport rice husk, previously regarded as a byproduct, back to the poultry farms in Northwest Arkansas to ensure full return loads in their trucks (Strausberg, 1995; Cothren, 2011). This became the foundation for a unique relationship between the two entities and contributed to the economic growth of both industries into the large corporations they have now become (Strausberg, 1995).
While a practical solution from an economic stand point, it remained to be determined whether poultry production systems would be negatively influenced by using rice husk as a litter source. Several studies since then have investigated if rice husk-based litter would impact bird growth and performance. Swain and Sundaram (2000) raised birds to day 42 on coir dust, saw dust, and rice husk and found no changes in body weight gain or FCR. Anisuzzaman and Chowdhury (1996) found that Shaver broiler chicks reared on rice husk for 52 d exhibited the greatest weight gain and FCR compared to sawdust, paddy straw, and rice husk. Birds on rice husk weighed 1,634 g on average compared to 1,520 g for sawdust and 1,466 g for paddy straw. The FCR for birds reared on rice husk was 2.05, which was statistically similar to the FCR for birds reared on sawdust (2.08) but significantly different from those raised on paddy straw (2.10) or sand (2.13) (P < 0.05).
Rice husk may also have utility a poultry and animal feed additive. Tough rice husk has minimal food uses, but it is a common additive to pet foods. It provides a source of fiber for pets and meets the sensation of feeling fully fed (Kennedy and Burlingame, 2003). Rice hulls can also be added to food to avoid clumping as an anticaking agent (Kennedy and Burlingame, 2003). Only two studies have investigated the use of rice husk in poultry feeds. Anak broilers were given 20% rice husk, and the final body weight on day 42 did not vary significantly (Ugwu et al., 2008). Feed conversion ratio (FCR) was negatively impacted by the addition of rice husk (3.46 vs. 2.67 in the control) and increased feed cost. Leeson et al. (1991) evaluated starter diets using 25, 40, or 55% ground rice hulls fed to broilers from day 4 to 11. Significant weight reductions were observed in all three diets supplemented with rice hulls by day 11. However, when the birds were switched to a conventional grower and finisher diet, body weight gain recovered to match birds fed a conventional diet throughout by day 42.
After rice is polished, it is graded according to size and rice that does not meet the required size is considered broken, usually less than three fourths of the whole kernel length (Van Dalen, 2004). Broken rice is divided into three groups according to the United States Department of Agriculture (USDA), and referred to as second heads, screenings, and brewers. These names represent categories of large, intermediate, and small fragments, respectively (United States Department of Agriculture, 2009). Broken rice is generally either sold without prior separation or consolidated and ground into flour, regardless of the size (Mukhopadhyay and Siebenmorgen, 2017). Currently, the USDA has reported that 10% of the total rice consumed in the US is used in both pet food and brewery industry, most of which is broken rice (United States Department of Agriculture, 2016). There is low economic value in broken rice as compared to head rice, which makes it a great choice to utilize as a byproduct. Medium and large rice mills generate 10 to 15% broken kernels and this can be as high as 25% in small mills and depends on moisture absorption, chalkiness, immature concerns, relative humidity, insect infection and other factors (Siebenmorgen et al., 1998; Muthayya et al., 2014; Bruce and Atungulu, 2018). Broken rice is worth 60–80% the value of the head rice, which can significantly impact profits (Mukhopadhyay and Siebenmorgen, 2017).
Broken rice is processed into flour and utilized as a food additive because of its human nutritional benefits (Kim et al., 2012). According to Hartmann et al. (2006), there is an increase in celiac disease, to which gluten is a major contributor. Gluten is found in common foods consumed daily, such as baked goods, snacks, cereals, and pasta. Rice flour is gluten-free; therefore, it is an alternative for producing gluten-free products (Quiñones et al., 2015). Rice flour is also hypoallergenic (Marcoa and Rosell, 2008). For baby food, puddings, and other food products; food companies prefer rice flour to other flours because of decreased risk for people with sensitivities (Gujaral and Rosell, 2004). Thus, it has become more economically justifiable to grind broken rice to produce flour for such applications (Qian and Zhang, 2013).
There has been a steady increase in the use of broken rice within the United States pet food industry. Brewer's rice is a common ingredient used in pet foods and broken kernels can be substituted (Buff et al., 2014). It is a popular additive as it can help with bowel movements in pets because of its high fiber content while also satisfying hunger rapidly (De Godoy et al., 2013). Nutrient analysis of brewer's rice indicates a high concentration of fiber (0.5%), phosphorus (1.55%) and potassium (1.48%); however, brown rice, which is also utilized in dog food, contains higher concentrations of all three, 1.24, 3.25, and 2.94%, respectively (Buff et al., 2014). In addition, broken rice is used for food consumption in humans. In Vietnam, broken rice dishes are popular (Nagano et al., 2000). Known as Banh Da Nem, broken rice kernels are further broken down and combined with water to create a film known as rice paper which is utilized to form traditional Spring rolls (Nagano et al., 2000).
Brewer's rice is often used as an ingredient for beer brewing, which gives it the name. This particular rice source lends flavor, aroma, color, and mouthfeel to beer (Marconi et al., 2017). Brewer's rice also provides the raw ingredients needed as substrates for the yeast to ferment and generate alcohol. Rice bran was the grain of choice for many beer companies because it is more economical than barley malt; however, beers with rice result in a very neutral, dry flavor of beer with a light color (Marconi et al., 2017).
Broken rice kernels have not been tested as a feed source for animal and poultry production; however, whole rice kernels have been evaluated. While broken rice kernels are functionally similar to unbroken rice but are undesirable for human consumption due to lack of visual appeal, they could potentially be utilized in conventional and alternative poultry production systems at a lower cost than unbroken rice kernels. White rice and parboiled rice were utilized as a replacement for corn in a corn-soybean diet and provided to broilers from day 1 to 7 or day 1 to 21 (Ebling et al., 2015). In the pre-starter (day 1–7) and starter feed (day 8–21), white rice or parboiled rice represented ~50% of the feed, depending on the trial. These concentrations were based on balancing appropriate nutrient levels according to broiler requirements. For birds provided white rice from day 1 to 21, the FCR was significantly different on day 21. Birds fed a corn diet exhibited an FCR of 1.286 compared to 1.163 for white rice and 1.205 for parboiled rice. For birds provided white and parboiled rice only from day 1 to 7, FCR on day 33 was significantly different for trial 1 but not trial 2. The difference between trial 1 and trial 2 was the inclusion of 4.02% soybean oil, which may be a confounding factor. Changes in body weight and FCR were not reported at the time of sacrifice. This experiment indicates that corn can be replaced by rice in pre-starter and potentially starter diets for improved or equal improved FCR.
Another study evaluated the changes in the physiological properties of the gastrointestinal tract (GIT) of broilers fed a diet of corn or rice (61%) (González-Alvarado et al., 2008). Compared to corn, broilers fed a rice diet increased gizzard digesta concentration and pH. Additionally, the intestinal digesta concentration and pH, along with the relative weights of the gizzard and proventriculus, respectively were reduced. This study indicates that the inclusion of sustainably grown rice in poultry diets may alter the physiology of the GIT and should be further investigated for its effects on pathogen levels and bird performance. Application of 16S rDNA-based microbiome sequencing technologies and metabolomics profiling would potentially reveal more in-depth characterization of the impact of these rice diets and changes of physiological conditions on GIT microbial community (Park et al., 2013; Ricke et al., 2017). Generating this level of data could lead to the means for optimizing dietary combinations that are optimal for inclusion of rice in the poultry diet.
Rice bran composition is largely dependent on the type of rice and efficiency of the milling system (Sharif et al., 2014). It is estimated that the world's annual production of rice bran amounts to 76 million tons (Kahlon, 2009). To obtain the bran itself, the outer layer of the rice kernel is removed during milling. Rice bran is ~5–8% of the whole rice kernel and is considered nutritionally rich as it is composed of 15–22% lipids, 34–52% carbohydrates, 7–11% fiber, 6–10% ash, 8–12% moisture, and 10–16% protein (Luh, 1991). Studies have shown that pigmented bran has more nutritional value than white bran because it possesses more bioactive compounds (Foo and Hameed, 2009; Friedman, 2013). These percentages demonstrate that rice bran can potentially serve as a viable source of protein, fat, and fiber that could have positive impacts on human health. Rice bran is a rich source of E complex vitamins (tocopherols and tocotrienols) and B complex vitamins (niacin, thiamine, pantothenic acid, and pyridoxine), while being the only natural source of γ-oryzanol. Historically, rice bran was typically considered a waste component and was either discarded or utilized in composting (Patel, 2007; Khan et al., 2009).
The milling process generates crude rice bran; therefore, it must be processed and improved before it can be utilized. During the milling process, microbial activity is involved which produces lipase hydrolysates (Kim et al., 2012; Gul et al., 2015). This turns the RBO into glycerol and free fatty acids which gives the product its rancid smell and bitter taste that renders the bran unsuitable for consumption. Rice bran undergoes stabilization, which is the inactivation of the lipase enzyme that causes it to denature in as short as 6 h (Rohman et al., 2014). Stabilization of rice bran can be achieved through cold storage, heat treatment, control of relative humidity, sun-drying, steaming, and expelling (Rohman et al., 2014). The most common method used is a combination of heat, water, and pressure, which does not denature the nutritional value of the bran (Khan et al., 2011). Milling is critical because it determines the texture of the rice bran produced.
Rice bran as a source of value-added food product is an underutilized byproduct of rice; however, as demand for food consumption increases, new and novel ways to utilize the previously unassuming waste material are necessary to investigate. Research has shown that rice bran has commercial benefits based on its characteristics (Nagendra Prasad et al., 2011), and possesses several unique properties that render its suitability for niche markets, such as food, nutraceutical, and pharmaceutical industries. Current applications and potential opportunities of utilization for rice bran are discussed in the following sections.
Several studies have suggested that rice bran possesses unique properties that allow it to be administered as an anticancer agent (Fabian and Ju, 2011; Ling-Tan and Norhaizan, 2017). Several phenolic compounds, such as caffeic acid, cycloartenyl ferulate, and ferulic acid have been reported to inhibit the growth of human breast and colon cancer cells (Verschoyle et al., 2007), although there is a negative relationship with dietary fiber consumption and incidence of colorectal cancer (Ling-Tan and Norhaizan, 2017). Rice bran components, such as the phenols tricin and ferulic acid have been demonstrated to exhibit induced apoptosis, alter cell cycle progression, and inhibit cell proliferation of cancer cells (Hudson et al., 2000; Henderson et al., 2012). It would be of interest to examine the response of the colonic microbial environment to the presence of rice bran in the digesta and whether fermentation patterns and microbial composition are altered in such a way to influence the development of colon cancer.
According to several studies, rice bran also has the potential to prevent a range of chronic diseases (Kim et al., 2012; Gul et al., 2015). The bioactive components and their impacts on rice, notably their human health benefits, were extensively reviewed by Ryan (2011). Rice bran is rich in dietary fiber, which helps maintain a healthy weight and prevent overeating by providing a feeling of fullness during consumption (Gul et al., 2015). Fiber rich diets have shown to lower cholesterol and lower blood pressure in humans. By lowering blood level cholesterol and improving insulin sensitivity, rice bran consumption can reduce the risk of coronary heart disease (CHD) (Al-Khalaf and Yousif, 1984; Kennedy and Burlingame, 2003; Zareei et al., 2017). In addition, γ-oryzanol is the main component in rice bran with anti-atherogenic potential. The chemical components of γ-oryzanol include transferulic acid esters, triterpene alcohols, and sterols (Sohail et al., 2017). This naturally occurring γ-oryzanol, along with vitamin E, exhibits pronounced free radical scavenging activity, which is helpful to protect cells from oxidative stress (Nayik et al., 2015).
Rice bran is an ideal mixture for pastries and baked goods because it can be used to produce acceptable low-fat, high-fiber products (Sharif et al., 2014). In food, it can be applied as defatted rice bran which is converted into flour, as well as in the forms of RBO and protein concentrates (Prakash and Ramaswamy, 1996). Rice bran possesses a characteristic nutty flavor similar to peanut oil, which is essential for industry use for snacks as well as baked goods (Sarkar and Bhattacharyya, 1991). Baked products, such as cookies, muffins, pastries have all shown a better nutritional value with the use of rice bran flour compared to wheat flour (Sarkar and Bhattacharyya, 1991). The addition of 10, 15, and 20% rice bran into wheat flour increased the protein content by 0.93, 1.5, and 3.43% in baked cookies compared to cookies with 100% wheat flour, without impacting sensory ratings (Younas et al., 2011). Rice bran oil is also used in baked goods as a shortening replacer because baked goods need fat and an emulsifier due to their water and fat absorption properties (Sharif et al., 2005). The quality of food is improved with the use of rice bran especially color, appearance, taste, and texture (Prakash and Ramaswamy, 1996; Esa et al., 2013; Sharif et al., 2014). The consumer concern for better health and eating habits render rice bran an optimal nutritional and dietary supplement for overall health maintenance in food use (Issara and Rawdkuen, 2017; Iriondo-DeHond et al., 2018); however, more studies are needed to provide the detailed information needed to make more specific recommendations to individual consumer requirements.
In the produce market, rice bran wax provides an inexpensive option to coat fruit and vegetables by serving as a protective outer layer (Dhall, 2013). When rice bran wax was added to sweet pepper at 10% as a covering, it reduced weight loss of the pepper after 7 days (Jutamongkon et al., 2011). Rice bran wax has also been shown to protect physicochemical properties, such as firmness, total acidity, pH and juice content in fruits and vegetable (Jutamongkon et al., 2011; Zhang et al., 2016). Various studies have also demonstrated the application of rice bran wax as an edible coating to candy, such as chocolate and gum (Sabale et al., 2007; Dhall, 2013). Dassanayake et al. (2009) investigated the thermal behavior and the crystal morphology of rice bran wax. Compared to candelilla wax and carnauba wax, rice bran wax showed a better ability to structure oils at lower concentration, with a minimum of 0.5% of rice bran being necessary for gelation to occur, compared to 2% for candelilla wax or 4% for carnauba wax (Dassanayake et al., 2009). Although considered as a food additive, rice bran wax currently has restrictions on its use; therefore, more research is needed to fully understand its complete utilization and to potentially overcome some of the current restrictions.
Rice bran is recognized as a useful energy source and a viable source of feed ingredient for broilers chickens and can also be fed to other livestock (Farrell, 1994; Gallinger et al., 2004; Amaefule et al., 2006). When fed to rats, the rice bran diet consisting of 15.8% protein, 26.1% fat, and 58.1% carbohydrate was found to yield higher concentrations of volatile fatty acids in rat ceca compared to rats given a wheat bran diet (Topping et al., 1990). Regular and defatted rice bran were also provided to mice and pigs and growth responses were examined (Warren and Farrell, 1990). Growth rates of pigs or rats were not significantly affected by the inclusion of rice bran up to 300 g/kg in the feed during the growth period which was 2 weeks long for rats (day 7–21) and 5 weeks for pigs (day 7–48).
When broiler chickens were fed diets amended with 600 g rice bran/kg, weight gain and feed efficiency were improved up to 14 days of age; however, by the end of the 42 day rearing period this effect was not observed (Sayre et al., 1988). Palo and Sell (1996) observed that the inclusion of 22.5% defatted rice bran did not significantly reduce the FCR of broilers. Defatted rice bran is the byproduct of bran when RBO is extracted (Palo and Sell, 1996). While this product did not improve growth performance, it does appear to serve as a potential low-cost alternative filler for the poultry feed.
Defatted rice bran also contains high concentrations of fiber including lignin, which has been evaluated in broilers both for the impact on performance as well as the microbial populations (Warren and Farrell, 1990; Baurhoo et al., 2008; Ricke et al., 2013). Lignin is generally considered indigestible but can nutritionally influence bird performance and GIT microbial composition, fermentation patterns, and mineral absorption (Ricke et al., 1982, 2013; Jung and Fahey, 1983; Van der Aar et al., 1983; Dunkley et al., 2007; Baurhoo et al., 2008). Indulin, purified lignin, was shown to significantly improve weight gain by 2 g/bird/day compared to the control (Ricke et al., 1982). Salmonella Enteritidis in layer hens is limited when high fiber sources are utilized (Ricke, 2003; Woodward et al., 2005; Ricke et al., 2013). Salmonella Enteritidis can be a particular issue in layer hens as the risk of colonization increases during feed withdrawal, such as during a molt (Ricke, 2003). In Woodward et al. (2005), 50-weeks old white leghorn hens were provided access to alfalfa during a molt and feed withdrawal. On day 4 of the molting process birds were inoculated with 5.6 × 104 CFU of a nalidixic acid resistant strain of S. Enteritidis. By day 9, Salmonella cecal concentrations were reduced in molted hens provided alfalfa in 3 of the 4 trials. A 2–3 log CFU/g reductions of Salmonella was observed when the high dietary amendment was provided. Rice bran has also been used as a component of a low energy–low protein molt diet by Soe et al. (2007). They demonstrated that this molt diet induced molt in layers and increased post-molt production. How such a diet might impact Salmonella colonization and infection of birds undergoing molt remains to be determined.
Given the general influence that fiber components have on the poultry GIT it would be of interest to fractionate rice bran fiber and examine responses of the microbial populations in the bird to various fractions, such as lignin. It is conceivable that forage fiber sources, such as alfalfa would behave differently in the bird GIT than a cereal grain fiber source, such as rice bran. Cereal grain source may be important as well. Knudsen (2014) has pointed out that heterogeneity in cereal grain cell wall fiber components, such as cellulose, occur among cereal grains commonly used in broiler diets and could influence exogenous enzyme efficiency. If rice bran were to become a dietary staple in poultry diets some compositional characterization of the various fractions and subsequent screening with in vitro cecal incubations to assess cecal microbial responses, followed by bird trials would need to be conducted.
Fiber, in general, possess prebiotic-like effects in poultry (Hutkins et al., 2016; Micciche et al., 2018b; Ricke, 2018). Organic acids, such as short-chain fatty acids (SCFAs), are an outcome of fiber fermentation in the GIT and these have been documented as inhibiting Salmonella growth (Jørgensen et al., 1996; Ricke, 2003). Organic acids, such as butyrate, propionate, and acetate, have been demonstrated to reduce Salmonella concentrations within poultry (Durant et al., 2000a,b,c; Cummings and Macfarlane, 2002; Ricke, 2003; Dittoe et al., 2018; Micciche et al., 2018b); however, regulatory agencies do not currently consider fiber to be a prebiotic as it must be resistant to GIT absorption and selectively stimulate the growth or activity of beneficial bacteria (Roberfroid, 2007; Van Loveren et al., 2012; Roto et al., 2015; Gibson et al., 2017). These studies have not investigated the use of fiber sources from defatted rice bran, and this source should be investigated for impacts on feed efficiency and GIT microbial composition.
The application of bran as animal feed has limited use, because of its chemical composition that makes it prone to rancidity; however, at a low level of rice bran in the feed, it can serve as a potential nutritional feed supplement. Furthermore, even when rice bran rancidity is low, Gallinger et al. (2004) found that when given to broilers on day 1, rice bran diets supplemented with 20% or higher concentrations resulted in poor FCR and reductions in body weight growth by day 42 (P < 0.05). By day 40, broilers fed diets with 10% rice bran exhibited significantly improved FCR compared to the control (1.77 vs. 1.84).
Due to the components within rice bran, such as fiber and lignin, there is potential for it to modulate the microbiome of the broiler GIT and elicit prebiotic-like effects (Sheflin et al., 2015; Micciche et al., 2018b). The International Scientific Association for Probiotics and Prebiotics (ISAPP) defines prebiotics as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (Gibson et al., 2017). Mice, 4–6 weeks of age, were fed diets containing 0, 10, or 20% Neptune rice bran 1 week before oral infection with 2 × 107 CFU S. Typhimurium. Nine days after inoculation fecal concentrations of Salmonella were reduced significantly by ~2 log CFU/g of the fecal sample compared to the control group which had 6.5 logs CFU/g of S. Typhimurium. However, by day 14 no significant differences in fecal concentrations were observed between the control and the rice bran treatments (P > 0.05). Across the 14-days trial decreased concentrations of pro-inflammatory cytokines, such as TNF alpha, IFN-gamma, and IL-12, were observed. Increased concentration of native Lactobacillus spp. in fecal samples was also observed. Similar reductions were observed when rice bran from IAC600, IL 121-1-1, and Red Wells was utilized during a trial which measured shedding at day 2, 4, and 6 post-infection (Goodyear et al., 2015). However, when using rice bran from Jasmine 85 and Red Wells and Shufeng, fecal shedding of Salmonella in mice was not significantly affected (P > 0.05). This may in part be due to the ability for IAC600 and Red Wells rice bran to significantly improve the small intestinal representation of neutrophils, macrophages, interdigitating dendritic cells, CD8+, and regulatory T cells compared to Wells and Shufeng rice bran. This implies that the type of rice bran utilized effects its impact on the intestinal immune system and pathogen reduction within mice and potentially other animals (Kumar et al., 2012).
Calrose Rice bran was used as a nutrient source for Lactobacillus paracasei, and the cell-free supernatant of Lactobacillus paracasei was collected and tested against Salmonella in vitro (Nealon et al., 2017). Either 100, 50, or 25 μL of the supernatant of L. paracasei was added to 200 μL of Luria Bertani (LB) containing 2 × 106 CFU of S. Typhimurium. After 16 h of incubation concentrations of Salmonella were determined based on culture plating estimations. Compared to the supernatant of L. paracasei grown in De Man, Rogos and Sharpe broth (MRS) a 13.1% reduction in Salmonella concentration was observed. This indicates that rice bran has potential prebiotic-like effects in vitro.
More recently, in vitro broiler cecal incubation work has demonstrated that rice bran from certain varieties of rice may be inhibitory to Salmonella when fermented by cecal microbiota (Rubinelli et al., 2017). Cecal contents were diluted in anaerobic culture medium with starter feed. Jasmine, Red Wells, or Calrose rice bran was added to these mixtures at a concentration of 1% and anaerobic mixtures were inoculated with 107 CFU/mL of Salmonella Typhimurium immediately after initial cecal content addition. No reductions in concentrations were observed in the presence of rice bran at 24 or 48 h. However, when S. Typhimurium was added 24 h after the cecal contents, S. Typhimurium was reduced in Calrose rice bran cecal incubations by 4 log CFU/mL compared to the control, and by 48 h Salmonella concentrations were below the limit of detection (10 CFU/mL). The impact on the cecal microbiome was also investigated by Rubinelli et al., (2017). In the Calrose treatment, the relative abundance of Firmicutes and the Clostridia genus were significantly increased compared to the feed plus cecal control group. The shift in microbiome profiles, and therefore fermentation pathways, by Calrose rice bran, may have played a role in Salmonella reduction. As such, cultivars of rice bran could possess prebiotic-like properties. Further studies are needed to screen individual fractions of the rice bran in the in vitro cecal model to identify which fractions are contributing to the reduction in S. Typhimurium. Finally, once fractions of rice bran have been identified poultry performance studies need to be conducted to assess whether the rice bran and/or individual fractions would have deleterious impact on bird health and performance or potentially be beneficial.
Rice bran as a dietary supplement has been examined in other animal species in addition to poultry. For example, in pigs, rice bran (10%) was provided within the diet after day 5 until euthanization (Yang et al., 2014). Rotavirus was administered to pigs on day 28 at a concentration of 1 × 105 focus forming units (FFU). One week post-infection, diarrhea induced by rotavirus was reduced, and in groups administered the human rotavirus vaccine its protective effects were improved. Rice bran increased CD4 and CD8 T cells and increased total IgM- and IgA-secreting cells, total serum IgM, IgG, and IgA titers, and HRV-specific IgA titers in intestinal contents. For instance, IgA in the blood was significantly improved to 150 immunoglobulin-secreting cells per 5 × 105 mononuclear cells compared to 25 in the vaccine only group. This study indicates that rice bran may have immunogenic benefits, but this type of experiment has not been performed with other viruses or other animals.
The inclusion of rice bran in the diets of animals range from 10 to 40% depending on the target animal (Gallinger et al., 2004). An added advantage of rice bran is its high content of linoleic acid that helps improve egg weight and laying potential of chickens (Szymczyk et al., 2001; Gallinger et al., 2004); however, when provided to broilers, concentrations below 1% did not improve growth performance and concentrations from 2 to 5% significantly reduced body weight gain (Szymczyk et al., 2001; Du and Ahn, 2002; Badinga et al., 2003). This indicates that not every component of rice bran is beneficial to animal growth, performance, and health, and rice bran must be further characterized to understand its beneficial qualities. By breaking down rice bran into its bioactive component's beneficial qualities can be determined, as well as potential synergism among the components (Ling-Tan and Norhaizan, 2017).
On average, rice bran contains ~10–23% RBO (Kennedy and Burlingame, 2003; Friedman, 2013). Rice bran oil is rich in unsaturated linoleic and oleic fatty acids and bioactive compounds, such as γ-oryzanol, phytosterols, tocopherols, and tocotrienols that have been described as nutritionally beneficial (Gul et al., 2015). After the bran is stabilized, it goes through further processing to make it oil edible (Rafe et al., 2017).
There are three common methods used for RBO extraction namely, hydraulic pressing, X-M milling, and solvent extraction (Nagendra Prasad et al., 2011). Solvent extraction is the most commonly used method for oil extraction. This is because yields have been demonstrated to be as high as 0.549 g RBO/g bran and the solvent can be easily removed through several purification steps (Chiou et al., 2013). The solvent used in most commercial extractions that yields the most oil is hexane. Although widely accepted, hexane does have drawbacks as it can affect the color and is considered hazardous (Nagendra Prasad et al., 2011). After extraction, rice bran is divided into two groups: crude bran oil and defatted rice. Crude RBO consists of 4% unsaponifiable (wax, fat, and oil), 4% free fatty acids, and 90% lipids. Crude RBO is refined through the removal of free fatty acids and is necessary to improve rancidity and sensory properties (Khan et al., 2011; Vaisali et al., 2015).
In the past, rice bran was considered a waste artifact (Sharif et al., 2014). However, its nutritional benefits have been studied or explored (Mishira, 2017). Rice bran oil is a very common food additive in countries, such as Japan, China, and India because of its beneficial features to human health and it is referred to as “wonder oil” (Esa et al., 2013; Nayik et al., 2015). Rice bran oil is hypoallergenic, can be produced sustainably, and can be used as an alternative cooking oil for those who are allergic to traditional oils (Nayik et al., 2015). It also provides a better coating for foods that need to be fried because RBO requires less oil to cook creating fewer polymers, which correlates to more flavor in food because of the lesser amount of fat consumed during frying (Zareei et al., 2017).
With the increase in consumer desire for natural products along with olive oil becoming scarce to produce, RBO has become very prominent economically (Sohail et al., 2017). One reason for the rise in rice bran industry is that it has shown to be linked to the reduction of cholesterol (Nagendra Prasad et al., 2011). Rice bran nutrients, such as dietary fiber, essential fatty acids, γ-oryzanol, tocopherols, and tocotrienols have potential health benefits. Rice bran oil is a good source of oryzanol, which can increase bile and neutral sterol excretion induced by catabolism of cholesterol (Aggarwal et al., 2010). Chou et al. (2009) determined that RBO can suppress the hyperinsulinemic effect, which is caused by excess levels of insulin and reduced the atherogenic index that measures the ratio of triglycerides to HDL-cholesterol and is a measure of cardiovascular disease (Dobiasova, 2006). It also helps to reduce triglycerides and improve the high-density lipoprotein cholesterol to low-density lipoprotein cholesterol ratio (HDL/LDL). According to Sugano and Tsuji (1996), RBO has been demonstrated to reduce cholesterol ~7–10% because of its effective reduction outcome on LDL. Unsaponifiables present in the rice bran have also been shown to significantly reduce liver cholesterol levels (Sugano and Tsuji, 1996).
Rice bran oil has also been linked to anti-inflammatory properties giving it the ability to reduce skin irritation, help with eczema, and heal skin after damage from cracking or after a sunburn (Ammar et al., 2012; Friedman, 2013). A sunscreen containing oryzanol and ferulic acid has been developed because of this property (Ammar et al., 2012). Rice bran oil is used in sunscreen and lotion because it protects the skin against UV rays (Mukhopadhyay and Siebenmorgen, 2017). Vitamin E, mainly the tocotrienols, provide nourishment to skin cells and prevent the pores from clogging, which aids in skin regeneration and slows down the aging process (Nagendra Prasad et al., 2011; Nayik et al., 2015).
Rice bran oil has been suggested to overcome potential rancidity of lipids in poultry feed (Goffman and Bergman, 2003). Oil content of the bran aids with the binding of animal feed, which reduces feed waste because it keeps the bran submerged during feeding. Since it is inexpensive, over 90% of the rice bran produced worldwide is sold as animal feed (Kahlon, 2009). Broilers provided feeds amended with 1–5% crude RBO did not significantly improve body weight gain over a 42-days rearing period; however, utilizing 3% of RBO significantly improved FCR compared to the control (2.21 vs. 2.32) (Anitha et al., 2006). This study did not evaluate the cost-benefit to the application of RBO, and this information would be critical for determining the effective use by farmers.
Rice milling is a significant post-harvest process as it changes rice nutritional quality. Milling determines which kernels are designated for consumer consumption and which kernels are determined to be a byproduct. Lately, there has been an increase in the consumption and utilization of the byproducts of the milling process, particularly using broken kernels (Esa et al., 2013; Triratanasirichai et al., 2017). Studies have shown that rice bran, RBO, rice husk, and broken rice have potential health, animal, and alternative food uses and can be produced sustainably as they are considered renewables (Nagano et al., 2000; Ammar et al., 2012; De Godoy et al., 2013; Esa et al., 2013). Rice bran, husk, and broken rice have a variety of applications for the mechanical, food, cosmetic, agricultural, and fuel industries. With future studies, rice milling byproducts can be a cost-effective substitute for certain processes in these industries. Furthermore, countries with power plants, such as Thailand and Japan use rice husk byproduct to help fuel their power plants as well as decrease the sensitivity degree coefficient in the mining and petroleum sectors (Kunimitsu and Ueda, 2009). In Vietnam, rice husk firewood is popular because it is more affordable over traditional firewood (Truong et al., 2016). Their rice husk pellet exports have boosted the economy because of the convenience of shipping of the material (Truong et al., 2016).
In poultry production, an emerging concern is the rising cost of feed as 60–70% of the cost of poultry operation is due to purchasing and formulating feed (Thirumalaisamy et al., 2016; Randall, 2018). In 2018 the price of soybean meal, the dominant nutritional supplement in feed, rose by 14% (Jacob, 2015; Randall, 2018). These price increases can be especially detrimental to alternative and small scale production operations due to the economic impact on both. Furthermore, Farrell (2005) has pointed out that in some countries where the standard feedstocks of corn and soybeans are not readily available and/or affordable alternative feed sources, such as rice bran may be needed for poultry production. It is intuitive that rice bran as a source of feed amendment for reducing Salmonella in poultry could be translated into economic gain as well. Before such evaluations take place, further studies must be performed to quantitatively determine the general level of Salmonella reduction that could be expected. Once that is known, risk assessment appraisal could be applied and if bird performance parameters are also improved, then it will be formulated into economic benefit as well.
Finding economically viable avenues for rice milling by products, such as within alternative poultry production, is essential to reduce waste and improve efficiency. Rice bran, husk, and broken kernels may fill this niche as they appear to have no negative effects on FCR if used in low enough concentrations. However, future studies must be performed to find the optimal concentration and indicate any potential prebiotic-like effects of these byproducts possess. Once established, by products of rice milling may be efficiently utilized in various industries including sustainable alternative poultry production.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AM was supported by a Distinguished Doctoral Fellowship and support from the Department of Food Science at the University of Arkansas. Deandrae Smith is acknowledged for the creation and use of Figure 1.
Aggarwal, B. B., Sundaram, C., Prasad, S., and Kannappan, R. (2010). Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem. Pharmacol. 80, 1613–1631. doi: 10.1016/j.bcp.2010.07.043
Al-Khalaf, M. N., and Yousif, H. A. (1984). Use of rice husk ash in concrete. Int. J. Cem. Compos. Lightweight Concr. 6, 241–248. doi: 10.1016/0262-5075(84)90019-8
Amaefule, K. U., Iheukwumere, F. C., Lawal, A. S., and Ezekwonna, A. A. (2006). The effect of treated rice milling waste on performance, nutrient retention, carcass and organ characteristics of finisher broilers. Int. J. Poult. Sci. 5, 51–55. doi: 10.3923/ijps.2006.51.55
Ammar, H. O., Al-Okbi, S. Y., Mostafa, D. M., and Helal, A. M. (2012). Rice bran oil: preparation and evaluation of novel liquid, solid and semisolid formulations. Int. J. Pharm. Compd. 16, 516–523.
Anisuzzaman, M., and Chowdhury, S. D. (1996). Use of four types of litter for rearing broilers. Br. Poult. Sci. 37, 541–545. doi: 10.1080/00071669608417883
Anitha, B., Moorthy, M., and Viswanathan, K. (2006). Production performance of broilers fed with crude rice bran oil. Int. J. Poult. Sci. 5, 1046–1052. doi: 10.3923/ijps.2006.1046.1052
Asavapisit, S., and Ruengrit, N. (2005). The role of RHA-blended cement in stabilizing metal-containing wastes. Cem. Concr. Compos. 27, 782–787. doi: 10.1016/j.cemconcomp.2005.03.003
Atungulu, G. G., and Pan, Z. (2014). Rice industrial processing worldwide and impact on macro-and micronutrient content, stability, and retention. Ann. N. Y. Acad. Sci. 1324, 15–28. doi: 10.1111/nyas.12492
Badar, R., and Qureshi, S. A. (2014). Composted rice husk improves the growth and biochemical parameters of sunflower plants. J. Bot. 20, 1–6. doi: 10.1155/2014/427648
Badinga, L., Selberg, K. T., Dinges, A. C., Corner, C. W., and Miles, R. D. (2003). Dietary conjugated linoleic acid alters hepatic lipid content and fatty acid composition in broiler chickens. Poult. Sci. 82, 111–116. doi: 10.1093/ps/82.1.111
Baurhoo, B., Ruiz-Feria, C. A., and Zhao, X. (2008). Purified lignin: nutritional and health impacts on farm animals—a review. Anim. Feed Sci. Technol. 144, 175–184. doi: 10.1016/j.anifeedsci.2007.10.016
Bradshaw, J. P. (2003). Cationic antimicrobial peptides. BioDrugs 17, 233–240. doi: 10.2165/00063030-200317040-00002
Bruce, R. M., and Atungulu, G. G. (2018). Assessment of pasting characteristics of size fractionated industrial parboiled and non-parboiled broken rice. Cereal Chem. 95, 889–899. doi: 10.1002/cche.10107
Buff, P. R., Carter, R. A., Bauer, J. E., and Kersey, J. H. (2014). Natural pet food: A review of natural diets and their impact on canine and feline physiology. J. Anim. Sci. 92, 3781–3791. doi: 10.2527/jas.2014-7789
Chao-Lung, H., Le Anh-Tuan, B., and Chun-Tsun, C. (2011). Effect of rice husk ash on the strength and durability characteristics of concrete. Constr. Build. Mater. 25, 3768–3772. doi: 10.1016/j.conbuildmat.2011.04.009
Chiou, T. Y., Ogino, A., Kobayashi, T., and Adachi, S. (2013). Characteristics and antioxidative ability of defatted rice bran extracts obtained using several extractants under subcritical conditions. J. Oleo Sci. 62, 1–8. doi: 10.5650/jos.62.1
Chou, T. W., Ma, C. Y., Cheng, H. H., Chen, Y. Y., and Lai, M. H. (2009). A rice bran oil diet improves lipid abnormalities and suppress hyperinsulinemic responses in rats with streptozotocin/nicotinamide-induced type 2 diabetes. J. Clin. Biochem. Nutr. 45, 29–36. doi: 10.3164/jcbn.08-257
Choudhury, A. T. M. A., and Kennedy, I. R. (2004). Prospects and potentials for systems of biological nitrogen fixation in sustainable rice production. Biol. Fertil. Soils 39, 219–227. doi: 10.1007/s00374-003-0706-2
Cleveland, J., Montville, T. J., Nes, I. F., and Chikindas, M. L. (2001). Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 71, 1–20. doi: 10.1016/S0168-1605(01)00560-8
Cothren, Z. (2011). J. B. Hunt. Available online at: http://www.encyclopediaofarkansas.net/encyclopedia/entry-detail.aspx?entryID=2138 (accessed March 9, 2019).
Cummings, J. H., and Macfarlane, G. T. (2002). Gastrointestinal effects of prebiotics. Br. J. Nutr. 87, S145–S151. doi: 10.1079/BJN/2002530
Dassanayake, L. S. K., Kodali, D. R., Ueno, S., and Sato, K. (2009). Physical properties of rice bran wax in bulk and organogels. J. Am. Oil Chem. Soc. 86, 1163–1174. doi: 10.1007/s11746-009-1464-6
De Godoy, M. R., Kerr, K. R., and Fahey, G. C. (2013). Alternative dietary fiber sources in companion animal nutrition. Nutrients 5, 3099–3117. doi: 10.3390/nu5083099
Dewey-Mattia, D., Manikonda, K., Hall, A. J., Wise, M. E., and Crowe, S. J. (2018). Surveillance for foodborne disease outbreaks—United States, 2009–2015. MMWR Surveill. Summ. 67:1. doi: 10.15585/mmwr.ss6710a1
Dhall, R. K. (2013). Advances in edible coatings for fresh fruits and vegetables: a review. Crit. Rev. Food Sci. Nutr. 53, 435–450. doi: 10.1080/10408398.2010.541568
Dittoe, D. K., Ricke, S. C., and Kiess, A. S. (2018). Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 5:216. doi: 10.3389/fvets.2018.00216
Dobiasova, M. (2006). AIP–atherogenic index of plasma as a significant predictor of cardiovascular risk: from research to practice. Vnitr. Lek. 2, 64–71.
Du, M., and Ahn, D. U. (2002). Effect of dietary conjugated linoleic acid on the growth rate of live birds and on the abdominal fat content and quality of broiler meat. Poult. Sci. 81, 428–433. doi: 10.1093/ps/81.3.428
Dunkley, K. D., Dunkley, C. S., Njongmeta, N. L., Callaway, T. R., Hume, M. E., Kubena, L. F., et al. (2007). Comparison of in vitro fermentation and molecular microbial profiles of high-fiber feed substrates incubated with chicken cecal inocula. Poult. Sci. 86, 801–810. doi: 10.1093/ps/86.5.801
Durant, J. A., Corrier, D. E., and Ricke, S. C. (2000a). Short-chain volatile fatty acids modulate the expression of the hilA and invF genes of Salmonella Typhimurium. J. Food Prot. 63, 573–578. doi: 10.4315/0362-028X-63.5.573
Durant, J. A., Lowry, V. K., Nisbet, D. J., Stanker, L. H., Corrier, D. E., and Ricke, S. C. (2000b). Short chain fatty acids alter HEp-2 cell association and invasion by stationary growth phase Salmonella Typhimurium. J. Food Sci. 65, 1206–1209. doi: 10.1111/j.1365-2621.2000.tb10266.x
Durant, J. A., Lowry, V. K., Nisbet, D. J., Stanker, L. H., Corrier, D. E., and Ricke, S. C. (2000c). Late logarithmic Salmonella Typhimurium Hep-2 cell association and invasion response to short-chain fatty acid addition. J. Food Saf. 20, 1–11. doi: 10.1111/j.1745-4565.2000.tb00284.x
Ebling, P. D., Kessler, A. M., Villanueva, A. P., Pontalti, G. C., Farina, G., and Ribeiro, A. M. L. (2015). Rice and soy protein isolate in pre-starter diets for broilers. Poult. Sci. 94, 2744–2752. doi: 10.3382/ps/pev279
Esa, N. M., Ling, T. B., and Peng, L. S. (2013). By-products of rice processing: an overview of health benefits and applications. Rice Res. 1, 1–11. doi: 10.4172/jrr.1000107
Fabian, C., and Ju, Y. H. (2011). A review on rice bran protein: its properties and extraction methods. Crit. Rev. Food Sci. Nutr. 51, 816–827. doi: 10.1080/10408398.2010.482678
Faccin, G. L., Miotto, L. A., Do Nascimento Vieira, L., Barreto, P. L. M., and Amante, E. R. (2009). Chemical, sensorial and rheological properties of a new organic rice bran beverage. Rice Sci. 16, 226–234. doi: 10.1016/S1672-6308(08)60083-9
FAOSTAT (2017). Food and Agriculture Organization of the United Nations: Preliminary Agricultural Production data. Available online at: http://www.fao.org/economic/est/publications/rice-publications/rice-market-monitor-rmm/en/ (accessed March 20, 2019).
Farrell, D. J. (1994). Utilization of rice bran in diets for domestic fowl and ducklings. Worlds Poult. Sci. J. 50, 115–131. doi: 10.1079/WPS19940012
Farrell, D. J. (2005). Matching poultry production with available feed resources: issues and constraints. J. World's Poult. Sci. 61:298–307. doi: 10.1079/WPS200456
Foo, K. Y., and Hameed, B. H. (2009). Utilization of rice husk ash as novel adsorbent: a judicious recycling of the colloidal agricultural waste. Adv. Colloid Interface Sci. 152, 39–47. doi: 10.1016/j.cis.2009.09.005
Friedman, W. (2013). Rice brans, rice bran oils, and rice hulls: composition, food and industrial uses, and bioactivities in humans, animals, and cells. J. Agric. Food Chem. 61, 10626–10641. doi: 10.1021/jf403635v
Gallinger, C. I., Suárez, D. M., and Irazusta, A. (2004). Effects of rice bran inclusion on performance and bone mineralization in broiler chicks. J. Appl. Poult. Res. 13, 183–190. doi: 10.1093/japr/13.2.183
Gibson, G. R., Hutkins, R. W., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., et al. (2017). The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14:491. doi: 10.1038/nrgastro.2017.75
Goffman, F. D., and Bergman, C. (2003). Relationship between hydrolytic rancidity, oil concentration, and esterase activity in rice bran. Cereal Chem. 80, 689–692. doi: 10.1094/CCHEM.2003.80.6.689
González-Alvarado, J. M., Jiménez-Moreno, E., Valencia, D. G., Lázaro, R., and Mateos, G. G. (2008). Effects of fiber source and heat processing of the cereal on the development and pH of the gastrointestinal tract of broilers fed diets based on corn or rice. Poult. Sci. 87, 1779–1795. doi: 10.3382/ps.2008-00070
Goodyear, A., Kumar, A., Ehrhart, E. J., Kelly, S., Swanson, K. S., and Grusak, M. A. (2015). Dietary rice bran supplementation prevents Salmonella colonization differentially across varieties and by priming intestinal immunity. J. Funct. Foods 18, 653–664. doi: 10.1016/j.jff.2015.08.027
Gujaral, H. S., and Rosell, C. M. (2004). Improvement of breadmaking quality of rice flour by glucose oxidase. Food Res. Int. 37, 75–81. doi: 10.1016/j.foodres.2003.08.001
Gul, K., Yousuf, B., Singh, A. K., Singh, P., and Wan, A. (2015). A rice bran: nutritional values and its emerging potential for development of functional food—a review. Bioact. Carbohydr. Dietary Fibre 6, 24–30. doi: 10.1016/j.bcdf.2015.06.002
Hartmann, G., Koehler, P., and Wieser, H. (2006). Rapid degradation of gliadin peptides toxic for coeliac disease patients by proteases from germinating cereals. J. Cereal Sci. 44, 368–371. doi: 10.1016/j.jcs.2006.10.002
Henderson, A. J., Ollila, C. A., Kumar, A., Borresen, E. C., Raina, K., Agarwal, R., et al. (2012). Chemopreventive properties of dietary rice bran: current status and future prospects. Adv. Nutr. 3, 643–653. doi: 10.3945/an.112.002303
Hossain, S. S., Mathur, L., and Roy, P. K. (2018). Rice husk/rice husk ash as an alternative source of silica in ceramics: a review. J. Asian Ceram. Soc. 6, 299–313. doi: 10.1080/21870764.2018.1539210
Hudson, E. A., Dinh, P. A., Kokubun, T., Simmonds, M. S., and Gescher, A. (2000). Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomarkers Prev. 9, 1163–1170.
Hutkins, R. W., Krumbeck, J. A., Bindels, L. B., Cani, P. D., Fahey, G. C., Goh, Y. J., et al. (2016). Prebiotics: why definitions matter. Curr. Opin. Biotechnol. 37, 1–7. doi: 10.1016/j.copbio.2015.09.001
International Rice Research Institute (IRRI) (2016). Milling Byproducts. Manila: International Rice Research Institute (IRRI). Available online at: http://www.knowledgebank (accessed March 21, 2019).
Iriondo-DeHond, M., Miguel, E., and Del Castillo, M. D. (2018). Food byproducts as sustainable ingredients for innovative and healthy dairy foods. Nutrients 10, 1358–1384. doi: 10.3390/nu10101358
Issara, U., and Rawdkuen, S. (2017). Rice bran: a potential of main ingredient in healthy beverage. Int. Food Res. J. 23, 2306–2231.
Jacob, J. (2015). Feeding Soybean to Poultry. Available online at: https://articles.extension.org/pages/67352/feeding-soybean-to-poultry (accessed February 22, 2019).
Janes, M. E., Nannapaneni, R., Proctor, A., and Johnson, M. G. (1998). Rice hull ash and silicic acid as adsorbents for concentration of bacteriocins. Appl. Environ. Microbiol. 64, 4403–4409.
Joerger, R. D. (2003). Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 82, 640–647. doi: 10.1093/ps/82.4.640
Jørgensen, H., Zhao, X. Q., Knudsen, K. E. B., and Eggum, B. O. (1996). The influence of dietary fibre source and level on the development of the gastrointestinal tract, digestibility and energy metabolism in broiler chickens. Br. J. Nutr. 75, 379–395. doi: 10.1079/BJN19960141
Jung, H. G., and Fahey, G. C. Jr. (1983). Nutritional implications of phenolic monomers and lignin: a review 1. J. Anim. Sci. 57, 206–219. doi: 10.2527/jas1983.571206x
Jutamongkon, R., Praditdoung, S., and Vananuvat, N. (2011). Effect of rice bran waxing on fruit and vegetable storage. Kasetsart J. Nat. Sci. 45, 1115–1126.
Kahlon, T. S. (2009). “Chapter 14. Rice bran: Production, composition, functionality and food applications, physiological benefits,” in Fiber Ingredients: Food Applications and Health Benefits, eds S. S. Cho and P. Samuel (Boca Raton, FL: CRC Press), 305–321. doi: 10.1201/9781420043853-c14
Kalapathy, U., Proctor, A., and Shultz, J. (2000). A simple method for production of pure silica from rice hull ash. Bioresour. Technol. 73, 257–262. doi: 10.1016/S0960-8524(99)00127-3
Kapur, T., Kandpal, T. C., and Garg, H. P. (1998). Electricity generation from rice husk in Indian rice mills: potential and financial viability. Biomass Bioenergy 14, 573–583. doi: 10.1016/0961-9534(95)00116-6
Kennedy, G., and Burlingame, B. (2003). Analysis of food composition data on rice from a plant genetic resources perspective. Food Chem. 80, 589–596. doi: 10.1016/S0308-8146(02)00507-1
Khan, M. A. I., Ueno, K., Horimoto, S., Komai, F., Tanaka, K., and Ono, Y. (2009). Physicochemical, including spectroscopic, and biological analyses during composting of green tea waste and rice bran. Biol. Fertil. Soils 45, 305–313. doi: 10.1007/s00374-008-0335-x
Khan, S. H., Butt, M. S., Sharif, M. K., Sameen, A., Mumtaz, S., and Sultan, M. T. (2011). Functional properties of protein isolates extracted from stabilized rice bran by microwave, dry heat, and parboiling. J. Agric. Food Chem. 59:2416–2420. doi: 10.1021/jf104177x
Kim, H. Y., Hwang, I. G., Kim, T. M., Woo, K. W., Park, D. S., Kim, J. S., et al. (2012). Chemical and functional components in different parts of rough rice (Oryza sativa L.) before and after germination. Food Chem. 134, 288–293. doi: 10.1016/j.foodchem.2012.02.138
Klotzbücher, T., Marxen, A., Vetterlein, D., Schneiker, J., Türke, M., Van Sinh, N., et al. (2015). Plant-available silicon in paddy soils as a key factor for sustainable rice production in Southeast Asia. Basic Appl. Ecol. 16, 665–673. doi: 10.1016/j.baae.2014.08.002
Knudsen, K. E. B. (2014). Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 93, 2380–2393. doi: 10.3382/ps.2014-03902
Kumar, A., Henderson, A., Forster, G. M., Goodyear, A. W., Weir, T. L., Leach, J. E., et al. (2012). Dietary rice bran promotes resistance to Salmonella enterica serovar Typhimurium colonization in mice. BMC Microbiol. 12:71. doi: 10.1186/1471-2180-12-71
Kumar, S., Sangwan, P., Dhankhar, R. M. V., and Bidra, S. (2013). Utilization of rice husk and their ash: a review. Res. J. Chem. Environ. Sci. 1, 126–129.
Kunimitsu, Y., and Ueda, T. (2009). Macro-Economic Impacts of Installing Rice Husk Electricity Power Plants in Thailand. National Institute for Rural Engineering (Tsukuba), 1–18.
Leeson, S., Summers, J. D., and Caston, L. J. (1991). Diet dilution and compensatory growth in broilers. Poult. Sci. 70, 867–873. doi: 10.3382/ps.0700867
Lim, J. S., Abdul Manan, Z, Wan Alwi, S. R., and Hashim, H. (2012). A review on utilisation of biomass from rice industry as a source of renewable energy. Renew. Sustain. Energy Rev. 16, 3084–3094. doi: 10.1016/j.rser.2012.02.051
Ling-Tan, B. L., and Norhaizan, M. E. (2017). Scientific evidence of rice by-products for cancer prevention: chemopreventive properties of waste products from rice milling on carcinogenesis in vitro and in vivo. BioMed Res. Int. 20, 1–18. doi: 10.1155/2017/9017902
Linscombe, S. (2006). Rice Quality Determines Payment. Available online at: https://www.lsuagcenter.com/portals/our_offices/research_stations/rice/features/publications/rice-quality-determines-payment (accessed February 17, 2019).
Luh, B. S. (1991). “Chapter 13: rice oil,” in Rice Production, 2nd Edn., ed S. B. Luh (New York City, NY: Springer Publishing), 714–730. doi: 10.1007/978-1-4899-3754-4
Marcoa, C., and Rosell, C. M. (2008). Effect of different protein isolates and transglutaminase on rice flour properties. J. Food Eng. 84, 132–139. doi: 10.1016/j.jfoodeng.2007.05.003
Marconi, O., Sileoni, V., Ceccaroni, D., and Perretti, G. (2017). “Chapter 4: the use of rice in brewing,” in Advances in International Rice Research, ed Marconi (London: International Rice Research Tech.), 50–64. doi: 10.5772/66450
Micciche, A. C., Foley, S. L., Pavlidis, H. O., McIntyre, D. R., and Ricke, S. C. (2018b). A review of prebiotics against Salmonella in poultry: current and future potential for microbiome research applications. Front. Vet. Sci. 5:191. doi: 10.3389/fvets.2018.00191
Micciche, A. C., Rubinelli, P. M., Wages, J. A., and Ricke, S. C. (2018a). Source of water and potential sanitizers and biological antimicrobials for alternative poultry processing food safety applications. Front. Sustain. Food Syst. 2:82. doi: 10.3389/fsufs.2018.00082
Mishira, N. (2017). Utilization of waste defatted rice bran in formulation of functional cookies and its effect on physiochemical characteristic of cookies. Int. J. Adv. Sci. Res. 2, 64–68.
Mukhopadhyay, S., and Siebenmorgen, T. J. (2017). Physical and functional characteristics of broken rice kernels caused by moisture-adsorption fissuring. Cereal Chem. 94, 539–545. doi: 10.1094/CCHEM-08-16-0214-R
Muthayya, S., Sugimoto, J. D., Montgomery, S., and Maberly, G. F. (2014). An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 1324, 7–14. doi: 10.1111/nyas.12540
Nagano, H., Shoji, Z., Tamura, A., and Kato, M. (2000). Some characteristics of rice paper of Vietnamese traditional food (Vietnamese spring rolls). Food Sci. Technol. Res. 6, 102–105. doi: 10.3136/fstr.6.102
Nagendra Prasad, M. N., Sanjay, K. R., Shravya Khatokar, M., Vismaya, M. N., and Nanjunda Swamy, S. (2011). Health benefits of rice bran–a review. J. Nutr. Food Sci. 1, 1–7. doi: 10.4172/2155-9600.1000108
Nayik, G. A., Majid, I., Gull, A., and Muzaffar, K. (2015). Rice bran oil, the future edible oil of India: a mini review. J. Rice Res. 3, 1–3. doi: 10.4172/2375-4338.1000151
Nealon, N. J., Worcester, C. R., and Ryan, E. P. (2017). Lactobacillus paracasei metabolism of rice bran reveals metabolome associated with Salmonella Typhimurium growth reduction. J. Appl. Microbiol. 122, 1639–1656. doi: 10.1111/jam.13459
Odior, A. O., and Oyawale, F. A. (2011). Application of time study model in rice milling firm: a case study. J. Appl. Sci. Environ. Manage. 15, 501–505.
Palo, P. E., and Sell, J. L. (1996). Utilization of defatted rice bran by broiler chickens. Poult. Sci. 75, 1012–1017. doi: 10.3382/ps.0751012
Park, S. H., Hanning, I., Perrota, A., Bench, B. J., Alm, E., and Ricke, S. C. (2013). Modifying the gastrointestinal ecology in alternatively raised poultry and the potential for molecular and metabolomic assessment. Poult. Sci. 92, 546–561. doi: 10.3382/ps.2012-02734
Patel, R. M. (2007). Stabilized rice bran. The functional food of the 21st century. Agro Food Ind. Hi Tech. 18, 39–41.
Prabhakaran, P., Ranganathan, R., Kumar, V. M., Rajasekar, R., Devakumar, L., and Pal, S. K. (2017). Review on parameters influencing the rice breakage and rubber roll wear in sheller. Arch. Metall. Mater. 62, 1875–1880. doi: 10.1515/amm-2017-0284
Pradhan, A., Ali, S. M., and Dash, R. (2013). Biomass gasification by the use of rice husk gasifier. Special Issue Int. J. Adv. Comput. Theory Eng. 2, 14–17.
Prakash, J., and Ramaswamy, H. S. (1996). Rice bran proteins: properties and food uses. Crit. Rev. Food Sci. Nutr. 36, 537–552. doi: 10.1080/10408399609527738
Prasara, A. J., and Gheewala, S. H. (2017). Sustainable utilization of rice husk ash from power plants: a review. J. Clean. Prod. 167, 1020–1028. doi: 10.1016/j.jclepro.2016.11.042
Qian, H., and Zhang, H. (2013). “Rice flour and related products,” in Handbook of Food Powders: Processes and Properties, eds B. Bhandari, N. Bansal, M. Zhang M, and P. Schuck (Philadelphia, PA: Woodhead Publishing), 553–575. doi: 10.1533/9780857098672.3.553
Quiñones, R. S., Macachor, C. P., and Quiñones, H. G. (2015). Development of gluten-free composite flour blends. Trop. Technol. J. 19. 1–4. doi: 10.7603/s40934-015-0003-3
Rafe, A., Sadeghian, A., and Hoseini-Yazdi, S. Z. (2017). Physicochemical, functional, and nutritional characteristics of stabilized rice bran form tarom cultivar. Food Sci. Nutr. 5, 407–414. doi: 10.1002/fsn3.407
Randall, K. (2018). Market Prices: Spike in Feed Cost Sees Rations Rise. Available online at: https://www.poultryworld.net/UK/Articles/2018/3/Market-trends-Spike-in-feed-cost-sees-rations-rise-263570E (accessed February 22, 2019).
Rice Bran Oil Market (2016). Rice Bran Oil Market Size, Industry Outlook Report, Regional Analysis (U.S., Germany, UK, Italy, Russia, China, India, Japan, South Korea, Brazil, Mexico, Saudi Arabia, UAE, South Africa), Downstream Application Development, Price Trends, Competitive Market Share & Forecast, 2017–2021. Part 5 Global Market Landscape. Available online at: http://thenewsmates.com/rice-bran-oil-market-2019-with-top-countries-data-size-production-prospects-consumption-cost-structure-and-forecast-to-2023/114110/ (accessed March 20, 2019).
Ricke, S. C. (2003). Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 82, 632–639. doi: 10.1093/ps/82.6.1003
Ricke, S. C. (2018). Impact of prebiotics on poultry production and food safety. Yale J. Biol. Med. 91:151.
Ricke, S. C., Dunkley, C. S., and Durant, J. A. (2013). A review on development of novel strategies for controlling Salmonella enteritidis colonization in laying hens: fiber-based molt diets. Poult. Sci. 92, 502–525. doi: 10.3382/ps.2012-02763
Ricke, S. C., Hacker, J., Yearkey, K., Shi, Z., Park, S. H., and Rainwater, C. (2017). “Chapter 19. Unravelling food production microbiomes: concepts and future directions,” in Food and Feed Safety Systems and Analysis, eds S. C. Ricke, G. G. Atungulu, S. H. Park, and C. E. Rainwater (San Diego, CA: Elsevier Inc.), 347–374. doi: 10.1016/B978-0-12-811835-1.00019-1
Ricke, S. C., Van der Aar, P. J., Fahey, G. C. Jr., and Berger, L. L. (1982). Influence of dietary fibers on performance and fermentation characteristics of gut contents from growing chicks. Poult. Sci. 61, 1335–1343. doi: 10.3382/ps.0611335
Roberfroid, M. (2007). Prebiotics: the concept revisited. J. Nutr. 137, 830S−837S. doi: 10.1093/jn/137.3.830S
Rohman, A., Helmiyati, S, Hapsari, M., and Setyaningrum, D. L. (2014). Rice in health and nutrition. Int. Food Res. J. 21, 13–24.
Roto, S. M., Rubinelli, P. M., and Ricke, S. C. (2015). An introduction to the avian gut microbiota and the effects of yeast-based prebiotic-type compounds as potential feed additives. Front. Vet. Sci. 2:28. doi: 10.3389/fvets.2015.00028
Roy, P., Shimizu, N., Shiina, T., and Kimura, T. (2006). Energy consumption and cost analysis of local parboiling processes. J. Food Eng. 76, 646–655. doi: 10.1016/j.jfoodeng.2005.06.034
Rubinelli, P. M., Kim, S. A., Park, S. H., Roto, S. M., Nealon, N. J., Ryan, E. P., et al. (2017). Differential effects of rice bran cultivars to limit Salmonella Typhimurium in chicken cecal in vitro incubations and impact on the cecal microbiome and metabolome. PLoS ONE 12:e0185002. doi: 10.1371/journal.pone.0185002
Ryan, E. P. (2011). Bioactive food components and health properties of rice bran. J. Am. Vet. Med. Assoc. 238, 593–600. doi: 10.2460/javma.238.5.593
Sabale, V., Sabale, P. M., and Lakhotiya, C. L. (2007). In vitro studies on rice bran oil wax as skin moisturizer. Indian J. Pharm. Sci. 69, 215–218. doi: 10.4103/0250-474X.33146
Sanchez, J., Thanabalan, A., Khanal, T., Patterson, R., Slominski, B. A., and Kiarie, E. (2018). Effects of feeding broiler chickens up to 11% rice bran in a corn-soybean meal diet without or with a multi-enzyme supplement. Anim. Nutr. 5, 41–48. doi: 10.1016/j.aninu.2018.12.001
Sarkar, S., and Bhattacharyya, D. K. (1991). Nutrition of rice bran oil in relation to its purification. J. Am. Oil Chem. Soc. 68, 956–962. doi: 10.1007/BF02657543
Savant, N. K., Snyder, G. H., and Datnoff, L. E. (1996). Silicon management and sustainable rice production. Adv. Agron. 58, 151–199. doi: 10.1016/S0065-2113(08)60255-2
Sayre, R. N., Earl, L., Kratzer, F. H., and Saunders, R. M. (1988). Effect of diets containing raw and extrusion-cooked rice bran on growth and efficiency of food utilization of broilers. Br. Poult. Sci. 29, 815–823. doi: 10.1080/00071668808417110
Schwartz, M. (1992). J. B. Hunt: The Long Haul to Success. Fayetteville AR: University of Arkansas Press.
Sharif, K., Butt, M. S., Anjum, F. M., and Nasir, M. (2005). Improved quality of baked products by rice bran oil. Internet J. Food Saf. 5, 1–8.
Sharif, M., Butt, M. S., Anjum, F. M., and Khan, S. H. (2014). Rice bran: a novel functional ingredient. Crit. Rev. Food Sci. Nutr. 54, 807–816. doi: 10.1080/10408398.2011.608586
Sheflin, A. M., Borresen, E. C., Wdowik, M. J., Rao, S., Brown, R. J., and Heuberger, A. L. (2015). Pilot dietary intervention with heat-stabilized rice bran modulates stool microbiota and metabolites in healthy adults. Nutrients 7, 1282–1300. doi: 10.3390/nu7021282
Siebenmorgen, T. J., Counce, P. A., and Wilson, C. E. (2011). Factors Affecting Rice Milling Quality. Available online at: https://www.uaex.edu/publications/pdf/FSA-2164.pdf (accessed March 20, 2019).
Siebenmorgen, T. J., Nehus, Z. T., and Archer, T. R. (1998). Milled rice breakage due to environmental conditions. Cereal Chem. 75, 149–152. doi: 10.1094/CCHEM.1998.75.1.149
Sikarwar, V. S., Zhao, M., Clough, P., Yao, J., Zhong, X., Memon, M. Z, Shah, N., et al. (2016). An overview of advances in biomass gasification. Energy Environ. Sci. 9, 2939–2977. doi: 10.1039/C6EE00935B
Singh, D., Kumar, R., Kumar, A., and Rai, K. N. (2008). Synthesis and characterization of rice husk silica, silica-carbon composite and H3PO4 activated silica. Ceramica 54, 203–212. doi: 10.1590/S0366-69132008000200011
Smith, D. (2019). Presentation: “Heat and Mass Transfer in Parboiled Rice During Heating With 915 MHz Microwave Energy and Impacts on Milled Rice Properties”. Fayetteville, NC: University of Arkansas.
Soe, H. Y., Makino, Y., Uozumi, N., Yayota, M., and Ohtani, S. (2007). Evaluation of non-feed removal induced molting in laying hens. J. Poult. Sci. 44, 153–160. doi: 10.2141/jpsa.44.153
Sohail, M., Rakha, A., Butt, M. S., Iqbal, M. J., and Rashid, S. (2017). Rice bran nutraceutics: a comprehensive review. Crit. Rev. Food Sci. Nutr. 57, 3771–3780. doi: 10.1080/10408398.2016.1164120
Soltani, N., Bahrami, M., Pech-Canul, I., and Gonzalez, A. (2015). Review on the physicochemical treatments of rice husk for production of advanced materials. Chem. Eng. J. 264, 899–935. doi: 10.1016/j.cej.2014.11.056
Strausberg, S. F. (1995). “Chapter 9. Light and shadows challenges and achievements,” in From Hills and Hollers: Rise of the Poultry Industry in Arkansas, ed M. Shult and C. Scifres (Fayetteville, AR: University of Arkansas, 131–146.
Swain, B. K., and Sundaram, R. N. S. (2000). Effect of different types of litter material for rearing broilers. Br. Poult. Sci. 41, 261–262. doi: 10.1080/713654931
Szymczyk, B., Pisulewski, P. M., Szczurek, W., and Hanczakowski, P. (2001). Effects of conjugated linoleic acid on growth performance, feed conversion efficiency, and subsequent carcass quality in broiler chickens. Br. J. Nutr. 85, 465–473. doi: 10.1079/BJN2000293
Thirumalaisamy, G., Muralidharan, J., Senthilkumar, S., Hema Sayee, R., and Priyadharsini, M. (2016). Cost-effective feeding of poultry. Int. J. Sci. Environ. Technol. 5, 3997–4005.
Topping, D. L., Illman, R. J., Roach, P. D., Trimble, R. P., Kambouris, A., and Nestel, P. J. (1990). Modulation of the hypolipidemic effect of fish oils by dietary fiber in rats: studies with rice and wheat bran. J. Nutr. 120, 325–330. doi: 10.1093/jn/120.4.325
Triratanasirichai, K., Singh, M., and Anal, A. K. (2017). “Value-added by-products from rice processing industries,” in Food Processing By-Products and Their Utilization, ed A. K. Anal (Hoboken, NJ: Wiley & Sons), 277–293. doi: 10.1002/9781118432921.ch12
Truong, A. H., Tran, H. A., and Ha-Duong, M. (2016). “Socio-economic impacts of co-firing in Vietnam: the case of Ninh Binh Coal Power Plant,” in Handbook of Biomass Investment in Vietnam Vietnam Energy Association, ed M. Ha-Duong (Hanoi: Vietnam Energy Association), 1–17.
Ugwu, S. O. C., Onyimonyi, A. E., and Ozonoh, C. I. (2008). Comparative performance and haematological indices of finishing broilers fed palm kernel cake, bambara offal and rice husk as partial replacement for maize. Int. J. Poult. Sci. 7, 299–303. doi: 10.3923/ijps.2008.299.303
United States Department of Agriculture (2009). United States Standards for Rice. Available online at: https://www.gipsa.usda.gov/fgis/standards/ricestandards.pdf (accessed February 19, 2019).
United States Department of Agriculture (2016). Overview of Rice. Available online at: https://www.ers.usda.gov/topics/crops/rice/ (accessed February 19, 2019).
United States Department of Agriculture (2017). Certified Organic Survey 2016 Summary. Available online at: https://downloads.usda.library.cornell.edu/usda-esmis/files/zg64tk92g/70795b52w/4m90dz33q/OrganicProduction-09-20-2017_correction.pdf (accessed February 19, 2019).
Vaisali, C., Charanyaa, S., Belur, P. D., and Regupathi, I. (2015). Refining of edible oils: a critical appraisal of current and potential technologies. Int. J. Food Sci. Technol. 50, 13–23. doi: 10.1111/ijfs.12657
Van Dalen, G. (2004). Determination of the size distribution and percentage of broken kernels of rice using flatbed scanning and image analysis. Food Res. Int. 37, 51–58. doi: 10.1016/j.foodres.2003.09.001
Van der Aar, P. J., Fahey, G. C. Jr., Ricke, S. C., Allen, S. E., and Berger, L. L. (1983). Effects of dietary fibers on mineral status of chicks. J. Nutr. 113, 653–661. doi: 10.1093/jn/113.3.653
Van Loveren, H., Sanz, Y., and Salminen, S. (2012): Health claims in Europe: probiotics prebiotics as case examples. Annu. Rev. Food Sci. Technol. 3, 247–261. doi: 10.1146/annurev-food-022811-101206
Verschoyle, R. D., Greaves, P., Cai, H., Edwards, R. E., Steward, W. P., and Gescher, A. J. (2007). Evaluation of the cancer chemopreventive efficacy of rice bran in genetic mouse models of breast, prostate and intestinal carcinogenesis. Br. J. Cancer 96, 248. doi: 10.1038/sj.bjc.6603539
Warren, B. E., and Farrell, D. J. (1990). The nutritive value of full-fat and defatted Australian rice bran. II. Growth studies with chickens, rats and pigs. Anim. Feed Sci. Technol. 27, 229–246. doi: 10.1016/0377-8401(90)90085-M
Woodward, C. L., Kwon, Y. M., Kubena, L. F., Byrd, J. A., Moore, R. W., Nisbet, D. J., et al. (2005). Reduction of Salmonella enterica serovar Enteritidis colonization and invasion by an alfalfa diet during molt in Leghorn hens. Poult. Sci. 84, 185–193. doi: 10.1093/ps/84.2.185
Yang, X., Wen, K., Tin, C., Li, G., Wang, H., Kocher, J., et al. (2014). Dietary rice bran protects against rotavirus diarrhea and promotes Th1-type immune responses to human rotavirus vaccine in gnotobiotic pigs. Clin. Vaccine Immunol. 21, 1396–1140. doi: 10.1128/CVI.00210-14
Younas, A., Bhatti, M. S., Ahmed, A., and Randhawa, M. A. (2011). Effect of rice bran supplementation on cookie baking quality. Pak. J. Agric. Sci. 48, 133–138.
Zareei, S. A., Ameri, F., Dorostkar, F., and Ahmadi, M. (2017). Rice husk ash as a partial replacement of cement in high strength concrete containing micro silica: evaluating durability and mechanical properties. Case Stud. Constr. Mater. 7, 73–81. doi: 10.1016/j.cscm.2017.05.001
Keywords: rice milling, byproducts, utilization trends, sustainability, market drivers, alternative poultry production
Citation: Bodie AR, Micciche AC, Atungulu GG, Rothrock MJ Jr and Ricke SC (2019) Current Trends of Rice Milling Byproducts for Agricultural Applications and Alternative Food Production Systems. Front. Sustain. Food Syst. 3:47. doi: 10.3389/fsufs.2019.00047
Received: 22 March 2019; Accepted: 10 June 2019;
Published: 27 June 2019.
Edited by:
Christos Damalas, Democritus University of Thrace, GreeceReviewed by:
Irene Hanning, The University of Tennessee, Knoxville, United StatesCopyright © 2019 Bodie, Micciche, Atungulu, Rothrock and Ricke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven C. Ricke, c3JpY2tlQHVhcmsuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.