
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Oncol. , 09 January 2024
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1308460
This article is part of the Research Topic Advances in the use of EGFR TKIs in the Treatment of NSCLC View all 21 articles
Lung cancer remains the leading cause of cancer-related deaths worldwide, surpassing the mortality of breast, colorectal and prostate cancers combined. The 5-year overall survival is around 18% including all stages of non-small cell lung cancer (NSCLC). It is likely that screening, targeted therapy, and immunotherapy will improve this poor prognosis in the coming years (1).
Currently, the choice of the treatment for patients with advanced NSCLC is based on the integrated evaluation of some parameters: histology (squamous versus non-squamous); presence of driver molecular alterations (sensitizing mutations of EGFR and/or BRAF, and rearrangements of ALK and/or ROS1 and/or NTRK); PD-L1 expression level; patient clinical characteristics such as age, performance status (PS) and comorbidities. The presence or absence of those driver molecular alterations allows distinguishing oncogene-addicted disease from non-oncogene-addicted disease, which presents different therapeutic approaches (2).
Many driver molecular alterations have been known in NSCLC till now. Some of these can be already targeted by means of specific drugs, while others could be targetable. These include: KRAS gene mutations (20-30%), EGFR (10-15% of Caucasian patients and up to 40% of Asian patients), BRAF (2-4%), ALK rearrangements (3-7%), ROS1 (1-2%), RET (1-2%), NTRK (0.5-1%), HER2 gene mutations (1-2%) and MET gene amplifications or mutations (2-4%) (3).
EGFR tyrosine kinase inhibitors (TKIs) represent the recommended first-line treatment in patients with advanced NSCLC and common mutations, i.e. exon 19 deletions (Ex19dels) and exon 21 point mutation (L858R). Many randomized phase III trials showed, in patients with advanced NSCLC and common EGFR mutations, the superiority of EGFR TKIs, i.e. gefitinib, erlotinib, afatinib, in the first line of treatment compared to standard platinum-based chemotherapy, in terms of both RR and PFS (4). The majority of patients treated with these drugs, around a half, develop the resistance point mutation T790M. To overcome this resistance mechanism, osimertinib was developed as second-line treatment. Subsequently its superiority in terms of overall survival (OS) over first- and second-generation TKIs was demonstrated also in the first-line setting (5, 6). As a consequence, osimertinib had been the best option for the treatment of advanced NSCLC patients with activating EGFR mutations until now (2). However, the recent results of FLAURA-2 trial and MARIPOSA trial showed that upfront combination strategies delayed resistance occurrence. Indeed, osimertinib plus platinum-pemetrexed and amivantamab plus lazertinib, respectively, achieved longer PFS compared to single agent osimertinib (7, 8).
Osimertinib has largely improved the survival of advanced NSCLC patients with EGFR mutation both in the first- or second-line setting, but the emergence of acquired resistance remains a great need.
The resistance mechanisms to osimertinib may be different depending on whether this drug is administered in the first or in the subsequent lines. In fact, osimertinib was initially developed exclusively for patients who had developed a T790M mutation during treatment with first or second generation TKIs. In this setting, the most frequent on-target resistance mechanism (approximately 14%) is the point mutation of EGFR exon 20, C797S. Instead, MET amplification occurred in 19% of patients, in 7% in conjunction with the C797S mutation. Furthermore, HER2 and PIK3CA amplifications, RET and NTRK rearrangements, and BRAF V600E mutation were each observed in 3–5% of cases (9, 10).
As regards the use of osimertinib as first-line treatment, some literature data reported an intrinsic resistance. The relative mechanisms include the HER2 and MET amplifications, as observed in in vitro studies, but also the combination of the KRAS G12D mutation and PTEN loss, and CDCP1 or AXL RNA overexpression, as reported in some NSCLC patients (11–13).
The main information about the acquired resistance to first-line osimertinib derives from the phase III trial FLAURA, while some other literature data are from case reports or small case series. Cell-free DNA from blood samples of patients included in FLAURA trial was analyzed via NGS (14). The authors did not find emergent T790M mutation. The most common resistance mechanism was MET amplification (15%), followed by less frequent EGFR amplification (9%), as well as C797S mutation (7%). S768I mutation or other combined EGFR mutations, such as exon 19 deletion + G724S (exon 18), L718Q (exon 18) + EGFR exon 20 insertion (exon 18 + 20), L718Q + C797S or L718Q + L797S (exon 18 + 20), are very rare (<1%). These findings suggest that secondary EGFR mutations are not the main mechanism of resistance to osimertinib.
Many EGFR-independent mechanisms were observed when resistance to first- or second-line osimertinib developed. These mechanisms are more evident with osimertinib than on-target mutations possibly because of its stronger EGFR inhibition compared to first or second-generation TKIs (15). Moreover, during the treatment with osimertinib EGFR-independent resistance mechanisms tend to occur earlier than EGFR-dependent ones, maybe because of pre-existent subclones rapidly arising under the selective pressure of treatment (16). This kind of mechanisms includes MET amplifications, HER2 amplifications, PI3KCA, BRAF and RAS mutations, ALK or RET rearrangements, cell cycle gene alterations (17). The ongoing ELIOS trial (NCT03239340) is designed to investigate prospectively the mechanisms of acquired resistance to first-line osimertinib, given that paired tissue biopsies (pre-treatment and at progression) are collected. Moreover, HER3 expression was found in around 80% of NSCLC and in more than 85% of those harboring EGFR activating mutations. It emerged as a further mechanism of resistance to EGFR TKIs and favors metastatic progression and worse prognosis (18).
Finally, phenotypic changes were also observed as resistance mechanisms in up to 15% of patients treated with first- or later-line osimertinib. These include the epithelial-mesenchymal transition and the transformation of adenocarcinoma to small-cell carcinoma, this latter associated with RB1 and TP53 mutations (19).
Patients experiencing progression during first-line osimertinib should receive chemotherapy regimens used in non-squamous NSCLC. The combination of carboplatin, paclitaxel, bevacizumab and atezolizumab could represent a treatment option for them (20). Anti-PD-1/PD-L1 drugs as monotherapy have not proven to be a successful option in this subgroup of patients (21). However, three phase III trials studied chemoimmunotherapy after osimertinib resistance. CheckMate-722 confirmed the lack of benefit in these patients (22). KEYNOTE-789 and ORIENT-31 are still ongoing.
To prolong the benefit obtainable with osimertinib, similarly to first-generation TKIs, this drug could be continued “beyond progression” in selected cases with some conditions, such as exclusively radiological progression of mild entity, slow tumor kinetics, absence of clinical symptoms, and in case of oligoprogressive disease local therapies can be associated (23).
Here, we discuss the evolution of treatment strategies to achieve the best outcomes for patients progressing during first-line osimertinib. The majority of the studies addressing this aim are still ongoing.
Many trials were designed to combine osimertinib with other new targeted agents directed against the emerging resistance mechanisms. These drugs can be classified in specific inhibitors, antibody-drug conjugates, and bispecific antibodies (24) (Table 1).
Given that MET alterations are the most frequent resistance mechanism to osimertinib, three MET inhibitors were developed and combined with osimertinib, i.e. savolitinib, tepotinib and capmatinib. However, other specific inhibitors are available against MEK (selumetinib), AXL (DS-1205c), ERK (ERAS-007), mTOR (sapanisertib), PI3Kα/δ (TQ-B3525), JAK1 (itacitinib), CDK4/6 (abemaciclib), AURKA (alisertib).
Antibody-drug conjugates (ADC) are composed of a link between a specific monoclonal antibody directed against a tumor molecule and a cytotoxic payload. After the bond of the monoclonal antibody with its own target, the drug is internalized and the linker is degenerated releasing the cytotoxic payload, with consequent antitumor effect. Some of these drugs have been already available in trials for NSCLC patients and specifically target MET (telisotuzumab vedotin), HER2 (trastuzumab deruxtecan), HER3 (patritumab deruxtecan), TROP2 (datopotamab deruxtecan and SKB264). All these antibody-drug conjugates are studied after osimertinib resistance. However, only patritumab deruxtecan and telisotuzumab vedotin are combined with osimertinib. Interestingly, the efficacy results from phase I and phase II trials with patritumab deruxtecan were achieved across a broad range of pretreatment HER3 expression in the tumor membrane and across the various EGFR-TKI resistance mechanisms (25, 26).
Finally, the category of bispecific antibodies in this setting is represented only by amivantamab until now. This is a fully humanized antibody directed against mutated EGFR and mutated or amplified MET. It exerts its function both via the inhibition of the ligand binding and antibody-dependent cellular cytotoxicity (27). This drug is combined with lazertinib, a potent brain-penetrant third-generation EGFR TKI, and investigated in three clinical trials (CHRYSALIS, CHRYSALIS-2, MARIPOSA-2) for patients progressing during or after osimertinib.
ORCHARD is a biomarker-directed Phase II platform trial. This study is evaluating the optimal treatment strategy depending on the underlying resistance mechanism to first-line osimertinib. For this reason, treatment assignment is based on a molecular tumor characterization from a tissue biopsy performed at progression during osimertinib. Each patient will be assigned to treatment with the combination of osimertinib and a specific targeted drug for the resistance mechanism detected, e.g. osimertinib + savolitinib for MET amplification, osimertinib + necitumumab for EGFR amplification, and so on. If a resistance mechanism is not found, the patient will be assigned to platinum-based chemotherapy with durvalumab. Those patients with resistance mechanisms not targetable will be treated according to local practice. This trial also includes an adaptive design to allow the addition of new emerging drugs (28). Recently, an interim analysis of safety and efficacy showed that osimertinib + necitumumab in patients with a secondary gene alteration in EGFR, i.e. amplification, L718 or G724 mutation, exon 20 insertion, met futility criterion so that recruitment for this treatment was closed (29).
The therapeutic strategies for the acquired resistance to osimertinib we discussed before are suitable for EGFR-independent resistance mechanisms, which are more frequent for patients treated with first-line osimertinib. However, some of these patients develop on-target EGFR mutations, such as C797S point mutation in exon 20, mainly in cis with the activating mutation. To target this alteration a specific drug does not yet exist. Brigatinib, an ALK inhibitor showed activity against cells with this mutation, but it needed the combination with cetuximab to be effective in vivo (30). In a retrospective analysis including 15 patients who developed resistance to osimertinib because of C797S mutation, brigatinib plus cetuximab achieved the 10% objective response rate and 60% disease control rate (31).
Among the various EGFR inhibitors under development, there are new TKIs, which are “fourth generation” or allosteric EGFR inhibitors (Table 1). It means that these drugs bind to the receptor outside the ATP pocket of the kinase domain. This bond selectively alters EGFR conformation and bypasses the resistance mechanisms mediated by new mutations in the ATP-binding domain, mainly C797S (32). Among these drugs, a limited activity was obtained with EAI-045, the first one identified in this class, when used as a single agent, but it has to be combined with cetuximab to be effective in cell lines with triple EGFR mutations (L858R/T790M/C797S). Similarly, JBJ-04-125-02 achieved increased cell death and more effective inhibition of cell proliferation when combined with osimertinib, compared with single agent alone (33). CH7233163, a further fourth generation EGFR TKI, exhibited even more potent antitumor activity against the EGFR triple mutation (ex19del/T790M/C797S) (34). Some other allosteric EGFR inhibitors were included in the design of phase I/II clinical trials, i.e. BLU-701 (HARMONY trial), BLU-945 (SYMPHONY trial), BBT-176, JIN-A02. The first two of these are studied both as single agent and in combination with osimertinib in patients who progressed during or after a previous EGFR TKI.
From the scenario of these new therapeutic strategies emerges the possibility of avoiding or delaying the chemotherapy option for patients who develop resistance to first-line osimertinib. We still do not have sufficient efficacy data available to allow considering some therapeutic strategies over others. However, based on the molecular targets at which these new drugs are directed, when patients treated with osimertinib become refractory to this drug, it is necessary to evaluate which resistance mechanism has developed. The search for these resistance mechanisms involves the need to carry out tissue biopsies, possibly associated with a blood sample for the detection of gene alterations in cell-free DNA. This combined, liquid and tissue, evaluation would allow defining the prevailing resistance mechanism, on the basis of which the most appropriate molecular treatment can be indicated. In principle, the therapeutic choice could be oriented towards combination strategies of osimertinib plus a specific inhibitor or antibody-drug conjugate or bispecific antibody in the case of EGFR-independent resistance mechanisms (e.g. MET overexpression or MET amplification, as highlighted in the biomarker analysis of CHRYSALIS-2 trial) (35). Instead, the use of allosteric EGFR TKIs would be more suitable in case of the prevalence of new EGFR mutations, especially at the ATP-binding site. A relevant issue that could be resolved by the results of these ongoing clinical trials concerns the usefulness of combining these new drugs with the continuation of osimertinib versus its suspension. We summarized a possible future scenario in Figure 1 to attribute a next-generation therapeutic option to each of the more frequent mechanisms of resistance to first-line osimertinib. On the basis of the findings that are emerging from many clinical trials, we suppose that in the next future oncologists will be able to address each patient, who experience resistance to upfront osimertinib, toward a different treatment strategy according to the molecular alterations highlighted via circulating cell-free DNA and/or DNA from tumor tissue biopsy. However, the results of upfront combination strategies, such as those investigated in FLAURA-2 and MARIPOSA trial, will further change the scenario, and we think that a different spectrum of resistance mechanisms could emerge. To face with the complexity of gene alterations that can lead to the resistance to first-line treatment, i.e. osimertinib single agent or combination strategies, liquid biopsy will be mandatory and, if not informative, it can be completed with tissue biopsy. However, we believe that the design of platform trials could be the best option to manage the complexity of resistance occurrence.
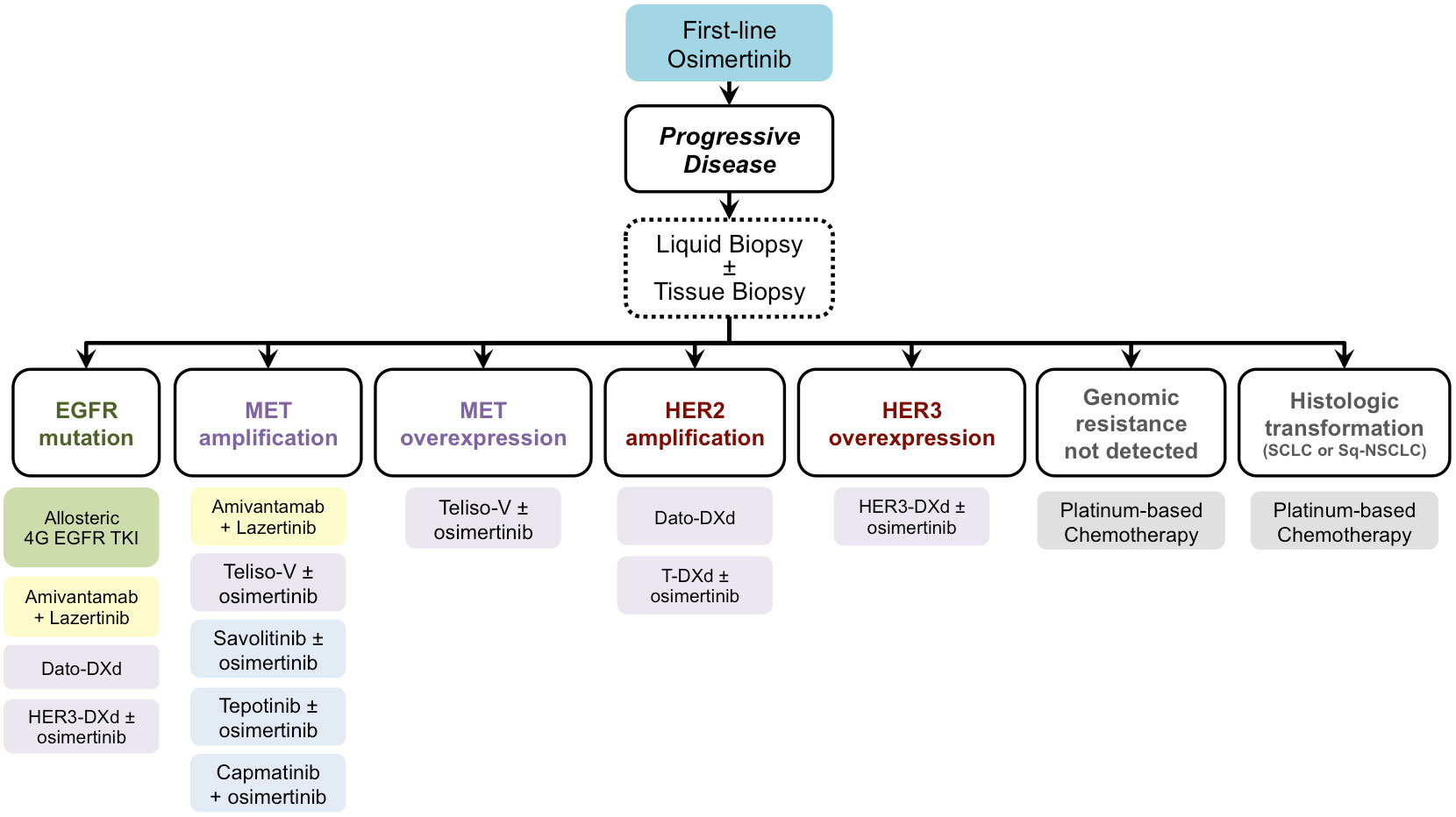
Figure 1 Future scenario for treating patients who developed resistance during or after osimertinib according to post-progression molecular characterization. EGFR, Epidermal Growth Factor Receptor; MET, MET Proto-Oncogene, Receptor Tyrosine Kinase; HER2, Human Epidermal Growth Factor Receptor 2; HER3, Human Epidermal Growth Factor Receptor 3; 4G, fourth generation; Dato-DXd, Datopotamab Deruxtecan; HER3-DXd, Patritumab Deruxtecan; Teliso-V, Telisotuzumab Vedotin; T-DXd, Trastuzumab Deruxtecan; SCLC, small cell lung cancer; Sq-NSCLC, squamous non-small cell lung cancer.
GB: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. AB: Data curation, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing. LCa: Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. LCr: Conceptualization, Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
LCa has served as consultant or advisor to Bristol-Myers Squibb, Merck Sharp and Dohme, and has received compensated educational activities from Bristol Myers Squibb, Astrazeneca, and Sanofi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med (2020) 383:640–9. doi: 10.1056/NEJMoa1916623
2. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol (2023) 34:358–76. doi: 10.1016/j.annonc.2022.12.013
3. Hendriks LE, Kerr KM, Menis J, Mok S, Nestle U, Passaro A, et al. EGC. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol (2023) 34:339–57. doi: 10.1016/j.annonc.2022.12.009
4. Lee CK, Davies L, Wu Y-L, Mitsudomi T, Inoue A, Rosell R, et al. Gefitinib or erlotinib vs chemotherapy for EGFR mutation-positive lung cancer: individual patient data meta-analysis of overall survival. J Natl Cancer Inst (2017) 109(6). doi: 10.1093/jnci/djw279
5. Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med (2017) 376:629–40. doi: 10.1056/NEJMoa1612674
6. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med (2020) 382:41–50. doi: 10.1056/NEJMoa1913662
7. Planchard D, Jänne PA, Cheng Y, Yang JC-H, Yanagitani N, Kim S-W, et al. Osimertinib with or without chemotherapy in EGFR-mutated advanced NSCLC. N Engl J Med (2023) 389:1935–48. doi: 10.1056/NEJMoa2306434
8. Cho BC, Felip E, Spira AI, Girard N, Lee J, Lee S, et al. LBA14 - Amivantamab plus lazertinib vs osimertinib as first-line treatment in patients with EGFR-mutated, advanced non-small cell lung cancer (NSCLC): Primary results from MARIPOSA, a phase III, global, randomized, controlled trial. Ann Oncol (2023) 34:S1254–335. doi: 10.1016/annonc/annonc1358
9. Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med (2015) 21:560–2. doi: 10.1038/nm.3854
10. Oxnard GR, Hu Y, Mileham KF, Husain H, Costa DB, Tracy P, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol (2018) 4:1527–34. doi: 10.1001/jamaoncol.2018.2969
11. Santoni-Rugiu E, Melchior LC, Urbanska EM, Jakobsen JN, de SK, Grauslund M, et al. Intrinsic resistance to EGFR-tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: differences and similarities with acquired resistance. Cancers (Basel) (2019) 11(7):923. doi: 10.3390/cancers11070923
12. Karachaliou N, Chaib I, Cardona AF, Berenguer J, Bracht JWP, Yang J, et al. Common co-activation of AXL and CDCP1 in EGFR-mutation-positive non-smallcell lung cancer associated with poor prognosis. EBioMedicine (2018) 29:112–27. doi: 10.1016/j.ebiom.2018.02.001
13. Ortiz-Cuaran S, Scheffler M, Plenker D, Dahmen L, Scheel AH, Fernandez-Cuesta L, et al. Heterogeneous mechanisms of primary and acquired resistance to third-generation EGFR inhibitors. Clin Cancer Res an Off J Am Assoc Cancer Res (2016) 22:4837–47. doi: 10.1158/1078-0432.CCR-15-1915
14. Gray JE, Okamoto I, Sriuranpong V, Vansteenkiste J, Imamura F, Lee JS, et al. Tissue and plasma EGFR mutation analysis in the FLAURA trial: osimertinib versus comparator EGFR tyrosine kinase inhibitor as first-line treatment in patients with EGFR-mutated advanced non-small cell lung cancer. Clin Cancer Res an Off J Am Assoc Cancer Res (2019) 25:6644–52. doi: 10.1158/1078-0432.CCR-19-1126
15. Schoenfeld AJ, Yu HA. The evolving landscape of resistance to osimertinib. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2020) 15:18–21. doi: 10.1016/j.jtho.2019.11.005
16. Schoenfeld AJ, Chan JM, Kubota D, Sato H, Rizvi H, Daneshbod Y, et al. Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin Cancer Res an Off J Am Assoc Cancer Res (2020) 26:2654–63. doi: 10.1158/1078-0432.CCR-19-3563
17. Chmielecki J, Gray JE, Cheng Y, Ohe Y, Imamura F, Cho BC, et al. Candidate mechanisms of acquired resistance to first-line osimertinib in EGFR-mutated advanced non-small cell lung cancer. Nat Commun (2023) 14:1070. doi: 10.1038/s41467-023-35961-y
18. Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Joon OP, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science (2007) 316:1039–43. doi: 10.1126/science.1141478
19. Offin M, Chan JM, Tenet M, Rizvi HA, Shen R, Riely GJ, et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at risk for Histologic Transformation and Inferior Clinical Outcomes. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2019) 14:1784–93. doi: 10.1016/j.jtho.2019.06.002
20. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med (2019) 7:387–401. doi: 10.1016/S2213-2600(19)30084-0
21. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol Off J Eur Soc Med Oncol (2019) 30:1321–8. doi: 10.1093/annonc/mdz167
22. Lee ATM, Nagasaka M. CheckMate-722: the rise and fall of nivolumab with chemotherapy in TKI-refractory EGFR-mutant NSCLC. Lung Cancer (Auckland NZ) (2023) 14:41–6. doi: 10.2147/LCTT.S408886
23. Bennouna J, Girard N, Audigier-Valette C, le Thuaut A, Gervais R, Masson P, et al. Phase II study evaluating the mechanisms of resistance on tumor tissue and liquid biopsy in patients with EGFR-mutated non-pretreated advanced lung cancer receiving osimertinib until and beyond radiologic progression: the MELROSE trial. Clin Lung Cancer (2020) 21:e10–4. doi: 10.1016/j.cllc.2019.09.007
24. Araki T, Kanda S, Horinouchi H, Ohe Y. Current treatment strategies for EGFR-mutated non-small cell lung cancer: from first line to beyond osimertinib resistance. Jpn J Clin Oncol (2023) 53:547–61. doi: 10.1093/jjco/hyad052
25. Jänne PA, Baik C, Su W-C, Johnson ML, Hayashi H, Nishio M, et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated non-small cell lung cancer. Cancer Discovery (2022) 12:74–89. doi: 10.1158/2159-8290.CD-21-0715
26. Yu HA, Goto Y, Hayashi H, Felip E, Chih-Hsin Yang J, Reck M, et al. HERTHENA-lung01, a phase II trial of patritumab deruxtecan (HER3-DXd) in epidermal growth factor receptor-mutated non-small-cell lung cancer after epidermal growth factor receptor tyrosine kinase inhibitor therapy and platinum-based chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol (2023) 41(35):5363–5375. doi: 10.1200/JCO.23.01476
27. Cho BC, Simi A, Sabari J, Vijayaraghavan S, Moores S, Spira A. Amivantamab, an epidermal growth factor receptor (EGFR) and mesenchymal-epithelial transition factor (MET) bispecific antibody, designed to enable multiple mechanisms of action and broad clinical applications. Clin Lung Cancer (2023) 24:89–97. doi: 10.1016/j.cllc.2022.11.004
28. Yu HA, Goldberg SB, Le X, Piotrowska Z, Goldman JW, De Langen AJ, et al. Biomarker-directed phase II platform study in patients with EGFR sensitizing mutation-positive advanced/metastatic non-small cell lung cancer whose disease has progressed on first-line osimertinib therapy (ORCHARD). Clin Lung Cancer (2021) 22:601–6. doi: 10.1016/j.cllc.2021.06.006
29. Riess JW, De Langen JA, Piotrowska Z, Goldberg SB, Goldman JW, Okamoto I, et al. 329P ORCHARD: Osimertinib + necitumumab in patients (pts) with advanced NSCLC whose disease progressed on first-line (1L) osimertinib. Ann Oncol (2022) 33:S1571–2. doi: 10.1016/j.annonc.2022.10.368
30. Uchibori K, Inase N, Araki M, Kamada M, Sato S, Okuno Y, et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun (2017) 8:14768. doi: 10.1038/ncomms14768
31. Wang Y, Yang N, Zhang Y, Li L, Han R, Zhu M, et al. Effective treatment of lung adenocarcinoma harboring EGFR-activating mutation, T790M, and cis-C797S triple mutations by brigatinib and cetuximab combination therapy. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2020) 15:1369–75. doi: 10.1016/j.jtho.2020.04.014
32. Jia Y, Yun C-H, Park E, Ercan D, Manuia M, Juarez J, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature (2016) 534:129–32. doi: 10.1038/nature17960
33. To C, Jang J, Chen T, Park E, Mushajiang M, De Clercq DJH, et al. Single and dual targeting of mutant EGFR with an allosteric inhibitor. Cancer Discovery (2019) 9:926–43. doi: 10.1158/2159-8290.CD-18-0903
34. Kashima K, Kawauchi H, Tanimura H, Tachibana Y, Chiba T, Torizawa T, et al. CH7233163 overcomes osimertinib-resistant EGFR-del19/T790M/C797S mutation. Mol Cancer Ther (2020) 19:2288–97. doi: 10.1158/1535-7163.MCT-20-0229
35. Besse B, Baik CS, Marmarelis ME, Sabari JK, Goto K, Shu CA, et al. Predictive biomarkers for treatment with amivantamab plus lazertinib among EGFR-mutated NSCLC in the post-osimertinib setting: Analysis of tissue IHC and ctDNA NGS. J Clin Oncol (2023) 41(16):S9013. doi: 10.1200/JCO.2023.41.16_suppl.9013
Keywords: non-small cell lung cancer, osimertinib, resistance, tyrosine kinase inhibitor, allosteric inhibitor
Citation: Bronte G, Belloni A, Calabrò L and Crinò L (2024) The great need to overcome osimertinib resistance in advanced non-small cell lung cancer: from combination strategies to fourth-generation tyrosine kinase inhibitors. Front. Oncol. 13:1308460. doi: 10.3389/fonc.2023.1308460
Received: 06 October 2023; Accepted: 26 December 2023;
Published: 09 January 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Gonzalo Recondo, Norberto Quirno Medical Education and Clinical Research Center (CEMIC), ArgentinaCopyright © 2024 Bronte, Belloni, Calabrò and Crinò. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Bronte, g.bronte@staff.univpm.it
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.