- 1Division of Thoracic Tumor Multimodality Treatment, Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Department of Radiotherapy Physics & Technology, West China Hospital, Sichuan University, Chengdu, China
Background: The distant metastasis of lung cancer primarily occurs in the bones, liver, brain, and lungs, while the breast is an extremely rare site of metastasis. There is very limited literature on the occurrence of breast metastasis from lung cancer, and metastatic lesions in the breast are prone to being misdiagnosed as primary breast cancer, requiring careful attention and differentiation in the clinical diagnostic and treatment process.
Case summary: The patient, a 63-year-old female, initially presented with an EGFR exon 21 L858R mutated left lung adenocarcinoma in 2017, treated successfully with surgical resection and subsequent monitoring. The relapse of disease occurred in January 2020. Despite maintaining a prolonged progression-free survival (PFS) with first-generation EGFR-TKI Afatinib, disease progression occurred in 2022 without detectable resistance mutations. Transition to second-generation TKI Furmonertinib resulted in poor control, with rapid progression including unusual bilateral breast metastases that exhibited inflammatory breast cancer-like peau d’orange changes. Standard chemotherapy achieved only short-term stability. Upon detecting a MET amplification mutation, treatment with Savolitinib was initiated. Remarkably, this led to significant clinical and radiographic improvement, notably resolving the peau d’orange appearance and reducing multiple lesions across the body.
Conclusion: This case underscores the importance of continuous genetic profiling and tailored treatment approaches in managing advanced lung adenocarcinoma, particularly when presenting with rare metastatic sites and complex genetic landscapes. The successful application of Savolitinib following the identification of a MET amplification mutation highlights its potential in overcoming resistance mechanisms in NSCLC, providing a significant therapeutic option for similarly challenging cases.
Introduction
Lung cancer is the most common malignant tumor worldwide and one of the deadliest cancers (1). Among them, non-small cell lung cancer (NSCLC) accounts for more than 85% of lung cancer cases, with lung adenocarcinoma being the most common subtype (2). Distant metastasis of lung cancer mainly occurs in the bones, liver, brain, and lungs, while the breast is an extremely rare site for metastasis (3). Breast tissue is considered an unconventional site for lung cancer metastasis, which may be related to the rich lymphatic system and capillary network in the breast. Its physiological characteristics may influence the tendency of lung cancer metastasis (4). Existing literature reports very few cases of breast metastasis in lung cancer.
Savolitinib is a highly selective mesenchymal-epithelial transition factor (MET) tyrosine kinase inhibitor (TKI) used primarily for treating NSCLC with MET exon 14 skipping mutations (5). It operates by blocking the MET signaling pathway, which is crucial for tumor growth and metastasis. Clinical studies have shown that Savolitinib, particularly in combination with other epidermal growth factor receptor (EGFR) inhibitors, can overcome resistance mechanisms in NSCLC that have failed previous treatments (6).
This reports a case of advanced lung adenocarcinoma with multiple metastases, including metastasis to the breast, with EGFR and MET mutation. We discuss this rare case in conjunction with existing relevant literature, aiming to enhance understanding of such phenomena and provide support for clinical decision-making. Additionally, through the report and review of this rare case, we hope to uncover more characteristics of lung cancer metastasis to the breast, providing further clues for future clinical decision-making and research.
Case presentation
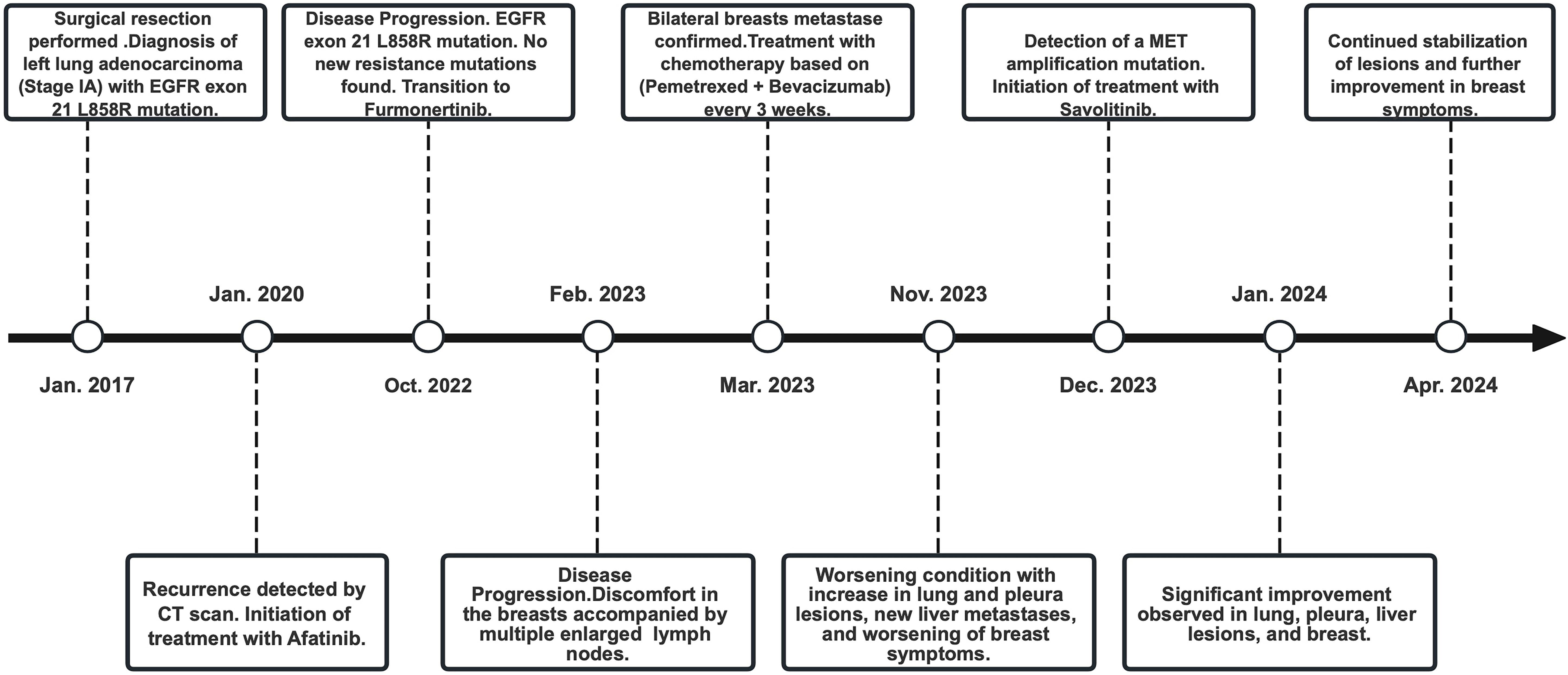
The patient, a 63-year-old elderly female, was identified with an occupying lesion in the left lung in January 2017, which was subsequently excised. The postoperative pathology revealed lung invasive adenocarcinoma. Genetic testing of the postoperative tissue showed an EGFR exon 21 L858R mutation. As the patient’s disease was staged at IA (according to AJCC 8th edition criteria) (7), postoperative targeted therapy was not administered. From July 2017 to January 2020, regular follow-up examinations showed no recurrence of the disease. In January 2020, a new solid nodule was detected in the left lung during a follow-up, suggesting recurrence, and the patient began treatment with afatinib. From January 2020 to October 2022, during the treatment period, regular examinations showed no disease progression.
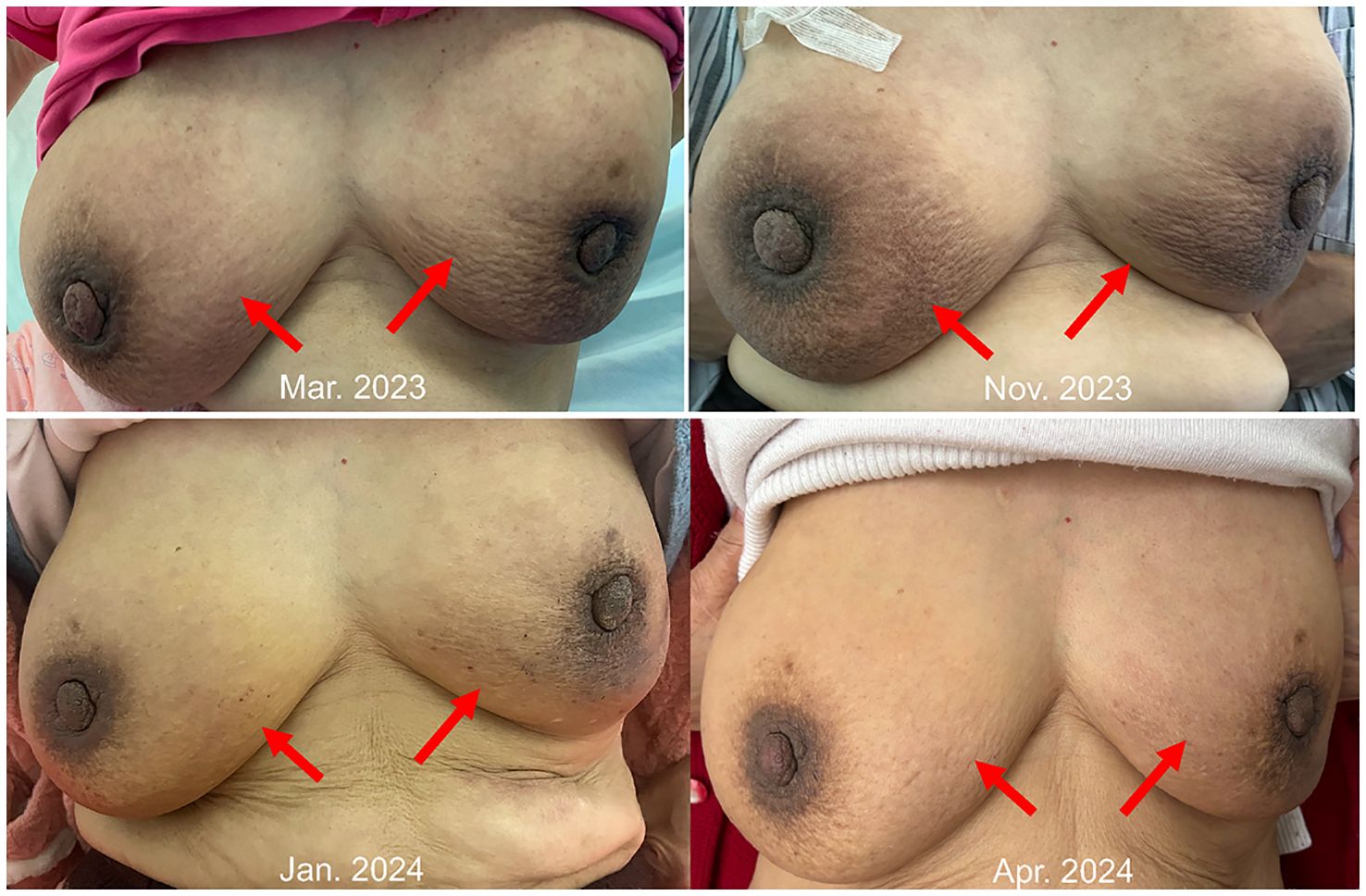
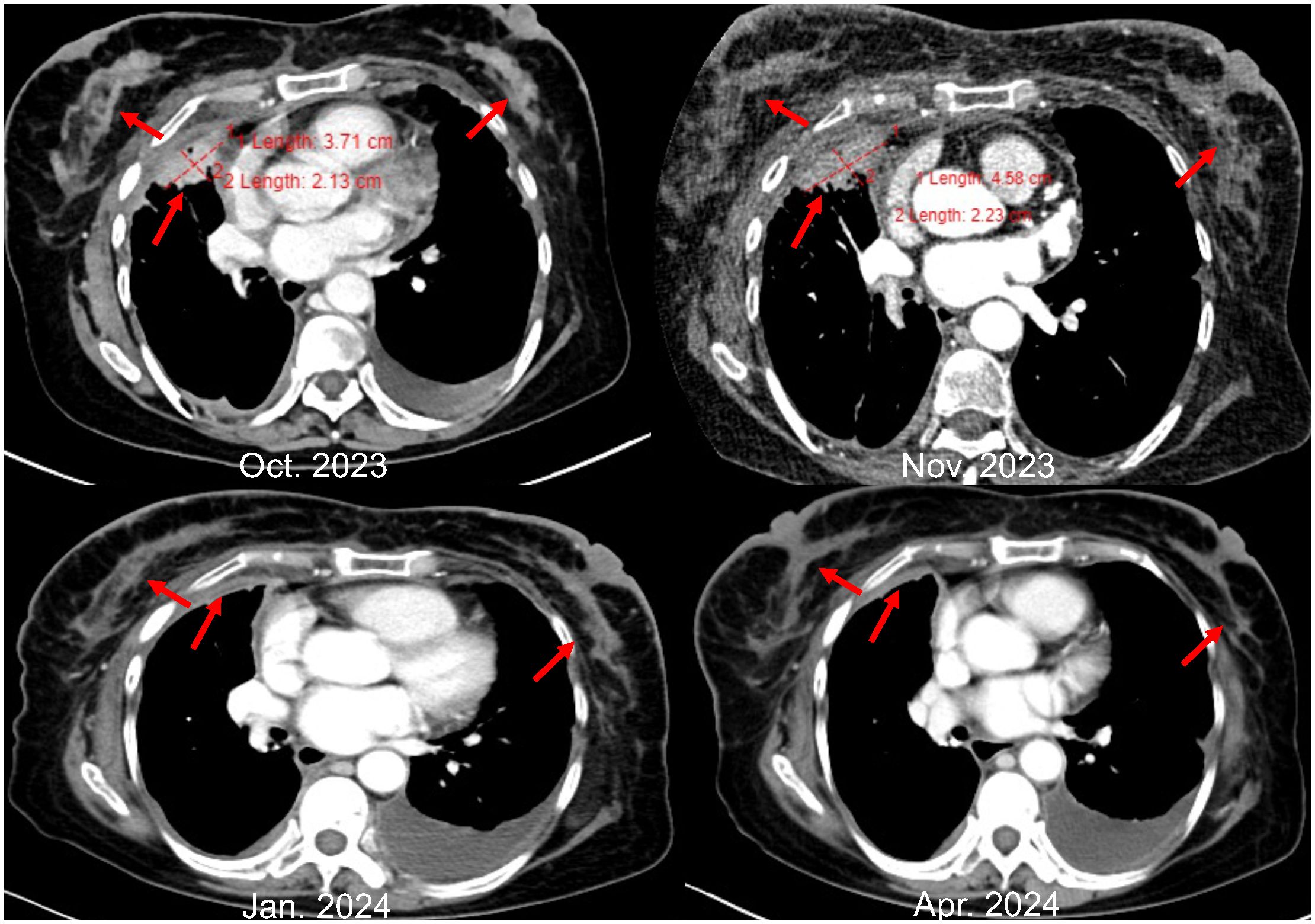
However, in October 2022, multiple new lesions were found in both lungs during a check-up, leading to a determination of disease progression (PD) according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Re-testing still showed the EGFR exon 21 L858R mutation without new resistance mutations. Considering the ineffectiveness of afatinib treatment, the patient was switched to Furmonertinib for targeted therapy. In January 2023, the patient experienced discomfort in the breasts, but visual examination of the skin showed no abnormalities. Breast ultrasound indicated ductal ectasia with Breast Imaging-Reporting and Data System (BI-RADS) category 4A, accompanied by multiple abnormal enlarged axillary lymph nodes. Further imaging studies in February 2023 revealed additional lymph node metastases in the cervical, axillary, mediastinal, and retroperitoneal regions, as well as breast and bone metastases. The therapeutic effect of Furmonertinib was poor, and the patient’s condition progressed comprehensively. In March 2023, the patient’s bilateral breast skin began to show radiating streaks with pigmentation, presenting a peau d’orange appearance (Figure 1). Biopsy of the left breast confirmed poorly differentiated adenocarcinoma consistent with pulmonary origin (Figure 2). Due to the ineffectiveness of Furmonertinib, from March 2023 to October 2023, the patient underwent chemotherapy based on pemetrexed combined with bevacizumab. The overall therapeutic effect during chemotherapy was stable disease (SD).
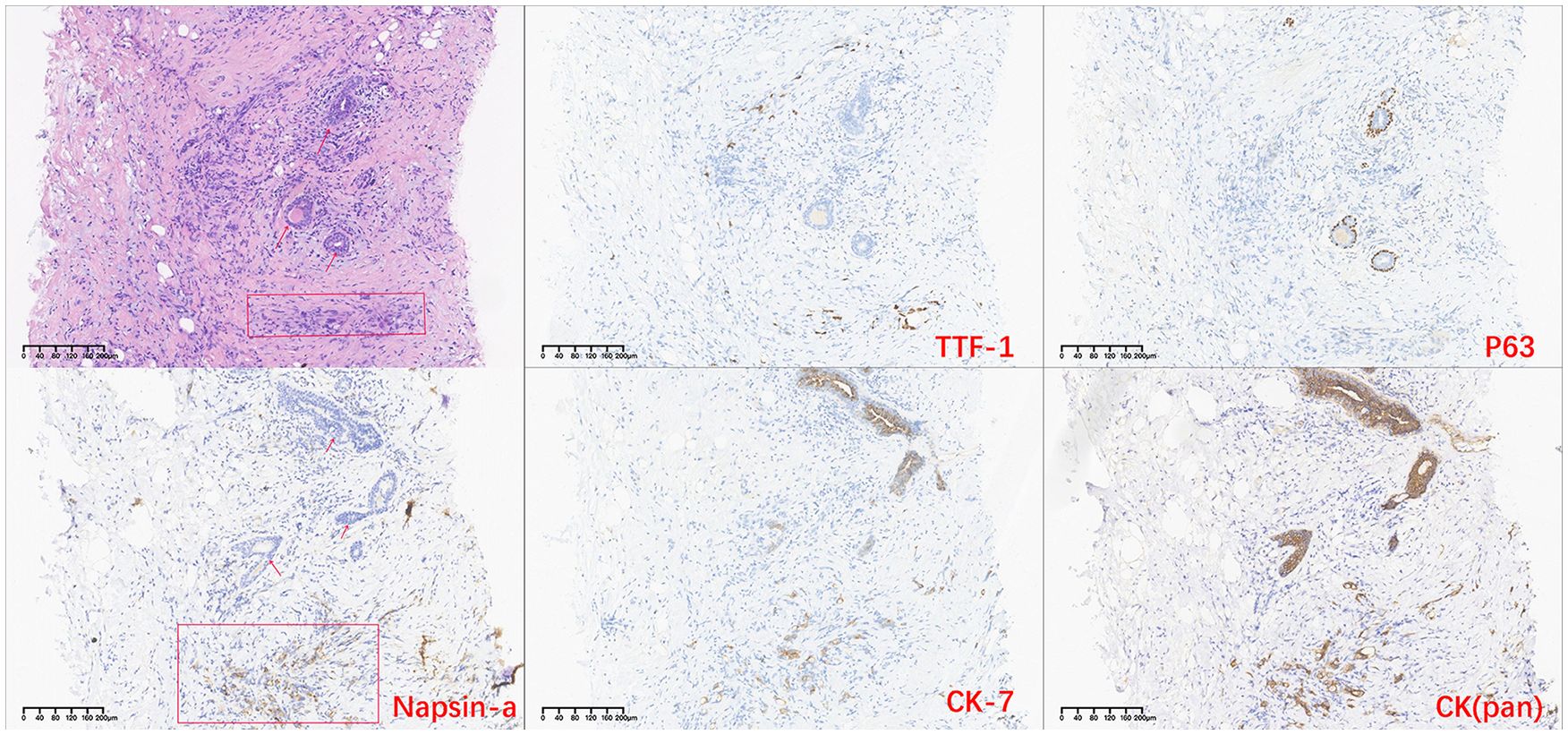
Figure 2. H-E stained histopathological and immunohistochemistry images of breast tissue. (The arrow indicates breast duct epithelium, and the rectangular box indicates tumor cells site).
Yet again, in November 2023, enhanced imaging examinations revealed an increase in the size of the lesions in both lungs and the pleura (Figure 3), new liver metastases, and worsening of the peau d’orange appearance of the breast, indicating further disease progression. Subsequent tissue biopsy and next-generation sequencing revealed, besides the EGFR L858R mutation, an additional MET amplification mutation. Consequently, from December 2023, the patient commenced treatment with Savolitinib. One month later, imaging showed significant reduction in the lesions in the lungs, pleura, and liver, achieving partial remission (PR), and a marked improvement in the peau d’orange changes of the breast. Three months later, imaging indicated stabilization of lesions throughout the body including pleura, with further improvement in the breast’s peau d’orange appearance. The timeline of this patient’s treatment journey was shown in Figure 4. As of the last update, the patient’s PFS with Savolitinib treatment has been maintained for five months.
Discussion
A total of 29 cases of breast metastasis were reported in the 23 years from 2000 to 2023 (8). Although the metastasis of lung cancer to the breast is rare, when it occurs, it poses a series of challenges in terms of diagnosis and treatment. In many cases, this type of metastasis may be misdiagnosed as breast cancer, requiring clear pathological diagnosis and genetic analysis to guide the optimal treatment strategy (9). In certain instances, breast metastasis from lung cancer may exhibit a favorable response to standard treatment approaches for lung cancer (10). This further emphasizes the importance of understanding and identifying such rare metastases.
At first, Lung cancer cells can metastasize to distant sites through vascular and lymphatic systems. Due to the rich lymphatic and blood supply in the breast, it is plausible that lung cancer cells exploit these networks to establish metastatic sites (11). The anatomical and physiological characteristics of the breast, such as its extensive vascular network, may facilitate the lodging and growth of metastatic lung cancer cells.
Secondly, the tumor microenvironment, influenced by interactions between tumor cells and surrounding stroma, can promote metastasis. Factors secreted by tumor cells, such as VEGF (vascular endothelial growth factor) and TGF-β (transforming growth factor-beta), can modify the microenvironment to support tumor invasion and metastasis (12). The microenvironment of the breast might provide a fertile ground for lung cancer cells due to its unique stromal composition, which can be influenced by hormonal status.
Finally, the molecular drivers, which are probably the most applicable to this case. Despite significant advancements in the treatment of EGFR-mutated lung adenocarcinoma, such as targeted therapies for this mutation, its performance and mechanisms in breast metastasis remain unclear (13). Mutations in EGFR can activate downstream signaling pathways, such as PI3K/Akt and MAPK, enhancing the metastatic potential of tumor cells to colonize distant organs, including the breast (14). The EGFR mutation in lung adenocarcinoma patients may also influence the pattern of metastasis. By altering the interaction between tumor cells and the microenvironment, as well as affecting the invasiveness and migratory abilities of tumor cells, EGFR mutations can modify the metastatic patterns of lung adenocarcinoma (15). In certain case, a correlation has been explored between EGFR mutation and the metastasis of lung cancer to the breast (16). However, further research is still needed to determine the precise mechanisms underlying this relationship. The patient’s case, as highlighted, initially showed no additional genetic mutations when breast metastasis first appeared, suggesting that the breast lesions were primarily driven by the existing EGFR mutation. As the disease progressed, despite ongoing targeted therapy, a MET amplification mutation was eventually detected, indicating a shift in the driving force of the tumor’s biology.
MET amplification leads to overexpression of the MET protein, which in turn increases the activation of its tyrosine kinase domain. This activation triggers multiple downstream signaling cascades, notably the PI3K/Akt and MAPK pathways, which are crucial for cell survival, proliferation, and migration. These pathways are also involved in the epithelial-mesenchymal transition (EMT) (17, 18). MET and EGFR pathways exhibit significant crosstalk, which can contribute to compensatory signaling when one pathway is inhibited. When EGFR- tyrosine kinase inhibitor (TKI) therapies, such as erlotinib or gefitinib, are used to target EGFR mutations in NSCLC, cells with MET amplification can bypass the blocked EGFR signaling by activating alternative pathways through MET, leading to continued tumor growth and survival despite EGFR inhibition (19, 20). The crosstalk between MET and other receptor tyrosine kinases facilitates a bypass track for signaling, contributing to resistance against EGFR-TKIs. In clinical settings, patients initially responsive to EGFR-TKIs often develop resistance over time, which can frequently be traced back to secondary mutations or amplifications like MET. This resistance is characterized by a resurgence of proliferative and survival signaling despite ongoing EGFR blockade (21–23).
Besides resistance, MET amplification is directly linked to increased metastatic capabilities of tumor cells. MET-driven signaling enhances cell motility, invasion, and disruption of normal cellular adhesion, all of which are critical steps in the metastatic spread of cancer cells to distant organs (17, 18). Also, MET enhances the expression of vascular endothelial growth factor (VEGF), a key mediator of angiogenesis. The interaction between MET and VEGF signaling pathways boosts the vascularization of tumors, aiding their growth and the establishment of metastatic colonies (24). MET-driven signals can suppress immune surveillance mechanisms and modify the inflammatory response, creating a tumor-permissive environment that supports cancer cell survival and dissemination. This modulation of the tumor microenvironment not only aids in primary tumor growth but also plays a crucial role in preparing distant sites for metastatic colonization (25).
Therefore, it is pertinent to highlight the clinical significance of continuous genetic profiling (26). The dynamic nature of tumor genetics, exemplified in this case by the evolution from an EGFR mutation to an additional MET amplification, underscores the necessity of repeated genetic assessments. The development of resistance to targeted therapies, as observed in this patient with the initial response to EGFR-TKIs followed by progression, is a significant challenge in the treatment of lung adenocarcinoma. The resistance result required alternative therapeutic strategies, such as the use of MET inhibitors in cases where MET amplification is identified (19).
Savolitinib inhibits the activity of MET kinase, effectively blocking the MET signaling pathways. It targets the ATP binding site of the MET protein, which is essential for its kinase activity, thereby preventing the phosphorylation and activation of MET. This inhibition leads to a disruption in the downstream signaling pathways, particularly the PI3K/Akt and MAPK pathways, which are involved in promoting cell proliferation and survival. By inhibiting these pathways, Savolitinib impedes tumor growth and metastasis (6, 27). Savolitinib has progressed through various phases of clinical trials, showing beneficial efficacy and an acceptable safety profile in treating NSCLC patients with acquired resistance due to MET amplification. These studies highlight its potential not only as a monotherapy but also in combination with other targeted therapies, providing a robust strategy for tackling complex resistance mechanisms in advanced NSCLC (28–31).
In addition, there is a serious lack of understanding regarding effective treatment options for lung cancer metastasis to the breast. Due to the rarity of breast metastasis, it is difficult to conduct large-scale clinical trials to test different treatment strategies (32, 33). Currently, most treatment decisions are based on individual case reports and small case series. Each case contributes valuable insights into the potential responses to therapies and outcomes, forming a basis for future research and clinical guidelines. This is also one of the reasons why we report such rare case in this article, as we hope to provide more information for future understanding and treatment of similar cases.
Conclusions
In summary, this case report is a valuable addition to the existing literature, particularly in understanding the potential of Savolitinib in treating NSCLC with MET amplification and managing rare metastatic presentations like bilateral breast metastasis. It highlights the importance of genetic profiling in guiding treatment decisions and the potential for new therapies to improve outcomes in challenging cases of lung adenocarcinoma.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RD: Conceptualization, Writing – original draft. Y-YL: Conceptualization, Writing – review & editing. L-LB: Data curation, Writing – review & editing. LZ: Supervision, Writing – review & editing. Y-SW: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (award No. 11905150).
Acknowledgments
The authors thank the patient and families for their agreement to the publication of this report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, et al. Non-small-cell lung cancer. Nat Rev Dis Primers. (2015) 1:1–16. doi: 10.1038/nrdp.2015.9
3. Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic sites and survival in lung cancer. Lung Cancer. (2014) 86:78–84. doi: 10.1016/j.lungcan.2014.07.020
4. Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, et al. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol. (2012) 188:5954–61. doi: 10.4049/jimmunol.1103466
5. Markham A. Savolitinib: first approval. Drugs. (2021) 81:1665–70. doi: 10.1007/s40265-021-01584-0
6. Zhu X, Lu Y, Lu S. Landscape of savolitinib development for the treatment of non-small cell lung cancer with MET alteration—A narrative review. Cancers. (2022) 14:6122. doi: 10.3390/cancers14246122
7. Edition S, Edge S, Byrd D. AJCC cancer staging manual. New York, NY, USA: Springer-Verlag (2017).
8. Ding J, Gu H, Yang Z, Lu Y, Guo G. Breast metastasis from lung adenocarcinoma: a case report and review of the literature. Front Oncol. (2024) 14. doi: 10.3389/fonc.2024.1370453
9. Lee SK, Kim WW, Kim SH, Hur SM, Kim S, Choi JH, et al. Characteristics of metastasis in the breast from extramammary Malignancies. J Surg Oncol. (2010) 101:137–40. doi: 10.1002/jso.21453
10. Guedes H, Barroso A, João D, Furtado A, Costa T. Lung cancer and breast metastasis: A rare and atypical presentation. Pulmonology. (2023) 30(3):305–6. doi: 10.1016/j.pulmoe.2023.08.001
11. Majidpoor J, Mortezaee K. Steps in metastasis: an updated review. Med Oncol. (2021) 38:3. doi: 10.1007/s12032-020-01447-w
12. Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. (2009) 9:239–52. doi: 10.1038/nrc2618
13. Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. (2010) 277:301–8. doi: 10.1111/j.1742-4658.2009.07448.x
14. Uribe ML, Marrocco I, Yarden Y. EGFR in cancer: Signaling mechanisms, drugs, and acquired resistance. Cancers. (2021) 13:2748. doi: 10.3390/cancers13112748
15. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. New Engl J Med. (2010) 362:2380–8. doi: 10.1056/NEJMoa0909530
16. Valenza C, Porta FM, Rappa A, Guerini-Rocco E, Viale G, Barberis M, et al. Complex differential diagnosis between primary breast cancer and breast metastasis from EGFR-mutated lung adenocarcinoma: case report and literature review. Curr Oncol. (2021) 28:3384–92. doi: 10.3390/curroncol28050292
17. Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan C, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. (2018) 17:1–14. doi: 10.1186/s12943-017-0753-1
18. Organ SL, Tsao M-S. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. (2011) 3:S7–S19. doi: 10.1177/1758834011422556
19. Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. (2007) 316:1039–43. doi: 10.1126/science.1141478
20. Shi P, Oh Y-T, Zhang G, Yao W, Yue P, Li Y, et al. Met gene amplification and protein hyperactivation is a mechanism of resistance to both first and third generation EGFR inhibitors in lung cancer treatment. Cancer Lett. (2016) 380:494–504. doi: 10.1016/j.canlet.2016.07.021
21. Juchum M, Günther M, Laufer SA. Fighting cancer drug resistance: Opportunities and challenges for mutation-specific EGFR inhibitors. Drug Resistance Updates. (2015) 20:12–28. doi: 10.1016/j.drup.2015.05.002
22. Remon J, Moran T, Majem M, Reguart N, Dalmau E, Marquez-Medina D, et al. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: a new era begins. Cancer Treat Rev. (2014) 40:93–101. doi: 10.1016/j.ctrv.2013.06.002
23. Tartarone A, Lerose R. Clinical approaches to treat patients with non-small cell lung cancer and epidermal growth factor receptor tyrosine kinase inhibitor acquired resistance. Ther Adv Respir Dis. (2015) 9:242–50. doi: 10.1177/1753465815587820
24. You W-K, Sennino B, Williamson CW, Falcón B, Hashizume H, Yao L-C, et al. VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res. (2011) 71:4758–68. doi: 10.1158/0008-5472.CAN-10-2527
25. Zambelli A, Biamonti G, Amato A. HGF/c-Met signaling in the tumor microenvironment. Tumor Microenvironment: Signaling Pathways–Part B. (2021) 1270:31–44. doi: 10.1007/978-3-030-47189-7_2
26. Cagle PT, Chirieac LR. Advances in treatment of lung cancer with targeted therapy. Arch Pathol Lab Med. (2012) 136:504–9. doi: 10.5858/arpa.2011-0618-RA
27. Jones RD, Petersson K, Tabatabai A, Bao L, Tomkinson H, Schuller AG. Pharmacokinetic/pharmacodynamic analysis of savolitinib plus osimertinib in an EGFR mutation–positive, MET-amplified non–small cell lung cancer model. Mol Cancer Ther. (2023) 22:679–90. doi: 10.1158/1535-7163.MCT-22-0193
28. Gan HK, Millward M, Hua Y, Qi C, Sai Y, Su W, et al. First-in-human phase I study of the selective MET inhibitor, savolitinib, in patients with advanced solid tumors: safety, pharmacokinetics, and antitumor activity. Clin Cancer Res. (2019) 25:4924–32. doi: 10.1158/1078-0432.CCR-18-1189
29. Sequist LV, Han J-Y, Ahn M-J, Cho BC, Yu H, Kim S-W, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicenter, open-label, phase 1b study. Lancet Oncol. (2020) 21:373–86. doi: 10.1016/S1470-2045(19)30785-5
30. Yang J-J, Fang J, Shu Y-Q, Chang J-H, Chen G-Y, He JX, et al. A phase Ib study of the highly selective MET-TKI savolitinib plus gefitinib in patients with EGFR-mutated, MET-amplified advanced non-small-cell lung cancer. Investigational New Drugs. (2021) 39:477–87. doi: 10.1007/s10637-020-01010-4
31. Wang Y, Liu T, Chen G, Gong J, Bai Y, Zhang T, et al. Phase Ia/Ib study of the selective MET inhibitor, savolitinib, in patients with advanced solid tumors: safety, efficacy, and biomarkers. Oncologist. (2022) 27:342–e83. doi: 10.1093/oncolo/oyab066
32. Parker AL, Benguigui M, Fornetti J, Goddard E, Lucotti S, Insua-Rodríguez J, et al. Current challenges in metastasis research and future innovation for clinical translation. Clin Exp Metastasis. (2022) 39:263–77. doi: 10.1007/s10585-021-10144-5
Keywords: case report, advanced lung adenocarcinoma, MET mutation, breast metastasis, Savolitinib
Citation: Deng R, Li Y-y, Bai L-l, Zhou L and Wang Y-S (2024) Case report: A case of Savolitinib in the treatment of MET amplification mutation advanced lung adenocarcinoma with rare bilateral breast metastasis. Front. Oncol. 14:1450855. doi: 10.3389/fonc.2024.1450855
Received: 18 June 2024; Accepted: 19 July 2024;
Published: 13 August 2024.
Edited by:
Jin-Ming Yang, University of Kentucky, United StatesReviewed by:
Min Zhang, University of Kentucky, United StatesXinyi Wang, University of Kentucky, United States
Copyright © 2024 Deng, Li, Bai, Zhou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhou, bGkuemhvdUBzY3UuZWR1LmNu; Yong-Sheng Wang, d2FuZ3lzNzZAZ21haWwuY29t
 Rui Deng
Rui Deng Yan-ying Li1
Yan-ying Li1 Li Zhou
Li Zhou