- 1Department of Pathology, China-Janpan Friendship Hospital, Beijing, China
- 2Department of Pathology, Qinghai Provincial People’s Hospital, Xining, China
- 3Department of Oncology, Qinghai Provincial People’s Hospital, Xining, China
- 4Geneplus-Beijing, Beijing, China
Background: Anaplastic lymphoma kinase (ALK) rearrangement, the most common oncogenic rearrangement in lung adenocarcinoma, occurs in approximately 5% of non-small cell lung cancer (NSCLC) patients. EML4 gene is the most common partner of ALK rearrangement, and distinct EML4-ALK fusions differ in their responsiveness to ALK tyrosine kinase inhibitors. However, the concurrence of two ALK rearrangements in one patient and whose response to ALK-TKIs have rarely been reported so far.
Case presentation: A 47-year-old Chinese male was diagnosed with stage IV lung adenocarcinoma with multiple intracranial metastases and adrenal metastasis. After progression of two lines of chemotherapy combined with local radiotherapy regimens, his tumor tissue sample was sent to perform the DNA-based next-generation sequencing of 116 genes. Surprisingly, EML4-ALK (E13:A20) fusion and a novel SV2B-ALK (S6:A20) fusion were concurrently identified, which was confirmed using immunohistochemistry and fluorescence in-situ hybridization. Given the superior efficacy of alectinib, the patient received alectinib in the third-line setting with the progression-free survival over 14 months up to now. Moreover, through comprehensive review of previous literatures, a total of 22 patients with multiple ALK fusions and their response to ALK-TKIs were summarized.
Conclusion: This is the first report of a NSCLC patient with a novel SV2B-ALK, EML4-ALK double-fusion benefiting from alectinib. Alectinib may be an effective therapeutic option for both primary and metastatic lesions including brain metastases in the late-line setting in NSCLC patients with double-ALK fusion.
1 Introduction
Lung cancer is the leading cause of cancer-related mortality, among which non-small cell lung cancer (NSCLC) is the most predominant type (1). ALK rearrangement was found in 3% to 7% of NSCLC patients in previous studies and EML4 gene is the most common ALK rearrangement partner (2). Multiple ALK tyrosine kinase inhibitors (TKIs) have been proven to greatly improve the clinical outcome of ALK-rearranged NSCLC patients (3, 4). Currently, more and more ALK fusions have been reported with the wide application of comprehensive next-generation sequencing (NGS) (2, 5). However, reports on patients harboring double ALK fusions simultaneously were still rare, and the effectiveness of ALK-TKIs in these patients was also barely reported. Herein, we presented one NSCLC patient with extensive metastases and a novel synaptic vesicle protein 2B (SV2B) - ALK and EML4 - ALK double-fusion. This patient responded well to alectinib in the third-line setting with the progression-free survival (PFS) exceeding 14 months up to now. Moreover, the previous reports of ALK double fusions were summarized to facilitate clinicians to acquire the clinical evidences and make clinical decisions for these even rare patients with ALK double fusions.
2 Case presentation
A 47-year-old Chinese male with the smoking history of 30 pack-year came to Qinghai Provincial People’s Hospital complaining of coughing and expectoration. Chest computed tomography (CT) showed 4.0×3.3 cm mass in the lower lobe of the right lung with metastasis to hilum of right lung, mediastinal lymph node and supraclavicular lymph node. Magnetic resonance imaging (MRI) scans indicated multiple intracranial metastases and adrenal metastasis. Transbronchial biopsy under fiberscope revealed poorly differentiated adenocarcinoma (cT3N2M1c, stage IVb, Figures 1A, B). EGFR wild type was identified in the biopsy tumor tissue through DNA-based PCR. The mutations of other driver genes, including KRAS, ALK and ROS1, were not detected. This patient received chemotherapy (cisplatin/pemetrexed/bevacizumab) for 8 cycles and radiotherapy were added in lung metastases (60Gy/2Gy/30F), intracranial metastases (3000cGy/10Fx) and adrenal metastasis (6000cGy/30Fx). Progression occurred after 18 months of treatment and albumin-bound paclitaxel was administered as second-line chemotherapy. After 1 cycle of chemotherapy, the patient’s symptoms of chest tightness and shortness of breath were aggravated, and CT scan revealed enlargement of the lesions in right lung and bilateral adrenal metastases.
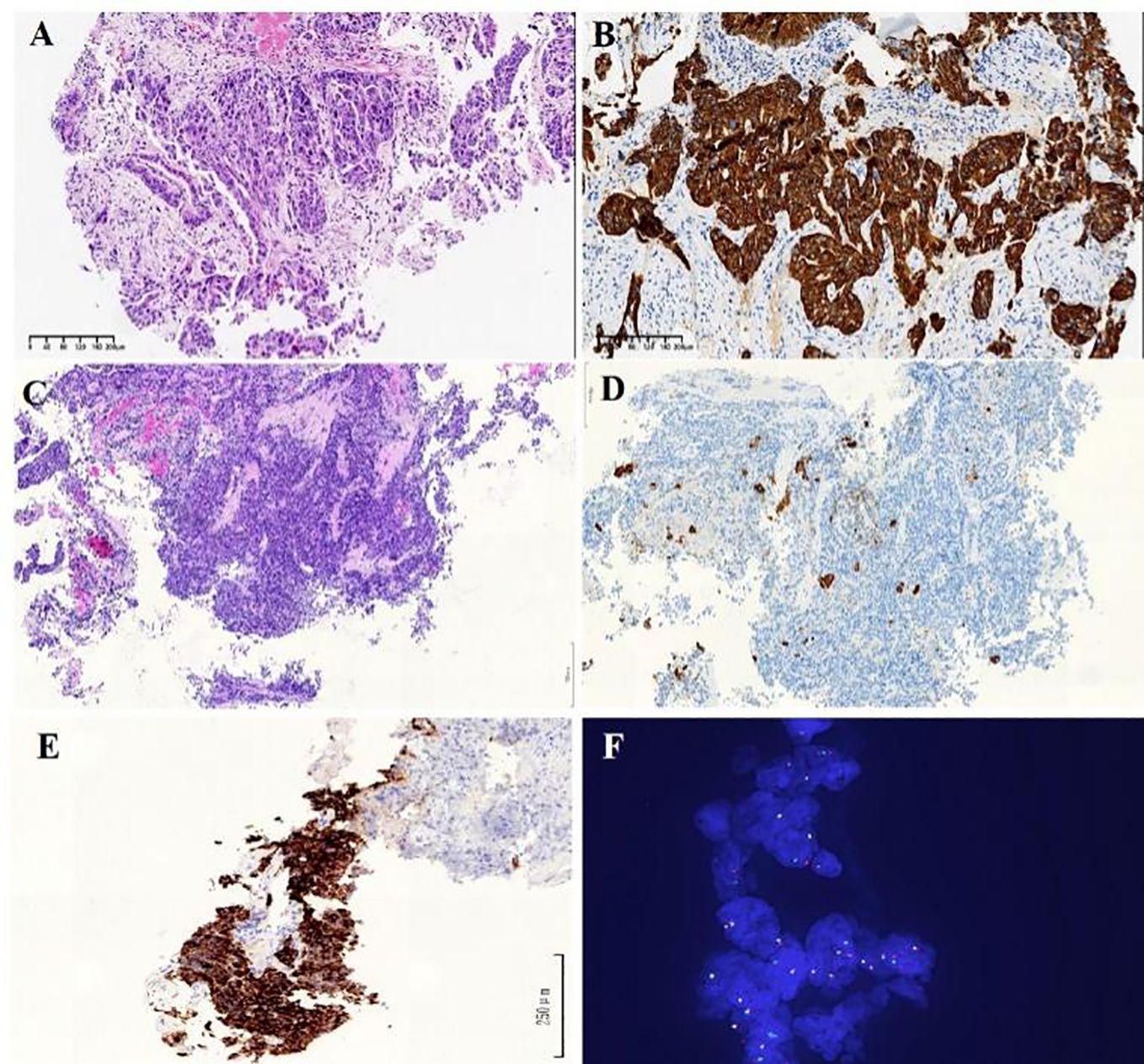
Figure 1. (A) HE staining of transbronchial biopsy under fiberscopic examination indicates poorly differentiated NSCLC Diagnosis of lung adenocarcinoma. (B) Immunohistochemistry analysis revealed immunoreactivity to CK7 (×100). (C) HE staining of re-biopsy of the lesion show as solid-pattern adenocarcinoma. (D) Immunohistochemistry analysis revealed immunoreactivity to CK7 (×100). (E) Immunohistochemistry staining showed strong ALK receptor tyrosine kinase protein expression in the re-biopsy tissue (×200). (F) Fluorescent in situ hybridization showed rearranged ALK gene through ALK gene isolation probe (×100).
A re-biopsy of the growing lung lesion was performed and pathological analysis confirmed it as solid-pattern adenocarcinoma. Immunohistochemistry analysis was positive for thyroid transcription factor 1, NapsinA, and 30% for Ki-67, but negative for chromograin A, CD56, and synaptophysin (Figures 1C, D). The tissue sample was also sent to perform the DNA-based NGS (Amoy Diagnostics, Xiamen, China) using a gene panel comprising of 116 lung cancer-related genes. Two ALK rearrangements, including EML4-ALK (E13:A20) and a novel SV2B-ALK (S6:A20), were concurrently identified with the abundance of 43.81% and 41.01%, respectively (Figure 2). Besides, a synonymous mutation in exon 4 of TP53 (c.375G>A, allelic frequency: 27.32%) and a nonsense mutation in exon 2 of CDKN2A (c.358G>T, allelic frequency: 20.64%) were also detected. To confirm the presence of ALK fusion, the expression of ALK protein was evaluated using a rabbit monoclonal antibody (Ventana D5F3, ROCHE, China) on a benchmark system (Figure 1E), which reveals strong expression of ALK protein in the lung lesion. Fluorescent in situ hybridization (FISH) through ALK gene isolation probe also confirmed the ALK rearrangement (Figure 1F).
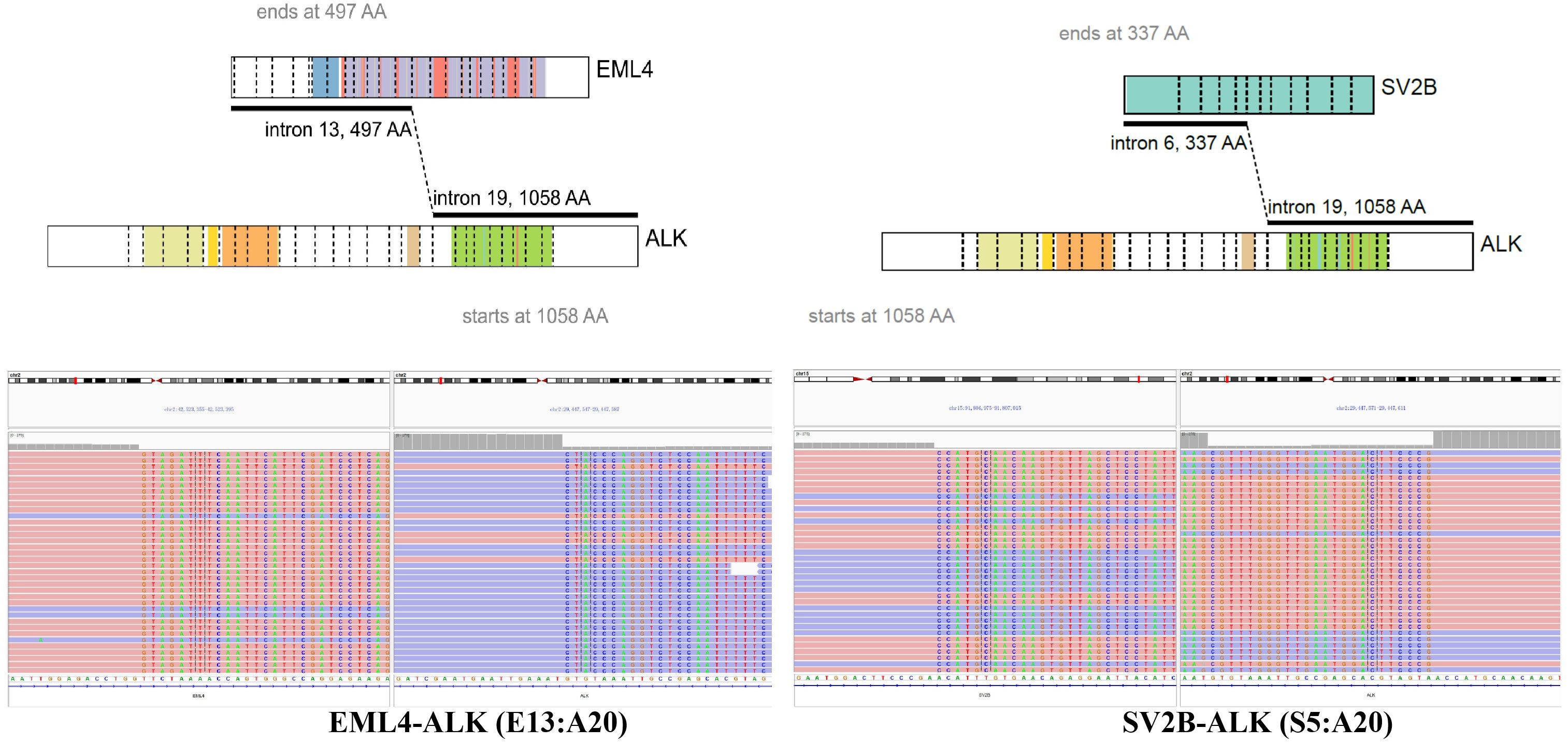
Figure 2. Identification of SV2B-ALK and EML4-ALK double-fusion. ALK, anaplastic lymphoma kinase; SV2B, neurobeachin; EML4, echinoderm microtubule-associated protein-like 4 gene.
Given the promising efficacy regarding both central nervous system (CNS) and non-CNS lesions and tolerability of alectinib, alectinib was administered orally at a dose of 600 mg twice per day as the third-line treatment from October 2021 to December 2022. Dynamic monitoring of serum tumor markers suggested that CEA, CA125 and CA19-9 dropped dramatically five month later (Figure 3A). A follow-up CT scan performed at 10 months after treatment found that the lesions in right lung obviously shrank from 3.3 cm * 3.0 cm to 0.9 cm * 1.0 cm, and the right adrenal metastasis was also smaller than before (4.9 cm *3.0 cm to 4.1 cm * 2.0 cm). Meanwhile, MRI examination showed the intracranial metastases in bilateral frontal lobe and parietal lobe were not clearly displayed, and the left occipital lobe lesions were smaller than before (Figure 3B). According to RECIST 1.1, partial response was achieved. The patient’s chest tightness, and shortness of breath were significantly relieved. The patient tolerated the treatment well with no significant adverse events until May 2023, when the patient succumbed to respiratory complications secondary to COVID-19 infection.
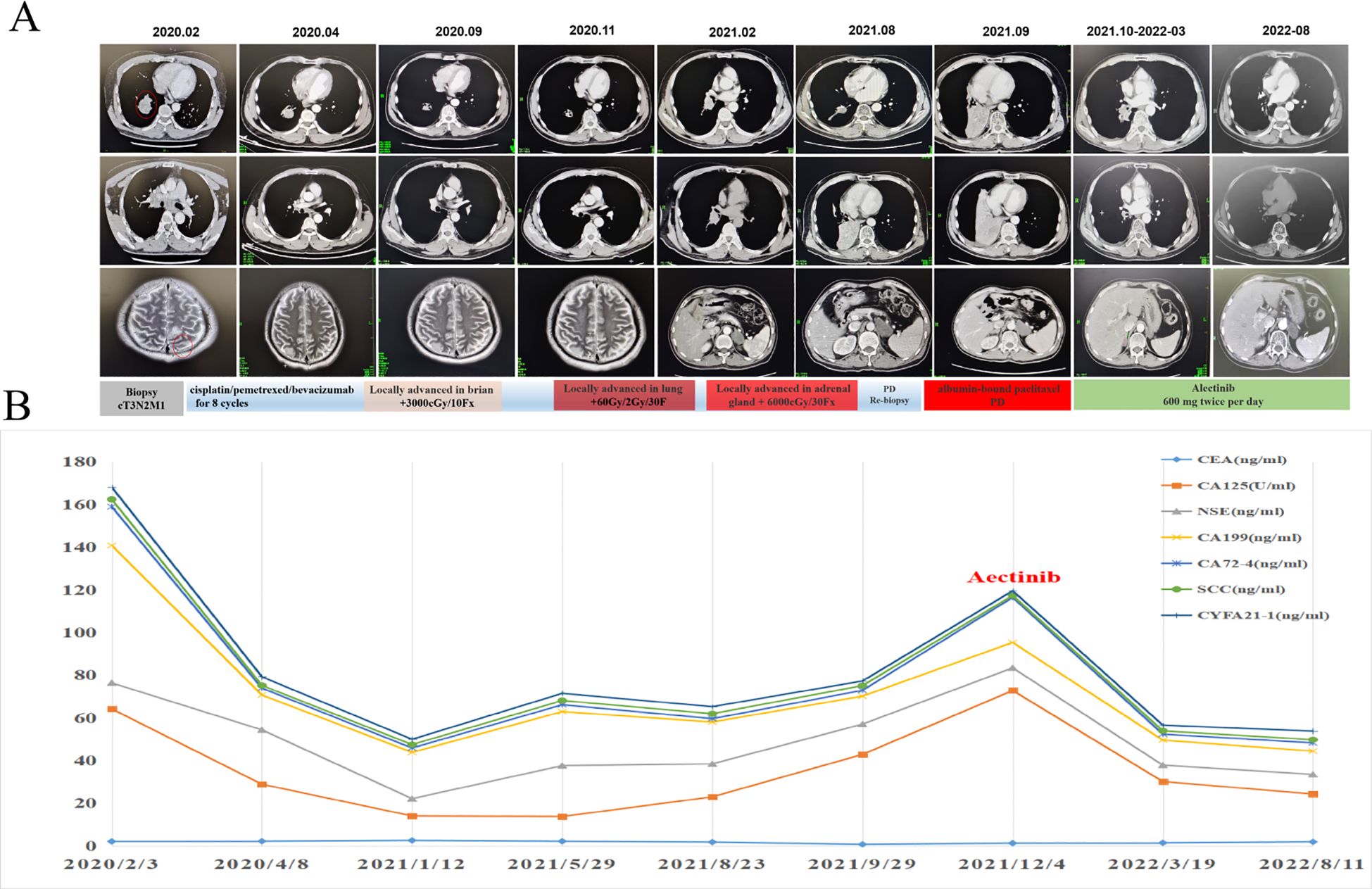
Figure 3. Dynamic changes of plasma tumor markers and tumor lesions during the alectinib treatment. (A) CT/MRI scans before and during alectinib treatment. (B) Dynamic monitoring of tumor markers.
3 Discussion
EML4-ALK rearrangement defines a unique molecular subtype of NSCLC, of which the patients could benefit from multiple ALK inhibitors. With the wide application of extensive genomic sequencing, more than 50 fusion partners of ALK have been found in NSCLC (6). It is worthwhile to report the uncommon ALK partners and their sensitiveness to ALK inhibitors, which may provide the clinical evidences of treatment options to patients with the same rare fusion. To our knowledge, this is the first study to report a lung adenocarcinoma patient with a novel SV2B-ALK and EML4-ALK double fusion who had a durable and remarkable response to alectinib in the third-line setting.
Alectinib is a second-generation, highly-selective ALK inhibitor and is highly recommended by NSCLC NCCN guideline due to its excellent efficacy in ALK-rearranged NSCLC patients. In the first-line setting, PFS was significantly prolonged with alectinib vs. crizotinib (median PFS: 34.8 months vs. 10.9 months) (7). In Asian patients, the median PFS of alectinib and crizotinib was 41.6 months and 11.1 months, respectively. Patients with CNS metastases at baseline also respond well to alectinib with the median PFS of 42.3 months (8). In ALEX-J study, the median PFS of alectinib was 20.3 months in ALK inhibitor-naive, chemotherapy-treated NSCLC patients (9). The efficacy of alectinib in third-line setting has not been well studied. In our case, the patient received alectinib as the third-line therapy after treatment failure of chemotherapy and radiotherapy with the excellent efficacy in both non-CNS and CNS lesions, which suggested that alectinib could be considered in the third-line setting in patients with CNS metastases.
In our case, 1-13 exons of EML4 gene fused with 20-29 exons of ALK, which was a classic EML4-ALK v1 fusion. Moreover, 1-6 exons of SV2B gene fused with 20-29 exons of ALK gene, which retained the intact kinase domain of ALK protein. Synaptic vesicle protein (SV2) is a neuronal protein with three isoforms (SV2A, SV2B and SV2C), and plays an important role in exocytosis and in the secretory process of synaptic and endocrine cells. As a synaptic protein, SV2B is widely expressed in the nervous system, especially throughout the brain (10, 11). Thus, we speculated that the promoter of SV2B driven ALK kinase domain expression may contribute to brain metastasis of this patients, as well as to the excellent intracranial efficacy of alectinib, though we could not confirm its expression in the brain lesion. With similar dilemma of cases reporting multiple ALK fusions, we summarized previous literatures to facilitate future decision making. Up to now, only 22 cases have been reported (Table 1) (6, 12–31). Among them, 12 patients harbored the classic EML4-ALK fusion and an uncommon ALK fusion (6, 12, 13, 15, 18, 20, 22, 25, 27–29, 31). Other patients had two or more uncommon ALK fusions. STRN-ALK fusion was reported in two cases (14, 15), while other uncommon fusions occurred only once. In several studies, these fusions were confirmed using other techniques, including FISH, IHC, Sanger sequencing and PCR. Most patients were confirmed to be positive for ALK rearrangement or expression, whilst P022 had negative result for FISH testing (31). In previous reports, most patients received crizotinib as the systematic therapy with the longest PFS over 31 months (13). In the past two years, alectinib was also used in 5 cases with the immature PFS data in 4 of them. It’s worth noting that P21 received crizotinib as second-line therapy. CT scans after 3 months of treatment showed a significant peripheral response, but growing brain metastases (30). Considering the superior efficacy of aletinib over crizotinib and its efficacy against brain metastases, alectinib was selected as the third-line therapy in this case, with the PFS over 14 months and prominent response in brain metastases.
In conclusion, this is the first report of a novel SV2B-ALK and EML4-ALK double-fusion in a lung adenocarcinoma patient with extensive metastases. Our patient responded well to alectinib in both CNS and non-CNS lesions in the third line setting. By reviewing literature, we concluded that comprehensive NGS is crucial to detect the novel fusions in NSCLC, which may affect the sensitivity of targeted therapy and thus the decision-making of treatment regimens.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by ethics committee of China-Japan Friendship Hospital (2023-KY-023) . The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HC: Investigation, Visualization, Writing – original draft. MZ: Resources, Writing – review & editing. LB: Resources, Writing – review & editing. YN: Resources, Writing – review & editing. XW: Resources, Writing – review & editing. RJ: Resources, Writing – review & editing. YW: Resources, Writing – review & editing. QF: Resources, Writing – review & editing. BW: Resources, Writing – review & editing. TD: Resources, Writing – review & editing. MY: Writing – review & editing. RC: Writing – review & editing. YQ: Writing – review & editing. DZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National High Level Hospital Clinical Research Funding (grant no. 2022-NHLHCRF-LX-01-0206), CAMS Innovation Fund for Medical Sciences (grant no. 2021-I2M-1−012), Qinghai Provincial People's Hospital Oncology Department Provincial-level Clinical Core Specialty Construction Project (grant no. 2022-109).
Conflict of interest
MY and RC are employees of Geneplus-Beijing Beijing, China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
2. Du X, Shao Y, Qin HF, Tai YH, Gao HJ. ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac Cancer. (2018) 9:423–30. doi: 10.1111/tca.2018.9.issue-4
3. Cameron LB, Hitchen N, Chandran E, Morris T, Manser R, Solomon BJ, et al. Targeted therapy for advanced anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer. Cochrane Database systematic Rev. (2022) 1:Cd013453. doi: 10.1002/14651858
4. Remon J, Pignataro D, Novello S, Passiglia F. Current treatment and future challenges in ROS1- and ALK-rearranged advanced non-small cell lung cancer. Cancer Treat Rev. (2021) 95:102178. doi: 10.1016/j.ctrv.2021.102178
5. Cai C, Tang Y, Li Y, Chen Y, Tian P, Wang Y, et al. Distribution and therapeutic outcomes of intergenic sequence-ALK fusion and coexisting ALK fusions in lung adenocarcinoma patients. Lung Cancer. (2021) 152:104–8. doi: 10.1016/j.lungcan.2020.12.018
6. Liang Q, Xu H, Liu Y, Zhang W, Sun C, Hu M, et al. Coexistence of a novel NBEA-ALK, EML4-ALK double-fusion in a lung adenocarcinoma patient and response to alectinib: A case report. Lung Cancer. (2021) 162:86–9. doi: 10.1016/j.lungcan.2021.10.015
7. Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann oncology: Off J Eur Soc Med Oncol. (2020) 31:1056–64. doi: 10.1016/j.annonc.2020.04.478
8. Zhou C, Lu Y, Kim S, Baisamut T, Zhou J, Zhang Y, et al. LBA11 Alectinib (ALC) vs crizotinib (CRZ) in Asian patients (pts) with treatment-naïve advanced ALK+ non-small cell lung cancer (NSCLC): 5-year update from the phase III ALESIA study. Ann Oncol. (2022) 33:S1563. doi: 10.1016/j.annonc.2022.10.353
9. Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet (London England). (2017) 390:29–39. doi: 10.1016/S0140-6736(17)30565-2
10. Cortés-Algara A, Cárdenas-Rodríguez N, Lara-Padilla E, Floriano-Sánchez E, Martinez-Contreras R, Anaya-Ruiz M, et al. Synaptic vesicle protein isoforms (SV2A, SV2B, SV2C): Expression in breast cancer and their association with risk factors and metastasis in Mexican women. Int J Clin Exp Pathol. (2017) 10:1998–2004.
11. Detrait E, Maurice T, Hanon E, Leclercq K, Lamberty Y. Lack of synaptic vesicle protein SV2B protects against amyloid-beta(2)(5)(-)(3)(5)-induced oxidative stress, cholinergic deficit and cognitive impairment in mice. Behav Brain Res. (2014) 271:277–85. doi: 10.1016/j.bbr.2014.06.013
12. Zhang Y, Yang X, Zhu X-L, Hao J-Q, Bai H, Xiao Y-C, et al. Bioinformatics analysis of potential core genes for glioblastoma. Bioscience Rep. (2020) 40:BSR20201625. doi: 10.1042/BSR20201625
13. Tao H, Liu Z, Mu J, Gai F, Huang Z, Shi L. Concomitant novel ALK-SSH2, EML4-ALK and ARID2-ALK, EML4-ALK double-fusion variants and confer sensitivity to crizotinib in two lung adenocarcinoma patients, respectively. Diagn Pathol. (2022) 17:27. doi: 10.1186/s13000-022-01212-9
14. Ren K, Ding G, Xie S, Yang L. Long-term survival after salvage thoracic surgery on a patient with ALK-rearranged metastatic lung adenocarcinoma after progression on targeted therapy. Onco Targets Ther. (2021) 14:5221–5. doi: 10.2147/OTT.S325460
15. Zeng H, Li Y, Wang Y, Huang M, Zhang Y, Tian P, et al. Case report: identification of two rare fusions, PDK1-ALK and STRN-ALK, that coexist in a lung adenocarcinoma patient and the response to alectinib. Front Oncol. (2021) 11:722843. doi: 10.3389/fonc.2021.722843
16. Zeng Q, Gao H, Zhang L, Qin S, Gu Y, Chen Q. Coexistence of a secondary STRN-ALK, EML4-ALK double-fusion variant in a lung adenocarcinoma patient with EGFR mutation: a case report. Anticancer Drugs. (2021) 32:890–3. doi: 10.1097/CAD.0000000000001094
17. Jiao Y, Liu M, Luo N, Guo H, Li J. Novel MRPL13-ALK and PPP1CB-ALK double fusion as a potential mechanism of acquired resistance to first-line osimertinib in EGFR-mutant high-grade neuroendocrine tumor of the lung. JTO Clin Res Rep. (2020) 1:100079. doi: 10.1016/j.jtocrr.2020.100079
18. Zhao G, Chen L, Xiao M, Yang S. Rare coexistence of three novel CDCA7-ALK, FSIP2-ALK, ALK-ERLEC1 fusions in a lung adenocarcinoma patient who responded to Crizotinib. Lung Cancer. (2021) 152:189–92. doi: 10.1016/j.lungcan.2020.12.013
19. Zhao S, Liu W, Li S, Shi T, Chen Q, Li Q, et al. A case of simultaneously diagnosed lung adenocarcinoma and endobronchial inflammatory myofibroblastic tumor with two distinct types of ALK translocation. Cancer Res Treat. (2021) 53:601–6. doi: 10.4143/crt.2020.952
20. Wang YL, Wu ZZ, Zhang HR, Chen DS, Zhao X. Coexistence of a novel RGS18 downstream intergenic region ALK fusion and a THUMPD2-ALK fusion in a lung adenocarcinoma patient and response to crizotinib. Lung Cancer. (2021) 154:216–8. doi: 10.1016/j.lungcan.2021.02.008
21. Zhong JM, Zhang GF, Lin L, Li DY, Liu ZH. A novel EML4-ALK BIRC6-ALK double fusion variant in lung adenocarcinoma confers sensitivity to alectinib. Lung Cancer. (2020) 145:211–2. doi: 10.1016/j.lungcan.2020.04.030
22. Wu X, Zhou H, He Z, Zhang Z, Feng W, Zhao J, et al. Coexistence of a novel CCNY-ALK and ATIC-ALK double-fusion in one patient with ALK-positive NSCLC and response to crizotinib: a case report. Transl Lung Cancer Res. (2020) 9:2494–9. doi: 10.21037/tlcr-20-1049
23. Guo J, Shi J, Yao M, Jin Y, Liu D, Liu W, et al. A rare double ALK fusion variant EML4-ALK and CDK15-ALK in lung adenocarcinoma and response to crizotinib: A case report. Med (Baltimore). (2020) 99:e22631. doi: 10.1097/MD.0000000000022631
24. Cai C, Long Y, Li Y, Huang M. Coexisting of COX7A2L-ALK, LINC01210-ALK, ATP13A4-ALK and acquired SLCO2A1-ALK in a lung adenocarcinoma with rearrangements loss during the treatment of crizotinib and ceritinib: A case report. Onco Targets Ther. (2020) 13:8313–6. doi: 10.2147/OTT.S258067
25. Li Z, Li P, Yan B, Gao Q, Jiang X, Zhan Z, et al. Sequential ALK inhibitor treatment benefits patient with leptomeningeal metastasis harboring non-EML4-ALK rearrangements detected from cerebrospinal fluid: A case report. Thorac Cancer. (2020) 11:176–80. doi: 10.1111/1759-7714.13259
26. Wu X, Wang W, Zou B, Li Y, Yang X, Liu N, et al. Novel NLRC4-ALK and EML4-ALK double fusion mutations in a lung adenocarcinoma patient: A case report. Thorac Cancer. (2020) 11:1695–8. doi: 10.1111/1759-7714.13389
27. Pagan C, Barua S, Hsiao SJ, Mansukhani M, Saqi A, Murty V, et al. Targeting SLMAP-ALK-a novel gene fusion in lung adenocarcinoma. Cold Spring Harb Mol Case Stud. (2019) 5:a003939. doi: 10.1101/mcs.a003939
28. Qin BD, Jiao XD, Liu K, Wu Y, Zang YS. Identification of a novel EML4-ALK, BCL11A-ALK double-fusion variant in lung adenocarcinoma using next-generation sequencing and response to crizotinib. J Thorac Oncol. (2019) 14:e115–7. doi: 10.1016/j.jtho.2019.01.032
29. Luo J, Gu D, Lu H, Liu S, Kong J. Coexistence of a novel PRKCB-ALK, EML4-ALK double-fusion in a lung adenocarcinoma patient and response to crizotinib. J Thorac Oncol. (2019) 14:e266–8. doi: 10.1016/j.jtho.2019.07.021
30. Lin H, Ren G, Liang X. A novel EML6-ALK FBXO11-ALK double fusion variant in lung adenocarcinoma and response to crizotinib. J Thorac Oncol. (2018) 13:e234–6. doi: 10.1016/j.jtho.2018.07.011
Keywords: alectinib, ALK double fusions, CNS metastases, NSCLC, SV2B-ALK novel fusion
Citation: Chen H, Zhang M, Bai L, Niu Y, Wang X, Jiang R, Wang Y, Feng Q, Wang B, Dai T, Yuan M, Chen R, Qi Y and Zhong D (2024) Coexistence of a novel SV2B-ALK, EML4-ALK double-fusion in a lung poorly differentiated adenocarcinoma patient and response to alectinib: a case report and literature review. Front. Oncol. 14:1453259. doi: 10.3389/fonc.2024.1453259
Received: 22 June 2024; Accepted: 26 November 2024;
Published: 13 December 2024.
Edited by:
Yuyan Wang, Beijing Cancer Hospital, ChinaReviewed by:
Michael Shafique, Moffitt Cancer Center, United StatesLara Kujtan, University of Missouri–Kansas City, United States
Nathan Merrill, University of Michigan, United States
Copyright © 2024 Chen, Zhang, Bai, Niu, Wang, Jiang, Wang, Feng, Wang, Dai, Yuan, Chen, Qi and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yujuan Qi, cWl5dWp1YW4xMTA4QDEyNi5jb20=; Dingrong Zhong, NzQ4ODAzMDY5QHFxLmNvbQ==
 Huang Chen
Huang Chen Menglan Zhang
Menglan Zhang Liyan Bai
Liyan Bai Yun Niu1
Yun Niu1 Mingming Yuan
Mingming Yuan Rongrong Chen
Rongrong Chen