- 1Department of Pharmacy, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
- 2Department of Nutrition, Isfahan University of Medical Sciences, Isfahan, Iran
- 3Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
- 4School of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
- 5Maternal and Childhood Obesity Research Center, Urmia University of Medical Sciences, Urmia, Iran
Introduction: The findings of randomized controlled trials (RCTs) regarding the effect of flaxseed on adipokine concentrations are conflicting. Therefore, the present meta-analysis was conducted to provide definite and conclusive results.
Methods: Systematically, Scopus, Embase, PubMed, Web of Science databases, and Google Scholar were searched for relevant literature published up to December 2022. Based on random-effect models, standard mean differences (SMDs) were calculated for net changes in adipokine concentrations.
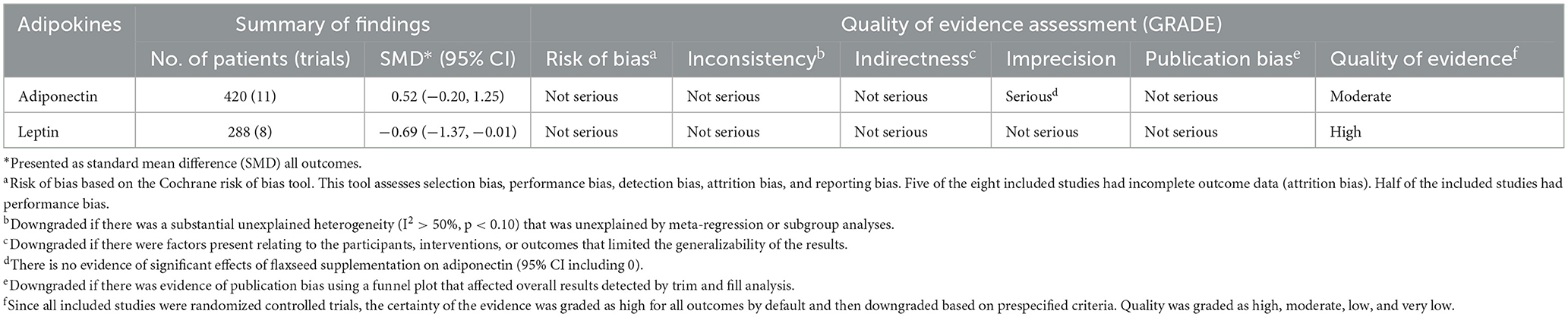
Results: Overall, 13 RCTs (15 arms) were eligible to be included. The results indicated that leptin was significantly reduced after the intervention with flaxseed supplement (SMD = −0.69, 95% CI: −1.37, −0.01; p = 0.048; I2 = 92.0%, p < 0.001). In addition, flaxseed supplements had no considerable effect on plasma adiponectin (SMD = 0.52, 95% CI: −0.20, 1.25, p = 0.159; I2 = 92.0%, p < 0.001).
Discussion: Flaxseed significantly improves leptin but does not affect adiponectin concentrations. Additional future well-designed trials are required to further assess the potential benefits of flaxseed on adipokines in humans.
1. Introduction
The most common circulating hormone secreted by adipocytes is adiponectin. Adiponectin regulates many metabolic pathways, including fatty acid modulation and glucose oxidation (1). The high-molecular weight (HMW) of adiponectin is also considered a risk factor for metabolic syndrome (MetS) (2, 3). Previous studies have demonstrated that adiponectin levels are lowered in people with type 2 diabetes (T2DM), MetS, and cardiovascular disease (CVD) (4). High plasma levels of adiponectin have also been associated with a lower risk of myocardial infarction in men (5). Leptin is a hormone that regulates energy intake and consumption. It may also have an effect on the pathways that regulate glycolytic enzyme activity, glucose uptake, and the production of inflammatory cytokines (6–8).
Flaxseed (Linum usitatissimum), which is an oil seed or grain, has been suggested as a possible functional food since it contains bioactive components (9). Alpha-linolenic acid (ALA), which makes up ~55% of the total fatty acid content, is present in high amounts. Lignans, a group of phytoestrogens, are also present. There is also dietary fiber that makes up 28% of the weight, and up to one-third is soluble fiber (10). These characteristics suggest that flaxseed may have anti-inflammatory effects and clinical intervention trials have been conducted to ascertain whether flaxseed and flaxseed-derived products (flaxseed oil, whole flaxseed, or lignans) are effective in reducing a variety of cardiovascular risk factors, especially inflammatory indicators such as C-reactive protein (11–14).
In addition to the potential anti-inflammatory capabilities of flaxseed, adiponectin expression has been found to be induced by several flaxseed components in preclinical animal models (15). Additionally, flaxseed oil increased the expression of hepatic adiponectin receptors and circulating adiponectin (16). Other investigations have reached the conclusion that variations in leptin expression may contribute to the possible cardioprotective benefits of flaxseed supplementation (17). According to experimental research, ALA can bind to peroxisome proliferator-activated receptor gamma (PPARγ), which can enhance adiponectin expression and levels in the blood (18, 19). Other clinical trials have reported that ALA enhances adiponectin; in fact, ALA and adiponectin production was found to have a dose–response relationship (20). However, randomized controlled trials (RCTs) have shown conflicting outcomes. The effects of flaxseed on adiponectin and leptin levels were evaluated in a previous meta-analysis published in 2020 (21); however, several trials did not fully measure changes in their concentration. We conducted an additional study on the effects of flaxseed on leptin and adiponectin levels in adults as a result of the contradictory findings of the previous studies and the lack of a comprehensive meta-analysis. To determine the effect of flaxseed supplementation and flaxseed-derived products on adiponectin and leptin levels, the present study performed a comprehensive systematic review and meta-analysis of all relevant RCTs in adults.
2. Methods
This systematic review and meta-analysis was carried out and reported under the Preferred Reporting Items of Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines (22).
2.1. Search strategy
We searched international databases, including Scopus, Embase, PubMed, Web of Science databases, and Google Scholar, from inception to December 2022, using the following keywords: (flax OR flaxseed OR linseed OR lignan OR whole flaxseed OR ground flaxseed OR flaxseed oil OR L. usitatissimum) AND (adiponectin OR adipocytokines OR leptin). The search strategy is presented in Supplementary Table S1. The search process was conducted by two researchers (VM and AHM). In addition, reference lists were searched from included studies.
2.2. Eligibility criteria
Retrieved studies were included in our meta-analysis if they met the following evidence-based PICOS criteria: (1) Patients: adult individuals >18 years old; (2) Intervention: flaxseed supplementation; (3) Control: placebo or control; (4) Outcomes: sufficient data for extraction regarding adiponectin and leptin levels; and (5) Study design: RCTs. In vitro, in vivo, and ex vivo studies, observational studies, quasi-experimental studies, and animal studies were excluded from this meta-analysis. Only articles in English were included in the study.
2.3. Data extraction
Two independent researchers (SA and VM) screened and extracted data from each qualified trial. First author's name, publication year, study location, study design, sample size in each group, dose and type of flaxseed, duration of intervention, average age, gender and baseline body mass index (BMI) of subjects, and mean and standard deviation (SD) of adipokines in both groups at baseline and at the end of the study and their changes from baseline were extracted from the selected RCTs. Any disagreement about the choice of studies was settled by consensus (AHF).
2.4. Quality assessment and assessment of the meta-evidence
The methodological quality assessments of each included study were performed independently by at least two researchers using the Cochrane Collaboration risk of bias tool, in which domains were judged as “low-risk, high-risk, or unclear” (23). The credibility of RCTs was evaluated using the Grading of Recommendations, Assessment, and Evaluation (GRADE) approach, which consisted of five factors as follows: risk of bias, consistency of results, directness, precision, and potential for publication bias. The evidence is categorized into four categories, namely high, moderate, low, or very low.
2.5. Statistical analysis
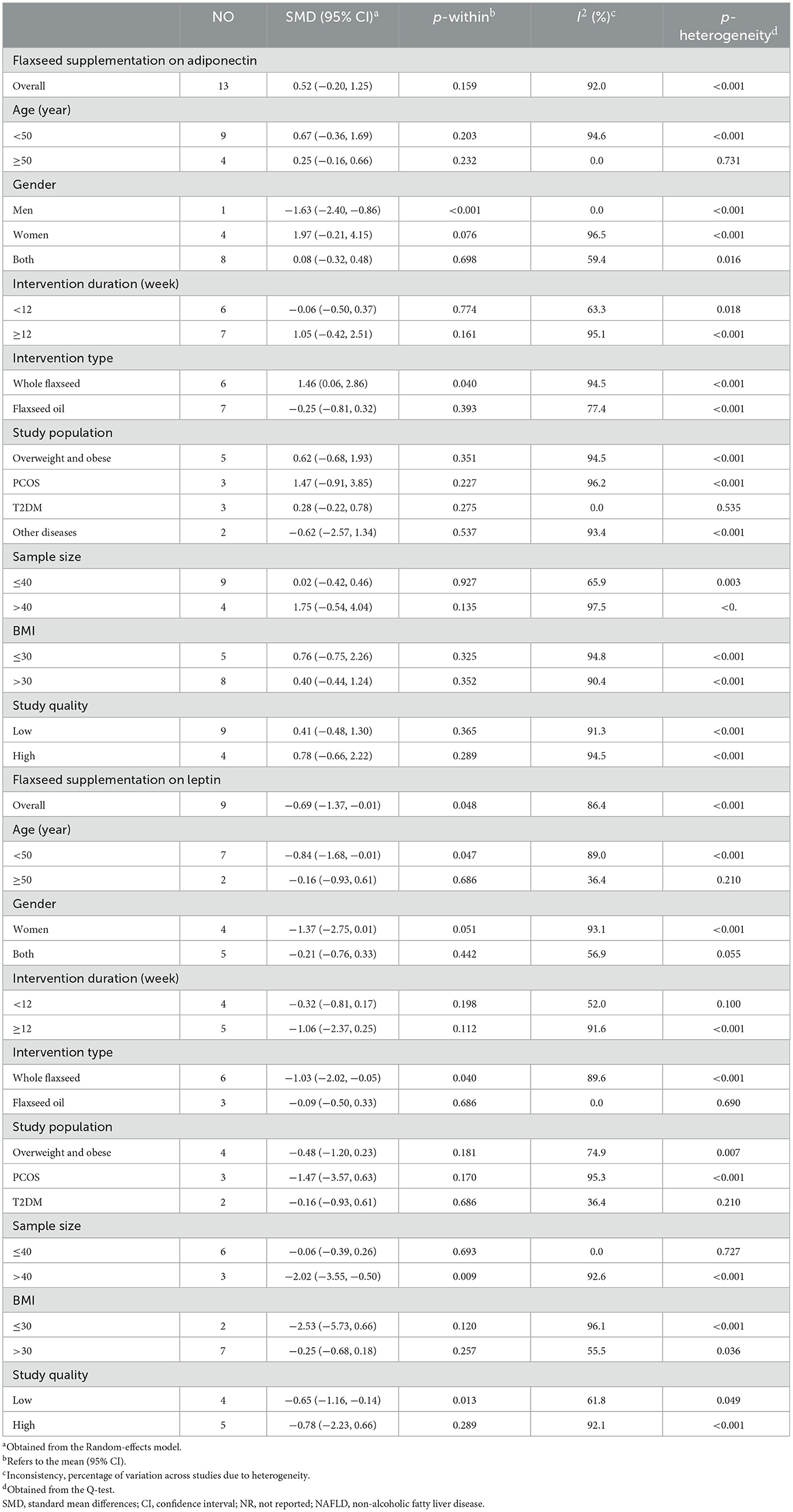
The STATA program (version 16) was used to conduct the statistical analysis (Stata Corp, College Station, TX). To assess the effect size for adipokines, SD and mean differences were determined for the two groups. Furthermore, a random-effects model was used to estimate standardized mean differences (SMDs) with 95% confidence intervals (CIs) (24). When standard error (SE) or confidence interval (CI) was reported, they were also transformed into SD. Heterogeneity between studies was assessed using I2 and the p-value of Cochran's Q-test. We performed a subgroup analysis according to baseline BMI (< 30, ≥30), study quality (high and low), intervention duration (< 12 and ≥12 weeks), type of flaxseed (whole flaxseed and flaxseed oil), sample size (≤ 40 and >40), the health condition [T2DM, polycystic ovary syndrome (PCOS), obesity, and others], gender (men, women, and both), and average age (< 50 and ≥50 years) to identify potential sources of heterogeneity. We also performed a sensitivity analysis to determine the effect of removing one particular study from the overall SMDs. Begg's adjusted rank correlation and Egger's regression asymmetry test were applied to examine the results of the small study effect (25, 26). Publication bias was assessed by visual inspection of funnel plots. If there was evidence of publication bias, the “trim and fill” method was carried out. All statistical tests were two-sided, and a p-value of < 0.05 was considered statistically significant.
3. Results
3.1. Flow and characteristics of included studies
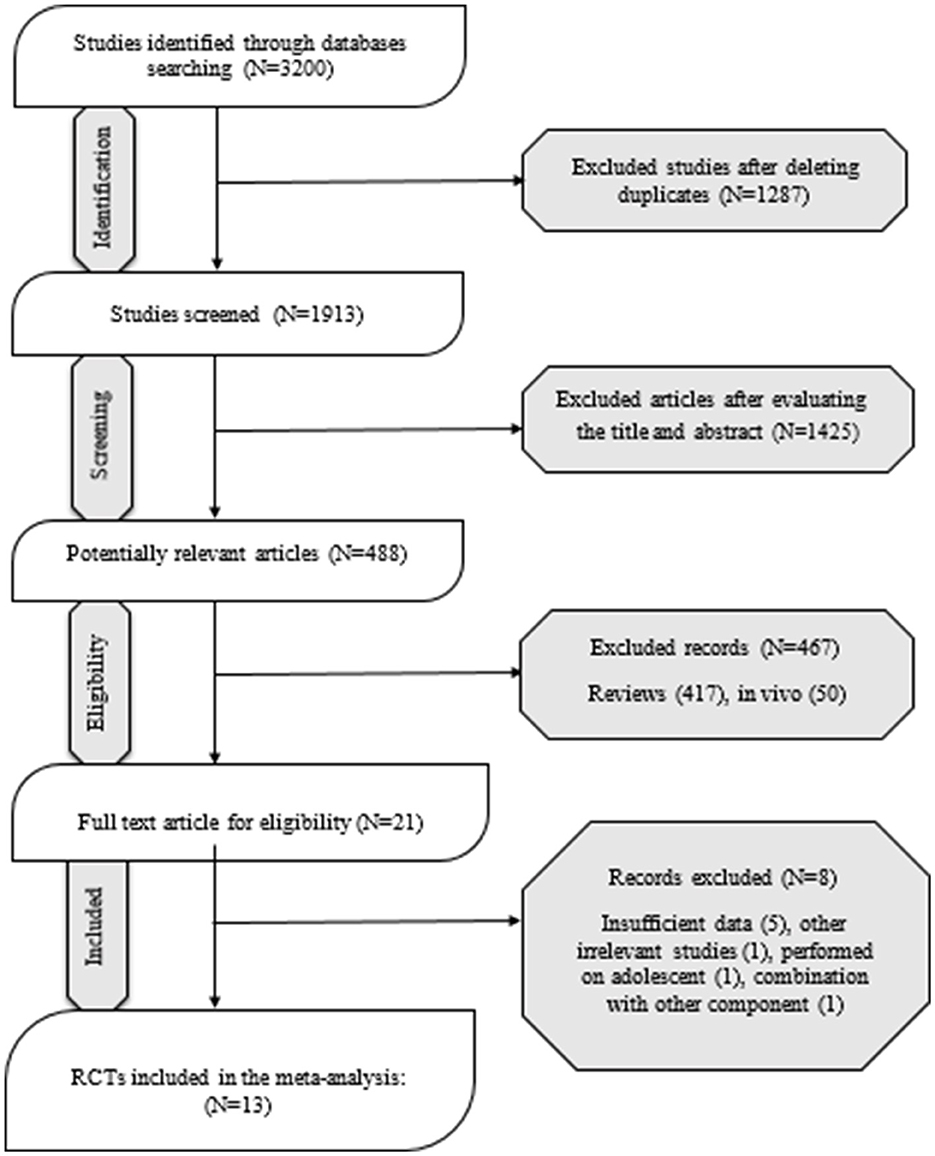
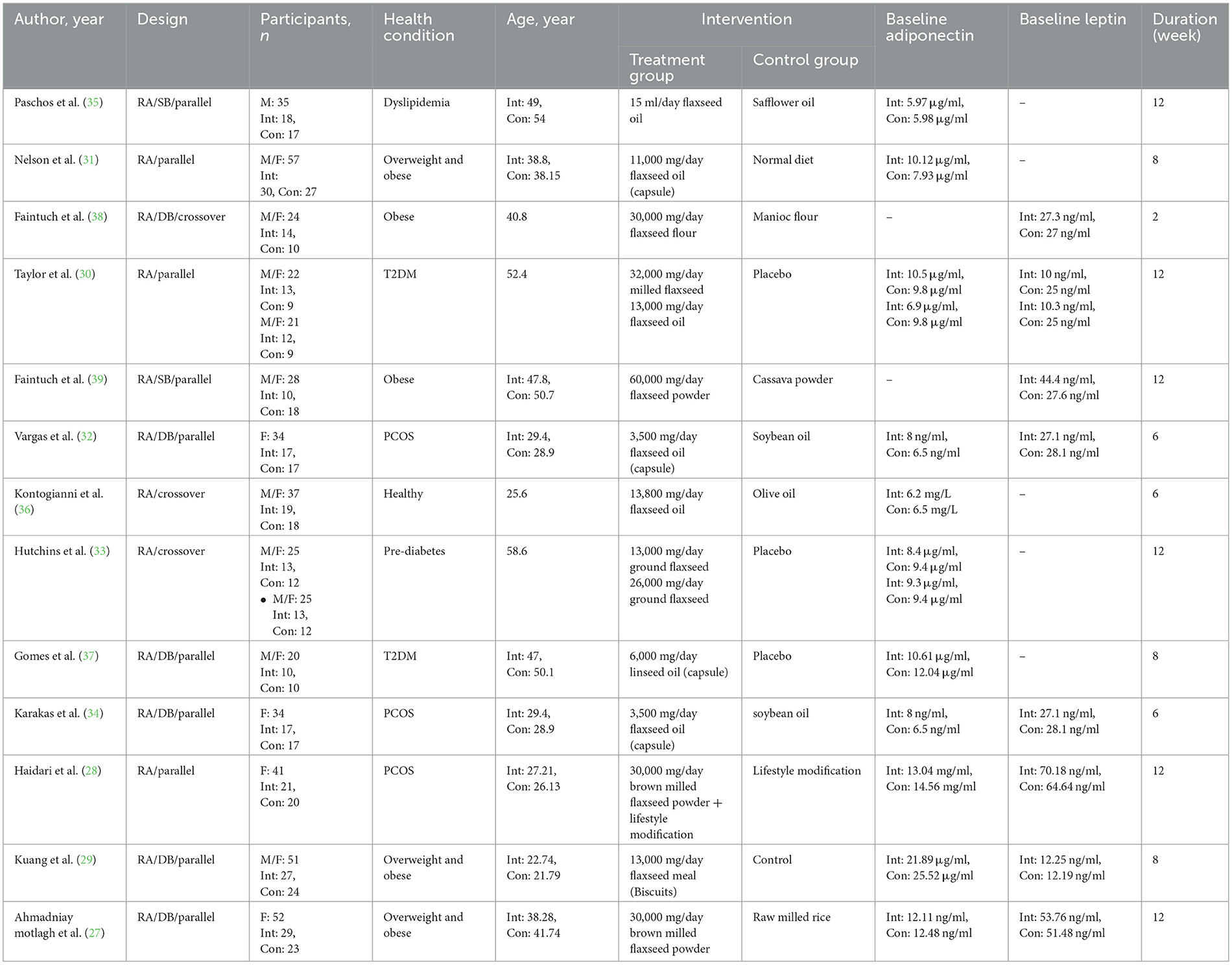
A total of 3,200 studies were identified in the databases, and 1,287 duplicates were excluded. In total, 1,425 studies were evaluated based on the title and abstract, and 467 were deemed irrelevant. There were 21 studies that went through a full-text evaluation, and 8 were omitted. Finally, 13 studies were included in the analysis. Figure 1 shows the selection process of the study. Studies were conducted in Iran (27, 28), China (29), Canada (30), USA (31–34), Greece (35, 36), and Brazil (37–39). The range of intervention periods varied from 2 to 12 weeks. Whole flaxseed (27–30, 38, 39) and ground flaxseed (33) were used in four RCTs with doses from 13,000 to 60,000 mg/day. In the other studies, flaxseed oil (30–32, 34–37) was used, with doses of 3,500 to 14,200 mg/day. In this study, different patient populations were examined in eligible RCTs. Included subjects were patients with obesity (27, 29, 31, 38, 39), dyslipidemia (35), T2DM (30, 37), pre-diabetes (33), and PCOS (28, 32, 34), and healthy people (36). Detailed characteristics of the included studies are summarized in Table 1.
3.2. Risk of bias assessment and quality of evidence
Random allocation of participants was mentioned in all included trials. Most of the included studies had a low/unclear risk of allocation concealment and reporting bias. In addition, most studies showed a high risk of other sources of bias and detection bias. Out of the 13 RCTs in the current study, five were of high quality (27–29, 32, 34), six were of moderate quality (33, 35–39), and two were of low quality (30, 31). Detailed information regarding the quality of the included RCTs based on the Cochrane risk of bias assessment is shown in Table 2. GRADE quality of evidence was high for leptin and moderate for adiponectin (Table 3).
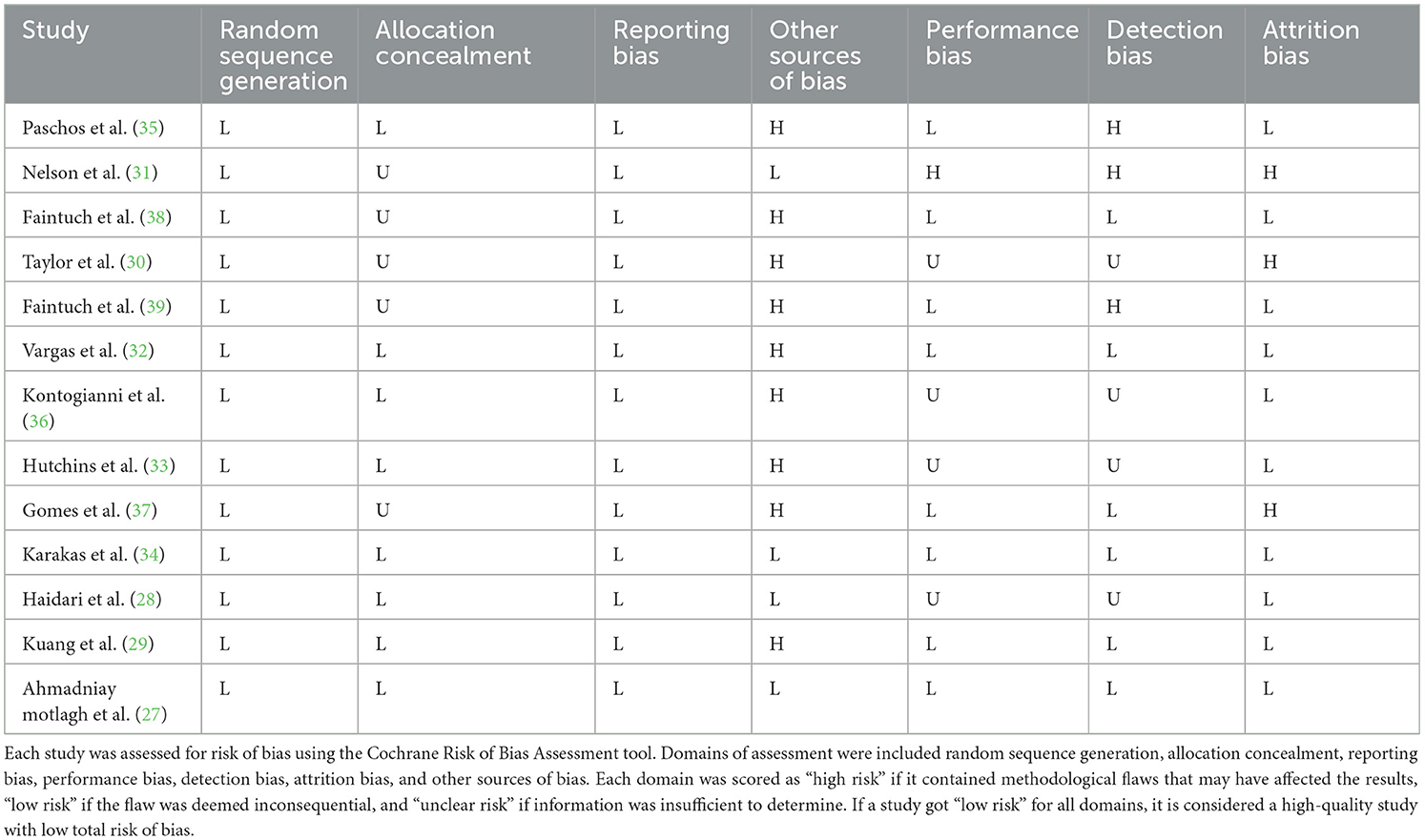
Table 2. Results of risk of bias assessment for randomized clinical trials included in the current meta-analysis on the effects of flaxseed supplementation on adipokines in adults.
3.3. Flaxseed on adiponectin concentrations
Based on the result of 11 RCTs comprising 13 treatment arms, flaxseed could not significantly affect circulating adiponectin in adults (SMD = 0.52, 95% CI: −0.20, 1.25, p = 0.159; Figure 2). The results were heterogeneous (I2 = 92.0%, p < 0.001), and the sensitivity analysis results revealed no significant change following the removal of each study. Subgroup analysis indicated significant effects on adiponectin in RCTs administered with whole flaxseed (Table 4). Egger's and Begg's tests showed significant small-study effects (p < 0.05). The trim and fill method was performed (without imputed study) following the uneven distribution of the funnel plot (Figure 3).
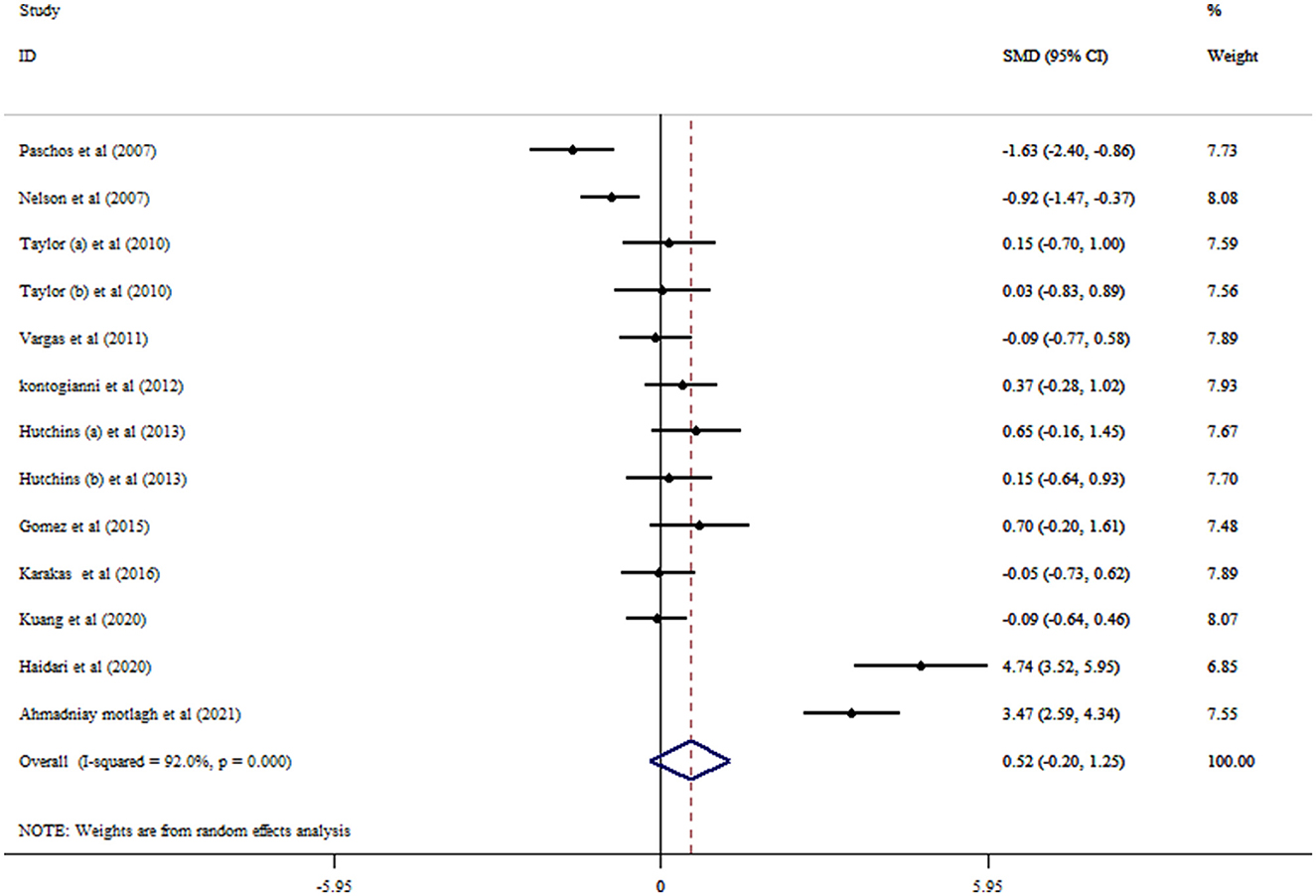
Figure 2. Forest plot detailing mean difference and 95% confidence intervals (CIs) and the effects of flaxseed supplementation on adiponectin levels.
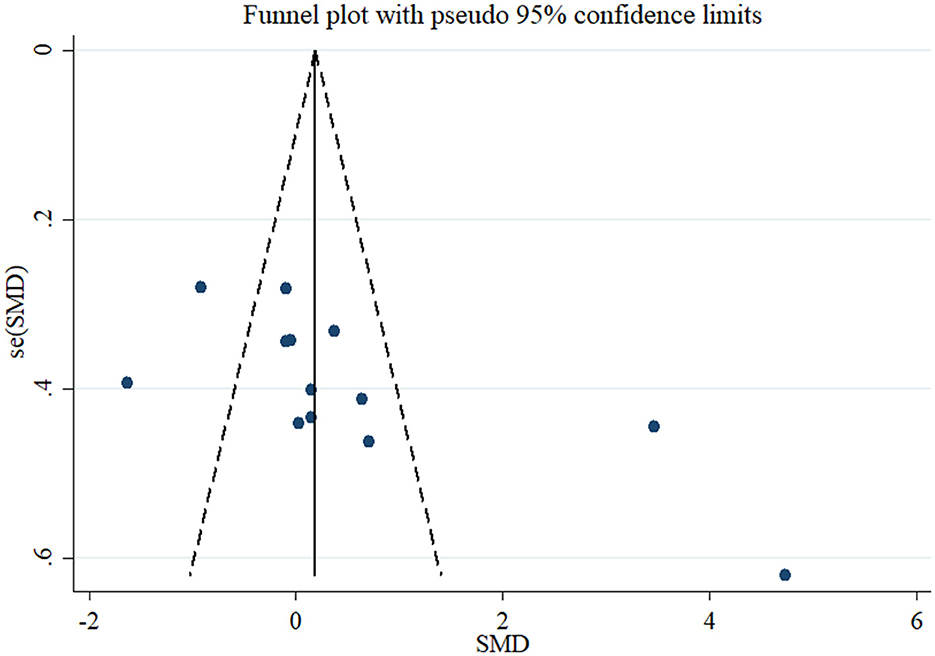
Figure 3. Funnel plot displaying publication bias in the studies reporting the effects of flaxseed supplementation on adiponectin levels.
3.4. Flaxseed on leptin concentrations
Eight RCTs with nine arms investigated the effect of flaxseed supplementation on leptin levels. The results indicated a significant reducing effect of flaxseed supplementation on leptin levels (SMD = −0.69, 95% CI: −1.37, −0.01; p = 0.048) with between-study heterogeneity (I2 = 86.4%, p < 0.001; Figure 4). Moreover, the overall effects of flaxseed on leptin were changed to not significantly impact by excluding studies using a one-study removal analysis (27–29, 32, 34). Whole flaxseed supplementation among RCTs with a sample size of >40 participants and age < 50 years contributed to a robust reduction in leptin concentrations (Table 4). Begg's tests showed no significant publication bias (p = 0.754).
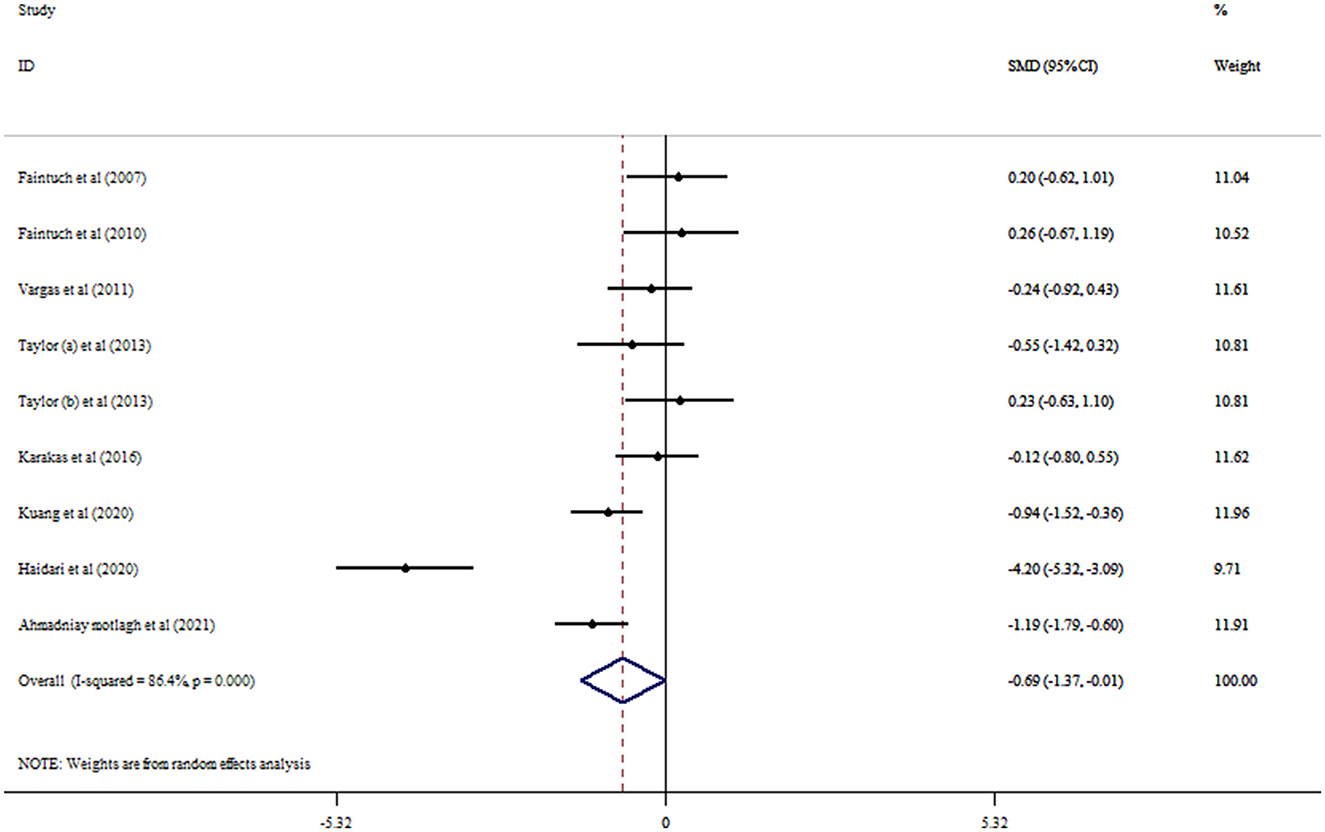
Figure 4. Forest plot detailing mean difference and 95% confidence intervals (CIs) and the effects of flaxseed supplementation on leptin levels.
4. Discussion
The results of our pooled analysis showed that flaxseed supplementation, despite its non-significant effect on adiponectin, caused a significant decrease in circulating leptin. However, subgroup analysis showed that flaxseed had no significant effect on leptin levels in high-quality studies, and only low-quality studies showed ameliorating effects of flaxseed on leptin. Consequently, the interpretation of this result should be accompanied by caution, and studies with appropriate designs and a low risk of bias are needed to confirm our results on leptin. In addition, the examination of other subgroups showed that flaxseed significantly increases adiponectin in the form of whole flaxseed. Regarding leptin, the whole flaxseed caused a significant decrease in people of < 50 years of age and in studies with a sample size of over 40. The results of the previous meta-analysis study in 2019 by Jalili et al. (21) showed that flaxseed supplementation has no significant effect on leptin and adiponectin levels and also on studied subgroups. However, the abovementioned study suggested that additional clinical trial studies should also be conducted for a definitive conclusion. Our study added four more clinical trials (27–29, 34) than Jalili et al.'s study of the pooled analysis which yielded different results in some aspects. In addition, the subgroup analysis of Jalili et al.'s study was only limited to the duration of the supplementation, the study population (healthy or unhealthy), and the type of intervention. However, our study added demographic variables (age and gender), sample size, body mass index, and study quality to subgroup analysis and examined the studied population more comprehensively than the aforementioned study in order to obtain generalizable results. In addition, unlike the abovementioned study, the quality of the obtained evidence in our investigation was checked with the GRADE tool.
In both studied biomarkers, whole flaxseed compared with flaxseed oil led to a significant improvement in leptin and adiponectin levels. Unlike flaxseed oil, which contains omega-3 fatty acids, especially polyunsaturated fatty acids (PUFAs), whole flaxseed contains PUFAs, soluble and insoluble fibers, proteins, various antioxidants, and phytoestrogenic lignans (40) that explain more improving effects on adipokines.
Studies have pointed out that the circulating levels of adiponectin and leptin are higher in women than in men (41, 42). Due to the existence of only one low-quality study (34) in the subgroup of men, the significant reduction of adiponectin in this subgroup cannot be a generalizable and valid result. No significant results have been reported for other gender subgroups either in leptin or adiponectin. However, it is suggested that future studies separate the effect of flaxseed supplements in men and women, in order to report a more accurate result. In terms of mean age, only one low-quality study (30) with two investigated arms included a < 50 years of age subgroup in the leptin-pooled analysis. Therefore, a significant decrease in leptin in this subgroup is not highly worth noting. However, this finding can be a sign for future studies to clarify the effect of flaxseed on this age group.
The sample size is another important factor determining the true effect of flaxseed on leptin. Subgroup analysis showed that studies with a >40 sample size reported a significant decrease in leptin levels following flaxseed supplementation. As a general principle in epidemiological studies, large sample sizes lead to high power to show a true effect (43). However, a very high sample size can also lead to false conclusions (44).
The various compounds found in whole flaxseed lead to beneficial effects on circulating levels of leptin and adiponectin. Fatty acids through interaction with transcription factors such as PPARγ, CCAAT/enhancer-binding protein (C/EBP), and sterol regulatory element-binding transcription factor 1 (SREBPF1) can alter the expression of leptin and adiponectin (45). Moreover, the anti-inflammatory properties of omega-3 fatty acids contribute to the regulation of adipokine production (46). Studies have reported that inflammatory conditions can lead to the inhibition of adiponectin production from adipocytes (47). In addition, pro-inflammatory cytokines have stimulating effects on leptin production (48). The main fatty acid of flaxseed oil is ALA, which is a poor activator of PPARγ compared with arachidonic acid as the main activator of PPARγ among fatty acids (49). This could explain the difference in the results observed between whole flaxseed and flaxseed oil. Dietary fibers can regulate the levels of adipokines in various ways, such as through changing body composition (50) and modifying gut microbiota (51). Due to the similar structure of phytoestrogens and estrogen, these compounds can bind to estrogen receptors (ERs) with a high affinity toward ERβ than ERα (52), leading to the inhibition of adipocyte differentiation and lipid accumulation in an in vivo model (53). However, phytoestrogens can directly bind to and activate PPARγ (52). This cross-talk between PPARγ and ERs focused on future studies to elucidate the precise effect of phytoestrogens on obesity-related pathways. The beneficial effects of plant polyphenols and antioxidants on the balance between different adipokines have been investigated in some studies (54, 55).
There were some limitations worth noting in our study that are suggested to cover in future studies. First, due to the lack of sufficient studies, an accurate comparison between men and women was not possible. As it is known, the expression of estrogen receptors between the two genders has a different pattern (56), and this can be effective in the effect of flaxseed on the circulating levels of adipokines. Second, it seems that the effect of flaxseed on other adipokines, such as visfatin and resistin, should also be taken into consideration in order to obtain a more comprehensive conclusion. Third, there were limited studied populations; therefore, additional studies on other diseases especially inflammatory conditions seem necessary.
Our study also has some worth noting strengths. First, the present study tried to cover all the limitations of the previous meta-analysis. Second, due to the low risk of bias in the included studies and the appropriate design of the current meta-analysis, the quality of our obtained results was moderate for adiponectin and high for leptin. Third, our study was registered in PROSPERO (code: CRD42023399735).
5. Conclusion
Whole flaxseed is effective in improving the levels of adiponectin and leptin. Flaxseed oil cannot change circulating levels of adipokines. The quality of our obtained results is moderate for adiponectin and high for leptin.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
VM was responsible for designing and coordinating the study. VM, KK, SA, HJ, and AH were responsible for the statistical study and writing of the manuscript. AF was responsible for reviewing the manuscript. KK was responsible for the statistical work and for writing the manuscript. All authors were responsible for data collection, data analysis, and data interpretation of the manuscript, and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1179089/full#supplementary-material
Supplementary Table S1. The search strategy.
References
1. Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab. (2007) 9:282–9. doi: 10.1111/j.1463-1326.2006.00610.x
2. Seino Y, Hirose H, Saito I, Itoh H. High-molecular-weight adiponectin is a predictor of progression to metabolic syndrome: a population-based 6-year follow-up study in Japanese men. Metabolism. (2009) 58:355–60. doi: 10.1016/j.metabol.2008.10.008
3. Zhu N, Pankow JS, Ballantyne CM, Couper D, Hoogeveen RC, Pereira M, et al. High-molecular-weight adiponectin and the risk of type 2 diabetes in the ARIC study. J Clin Endocrinol Metab. (2010) 95:5097–104. doi: 10.1210/jc.2010-0716
4. Ferrarezi DAF, Cheurfa N, Reis AF, Fumeron F, Velho G. Adiponectin gene and cardiovascular risk in type 2 diabetic patients: a review of evidences. Arq Bras Endocrinol Metabol. (2007) 51:153–9. doi: 10.1590/S0004-27302007000200003
5. Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol. (2007) 165:164–74. doi: 10.1093/aje/kwk001
6. Ahima RS. Revisiting leptin's role in obesity and weight loss. J Clin Invest. (2008) 118:2380–3. doi: 10.1172/JCI36284
7. Denroche HC, Huynh FK, Kieffer TJ. The role of leptin in glucose homeostasis. J Diabetes Investig. (2012) 3:115–29. doi: 10.1111/j.2040-1124.2012.00203.x
8. La Cava A. Leptin in inflammation and autoimmunity. Cytokine. (2017) 98:51–8. doi: 10.1016/j.cyto.2016.10.011
9. Musazadeh V, Jafarzadeh J, Keramati M, Zarezadeh M, Ahmadi M, Farrokhian Z, et al. Flaxseed oil supplementation augments antioxidant capacity and alleviates oxidative stress: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. (2021) 2021:4438613. doi: 10.1155/2021/4438613
10. Kajla P, Sharma A, Sood DR. Flaxseed—a potential functional food source. J Food Sci Technol. (2015) 52:1857–71. doi: 10.1007/s13197-014-1293-y
11. Bassett CMC, McCullough RS, Edel AL, Patenaude A, LaVallee RK, Pierce GN. The α-linolenic acid content of flaxseed can prevent the atherogenic effects of dietary trans fat. Am J Physiol Heart Circ Physiol. (2011) 301:H2220–6. doi: 10.1152/ajpheart.00958.2010
12. Khalesi S, Irwin C, Schubert M. Flaxseed consumption may reduce blood pressure: a systematic review and meta-analysis of controlled trials. J Nutr. (2015) 145:758–65. doi: 10.3945/jn.114.205302
13. Pan A, Yu D, Demark-Wahnefried W, Franco OH, Lin X. Meta-analysis of the effects of flaxseed interventions on blood lipids. Am J Clin Nutr. (2009) 90:288–97. doi: 10.3945/ajcn.2009.27469
14. Ren G-Y, Chen C-Y, Chen G-C, Chen W-G, Pan A, Pan C-W, et al. Effect of flaxseed intervention on inflammatory marker C-reactive protein: a systematic review and meta-analysis of randomized controlled trials. Nutrients. (2016) 8:136. doi: 10.3390/nu8030136
15. Fukumitsu S, Aida K, Ueno N, Ozawa S, Takahashi Y, Kobori M. Flaxseed lignan attenuates high-fat diet-induced fat accumulation and induces adiponectin expression in mice. Br J Nutr. (2008) 100:669–76. doi: 10.1017/S0007114508911570
16. Wang M, Zhang X-J, Feng K, He C, Li P, Hu Y-J, et al. Dietary α-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice. Sci Rep. (2016) 6:1–11. doi: 10.1038/srep26826
17. McCullough RS, Edel AL, Bassett CMC, Lavallée RK, Dibrov E, Blackwood DP, et al. The alpha linolenic acid content of flaxseed is associated with an induction of adipose leptin expression. Lipids. (2011) 46:1043–52. doi: 10.1007/s11745-011-3619-0
18. Marion-Letellier R, Savoye G, Ghosh S. Fatty acids, eicosanoids, hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. (1997) 11:779–91. doi: 10.1210/mend.11.6.0007
19. Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. (2001) 50:2094–9. doi: 10.2337/diabetes.50.9.2094
20. Duda MK, O'Shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, et al. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. (2009) 81:319–27. doi: 10.1093/cvr/cvn310
21. Jalili C, Pezeshki M, Askarpour M, Marx W, Hassani B, Hadi A, et al. The effect of flaxseed supplementation on circulating adiponectin and leptin concentration in adults: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. (2020) 34:1578–86. doi: 10.1002/ptr.6634
22. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1–9. doi: 10.1186/2046-4053-4-1
23. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
24. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
25. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
26. Irwig L, Macaskill P, Berry G, Glasziou P. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
27. Motlagh HA, Aalipanah E, Mazidi M, Faghih S. Effect of flaxseed consumption on central obesity, serum lipids, adiponectin level in overweight or obese women: a randomised controlled clinical trial. Int J Clin Pract. (2021) 75:e14592. doi: 10.22541/au.162045943.35168913/v1
28. Haidari F, Banaei-Jahromi N, Zakerkish M, Ahmadi K. The effects of flaxseed supplementation on metabolic status in women with polycystic ovary syndrome: a randomized open-labeled controlled clinical trial. Nutr J. (2020) 19:1–11. doi: 10.1186/s12937-020-0524-5
29. Kuang X, Kong Y, Hu X, Li K, Guo X, Liu C, et al. Defatted flaxseed flour improves weight loss and lipid profile in overweight and obese adults: a randomized controlled trial. Food Funct. (2020) 11:8237–47. doi: 10.1039/D0FO00838A
30. Taylor CG, Noto AD, Stringer DM, Froese S, Malcolmson L. Dietary milled flaxseed and flaxseed oil improve N-3 fatty acid status and do not affect glycemic control in individuals with well-controlled type 2 diabetes. J Am Coll Nutr. (2010) 29:72–80. doi: 10.1080/07315724.2010.10719819
31. Nelson TL, Stevens JR, Hickey MS. Adiponectin levels are reduced, independent of polymorphisms in the adiponectin gene, after supplementation with α-linolenic acid among healthy adults. Metabolism. (2007) 56:1209–15. doi: 10.1016/j.metabol.2007.04.017
32. Vargas ML, Almario RU, Buchan W, Kim K, Karakas SE. Metabolic and endocrine effects of long-chain versus essential omega-3 polyunsaturated fatty acids in polycystic ovary syndrome. Metabolism. (2011) 60:1711–8. doi: 10.1016/j.metabol.2011.04.007
33. Hutchins AM, Brown BD, Cunnane SC, Domitrovich SG, Adams ER, Bobowiec CE. Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: a randomized study. Nutr Res. (2013) 33:367–75. doi: 10.1016/j.nutres.2013.02.012
34. Karakas SE, Perroud B, Kind T, Palazoglu M, Fiehn O. Changes in plasma metabolites and glucose homeostasis during omega-3 polyunsaturated fatty acid supplementation in women with polycystic ovary syndrome. BBA Clin. (2016) 5:179–85. doi: 10.1016/j.bbacli.2016.04.003
35. Paschos GK, Zampelas A, Panagiotakos DB, Katsiougiannis S, Griffin BA, Votteas V, et al. Effects of flaxseed oil supplementation on plasma adiponectin levels in dyslipidemic men. Eur J Nutr. (2007) 46:315–20. doi: 10.1007/s00394-007-0668-5
36. Kontogianni MD, Vlassopoulos A, Gatzieva A, Farmaki A-E, Katsiougiannis S, Panagiotakos DB, et al. Flaxseed oil does not affect inflammatory markers and lipid profile compared to olive oil, in young, healthy, normal weight adults. Metabolism. (2013) 62:686–93. doi: 10.1016/j.metabol.2012.11.007
37. Gomes PM, Hollanda-Miranda WR, Beraldo RA, Castro AVB, Geloneze B, Foss MC, et al. Supplementation of α-linolenic acid improves serum adiponectin levels and insulin sensitivity in patients with type 2 diabetes. Nutrition. (2015) 31:853–7. doi: 10.1016/j.nut.2014.12.028
38. Faintuch J, Horie LM, Barbeiro HV, Barbeiro DF, Soriano FG, Ishida RK, et al. Systemic inflammation in morbidly obese subjects: response to oral supplementation with alpha-linolenic acid. Obes Surg. (2007) 17:341–7. doi: 10.1007/s11695-007-9062-x
39. Faintuch J, Bortolotto LA, Marques PC, Faintuch JJ, França JI, Cecconello I. Systemic inflammation and carotid diameter in obese patients: pilot comparative study with flaxseed powder and cassava powder. Nutr Hosp. (2011) 26:208–13.
40. Goyal A, Sharma V, Upadhyay N, Gill S, Sihag M. Flax and flaxseed oil: an ancient medicine & modern functional food. J Food Sci Technol. (2014) 51:1633–53. doi: 10.1007/s13197-013-1247-9
41. Hellström L, Wahrenberg H, Hruska K, Reynisdottir S, Arner P. Mechanisms behind gender differences in circulating leptin levels. J Intern Med. (2000) 247:457–62. doi: 10.1046/j.1365-2796.2000.00678.x
42. Manieri E, Herrera-Melle L, Mora A, Tomás-Loba A, Leiva-Vega L, Fernández DI, et al. Adiponectin accounts for gender differences in hepatocellular carcinoma incidence. J Exp Med. (2019) 216:1108–19. doi: 10.1084/jem.20181288
43. Serdar CC, Cihan M, Yücel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med. (2021) 31:010502. doi: 10.11613/BM.2021.010502
44. Faber J, Fonseca LM. How sample size influences research outcomes. Dental Press J Orthod. (2014) 19:27–9. doi: 10.1590/2176-9451.19.4.027-029.ebo
45. Nasir Y, Farzollahpour F, Mirzababaei A, Maghbooli Z, Mirzaei K. Associations of dietary fats intake and adipokines levels in obese women. Clin Nutr ESPEN. (2021) 43:390–6. doi: 10.1016/j.clnesp.2021.03.018
46. Albracht-Schulte K, Kalupahana NS, Ramalingam L, Wang S, Rahman SM, Robert-McComb J, et al. Omega-3 fatty acids in obesity and metabolic syndrome: a mechanistic update. J Nutr Biochem. (2018) 58:1–16. doi: 10.1016/j.jnutbio.2018.02.012
47. Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. (2007) 380:24–30. doi: 10.1016/j.cca.2007.01.026
48. Bruun JM, Pedersen SB, Kristensen K, Richelsen B. Effects of pro-inflammatory cytokines and chemokines on leptin production in human adipose tissue in vitro. Mol Cell Endocrinol. (2002) 190:91–9. doi: 10.1016/S0303-7207(02)00007-2
49. Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. (2011) 1812:1007–22. doi: 10.1016/j.bbadis.2011.02.014
50. Islam A, Civitarese AE, Hesslink RL, Gallaher DD. Viscous dietary fiber reduces adiposity and plasma leptin and increases muscle expression of fat oxidation genes in rats. Obesity. (2012) 20:349–55. doi: 10.1038/oby.2011.341
51. Queipo-Ortuño MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, et al. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE. (2013) 8:e65465. doi: 10.1371/journal.pone.0065465
52. Kuryłowicz A, Cakała-Jakimowicz M, Puzianowska-Kuznicka M. Targeting abdominal obesity and its complications with dietary phytoestrogens. Nutrients. (2020) 12:582. doi: 10.3390/nu12020582
53. Park HJ, Della-Fera MA, Hausman DB, Rayalam S, Ambati S, Baile CA. Genistein inhibits differentiation of primary human adipocytes. J Nutr Biochem. (2009) 20:140–8. doi: 10.1016/j.jnutbio.2008.01.006
54. Derdemezis CS, Kiortsis DN, Tsimihodimos V, Petraki MP, Vezyraki P, Elisaf MS, et al. Effect of plant polyphenols on adipokine secretion from human SGBS adipocytes. Biochem Res Int. (2011) 2011:285618. doi: 10.1155/2011/285618
55. Daneshzad E, Farsad-Naeimi A, Heshmati J, Mirzaei K, Maghbooli Z, Keshavarz S-A. The association between dietary antioxidants and adipokines level among obese women. Diabetes Metab Syndr. (2019) 13:1369–73. doi: 10.1016/j.dsx.2019.02.022
Keywords: flaxseed, adiponectin, leptin, meta-analysis, systematic review
Citation: Abbasi S, Karimi K, Hossein Moridpour A, Musazadeh V, Faghfouri AH and Jozi H (2023) Can flaxseed supplementation affect circulating adipokines in adults? An updated systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 10:1179089. doi: 10.3389/fnut.2023.1179089
Received: 03 March 2023; Accepted: 14 August 2023;
Published: 07 September 2023.
Edited by:
Jose Atilio Canas, Johns Hopkins All Children's Hospital, United StatesReviewed by:
Samantha Maurotti, Magna Græcia University, ItalyKailin Yang, Hunan University of Chinese Medicine, China
Copyright © 2023 Abbasi, Karimi, Hossein Moridpour, Musazadeh, Faghfouri and Jozi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amir Hossein Faghfouri, YW1pci5udXQ4OUBnbWFpbC5jb20=; Vali Musazadeh, bW9zYXphZGVoLnZhbGkwNUBnbWFpbC5jb20=
 Shaghayegh Abbasi1
Shaghayegh Abbasi1 Vali Musazadeh
Vali Musazadeh