- 1School of Acupuncture-Moxibustion and Tuina, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2School of Chinese Classics, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3School of Nursing, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4Department of Gynecology, School of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Endometrial cancer (EC) is a prevalent epithelial malignancy in the uterine corpus’s endometrium and myometrium. Regulating apoptosis of endometrial cancer cells has been a promising approach for treating EC. Recent in-vitro and in-vivo studies show that numerous extracts and monomers from natural products have pro-apoptotic properties in EC. Therefore, we have reviewed the current studies regarding natural products in modulating the apoptosis of EC cells and summarized their potential mechanisms. The potential signaling pathways include the mitochondria-dependent apoptotic pathway, endoplasmic reticulum stress (ERS) mediated apoptotic pathway, the mitogen-activated protein kinase (MAPK) mediated apoptotic pathway, NF-κB-mediated apoptotic pathway, PI3K/AKT/mTOR mediated apoptotic pathway, the p21-mediated apoptotic pathway, and other reported pathways. This review focuses on the importance of natural products in treating EC and provides a foundation for developing natural products-based anti-EC agents.
1 Introduction
Endometrial cancer (EC) refers to a prevalent epithelial malignancy occurring in the endometrium and myometrium of the uterine corpus. It is the most common gynecological malignancy in developed countries and the second most common in developing countries (Sung et al., 2021; Akazawa and Hashimoto, 2022). The morbidity of EC is estimated to increase by more than 50% worldwide by 2040 (Moore and Brewer, 2017; Zhang S. et al., 2019; Brooks et al., 2019). EC typically occurs in postmenopausal women, while a rising incidence is observed in the premenopausal population due to the increasing onset of obesity globally (Moore and Brewer, 2017). Conventional treatments for EC include surgical resection, radiotherapy, chemotherapy, and hormonotherapy, depending on the cancer stage (An et al., 2021). Though these treatment regimens benefit the patients, the outcomes and prognosis of those at the advanced and recurrent stage or with metastasis remain poor (Lu and Broaddus, 2020).
On the other hand, these options are often accompanied by adverse consequences. For example, a hysterectomy is recommended for patients with higher-grade EC or myometrium invasion, while these patients have to lose their childbearing ability (Lu and Broaddus, 2020). For patients receiving chemotherapy, the issue of drug resistance would not be ignored, which could compromise the therapeutic effects of the agents leading to treatment failure (Hashem et al., 2022). Various side effects during the treatments pose multiple challenges to the patients; they would suffer from a functional loss in different behavioral and life domains and psychosocial distress (Concin et al., 2021). Hence, it presents an urgent need to explore new treatment alternatives for EC to improve patient outcomes and prognosis.
The specific pathogenesis of EC remains to be fully elucidated. Several physical, clinical, and genetic variables, including age, race, proximity to the metabolic syndrome, unopposed estrogen exposure, and genetic predispositions, are thought to have a role in the unique etiology of EC (Cai et al., 2019; Passarello et al., 2019). EC can be typically categorized into type-I and type-II due to their molecular and histopathology features, based on a classification system produced by Bokhman in 1983. Type-I accounts for most EC cases (70%–80%). In the endometrium, periodic hyperplasia is delicately controlled by programmed cell growth and death. The long-term effects of estrogen without progestin antagonism, which cause endometrial hyperplasia and atypical hyperplasia, followed by carcinogenesis, may cause type-I EC. Endometrial hyperplasia is a significant problem, and also the associated risk factors include hyperinsulinemia, obesity, high estradiol levels, and advanced age. Endometrial hyperplasia without atypical has a low (5%) risk of progression to endometrial cancer over 20 years. However, atypical glandular hyperplasia has a 27.5% risk of progression over 20 years and up to 43% of such patients (Hutt et al., 2019). Atypical hyperplasia, related to abnormal growth and proliferation of endometrial cells, is a precursor lesion for type-I EC (Armstrong et al., 2012; Braun et al., 2016; Urick and Bell, 2019), while its molecular basis is still unclear (Terzic et al., 2021). Apoptosis is a multistep programmed cell death process critical in clearing senescent and aberrant cells. Studies have demonstrated that inhibited cellular apoptosis is closely associated with the pathogenesis of EC. Dysfunction or inhibition of cellular apoptosis in the endometrium causes uncontrolled cell proliferation, aberration, and carcinogenesis (Fisher, 1994; Zhang et al., 2020). Given this situation, regulating apoptosis of EC cells would be a promising target for developing effective anti-EC agents.
In recent years, natural products (NPs) have become a research hotspot in cancer treatment (Huang et al., 2019; Atanasov et al., 2021; Kim et al., 2021; Anjum et al., 2022; Liu et al., 2022; Huang et al., 2023; Yuan et al., 2023). NPs refer to components, isolated metabolites, and extracts from natural plants and be of multiple bioactivities, such as regulating oxidative stress, inflammatory response, and cellular apoptosis. These agents also reveal therapeutic effects on various cancers (Shanmugam et al., 2016) with low toxicity and few side effects (Torquato et al., 2017). The detailed mechanisms underlying the anti-cancer properties of NPs need to be further explored to facilitate the development of NP-based anti-cancer agents. Both in-vitro and in-vivo studies demonstrate that many NPs could effectively suppress EC cells’ growth, proliferation, and differentiation via regulating apoptosis (Liu et al., 2012), indicating the apoptosis-regulatory properties of NPs would be a promising direction for further exploration.
Therefore, we have performed a comprehensive search in Google Scholar, PubMed, China National Knowledge Infrastructure (CNKI), Wanfang Database, and VIP database, from the inception to 31 December 2022, for studies regarding NPs for the treatment of EC via inducing apoptosis, and have reviewed the relevant pathways including mitochondria-dependent apoptotic pathway, endoplasmic reticulum stress (ERS) mediated apoptotic pathway, mitogen-activated protein kinase (MAPK) mediated apoptotic pathway, NF-κB mediated apoptotic pathway, PI3K/Akt mediated apoptotic pathway, p21-mediated apoptotic pathway and others. We hope our work could provide inspiration and valuable references for future studies.
2 Overview of apoptosis
Cellular apoptosis is a genetically-regulated programmed cell death process that plays an essential role in cellular metabolism (Hengartner, 2000). Inadequate apoptosis could cause pathological changes like carcinogenesis, autoimmune diseases, and diabetes (Nair et al., 2014). It was first reported by Kerr et al., in 1972, describing it as characteristic morphological changes and a series of enzyme-dependent biochemical processes (Kerr et al., 1972). Apoptosis can be divided into the exogenous death receptor and endogenous mitochondrial apoptosis pathways (Ricci and El-Deiry, 2007; Zhang et al., 2022).
The intrinsic pathway refers to apoptotic cascades triggered by intracellular signals, such as DNA damage, aberrant cell metabolism, calcium overload, chemotherapeutic drugs, radiation, high levels of reactive oxygen species, and detachment from the extracellular matrix (Chaudhry and Asselin, 2009). In a typical situation, there is a dynamic balance between the expression of pro-apoptotic protein and anti-apoptotic protein, which regulates physiological apoptosis. Decreased expression of anti-apoptotic protein BCL-2 family members or increased expression of pro-apoptotic proteins in response to the various stimulus signals described previously leads to an unbalance in the BCL/BAX ratio, which in turn initiates the endogenous pathway. The intrinsic pathway is mainly mediated by the B Cell lymphoma-2 (BCL-2) gene family (Ashkenazi, 2008; Nair et al., 2014). Interactions between the BCL-2 protein family determine mitochondrial outer membrane permeability (Green, 2022). The pro-apoptotic BCL-2 effectors, such as BAX, BAK, BIM, BID, and PUMA, promote apoptosis by causing mitochondrial outer membrane permeabilization (MOMP), whereas anti-apoptotic BCL-2 effectors inhibit this process, such as BCL-2, BCL-XL, BCL-W, BCL-2-A1 and MCL1 (Carneiro and El-Deiry, 2020; Wolf et al., 2022). When BAX/BAK is inserted into the mitochondrial membrane, cytochrome c (Cyt-c) is released into the cytosol from the outer mitochondrial membrane. Cytochrome c’s release is critical in cell apoptosis (Santucci et al., 2019). Cytosolic cytochrome c combines with apoptotic protease activating factor-1 (Apaf–1) and recruit pro-caspase-9 to form the apoptosome, a multiprotein complex (Li et al., 2017). Apoptosome is a multiprotein platform of caspase-9 activation to execute apoptosis (Malladi et al., 2009; Bratton and Salvesen, 2010; Dorstyn et al., 2018; Avrutsky and Troy, 2021). Once activated, caspase-9 could cleave and activate downstream pro-caspase-3 and -7 in the apoptosome, which in turn triggers the activation of further caspase-9 (Qin et al., 1999, 1; McComb et al., 2019, 7). If caspase-9 successfully processes some caspase-3 or caspase-7 in this situation, XIAP can bind to and suppress these active effector caspases (Bratton and Salvesen, 2010).
The extrinsic pathway is mainly triggered by extracellular stimuli (Jan and Chaudhry, 2019; Kashyap et al., 2021). The extracellular ligands such as tumor necrosis factor (TNF), Fas ligand (Fas-L), death receptor3 ligand (DR3L), and TNF-related apoptosis-inducing ligand (TRAIL) recognize and bind to their cognate death receptors (such as TNFR, Fas, DR3, DR4 or DR5) (Mandal et al., 2020). Procaspase-8 binds to the exposed DED of death receptor-related FADD through a pocket in its DED1 to form a death-inducing signaling complex (DISC) (Jiang M. et al., 2021) and subsequently activate pro-caspase-8. It can cleave and activate the downstream targeted molecules, including executor caspase-3 and caspase-7 and turn on the exogenous apoptotic cell death response (Jiang M. et al., 2021). Activated caspase-8 is a crucial protein of cross-talk signal way and could cleave Bid into tBid. Bid is generally thought to be inactive as an apoptosis inducer. tBid could induce mitochondrial outer membrane permeabilization (MOMP) in cells and induce the release of cytochrome c (CytC) and Smac/DIABLO from the mitochondria. Eventually, tBid can initiate the mitochondrial apoptosis pathway and makes significant in the endogenous apoptotic pathway by activating caspase-9 (Kantari and Walczak, 2011).
3 Endometrial carcinogenesis
Carcinogenesis in the endometrium is a complex and multistep process. The specific mechanisms remain elusive while several physical, pathological, and genetic factors are considered to be involved, such as age, race, concomitance with metabolic syndrome, unopposed estrogen exposure, and genetic predispositions (Cai et al., 2019; Passarello et al., 2019). Dysregulation of cellular apoptosis in the endometrium causes uncontrolled cell proliferation, aberration, and carcinogenesis (Fisher, 1994; Mirakhor Samani et al., 2018; Zhang et al., 2020).
EC can be typically categorized into type-I and type-II due to their molecular and histopathological features (Rodríguez-Palacios et al., 2022; Karia et al., 2023), based on a classification system produced by Bokhman in 1983. The type-I EC, endometrioid tumors, accounts for most EC cases (70%–80%). The type-I EC is derived from a precancerous condition called endometrial hyperplasia, whereas the type-II is hormone-independent pathogenesis without known precursor lesions (Huvila et al., 2013). Hyperplasia is a significant problem, and the associated risk factors include hyperinsulinemia, obesity, high estradiol levels, and increasing age (Singh et al., 2020). Endometrial hyperplasia without atypical has a low (5%) risk of progression to endometrial cancer over 20 years. However, atypical glandular hyperplasia has a 27.5% risk of progression over 20 years and up to 43% of such patients (Hutt et al., 2019). Atypical hyperplasia may further evolve into complex atypical hyperplasia (CAH). CAH is a precursor lesion for endometrioid-type endometrial cancer and is related to abnormal growth and proliferation of endometrial cells (Armstrong et al., 2012; Braun et al., 2016; Urick and Bell, 2019).
Clinical studies have found that the normal apoptotic mechanisms of many malignant cells are inhibited, preventing the body from early clearance of cells that may be at risk of cancer. Endometrial periodic hyperplasia is under delicate control by programmed cell growth and death. Apoptosis typically occurs between the human endometrium’s late secretory and menstrual stages (Otsuki, 2001). Compared to the proliferating phase, the expression of BCL-2 and the activation of caspase-3, -8, and -9 are higher in secretory to menstruating stages (Otsuki, 2001). It is reported that EC patients are resistant to apoptosis due to the unbalance of the anti- and pro-apoptotic molecules. Increasing evidence has suggested that anti-apoptotic mediators, such as BCL-2, Mcl-2, and IAP (Ai et al., 2006), are downregulated in EC patients, whereas the pro-apoptotic proteins, such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), p53 (Kohlberger et al., 1996; Geisler et al., 1999; Edmondson et al., 2017) upregulated modulator.
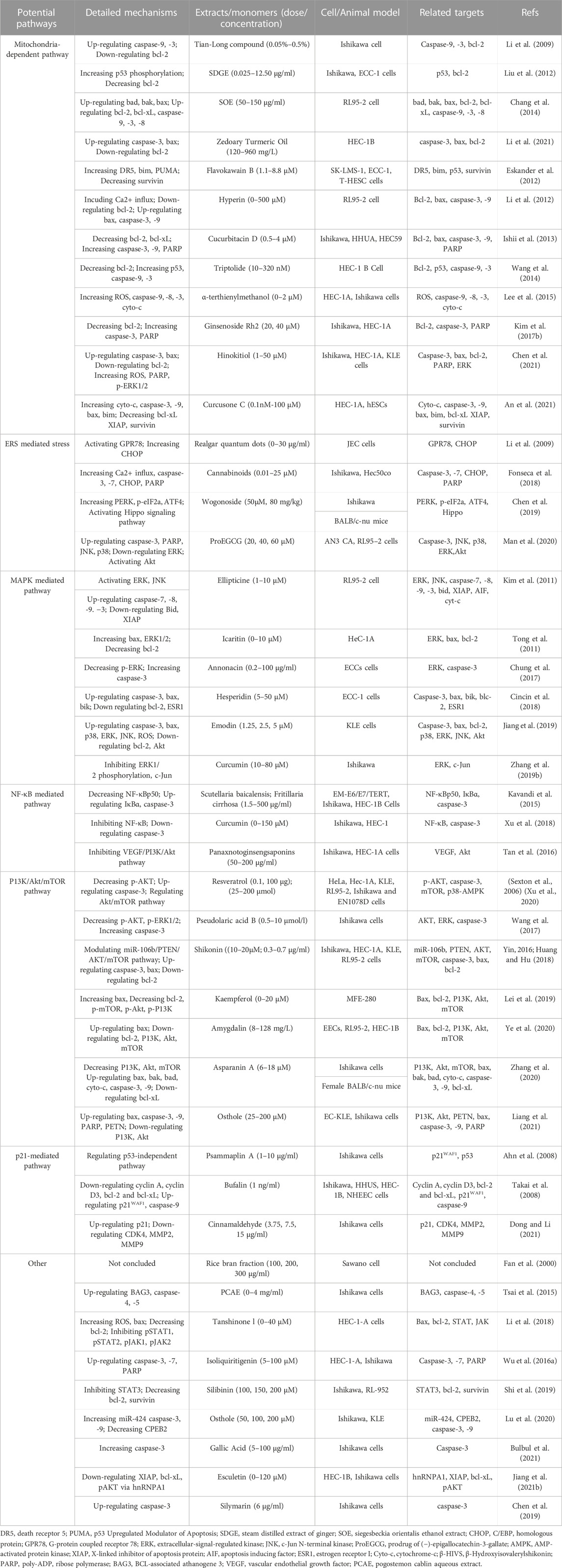
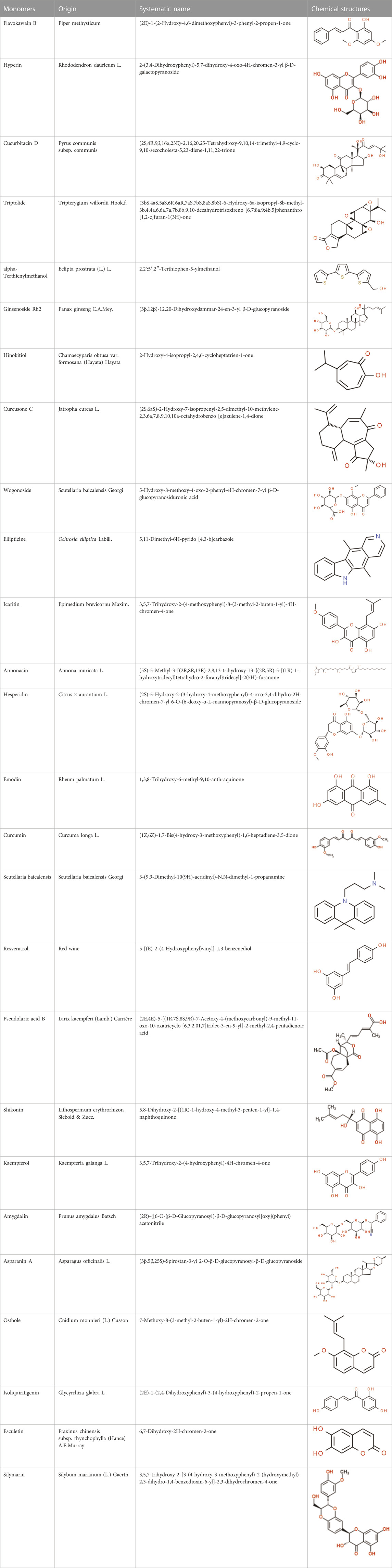
Cellular apoptosis is crucial in endometrial hyperplasia, atypical hyperplasia, complex atypical hyperplasia, and eventually endometrial cancer. Given this situation, regulating apoptosis of EC cells would be a promising target for developing effective anti-EC agents and could provide a possible direction for developing anti-EC drugs. In almost all cases, detailed information of NPs and their potential effects with mechanisms on modulating apoptosis in EC is illustrated in Table 1, and the chemical structures of isolated metabolites are summarized in Table 2.
4 Effects and mechanisms of NPs on apoptosis in EC
4.1 Mitochondria-dependent apoptotic pathway
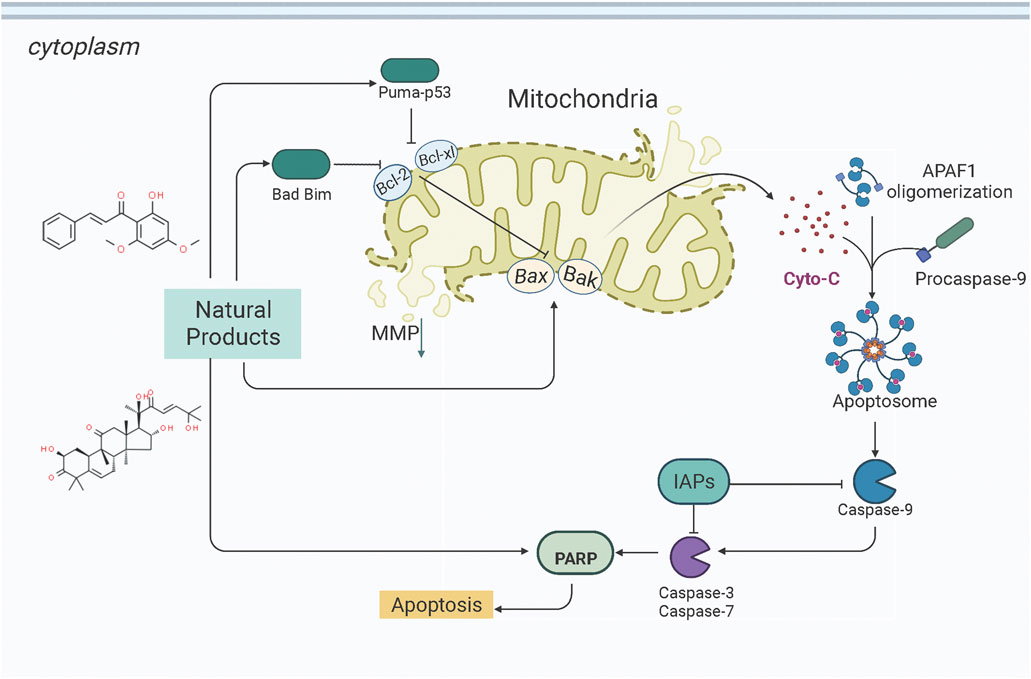
Mitochondria is the core organelle for energy synthesis and supply, thereby maintaining cellular function and managing cell life and death (Abate et al., 2020; Wang and Roh, 2020). Mitochondrial malfunction often triggers stress-mediated apoptosis. Since resistance to apoptosis is decisive for degenerative diseases and is a hallmark of cancer, the basis of cellular health is the correct functioning of mitochondria. Internal apoptotic signals, such as p53-PUMA or death receptor signal pathways, could alter the mitochondrial membrane permeability (MMP), releasing Cyto-c and other apoptosis-related factors into the cytosol to form the apoptosome. The apoptosome recruits and activates caspase-9, which in turn activates the effector caspases (caspase-3, -6, -7, etc.). Subsequently, the down-stream cascades by cleaving poly ADP-ribose polymerase (PARP) and actin substrates. Current studies demonstrate that some NPs effectively treat EC by modulating the mitochondria-dependent apoptotic pathway. All the relevant NPs that activate apoptosis via the mitochondria-dependent pathway are listed in Figure 1.
4.1.1 Extracts from NPs
In early 2009, Li et al. studied the anti-EC effects of the Tian-Long compound (TL compound) in vitro. They found that TL compound (0.05%–0.5%) could significantly suppress the proliferation of Ishikawa cells by activating the mitochondrial-dependent apoptotic pathway. The potential mechanisms could be the upregulation of caspase-9 and caspase-3 and the downregulation of BCL-2 (Li et al., 2009). Liu et al. reported that a Steam Distilled Extract of Ginger (SDGE, 0.025–12.50 μg/ml) could induce apoptosis in Ishikawa and ECC-1 cells. The possible mechanisms are closely related to up-regulating p53 phosphorylation and down-regulating BCL-2 (Liu et al., 2012, 5). Later in 2014, Chang et al. investigated the pro-apoptotic effects of Siegesbeckia orientalis Ethanol Extract (SOE, 50–150 μg/ml) on Human Endometrial RL-95 Cancer Cells and found that SOE was of significant anti-proliferative and apoptotic effects in RL95-2 cells via activating both intrinsic and extrinsic signaling pathways. A study on its specific mechanisms revealed that SOE could upregulate the expression of Bad, Bak, and Bax, caspase-3, -9, and -8, whereas downregulate that of BCL-2 and BCL-xL (Chang et al., 2014). In 2021, Li et al. reported that Zedoary Turmeric Oil (120–960 mg/L) could significantly inhibit the proliferation of HEC-1-B cells and induce apoptosis by up-regulating the expressions of Bax and caspase-3 and down-regulating the expression of BCL-2 (Li et al., 2021).
4.1.2 Monomers from NPs
In 2012, Zhou et al. reported that Flavokawain B (FKB, 1.1–8.8 μM) could significantly inhibit the growth of SK-LMS-1 and ECC-1 cell lines compared to non-malignant human endometrium fibroblast-like cells. The potential mechanisms might be associated with G2/M arrest and induction of mitochondrial-dependent apoptosis via upregulation of the pro-apoptotic proteins DR5, Puma, and Bim and downregulation of survivin, an inhibitor of apoptosis protein (IAP) (Eskander et al., 2012) and a promising therapeutic target as a new therapy for cancer treatment (Martínez-García et al., 2019). In 2012, Li et al. studied the anti-proliferative activity of Hyperin on RL952 cells. The results showed that Hyperin (0–200 μM) could suppress the viability of RL952 cells by inducing apoptosis, which would attribute to the regulation of Ca2+ influx, downregulation of BCL-2, and up-expression of bax, caspase-3,-8, and -9 (Li et al., 2012). In 2013, Cucurbitacin D (0.5–4 μM), extracted from Extrasynthese, proved the effect of induction of apoptosis via decreasing BCL-2, BCL-xL, and increasing caspase-3, caspase −9, PARP (Ishii et al., 2013). Triptolide (TP, 10–320 nM), a validated component purified from Tripterygium wilfordii Hook. f. showed to promote apoptosis via a p53-independent mitochondrial pathway. The possible mechanisms are closely related to the reactivation of the p53 to induce apoptosis via downregulation of the expression of BCL-2, and upregulation of caspase-9,-3 in HEC-1B Cells. In 2015, an in vitro study by Lee et al. suggested that α-terthienylmethanol (0–2 μM), isolated from Eclipta prostrata, and could induce apoptosis in HEC-1A and Ishikawa cells via increasing expression of Pro-caspase-3, 8, 9, and Cyto-c in a time-dependent manner and increasing ROS generation. The author also suggested that the apoptosis would be likely mediated by both the intrinsic and extrinsic pathways in ECCs (Lee et al., 2015). Later in 2017, Kim et al. found that the Ginsenoside Rh2 (20, 40 μM) could induce apoptosis in Ishikawa and HEC-1A cells via activation of caspase-3, PARP, and inhibition of BCL-2 (Kim J. H. et al., 2017). In 2021, Chen et al. observed that Hinokitiol (1–50 μM) could induce ROS-Mediated Apoptosis and p53-Driven Cell-Cycle Arrest in Endometrial Cancer Cell Lines (Ishikawa, HEC-1A, KLE) through up-regulating ROS,bax,caspase-3, PARP, p-ERK1/2, whereas down-regulating BCL-2 (Chen et al., 2021). In 2021, an in vivo study by Junxia et al. demonstrated that the Curcusone C (0.1 nM–100 μM) treatment caused significant anti-proliferative and apoptotic effects in Ishikawa and HEC-1A cells by inducing the release of Cytochrome c and increasing caspase-3,-9, Bax and bim, whereas decreasing X-linked inhibitor of apoptosis protein (XIAP), survivin, and BCL-xL (An et al., 2021).
4.2 Endoplasmic reticulum stress mediated pathway
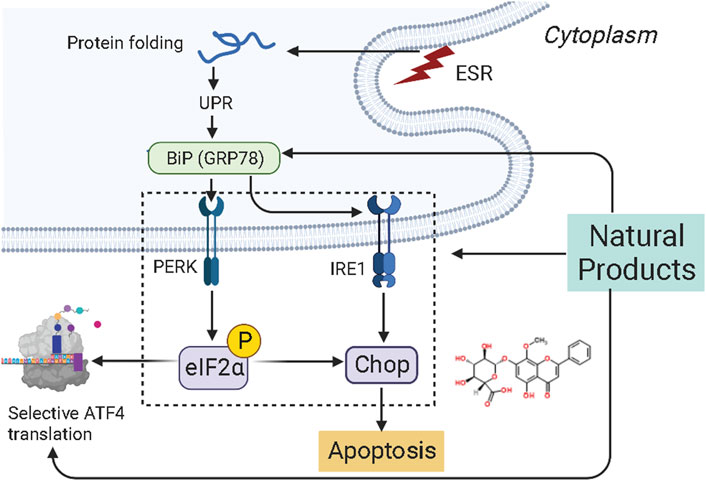
The endoplasmic reticulum (ER) is the central subcellular region for protein synthesis, folding, and transport. It also plays a vital role in intracellular Ca2+ homeostasis and various metabolic processes (Clarke et al., 2014; Wang et al., 2019). Cellular stress conditions can activate endoplasmic reticulum stress (ERS) to restore endoplasmic reticulum homeostasis and normal cellular function. In response to ER stress stimuli, such as the accumulation of unfolded/misfolded proteins in the ER above a critical threshold, the unfolded protein response (UPR) is initiated through three signaling cascades involving the protein kinase RNA-like ER kinase (PERK), inositol-requiring enzyme-1 (IRE1), and activating transcription factor-6 (ATF6) (Ron and Walter, 2007; Wang and Kaufman, 2016; Marciniak, 2019). However, if it fails, UPR triggers cell death (Wu F.-L. et al., 2016). ER stress and UPR have been shown to play critical roles in cancer pathogenesis, progression, and therapeutic response (Oakes et al., 2015). Increasing attention has been paid to ER stress’s essential role in endometrial carcinogenesis and the drug-resistance during chemotherapy. Several studies have demonstrated that NPs would be promising anti-cancer effects on EC via targeting ERS-mediated apoptosis. The potential effectiveness and mechanism of NPs on ERS-mediated apoptosis are summarized in Figure 2.
4.2.1 Extracts from NPs
In 2015, Wang et al. found that Realgar quantum dots (RQDs, 0–80 μg/ml) can induce apoptosis in vitro by increasing the expression level of GRP78 (BIP) and GADD153 (CHOP). Their studies demonstrated that RQDs could activate ER stress and mitochondrial pathways (Wang et al., 2015). Later in 2018, Fonseca et al. reported that Cannabinoids (0.01–25 μM) could induce apoptosis in vitro by activating TRPV1 and increasing caspase-3,-7, Ca2+ influx, CHOP, and cleaved PARP (Fonseca et al., 2018).
4.2.2 Monomers from NPs
In 2019, Chen et al. found that Wogonoside (50 μM, 80 mg/kg), a bioactive flavonoid component derived from Scutellaria baicalensis Georgi, can induce apoptosis and inhibit cell proliferation depending on the ER stress-Hippo signaling axis in vitro and in vivo (in Ishikawa and BALB/c-nu mice) via increasing the expression of protein kinase-like endoplasmic reticulum kinase (PERK), binding protein (Bip), p-eIF2a, and transcription factor 4 (TCF4) (Chen et al., 2019).
4.3 MAPK-mediated apoptotic pathway
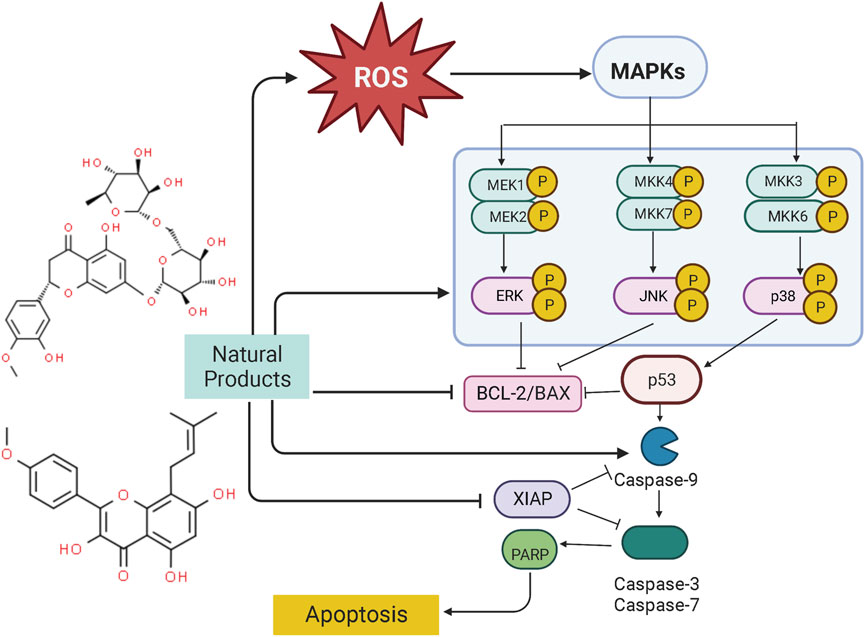
Mitogen-activated protein kinase (MAPK) pathway is an important signal transduction pathway in eukaryotic organisms. MAPK signaling pathways are involved in cell growth, migration, proliferation, differentiation, and apoptosis (Kim and Choi, 2010). Each MAPK signaling cascade consists of at least three layers of protein kinases: MAP3K, MAPKK, and MAPK. These cascades can be divided into extracellular signal-regulated kinase (ERK)1/2, c-Jun N-terminal kinase (JNK), P38 MAPK (P38), ERK3/4, and ERK7/8 (Chuderland and Seger, 2005; Dhillon et al., 2007). Among them, the JNK and p38 MAPK pathways are mainly related to cell stress and apoptosis, while ERK/MAPK signaling pathway is the most intensively studied MAPK signaling pathway, which is closely associated with cell proliferation and differentiation (Chuderland and Seger, 2005). However, the abnormal regulation of the MAPK signaling pathway plays a significant role in carcinogenesis. It is abundantly reported that NPs could be a promising way to treat EC to induce apoptosis through the MAPK pathway. The potential effectiveness and mechanism of NPs on MAPK-mediated apoptosis are summarized in Figure 3.
4.3.1 Extracts from NPs
In another study by Man GCW et al., in 2020, the apoptotic effects of a prodrug of (−)-epigallocatechin-3-gallate (ProEGCG, 20, 40, 60 μM) showed a highly anti-proliferative activity on tumor cells in both EC xenografts cultured in vivo and RL95–2 and AN3 CA EC cells in vitro via promoting apoptosis, which was associated with activation of Akt, Up-regulating caspase-3, PARP, JNK, p38 whereas down-regulating ERK (Man et al., 2020).
4.3.2 Monomers from NPs
Ellipticine (5,11-dimethyl-6H-pyrido [4,3-b]carbazole) is a bioactive component of Ochrosia elliptical, which has been demonstrated to be of pro-apoptotic effect on EC-RL95-2 cells (0.1–20 μM), and the potential mechanisms are related to the activation of ERK, JNA, as well as the increase of ROS generation. Ellipticine can also regulate the XIAP transcription and mediate the caspase cascade reaction to induce cellular apoptosis (Kim et al., 2011). In 2011, Tong et al. reported that Icaritin (0–10 μM), a compound from Epimedium Genus, possessed significant anti-proliferative and apoptosis-inducing activities in Hec1A cells, the potential mechanisms are correlated to increasing bax, ERK1/2 whereas decreasing BCL-2 (Tong et al., 2011). Another investigation in 2017 by Chung et al. studied the anti-proliferative effects of Annonacin (0.2–100 μg/ml) on both EC cell lines (ECC-1 and HEC-1A) and primary cells (EC6-ept and EC14-ept) and found that Annonacin has significant anti-proliferative activity via inhibition of ERK signaling pathway through down-regulating p-ERK whereas increasing caspase-3 (Chung et al., 2017). Hesperidin (Hsd) is the most active flavanone glycoside in citrus flavonoids. Studies in 2018 found that Hsd (5–50 μM) could downregulate MAPK, PI3K, STAT, and mTOR signal transduction pathways for regulating apoptotic and autophagic responses. The underlying mechanism may be related to up-regulating caspase-3, bax, and bik, whereas downregulate BCL-2 and ESR1 (Cincin et al., 2018). In 2018, Jiang et al. found that Emodin (1.25, 2.5, 5 μM), a significant component of rhubarb, can induce apoptosis in vivo (Xenograft Tumor Models) and in vitro in a time- and dose-dependent manner via inhibiting the PI3K/Akt pathways while activating MAPK signaling, and after Emodin treatment, caspase-3, bax, p38, ERK, JNK, and ROS were significantly upregulated whereas BCL-2 and Akt were downregulated (Jiang et al., 2019). Curcumin (10–80 μM), reported to have antioxidant, anti-inflammatory, liver protection, analgesia and antiarthritis, lipid modification, immune regulation, and anti-diabetic properties, could induce apoptosis via Inhibiting the Phosphorylation of ERK/c-Jun pathway via reducing mRNA expression of ERK2 and JUN genes (Zhang Z. et al., 2019).
4.4 NF-κB mediated apoptotic pathway
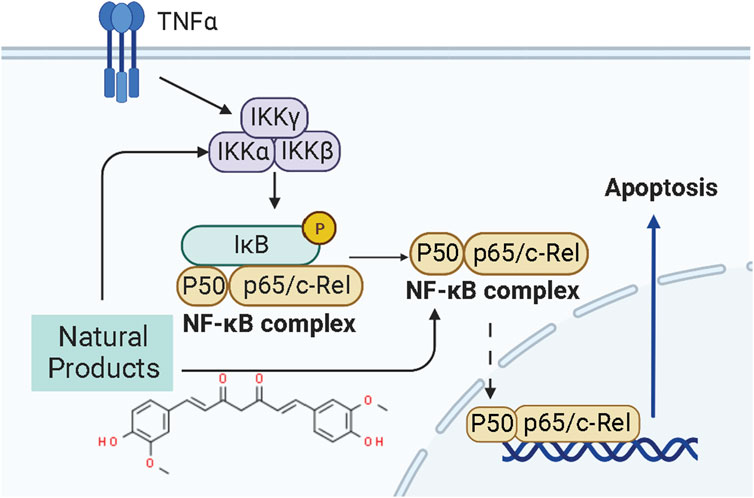
NF-κB is a transcription factor that usually exists as a dimer. p65/relA and p50 are the most common dimeric forms of NF-κB, and its dimers have two states: inactivation and activation. In the “resting” state of cell c, NF-κB is inactive and binds to the inhibitor IκBα on the cell membrane, preventing it from entering the nucleus to activate genes. When external signals stimulate the cell, IκBα is degraded, NF-κB is released, and its nuclear localization sequence (NLS) is exposed. NF-κB rapidly enters the nucleus from the cell membrane and binds to specific sequences on nuclear DNA to initiate or enhance transcription of related genes, which can control protein transcription and participate in physiological processes such as cell proliferation and apoptosis, stress response, and cytokine release. Recently, the NF-κB pathway has been considered a promising therapeutic target for EC therapy. Studies have shown NPs can induce apoptosis in ECCs and prevent endometrial hyperplasia. The potential mechanisms of NPs on NF-κB mediated apoptosis are summarized in Figure 4.
In 2015, it was reported by Kavandi et al. found that the anti-proliferative properties of the herbs Scutellaria baicalensis (SB) and Fritillaria cirrhosa (FC,1.5–500 μg/ml) on EM-E6/E7/TERT, Ishikawa, and HEC-1B Cells closely related to NF-κB pathway via regulation of decreasing NF-κB p50 whereas up-regulating IκBα and caspase-3 (Kavandi et al., 2015). Afterward, Xu et al., in 2018 recorded that Curcumin (0–150 μM) extracted from the rhizome of the plant Curcuma longa could induce apoptosis through negative regulation of the NF-κB pathway in vitro in vivo, and the molecular mechanisms might be related to inhibiting NF-κB and down-regulating caspase-3 (Xu et al., 2018).
4.5 PI3K/AKT/mTOR pathway
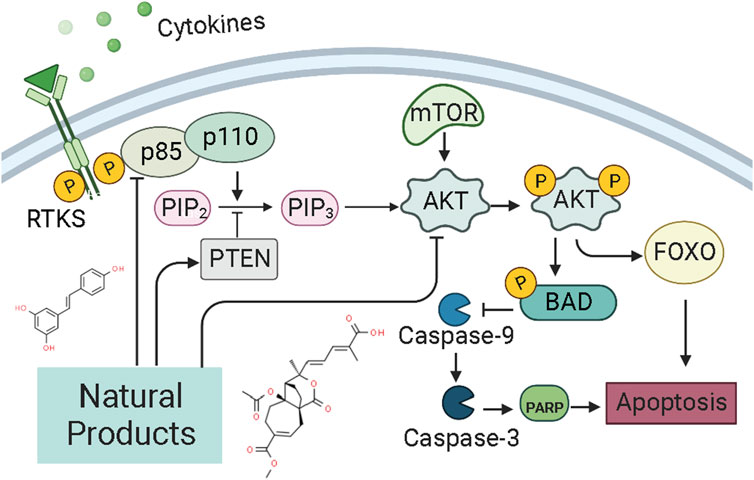
PI3K, or phosphatidylinositol 3-kinase, is a family of lipid kinases that control different processes in mammalian cells, including cell proliferation, survival, differentiation, activation of effector functions, and metabolism (Ali et al., 2015). The PI3K family consists of three classes of PI3Ks (I-III) (Narita et al., 2002). Class I can be further divided into class IA and class IB enzymes, and Class IA PI3K enzymes include a catalytic (p110) and a regulatory subunit (p85 or p101) (Hennessy et al., 2005; Sujobert and Sujobert, 2005; Fruman, 2008, 110; Piddock et al., 2017). Akt, also known as protein kinase B (PKB), is a serine/threonine-specific protein kinase (Yip, 2015). Signaling pathways determined by PI3K, AKT, and the mammalian target of rapamycin (mTOR) are critical for many features of cancer, such as cell growth, survival, metabolism, apoptosis, and angiogenesis (Ediriweera et al., 2019; Fattahi et al., 2020; Miricescu et al., 2020; Mirza-Aghazadeh-Attari et al., 2020). The PI3K/Akt/mTOR intracellular signaling cascade begins with activating RTKs and cytokine receptors, which generate phosphorylated tyrosine residues that provide anchor sites for recruiting PI3K to membrane translocation. Class IA PI3Ks can be activated by receptor tyrosine kinases (RTKs), G protein-coupled receptors (GPCRs) located on the cell surface membrane (Darici et al., 2020). Upon activation, The P110 catalytic subunit of PI3Ks could convert phosphorylate PI(4,5)P2 to PI(3,4,5)P3 (Denley et al., 2009), a second messenger. And then, PIP3 induces the activation of phosphoinositide-dependent kinase-1 (PDK1) and downstream targets of AKT (Pothongsrisit and Pongrakhananon, 2021). The levels of PI(3,4,5)P3 and PI(4,5)P2 could be regulated by PTEN (Chalhoub and Baker, 2009; Blanco et al., 2020). The PI3K/AKT/mTOR signaling pathway is the essential cell signaling pathway in animals, involved in regulating physiological processes such as cell growth, survival, proliferation, metabolism, and apoptosis. Alterations in the PI3K/AKT/mTOR pathway are now thought to be strongly associated with the carcinogenesis and progression of endometrial cancer (Slomovitz and Coleman, 2012; Chen et al., 2014). The pathway most frequently damaged in endometrial cancer is the PI3K/AKT/mTOR pathway (Crosbie et al., 2022). In recent years, it has been abundantly reported that NPs could exert pro-apoptosis via the PI3K/AKT/mTOR pathway (Mirza-Aghazadeh-Attari et al., 2020). The potential mechanisms of NPs on PI3K/AKT/mTOR mediated apoptosis are summarized in Figure 5.
4.5.1 Extracts from NPs
In 2016, Tan et al. reported that the intervention of Panaxnotoginsengsaponins (PNS, 50–200 μg/ml) could induce apoptosis in Ishikawa and HEC-1A cells via inhibiting the expression of VEGF, which may be related to inhibiting PI3K/AKT/mTOR signaling pathway (Tan et al., 2016).
4.5.2 Monomers from NPs
Resveratrol (3, 4, 5-trihydroxy-trans-stilbène), a natural phytoalexin present in grape skins, has considerable anti-proliferation effects and can induce apoptotic cell death in various types of cancers cell in vitro. In 2006, Émilie Sexton et al. reported that high-dose of resveratrol (0,10, and 100 μM) could inhibit cell growth and trigger apoptotic cell death in vitro via decreasing p-Akt, whereas up-regulating caspase-3 (Sexton et al., 2006). Another study in 2020 by Xu et al. also suggested that the anti-proliferative and pro-apoptotic effect of resveratrol might attribute to the regulation of the Akt/mTOR signaling pathway (Xu et al., 2020). Pseudolaric acid B (PAB) is the major bioactive component of Pseudolarix kaempferi Gorden. Studies in 2017 by Wang et al. have found that PAB (0.5–10 μmol/l) could inhibit Ishikawa cell proliferation and induces apoptosis in vitro. Its related molecular mechanisms may involve Akt-GSK-3β and ERK1/2 signaling pathways via decreasing p-Akt and p-ERK1/2 whereas increasing caspase-3 and p-GSK3β (Wang et al., 2017). Shikonin, an active biological component derived from the roots of the herb Lithospermu erythrorhizon, has considerable antitumor effects, including antioxidation, anti-inflammation, and anti-apoptosis. Studies reported in 2016 by Yin et al. and in 2017 by Huang et al. suggested that Shikonin could promote apoptosis in vitro by modulating the miR-106b/PTEN/Akt/mTOR pathway (Yin, 2016; Huang and Hu, 2018). In 2019, a study by Xia et al. suggested that Kaempferol (0–20 μM) can promote apoptosis via increasing bax whereas decreasing p-PI3K p-mTOR, p-Akt, and BCL-2 (Lei et al., 2019). Besides, from the results of Ye et al., Amygdalin (8–128 mg/L) could also induce apoptosis in vitro via regulation of the proteins related to the PI3K-Akt signal (Ye et al., 2020). Another study in 2020 by Zhang et al. first reported that Asparanin A (AA, 6–18 μM) could promote apoptosis in vitro and in vivo by activating the mitochondrial pathway and inhibiting PI3K/Akt signaling pathway (Zhang et al., 2020). Recently, a study by Liang et al., in 2021 showed that Osthole (25–200 μM) could suppress the growth in vitro and in vivo, which was associated with up-regulating bax, caspase-3, -9, PARP, PETN, whereas down-regulating PI3K and Akt (Liang et al., 2021).
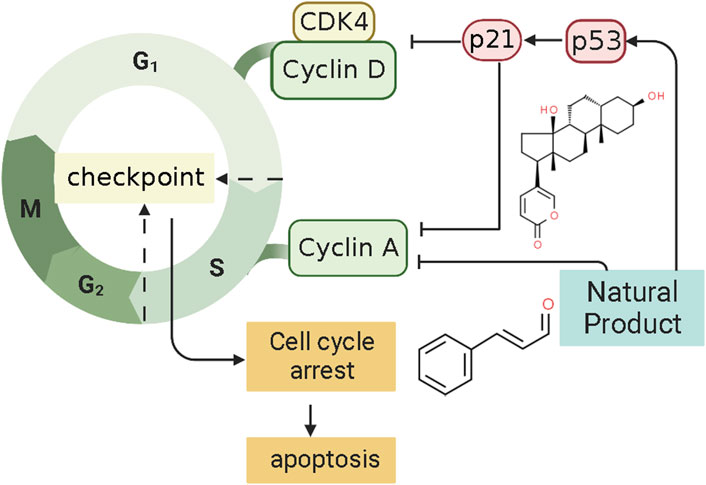
4.6 P21-mediated pathway
P21, also called P21WAF1/CIP1 or P21/CDKN 1a, is a small protein with 165 amino acids related to cell cycle progression (Karimian et al., 2016). In 1993, a finding found that P21, or wild-type p53-activated fragment 1 (WAF1), is directly regulated by P53 and can suppress tumor cell growth in culture (El-Deiry et al., 1993). However, P21 is a downstream mediator of the P53 transcription factor and can interact directly with P53 (Kim E. M. et al., 2017). Thus, P21 may be an essential p53 growth suppression pathway component. Some findings indicate that the p53/p21 complex regulates cell apoptosis by targeting Bcl-2 proteins (Kim et al., 2022). P21 protein, a cyclin-dependent kinase inhibitor (CKI), can bind to and inhibit the activity of CDK1, CDK2, and CDK4/6 enzyme complexes (Marchetti et al., 1996), thereby acting as a cell cycle regulator at the G1 and S phases (Kikuchi et al., 2022). When DNA is damaged, the increased expression of p53 could activate the transcription of gene p21 by binding to its response element within its promoter. P21WAF1 can decrease kinase activity and may be a key regulator of G0/G1 accumulation and G1 cell cycle arrest. Consequently, cell apoptosis was induced by p21. Several studies have shown that NPs can regulate the cell cycle of ECCs by mediating P21, thereby promoting the induction of apoptosis in EC. The potential effectiveness and mechanism of NPs on P21-mediated apoptosis are summarized in Figure 6.
In 2008, after the human endometrial Ishikawa cancer cell line was prepared, Mee et al. investigated the effect of the Psammaplin A (0.1–10 μg/ml), a natural histone deacetylase inhibitor, induces on the Ishikawa cells. They found that Psammaplin A (5 μg/ml) can notably inhibit the proliferation and induced cell cycle arrest or apoptosis in vitro. The molecular mechanisms might be related to the increased expression of p21WAF1 through a p53-independent pathway (Ahn et al., 2008). In the same year, Takai et al. first demonstrated that Bufalin (1 ng/ml) could inhibit proliferation and induce apoptosis in vitro. The mechanism may be related to an increase in cleaved caspase-9 expression caused by up-regulating the levels of p21WAF1 protein and down-regulating cyclin A, cyclin D3, BCL-2, and BCL-xL (Takai et al., 2008). Recently, Dong et al. investigated the effect of Cinnamaldehyde (3.75, 7.5, 15 μg/ml) on Ishikawa cells. The results showed that Cinnamaldehyde has notable pro-apoptotic effects via up-regulating p21WAF1, whereas down-regulating CDK4, MMP2, and MMP9 (Dong and Li, 2021).
4.7 Other reported pathways
In addition to the apoptotic pathways mentioned above, there are NPs reported to exert pro-apoptotic effects on EC through other mechanisms.
4.7.1 Extracts from NPs
In 2000, the Rice bran fraction (100, 200, 300 μg/ml) was reported to be a lipoprotein fraction that could induce apoptosis of the Sawano cells (Fan et al., 2000). In 2015, Tsai et al. studied the influence of Pogostemon cablin Aqueous Extract (PCAE, 0–4 mg/ml) on the induction of apoptosis. The results showed that PCAE induced apparent apoptosis in Ishikawa cells. In addition, further investigation revealed that the mechanism might be related to up-regulating BAG3,caspase-4, and caspase-5 (Tsai et al., 2015). In 2018, it was also reported that Tanshinone l (0–40 μM) could induce apoptosis and can increase ROS, bax. In contrast, downregulate BCL-2 and inhibit the phosphorylation of pSTAT1, pSTAT-2, pJAK1, and pJAk, inhibiting JAK/STAT pathway signal pathway and mitochondrial-mediated apoptosis in HEC-1-A cells (Li et al., 2018).
4.7.2 Monomers from NPs
In 2016, Wu et al. studied the inhibitory effect of Isoliquiritigenin (ISL, 5–100 μM), a licorice flavonoid, which was shown to could induce apoptosis and cell growth inhibition in vitro and in vivo via up-regulating caspase-3, caspase −7 and PARP (Wu C.-H. et al., 2016). Later in 2019, Shi et al. found that the Silibinin (SB, 100, 150, 200 μM), extracted from milk thistle seeds, can significantly inhibit the proliferation and promote apoptosis in a dose- and time-dependent manner via blocking pathways of STAT3 activation and SREBP1-mediated lipid accumulation, which is closely related to inhibiting STAT3, whereas decreasing BCL-2 and survivin (Shi et al., 2019). Lu et al., in 2019 recorded that Osthole (50, 100, 200 μM) could induce apoptosis in the Ishikawa and KLE cells, and after Osthole treatment,caspase-3, -9,miR-424 were significantly upregulated, and the CPEB2 were downregulated (Lu et al., 2020). A report in 2021 by Bulbul et al. studied the effect of Gallic Acid (3,4,5-tri hydroxybenzoic acid; GA; 5–100 μg/ml) and found it could induce apoptosis in Ishikawa cells by mitochondrial pathway via up-regulating caspase-3 (Bulbul et al., 2021). In 2021, Jiang reported that Esculetin (0–120 μM) could result in apoptosis and an arrest in proliferation in the HEC-1B, Ishikawa cells, and can target hnRNPA1, thereby downregulate the expression level BCL-XL, XIAP, and pAkt protein (Jiang R. et al., 2021). Recently, Hua et al. suggested that Silymarin (6 μg/ml) could induce apoptosis in Ishikawa cells via up-regulating caspase-3 (Chen et al., 2019).
5 Perspectives and conclusion
NPs are a wide range of bioactive components isolated from natural organisms, including plants, animals, insects, marine organisms (Manoharan and Perumal, 2022), and microorganisms. NPs are attractive sources for developing new medicinal and therapeutic agents (Thomford et al., 2018; Thompson and Lutsiv, 2023; Rao et al., 2019). For a long time, NPs have been regarded as a rich source of the active ingredients in new drugs. Moreover, the structural complexity and functional diversity of NPs are irreplaceable advantages compared to chemical drugs. These bioactive elements exert remarkable therapeutic effects on various diseases. NPs possess anti-cancer, anti-inflammatory, antioxidant, anti-bacterial, analgesic, anti-diabetic, and enzyme-inhibitory activities (Hassan et al., 2022). In recent years, the anti-cancer effects of NPs have drawn increasing attention (Hassan et al., 2022; Islam, 2022; Nuzzo et al., 2022), and we focus on their apoptosis-regulatory effect. Existing studies suggest that NPs can promote EC cell apoptosis through multiple pathways and thus exert anti-EC effects. Although existing research has reached a depth, some issues have not been well addressed and cannot be ignored to advance the development of NP-based anti-EC drugs.
First, the material basis of NPs for preventing and treating diseases is their active ingredients. Limited sources or meager amounts of bioactive molecules raw material is considered one of the most important obstacles to developing NPs into drugs. Many unexplored natural resources, especially uncultured marine organisms, will expand the sources of NPs because they can provide complex molecules with biologically active pharmacophores (Bilal and Iqbal, 2020). To better develop NPs, two different aspects may be involved: isolating additional structures directly from NPs, and modifying or improving these structures by chemical or biochemical methods (Li and Lou, 2018). These pathways may be helpful to facilitate the production of candidate molecules with lower costs, better efficacy, and less toxic side effects. Furthermore, the difficulty of extracting bioactive molecules is considered one of the significant obstacles to developing NPs into chemotherapeutic agents. Conventional extraction techniques frequently include preparatory fractionation of the parent material or crude extract, which limits their practical adoption on a large scale. Traditional extraction techniques also have other drawbacks, such as long extraction times, solvent purity issues, excessive solvent consumption and evaporation, shortened extraction yields, and thermal degradation of thermally degraded compounds. These limitations limit the development of NPs. Numerous modern extraction methods have been created and used, taking into account the structural and compositional characteristics of target sources, such as enzyme-assisted extraction (EAE), supercritical-fluid extraction (SFE), and microwave-assisted extraction (MAE), etc. (Bilal and Iqbal, 2020). Second, most studies have only focused on a single certain NP, and the combination of multiple NPs may help improve the efficacy and further explore the role of these NPs in the overall regulation of apoptosis, as well as their drug interrelationships. Third, the above studies were almost carried out via in vitro and in vivo approaches. Not all papers conducted in vivo experiments, so further investigation is suggested. Besides, the experimental data above almost explored a single pathway targeting the pro-apoptotic effects of NPs, and only a few NPs targeting cross-talk are available in studies. This may lead to the failure of drugs if this mechanism is interrupted or altered due to various cancer-related phenomena. This may also be a limitation and cause drug resistance to cancer. Clinical trials are also necessary to demonstrate whether the in vitro and in vivo animal data are reproduced in humans and to allow the application of NPs in cancer prevention and treatment. Most articles have analyzed their mechanism of action at the cellular and/or molecular level (Ekiert and Szopa, 2022). Fourth, NPs that are well tolerated and have less toxicity will help patients to achieve better treatment outcomes and improve their quality of life. The toxicity and pharmacokinetic selectivity of NPs should be further explored to validate their safety, which is a key step in the development of new drugs and can provide a strong basis for their translation to the clinic. Despite efforts to improve the therapeutic outcome for EC over the past decades, chemoresistance and side effects remain significant problems. The following clinical research stage must include a rational combination of agents that activate apoptotic signaling pathways and block pro-survival mechanisms while minimizing off-target toxicities. Furthermore, NPs that can treat various symptoms related to chemotherapeutics, such as nausea and vomiting, should be investigated. Exploration of the combination NPs with classical chemotherapeutic agents may be a possible way to enhance the susceptibility of cancer cells. Moreover, a study (Li et al., 2022) suggests that acupoint stimulation involves synergy with chemotherapy and can alleviate chemotherapeutic agents side effects.
NPs with anti-EC effects were classified and systematically organized by their inducing-apoptosis mechanisms and the sources in the review. The cell line, animal model, dose, efficacy, and mechanism of the NPs in each paper were covered clearly. The main related signal pathways are the mitochondrial-dependent apoptotic pathway, endoplasmic reticulum stress (ERS) mediated apoptotic pathway, mitogen-activated protein kinase (MAPK) mediated apoptotic pathway, NF-κB mediated apoptotic pathways, PI3K-Akt mediated apoptotic pathway, P21-mediated apoptotic pathway, and other reported pathways. In conclusion, we summarized the experiment-based molecular mechanisms and regulatory networks of NPs for EC. Hopefully, this review focuses on the importance of natural medicines in treating EC and provides a foundation for developing potential anti-EC drugs from natural therapies. There are more and more studies about NPs, and the depth of the research is increasing (Kirchmair, 2020; Naeem et al., 2022). NPs and their biological activities are currently a subject of great interest in the pharmaceutical (Ekiert and Szopa, 2020). Hopefully, the information presented in this review might be significant for further preclinical and clinical investigation.
Author contributions
All authors contributed to the article and approved the submitted version. JH, YZ, and YR conceived and designed this paper. XZ, YZ, RZ, YW, TL, SS, and SZ summarized and analyzed the data. XZ and YW drafted, revised, and edited the paper.
Funding
This research was supported by the Xinglin Scholar Research Promotion Project of Chengdu University of TCM (Grant no. QJRC2021019), and NSFC (National Natural Science Foundation of China, Grant no. 82004415).
Acknowledgments
We would like to acknowledge the assistance and contributions from our colleagues. I also would like to thank Xiang Zhang for helping me to export images on biorender.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1209412/full#supplementary-material
References
Abate, M., Festa, A., Falco, M., Lombardi, A., Luce, A., Grimaldi, A., et al. (2020). Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin. Cell Dev. Biol. 98, 139–153. doi:10.1016/j.semcdb.2019.05.022
Ahn, M. Y., Jung, J. H., Na, Y. J., and Kim, H. S. (2008). A natural histone deacetylase inhibitor, Psammaplin A, induces cell cycle arrest and apoptosis in human endometrial cancer cells. Gynecol. Oncol. 108, 27–33. doi:10.1016/j.ygyno.2007.08.098
Ai, Z., Yin, L., Zhou, X., Zhu, Y., Zhu, D., Yu, Y., et al. (2006). Inhibition of survivin reduces cell proliferation and induces apoptosis in human endometrial cancer. Cancer 107, 746–756. doi:10.1002/cncr.22044
Akazawa, M., and Hashimoto, K. (2022). Development and validation of machine learning models for the prediction of overall survival and cancer-specific survival in patients with endometrial cancer: An analysis of the surveillance, epidemiology, and end results (SEER) database by munetoshi Akazawa, kazunori Hashimoto SSRN. Available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4191367 (Accessed December 11, 2022).
Ali, A. K., Nandagopal, N., and Lee, S.-H. (2015). IL-15-PI3K-AKT-mTOR: A critical pathway in the life journey of natural killer cells. Front. Immunol. 6, 355. doi:10.3389/fimmu.2015.00355
An, J., Li, L., and Zhang, X. (2021). Curcusone C induces apoptosis in endometrial cancer cells via mitochondria-dependent apoptotic and ERK pathway. Biotechnol. Lett. 43, 329–338. doi:10.1007/s10529-020-03027-4
Anjum, J., Mitra, S., Das, R., Alam, R., Mojumder, A., Emran, T. B., et al. (2022). A renewed concept on the MAPK signaling pathway in cancers: Polyphenols as a choice of therapeutics. Mol. Cell Biochem. 477, 106398. doi:10.1016/j.phrs.2022.106398
Armstrong, A. J., Hurd, W. W., Elguero, S., Barker, N. M., and Zanotti, K. M. (2012). Diagnosis and management of endometrial hyperplasia. J. Minim. Invasive Gynecol. 19, 562–571. doi:10.1016/j.jmig.2012.05.009
Ashkenazi, A. (2008). Targeting the extrinsic apoptosis pathway in cancer. Cytokine & Growth Factor Rev. 19, 325–331. doi:10.1016/j.cytogfr.2008.04.001
Atanasov, A. G., Zotchev, S. B., Dirsch, V. M., and Supuran, C. T. (2021). Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 20, 200–216. doi:10.1038/s41573-020-00114-z
Avrutsky, M. I., and Troy, C. M. (2021). Caspase-9: A multimodal therapeutic target with diverse cellular expression in human disease. Front. Pharmacol. 12, 701301. doi:10.3389/fphar.2021.701301
Bilal, M., and Iqbal, H. M. N. (2020). Biologically active macromolecules: Extraction strategies, therapeutic potential and biomedical perspective. Int. J. Biol. Macromol. 151, 1–18. doi:10.1016/j.ijbiomac.2020.02.037
Blanco, J., Cameirao, C., López, M. C., and Muñoz-Barroso, I. (2020). Phosphatidylinositol-3-kinase-Akt pathway in negative-stranded RNA virus infection: A minireview. Arch. Virol. 165, 2165–2176. doi:10.1007/s00705-020-04740-1
Bratton, S. B., and Salvesen, G. S. (2010). Regulation of the apaf-1-caspase-9 apoptosome. J. Cell Sci. 123, 3209–3214. doi:10.1242/jcs.073643
Braun, M. M., Overbeek-Wager, E. A., and Grumbo, R. J. (2016). Diagnosis and management of endometrial cancer. Am. Fam. Physician 93, 468–474.
Brooks, R. A., Fleming, G. F., Lastra, R. R., Lee, N. K., Moroney, J. W., Son, C. H., et al. (2019). Current recommendations and recent progress in endometrial cancer. CA A Cancer J. Clin. 69, 258–279. doi:10.3322/caac.21561
Bulbul, M., Karabulut, S., Kalender, M., and Keskin, I. (2021). Effects of gallic acid on endometrial cancer cells in two and three dimensional cell culture models. Asian Pac J. Cancer Prev. 22, 1745–1751. doi:10.31557/APJCP.2021.22.6.1745
Cai, J., Huang, S., Yi, Y., and Bao, S. (2019). Downregulation of PTPN18 can inhibit proliferation and metastasis and promote apoptosis of endometrial cancer. Clin. Exp. Pharmacol. Physiol. 46, 734–742. doi:10.1111/1440-1681.13098
Carneiro, B. A., and El-Deiry, W. S. (2020). Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 17, 395–417. doi:10.1038/s41571-020-0341-y
Chalhoub, N., and Baker, S. J. (2009). PTEN and the PI3-kinase pathway in cancer. Annu. Rev. Pathol. Mech. Dis. 4, 127–150. doi:10.1146/annurev.pathol.4.110807.092311
Chang, C.-C., Hsu, H.-F., Huang, K.-H., Wu, J.-M., Kuo, S.-M., Ling, X.-H., et al. (2014). Anti-proliferative effects of Siegesbeckia orientalis ethanol extract on human endometrial RL-95 cancer cells. Molecules 19, 19980–19994. doi:10.3390/molecules191219980
Chaudhry, P., and Asselin, E. (2009). Resistance to chemotherapy and hormone therapy in endometrial cancer. Endocrine-Related Cancer 16, 363–380. doi:10.1677/ERC-08-0266
Chen, H.-Y., Cheng, W.-P., Chiang, Y.-F., Hong, Y.-H., Ali, M., Huang, T.-C., et al. (2021). Hinokitiol exhibits antitumor properties through induction of ROS-mediated apoptosis and p53-driven cell-cycle arrest in endometrial cancer cell lines (Ishikawa, HEC-1A, KLE). Int. J. Mol. Sci. 22, 8268. doi:10.3390/ijms22158268
Chen, J., Zhao, K.-N., Li, R., Shao, R., and Chen, C. (2014). Activation of PI3K/Akt/mTOR pathway and dual inhibitors of PI3K and mTOR in endometrial cancer. Curr. Med. Chem. 21, 3070–3080. doi:10.2174/0929867321666140414095605
Chen, S., Wu, Z., Ke, Y., Shu, P., Chen, C., Lin, R., et al. (2019). Wogonoside inhibits tumor growth and metastasis in endometrial cancer via ER stress-Hippo signaling axis. Acta Biochim. Biophys. Sin. (Shanghai) 51, 1096–1105. doi:10.1093/abbs/gmz109
Chuderland, D., and Seger, R. (2005). Protein–protein interactions in the regulation of the extracellular signal-regulated kinase. Mol. Biotechnol. 29, 57–74. doi:10.1385/MB:29:1:57
Chung, I., Yap, C., Subramaniam, K., and Khor, S. (2017). Annonacin exerts antitumor activity through induction of apoptosis and extracellular signal-regulated kinase inhibition. Phcog Res. 9, 378–383. doi:10.4103/pr.pr_19_17
Cincin, Z. B., Kiran, B., Baran, Y., and Cakmakoglu, B. (2018). Hesperidin promotes programmed cell death by downregulation of nongenomic estrogen receptor signalling pathway in endometrial cancer cells. Biomed. Pharmacother. 103, 336–345. doi:10.1016/j.biopha.2018.04.020
Clarke, H. J., Chambers, J. E., Liniker, E., Marciniak, S. J., Clarke, H. J., Chambers, J. E., et al. (2014). Endoplasmic reticulum stress in malignancy. Cancer Cell 25, 563–573. doi:10.1016/j.ccr.2014.03.015
Concin, N., Creutzberg, C. L., Vergote, I., Cibula, D., Mirza, M. R., Marnitz, S., et al. (2021). ESGO/ESTRO/ESP Guidelines for the management of patients with endometrial carcinoma. Virchows Arch. 478, 153–190. doi:10.1007/s00428-020-03007-z
Crosbie, E. J., Kitson, S. J., McAlpine, J. N., Mukhopadhyay, A., Powell, M. E., and Singh, N. (2022). Endometrial cancer. Lancet 399, 1412–1428. doi:10.1016/S0140-6736(22)00323-3
Darici, S., Alkhaldi, H., Horne, G., Jørgensen, H. G., Marmiroli, S., and Huang, X. (2020). Targeting PI3K/Akt/mTOR in AML: Rationale and clinical evidence. J. Clin. Med. 9, 2934. doi:10.3390/jcm9092934
Denley, A., Gymnopoulos, M., Kang, S., Mitchell, C., and Vogt, P. K. (2009). Requirement of phosphatidylinositol(3,4,5)Trisphosphate in phosphatidylinositol 3-kinase-induced oncogenic transformation. Mol. Cancer Res. 7, 1132–1138. doi:10.1158/1541-7786.MCR-09-0068
Dhillon, A. S., Hagan, S., Rath, O., and Kolch, W. (2007). MAP kinase signalling pathways in cancer. Oncogene 26, 3279–3290. doi:10.1038/sj.onc.1210421
Dong, Y., and Li, W. (2021). Effects of cinnamaldehyde on proliferation, apoptosis and invasion of endometrial carcinoma cell line Ishikawa (China). Prog. Anatomical Sci. 27, 248–251. doi:10.16695/j.cnki.1006-2947.2021.02.030
Dorstyn, L., Akey, C. W., and Kumar, S. (2018). New insights into apoptosome structure and function. Cell Death Differ. 25, 1194–1208. doi:10.1038/s41418-017-0025-z
Ediriweera, M. K., Tennekoon, K. H., and Samarakoon, S. R. (2019). Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Seminars Cancer Biol. 59, 147–160. doi:10.1016/j.semcancer.2019.05.012
Edmondson, R. J., Crosbie, E. J., Nickkho-Amiry, M., Kaufmann, A., Stelloo, E., Nijman, H. W., et al. (2017). Markers of the p53 pathway further refine molecular profiling in high-risk endometrial cancer: A trans portec initiative. Gynecol. Oncol. 146, 327–333. doi:10.1016/j.ygyno.2017.05.014
Ekiert, H. M., and Szopa, A. (2020). Biological activities of natural products. Molecules 25, 5769. doi:10.3390/molecules25235769
Ekiert, H. M., and Szopa, A. (2022). Biological activities of natural products II. Molecules 27, 1519. doi:10.3390/molecules27051519
El-Deiry, W. S., Tokino, T., Velculescu, V. E., Levy, D. B., Parsons, R., Trent, J. M., et al. (1993). WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825. doi:10.1016/0092-8674(93)90500-P
Eskander, R. N., Randall, L. M., Sakai, T., Guo, Y., Hoang, B., and Zi, X. (2012). Flavokawain B, a novel, naturally occurring chalcone, exhibits robust apoptotic effects and induces G2/M arrest of a uterine leiomyosarcoma cell line. J. Obstet. Gynaecol. Res. 38, 1086–1094. doi:10.1111/j.1447-0756.2011.01841.x
Fan, H., Morioka, T., and Ito, E. (2000). Induction of apoptosis and growth inhibition of cultured human endometrial adenocarcinoma cells (Sawano) by an antitumor lipoprotein fraction of rice bran. Gynecol. Oncol. 76, 170–175. doi:10.1006/gyno.1999.5669
Fattahi, S., Amjadi-Moheb, F., Tabaripour, R., Ashrafi, G. H., and Akhavan-Niaki, H. (2020). PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 262, 118513. doi:10.1016/j.lfs.2020.118513
Fisher, D. E. (1994). Apoptosis in cancer therapy: Crossing the threshold. Cell 78, 539–542. doi:10.1016/0092-8674(94)90518-5
Fonseca, B. M., Correia-da-Silva, G., and Teixeira, N. A. (2018). Cannabinoid-induced cell death in endometrial cancer cells: Involvement of TRPV1 receptors in apoptosis. J. Physiol. Biochem. 74, 261–272. doi:10.1007/s13105-018-0611-7
Fruman, D. (2008). Faculty Opinions recommendation of the p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma, 575678.
Geisler, J. P., Geisler, H. E., Wiemann, M. C., Zhou, Z., Miller, G. A., and Crabtree, W. (1999). p53 expression as a prognostic indicator of 5-year survival in endometrial cancer. Gynecol. Oncol. 74, 468–471. doi:10.1006/gyno.1999.5482
Green, D. R. (2022). The mitochondrial pathway of apoptosis Part II: The BCL-2 protein family. Cold Spring Harb. Perspect. Biol. 14, a041046. doi:10.1101/cshperspect.a041046
Hashem, S., Ali, T. A., Akhtar, S., Nisar, S., Sageena, G., Ali, S., et al. (2022). Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 150, 113054. doi:10.1016/j.biopha.2022.113054
Hassan, S. S. U., Abdel-Daim, M. M., Behl, T., and Bungau, S. (2022). Natural products for chronic diseases: A ray of hope. Molecules 27, 5573. doi:10.3390/molecules27175573
Hennessy, B. T., Smith, D. L., Ram, P. T., Lu, Y., Mills, G. B., Hennessy, B. T., et al. (2005). Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 4, 988–1004. doi:10.1038/nrd1902
Huang, C., and Hu, G. (2018). Shikonin suppresses proliferation and induces apoptosis in endometrioid endometrial cancer cells via modulating miR-106b/PTEN/AKT/mTOR signaling pathway. Biosci. Rep. 38, BSR20171546. doi:10.1042/BSR20171546
Huang, X., Yang, Z., Xie, Q., Zhang, Z., Zhang, H., and Ma, J. (2019). Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharmacother. 117, 109142. doi:10.1016/j.biopha.2019.109142
Huang, Y., Hou, Y., Qu, P., and Dai, Y. (2023). Editorial: Combating cancer with natural products: Non-coding RNA and RNA modification. Front. Pharmacol. 14, 1149777. doi:10.3389/fphar.2023.1149777
Hutt, S., Tailor, A., Ellis, P., Michael, A., Butler-Manuel, S., and Chatterjee, J. (2019). The role of biomarkers in endometrial cancer and hyperplasia: A literature review. Acta Oncol. 58, 342–352. doi:10.1080/0284186X.2018.1540886
Huvila, J., Talve, L., Carpén, O., Edqvist, P.-H., Pontén, F., Grénman, S., et al. (2013). Progesterone receptor negativity is an independent risk factor for relapse in patients with early stage endometrioid endometrial adenocarcinoma. Gynecol. Oncol. 130, 463–469. doi:10.1016/j.ygyno.2013.06.015
Ishii, T., Kira, N., Yoshida, T., and Narahara, H. (2013). Cucurbitacin D induces growth inhibition, cell cycle arrest, and apoptosis in human endometrial and ovarian cancer cells. Tumor Biol. 34, 285–291. doi:10.1007/s13277-012-0549-2
Islam, M. S. (2022). Natural products and disease prevention, relief and treatment. Nutrients 14, 2396. doi:10.3390/nu14122396
Jan, R., and Chaudhry, G.-S. (2019). Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 9, 205–218. doi:10.15171/apb.2019.024
Jiang, J., Zhou, N., Ying, P., Zhang, T., Liang, R., and Jiang, X. (2019). Emodin promotes apoptosis of human endometrial cancer through regulating the MAPK and PI3K/AKT pathways. Open Life Sci. 13, 489–496. doi:10.1515/biol-2018-0058
Jiang, M., Qi, L., Li, L., Wu, Y., Song, D., and Li, Y. (2021a). Caspase-8: A key protein of cross-talk signal way in “PANoptosis” in cancer. Int. J. Cancer 149, 1408–1420. doi:10.1002/ijc.33698
Jiang, R., Su, G., Chen, X., Chen, S., Li, Q., Xie, B., et al. (2021b). Esculetin inhibits endometrial cancer proliferation and promotes apoptosis via hnRNPA1 to downregulate BCLXL and XIAP. Cancer Lett. 521, 308–321. doi:10.1016/j.canlet.2021.08.039
Kantari, C., and Walczak, H. (2011). Caspase-8 and Bid: Caught in the act between death receptors and mitochondria. Biochimica Biophysica Acta 1813, 558–563. doi:10.1016/j.bbamcr.2011.01.026
Karia, P. S., Huang, Y., Tehranifar, P., Wright, J. D., Genkinger, J. M., Karia, P. S., et al. (2023). Racial and ethnic differences in type II endometrial cancer mortality outcomes: The contribution of sociodemographic, clinicopathologic, and treatment factors. Gynecol. Oncol. 168, 119–126. doi:10.1016/j.ygyno.2022.11.015
Karimian, A., Ahmadi, Y., Yousefi, B., Karimian, A., Ahmadi, Y., and Yousefi, B. (2016). Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 42, 63–71. doi:10.1016/j.dnarep.2016.04.008
Kashyap, D., Garg, V. K., and Goel, N. (2021). Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Adv. Protein Chem. Struct. Biol. 125, 73–120. doi:10.1016/bs.apcsb.2021.01.003
Kavandi, L., Lee, L. R., Bokhari, A. A., Pirog, J. E., Jiang, Y., Ahmad, K. A., et al. (2015). The Chinese herbsScutellaria baicalensisandFritillaria cirrhosatarget NFκB to inhibit proliferation of ovarian and endometrial cancer cells. Mol. Carcinog. 54, 368–378. doi:10.1002/mc.22107
Kikuchi, K., Haneda, M., Hayashi, S., Maeda, T., Nakano, N., Kuroda, Y., et al. (2022). P21 deficiency exhibits delayed endochondral ossification during fracture healing. Bone 165, 116572. doi:10.1016/j.bone.2022.116572
Kim, A., Ha, J., Kim, J., Cho, Y., Ahn, J., Cheon, C., et al. (2021). Natural products for pancreatic cancer treatment: From traditional medicine to modern drug discovery. Nutrients 13, 3801. doi:10.3390/nu13113801
Kim, E. K., and Choi, E.-J. (2010). Pathological roles of MAPK signaling pathways in human diseases. Biochimica Biophysica Acta 1802, 396–405. doi:10.1016/j.bbadis.2009.12.009
Kim, E. M., Jung, C.-H., Kim, J., Hwang, S.-G., Park, J. K., and Um, H.-D. (2017a). The p53/p21 complex regulates cancer cell invasion and apoptosis by targeting bcl-2 family proteins. Cancer Res. 77, 3092–3100. doi:10.1158/0008-5472.CAN-16-2098
Kim, J. H., Kim, M., Yun, S.-M., Lee, S., No, J. H., Suh, D. H., et al. (2017b). Ginsenoside Rh2 induces apoptosis and inhibits epithelial-mesenchymal transition in HEC1A and Ishikawa endometrial cancer cells. Biomed. Pharmacother. 96, 871–876. doi:10.1016/j.biopha.2017.09.033
Kim, J. Y., Lee, S. G., Chung, J.-Y., Kim, Y.-J., Park, J.-E., Koh, H., et al. (2011). Ellipticine induces apoptosis in human endometrial cancer cells: The potential involvement of reactive oxygen species and mitogen-activated protein kinases. Toxicology 289, 91–102. doi:10.1016/j.tox.2011.07.014
Kim, U., Kim, K. S., Park, J.-K., and Um, H.-D. (2022). Involvement of the p53/p21 complex in p53-dependent gene expression. Biochem. Biophysical Res. Commun. 621, 151–156. doi:10.1016/j.bbrc.2022.07.022
Kirchmair, J. (2020). Molecular informatics in natural products research. Mol. Inf. 39, e2000206. doi:10.1002/minf.202000206
Kohlberger, P., Gitsch, G., Loesch, A., Tempfer, C., Kaider, A., Reinthaller, A., et al. (1996). p53 protein overexpression in early stage endometrial cancer. Gynecol. Oncol. 62, 213–217. doi:10.1006/gyno.1996.0218
Lee, J.-S., Ahn, J.-H., Cho, Y.-J., Kim, H.-Y., Yang, Y.-I., Lee, K.-T., et al. (2015). α-Terthienylmethanol, isolated from Eclipta prostrata, induces apoptosis by generating reactive oxygen species via NADPH oxidase in human endometrial cancer cells. J. Ethnopharmacol. 169, 426–434. doi:10.1016/j.jep.2015.04.029
Lei, X., Guo, J., Wang, Y., Cui, J., Feng, B., Su, Y., et al. (2019). Inhibition of endometrial carcinoma by Kaempferol is interceded through apoptosis induction, G2/M phase cell cycle arrest, suppression of cell invasion and upregulation of m-TOR/PI3K signalling pathway. J. BUON 24, 1555–1561.
Li, F.-R., Yu, F.-X., Yao, S.-T., Si, Y.-H., Zhang, W., and Gao, L.-L. (2012). Hyperin extracted from manchurian Rhododendron leaf induces apoptosis in human endometrial cancer cells through a mitochondrial pathway. Asian Pac. J. Cancer Prev. 13, 3653–3656. doi:10.7314/apjcp.2012.13.8.3653
Li, G., and Lou, H.-X. (2018). Strategies to diversify natural products for drug discovery. Med. Res. Rev. 38, 1255–1294. doi:10.1002/med.21474
Li, P., Zhou, L., Zhao, T., Liu, X., Zhang, P., Liu, Y., et al. (2017). Caspase-9: Structure, mechanisms and clinical application. Oncotarget 8, 23996–24008. doi:10.18632/oncotarget.15098
Li, Q., Zhang, J., Liang, Y., Mu, W., Hou, X., Ma, X., et al. (2018). Tanshinone l exhibits anticancer effects in human endometrial carcinoma HEC-1-A cells via mitochondrial mediated apoptosis, cell cycle arrest and inhibition of JAK/STAT signalling pathway. J. BUON 23, 1092–1096.
Li, S., Zhao, S., Guo, Y., Yang, Y., Huang, J., Wang, J., et al. (2022). Clinical efficacy and potential mechanisms of acupoint stimulation combined with chemotherapy in combating cancer: A review and prospects. Front. Oncol. 12, 864046. doi:10.3389/fonc.2022.864046
Li, W., Tian, L., and Liu, J. (2021). The effects of zedoary turmeric Oil on proliferation and apoptosis and expressions of caspase-3 and bax and bcl-2 in HEC-1B (China). Henan Tradit. Chin. Med. 41, 384–387.
Li, Z.-L., Morishima, S., Tang, J.-T., and Otsuki, Y. (2009). Apoptotic effects of Tian-Long compound on endometrial adenocarcinoma cells in vitro. Med. Mol. Morphol. 42, 32–39. doi:10.1007/s00795-008-0424-9
Liang, L., Yang, B., Wu, Y., and Sun, L. (2021). Osthole suppresses the proliferation and induces apoptosis via inhibiting the PI3K/AKT signaling pathway of endometrial cancer JEC cells. Exp. Ther. Med. 22, 1171. doi:10.3892/etm.2021.10605
Liu, C., Zeng, Y., Wen, Y., Huang, X., and Liu, Y. (2022). Natural products modulate cell apoptosis: A promising way for the treatment of ulcerative colitis. Front. Pharmacol. 13, 806148. doi:10.3389/fphar.2022.806148
Liu, Y., Whelan, R. J., Pattnaik, B. R., Ludwig, K., Subudhi, E., Rowland, H., et al. (2012). Terpenoids from Zingiber officinale (Ginger) induce apoptosis in endometrial cancer cells through the activation of p53. PLoS One 7, e53178. doi:10.1371/journal.pone.0053178
Lu, K. H., and Broaddus, R. R. (2020). Endometrial cancer. N. Engl. J. Med. 383, 2053–2064. doi:10.1056/NEJMra1514010
Lu, K., Lin, J., and Jiang, J. (2020). Osthole inhibited cell proliferation and induced cell apoptosis through decreasing CPEB2 expression via up-regulating miR-424 in endometrial carcinoma. J. Recept. Signal Transduct. 40, 89–96. doi:10.1080/10799893.2019.1710846
Malladi, S., Challa-Malladi, M., Fearnhead, H. O., and Bratton, S. B. (2009). The Apaf-1·procaspase-9 apoptosome complex functions as a proteolytic-based molecular timer. EMBO J. 28, 1916–1925. doi:10.1038/emboj.2009.152
Man, G. C. W., Wang, J., Song, Y., Wong, J. H., Zhao, Y., Lau, T. S., et al. (2020). Therapeutic potential of a novel prodrug of green tea extract in induction of apoptosis via ERK/JNK and Akt signaling pathway in human endometrial cancer. BMC Cancer 20, 964. doi:10.1186/s12885-020-07455-3
Mandal, R., Barrón, J. C., Kostova, I., Becker, S., and Strebhardt, K. (2020). Caspase-8: The double-edged sword. Biochimica Biophysica Acta 1873, 188357. doi:10.1016/j.bbcan.2020.188357
Manoharan, S., and Perumal, E. (2022). Potential role of marine bioactive compounds in cancer signaling pathways: A review. Eur. J. Pharmacol. 936, 175330. doi:10.1016/j.ejphar.2022.175330
Marchetti, A., Doglioni, C., Barbareschi, M., Buttitta, F., Pellegrini, S., Bertacca, G., et al. (1996). P21 RNA and protein expression in non-small cell lung carcinomas: Evidence of p53-independent expression and association with tumoral differentiation. Oncogene 12, 1319–1324.
Marciniak, S. J. (2019). Endoplasmic reticulum stress: A key player in human disease. FEBS J. 286, 228–231. doi:10.1111/febs.14740
Martínez-García, D., Manero-Rupérez, N., Quesada, R., Korrodi-Gregório, L., and Soto-Cerrato, V. (2019). Therapeutic strategies involving survivin inhibition in cancer. Med. Res. Rev. 39, 887–909. doi:10.1002/med.21547
McComb, S., Chan, P. K., Guinot, A., Hartmannsdottir, H., Jenni, S., Dobay, M. P., et al. (2019). Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7. Sci. Adv. 5, eaau9433. doi:10.1126/sciadv.aau9433
Mirakhor Samani, S., Ezazi Bojnordi, T., Zarghampour, M., Merat, S., Fouladi, D. F., Mirakhor Samani, S., et al. (2018). Expression of p53, bcl-2 and bax in endometrial carcinoma, endometrial hyperplasia and normal endometrium: A histopathological study. J. Obstetrics Gynaecol. 38, 999–1004. doi:10.1080/01443615.2018.1437717
Miricescu, D., Totan, A., Stanescu-Spinu, I.-I., Badoiu, S. C., Stefani, C., and Greabu, M. (2020). PI3K/AKT/mTOR signaling pathway in breast cancer: From molecular landscape to clinical aspects. Int. J. Mol. Sci. 22, 173. doi:10.3390/ijms22010173
Mirza-Aghazadeh-Attari, M., Ekrami, E. M., Aghdas, S. A. M., Mihanfar, A., Hallaj, S., Yousefi, B., et al. (2020). Targeting PI3K/Akt/mTOR signaling pathway by polyphenols: Implication for cancer therapy. Life Sci. 255, 117481. doi:10.1016/j.lfs.2020.117481
Moore, K., and Brewer, M. A. (2017). Endometrial cancer: Is this a new disease? Am. Soc. Clin. Oncol. Educ. Book 37, 435–442. doi:10.1200/EDBK_175666
Naeem, A., Hu, P., Yang, M., Zhang, J., Liu, Y., Zhu, W., et al. (2022). Natural products as anticancer agents: Current status and future perspectives. Molecules 27, 8367. doi:10.3390/molecules27238367
Nair, P., Lu, M., Petersen, S., and Ashkenazi, A. (2014). “Chapter five - apoptosis initiation through the cell-extrinsic pathway,” in Methods in enzymology regulated cell death Part A: Apoptotic mechanisms. Editors A. Ashkenazi, J. Yuan, and J. A. Wells (Cambridge: Academic Press), 99–128.
Narita, M., Ohnishi, O., Nemoto, M., Yajima, Y., and Suzuki, T. (2002). Implications of phosphoinositide 3-kinase in the μ- and δ-opioid receptor-mediated supraspinal antinociception in the mouse. Neuroscience 113, 647–652. doi:10.1016/S0306-4522(02)00197-5
Nuzzo, G., Senese, G., Gallo, C., Albiani, F., Romano, L., d’Ippolito, G., et al. (2022). Antitumor potential of immunomodulatory natural products. Mar. Drugs 20, 386. doi:10.3390/md20060386
Oakes, S. A., Papa, F. R., Oakes, S. A., and Papa, F. R. (2015). The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. Mech. Dis. 10, 173–194. doi:10.1146/annurev-pathol-012513-104649
Otsuki, Y. (2001). Apoptosis in human endometrium: Apoptotic detection methods and signaling. Med. Electron Microsc. 34, 166–173. doi:10.1007/s007950100011
Passarello, K., Kurian, S., and Villanueva, V. (2019). Endometrial cancer: An overview of pathophysiology, management, and care. Seminars Oncol. Nurs. 35, 157–165. doi:10.1016/j.soncn.2019.02.002
Piddock, R., Bowles, K., and Rushworth, S. (2017). The role of PI3K isoforms in regulating bone marrow microenvironment signaling focusing on acute myeloid leukemia and multiple myeloma. Cancers 9, 29. doi:10.3390/cancers9040029
Pothongsrisit, S., and Pongrakhananon, V. (2021). Targeting the PI3K/AKT/mTOR signaling pathway in lung cancer: An update regarding potential drugs and natural products. Molecules 26, 4100. doi:10.3390/molecules26134100
Qin, H., Srinivasula, S. M., Wu, G., Fernandes-Alnemri, T., Alnemri, E. S., and Shi, Y. (1999). Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature 399, 549–557. doi:10.1038/21124
Rao, T., Tan, Z., Peng, J., Guo, Y., Chen, Y., Zhou, H., et al. (2019). The pharmacogenetics of natural products: A pharmacokinetic and pharmacodynamic perspective. Pharmacol. Res. 146, 104283. doi:10.1016/j.phrs.2019.104283
Ricci, M. S., and El-Deiry, W. S. (2007). “The extrinsic pathway of apoptosis,” in Apoptosis, senescence, and cancer (New York City: Springer), 31–54.
Rodríguez-Palacios, D. Á., Colorado-Yohar, S. M., Velten, M., Vaamonde-Martín, R. J., Ballesta, M., Chirlaque, M.-D., et al. (2022). Incidence and trend of type I and II endometrial cancer in women from two population-based European cancer registries (1998–2012). Int. J. Environ. Res. Public Health 19, 3789. doi:10.3390/ijerph19073789
Ron, D., and Walter, P. (2007). Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529. doi:10.1038/nrm2199
Santucci, R., Sinibaldi, F., Cozza, P., Polticelli, F., and Fiorucci, L. (2019). Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 136, 1237–1246. doi:10.1016/j.ijbiomac.2019.06.180
Sexton, É., Van Themsche, C., Leblanc, K., Parent, S., Lemoine, P., and Asselin, E. (2006). Resveratrol interferes with AKT activity and triggers apoptosis in human uterine cancer cells. Mol. Cancer 5, 45. doi:10.1186/1476-4598-5-45
Shanmugam, M. K., Lee, J. H., Chai, E. Z. P., Kanchi, M. M., Kar, S., Arfuso, F., et al. (2016). Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Seminars Cancer Biol. 40–41, 35–47. doi:10.1016/j.semcancer.2016.03.005
Shi, Z., Zhou, Q., Gao, S., Li, W., Li, X., Liu, Z., et al. (2019). Silibinin inhibits endometrial carcinoma via blocking pathways of STAT3 activation and SREBP1-mediated lipid accumulation. Life Sci. 217, 70–80. doi:10.1016/j.lfs.2018.11.037
Singh, S., Pavuluri, S., Jyothi Lakshmi, B., Biswa, B. B., Venkatachalam, B., Tripura, C., et al. (2020). Molecular characterization of Wdr13 knockout female mice uteri: A model for human endometrial hyperplasia. Sci. Rep. 10, 14621. doi:10.1038/s41598-020-70773-w
Slomovitz, B. M., and Coleman, R. L. (2012). The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin. Cancer Res. 18, 5856–5864. doi:10.1158/1078-0432.CCR-12-0662
Sujobert, P., Sujobert, P., Cornillet-Lefebvre, P., Hayflick, J. S., Prie, N., Verdier, F., et al. (2005). Essential role for the p110delta isoform in phosphoinositide 3-kinase activation and cell proliferation in acute myeloid leukemia. Blood 106, 1063–1066. doi:10.1182/blood-2004-08-3225
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Takai, N., Ueda, T., Nishida, M., Nasu, K., and Narahara, H. (2008). Bufalin induces growth inhibition, cell cycle arrest and apoptosis in human endometrial and ovarian cancer cells. Int. J. Mol. Med. 21, 637–643. doi:10.3892/ijmm.21.5.637
Tan, H., Chu, G., Hu, C., and Zhang, E. (2016). Effects of Panaxnotoginsengsaponins on proliferation, invasion, apoptosis of endometrial cancercell lines Ishikawa and HEC-1A (China). China Med. Her. 13, 13–16.
Terzic, M., Aimagambetova, G., Kunz, J., Bapayeva, G., Aitbayeva, B., Terzic, S., et al. (2021). Molecular basis of endometriosis and endometrial cancer: Current Knowledge and future perspectives. Int. J. Mol. Sci. 22, 9274. doi:10.3390/ijms22179274
Thomford, N., Senthebane, D., Rowe, A., Munro, D., Seele, P., Maroyi, A., et al. (2018). Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 19, 1578. doi:10.3390/ijms19061578
Thompson, H. J., and Lutsiv, T. (2023). Natural products in precision oncology: Plant-based small molecule inhibitors of protein kinases for cancer chemoprevention. Nutrients 15, 1192. doi:10.3390/nu15051192
Tong, J.-S., Zhang, Q.-H., Huang, X., Fu, X.-Q., Qi, S.-T., Wang, Y.-P., et al. (2011). Icaritin causes sustained ERK1/2 activation and induces apoptosis in human endometrial cancer cells. PLoS ONE 6, e16781. doi:10.1371/journal.pone.0016781
Torquato, H., Goettert, M., Justo, G., and Paredes-Gamero, E. (2017). Anti-cancer phytometabolites targeting cancer stem cells. Curr. Genomics 18, 156–174. doi:10.2174/1389202917666160803162309
Tsai, C.-C., Chang, Y.-H., Chang, C.-C., Cheng, Y.-M., Ou, Y.-C., Chien, C.-C., et al. (2015). Induction of apoptosis in endometrial cancer (Ishikawa) cells by Pogostemon cablin aqueous extract (PCAE). Int. J. Mol. Sci. 16, 12424–12435. doi:10.3390/ijms160612424
Urick, M. E., and Bell, D. W. (2019). Clinical actionability of molecular targets in endometrial cancer. Nat. Rev. Cancer 19, 510–521. doi:10.1038/s41568-019-0177-x
Wang, D., Tian, Y., Feng, W., Zhao, L., Zhao, M., Liu, J., et al. (2017). Pseudolaric acid B induces endometrial cancer Ishikawa cell apoptosis and inhibits metastasis through AKT-GSK-3β and ERK1/2 signaling pathways. Anti-Cancer Drugs 28, 603–612. doi:10.1097/CAD.0000000000000500
Wang, F., and Roh, Y. S. (2020). Mitochondrial connection to ginsenosides. Arch. Pharm. Res. 43, 1031–1045. doi:10.1007/s12272-020-01279-2
Wang, H., Liu, Z., Gou, Y., Qin, Y., Xu, Y., Liu, J., et al. (2015). Apoptosis and necrosis induced by novel realgar quantum dots in human endometrial cancer cells via endoplasmic reticulum stress signaling pathway. Int. J. Nanomedicine 10, 5505–5512. doi:10.2147/IJN.S83838
Wang, M., and Kaufman, R. J. (2016). Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529, 326–335. doi:10.1038/nature17041
Wang, X.-F., Zhao, Y.-B., Wu, Q., Sun, Z.-H., and Li, H.-J. (2014). Triptolide induces apoptosis in endometrial cancer via a p53-independent mitochondrial pathway. Mol. Med. Rep. 9, 39–44. doi:10.3892/mmr.2013.1783
Wang, Y., Wang, K., Jin, Y., and Sheng, X. (2019). Endoplasmic reticulum proteostasis control and gastric cancer. Cancer Lett. 449, 263–271. doi:10.1016/j.canlet.2019.01.034
Wolf, P., Schoeniger, A., and Edlich, F. (2022). Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane. Biochim. Biophys. Acta Mol. Cell Res. 1869, 119317. doi:10.1016/j.bbamcr.2022.119317
Wu, C.-H., Chen, H.-Y., Wang, C.-W., Shieh, T.-M., Huang, T.-C., Lin, L.-C., et al. (2016a). Isoliquiritigenin induces apoptosis and autophagy and inhibits endometrial cancer growth in mice. Oncotarget 7, 73432–73447. doi:10.18632/oncotarget.12369
Wu, F.-L., Liu, W.-Y., Van Poucke, S., Braddock, M., Jin, W.-M., Xiao, J., et al. (2016b). Targeting endoplasmic reticulum stress in liver disease. Expert Rev. Gastroenterology Hepatology 10, 1041–1052. doi:10.1080/17474124.2016.1179575
Xu, H., Gong, Z., Zhou, S., Yang, S., Wang, D., Chen, X., et al. (2018). Liposomal Curcumin targeting endometrial cancer through the NF-κB pathway. Cell Physiol. Biochem. 48, 569–582. doi:10.1159/000491886
Xu, H., Zhang, J., Wang, Q., Li, Y., and Zhang, B. (2020). Effect of resveratrol on the expression of molecules related to the mTOR signaling pathway in endometrial cancer. Chin. J. Birth Health Hered. 28, 533–536+577. doi:10.13404/j.cnki.cjbhh.2020.05.003
Ye, Wenwei, Pan, Dan, and Yang, Jingjin (2020). Effects of Amygdalin on proliferation, invasion and apoptosis of endometrial cancer cells through inhibiting PI3K/Akt/mTOR pathway (China). Zhejiang JITCWM 30, 795–799.
Yin, W. (2016). Effects and mechanism of Shikonin on the proliferation and apoptosis of endometrial Ishikawa cancer cell (China). Mod. J. Integr. Traditional Chin. West. Med. 25, 3548–3551.
Yip, P. Y. (2015). Phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin (PI3K-Akt-mTOR) signaling pathway in non-small cell lung cancer. Transl. Lung Cancer Res. 4, 165–176. doi:10.3978/j.issn.2218-6751.2015.01.04
Yuan, Y., Long, H., Zhou, Z., Fu, Y., and Jiang, B. (2023). PI3K–AKT-Targeting breast cancer treatments: Natural products and synthetic compounds. Biomolecules 13, 93. doi:10.3390/biom13010093
Zhang, F., Zhang, Y.-Y., Sun, Y.-S., Ma, R.-H., Thakur, K., Zhang, J.-G., et al. (2020). Asparanin A from Asparagus officinalis L. Induces G0/G1 cell cycle arrest and apoptosis in human endometrial carcinoma Ishikawa cells via mitochondrial and PI3K/AKT signaling pathways. J. Agric. Food Chem. 68, 213–224. doi:10.1021/acs.jafc.9b07103
Zhang, S., Gong, T.-T., Liu, F.-H., Jiang, Y.-T., Sun, H., Ma, X.-X., et al. (2019a). Global, regional, and national burden of endometrial cancer, 1990–2017: Results from the global burden of disease study, 2017. Front. Oncol. 9, 1440. doi:10.3389/fonc.2019.01440
Zhang, S., Rao, S., Yang, M., Ma, C., Hong, F., and Yang, S. (2022). Role of mitochondrial pathways in cell apoptosis during He-patic ischemia/reperfusion injury. Int. J. Mol. Sci. 23, 2357. doi:10.3390/ijms23042357
Keywords: natural products, endometrial cancer, apoptosis, signal pathway, anti-cancer effects
Citation: Zhou X, Zeng Y, Zheng R, Wang Y, Li T, Song S, Zhang S, Huang J and Ren Y (2023) Natural products modulate cell apoptosis: a promising way for treating endometrial cancer. Front. Pharmacol. 14:1209412. doi: 10.3389/fphar.2023.1209412
Received: 20 April 2023; Accepted: 30 May 2023;
Published: 08 June 2023.
Edited by:
Ayaz Shahid, Western University of Health Sciences, United StatesReviewed by:
Suryaa Manoharan, Bharathiar University, IndiaMuhammad Khan, University of the Punjab, Pakistan
Copyright © 2023 Zhou, Zeng, Zheng, Wang, Li, Song, Zhang, Huang and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinzhu Huang, huangjinzhu@cdutcm.edu.cn; Yulan Ren, renxg2468@163.com
†These authors have contributed equally to this work and share first authorship
 Xin Zhou
Xin Zhou