- 1Department of General & Neonatal Surgery, Children’s Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Structural Birth Defect and Reconstruction, Chongqing, China
- 2Department of Urology, Children’s Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Structural Birth Defect and Reconstruction, Chongqing, China
Background: Nutritional problem after surgery for Hirschprung’s disease (HSCR) was not optimistic. This study aimed to analyze the risk factors of postoperative undernutrition for patients with HSCR and establish a scoring system for predicting postoperative undernutrition.
Methods: Retrospective review of 341 patients with HSCR who received Laparoscopic-assisted pull-through surgery in a tertiary-level pediatric hospital was conducted with assessments of clinical data. Univariate/multivariate Logistic regression analysis was used to identify independent factors of postoperative undernutrition, and establish a scoring system for predicting postoperative nutritional status based on the sum of adjusted odds ratios (ORs).
Results: The postoperative undernutrition of 341 patients with HSCR was 29.9%. Multivariate Logistic regression analysis showed that non-breast feeding (mixed: OR = 6.116, artificial: OR = 12.00), preoperative undernutrition (risk of malnutrition: OR = 7.951, malnutrition: OR = 8.985), non-parental caregivers (OR = 3.164), long-segment HSCR (OR = 12.820), postoperative complications within 30 days (grade 1 ~ 2: OR = 2.924, Grade 3 ~ 4: OR = 6.249), and surgery for other systemic malformation (OR = 5.503) were risk factors for postoperative undernutrition (all p < 0.05), and scoring system was developed based on these determinants. The area under the receiver operator characteristic curve of the derivation sample was 0.887 (95% confidence interval [CI]: 0.839–0.934) and that of the validation sample was 0.846 (95% CI: 0.772 ~ 0.920) with the optimal cut-off value of 12; calibration curves of the derivation sample showed considerable predictive performance for postoperative undernutrition.
Conclusion: Risk factors identified affecting postoperative undernutrition should be taken seriously in patients with HSCR. We successfully developed a desirable scoring system to predict postoperative nutritional status, which might be helpful for clinical practice.
Introduction
Hirschprung’s disease (HSCR) is a congenital intestinal motility disorder caused by the absence of enteric ganglion cells in the myenteric and submucosal plexuses of the distal intestine, with a prevalence of approximately 1:5,000 and a male to female predominance of 4:1 (1). According to the length of ganglionotic segment, pediatric surgeons primarily categorize HSCR into three types: short-segment (S-HSCR, aganglionosis extending to the rectosigmoid), long-segment (L-HSCR, aganglionosis extending proximal to the sigmoid), and total colonic aganglionosis (TCA, aganglionosis involving the entire colon with or without extension to the ileum), and 80–85% of cases are limited to the rectosigmoid colon (2, 3). Surgical resection of the abnormally innervated bowel is the preferred management for HSCR, and temporary diverting enterostomy is indicated for patient with failed rectal irrigation (mainly L-HSCR and TCA) (4, 5). Over the past several decades, the development of nursing care and surgical techniques have significantly reduced the mortality of HSCR, but a series of postoperative problems have been gradually concerned, the hottest topic of which is Hirschsprung-associated enterocolitis (HAEC) (6). However, little exists in the current literature on postoperative nutritional assessment and influencing factors in patients with HSCR.
As a tertiary referral center, we have observed that nutritional problem was not optimistic and may be in the standardized and systematic follow-up of those patients. Nutritional problem negatively affects the growth and development of the child, even into adulthood (7). Pediatric surgeons need to be aware of this postoperative complication and elucidate the determinants of disparities in order to provide early intervention and parental counseling. Therefore, by collecting clinical data of patients with HSCR, this study aim to screen the risk factors of postoperative undernutrition and develop simple scoring system to identify patients at high risk of postoperative nutritional problem.
Materials and methods
Study approval
This study was approved by the Institutional Research Ethics Board of Children’s Hospital affiliated Chongqing Medical University (Date: 2021/No: 391) and complies with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Because this study was retrospective, the requirement for informed consent was waived.
Study population
We reviewed the files of patients diagnosed with HSCR who had been received Laparoscopic-assisted pull-through surgery in Gastrointestinal Neonatal Surgery Department of Children’s Hospital affiliated Chongqing Medical University (a tertiary pediatric hospital and National Clinical Research Center for Child Health and Disorders in China), between February 2016 to June 2021. The inclusion criteria were: (1) patients was diagnosed with HSCR based on histopathological examination, (2) received one-stage Laparoscopic-assisted pull-through surgery, (3) cooperated with the follow-up process for at least 3 years, and (4) had complete medical records. The exclusion criteria were as follows: (1) patients accompanied with other severe malformation that affect the management process of HSCR (7 cases); (2) those identified as cognitive disability (4 cases); and (3) those missed clinical data (22 cases). It needs to be stated that partial patients underwent temporary enterostomy, mainly L-HSCR and TCA, were not included in the study.
In our hospital, patients with HSCR underwent surgery by the permanent team (Yi Wang and Wei Liu, two expert pediatric surgeons with extensive experience), as described detailedly in our previous report (8). All patients received standardized and systematic follow-up by telephone, internet, or clinic visit.
Clinical variables
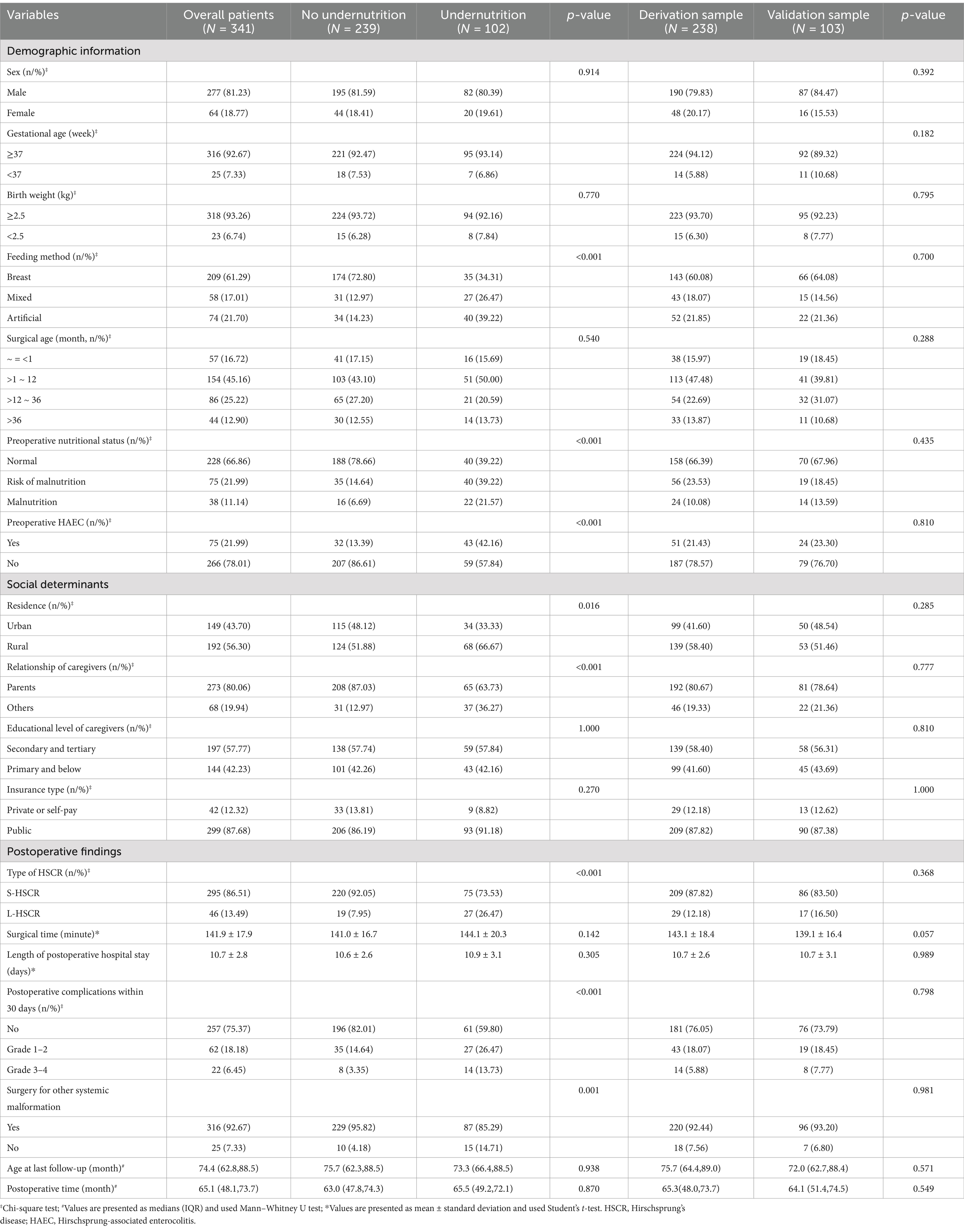
According to relevant literature and clinical practice, variables that may potentially influence postoperative nutritional status were retrospectively collected. Clinical data included the following: (1) demographic information: sex, gestational age, birth weight, feeding method, surgical age, preoperative comorbidities (e.g., nutritional status, HAEC), and surgery for other systemic malformation; (2) social determinants: residence, relationship of caregivers, educational level of caregivers, and insurance type; (3) postoperative findings: type of HSCR, surgical time, length of postoperative hospital stay, postoperative complications within 30 days [graded based on Clavien-Dindo classification system (CCS) (9)]. Furthermore, height and weight were measured at the last follow-up visits to allow for postoperative nutritional assessment.
Definition
We calculated continuous outcomes (height for-age Z-score, HAZ; weight-for-age Z-score, WAZ) using the Chinese child growth standards for nutritional assessment. Categorical outcomes were defined as follows: stunting, HAZ < −2; at risk of stunting, HAZ ≥ -2 and <-1; underweight, WAZ < −2; at risk of underweight, WAZ ≥ -2 and <-1; malnutrition, HAZ and (or) WAZ<-2; at risk of malnutrition, HAZ and (or) WAZ ≥ -2 and <-1, based on the approach recommended by the World Health Organization (10). It is important to note that this study focused on the outcome of undernutrition, so patients who did not match the above definition were classified as “normal.” For better application in clinical practice and the convenience of statistical analysis, we classified patients at risk of malnutrition and malnutrition as the undernourished group.
HAEC was diagnosed when all four of the following criteria were met: the presence of (1) vomiting or explosive diarrhea; (2) abdominal distension; (3) fever (core body temp ≥ 38.5°C) and/or (4) leukocytosis along with radiographic findings and treatment with antibiotics (11, 12). These four clinical features have been shown to be consistently present in patients with HAEC.
Statistical analysis
Data were analyzed using the IBM SPSS 27.0 and GraphPad Prism 9.0. To ensure balanced representation, the enrolled patients were randomly divided into a training set (n = 238) and a validation set (n = 103) using a 7:3 ratio. The clinical data of patients in the training sample were used to establish the prediction model of postoperative undernutrition, and then validated this model in the validation sample. Categorical data were expressed by n (%), and the chi-squared test was used for comparison. The numerical data were assessed for normality by Shapiro–Wilk test: if they matched, they were expressed as mean ± standard deviation (SD) with Student’s t-test; if not, they were expressed as median (interquartile range) with Mann–Whitney test. Univariate/multivariate Logistic regression analysis was used to identify independent factors for postoperative undernutrition. Subsequently, a scoring system to predict postoperative undernutrition was established based on these identified independent predictors. Rounded weights were added to the final model based on the odds ratios (ORs) in the scoring system. Finally, a receiver operator characteristic (ROC) curve was obtained to identify the optimal cut-off value based on Youden index (Youden index = sensitivity + specificity −1) yielding the best performance of prediction. Furthermore, the calibration curve were used to evaluate the calibration of this scoring system. Usually, factor with an area under the ROC curve (AUC) above 0.70 was considered to be useful, while an AUC between 0.80 and 0.90 indicated great diagnostic accuracy (13). p < 0.05 with 95% confidence interval (CI) and 5% margin of error was considered statistically significant.
Results
General data
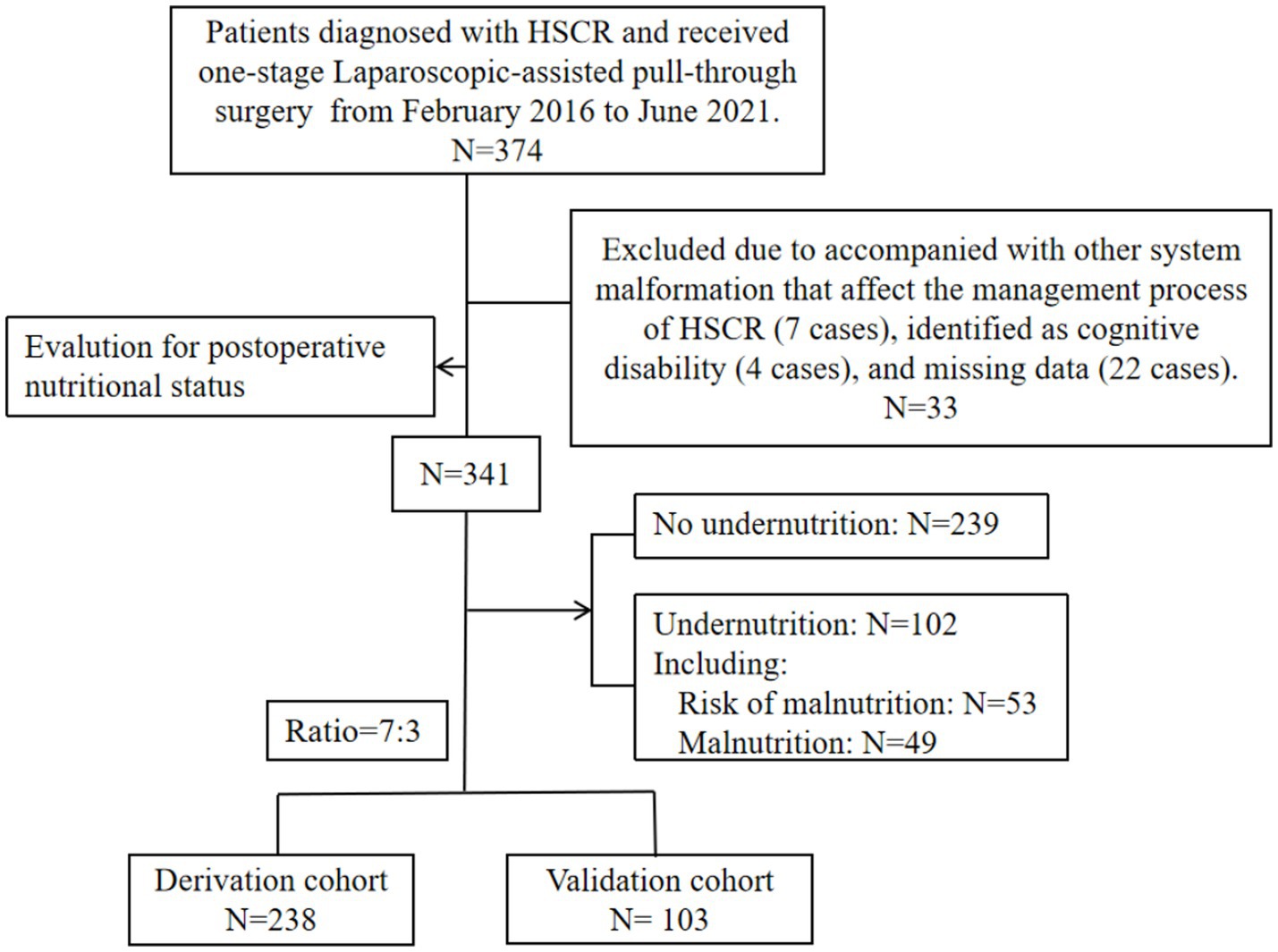
The entire number of patients met the in- and exclusion criteria during the time frame was 341 (S-HSCR: 295 cases; L-HSCR: 46 cases). Then, based on a training set ratio verification set of approximatively 7: 3, 238 patients were divided into the derivation sample and 103 patients were included in the validation sample (Figure 1). The clinical data for overall patients was shown in Table 1. Most patients underwent surgery at the age of 1 ~ 12 months (156/341), and the median postoperative time of follow-up was 65.1 (48.1, 73.7) months. Furthermore, 102 cases (29.9%) met the criteria of undernutrition [risk of malnutrition: 53 cases (15.5%); malnutrition: 49 cases (14.4%)], including 74 cases in the derivation sample and 28 cases in the validation sample, no statistical difference existed in the incidence of undernutrition between the two groups (p > 0.05).
Independent factors for postoperative undernutrition
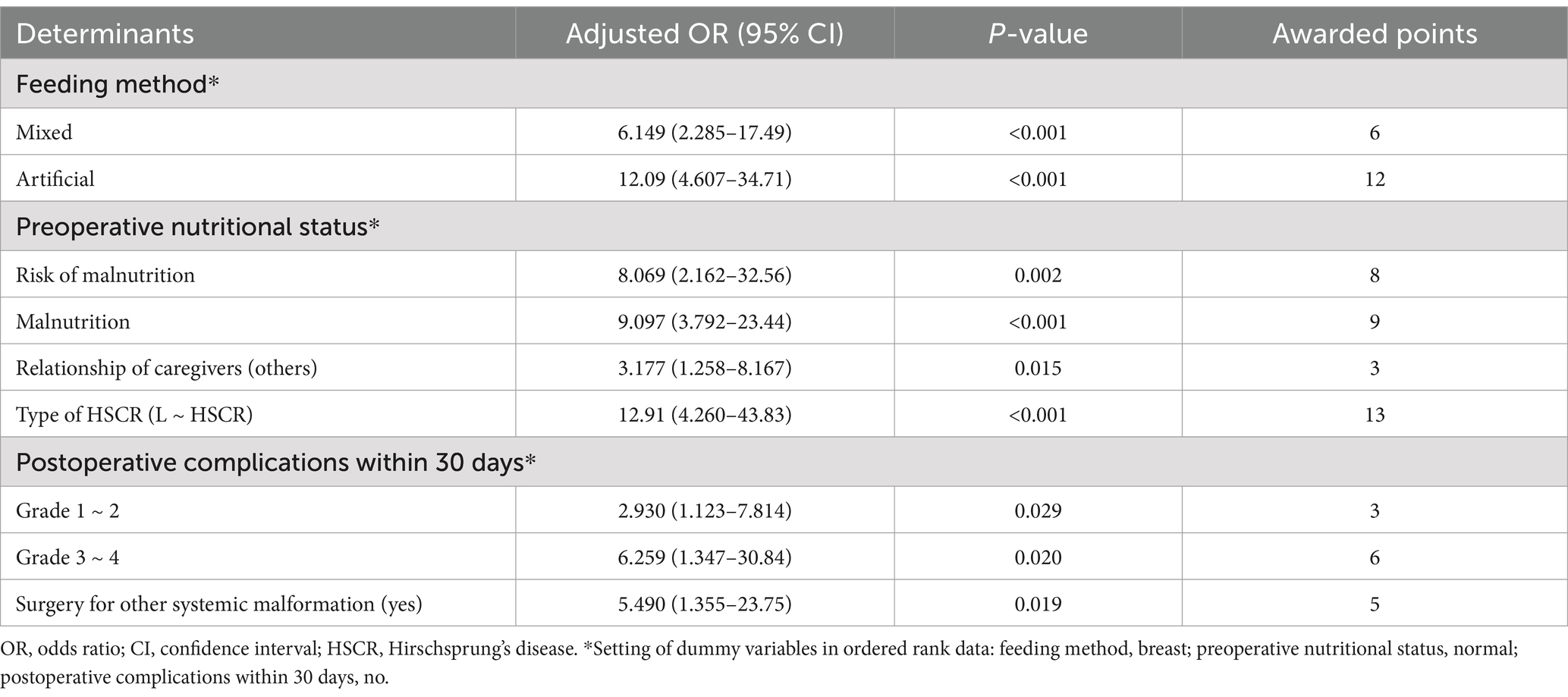
Univariate Logistic regression analysis of clinical data on the postoperative undernutrition in derivation sample are listed in Table 2. No significant differences existed between non-undernourished and undernourished groups in the following variables: sex, gestational age, birth weight, surgical age, educational level of caregivers, insurance type, surgical time, age at last follow-up, and postoperative time (all p > 0.05). However, patients with postoperative undernutrition had higher proportions of non-breast feeding (reference: breast; mixed: OR [95% CI] = 5.597 [2.765–11.680]; artificial: OR [95% CI] = 5.951 [2.991–12.11]), preoperative undernutrition (reference: normal; risk of malnutrition: OR [95% CI] = 6.769 [3.477–13.49]; malnutrition: OR [95% CI] = 10.150 [4.045–27.43]), preoperative HAEC (OR [95% CI] = 3.732[1.967–7.167]), non-parental caregivers (OR [95% CI] = 4.937 [2.528–9.879]), L-HSCR (OR [95% CI] = 7.726 [3.345–19.53]), postoperative complications within 30 days (reference: no; Grade 1 ~ 2: OR [95% CI] = 2.885 [1.449–5.756]; Grade 3 ~ 4: OR [95% CI] = 4.030 [1.332–12.83]), and surgery for other systemic malformation (OR [95% CI] = 3.916 [1.476–11.070]) (p < 0.05 for all). Diagnosis of collinearity (tolerance and variance expansion factor) for the above determinants was performed (Supplementary Table S1), and the results indicated that there was no multiple collinearity relationship existed.
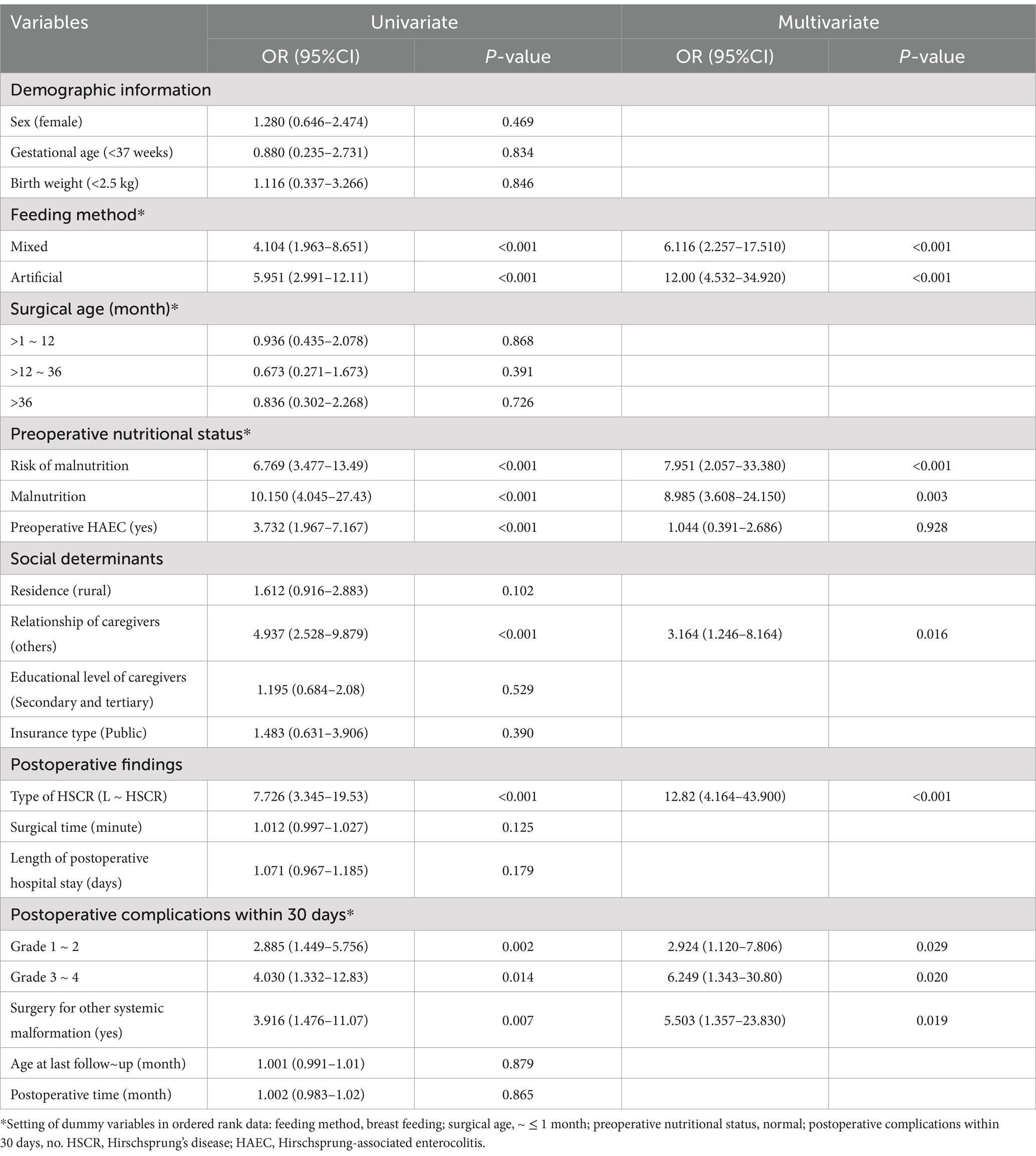
Table 2. Univariate/multivariate logistic regression analysis of clinical data on the postoperative nutritional status (derivation sample: N = 212).
Significant influenced factors were included in the multivariate Logistic regression analysis, and the results for each category of determinants are listed in Table 2. Feeding method, preoperative nutritional status, relationship of caregivers, type of HSCR, postoperative complications within 30 days, and surgery for other systemic malformation were independent factors for postoperative undernutrition (all p < 0.05).
Scoring system development
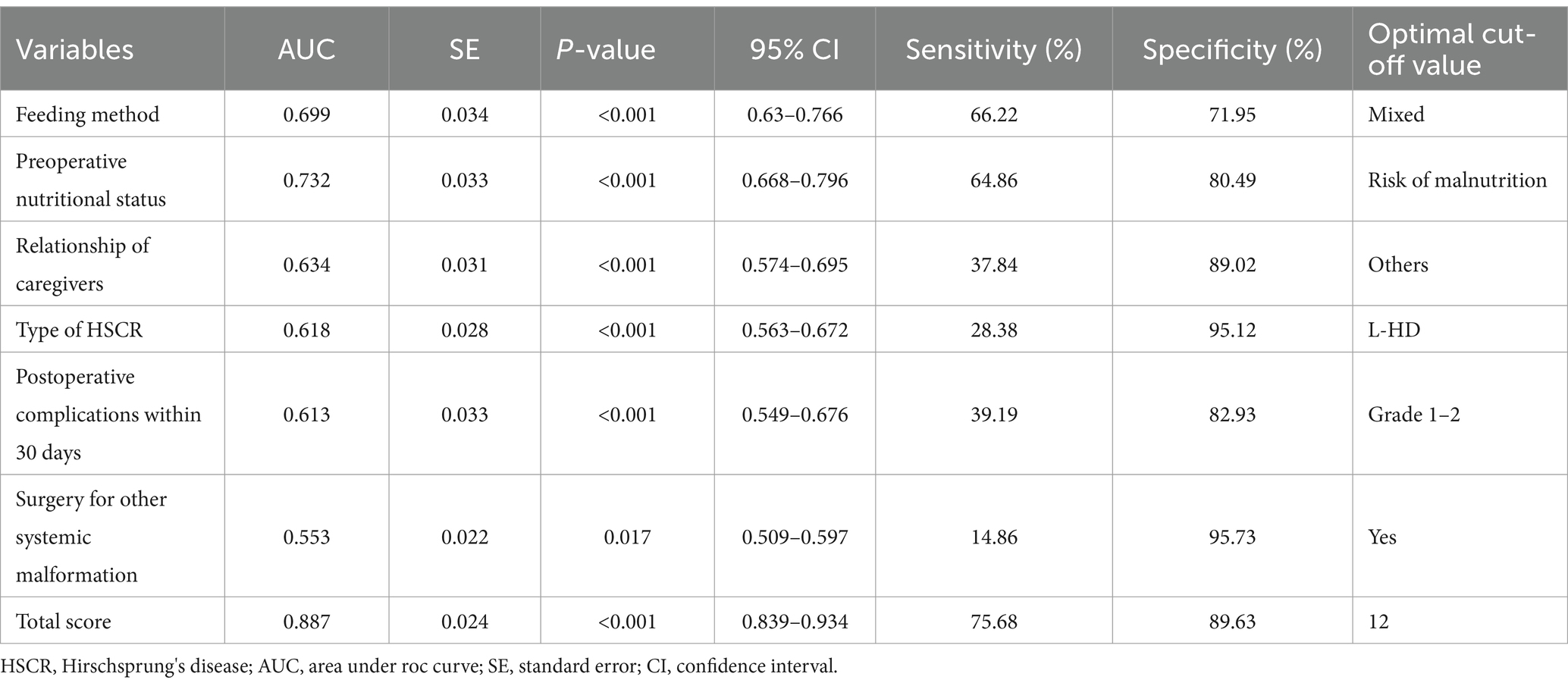
Based on the results of multivariate Logistic regression analysis, we explored the predictive value of these determinants for postoperative undernutrition (Figure 2). ROC curve analysis of feeding method, preoperative nutritional status, relationship of caregivers, type of HSCR, postoperative complications within 30 days, and surgery for other systemic malformation resulted in area under the curves of 0.669 (95% CI: 0.632–0.766), 0.732 (95%CI: 0.668–0.796), 0.634 (95%CI: 0.574–0.695), 0.618 (95%CI: 0.563–0.672), 0.613 (0.549–0.676), and 0.553 (0.509–0.597), respectively. The predictive values of each determinant are shown in Table 3. The preoperative nutritional status showed a clearly better predictive performance for identifying the patients who were undernourished, with 64.86% sensitivity and 80.49% specificity.
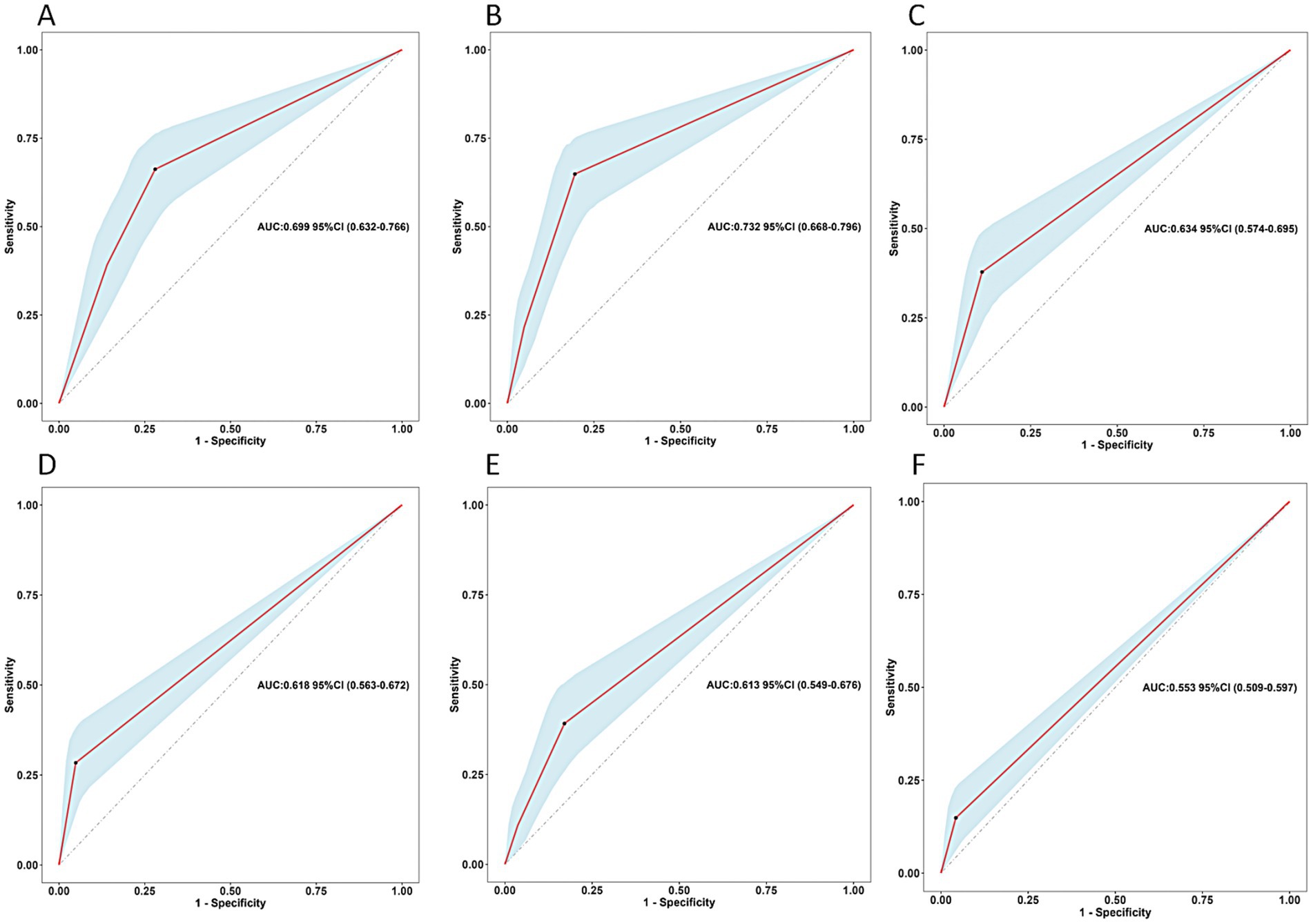
Figure 2. Predictive assessment of the screened variables (A: feeding method, B: preoperative nutritional status, C: relationship of caregivers, D: type of HSCR, E: postoperative complications within 30 days, F: surgery for other systemic malformation) for postoperative undernutrition with ROC curve analysis (derivation sample). ROC, receiver operating characteristics; AUC, area under the curve.
From the above analysis, we found that the predictive value (sensitivity and specificity) of a single determinant was not ideal, thus we established the scoring system. These six determinants for postoperative undernutrition were selected for our scoring system and were entered into the final regression model, which explained 86.1 of the variance. Based on the adjusted ORs, we awarded points to these determinants (Table 4). The maximum possible score of our model is 48 points. ROC curve analysis of the scoring system resulted in an AUC of 0.887 (95% CI: 0.839–0.934, p < 0.05, Figure 3A). The calibration plot for the scoring system (Predicted) and actual situation (Observed) is demonstrated in Figure 3B. The threshold was set on a value of 12, as this was the score with the best predictive value: 75.68% sensitivity, 89.63% specificity, 78.08% positive predictive value (PPV), and 89.70% negative predictive value (NPV). Compared with total score ≤ 12, a score of more than 12 had a 31.015 times higher chance of postoperative undernutrition (95% CI: 14.681–65.523).
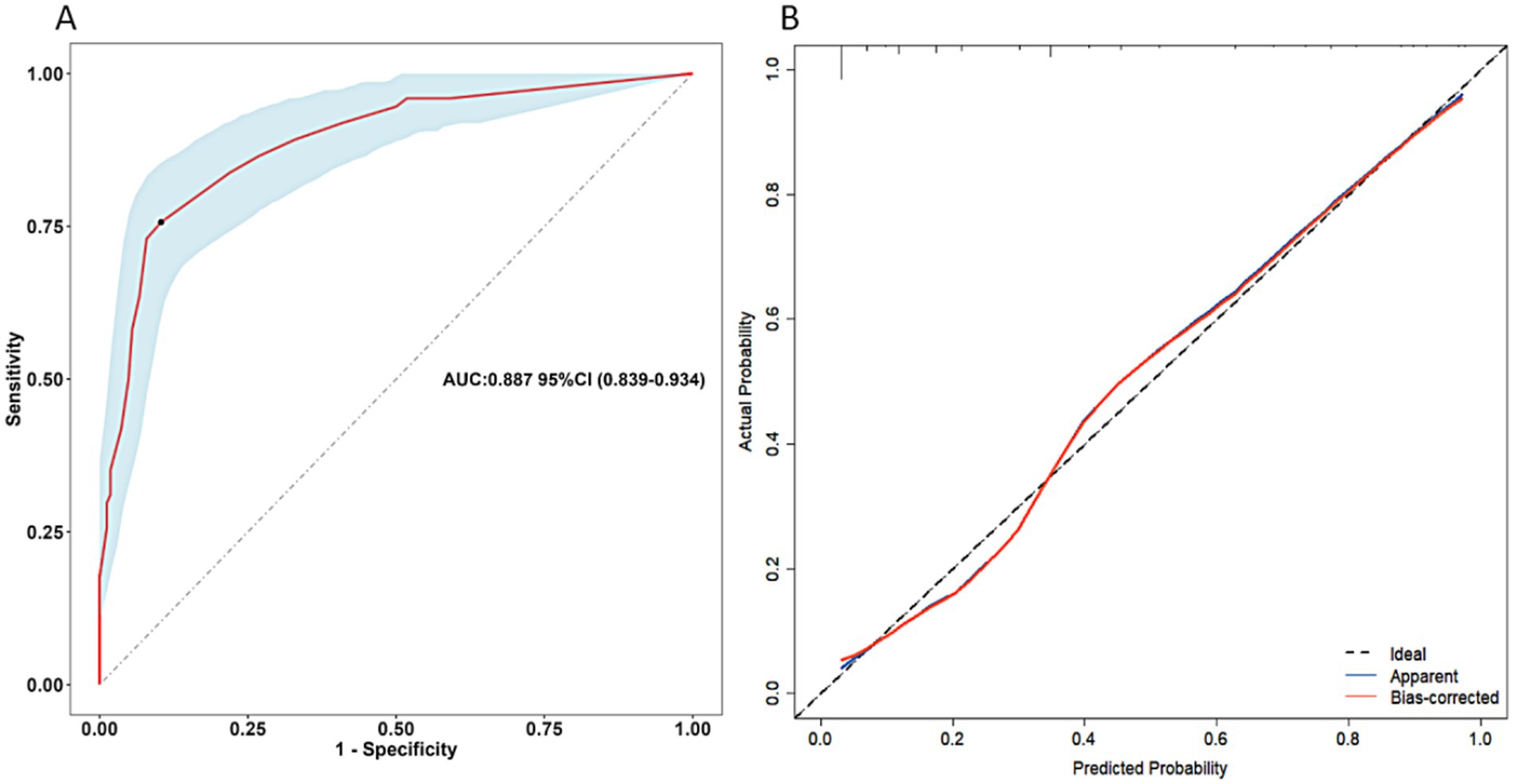
Figure 3. Receiver operating characteristics curve of the scoring system for postoperative undernutrition (A) and calibration curve (B) for this scoring system (derivation sample). AUC, area under the curve; CI, confidence interval.
Scoring system validation
Clinical data for validation of the scoring system were available for 103 patients, 27.2% of whom had postoperative undernutrition. In validation sample, undernourished patients were more frequently reported non-breast feeding, preoperative undernutrition, non-parental caregivers, L-HSCR, postoperative complications within 30 days, and surgery for other systemic malformation than those without undernutrition (all p < 0.05) (Table 5). Certainly, the proportion of total score > 12 calculated by the scoring system was significantly higher in the undernourished group than that in the no undernourished group (58.67 vs. 14.29%). The AUC of scoring system in validation sample was 0.846 (95% CI: 0.772 ~ 0.920, p < 0.05) (Figure 4). Our scoring system was shown to have a sensitivity of 89.29%, a specificity of 80.00%, a PPV of 62.50%, an NPV of 95.24%, a positive likelihood ratios (LR) of 4.46, and a negative LR of 0.13, respectively. The diagnostic accuracy of the prediction model was 88.0%.
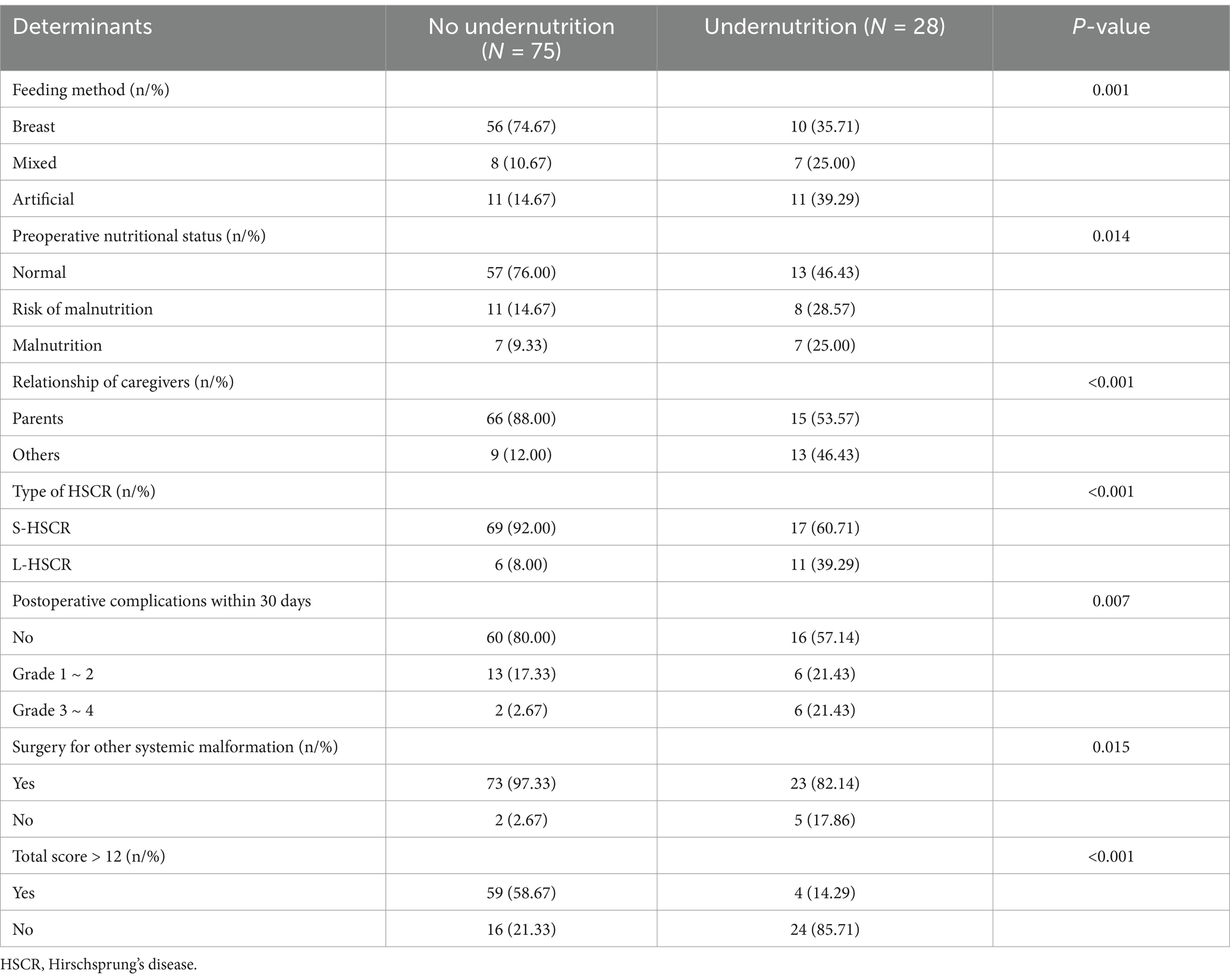
Table 5. The screened variables and scoring system on postoperative nutritional status (validation sample).
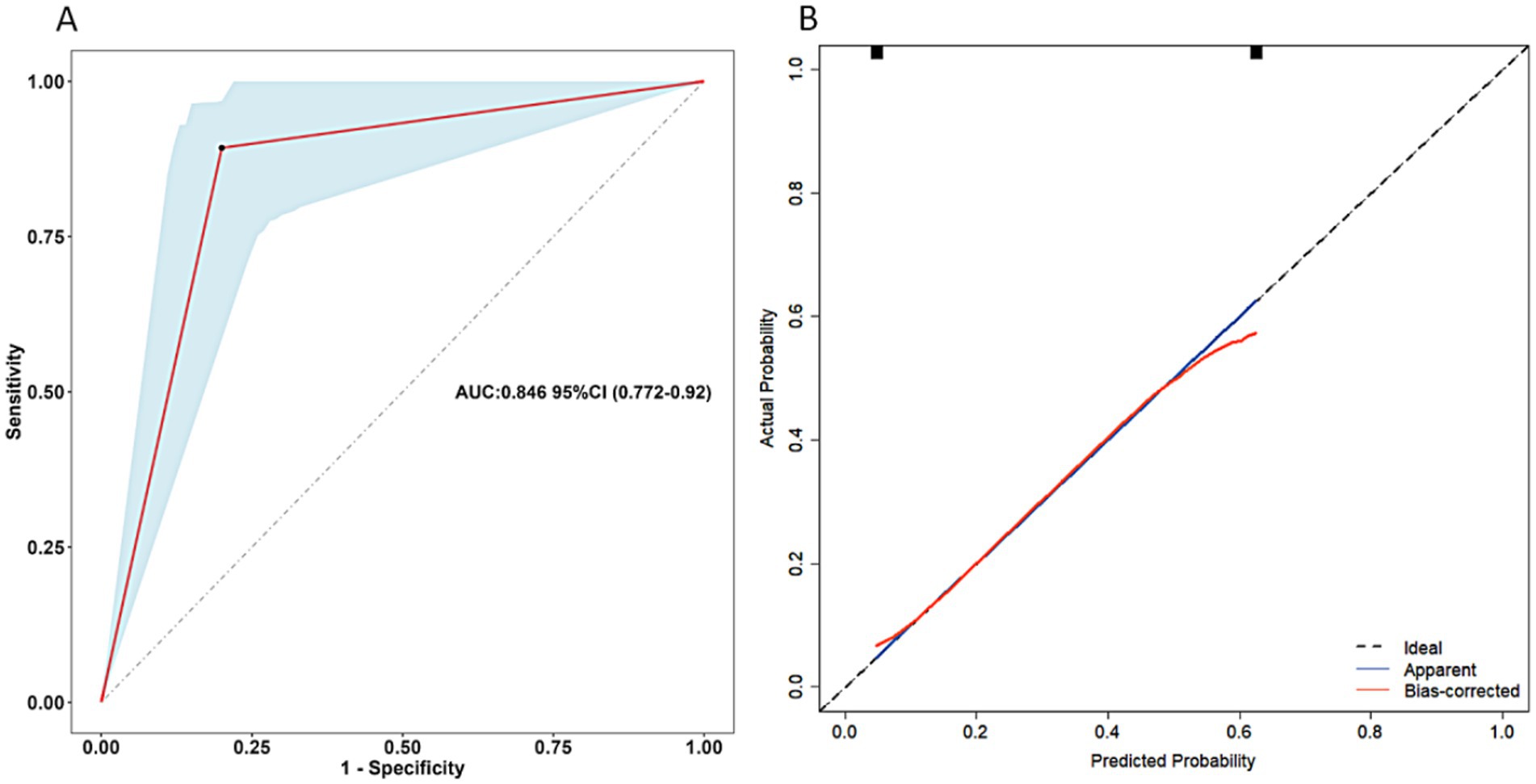
Figure 4. Receiver operating characteristics curve of the scoring system for postoperative undernutrition (A) and calibration curve (B) for this scoring system (validation sample). AUC, area under the curve; CI, confidence interval.
Discussion
HSCR is a challenge for pediatric surgeons and requires surgical procedure to respect the abnormally innervated bowel. If rectal irrigations do not sufficiently decompress the bowel, or there are severe complications such as bowel perforation, staged procedure is necessary (4). During the study period, 38 cases (mainly L-HSCR and TCA) received staged procedure in our department, which was not included in analysis due to the small sample size and inconsistent surgical strategies. Postoperative HAEC and defecation function have always been the focus of our team’s regular follow-up of patients, but during this process, we found that the nutritional problem was not optimistic. Undernutrition puts patients in a state of disease-carrying, unable to lead a normal life, placing a heavy financial and psychological burden on themselves and their families (14). Currently, few relevant studies have reported the postoperative nutritional problem in patients with HSCR. Therefore, we conducted this study to investigate the postoperative nutritional status and its influencing factors in these patients. It might be helpful for formulating individualized treatment plan and helping physicians to fully communicate complications with family members.
Nutritional problem is one of the significant morbidity in patients after surgery for HSCR, but have not received enough attention from pediatric surgeons. In this retrospective study, postoperative undernutrition occurs in 29.9% of patients, and even 14.4% being malnourished, which has not been reported in other publications. Furthermore, we found that feeding method (mixed/artificial), preoperative nutritional status (undernutrition), relationship of caregivers (others), type of HSCR (L-HSCR), postoperative complications within 30 days (yes), and surgery for other systemic malformation (yes) were independent risk factors for postoperative undernutrition, and developed a scoring system based on these six determinants, aiming to identify high-risk patients with nutritional problems. The scoring system showed desirable predictive accuracy and might be used to aid the differentiation of patients with nutritional problem for individualized prevention and intervention.
This study found that patients who received non-breast feeding were more likely to suffer from postoperative undernutrition than those who were breastfed. Breast feeding is widely considered the optimal mode of nutrition for infants, providing substantial amounts nutrient substances and improving appetite and growth (15). Several studies have shown that breast feeding reduces the risk of malnutrition and increase HAZ score in childhood, thereby eliminating linear growth stagnation in age-specific child and reducing child morbidity and mortality during periods of childhood illness (16, 17). Furthermore, the long-term benefits of breast feeding are also reflected in the protective effect of an array of immunomodulatory components on children, which is conducive to reducing the occurrence of infectious and allergic diseases, especially for patients ≤1 year of age (18). Furthermore, breast feeding was associated with a lowered risk for HAEC potentially mediated by modulating the gut microbiome composition (19). In brief, breast feeding is recommended for patients with HSCR, and may be beneficial in improving the postoperative nutritional status.
As is well-known that Preoperative undernutrition is a major risk factor for increased postoperative morbidity and mortality (20). Preoperative undernutrition will reduce the tolerance of patients to surgical trauma and make patients unable to maintain the body’s effective metabolism and organ/tissue function. Due to intestinal lesions leading to insufficient nutritional intake and/or increased energy expenditure, patients with HSCR have a high risk of preoperative undernutrition. In addition, stress and metabolic changes resulting from various perioperative traumas, such as the release of endocrine hormones and inflammatory mediators, catabolism of glycogen, fat and protein, and the need for additional energy to repair the trauma, etc., further aggravate the postoperative nutrition problem of those patients (21).Therefore, Optimal perioperative metabolic conditioning and nutritional management can reduce the state of decomposition and loss of lean tissue, promote protein synthesis, thereby reducing complications and providing protection for optimal wound healing and recovery (22).
Among the social factors included in this study, only non-parental caregivers was screened as an independent risk factor for postoperative undernutrition, while there was no statistical difference in residence and education level, which have been reported to affect children’s nutritional status in large sample population studies (23–25). During the follow-up, we found that non-parental caregivers were not familiar with the disease situation of the patient in infancy and could not provide a balanced nutritional requirement (26, 27). Conversely, caregivers who are parents may receive more attentive care and individualized feeding (28). In fact, we usually recommend that patients be cared for by their parents after surgery, as we empirically believe that parents are able to be more attentive to anal care, defecation function training, nutritional feeding, and psychological construction. However, for practical reasons, mainly economic factor, patients were often not raised by their parents. Due to the relatively limited sample size of this study, the influence of residence and education level on nutrition in these patients needs to be further explored.
Our study found a strong association between long-segment disease and development of postoperative undernutrition, in accordance with our expectations. Compared with S-HSCR, L-HSCR has the following characteristics, which may explain the differences in postoperative nutrition. First, a longer aganglionic segment involves a wider range of bowel resections which may lead to unbalanced gut microbiota homeostasis and immunity, and provoke postoperative recovery to a greater negative extent than the shorter aganglionic segment (29, 30). Furthermore, it can be assumed that even after definitive surgery, the longer aganglionic lesion is considered to affect intestinal motility. Subsequently, repeated dietary modifications and decreased appetite leads to a more suitable environment for undernutrition development. Most importantly, long-segment disease has been demonstrated by several studies to be associated with an increased risk of postoperative HAEC and defecation dysfunction (29, 31), which were also shown to be independent risk factors for undernutrition in this study. For patients with L-HSCR, dynamic assessment of postoperative nutritional status should be paid attention to during follow-up in order to identify high-risk patients with nutritional problem early and to intervene in a timely manner.
Many researchers believe that the 30-day period following surgery is a significant time window for evaluating postoperative outcomes (32). Although most patients recover well after surgery, postoperative complications within 30 days can lead to secondary surgery and comorbidities and have an adverse impact on quality of life. To better compare postoperative complications across different centers and enhance transparency, we adopted the CCS, an objective classification and scoring system aimed at objectively comparing different severities of complications (33). Since postoperative complications, graded higher than 3, would be treated by invasive procedures suggesting severe conditions, thus we classified complications based on CCS. Hypothetically, the patient is in a situation of acute stress and decreased resistance after radical surgery, which can be aggravated if complications occur. The more severe the complications, the more trauma and nutritional consumption of the body. Therefore, study of postoperative complications deserve more in-depth exploration in favor of reducing the occurrence of postoperative undernutrition.
It found that patients who underwent surgery for other systemic malformation were more likely to suffer from postoperative undernutrition. After multiple surgeries, the body requires additional energy and nutrients for repair and recovery, and this process can lead to an imbalance of nutrients without individualized nutritional management (34). In addition, 25 patients underwent other surgery, among which cardiac surgery was the most common (17 cases). Significant cardiac shunting results in poor systemic perfusion and reduced blood flow available for the gastrointestinal tract, ultimately leading to impaired nutrient absorption (35). Therefore, we recommend that pediatric surgeons and dietitians should pay attention to individualized nutritional assessment and management of patients before each surgery and provide adequate nutritional support in the postoperative period.
The reason for us to furnish a scoring system was to assess the possibility of postoperative undernutrition so that we can communicate postoperative complications with family members fully, as well as target limited medical resources for those at the highest risk. Thus, we added rounded weights to the final model based on the odds ratios of independent risk factors to make the scoring model simple and easy to application. Postoperative undernutrition was identified as the primary outcome of this study for reasons of clinical practicality, with the aim of early identification of a trend risk of malnutrition, enabling timely prevention. It suggests an important point, that patients with higher scores in the scoring system were more likely to develop more severe nutritional problems. Therefore, with reference to the independent risk factors screened, targeted clinical interventions, e.g., breast feeding, may be beneficial in reducing the incidence of postoperative undernutrition. Furthermore, it is recommended that the new prediction model needs to be verified by validation samples of the center or other centers to truly reflect the prediction performance of the model (36). We conducted an external validation of the scoring system and the results showed that the system was a simple and efficient method that aids the differentiation of nutrition problem, with AUC of 0.846 (95% CI: 0.772–0.920) for undernutrition.
Limitations
However, several potential limitations of this study should be noted when interpreting the results. The retrospective study design is an inherent weakness, non-standardization of data collection may have resulted in other statistically significant factors not being shown in this analysis. We intended to collect as many variables associated with postoperative nutritional status as possible, so a prospective study design with larger sample size would have been preferable. Another, considering that most of the patients included in this study originated from southwestern China (underdeveloped region), with a relatively high incidence of postoperative nutritional problem. Finally, only patients who underwent one-staged procedure (Laparoscopic-assisted pull-through surgery) were included, while patients who underwent staged procedure were excluded from the study, these patients may be more prone to postoperative undernutrition. Therefore, further investigation is planned to elucidate fully in the subsequent phase.
Conclusion
To our knowledge, this study is the first to analyze the risk factors and establish scoring system for postoperative undernutrition of HSCR. It identified that feeding method, preoperative nutritional status, relationship of caregivers, type of HSCR, postoperative complications within 30 days, and surgery for other systemic malformation were independently associated with postoperative undernutrition, and the scoring system based on these determinants showed desirable discrimination to assess the individualized prediction. Those patients suspected of having postoperative undernutrition need timely intervention to prevent the complications that would otherwise invariably ensue. However, further studies are required to confirm the feasibility of this scoring system and to increase the accuracy of predicting postoperative undernutrition.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The datasets generated and analyzed during the current study are not publicly available due to the ongoing analysis in other directions but are available from the corresponding author (Wei Feng and Dawei He) on reasonable request. Requests to access these datasets should be directed to Wei Feng, weif600@163.com.
Ethics statement
The studies involving humans were approved by the Institutional Research Ethics Board of Children’s Hospital Affiliated Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study was retrospective, the requirement for informed consent was waived.
Author contributions
XD: Writing – original draft. WF: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. AS: Data curation, Formal analysis, Writing – review & editing. WL: Formal analysis, Methodology, Writing – review & editing. YW: Funding acquisition, Methodology, Resources, Writing – review & editing. ZG: Supervision, Writing – review & editing. DH: Conceptualization, Investigation, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by research grants from the National Clinical Research Center for Child Health and Disorders, Children’s Hospital of Chongqing Medical University (No. NCRCCHD-2022-GP-03) and Program for Youth Innovation in Future Medicine, Chongqing Medical University (No. W0125).
Acknowledgments
The authors want to thank the nurses and medical officers of Gastrointestinal Neonatal Surgery Department of Children’s Hospital affiliated Chongqing Medical University for their support. Furthermore, we would like to express our sincere gratitude to the National Clinical Key Specialty Construction Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1441104/full#supplementary-material
References
1. Montalva, L, Cheng, LS, Kapur, R, Langer, JC, Berrebi, D, Kyrklund, K, et al. Hirschsprung disease. Nat Rev Dis Primers. (2023) 9:54. doi: 10.1038/s41572-023-00465-y
2. Roorda, D, Oosterlaan, J, van Heurn, E, and Derikx, JPM. Risk factors for enterocolitis in patients with Hirschsprung disease: a retrospective observational study. J Pediatr Surg. (2021) 56:1791–8. doi: 10.1016/j.jpedsurg.2021.04.020
3. Yan, J, Sun, J, Wu, R, Tan, SS, Chen, Y, Peng, Y, et al. Barium enema findings in total colonic aganglionosis: a single-center, retrospective study. BMC Pediatr. (2020) 20:499. doi: 10.1186/s12887-020-02403-3
4. Kyrklund, K, Sloots, C, de Blaauw, I, Bjørnland, K, Rolle, U, Cavalieri, D, et al. ERNICA guidelines for the management of rectosigmoid Hirschsprung's disease. Orphanet J Rare Dis. (2020) 15:164. doi: 10.1186/s13023-020-01362-3
5. Wood, RJ, and Garrison, AP. Total colonic Aganglionosis in Hirschsprung disease. Semin Pediatr Surg. (2022) 31:151165. doi: 10.1016/j.sempedsurg.2022.151165
6. Li, S, Zhang, Y, Li, K, Liu, Y, Chi, S, Wang, Y, et al. Update on the pathogenesis of the Hirschsprung-associated enterocolitis. Int J Mol Sci. (2023) 24:4602. doi: 10.3390/ijms24054602
7. Belluscio, LM, Berardino, BG, Ferroni, NM, Ceruti, JM, and Cánepa, ET. Early protein malnutrition negatively impacts physical growth and neurological reflexes and evokes anxiety and depressive-like behaviors. Physiol Behav. (2014) 129:237–54. doi: 10.1016/j.physbeh.2014.02.051
8. Feng, W, Zhang, B, Fan, L, Song, A, Hou, J, Die, X, et al. Clinical characteristics and influence of postoperative Hirschsprung-associated enterocolitis: retrospective study at a tertiary children's hospital. Pediatr Surg Int. (2024) 40:106. doi: 10.1007/s00383-024-05688-y
9. Rassweiler, JJ, Rassweiler, MC, and Michel, MS. Classification of complications: is the Clavien-Dindo classification the gold standard? Eur Urol. (2012) 62:256–8. discussion: 259–260. doi: 10.1016/j.eururo.2012.04.028
10. Mertens, A, Benjamin-Chung, J, Colford, JM Jr, Hubbard, AE, van der Laan, MJ, Coyle, J, et al. Child wasting and concurrent stunting in low- and middle-income countries. Nature. (2023) 621:558–67. doi: 10.1038/s41586-023-06480-z
11. Chantakhow, S, Tepmalai, K, Singhavejsakul, J, Tantraworasin, A, and Khorana, J. Prognostic factors of postoperative Hirschsprung-associated enterocolitis: a cohort study. Pediatr Surg Int. (2023) 39:77. doi: 10.1007/s00383-023-05364-7
12. Chung, P, Yu, M, Wong, K, and Tam, PKH. Risk factors for the development of post-operative enterocolitis in short segment Hirschsprung's disease. Pediatr Surg Int. (2019) 35:187–91. doi: 10.1007/s00383-018-4393-3
13. Giannini, EG, Zaman, A, Kreil, A, Floreani, A, Dulbecco, P, Testa, E, et al. Platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices: results of a multicenter, prospective, validation study. Am J Gastroenterol. (2006) 101:2511–9. doi: 10.1111/j.1572-0241.2006.00874.x
14. Bagri, NK, Jose, B, Shah, SK, Bhutia, TD, Kabra, SK, and Lodha, R. Impact of malnutrition on the outcome of critically ill children. Indian J Pediatr. (2015) 82:601–5. doi: 10.1007/s12098-015-1738-y
15. Charton, E, Bourgeois, A, Bellanger, A, le-Gouar, Y, Dahirel, P, Romé, V, et al. Infant nutrition affects the microbiota-gut-brain axis: comparison of human milk vs. infant formula feeding in the piglet model. Front Nutr. (2022) 9:976042. doi: 10.3389/fnut.2022.976042
16. Hanley-Cook, G, Argaw, A, Dahal, P, Chitekwe, S, and Kolsteren, P. Infant and young child feeding practices and child linear growth in Nepal: regression-decomposition analysis of national survey data, 1996-2016. Matern Child Nutr. (2022) 18:e12911. doi: 10.1111/mcn.12911
17. Chee Din, MA, Mohd Fahmi Teng, NI, and Abdul, MZ. Maternal depression and child feeding practices: determinants to malnutrition among young children in Malaysian rural area. Womens Health. (2023) 19:17455057221147800. doi: 10.1177/17455057221147800
18. Libuda, L, Filipiak-Pittroff, B, Standl, M, Schikowski, T, von Berg, A, Koletzko, S, et al. Full breastfeeding and allergic diseases-long-term protection or rebound effects? Nutrients. (2023) 15:2780. doi: 10.3390/nu15122780
19. Tang, W, Su, Y, Yuan, C, Zhang, Y, Zhou, L, Peng, L, et al. Prospective study reveals a microbiome signature that predicts the occurrence of post-operative enterocolitis in Hirschsprung disease (HSCR) patients. Gut Microbes. (2020) 11:842–54. doi: 10.1080/19490976.2020.1711685
20. Cerantola, Y, Grass, F, Cristaudi, A, Demartines, N, Schäfer, M, and Hübner, M. Perioperative nutrition in abdominal surgery: recommendations and reality. Gastroenterol Res Pract. (2011) 2011:739347. doi: 10.1155/2011/739347
21. Green Corkins, K, and Teague, EE. Pediatric nutrition assessment. Nutr Clin Pract. (2017) 32:40–51. doi: 10.1177/0884533616679639
22. Canada, NL, Mullins, L, Pearo, B, and Spoede, E. Optimizing perioperative nutrition in pediatric populations. Nutr Clin Pract. (2016) 31:49–58. doi: 10.1177/0884533615622639
23. Imam, A, Hassan-Hanga, F, Sallahdeen, A, and Farouk, ZL. Socio-demographic and household-level risk factors for severe acute malnutrition in pre-school children in North-Western Nigeria. J Trop Pediatr. (2020) 66:589–97. doi: 10.1093/tropej/fmaa018
24. Chowdhury, M, Khan, H, Mondal, M, and Kabir, R. Socio-demographic risk factors for severe malnutrition in children aged under five among various birth cohorts in Bangladesh. J Biosoc Sci. (2021) 53:590–605. doi: 10.1017/S0021932020000425
25. Khambalia, AZ, Lim, SS, Gill, T, and Bulgiba, AM. Prevalence and sociodemographic factors of malnutrition among children in Malaysia. Food Nutr Bull. (2012) 33:31–42. doi: 10.1177/156482651203300103
26. Asakura, K, Mori, S, Sasaki, S, and Nishiwaki, Y. A school-based nutrition education program involving children and their guardians in Japan: facilitation of guardian-child communication and reduction of nutrition knowledge disparity. Nutr J. (2021) 20:92. doi: 10.1186/s12937-021-00751-z
27. Rohit, A, Kirkham, R, McCarthy, L, Puruntatameri, V, Maple-Brown, L, and Brimblecombe, J. Exploring differences in perceptions of child feeding practices between parents and health care professionals: a qualitative study. BMC Public Health. (2021) 21:1449. doi: 10.1186/s12889-021-11493-2
28. Harvey, L, Bryant-Waugh, R, Watkins, B, and Meyer, C. Parental perceptions of childhood feeding problems. J Child Health Care. (2015) 19:392–401. doi: 10.1177/1367493513509422
29. Liu, Z, Zhang, Y, Li, S, Zhao, J, Yang, T, and Huang, J. Long-term bowel function after single-stage transanal endorectal pull-through in neonatal patients with Hirschsprung disease. Pediatr Surg Int. (2023) 39:255. doi: 10.1007/s00383-023-05517-8
30. Chantakhow, S, Khorana, J, Tepmalai, K, Boonchooduang, N, Chattipakorn, N, and Chattipakorn, SC. Alterations of gut Bacteria in Hirschsprung disease and Hirschsprung-associated enterocolitis. Microorganisms. (2021) 9:2241. doi: 10.3390/microorganisms9112241
31. Gosain, A, Frykman, PK, Cowles, RA, Horton, J, Levitt, M, Rothstein, DH, et al. Guidelines for the diagnosis and management of Hirschsprung-associated enterocolitis. Pediatr Surg Int. (2017) 33:517–21. doi: 10.1007/s00383-017-4065-8
32. Bell, M, Eriksson, LI, Svensson, T, Hallqvist, L, Granath, F, Reilly, J, et al. Days at home after surgery: an integrated and efficient outcome measure for clinical trials and quality assurance. EClinicalMedicine. (2019) 11:18–26. doi: 10.1016/j.eclinm.2019.04.011
33. Clavien, PA, Barkun, J, de Oliveira, ML, Vauthey, JN, Dindo, D, Schulick, RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
34. Torgersen, Z, and Balters, M. Perioperative nutrition. Surg Clin North Am. (2015) 95:255–67. doi: 10.1016/j.suc.2014.10.003
35. Farrell, AG, Schamberger, MS, Olson, IL, and Leitch, CA. Large left-to-right shunts and congestive heart failure increase total energy expenditure in infants with ventricular septal defect. Am J Cardiol. (2001) 87:1128–31. doi: 10.1016/s0002-9149(01)01479-5
Keywords: Hirschprung’s disease, undernutrition, risk factor, scoring system, prediction
Citation: Die X, Feng W, Song A, Liu W, Wang Y, Guo Z and He D (2024) Risk factors and simple scoring system for predicting postoperative nutritional status of Hirschsprung’s disease. Front. Nutr. 11:1441104. doi: 10.3389/fnut.2024.1441104
Edited by:
Benjamin D. Horne, Intermountain Healthcare, United StatesReviewed by:
Ana Luz Del Carmen Reyes Ramírez, National Institute of Medical Sciences and Nutrition Salvador Zubirán, MexicoYifan Fang, Fujian Children’s Hospital, China
Copyright © 2024 Die, Feng, Song, Liu, Wang, Guo and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Feng, weif600@163.com; Dawei He, hedawei@hospital.cqmu.edu.cn
 Xiaohong Die1
Xiaohong Die1