
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 18 October 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1276940
This article is part of the Research TopicNutrition and Sustainable Development Goal 3: Good Health and WellbeingView all 35 articles
Background: Oxidative stress is an important contributor to the progression of nonalcoholic fatty liver disease (NAFLD), but whether dietary and lifestyle pro- and antioxidants may have combined or independent effects on NAFLD, and advanced liver fibrosis (AHF) remains unclear. We aimed to elucidate the relationship between a well-established oxidative balance score (OBS) and NAFLD/AHF.
Methods: This was a cross-sectional study. We included adult participants with complete data from the National Health and Nutrition Examination Survey 1999–2018. Survey-weighted adjusted multivariate regression analyses were used to examine the association of all OBS with NAFLD/AHF. A combination of restricted cubic splines, mediation analysis, stratified analysis, and sensitivity analysis were used to further elucidate these associations.
Results: We included 6,341 eligible adult participants with prevalence of NAFLD and AHF of 30.2 and 13.9%, respectively. In the fully adjusted model, the highest quartile of OBS, dietary OBS, and lifestyle OBS were associated with 65, 55, and 77% reduced risk of NAFLD, respectively, compared with the reference population, respectively. However, all OBS were not associated with the risk of AHF. All OBS were nonlinearly associated with risk of NAFLD and had a more pronounced reduced risk for OBS, dietary OBS, and lifestyle OBS after exceeding 26, 21, and 5 points, respectively. OBS may exert a protective effect indirectly through inflammation, oxidative stress, and glycolipid metabolism markers. Stratification and sensitivity analyses demonstrate the robustness of our findings.
Conclusion: All OBS were nonlinearly and negatively associated with NAFLD risk. These effects may exert indirectly through inflammation, oxidative stress, and glycolipid metabolism markers.
Nonalcoholic fatty liver disease (NAFLD) is currently the most common chronic liver disease globally, with a course ranging from simple hepatic steatosis to the more severe form, nonalcoholic steatohepatitis (NASH), and a small percentage can progress to cirrhosis, liver cancer, and lead to death (1). The diagnosis of NAFLD is determined by the evidence of the presence of hepatic steatosis (accumulation of more than 5% fat in hepatocytes) confirmed by noninvasive markers/imaging/biopsy and the exclusion of secondary chronic liver injury (e.g., excessive alcohol consumption, viral hepatitis, etc.) (2). Generally, one in three adults worldwide may suffer from NAFLD, and its incidence continues to increase at an alarming rate (3). Better understanding of NAFLD/NASH and risk stratification by recognizing modifiable risk factors will help alleviate this public health burden.
Although the pathogenesis of NAFLD is still not fully understood, the accepted perspective is the ‘multiple hit’ hypothesis. Multiple factors including environmental exposures, unhealthy diet/lifestyle, and genetics/epigenetics have been shown to contribute to the following metabolic dysregulation, immune dysregulation, inflammation, and fibrosis through a variety of pathophysiological mechanisms (4). Oxidative stress caused by events such as mitochondrial dysfunction and endoplasmic reticulum stress has been well defined as an important hallmark for the progression from simple steatosis to NASH with more severe inflammation and fibrosis (5). Therefore, modulation of reactive oxygen species (ROS) production is currently an important research direction for therapies targeting NAFLD, especially NASH. Currently, several agents targeting anti-oxidative stress including vitamin E have shown improvement in NASH in preclinical experiments and clinical trials (6).
However, the impact of comprehensive dietary/lifestyle modifiable interventions related to oxidative stress on NAFLD and more severe cohorts such as those with advanced fibrosis is still lacking. Awareness of the impact of these modifiable risk factors and timely intervention may be of great value in the prevention of NAFLD progression and remission of NASH. To fill this knowledge gap, we employ a previously well-established oxidative balance score (OBS) in a nationally representative survey, the National Health and Nutrition Examination Survey (NHANES), to examine its effects on NAFLD and advanced liver fibrosis (AHF) for the first time.
The NHANES is a series of nationally representative, multi-stage, cross-sectional surveys with complex probability sampling. It was reviewed and approved by the NCHS Ethics Review Board, and informed consent was obtained from all participants. Further information can be found on the official website.
We conducted the analysis using ten consecutive cycles of NHANES 1999–2018. From the initial 101,316 individuals, we first excluded all those missing data for the US Fatty Liver Index (USFLI) (n = 71,814) and OBS components (n = 15,672). Second, we excluded those younger than 18 years or pregnant (n = 385), those with other liver diseases such as chronic hepatitis, excessive alcohol intake (≥3 drinks/day for men and ≥2 drinks/day for women), and cancer (n = 4,829), and those with extreme total energy intake (n = 1,065). Finally, we excluded populations with missing data on included covariates (n = 1,195) and those with missing mediating variables (n = 15). The final number of eligible adult participants was 6,341 (Figure 1).
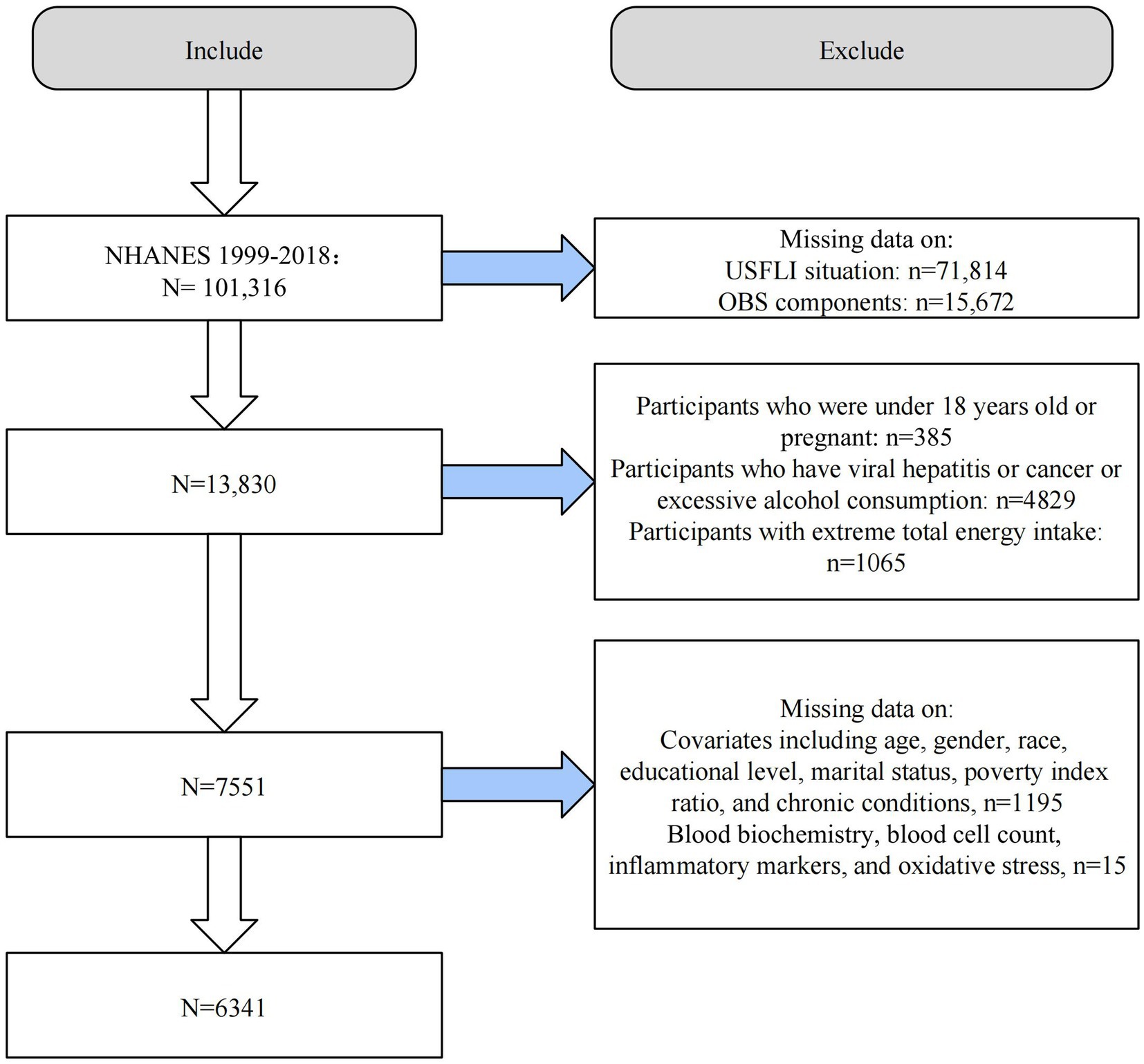
Figure 1. Flow chart of study population selection. USFLI, US fatty liver index; OBS, oxidative balance score; NHANES, the National Health and Nutrition Examination Survey.
We calculated the OBS based on previous well-established studies (7–9). The OBS used in this study consisted of 16 dietary factors and 4 lifestyle measures that have previously been shown to be associated with oxidative stress (Supplementary Table S1). These components were divided into pro-oxidants and antioxidants based on their effect on oxidative stress. Specifically, pro-oxidants consisted of dietary total fat, dietary iron intake, alcohol consumption, body mass index (BMI), and serum cotinine, while antioxidants included dietary fiber, carotenoids, riboflavin (vitamin B2), niacin, vitamin B6, total folate, vitamin B12, vitamin C, vitamin E, calcium, magnesium, zinc, copper, selenium intake, and physical activity. Dietary intake information was obtained from two 24 h dietary review questionnaires consisting of a 24 h dietary recall interview conducted at a Mobile Examination Center and a telephone follow-up 3–10 days later through the USDA’s Automated Multiple-Pass Method. The average of the two 24 h recall dietary interviews was used to proxy its intake. The OBS components and intakes for food or beverage consumed in the 24 h prior to the interview were collected and recorded by the NHANES computer-assisted dietary interview system. Instead, dietary nutrient intake was assessed according to the University of Texas Food Intake Analysis System and the USDA’s Food and Nutrient Database for Dietary Studies. We did not include intake of nutrients from dietary supplements and medications based on the previous study as these data were lacking or poorly provided in full in many cycles (9). Notably, we used serum nicotine as a proxy for smoking based on previous studies, as it incorporated both active and passive smoking levels. More information on the data collection can be found in the previous study (9).
Our allocation scheme is described in Table 1. Most of the components were assigned according to their sex-specific tertiles, with antioxidants assigned 0, 1, and 2 points from tertile 1 to 3, respectively, and pro-oxidants assigned the reverse. Instead, alcohol consumption was assigned a score based on recognized intake criteria (0 point for men ≥2 drinks/d and ≥1 drinks/d for women, 1 point for men <2 drinks/d and <1 drinks/d for women; and 2 points for <12 drinks/year). In addition, we divided OBS into dietary and lifestyle OBS according to the source of the components to explore their combined and independent effects on NAFLD/AHF, respectively.
Suspected NAFLD was defined according to the USFLI, which was developed using the NHANES database, which has moderately improved accuracy compared to the FLI in the multi-ethnic US population (10). USFLI was developed based on ethnicity, age, gamma glutamyltransferase (GGT), waist circumference (WC), fasting insulin, and fasting glucose with the following formula: USFLI = (e−0.8073 × non-Hispanic black + 0.3458 × Mexican American + 0.0093 × age + 0.6151 × ln (GGT) + 0.0249 × WC + 1.1792 × ln (insulin) + 0.8242 × ln (glucose)−14.7812)/(1 + e−0.8073 × non-Hispanic black + 0.3458 × Mexican American + 0.0093 × age + 0.6151 × ln (GGT) + 0.0249 × WC + 1.1792 × ln (insulin) + 0.8242 × ln (glucose)−14.7812) × 100. A USFLI ≥30 was considered to have putative NAFLD with an area under the receiver operating characteristic curve of 0.80 (10). AHF was defined as NAFLD fibrosis score (NFS) > 0.676 or fibrosis 4 index (FIB-4) > 2.67 or aspartate aminotransferase (AST)/platelet ratio index (APRI) > 1 in the presence of NAFLD (11). NFS has been validated to diagnose AHF non-invasively and accurately using the formula: NFS = −1.675 + 0.037 × age + 0.094 × BMI + 1.13 × impaired fasting glycemia or diabetes (yes = 1, no = 0) + 0.99 × AST/alanine aminotransferase (ALT) − 0.013 × platelet −0.66 × albumin (12). The formula for FIB-4 is: FIB-4 = (age × AST)/(Platelet counts × (SQRT(ALT))) (13). The formula for APRI is: APRI = ([AST/upper limit of normal (ULN)]/Platelet counts) × 100 (14). In the 1999–2000 cycle of NHANES, AST = 40 U/L was used as ULN, and 33 U/L was used as ULN in subsequent years (15).
Based on previous relevant studies, we selected several possible covariates, including age, gender (male or female), ethnicity (Mexican American, non-Hispanic black, non-Hispanic white, other Hispanic, or other races), education (<high school, high school, or >high school), family income to poverty ratio (PIR), marital status, total energy intake, and chronic comorbid conditions including a range of other cardiometabolic diseases, i.e., diabetes, hypertension, and self-report cardiovascular disease (CVD) (including coronary heart disease, stroke, heart attack, congestive heart failure, and angina). The criteria for diagnosing hypertension was that a doctor says someone has high blood pressure, is taking antihypertensive medication, or that their blood pressure values are in the hypertensive range (16). Diabetes was diagnosed based on if a doctor said that a person has diabetes, abnormal blood glucose/glucose tolerance test, or was taking related medication (17).
We speculated that OBS may have indirect effects on NAFLD through several other pathways. First, oxidative stress-related markers may be important mediators, and second, given the close association of oxidative stress with inflammation and metabolism, we considered the potential mediating effects of these markers (Figure 2). To investigate whether OBS acts indirectly on NAFLD through mediators related to inflammation, oxidative stress, and glycolipid metabolism, we performed a mediation analysis. We selected several important biomarkers of these relevant aspects in NAFLD based on the literature, including blood neutrophils (18), ALT/AST (19), serum albumin (20), uric acid (UA) (21), bilirubin (22), GGT (23), triglycerides (TG) (24), total cholesterol (TC) (24), high-density lipoprotein (HDL)-cholesterol (24), and fasting blood glucose (25). Detailed laboratory tests and data collection instruments are publicly available.1

Figure 2. Schematic diagram of the mediation effect. OBS, oxidative balance score; NAFLD, nonalcoholic fatty liver disease.
All analyses were conducted using EmpowerStats software and R version 4.1.3. Due to the complex design of NHANES, we appropriately weighted our data analysis according to the NHANES survey reporting guidelines for the survey. For continuous variables, we used the survey-weighted mean (95% confidence interval [CI]), and the value of p was calculated by survey-weighted linear regression. For categorical variables, we used survey-weighted percentages (95% CI), and value of ps were calculated by survey-weighted Chi-square tests. We used multivariate logistic regression analysis to examine the association between OBS and NAFLD/AHF. We constructed three multivariate regression models: first, we constructed model 1 (unadjusted model), which did not adjust for any covariates; model 2 adjusted for age, sex, race, marital status, education level, and energy intake (kcal/day); model 3 was based on model 2 and added adjustments for diabetes, hypertension, and CVD to model 2. Restricted cubic splines (RCS) were used to describe the nonlinear relationship between the OBS and NAFLD/AHF. The curve fitting term is defined by the RCS function from the rms package, and the degrees of freedom (or knots) are determined according to the magnitude of the P for nonlinear value.
To investigate whether OBS may influence the risk of NAFLD through other pathways, we performed a mediation analysis by selecting possible markers as mediating variables. The total effect of OBS on the risk of NAFLD (c) includes the direct effect of OBS (c′) and the indirect effect through mediating variables (a*b), and c = c′ + a*b. For the markers of inflammation, oxidative stress, and glycolipid metabolism included in this study, we performed individual mediator analyses based on previous studies and calculated direct effects in the association of OBS with risk of NAFLD and indirect effects through these markers. We calculated the proportion of the total effect mediated by the included mediating variables. All mediation analyses were adjusted for all covariates. In addition, stratified and sensitivity analyses were performed to test the consistency of the findings across subgroups and the stability of the results.
6,341 adult participants (representing a weighted US population of 129,919,267) were included in our study. 1,917 and 266 individuals were diagnosed with NAFLD and AHF (prevalence of 30.2 and 13.9%, respectively). We divided the quartiles according to OBS, and all indicators including PIR, gender, race, education level, marital status, daily energy intake, presence of diabetes, and hypertension were significantly different (all p < 0.05), except for age and presence of CVD, which did not differ between quartiles. As the quartiles of OBS gradually increased, participants were wealthier, had a higher proportion of women, a higher proportion of non-Hispanic whites, a higher level of education, a higher proportion of singles, a higher daily energy intake, and a lower prevalence of diabetes and hypertension (Table 2). We also divided the population according to the presence of NAFLD and AHF. Those with NAFLD differed significantly on all covariates except for PIR and daily energy intake, which did not differ from those without NAFLD. OBS (both dietary and lifestyle OBS) were significantly lower in the NAFLD population (all p < 0.0001). However, while OBS and dietary OBS differed in patients with AHF compared to non-AHF (1,917 patients with NAFLD had accessible diagnostic data), baseline lifestyle OBS showed no difference in the population (p = 0.786) (Supplementary Tables S2, S3).
Three models were constructed to explore the relationship between OBS and NAFLD/AHF when used as continuous or categorical variables. Model 1 was an unadjusted model, model 2 was adjusted for age, sex, race, marital status, education level, and daily energy intake, and model 3 was adjusted for all covariates. In all three models, OBS (continuous) (including dietary OBS and lifestyle OBS) was negatively associated with the risk of NAFLD (all p < 0.0001). When OBS was used as a categorical variable, the risk of NAFLD gradually decreased with increasing quartiles in all models (P for trend <0.0001). In the fully adjusted model, OBS was associated with a 65% lower risk of NAFLD in Q4 compared to Q1 (reference) (odds ratio [OR] (95% CI) = 0.352 (0.260, 0477), p < 0.0001). Similarly, in model 3, dietary OBS and lifestyle OBS in Q4 compared with Q1 reduced the risk of NAFLD by 55 and 77%, respectively, (all p < 0.0001) (Table 3). However, OBS (continuous and categorical) was only associated with the risk of AHF in the unadjusted model. There was no association with the risk of AHF in the adjusted models (all p > 0.05) (Supplementary Table S4).
We examined the nonlinear relationship between OBS and risk of NAFLD using RCS models. OBS, dietary OBS, and lifestyle OBS were all nonlinearly associated with risk of NAFLD (all P nonlinearity <0.0001). In addition, the inflection points of the nonlinear curves for the relationship between OBS, dietary OBS, and lifestyle OBS and NAFLD were 26, 21, and 5, respectively (Figure 3). We therefore performed a threshold effect analysis based on the inflection point. Interestingly, OBS (both dietary and lifestyle OBS) was significantly negatively associated with NAFLD risk both before and after the inflection point. After the inflection point, OBS was more significantly associated with a reduced risk of NAFLD, suggesting that OBS, dietary OBS, and lifestyle OBS were associated with a more significant reduction in NAFLD risk after exceeding 26, 21, and 5 points, respectively (Supplementary Table S5).
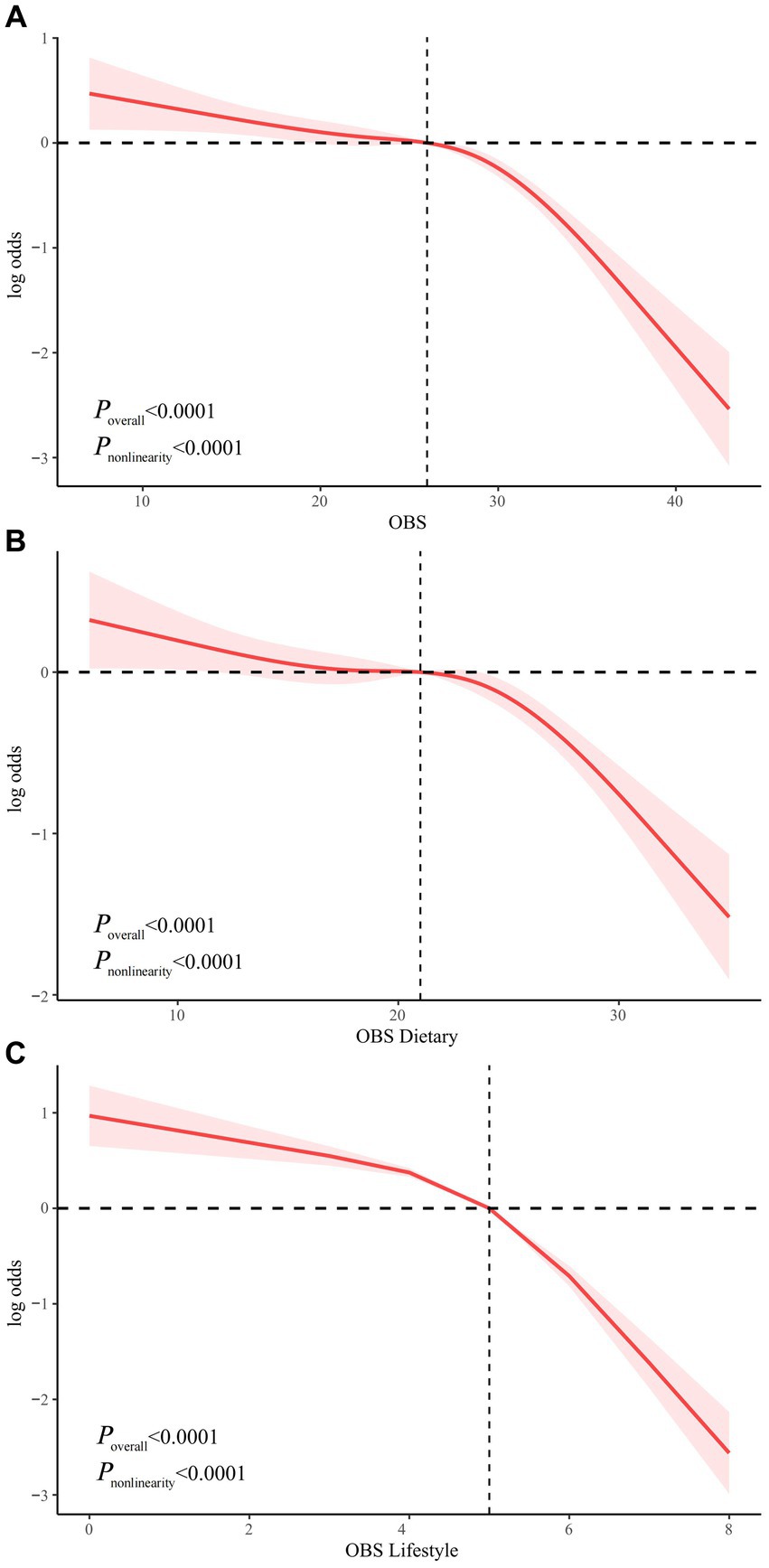
Figure 3. Non-linear relationship of all OBS with NAFLD. (A) Non-linear relationship between OBS and NAFLD. (B) Non-linear relationship between dietary OBS and NAFLD. (C) Non-linear relationship between lifestyle OBS and NAFLD. OBS, oxidative balance score; NAFLD, nonalcoholic fatty liver disease.
We then explored whether OBS indirectly affects NAFLD risk through markers related to inflammation, oxidative stress, and glycolipid metabolism. There was a significant direct effect of OBS with NAFLD risk in the presence of each individual mediating variable (all p < 0.0001). Indirect mediation effects were present for all included mediators (all p < 0.0001) except ALT and AST (all p > 0.05). The average mediated proportions of blood neutrophils, serum albumin, GGT, UA, bilirubin, TC, TG, HDL-cholesterol, and fasting glucose were 9.3, 7.6, 13.3, 13.9, 5.03, 0.9, 13.8, 29.3, and 3.6%, respectively. (All p < 0.0001, except for TC where P was 0.0120) (Table 4).
To investigate whether the association of OBS with the risk of NAFLD was consistent across subgroups, we performed a stratified analysis. Notably, the P for interaction was >0.05 for all subgroups (age, sex, race, education level, marital status, diabetes, hypertension, and CVD), indicating that our findings were consistent across all subgroups (Figure 4).
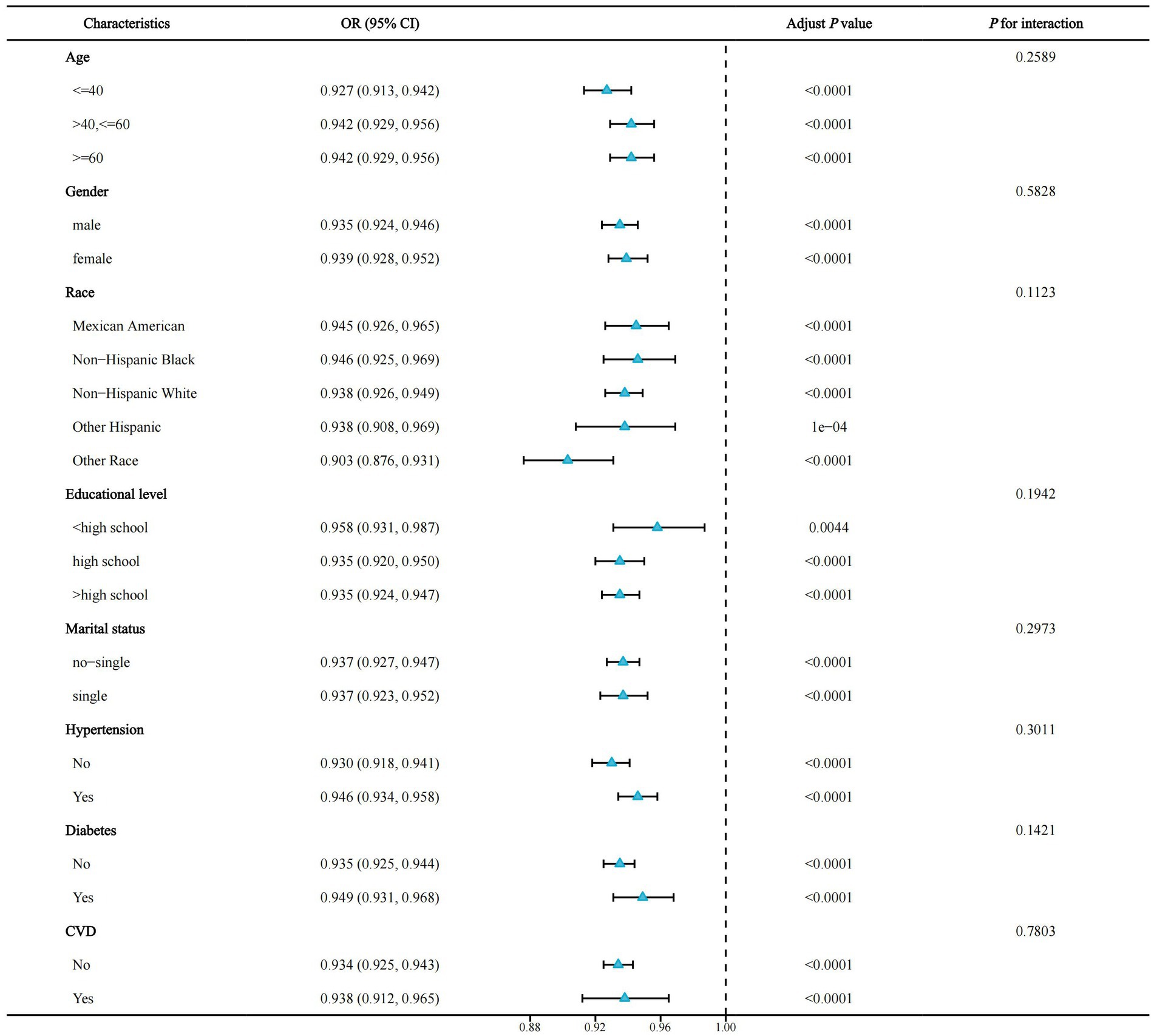
Figure 4. Forest plot for stratified analysis. OR, odds ratio; 95% CI, 95% confidence interval; CVD, cardiovascular disease.
To verify the stability of our results, we performed a sensitivity analysis. We obtained similar results after dividing the OBS (including dietary and lifestyle OBS) by tertile or quintile (Supplementary Table S6). Second, we did not adjust for daily energy intake in the multivariate regression analysis. Consistently, the associations of OBS, dietary OBS, and lifestyle OBS with NAFLD all remained similarly significant (Supplementary Table S7). These results indicate a robust stability of our findings.
To the best of our knowledge, this study is the first to elucidate the effects of dietary and lifestyle modifiable pro- and antioxidants on NAFLD/AHF in a real-world setting using a comprehensive composite indicator of oxidative stress, the OBS. By analyzing data from a US nationally representative cross-sectional study over 20 years, we showed that higher OBS scores are nonlinearly associated with lower risk of NAFLD and that similar effects are present for independent dietary OBS and lifestyle OBS, supporting the critical impact of OBS on the onset and progression of NAFLD. Furthermore, through mediation analysis, we conclude that OBS may indirectly mediate NAFLD risk through effects on inflammation, oxidative stress, and glycolipid metabolism. Notably, OBS (both dietary OBS and lifestyle OBS) were not associated with the risk of AHF in patients with NAFLD.
Impaired oxidative stress signaling is closely related to the pathogenesis of NAFLD/NASH based on the critical mechanism of overproduction of ROS leading to cellular damage, senescence, and death, and can lead to disruption of lipid metabolism, impaired insulin signaling, and dysregulation of innate immune signaling pathways (26). Mechanistically, oxidative stress is essentially due to an imbalance between pro-oxidants (e.g., superoxide anions, hydroxyl radical, and hydrogen peroxide) and antioxidants (superoxide dismutase, glutathione peroxidase, and vitamins A, C, E, etc.) in the body (27). In the pathophysiology of NAFLD/NASH, lipotoxicity in the liver leads to impaired function of several ROS-generating organelles, such as mitochondria, endoplasmic reticulum, and peroxisomes, resulting in the release of abundant ROS and causing an imbalance in redox signaling. Accumulating experimental evidence suggests that modulation of redox signaling in NAFLD/NASH may attenuate disease progression and can serve as a potential target. Targeting nuclear factor E2-related factor 2, a redox-regulated transcription factor, has been demonstrated to improve NASH by ameliorating lipotoxicity, inflammation, and oxidative stress (28). In addition, scattered clinical data suggest that supplementation with a variety of dietary antioxidants such as vitamin E, vitamin C, and hesperidin, as well as lifestyle changes such as exercise, may improve clinical indicators in part by reducing oxidative stress in patients with NAFLD/NASH (29–31).
As there are still no approved drugs for the treatment of NAFLD/NASH, dietary and lifestyle changes remain the cornerstone of NAFLD management (32). Because of the current lack of real-world evidence on whether dietary and lifestyle pro- and antioxidants have integrated and independent effects on the onset and progression of NAFLD, we used a previously well-established composite score that assesses the pro- and antioxidants properties of components of dietary and lifestyle sources. The OBS is a tool used to assess overall redox homeostasis in individuals and has been shown to be associated with colorectal cancer, CVD, and chronic kidney disease in previous epidemiological studies (33). More recent studies using NHANES have suggested that higher OBS is associated with improved cognitive function and depressive symptoms, and reduced risk of periodontitis and diabetes compared with those with the lowest OBS (7, 8, 34, 35). After adjustment for all included confounders, OBS treated as both a continuous and a categorical variable, was associated with the risk of NAFLD but not AHF. Compared to Q1, the risk of NAFLD was reduced by approximately 65% in the population with OBS located in Q4 (p trend <0.0001). Notably, both higher dietary OBS and lifestyle OBS were also independently associated with reduced risk of NAFLD, with approximately 55 and 77% reductions in the Q4 population compared to the reference population, respectively. Thus, lifestyle OBS appears to be associated with a greater reduction in NAFLD risk than dietary OBS. The underlying mechanisms remain unclear, but a recent study using NHANES III showed that physical activity is more important than diet for the prognosis of patients with metabolic-associated fatty liver disease (MAFLD) and sarcopenia (36). Future mechanistic studies are needed to further explore the relevant mechanisms.
We then further investigated the nonlinear relationship between OBS and risk of NAFLD. Both independent and combined dietary OBS and lifestyle OBS were nonlinearly associated with the risk of NAFLD (p nonlinearity <0.0001). The inflection points for OBS, dietary OBS, and lifestyle OBS were 26, 21, and 5 points, respectively. We used threshold effect analysis to show that OBS, dietary OBS, and lifestyle OBS were associated with a more pronounced reductions in NAFLD risk after 26, 21 and 5 points, respectively, which may have important implications for the dietary and lifestyle management of patients with NAFLD.
We then hypothesized that OBS may exert beneficial effects indirectly through relevant markers. Therefore, we next investigated whether OBS indirectly reduces the risk of NAFLD via these pathways of inflammation, oxidative stress, and glycolipid metabolism. Not surprisingly, most of these potential biomarkers may mediate the protective effect of OBS against NAFLD, although ALT and AST do not appear to mediate the effect. HDL-cholesterol, UA, and TG may have mediated the highest proportions, at 29.3, 13.9, and 13.8%, respectively. HDL-cholesterol mediates the regulation of lipid metabolism mainly by promoting reverse cholesterol transport, antioxidant, and anti-inflammatory mechanisms, and previous studies have demonstrated that both diet and lifestyle management can improve HDL-cholesterol levels (37). Conversely, TG has been shown to be a core biomarker of lipid dysregulation in patients with NAFLD (24). Moreover, the role of UA in anti-oxidative stress has been well established. Our study suggests that OBS may indirectly reduce the risk of NAFLD mainly through these markers, and future studies are needed to further validate our findings.
We performed a stratified analysis to elicit whether OBS still had relevant effects in different subgroups. In all subgroups, we did not observe significant differences between groups (all P for interaction >0.05), suggesting the consistency of our study across subgroups and that OBS may reduce NAFLD risk in people with different characteristics.
To test the stability of our results, we performed a sensitivity analysis. After dividing OBS (including dietary OBS and lifestyle OBS) into tertiles and quintiles, we obtained similar significant negative correlations with NAFLD risk. Consistently, a similar effect remained after not adjusting for daily energy intake in multivariate regression analysis. These findings justify the stability of our results, and the conclusion that OBS is associated with a reduced risk of NAFLD is robust.
Our results show that in the real world, healthy dietary and lifestyle habits with higher antioxidant properties are associated with a significant reduction in the risk of NAFLD. In fact, healthy eating habits have been widely shown to be associated with a reduced risk of NAFLD/MAFLD and liver fibrosis. A variety of dietary quality indices, including the Dietary Inflammatory Index (DII), the Mediterranean diet, the Dietary Approach to Stop Hypertension, the Alternate Healthy Eating Index diet, and the Healthy Eating Index-2015 are associated with the risk of NAFLD/MAFLD and liver fibrosis, with the higher DII being associated with lower diet quality, while the rest of the indices are considered healthy eating indices (38–40). Lifestyle changes are also recognized as having a fundamental role in the improvement of NAFLD. Obesity is a major contributor to the development and progression of NAFLD and there is strong evidence that a 10% weight loss is effective in improving the histological characteristics of NASH patients (41). Physically active individuals have an approximately 30% reduced risk of NAFLD compared to inactive individuals (40). In addition, smoking and low-moderate alcohol consumption have been suggested to be associated with the development and severity of NAFLD (42–44). Our results highlight that both independent and combined healthy diet and lifestyle in terms of oxidative stress can significantly reduce the incidence and development of NAFLD.
Interestingly, OBS did not appear to be associated with progression of fibrosis in patients with NAFLD. An important randomized controlled clinical trial showed that vitamin E was associated with improved histological features including hepatic steatosis and lobular inflammation and reduced biochemical markers such as ALT and AST, but not with improvement in fibrosis, in patients with NASH (45). However, a recent meta-analysis concluded that vitamin E was associated with at least ≥1 fibrosis stage improvement compared with placebo (46). Using NHANES, a study showed an association between serum vitamin C, choline, alpha-carotene, and vitamin B12 and liver fibrosis (47). Similarly, other antioxidants may be associated with fibrosis improvement in patients with NAFLD (48). Currently, the underlying mechanisms remain unclear, but an important reason may be that OBS is a composite index that includes both pro-oxidants and antioxidants, and therefore its combined effect cannot be explained by the alleviation of fibrosis by antioxidants alone. Therefore, future high-quality prospective studies are needed to further confirm our findings.
A point that warrants the need for cautious interpretation is the possibility of selection bias and compromised generalizability of the results as our study population went from an initial population of 101,316 to a final population of 6,341. However, several explanations can be offered as follows. First, the exclusion criteria implemented in our study were all rigorous. The fasting blood glucose and insulin tests required for the diagnosis of USFLI were only performed in a subset of participants, yet this also ensured the reliability of the NAFLD diagnosis (compared to FLI) because the fasting blood tests excluded dietary interferences and ensured that the samples were fresh. We excluded the population with missing covariates, which is more likely to ensure the reliability of the results in subsequent regression analyses adjusting for confounders. Finally, and most importantly, our analyses were all appropriately weighted and considered complex sampling design according to the NHANES study guidelines (49), i.e., probabilistic sampling to ensure that the overall sample was represented by a smaller sample. Therefore, we believe that weighting all analyses to represent the overall population and rigorous exclusion criteria ensure the reliability and generalizability of the results. More large-sample studies are needed in the future to validate our conclusions.
Our study has several important strengths. First, we used a nationally representative population-based study to conduct our investigation, and the results are generalizable and representative. Second, we included possible covariates based on previous studies, which greatly reduced the effect of confounding factors. We further explored the direct and indirect effects of OBS on NAFLD using mediation analysis, which showed potential beneficial effects of OBS through inflammation, oxidative stress, and glycolipid metabolism. Stratified and sensitivity analyses highlighted the robustness and stability of our findings. However, our study also has limitations. First, our study is a cross-sectional study, and thus residual confounders may still exist, and causal relationships cannot be drawn. Second, our study was conducted in the US population, and findings for other races and countries need to be further explored in future studies. Finally, we diagnosed NAFLD/AHF using non-invasive markers rather than liver biopsy, and thus may lack accuracy. However, because of the cost of liver biopsy, the potential risks of the operation, and the inability to generalize in large population-based studies, as well as the proven good accuracy of non-invasive scores, diagnosis using non-invasive markers is therefore also well characterized and these effects are unlikely to affect the reliability of the results.
Dietary and lifestyle OBS had joint and independent protective effects on the risk of NAFLD in a nationally representative cross-sectional study and showed a non-linear relationship. However, no association was found between OBS and AHF risk. The population with OBS in the highest quartile had a 65% reduction in NAFLD risk compared to the lowest quartile. OBS may indirectly affect the development of NAFLD through inflammation, oxidative stress, and glycolipid metabolism. These findings may be instrumental in stratifying the risk of NAFLD in the general population and allowing for interventions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The National Center for Health Statistics (NCHS) Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YL: Conceptualization, Formal analysis, Software, Writing – original draft. MC: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank all the participants in the NHANES for providing data for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1276940/full#supplementary-material
NAFLD, Nonalcoholic fatty liver disease; AHF, Advanced liver fibrosis; OBS, Oxidative balance score; ROS, Reactive oxygen species; NHANES, National Health and Nutrition Examination Survey; NCHS, National Center for Health Statistics; USFLI, US fatty liver index; BMI, Body mass index; GGT, Gamma glutamyltransferase; WC, Waist circumference; NFS, NAFLD fibrosis score; FIB-4, Fibrosis 4 index; AST, Aspartate aminotransferase; APRI, AST/platelet ratio index; ALT, Alanine aminotransferase; ULN, Upper limit of normal; PIR, Family income to poverty ratio; CVD, Cardiovascular disease; UA, Uric acid; TG, Triglycerides; TC, Total cholesterol; HDL, High-density lipoprotein; 95% CI, 95% confidence interval; RCS, Restricted cubic splines; OR, Odds ratio; DII, Dietary Inflammatory Index.
1. Pouwels, S, Sakran, N, Graham, Y, Leal, A, Pintar, T, Yang, W, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. (2022) 22:63. doi: 10.1186/s12902-022-00980-1
2. Chalasani, N, Younossi, Z, Lavine, JE, Diehl, AM, Brunt, EM, Cusi, K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. (2012) 142:1592–609. doi: 10.1053/j.gastro.2012.04.001
3. Riazi, K, Azhari, H, Charette, JH, Underwood, FE, King, JA, Afshar, EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2022) 7:851–61. doi: 10.1016/s2468-1253(22)00165-0
4. Lee, KC, Wu, PS, and Lin, HC. Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis. Clin Mol Hepatol. (2023) 29:77–98. doi: 10.3350/cmh.2022.0237
5. Karkucinska-Wieckowska, A, Simoes, ICM, Kalinowski, P, Lebiedzinska-Arciszewska, M, Zieniewicz, K, Milkiewicz, P, et al. Mitochondria, oxidative stress and nonalcoholic fatty liver disease: a complex relationship. Eur J Clin Investig. (2022) 52:e13622. doi: 10.1111/eci.13622
6. Xu, X, Poulsen, KL, Wu, L, Liu, S, Miyata, T, Song, Q, et al. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct Target Ther. (2022) 7:287. doi: 10.1038/s41392-022-01119-3
7. Li, H, Song, L, Cen, M, Fu, X, Gao, X, Zuo, Q, et al. Oxidative balance scores and depressive symptoms: mediating effects of oxidative stress and inflammatory factors. J Affect Disord. (2023) 334:205–12. doi: 10.1016/j.jad.2023.04.134
8. Song, L, Li, H, Fu, X, Cen, M, and Wu, J. Association of the oxidative balance score and cognitive function and the mediating role of oxidative stress: evidence from the National Health and nutrition examination survey (NHANES) 2011–2014. J Nutr. (2023) 5:14. doi: 10.1016/j.tjnut.2023.05.014
9. Zhang, W, Peng, SF, Chen, L, Chen, HM, Cheng, XE, and Tang, YH. Association between the oxidative balance score and telomere length from the National Health and nutrition examination survey 1999–2002. Oxidative Med Cell Longev. (2022) 2022:1345071. doi: 10.1155/2022/1345071
10. Ruhl, CE, and Everhart, JE. Fatty liver indices in the multiethnic United States National Health and nutrition examination survey. Aliment Pharmacol Ther. (2015) 41:65–76. doi: 10.1111/apt.13012
11. Li, L, Huang, Q, Yang, L, Zhang, R, Gao, L, Han, X, et al. The association between non-alcoholic fatty liver disease (NAFLD) and advanced fibrosis with serological vitamin B12 markers: results from the NHANES 1999-2004. Nutrients. (2022) 14:61224. doi: 10.3390/nu14061224
12. Angulo, P, Hui, JM, Marchesini, G, Bugianesi, E, George, J, Farrell, GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. (2007) 45:846–54. doi: 10.1002/hep.21496
13. Vallet-Pichard, A, Mallet, V, Nalpas, B, Verkarre, V, Nalpas, A, Dhalluin-Venier, V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. (2007) 46:32–6. doi: 10.1002/hep.21669
14. Wai, CT, Greenson, JK, Fontana, RJ, Kalbfleisch, JD, Marrero, JA, Conjeevaram, HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. (2003) 38:518–26. doi: 10.1053/jhep.2003.50346
15. Udompap, P, Mannalithara, A, Heo, NY, Kim, D, and Kim, WR. Increasing prevalence of cirrhosis among U.S. adults aware or unaware of their chronic hepatitis C virus infection. J Hepatol. (2016) 64:1027–32. doi: 10.1016/j.jhep.2016.01.009
16. Ng, CH, Wong, ZY, Chew, NWS, Chan, KE, Xiao, J, Sayed, N, et al. Hypertension is prevalent in non-alcoholic fatty liver disease and increases all-cause and cardiovascular mortality. Front Cardiovasc Med. (2022) 9:942753. doi: 10.3389/fcvm.2022.942753
17. Barb, D, Repetto, EM, Stokes, ME, Shankar, SS, and Cusi, K. Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Obesity. (2021) 29:1950–60. doi: 10.1002/oby.23263
18. Xia, Y, Zhang, Q, Liu, L, Meng, G, Wu, H, Bao, X, et al. Intermediary effect of inflammation on the association between dietary patterns and non-alcoholic fatty liver disease. Nutrition. (2020) 71:110562. doi: 10.1016/j.nut.2019.110562
19. Świderska, M, Maciejczyk, M, Zalewska, A, Pogorzelska, J, Flisiak, R, and Chabowski, A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free Radic Res. (2019) 53:841–50. doi: 10.1080/10715762.2019.1635691
20. Duran-Güell, M, Garrabou, G, Flores-Costa, R, Casulleras, M, López-Vicario, C, Zhang, IW, et al. Essential role for albumin in preserving liver cells from TNFα-induced mitochondrial injury. FASEB J. (2023) 37:e22817. doi: 10.1096/fj.202201526R
21. Copur, S, Demiray, A, and Kanbay, M. Uric acid in metabolic syndrome: does uric acid have a definitive role? Eur J Intern Med. (2022) 103:4–12. doi: 10.1016/j.ejim.2022.04.022
22. Chen, W, Tumanov, S, Fazakerley, DJ, Cantley, J, James, DE, Dunn, LL, et al. Bilirubin deficiency renders mice susceptible to hepatic steatosis in the absence of insulin resistance. Redox Biol. (2021) 47:102152. doi: 10.1016/j.redox.2021.102152
23. Banderas, DZ, Escobedo, J, Gonzalez, E, Liceaga, MG, Ramírez, JC, and Castro, MG. γ-Glutamyl transferase: a marker of nonalcoholic fatty liver disease in patients with the metabolic syndrome. Eur J Gastroenterol Hepatol. (2012) 24:805–10. doi: 10.1097/MEG.0b013e328354044a
24. Marra, F, and Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. (2018) 68:280–95. doi: 10.1016/j.jhep.2017.11.014
25. Mota, M, Banini, BA, Cazanave, SC, and Sanyal, AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. (2016) 65:1049–61. doi: 10.1016/j.metabol.2016.02.014
26. Chen, Z, Tian, R, She, Z, Cai, J, and Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. (2020) 152:116–41. doi: 10.1016/j.freeradbiomed.2020.02.025
27. Arroyave-Ospina, JC, Wu, Z, Geng, Y, and Moshage, H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: implications for prevention and therapy. Antioxidants. (2021) 10:20174. doi: 10.3390/antiox10020174
28. Bathish, B, Robertson, H, Dillon, JF, Dinkova-Kostova, AT, and Hayes, JD. Nonalcoholic steatohepatitis and mechanisms by which it is ameliorated by activation of the CNC-bZIP transcription factor Nrf2. Free Radic Biol Med. (2022) 188:221–61. doi: 10.1016/j.freeradbiomed.2022.06.226
29. Rives, C, Fougerat, A, Ellero-Simatos, S, Loiseau, N, Guillou, H, Gamet-Payrastre, L, et al. Oxidative stress in NAFLD: role of nutrients and food contaminants. Biomol Ther. (2020) 10:21702. doi: 10.3390/biom10121702
30. Cheraghpour, M, Imani, H, Ommi, S, Alavian, SM, Karimi-Shahrbabak, E, Hedayati, M, et al. Hesperidin improves hepatic steatosis, hepatic enzymes, and metabolic and inflammatory parameters in patients with nonalcoholic fatty liver disease: a randomized, placebo-controlled, double-blind clinical trial. Phytother Res. (2019) 33:2118–25. doi: 10.1002/ptr.6406
31. Farzanegi, P, Dana, A, Ebrahimpoor, Z, Asadi, M, and Azarbayjani, MA. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): roles of oxidative stress and inflammation. Eur J Sport Sci. (2019) 19:994–1003. doi: 10.1080/17461391.2019.1571114
32. Romero-Gómez, M, Zelber-Sagi, S, and Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. (2017) 67:829–46. doi: 10.1016/j.jhep.2017.05.016
33. Hernández-Ruiz, Á, García-Villanova, B, Guerra-Hernández, E, Amiano, P, Ruiz-Canela, M, and Molina-Montes, E. A review of a priori defined oxidative balance scores relative to their components and impact on health outcomes. Nutrients. (2019) 11:40774. doi: 10.3390/nu11040774
34. Qu, H . The association between oxidative balance score and periodontitis in adults: a population-based study. Front Nutr. (2023) 10:1138488. doi: 10.3389/fnut.2023.1138488
35. Wu, C, Ren, C, Song, Y, Gao, H, Pang, X, and Zhang, L. Gender-specific effects of oxidative balance score on the prevalence of diabetes in the US population from NHANES. Front Endocrinol. (2023) 14:1148417. doi: 10.3389/fendo.2023.1148417
36. Yi, Y, Wang, C, Ding, Y, He, J, Lv, Y, and Chang, Y. Diet was less significant than physical activity in the prognosis of people with sarcopenia and metabolic dysfunction-associated fatty liver diseases: analysis of the National Health and nutrition examination survey III. Front Endocrinol. (2023) 14:1101892. doi: 10.3389/fendo.2023.1101892
37. Montemayor, S, Bouzas, C, Mascaró, CM, Casares, M, Llompart, I, Abete, I, et al. Effect of dietary and lifestyle interventions on the amelioration of NAFLD in patients with metabolic syndrome: the FLIPAN study. Nutrients. (2022) 14:112223. doi: 10.3390/nu14112223
38. Tian, T, Zhang, J, Xie, W, Ni, Y, Fang, X, Liu, M, et al. Dietary quality and relationships with metabolic dysfunction-associated fatty liver disease (MAFLD) among United States adults, results from NHANES 2017–2018. Nutrients. (2022) 14:4505. doi: 10.3390/nu14214505
39. Gao, V, Long, MT, Singh, SR, Kim, Y, Zhang, X, Rogers, G, et al. A healthy diet is associated with a lower risk of hepatic fibrosis. J Nutr. (2023) 153:1587–96. doi: 10.1016/j.tjnut.2023.03.038
40. Vilar-Gomez, E, Nephew, LD, Vuppalanchi, R, Gawrieh, S, Mladenovic, A, Pike, F, et al. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology. (2022) 75:1491–506. doi: 10.1002/hep.32207
41. Vilar-Gomez, E, Martinez-Perez, Y, Calzadilla-Bertot, L, Torres-Gonzalez, A, Gra-Oramas, B, Gonzalez-Fabian, L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic Steatohepatitis. Gastroenterology. (2015) 149:367. doi: 10.1053/j.gastro.2015.04.005
42. Yuan, S, Chen, J, Li, X, Fan, R, Arsenault, B, Gill, D, et al. Lifestyle and metabolic factors for nonalcoholic fatty liver disease: Mendelian randomization study. Eur J Epidemiol. (2022) 37:723–33. doi: 10.1007/s10654-022-00868-3
43. Blomdahl, J, Nasr, P, Ekstedt, M, and Kechagias, S. Moderate alcohol consumption is associated with significant fibrosis progression in NAFLD. Hepatol Commun. (2023) 7:e0003. doi: 10.1097/hc9.0000000000000003
44. Magherman, L, Van Parys, R, Pauwels, NS, Verhelst, X, Devisscher, L, Van Vlierberghe, H, et al. Meta-analysis: the impact of light-to-moderate alcohol consumption on progressive non-alcoholic fatty liver disease. Aliment Pharmacol Ther. (2023) 57:820–36. doi: 10.1111/apt.17388
45. Sanyal, AJ, Chalasani, N, Kowdley, KV, McCullough, A, Diehl, AM, Bass, NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. (2010) 362:1675–85. doi: 10.1056/NEJMoa0907929
46. Majzoub, AM, Nayfeh, T, Barnard, A, Munaganuru, N, Dave, S, Singh, S, et al. Systematic review with network meta-analysis: comparative efficacy of pharmacologic therapies for fibrosis improvement and resolution of NASH. Aliment Pharmacol Ther. (2021) 54:880–9. doi: 10.1111/apt.16583
47. Liu, X, Shen, H, Chen, M, and Shao, J. Clinical relevance of vitamins and carotenoids with liver steatosis and fibrosis detected by transient elastography in adults. Front Nutr. (2021) 8:760985. doi: 10.3389/fnut.2021.760985
48. Kim, D, Konyn, P, Cholankeril, G, and Ahmed, A. Physical activity is associated with nonalcoholic fatty liver disease and significant fibrosis measured by FibroScan. Clin Gastroenterol Hepatol. (2022) 20:e1438. doi: 10.1016/j.cgh.2021.06.029
Keywords: nonalcoholic fatty liver disease, NHANES, advanced liver fibrosis, oxidative stress, antioxidant
Citation: Liu Y and Chen M (2023) Dietary and lifestyle oxidative balance scores are independently and jointly associated with nonalcoholic fatty liver disease: a 20 years nationally representative cross-sectional study. Front. Nutr. 10:1276940. doi: 10.3389/fnut.2023.1276940
Received: 13 August 2023; Accepted: 06 October 2023;
Published: 18 October 2023.
Edited by:
Mauro Serafini, University of Teramo, ItalyReviewed by:
Ronny Westerman, Bundesinstitut für Bevölkerungsforschung, GermanyCopyright © 2023 Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingkai Chen, chenmingkai@whu.edu.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.