- 1Neuroscience Division, Department of Neurology and Neurosurgery, Escola Paulista de Medicina, Federal University of São Paulo, São Paulo, Brazil
- 2Biomedical Engineering Division, Institute of Science and Technology, Federal University of São Paulo, São José dos Campos, Brazil
- 3Department of Gynecology, Federal University of São Paulo, São Paulo, Brazil
- 4Department of Gynecology and Neuropelveology, Increasing-Institute of Care and Rehabilitation in Neuropelveology and Gynecology, São Paulo, Brazil
- 5Department of Obstetrics and Gynecology, Santa Casa de São Paulo School of Medical Sciences, São Paulo, Brazil
- 6Department of Obstetrics and Gynecology, Faculty of Medicine, University of Toronto, Toronto, ON, Canada
Spinal cord injury (SCI) can cause significant motor, sensory, and autonomic dysfunction by disrupting neural connections. As a result, it is a global health challenge that requires innovative interventions to improve outcomes. This review assesses the wide-ranging impacts of SCI and focuses on the laparoscopic implantation of neuroprosthesis (LION) as an emerging and promising rehabilitation technique. The LION technique involves the surgical implantation of electrodes on lumbosacral nerves to stimulate paralyzed muscles. Recent findings have demonstrated significant improvements in mobility, sexual function, and bladder/bowel control in chronic SCI patients following LION therapy. This manuscript revisits the potential physiological mechanisms underlying these results, including neuroplasticity and modulation of autonomic activity. Additionally, we discuss potential future applications and amendments of LION therapy. This study emphasizes the potential of neuromodulation as a complementary approach to traditional rehabilitation, that can provide a beacon of hope for improving functionality and quality of life for individuals with SCI.
Introduction
Spinal cord injuries (SCI) are debilitating conditions that significantly impair quality of life and demand specialized care and innovative treatment approaches (Ahuja et al., 2017; Alizadeh et al., 2019; Ambrozaitis et al., 2006). According to 2016 data, the incidence of SCI is substantial, affecting 13 (11 to 16) individuals per 100,000 residents globally (Safdarian et al., 2023; GBD 2019 Diseases and Injuries Collaborators, 2020). These indices show how SCI impacts a notable amount of people globally.
The etiology of SCI can be categorized into traumatic, caused by external forces, and non-traumatic, resulting from neurodegenerative and/or ischemic processes, each leading to potentially irreversible spinal cord damage (Fehlings et al., 2017; Rouanet et al., 2017). Understanding the etiology of SCI is crucial for developing effective treatments and interventions for individuals with spinal cord injuries.
In a SCI, a physical interruption of neural connections directly affects the flow of information in the cortico-motor circuit, leading to motor, sensory, and autonomic dysfunctions, which will be more severe the more proximal and more extensive the injury is (Ahuja et al., 2017; Alizadeh et al., 2019). The most common impairments are spasticity, rigidity, hypotonicity, spasms, loss of control, phantom sensations, itches, and loss of sensibility (Rouanet et al., 2017; Perrouin-Verbe et al., 2021; Guest et al., 2022). The American Spinal Cord Injury Association (ASIA) quantifies the severity of the injury through the ASIA Impairment Scale (AIS). Complete injuries (AIS A) are characterized by complete interruption of motoric and sensory communication at the level of the injury. In motoric complete injuries (AIS B), sensory signals are still transmitted, but no voluntary motor function can be found below the level of injury. While, in incomplete injuries (AIS C-D), motoric and sensory functions are partially preserved below the level of injury (Fehlings et al., 2017; Nandoe Tewarie et al., 2010).
In the early stages of spinal cord injury, known as spinal shock phase, there is a reduction or complete abolition of the conduction of impulses (Rogers and Todd, 2016). During the chronic phase, the formation of the glial scars acts as a physical obstruction to axonal growth, leading to a reduction in functionality proportional to the extent of the injury (Atkinson and Atkinson, 1996; Ashby et al., 1974; Ditunno et al., 2004). Additionally, the autonomic effects can vary greatly from the acute to chronic phases, ranging from autonomic dysreflexia to hemodynamic instability, which may have negative implications for cardiovascular function and neurological recovery, thereby endangering both survival and quality of life (Eldahan and Rabchevsky, 2018; Spinal Cord Injury Centre of Western Denmark, 2021; Wulf and Tom, 2023; Goudman et al., 2022).
After a SCI, the thalamus recalibrates the integration of sensory and motor inputs, potentially altering the synchronization dynamics of the entire circuit (Alonso-Calviño et al., 2016; Murray Sherman and Guillery, 2002). This can lead to disruptions in the functioning of the cortico-thalamic system, resulting in impairments in behavior or neurocognitive functions in the long term. Afferent fibers play a crucial role in providing sensory feedback for precise movement control. Any delay in this signal, caused by partial or total deafferentation in the spinal cord, can lead to desynchronization of electrophysiological activity in the central nervous system regions responsible for decoding movement. It is important to note that this disruption can have significant consequences on movement coordination and control (Alonso-Calviño et al., 2016; Murray Sherman and Guillery, 2002; Murray Sherman, 2001).
Besides motor impairments, recent clinical studies have confirmed that patients with SCI also exhibit symptoms of attention decrease, loss of concentration and memory, and learning deficits (Distel et al., 2020; Molina et al., 2018; Sachdeva et al., 2018), with compromising effects on medial prefrontal cortex, and anterior cingulate, which are critical regions for emotional processing and attention modulation (Molina et al., 2018; Bouton et al., 2016; Moxon et al., 2014). Additionally, epidemiological studies suggest that SCI patients are at a heightened risk for developing dementia and mood disorders such as depression, anxiety, and PTSD (Perrouin-Verbe et al., 2021; Li et al., 2020; Budd et al., 2022; Huang et al., 2017).
Up to this moment, SCIs are only partially reversible, depending on the type and extension of the lesion (Rouanet et al., 2017; Rath and Balain, 2017; Flack et al., 2022). However, many new neurostimulation procedures are currently showing great progress and promising results in the medium term. These neurostimulation protocols can be used in conjunction with traditional rehabilitation therapies, such as physical therapy and occupational therapy (Carè et al., 2024; Capogrosso et al., 2018; Chalif et al., 2024; Alam et al., 2016). They can help to promote neuroplasticity (Bouton et al., 2016) or assist respiratory pacing and bladder control (Stampas et al., 2019; de Cássia Meine Azambuja et al., 2018), still allowing volitional and functional movements (Bouton et al., 2016; Grahn et al., 2014; James et al., 2018).
A promising neuromodulation technique is the LION (Laparoscopic Implantation of Neuroprosthesis) procedure1—the implantation of four stimulation electrodes onto the sciatic, pudendal, and femoral nerves bilaterally, allowing for continuous and on demand stimulation of those nerves (Possover, 2022; Possover, 2009; Possover et al., 2007a).
Recently, Lemos et al. (2023) assessed the impact of the LION procedure on mobility, sexual, urinary, and anorectal functions of 30 subjects with chronic spinal cord injury (SCI), showing that the procedure improves mobility and genital sensitivity and reduces the number of urinary and fecal incontinence episodes (Spinal Cord Injury Centre of Western Denmark, 2021). These results reinforced the establishment of neuromodulation with the LION procedure as an additional therapeutic resource for rehabilitating patients with chronic SCI.
On this narrative review, we will broadly evaluate the possible physiological mechanisms involved during neuromodulation-augmented rehabilitation strategies. We will then analyze the main LION neuromodulation findings and discuss the implications, perspectives, and limitations regarding the results, in light of the overall understanding built on neuromodulation in general. Finally, we will assess possible extensions of this technique and propose directions for future research.
Neuromodulation protocols for SCI patients
In this manuscript, we will use the term neuromodulation as any deliberate modulation of the nervous system’s activity through the application of electrical current, voltage difference, or directed magnetic/electric field near or onto neuronal cells (Carè et al., 2024; Deer et al., 2017; Sun and Morrell, 2014).
Neuromodulation can be either invasive or non-invasive. Invasive neuromodulation involves the surgical implantation of electrodes into the brain, spinal cord, nerve roots or peripheral nerves; while non-invasive involves external devices that stimulate the nervous system from outside the body (Capogrosso et al., 2018; Sun and Morrell, 2014; Kumar et al., 2005). Semi-invasive neuromodulation refers to methods involving simpler electrode implantation procedures, typically using an external generator (Dolbow et al., 2021). Table 1 provides an overview of some types of neuromodulations (protocols and devices). The main techniques are deep brain stimulation (DBS) (Gielen and Molnar, 2012; Benabid et al., 2009; Vachez and Creed, 2020), Vagus nerve stimulation (VNS) (Goudman et al., 2022; Terra et al., 2013; Rush et al., 2005), transcranial magnetic stimulation (TMS) (Alexeeva and Calancie, 2016; Rossi et al., 2021; Rossi et al., 2009), transcranial direct current stimulation (tDCS) (Thair et al., 2017; Arora et al., 2022; Li et al., 2021), spinal cord stimulation (SCS) (Chalif et al., 2024; Grahn et al., 2014; Kumar et al., 2005; Li et al., 2021; Gad et al., 2013), surface functional electrical stimulation (sFES) (Peckham and Knutson, 2005; Popović, 2014; Selfslagh et al., 2019; Westerveld et al., 2013), peripheral nerve stimulation (PNS) (Abd-Elsayad and Trescot, 2022; St Clair et al., 2003; Rossignol et al., 2008), neuromuscular electrical stimulation (NMES) (de Cássia Meine Azambuja et al., 2018; Lake, 1992; Ethier et al., 2015) and, more recently, LION (Spinal Cord Injury Centre of Western Denmark, 2021; Possover, 2022; Lemos et al., 2023; Lemos and Possover, 2015). These neuromodulation techniques are employed in diverse applications, treating conditions ranging from chronic pain and Parkinson’s disease to muscle spasticity and various psychiatric disorders (Benabid et al., 2009; Terra et al., 2013; Deer et al., 2019; Hofstoetter et al., 2014; Taylor et al., 2018).
Considering sensorimotor conditions, currently, two important new protocols use epidural spinal stimulation (Chalif et al., 2024; Choi et al., 2021; Greiner et al., 2021) (with surgical implantation of electrodes above the dorsal surface of the spinal cord) or transcutaneous spinal cord stimulation (Hofstoetter et al., 2014; Hofstoetter et al., 2020; Shkorbatova et al., 2020) (with non-invasive electrodes placement the patient’s skin over the vertebral column) to enhance spinal excitability. These methods have shown promising results in restoring voluntary motor output in SCI and post-stroke patients (Owolabi et al., 2022; Taylor et al., 2021).
Direct brain stimulation approaches modulate supraspinal circuits by increasing descending activity. They have also shown improvements onto upper and lower limbs motor output after SCI (Rossi et al., 2009; Arora et al., 2022; Li et al., 2021; Nitsche and Paulus, 2000). TMS and tDCS are the most used techniques for this purpose. TMS is a non-invasive brain stimulation technique that induces directed magnetic fields through the scalp and skull, evoking neuro-electrophysiological responses. Although most studies using repetitive TMS in individuals with para/tetraplegia have a small sample sizes, the results are promising (Alexeeva and Calancie, 2016; Rossi et al., 2021). Stimulation of the peripheral nervous system can be performed by semi-invasive procedures, using PNS and NMES (Carè et al., 2024; Deer et al., 2017; Abd-Elsayad and Trescot, 2022; St Clair et al., 2003), or non-invasive using sFES (Peckham and Knutson, 2005; Popović, 2014; Teferra, 2017). These techniques are currently the most established, clinically accessible, and reliable form of neuromodulation for SCI patients (with many devices commercially available and used in the clinic) (James et al., 2018; Wenger et al., 2014). Finally, the LION protocol brought a new perspective by stimulating the femoral, sciatic, and pudendal nerves of SCI patients. By associating a systematic activation of residual pathways through pre-parametrized electric stimuli on pelvic nerves, conjugated with standard rehabilitation procedures, the technique showed to be very promising (Spinal Cord Injury Centre of Western Denmark, 2021; Lemos et al., 2023).
Stimulation effects on lumbosacral roots
The lumbosacral plexus comprises the nerves of the lumbar (L1–L5) and sacral (S1–S5) plexuses, which supports all motor and sensory innervation of the lower extremity, as well as the pelvic floor and abdominal muscles, the kidneys, bladder, sexual organs, colon, and rectum through both somatic and autonomic nerve pathways (Moore et al., 2013; Harkema et al., 2011; Possover et al., 2007c).
Possover et al. (2007a,b) described the first laparoscopic implantation of electrodes onto the intrapelvic portion of lumbosacral nerves for treating refractory pelvic neuropathic pain and dysfunction of the urinary tract and bladder, naming it as the LION procedure. Since then, this procedure has been adapted to improve bowel function, sexual function, and gait in patients with SCI (Possover, 2022; Lemos and Possover, 2015).
The LION protocols comprise a personalized set of programs to be used in specific situations of patient daily life. The fundamental programming encompasses a low-frequency baseline program of stimulus (5–20 Hz), continually operative throughout the day to inhibit detrusor overactivity, improve baseline muscle tone and modulate spasticity of the muscles of the lower limbs, while stimulating neuroplasticity in the mid-long term (Harkema et al., 2011; Brindley, 1974; Billington et al., 2022). Individual mid-frequency protocols (20–40 Hz) are activated at the patient’s request to promote intermittent contractions of the quadriceps, deep gluteal, and pelvic floor muscles for the purposes of training and physiotherapy (Lemos and Possover, 2015; Laufer et al., 2001). In addition, there is a continuous stimulation protocol for orthostatism and gait training. The patient can use a remote control to select and activate programs as needed. Other programs can occasionally be established upon patient indication or request to aid specific purposes, such as penile erection, enhance the absorption of lower limb oedema, and aid in specific transfer requirements (Spinal Cord Injury Centre of Western Denmark, 2021; Lemos et al., 2023). The lowest frequency to induce a harmonic contraction (without tremor/vibration) was used for the programs stimulating the femoral, sciatic and gluteus maximus nerves, as higher frequencies induce rapid onset of muscle fatigue (Possover et al., 2007c; Laufer et al., 2001; Lemos et al., 2018; Lemos et al., 2021).
Frequencies of less than 40 to 50 Hz are known to preferentially activate slow twitch type I muscle fibers, which demonstrate higher resistance to muscle fatigue, while higher frequencies lead to the recruitment of fast twitch type IIa and type IIb muscle fibers, which fatigue easily. To train the muscles of the pelvic floor, the same approach was taken, using lower frequencies (30 Hz) to train the slow fibers and higher frequencies (50 Hz) to train the fast fibers. Pudendal neuromodulation in the range of 10 to 15 Hz was also employed to enhance bladder compliance and reduce neurogenic detrusor overactivity (Lemos and Possover, 2015; Kasch et al., 2022; Lemos et al., 2021; Billot et al., 2020) (see Figure 1).
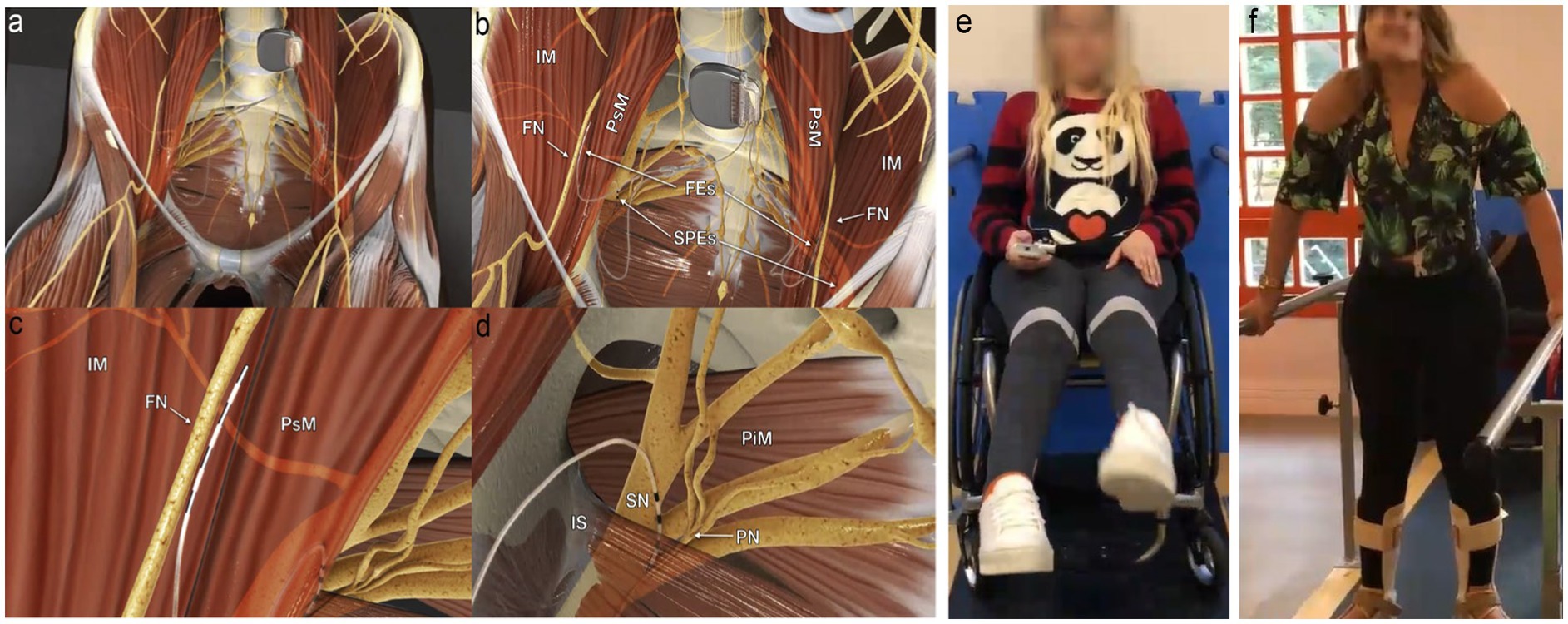
Figure 1. Electrodes placement and nerve stimulation. (A) Panoramic view of the system; the pulse generator is implanted into a paraumbilical subcutaneous pocket, and the electrodes run retroperitoneally down to the intrapelvic portions of the femoral (FN), sciatic (SN), and pudendal nerves (PN) bilaterally. (B) Panoramic view of femoral electrodes (FEs) and sciatic and pudendal electrodes (SPEs) positioning. (C) Detailed view of the right femoral electrode over the nerve between the iliac (IM) and the psoas (PsM) muscles. (D) Detailed view of the right sacral electrodes placed with half of its poles in the Alcock’s canal over the PN and the remaining poles over the SN. IS, ischial spine; PiM, piriformis muscle. (E) Extension of knees through femoral nerve stimulation using a remote activation. (F) Weight transfer and walking on parallel bars. Figure adapted from Lemos et al. (2023), with permission from the authors.
Additionally, pulse width (the duration of the electrical pulse applied to the muscle) was 60 μs for the femoral and sciatic nerves and 210 μs. The combination of frequency and pulse width is crucial in determining the strength and duration of muscle contraction. In 2022, Lemos et al. (2023) longitudinally assessed the impact of the LION procedure on mobility (Possover, 2014), sexual, urinary, and anorectal functions of 30 people with chronic spinal cord injury (SCI) (Lemos et al., 2023). Through a pre-programmed electrical stimulation protocol, combined with a specific rehabilitation program, they showed a significant improvement of different physiological functions. The most important were:
Impact on mobility
Lemos et al. (2023) showed that patients who underwent the LION procedure were able to regain walker-assisted gait, especially in their homes. The patients also became less dependent on adapted environments, such as being able to get up and reach objects on shelves or enter a non-adapted shower and sit on a standard chair to take a shower. After 1 year of follow-up, 72% of patients with thoracic injuries and 60% of patients with cervical injuries were able to walk with a walker using specific protocol that was activated on demand. Similarly, a randomized controlled trial (RCT) published by Kasch et al. (2022) further reinforced the potential of the LION procedure in enhancing gait ability. This study observed a significant improvement in the WISCI II scale for patients in the LION group, without changes in the control group. Notably, in this study, the LION participants were more homogeneous in terms of SCI level and injury type focusing on individuals with at least 12 months post-injury. The increase in WISCI II scores was clinically significant, aligning with previous case series reporting improvements in mobility over extended follow-up periods.
Impact on urinary function
47.8% of patients improved their urinary incontinence category, meaning that they experienced fewer episodes of leakage. The remaining 52.2% remained unchanged, and none of the patients’ incontinence worsened. Nighttime urinary incontinence improved in 30.4% of patients (Lemos et al., 2023). These findings align with evidence from studies on sacral root stimulation, which has been shown to activate bladder function effectively. For instance, the sacral anterior root stimulator (SARS) demonstrated significant reductions in urinary infections, from a median of seven to one per year, in a cohort of 20 participants (Brindley, 1994). Furthermore, 18 of these participants became catheter-free, and detrusor hyperreflexia was successfully abolished, enabling discontinuation of anticholinergic medications and reducing associated side effects such as constipation, dry mouth, and drowsiness. Over 85% of patients achieved continence, largely due to improved bladder compliance following posterior rhizotomy (Brindley, 1994; Creasey et al., 2001; Creasey and Dahlberg, 2001).
Impact on bowel function
In Lemos et al. (2023) study, before the surgery, 52% of patients needed more than 30 min to have a bowel movement. At follow-up, 65% of patients had reduced their bowel routine to less than 30 min. These results are consistent with findings from SARS studies, where bowel management was significantly enhanced (Creasey and Dahlberg, 2001). In these studies, the volunteers reported reduction of constipation and decreased reliance on laxatives and stool softeners. Additionally, bowel emptying time was reduced by 75%, offering substantial patient comfort. The intermittent stimulation used for micturition was adapted successfully for bowel evacuation, demonstrating the versatility of sacral stimulation in addressing multiple physiological functions (Creasey and Dahlberg, 2001).
Impact on sexual function
The International Index for Erectile Function Questionnaire showed improvement in erection in male patients. Additionally, 71% of patients of both sexes reported improved sensitivity in the genital area (Lemos et al., 2023).
Although these results show great improvements in a range of physiological functions for SCI patients, there is still limited knowledge about the physiological mechanisms involved during and after sacral nerve stimulation. Important questions remain unanswered, such as how stimuli from the lumbosacral plexus reach the somatosensory cortex, and how the afferent neural pathway is activated under different types of injuries (Spinal Cord Injury Centre of Western Denmark, 2021; Alonso-Calviño et al., 2016). It is also important to know how sequential stimulation over time in the lumbosacral plexus affect the cortical activity (Laufer et al., 2001; Chou and Binder-Macleod, 2007). Finally, it is necessary to investigate any potential side effects resulting from lumbosacral nerve stimulation in the acute and chronical phase of SCIs (Harkema et al., 2011; Lemos et al., 2018; Kasch et al., 2022).
Cortical response from pelvic nerve stimulation
One of the most intriguing points in the research by Lemos et al. (2023) was the patients’ report of partially recovering tactile sensitivity in different regions of their bodies, previously insensitive. These reports (also described by Possover, 2014) suggest there is a passage of afferent information from the pelvic region to the cortex, especially to the somatosensory cortex.
Even under complete or partial spinal deafferentation, alternative neural pathways, initially dormant, can be reactivated through neuroplasticity, induced by different therapeutic modalities. Recent research on the spinal cord’s dorsal horn has highlighted its critical role in structural reorganization of both local and remote neural networks. This process allows the nervous system to adapt to the loss of sensory input and facilitate functional recovery (Seki et al., 2003; Darian-Smith et al., 2014; Fisher et al., 2018). Moxon et al. (2014), for instance, demonstrated that sensory inputs and neurotrophic factors enable neuroplasticity around the lesion supporting functional healing in sensory pathways even after significant injuries. Additionally, Jones and Pons (1998) and Kaas (2000) highlighted the reorganization of cortical and thalamic sensory maps after injury, underscoring the capacity for adaptive changes in response to altered sensory inputs.
These residual pathways, often underutilized or inactive, may retain a capacity for information conduction that, with appropriate stimulation, can re-establish functional sensory connections. There is evidence that proper electric stimulation below non-responsive regions can result to cortical responses in clinically complete injuries, indicating the presence of residual afferent connections (Awad et al., 2020). By accounting for cortico-cortical modulation, these responses most likely reflect preserved afferent activations through residual somatosensory pathways, referred to as “sensory discomplete” injuries (Dimitrijević, 1988). This type of injury represents an intermediate level between complete and incomplete lesions, with potential for reactivating latent connections.
Pelvic stimulation, as used in the LION procedure, may leverage these pathways by facilitating connections with higher centers, especially the somatosensory cortex (Cardoso Melo et al., 2022). This process would involve both the sprouting of spared afferents and the cortical modulation of sensory inputs, enabling sensory recovery through circuits that would otherwise remain inactive. While this likely results from the activation of residual neural pathways linking these circuits, the lack of sensitivity prior to the LION procedure indicates that the injury initially prevented communication between them (Spinal Cord Injury Centre of Western Denmark, 2021; Alonso-Calviño et al., 2016; Rossignol et al., 2008).
In general, sensory information travels to the spinal cord and is then relayed to sensory centers in the cortex, thalamus, and cerebellum through the dorsal medial lemniscus and the spinocerebellar pathway, respectively. The cerebellum receives somatosensory information and integrates it with a copy of efferent motor commands transmitted by pontine nuclei located in the brainstem to estimate the sensory consequences of movements, where the cerebellum provides feedback to cortical areas through the thalamus (Murray Sherman and Guillery, 2002; Murray Sherman, 2001). On the other side, the motor cortex has loops with different cortical areas, including basal ganglia, cerebellum, and brainstem. The information provided by these loops is used to shape the final motor command, and the output is sent to the neurons of the ventral root of the spinal cord, which command the muscles to generate movement (Shkorbatova et al., 2020; Wenger et al., 2014; Ito, 2006; Courtine et al., 2009). Ozdemir and Perez (2018) and Raineteau and Schwab (2001) observed that, even in injured states, the spinal cord and cortex retain some capacity for adaptive responses, allowing pathways to reorganize and re-establish functional connections when provided with appropriate stimuli.
Therefore, since the intrinsic circuitry of the spinal cord is responsible for the coordination of sensory and motor information, by comparing the changes in power and phase of electrophysiological signals, in people with and without SCI, researchers could gain insights into the neural mechanisms underlying activity in spinal cord injury.
In 2023, Cardoso Melo et al. (2022) proposed a case series protocol to try to stablish a relationship between neuro-electrophysiological activities, via electroencephalography (EEG), and pelvic nerves stimulations (López-Larraz et al., 2015; Pfurtscheller and da Silva, 2017). By analyzing the temporal and spectral patterns of neural activity in patients with SCI, compared to subjects without SCI, specifically focusing on the cortical activity in the sensorimotor area, this preliminary study evaluates the effects of the Possover-LION neuromodulator on these groups (López-Larraz et al., 2015). In this protocol it was evaluated four SCI individuals who had undergone the LION procedure, analyzing their EEG-activity in resting state with the neuromodulator turned on and off, with open and closed eyes.
The study found that the subjects with the LION procedure showed increased activity levels on delta and theta bands and reduced activation levels on alpha and beta bands for both eyes open and closed conditions. These effects were further amplified when the neuromodulator was activated. While further research requires larger sample sizes and correlation assessments with factors like lesion duration, injury level, time with the neuromodulator, and additional physiotherapy treatments, these findings provide initial evidence of EEG rhythm alterations due to a direct LION neuromodulation. The shifts in delta and theta bands, commonly associated with states of neuroplasticity and adaptive changes, indicate the potential for this technique to facilitate cortical reorganization and sensory recovery (Cardoso Melo et al., 2022; Harmony, 2013).
Discussion
In this review, we outlined the etiology, physiological consequences, and long-term impacts of SCI under neuromodulation therapies. We focused on the potential of the laparoscopic implantation of neuroprosthesis (LION) procedure as an innovative approach for SCI rehabilitation.
Although growing evidence suggests that the LION neuromodulation interventions for SCI patients can improve a range of functions, some challenges relate to the accessibility, affordability, durability, feasibility, and scalability of the approaches still need to be improved. Additionally, the LION procedure often requires individualized programming to account for the specific injury characteristics and functional goals of each patient, which can present challenges in standardizing the approach. Patient variability, including differences in injury level, completeness, and chronicity, also influences response to stimulation, necessitating personalized adjustments to optimize outcomes. More critically, a more in-depth understanding of mechanisms is also crucial.
The main difference between the LION-based strategy and the other strategies available and tested is its unique combination of FES-like directly induced movements and chronic stimulation, which can potentially produce neuroplasticity effects similar to that observed in spinal cord stimulation protocols.
Stimulation of lumbosacral nerves, specifically the femoral, sciatic and pudendal nerves, can activate different sensory and motor cortical regions. However, different types of spinal cord injuries can produce different patterns of rhythmic activity in the cortex and different types of desynchronizations. Each pattern of activity can impact how the brain adapts to the type of injury and rehabilitation therapies. Neuromodulation has been shown to result in gains in muscle mass, improved control of urinary and bowel sphincters, and increased mobility using pelvic gait.
Lemos et al. (2023) demonstrated how the LION procedure, conjugated with rehabilitation, can rescue different physiological functions of patients with spinal cord injury. The procedure had a significant impact on mobility measures, with all patients showing improvement in mobility and most patients able to initiate gait training.
Observations also revealed that patients with complete SCI experienced measurable recovery in tactile sensitivity after undergoing the LION procedure. Additionally, patients with incomplete spinal cord injury have been found to experience a reclassification of the ASIA sensory scores. These results suggest there must be some flow of ascending information from the stimulated nerves to the somatosensory cortex. Overall, these findings highlight the potential benefits of neuromodulation in improving sensory and motor function in patients with SCI.
Another potential extension of the LION procedure is its integration with brain-computer interface (BCI) approaches (Maiseli et al., 2023; Lebedev and Nicolelis, 2006; Yin et al., 2023). This integration could eliminate the need for the remote-control commands (of the neuromodulators) that is currently used to switch between programs, creating a direct link between the decoding of neural signals related to motor intention and sensory feedback (Wenger et al., 2014; Bockbrader, 2019). Real-time monitoring of neural signals can potentially open the way for new closed-loop feedback mechanisms, where stimulation parameters are dynamically adjusted based on the individual’s neural activity and motor intentions. This closed-loop dynamics may optimize rehabilitation outcomes by tailoring interventions to the specific needs and progress of each patient, offering more natural and intuitive control over movements (Alam et al., 2016; Sun and Morrell, 2014). Finally, the improved tactile sensitivity reported after the LION procedure could be further leveraged by incorporating BCI-generated sensory feedback (Lundell et al., 2011; Fong et al., 2021). In this way, the combined approach may create a synergistic environment for enhanced neuroplastic changes, potentially accelerating the recovery process.
The LION procedure represents a promising avenue for advancing SCI rehabilitation. This approach has the potential to revolutionize treatment strategies, offering more personalized, adaptive, and effective rehabilitation for individuals with SCI. Addressing these limitations through advanced programming algorithms and real-time adaptive feedback systems may be promising for broader applicability. Continued research and clinical trials are crucial to determine the feasibility and enhance the synergistic effects of this innovative technique.
Author contributions
RL: Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing, Formal analysis, Investigation, Visualization. DV: Methodology, Project administration, Writing – original draft, Writing – review & editing, Investigation, Visualization. MM: Writing – original draft, Writing – review & editing. GF: Writing – original draft, Writing – review & editing. NL: Writing – original draft, Writing – review & editing. JF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was partially supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), through the program MCT-Instituto Nacional de Neurociência Translacional (INNT), grant number 573604/2008-8.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^Although there are other electrical stimulation techniques for lumbosacral nerves, in this article we will focus on describing the results using the LION technique specifically.
References
Abd-Elsayad, A., and Trescot, A. M. (2022). Peripheral nerve stimulation: a comprehensive guide. Amsterdam: Elsevier.
Ahuja, C. S., Wilson, J. R., Nori, S., Kotter, M. R. N., Druschel, C., Curt, A., et al. (2017). Traumatic spinal cord injury. Nat. Rev. Dis. Primers 3:17018. doi: 10.1038/nrdp.2017.18
Alam, M., Rodrigues, W., Pham, B. N., and Thakor, N. V. (2016). Brain-machine interface facilitated neurorehabilitation via spinal stimulation after spinal cord injury: recent progress and future perspectives. Brain Res. 1646, 25–33. doi: 10.1016/j.brainres.2016.05.039
Alexeeva, N., and Calancie, B. (2016). Efficacy of QuadroPulse rTMS for improving motor function after spinal cord injury: three case studies. J. Spinal Cord Med. 39, 50–57. doi: 10.1179/2045772314Y.0000000279
Alizadeh, A., Dyck, S. M., and Karimi-Abdolrezaee, S. (2019). Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 10:282. doi: 10.3389/fneur.2019.00282
Alonso-Calviño, E., Martínez-Camero, I., Fernández-López, E., Humanes-Valera, D., Foffani, G., and Aguilar, J. (2016). Increased responses in the somatosensory thalamus immediately after spinal cord injury. Neurobiol. Dis. 87, 39–49. doi: 10.1016/j.nbd.2015.12.003
Ambrozaitis, K. V., Kontautas, E., Spakauskas, B., and Vaitkaitis, D. (2006). Pathophysiology of acute spinal cord injury. Medicina 42, 255–261.
Arora, T., O’Laughlin, K., Potter-Baker, K., Kirshblum, S., Kilgore, K., Forrest, G. F., et al. (2022). Safety and efficacy of transcranial direct current stimulation in upper extremity rehabilitation after tetraplegia: protocol of a multicenter randomized, clinical trial. Spinal Cord 60, 774–778. doi: 10.1038/s41393-022-00768-z
Ashby, P., Verrier, M., and Lightfoot, E. (1974). Segmental reflex pathways in spinal shock and spinal spasticity in man. J. Neurol. Neurosurg. Psychiatry 37, 1352–1360. doi: 10.1136/jnnp.37.12.1352
Atkinson, P. P., and Atkinson, J. L. D. (1996). Spinal shock. Mayo Clin. Proc. 71, 384–389. doi: 10.4065/71.4.384
Awad, A., Levi, R., Waller, M., Westling, G., Lindgren, L., and Eriksson, J. (2020). Preserved somatosensory conduction in complete spinal cord injury: discomplete SCI. Clin. Neurophysiol. 131, 1059–1067. doi: 10.1016/j.clinph.2020.01.017
Bax, L., Staes, F., and Verhagen, A. (2005). Does neuromuscular electrical stimulation strengthen the quadriceps femoris? A systematic review of randomized controlled trials. Arch. Phys. Med. Rehabil. 86, 543–557.doi: 10.2165/00007256-200535030-00002
Benabid, A. L., Chabardes, S., Mitrofanis, J., and Pollak, P. (2009). Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 8, 67–81. doi: 10.1016/S1474-4422(08)70291-6
Billington, Z. J., Henke, A. M., and Gater, D. R. (2022). Spasticity management after spinal cord injury: the Here and now. J. Pers. Med. 12:808. doi: 10.3390/jpm12050808
Billot, M., Naiditch, N., Brandet, C., Lorgeoux, B., Baron, S., Ounajim, A., et al. (2020). Comparison of conventional, burst and high-frequency spinal cord stimulation on pain relief in refractory failed back surgery syndrome patients: study protocol for a prospective randomized double-blinded cross-over trial (MULTIWAVE study). Trials 21, 1–12. doi: 10.1186/s13063-020-04587-6
Bockbrader, M. (2019). Upper limb sensorimotor restoration through brain-computer interface technology in tetraparesis. Curr. Opin. Biomed. Eng. 11, 85–101. doi: 10.1016/j.cobme.2019.09.002
Bouton, C. E., Shaikhouni, A., Annetta, N. V., Bockbrader, M. A., Friedenberg, D. A., Nielson, D. M., et al. (2016). Restoring cortical control of functional movement in a human with quadriplegia. Nature 533, 247–250. doi: 10.1038/nature17435
Brindley, G. S. (1974). Emptying the bladder by stimulating sacral ventral roots. J. Physiol. 237, 15P–16P
Brindley, G. (1994). The first 500 patients with sacral anterior root stimulator implants: general description. Spinal Cord 32, 795–805. doi: 10.1038/sc.1994.126
Budd, M. A., Gater, D. R., and Channell, I. (2022). Psychosocial consequences of spinal cord injury: a narrative review. J. Pers. Med. 12:1178. doi: 10.3390/jpm12071178
Capogrosso, M., Wagner, F. B., Gandar, J., Moraud, E. M., Wenger, N., Milekovic, T., et al. (2018). Configuration of electrical spinal cord stimulation through real-time processing of gait kinematics. Nat. Protoc. 13, 2031–2061. doi: 10.1038/s41596-018-0030-9
Cardoso Melo, M., Pinheiro, D. J. L. L., Dantas, R. L. M., Athanazio, N. M. A., Morozini, C. F., dos Santos, G. C., et al. (2022). Evidence of cortical activation alterations of paraplegics with pelvic nerves stimulation on resting state: a case series. IX Latin American Congress on Biomedical Engineering and XXVIII Brazilian Congress on Biomedical Engineering.
Carè, M., Chiappalone, M., and Cota, V. R. (2024). Personalized strategies of neurostimulation: from static biomarkers to dynamic closed-loop assessment of neural function. Front. Neurosci. 18:1363128. doi: 10.3389/fnins.2024.1363128
Chalif, J. I., Chavarro, V. S., Mensah, E., Johnston, B., Fields, D. P., Chalif, E. J., et al. (2024). Epidural spinal cord stimulation for spinal cord injury in humans: a systematic review. J. Clin. Med. 13:1090. doi: 10.3390/jcm13041090
Choi, E. H., Gattas, S., Brown, N. J., Hong, J. D., Limbo, J. N., Chan, A. Y., et al. (2021). Epidural electrical stimulation for spinal cord injury. Neural Regen. Res. 16, 2367–2375. doi: 10.4103/1673-5374.313017
Chou, L.-W., and Binder-Macleod, S. A. (2007). The effects of stimulation frequency and fatigue on the force-intensity relationship for human skeletal muscle. Clin. Neurophysiol. 118, 1387–1396. doi: 10.1016/j.clinph.2007.02.028
Courtine, G., Gerasimenko, Y., van den Brand, R., Yew, A., Musienko, P., Zhong, H., et al. (2009). Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 12, 1333–1342. doi: 10.1038/nn.2401
Creasey, G. H., and Dahlberg, J. E. (2001). Economic consequences of an implanted neuroprosthesis for bladder and bowel management. Arch. Phys. Med. Rehabil. 82, 1520–1525. doi: 10.1053/apmr.2001.25912
Creasey, G. H., Grill, J. H., Korsten, M., Hoi Sang, U., Betz, R., Anderson, R., et al. (2001). An implantable neuroprosthesis for restoring bladder and bowel control to patients with spinal cord injuries: a multicenter trial. Arch. Phys. Med. Rehabil. 82, 1512–1519. doi: 10.1053/apmr.2001.25911
Darian-Smith, C., Lilak, A., Garner, J., and Irvine, K. A. (2014). Corticospinal sprouting differs according to spinal injury location and cortical origin in macaque monkeys. J. Neurosci. 34, 12267–12279. doi: 10.1523/JNEUROSCI.1593-14.2014
de Cássia Meine Azambuja, A., de Almeida Kuhn, A., dos Santos Américo, L., da Silva, M. C., dos Santos, P. P., dos Santos, L. J., et al. (2018). Neuromuscular electrical stimulation and transcutaneous electrical diaphragmatic stimulation in hospitalized patients with chronic cardiorespiratory diseases: a randomized clinical trial. J. Respir. Cardiovasc Phys. Ther. 7, 3–12.
Dedoncker, J., Brunoni, A. R., Baeken, C., and Vanderhasselt, M. A. (2016). A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and clinical samples: influence of stimulation parameters. Brain Stimul. 9, 501–517. doi: 10.1016/j.brs.2016.04.006
Deer, T. R., Jain, S., Hunter, C., and Chakravarthy, K. (2019). Neurostimulation for intractable chronic pain. Brain Sci. 9:23. doi: 10.3390/brainsci9020023
Deer, T. R., Lamer, T. J., Pope, J. E., Falowski, S. M., Provenzano, D. A., Slavin, K., et al. (2017). The neurostimulation appropriateness consensus committee (NACC) safety guidelines for the reduction of severe neurological injury. Neuromodulation Technol. Neural Interface 20, 15–30. doi: 10.1111/ner.12564
Deer, T. R., Pope, J. E., Benyamin, R., Vallejo, R., Friedman, A., Caraway, D., et al. (2016). Peripheral nerve stimulation for the treatment of chronic pain: safe and effective therapy in a cohort of more than 500 patients. Neuromodulation 19, 91–100. doi: 10.1111/ner.12381
Dimitrijević, M. R. (1988). Residual motor functions in spinal cord injury. Adv. Neurol. 47, 138–155
Distel, D. F., Amodeo, M., Joshi, S., and Abramoff, B. A. (2020). Cognitive dysfunction in persons with chronic spinal cord injuries. Phys. Med. Rehabil. Clin. N. Am. 31, 345–368. doi: 10.1016/j.pmr.2020.04.001
Ditunno, J. F., Little, J. W., Tessler, A., and Burns, A. S. (2004). Spinal shock revisited: a four-phase model. Spinal Cord 42, 383–395. doi: 10.1038/sj.sc.3101603
Dolbow, D. R., Gorgey, A. S., Sutor, T. W., Bochkezanian, V., and Musselman, K. (2021). Invasive and non-invasive approaches of electrical stimulation to improve physical functioning after spinal cord injury. J. Clin. Med. 10:5356. doi: 10.3390/jcm10225356
Doucet, B. M., Lam, A., and Griffin, L. (2012). Neuromuscular electrical stimulation for skeletal muscle function. Yale J. Biol. Med. 85, 201–215
Eldahan, K. C., and Rabchevsky, A. G. (2018). Autonomic dysreflexia after spinal cord injury: systemic pathophysiology and methods of management. Auton. Neurosci. 209, 59–70. doi: 10.1016/j.autneu.2017.05.002
Estores, I. M., Cheng, J., and Shi, Y. (2021). Transcutaneous spinal direct current stimulation for pain and spasticity: A clinical review. Neurosurg. Clin. N. Am. 32, 165–174. doi: 10.3389/fpsyt.2012.00063
Ethier, C., Gallego, J. A., and Miller, L. E. (2015). Brain-controlled neuromuscular stimulation to drive neural plasticity and functional recovery. Curr. Opin. Neurobiol. 33, 95–102. doi: 10.1016/j.conb.2015.03.007
Fehlings, M. G., Martin, A. R., Tetreault, L. A., Aarabi, B., Anderson, P., Arnold, P. M., et al. (2017). A clinical practice guideline for the Management of Patients with acute spinal cord injury: recommendations on the role of baseline magnetic resonance imaging in clinical decision making and outcome prediction. Global Spine J. 7, 221S–230S. doi: 10.1177/2192568217703089
Fisher, K. M., Lilak, A., Garner, J., and Darian-Smith, C. (2018). Extensive somatosensory and motor corticospinal sprouting occurs following a central dorsal column lesion in monkeys. J. Comp. Neurol. 526, 2373–2387. doi: 10.1002/cne.24491
Flack, J. A., Sharma, K. D., and Xie, J. Y. (2022). Delving into the recent advancements of spinal cord injury treatment: a review of recent progress. Neural Regen. Res. 17, 283–291. doi: 10.4103/1673-5374.317961
Fong, K. N. K., Ting, K. H., Zhang, J. J. Q., Yau, C. S. F., and Li, L. S. W. (2021). Event-related desynchronization during mirror visual feedback: a comparison of older adults and people after stroke. Front. Hum. Neurosci. 15:629592. doi: 10.3389/fnhum.2021.629592
Gad, P., Choe, J., Nandra, M. S., Zhong, H., Roy, R. R., Tai, Y. C., et al. (2013). Development of a multi-electrode array for spinal cord epidural stimulation to facilitate stepping and standing after a complete spinal cord injury in adult rats. J. Neuroeng. Rehabil. 10:2. doi: 10.1186/1743-0003-10-2
GBD 2019 Diseases and Injuries Collaborators (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 39, 1204–1222. doi: 10.1016/S0140-6736(20)30925-9
Gielen, F. L. H., and Molnar, G. C. (2012). “Basic principles of deep brain stimulation” in Deep brain stimulation: a new frontier in psychiatry. eds. D. Denys, M. Feenstra, and R. Schuurman (Berlin: Springer), 1–10.
Goudman, L., de Smedt, A., Louis, F., Stalmans, V., Linderoth, B., Rigoard, P., et al. (2022). The link between spinal cord stimulation and the parasympathetic nervous system in patients with failed back surgery syndrome. Neuromodulation 25, 128–136. doi: 10.1111/ner.13400
Grahn, P. J., Mallory, G. W., Berry, B. M., Hachmann, J. T., Lobel, D. A., and Lujan, J. L. (2014). Restoration of motor function following spinal cord injury via optimal control of intraspinal microstimulation: toward a next generation closed-loop neural prosthesis. Front. Neurosci. 8:296. doi: 10.3389/fnins.2014.00296
Greiner, N., Barra, B., Schiavone, G., Lorach, H., James, N., Conti, S., et al. (2021). Recruitment of upper limb motoneurons with epidural electrical stimulation of the cervical spinal cord. Nat. Commun. 12:435. doi: 10.1038/s41467-020-20703-1
Guest, J., Datta, N., Jimsheleishvili, G., and Gater, D. R. Jr. (2022). Pathophysiology, classification and comorbidities after traumatic spinal cord injury. J. Pers. Med. 12:1126. doi: 10.3390/jpm12071126
Harkema, S., Gerasimenko, Y., Hodes, J., Burdick, J., Angeli, C., Chen, Y., et al. (2011). Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–1947. doi: 10.1016/S0140-6736(11)60547-3
Harmony, T. (2013). The functional significance of delta oscillations in cognitive processing. Front. Integr. Neurosci. 7:83. doi: 10.3389/fnint.2013.00083
Hofstoetter, U. S., Freundl, B., Danner, S. M., Krenn, M. J., Mayr, W., Binder, H., et al. (2020). Transcutaneous spinal cord stimulation induces temporary attenuation of spasticity in individuals with spinal cord injury. J. Neurotrauma 37, 481–493. doi: 10.1089/neu.2019.6588
Hofstoetter, U. S., McKay, W. B., Tansey, K. E., Mayr, W., Kern, H., and Minassian, K. (2014). Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J. Spinal Cord Med. 37, 202–211. doi: 10.1179/2045772313Y.0000000149
Huang, S.-W., Wang, W.-T., Chou, L.-C., Liou, T.-H., and Lin, H.-W. (2017). Risk of dementia in patients with spinal cord injury: a nationwide population-based cohort study. J. Neurotrauma 34, 615–622. doi: 10.1089/neu.2016.4525
lfeld, B. M., Gilmore, C. A., Grant, S. A., Bolognesi, M. P., Del Gaizo, D. J., Wongsarnpigoon, A., et al. (2019). Ultrasound-guided percutaneous peripheral nerve stimulation for analgesia following total knee arthroplasty: a randomized, double-blind, placebo-controlled pilot study. J. Orthop. Surg. Res. 12:4. doi: 10.1186/s13018-016-0506-7
Ito, M. (2006). Cerebellar circuitry as a neuronal machine. Prog. Neurobiol. 78, 272–303. doi: 10.1016/j.pneurobio.2006.02.006
James, N. D., McMahon, S. B., Field-Fote, E. C., and Bradbury, E. J. (2018). Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol. 17, 905–917. doi: 10.1016/S1474-4422(18)30287-4
Jones, E. G., and Pons, T. P. (1998). Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science 282, 1121–1125. doi: 10.1126/science.282.5391.1121
Kaas, J. H. (2000). The reorganization of sensory and motor maps in adult mammals after injuries of the central and peripheral sensory systems. Annu. Rev. Neurosci.
Kasch, H., Løve, U. S., Jønsson, A. B., Severinsen, K. E., Possover, M., Elmgreen, S. B., et al. (2022). Effect of pelvic laparoscopic implantation of neuroprosthesis in spinal cord injured subjects: a 1-year prospective randomized controlled study. Spinal Cord 60, 251–255. doi: 10.1038/s41393-021-00693-7
Kriek, N., Groenen, S., Stronks, D. L., et al. (2022). Efficacy and safety of spinal cord stimulation for treatment of chronic pain in patients with complex regional pain syndrome: a systematic review. Neurosurgery 90, 251–260. doi: 10.1016/j.neurom.2023.05.003
Kumar, K., North, R., Taylor, R., Sculpher, M., van den Abeele, C., Gehring, M., et al. (2005). Spinal cord stimulation vs. conventional medical management: a prospective, randomized, controlled, multicenter study of patients with failed back surgery syndrome (PROCESS study). Neuromodulation 8, 213–218. doi: 10.1111/j.1525-1403.2005.00027.x
Lake, D. A. (1992). Neuromuscular electrical stimulation. An overview and its application in the treatment of sports injuries. Sports Med. 13, 320–336. doi: 10.2165/00007256-199213050-00003
Laufer, Y., Ries, J. D., Leininger, P. M., and Alon, G. (2001). Quadriceps femoris muscle torques and fatigue generated by neuromuscular electrical stimulation with three different waveforms. Phys. Ther. 81, 1307–1316. doi: 10.1093/ptj/81.7.1307
Lebedev, M. A., and Nicolelis, M. A. L. (2006). Brain-machine interfaces: past, present, and future. Trends Neurosci. 29, 536–546. doi: 10.1016/j.tins.2006.07.004
Lefaucheur, J. P., Aleman, A., Baeken, C., Benninger, D. H., Brunelin, J., di Lazzaro, V., et al. (2020). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 131, 474–528. doi: 10.1016/j.clinph.2019.11.002
Lemos, N., Fernandes, G. L., Morgado-Ribeiro, A., Souza, P., Lira, M., Contiero, W., et al. (2018) One-year urodynamics and mobility outcomes of patients submitted to femoral, sciatic and pudendal neuromodulation with the laparoscopic implantation of neuroprosthesis (LION) procedure. Open Discussion ePosters: Scientific Open Discussion Session 28
Lemos, N., Fernandes, G. L., Ribeiro, A. M., Maia-Lemos, P. S., Contiero, W., Croos-Bezerra, V., et al. (2023). Rehabilitation of people with chronic spinal cord injury using a laparoscopically implanted neurostimulator: impact on mobility and urinary, anorectal, and sexual functions. Neuromodulation 26, 233–245. doi: 10.1016/j.neurom.2022.01.010
Lemos, N., and Possover, M. (2015). Laparoscopic approach to intrapelvic nerve entrapments. J. Hip Preserv. Surg. 2, 92–98. doi: 10.1093/jhps/hnv030
Lemos, N., Sermer, C., Fernandes, G., Morgado-Ribeiro, A., Rossos, A., Zhao, Z. Y., et al. (2021). Laparoscopic approach to refractory extraspinal sciatica and pudendal pain caused by intrapelvic nerve entrapment. Sci. Rep. 11:10820. doi: 10.1038/s41598-021-90319-y
Li, Y., Cao, T., Ritzel, R. M., He, J., Faden, A. I., and Wu, J. (2020). Dementia, depression, and associated brain inflammatory mechanisms after spinal cord injury. Cells 9:1420. doi: 10.3390/cells9061420
Li, C., Jirachaipitak, S., Wrigley, P., Xu, H., and Euasobhon, P. (2021). Transcranial direct current stimulation for spinal cord injury-associated neuropathic pain. Korean J. Pain 34, 156–164. doi: 10.3344/kjp.2021.34.2.156
López-Larraz, E., Montesano, L., Gil-Agudo, Á., Minguez, J., and Oliviero, A. (2015). Evolution of EEG motor rhythms after spinal cord injury: a longitudinal study. PLoS One 10:e0131759. doi: 10.1371/journal.pone.0131759
Lundell, H., Barthelemy, D., Skimminge, A., Dyrby, T. B., Biering-Sørensen, F., and Nielsen, J. B. (2011). Independent spinal cord atrophy measures correlate to motor and sensory deficits in individuals with spinal cord injury. Spinal Cord 49, 70–75. doi: 10.1038/sc.2010.87
Maiseli, B., Abdalla, A. T., Massawe, L. V., Mbise, M., Mkocha, K., Nassor, N. A., et al. (2023). Brain-computer interface: trend, challenges, and threats. Brain Inform. 10:20. doi: 10.1186/s40708-023-00199-3
Molina, B., Segura, A., Serrano, J. P., Alonso, F. J., Molina, L., Pérez-Borrego, Y. A., et al. (2018). Cognitive performance of people with traumatic spinal cord injury: a cross-sectional study comparing people with subacute and chronic injuries. Spinal Cord 56, 796–805. doi: 10.1038/s41393-018-0076-0
Moore, K. L., Dalley, A. F., and Agur, A. M. R. (2013). Clinically oriented anatomy. Philadelphia, PA: Lippincott Williams & Wilkins.
Moxon, K. A., Oliviero, A., Aguilar, J., and Foffani, G. (2014). Cortical reorganization after spinal cord injury: always for good? Neuroscience 283, 78–94. doi: 10.1016/j.neuroscience.2014.06.056
Murray Sherman, S. (2001). “Chapter 4 thalamic relay functions” in Progress in brain research (Amsterdam: Elsevier), 51–69.
Murray Sherman, S., and Guillery, R. W. (2002). The role of the thalamus in the flow of information to the cortex. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 357, 1695–1708. doi: 10.1098/rstb.2002.1161
Nandoe Tewarie, R. D. S., Hurtado, A., Bartels, R. H. M. A., Grotenhuis, J. A., and Oudega, M. (2010). A clinical perspective of spinal cord injury. NeuroRehabilitation 27, 129–139. doi: 10.3233/NRE-2010-0589
Nielsen, J. F. (2002). Improvement of spasticity and muscle strength in multiple sclerosis patients after treatment with repetitive transcranial magnetic stimulation: a placebo-controlled study. Mult. Scler. J. 8, 284–287. doi: 10.1177/135245859600200503
Nitsche, M. A., and Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 527, 633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x
North, R. B., Kidd, D. H., Olin, J. C., and Sieracki, J. N. (1996). Spinal cord stimulation for axial low back pain: a prospective, controlled trial comparing dual with single percutaneous electrodes. Spine 21, 330–335.
Owolabi, M. O., Thrift, A. G., Mahal, A., Ishida, M., Martins, S., Johnson, W. D., et al. (2022). Primary stroke prevention worldwide: translating evidence into action. Lancet Public Health 7, e74–e85. doi: 10.1016/S2468-2667(21)00230-9
Ozdemir, R. A., and Perez, M. A. (2018). Afferent input and sensory function after human spinal cord injury. J. Neurophysiol. 119, 134–144. doi: 10.1152/jn.00354.2017
Peckham, P. H., and Knutson, J. S. (2005). Functional electrical stimulation for neuromuscular applications. Annu. Rev. Biomed. Eng. 7, 327–360. doi: 10.1146/annurev.bioeng.6.040803.140103
Perrouin-Verbe, B., Lefevre, C., Kieny, P., Gross, R., Reiss, B., and le Fort, M. (2021). Spinal cord injury: A multisystem physiological impairment/dysfunction. Rev. Neurol. 177, 594–605. doi: 10.1016/j.neurol.2021.02.385
Pfurtscheller, G., and da Silva, F. L. (2017). “C40EEG event-related desynchronization and event-related synchronization” in Niedermeyer’s electroencephalography: basic principles, clinical applications, and related fields. 7th ed (New York: Oxford Academic).
Popović, D. B. (2014). Advances in functional electrical stimulation (FES). J. Electromyogr. Kinesiol. 24, 795–802. doi: 10.1016/j.jelekin.2014.09.008
Possover, M. (2009). Laparoscopic management of neural pelvic pain in women secondary to pelvic surgery. Fertil. Steril. 91, 2720–2725. doi: 10.1016/j.fertnstert.2008.04.021
Possover, M. (2014). Recovery of sensory and supraspinal control of leg movement in people with chronic paraplegia: a case series. Arch. Phys. Med. Rehabil. 95, 610–614. doi: 10.1016/j.apmr.2013.10.030
Possover, M. (2022). The ‘Possover-LION procedure’ to the pelvic somatic nerves in people with a spinal cord injury. J. Minim. Invasive Gynecol. 29:340. doi: 10.1016/j.jmig.2021.12.004
Possover, M., Baekelandt, J., and Chiantera, V. (2007a). The laparoscopic implantation of neuroprothesis (LION) procedure to control intractable abdomino-pelvic neuralgia. Neuromodulation 10, 18–23. doi: 10.1111/j.1525-1403.2007.00083.x
Possover, M., Baekelandt, J., and Chiantera, V. (2007b). The laparoscopic approach to control intractable pelvic neuralgia: from laparoscopic pelvic neurosurgery to the LION procedure. Clin. J. Pain 23, 821–825. doi: 10.1097/AJP.0b013e31815349a8
Possover, M., Baekelandt, J., Flaskamp, C., Li, D., and Chiantera, V. (2007c). Laparoscopic neurolysis of the sacral plexus and the sciatic nerve for extensive endometriosis of the pelvic wall. Minim. Invasive Neurosurg. 50, 33–36. doi: 10.1055/s-2007-970075
Possover, M., and Forman, A. (2015). Neuromodulation in chronic pelvic pain: laparoscopic implantation of neuroprostheses to pelvic nerves. Neuromodulation Technol. Neural Interface 18, 317–322. doi: 10.1007/s10397-014-0838-4
Possover, M., and Forman, A. (2017). Recovery of supraspinal control of leg movement in a chronic complete flaccid paraplegic man after continuous low-frequency pelvic nerve stimulation and FES-assisted training. Spinal Cord Ser. Cases 3:16034. doi: 10.1038/scsandc.2016.34
Raineteau, O., and Schwab, M. E. (2001). Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2, 263–273. doi: 10.1038/35067570
Rath, N., and Balain, B. (2017). Spinal cord injury—the role of surgical treatment for neurological improvement. J. Clin. Orthop. Trauma 8, 99–102. doi: 10.1016/j.jcot.2017.06.016
Rogers, W. K., and Todd, M. (2016). Acute spinal cord injury. Best Pract. Res. Clin. Anaesthesiol. 30, 27–39. doi: 10.1016/j.bpa.2015.11.003
Rossi, S., Antal, A., Bestmann, S., Bikson, M., Brewer, C., Brockmöller, J., et al. (2021). Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin. Neurophysiol. 132, 269–306. doi: 10.1016/j.clinph.2020.10.003
Rossi, S., Hallett, M., Rossini, P. M., and Pascual-Leone, A.Safety of TMS Consensus Group (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 120, 2008–2039. doi: 10.1016/j.clinph.2009.08.016
Rossignol, S., Barrière, G., Frigon, A., Barthélemy, D., Bouyer, L., Provencher, J., et al. (2008). Plasticity of locomotor sensorimotor interactions after peripheral and/or spinal lesions. Brain Res. Rev. 57, 228–240. doi: 10.1016/j.brainresrev.2007.06.019
Rouanet, C., Reges, D., Rocha, E., Gagliardi, V., and Silva, G. S. (2017). Traumatic spinal cord injury: current concepts and treatment update. Arq. Neuropsiquiatr. 75, 387–393. doi: 10.1590/0004-282x20170048
Rush, A. J., Marangell, L. B., Sackeim, H. A., George, M. S., Brannan, S. K., Davis, S. M., et al. (2005). Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol. Psychiatry 58, 347–354. doi: 10.1016/j.biopsych.2005.05.025
Sachdeva, R., Gao, F., Chan, C. C. H., and Krassioukov, A. V. (2018). Cognitive function after spinal cord injury. Neurology 91, 611–621. doi: 10.1212/WNL.0000000000006244
Safdarian, M., Trinka, E., Rahimi-Movaghar, V., Thomschewski, A., Aali, A., Abady, G. G., et al. (2023). Global, regional, and national burden of spinal cord injury, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 22, 1026–1047. doi: 10.1016/S1474-4422(23)00287-9
Seki, K., Perlmutter, S., and Fetz, E. (2003). Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat. Neurosci. 6, 1309–1316. doi: 10.1038/nn1154
Selfslagh, A., Shokur, S., Campos, D. S. F., Donati, A. R. C., Almeida, S., Yamauti, S. Y., et al. (2019). Non-invasive, brain-controlled functional electrical stimulation for locomotion rehabilitation in individuals with paraplegia. Sci. Rep. 9:6782. doi: 10.1038/s41598-019-43041-9
Shkorbatova, P., Lyakhovetskii, V., Pavlova, N., Popov, A., Bazhenova, E., Kalinina, D., et al. (2020). Mapping of the spinal sensorimotor network by transvertebral and transcutaneous spinal cord stimulation. Front. Syst. Neurosci. 14:555593. doi: 10.3389/fnsys.2020.555593
Snyder-Mackler, L., Delitto, A., Stralka, S. W., and Bailey, S. L. (1994). Use of electrical stimulation in the treatment of quadriceps inhibition in athletes. J. Orthop. Sports Phys. Ther. 19, 282–288.
Spinal Cord Injury Centre of Western Denmark. (2021). Motor, sensory, and autonomic function in traumatic spinal cord injury after the LION procedure. (SCI-LION). Available at: https://clinicaltrials.gov/study/NCT03441256. (Accessed September 28, 2021)
St Clair, W. H., Arnold, S. M., Sloan, A. E., and Regine, W. F. (2003). Spinal cord and peripheral nerve injury: current management and investigations. Semin. Radiat. Oncol. 13, 322–332. doi: 10.1016/S1053-4296(03)00025-0
Stampas, A., Gustafson, K., Korupolu, R., Smith, C., Zhu, L., and Li, S. (2019). Bladder neuromodulation in acute spinal cord injury via transcutaneous tibial nerve stimulation: cystometrogram and autonomic nervous system evidence from a randomized control pilot trial. Front. Neurosci. 13:119. doi: 10.3389/fnins.2019.00119
Sun, F. T., and Morrell, M. J. (2014). Closed loop Neurostimulation: the clinical experience. Neurotherapeutics 11, 553–563. doi: 10.1007/s13311-014-0280-3
Taylor, R., Galvez, V., and Loo, C. (2018). Transcranial magnetic stimulation (TMS) safety: a practical guide for psychiatrists. Australas. Psychiatry 26, 189–192. doi: 10.1177/1039856217748249
Taylor, C., McHugh, C., Mockler, D., Minogue, C., Reilly, R. B., and Fleming, N. (2021). Transcutaneous spinal cord stimulation and motor responses in individuals with spinal cord injury: A methodological review. PLoS One 16:e0260166. doi: 10.1371/journal.pone.0260166
Teferra, M. (2017). Functional electrical stimulation (FES): review. Int. J. Latest Res. Eng. Technol. 3, 95–101.
Terra, V. C., Amorim, R., Silvado, C., Oliveira, A. J., Jorge, C. L., Faveret, E., et al. (2013). Vagus nerve stimulator in patients with epilepsy: indications and recommendations for use. Arq. Neuropsiquiatr. 71, 902–906. doi: 10.1590/0004-282X20130116
Thair, H., Holloway, A. L., Newport, R., and Smith, A. D. (2017). Transcranial direct current stimulation (tDCS): A beginner’s guide for design and implementation. Front. Neurosci. 11:641. doi: 10.3389/fnins.2017.00641
Vachez, Y. M., and Creed, M. C. (2020). Deep brain stimulation of the subthalamic nucleus modulates reward-related behavior: a systematic review. Front. Hum. Neurosci. 14:578564. doi: 10.3389/fnhum.2020.578564
Wenger, N., Moraud, E. M., Raspopovic, S., Bonizzato, M., DiGiovanna, J., Musienko, P., et al. (2014). Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci. Transl. Med. 6:255ra133. doi: 10.1126/scitranslmed.3008325
Westerveld, A. J., Schouten, A. C., Veltink, P. H., and van der Kooij, H. (2013). Control of thumb force using surface functional electrical stimulation and muscle load sharing. J. Neuro Engineering Rehabil. 10:104. doi: 10.1186/1743-0003-10-104
Wulf, M. J., and Tom, V. J. (2023). Consequences of spinal cord injury on the sympathetic nervous system. Front. Cell. Neurosci. 17:999253. doi: 10.3389/fncel.2023.999253
Yadav, A. P., and Ahmed, T. R. (2018). Transcutaneous electrical spinal cord stimulation for management of chronic neuropathic pain: a review of clinical efficacy. Pain Manag. 8, 425–432. doi: 10.1038/s41393-022-00776-z
Keywords: neuromodulation, spinal cord injury, laparoscopic neuroprosthesis, sensorimotor rehabilitation, neural stimulation
Citation: Marques Dantas RL, Vilela DN, Melo MC, Fernandes G, Lemos N and Faber J (2024) Neurostimulation on lumbosacral nerves as a new treatment for spinal cord injury impairments and its impact on cortical activity: a narrative review. Front. Hum. Neurosci. 18:1478423. doi: 10.3389/fnhum.2024.1478423
Edited by:
Hari S. Sharma, Uppsala University, SwedenReviewed by:
Huan Gao, Zhejiang University, ChinaCopyright © 2024 Marques Dantas, Vilela, Melo, Fernandes, Lemos and Faber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rodrigo Lantyer Marques Dantas, cm9kcmlnby5sYW50eWVyQHVuaWZlc3AuYnI=
 Rodrigo Lantyer Marques Dantas
Rodrigo Lantyer Marques Dantas Diego N. Vilela
Diego N. Vilela Mariana Cardoso Melo
Mariana Cardoso Melo Gustavo Fernandes
Gustavo Fernandes Nucelio Lemos
Nucelio Lemos Jean Faber
Jean Faber