- 1Department of Gastroenterology, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, China
- 2Health Management Center, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, China
Background: The effect of Helicobacter pylori (H. pylori) on nonalcoholic fatty liver disease (NAFLD) in the population is still controversial. Diabetes and NAFLD are both metabolically related diseases, and no studies have classified the population to study the effect of H. pylori infection on NAFLD in diabetics.
Methods: A population of people who were examined in the Taizhou Hospital Health Examination Center from 2017 to 2022 was included, and hematological indicators, body parameters, ultrasound data, and H. pylori detection by urea nitrogen test were collected from patients. All physical examination populations were divided into diabetic and non-diabetic populations.
Results: After multivariate logistic regression, H. pylori infection remained an independent risk factor for NAFLD in diabetics, but it had no significant effect on NAFLD in non-diabetic population. Additionally, there was a nonlinear relationship between glycosylated hemoglobin and H. pylori infection in diabetic population. Moreover, the incidence of NAFLD in diabetics increased with persistent H. pylori infection.
Conclusion: In the diabetic population, H. pylori infection does increase the risk of developing NAFLD. Glycemic control and eradication of H. pylori infection may have positive implications for reducing the incidence of NAFLD in diabetic population.
1. Introduction
Helicobacter pylori (H. pylori) is a Gram-negative spirochete pathogen that infects approximately half of adults population (1). The prevalence rate of H. pylori infection varies widely across the globe, with infection rates in Asia ranging from approximately 50–65% (2). As one of the most common infections in humans, H. pylori plays a major role in the development and progression of peptic ulcers, gastric mucosa-associated lymphomas and gastric cancers (3). Increasing evidence shows that H. pylori infection is linked to many extragastric diseases, such as liver disease, hematologic, respirator, cardiovascular and metabolism-related diseases (4, 5).
Non-alcoholic fatty liver disease (NAFLD) is becoming a leading cause of chronic liver diseases, with a 25–45% global prevalence, and is characterized by a wide range of liver conditions from steatosis to non-alcoholic steatohepatitis (NASH), cirrhosis and liver cancer. NAFLD has long been considered a manifestation of metabolic syndrome in the liver, but now there is growing evidence linking it to obesity, diabetes and insulin resistance (6–9).
Nowadays, the scientific community is increasingly interested in the relationship between NAFLD and H. pylori in humans (4, 10). However, it is controversial whether H. pylori infection contributes to the increased risk of NAFLD. Some studies suggest that H. pylori infection may have an impact on the development of NAFLD, possibly by increasing insulin resistance (IR), inflammatory mediator release and affecting lipid metabolism, while the eradication of H. pylori may play a role in reducing the risk of NAFLD (11–16). In contrast, in recent studies, many people have raised different opinions, there were two cross-sectional studies incorporating 21,456 and 71,633 healthy Chinese population found that H. pylori infection was not listed as a risk factor for NAFLD, besides, another observational study found no association between H. pylori infection and diagnosis of NAFLD in a Central European cohort (17–20). The reason for the different conclusions may be the lack of classification of the population. Some literatures have mentioned that H. pylori contributes to the pathogenesis of IR, a condition is closely related to NAFLD and diabetes (15, 21). In addition, diabetes and NAFLD are both metabolic syndromes, and they affect each other (22–25).
In this study, we conducted a cross-sectional survey and cohort investigation to examine whether H. pylori infection contributes to NAFLD risk in the diabetic population in China. Thus, we further examined the effect of H. pylori infection on NAFLD in the diabetic setting and explored the association between H. pylori infection, diabetes mellitus and NAFLD.
2. Materials and methods
2.1. Study population
The participants in this study were the people who went to Taizhou Hospital Health Examination Center for physical examination from June 2017 to June 2022. Patients with complete clinical information, such as age, gender, laboratory indices, and blood pressure, were required for the study population. All people underwent a urea nitrogen breath test, as well as liver ultrasound. Laboratory indicators include total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), alanine aminotransferase (ALT), aspartate transaminase (AST), gamma-glutamyl transpeptidase (GGT), glycated hemoglobin A1c (HbA1c) and fasting blood glucose (FBG). People with viral hepatitis, alcoholic liver disease, autoimmune liver disease, or with thyroid function problems, history of malignancy and incomplete clinical information were excluded. In total, 52,032 people were included in the study for analysis. People with diabetes mellitus who have been treated with oral medicine, FBG ≥ 7.0 mmol/l, and HbA1c ≥ 6.5% were defined as diabetic population, including 7,190 patients with diabetes. In addition, 778 of these diabetic patients who underwent multiple physical examinations (interval between physical examinations > 6 months) were categorized as H. pylori persistent negative, persistent infection, eradicated infection, and new infection according to their H. pylori infection status at the first and last physical examinations, respectively. This study was approved by the Ethics Committee of Taizhou Hospital (K20220790).
2.2. Collection of clinical indicators
The Health Management Center nurses measured and recorded the age, gender and blood pressure of the population. The venous blood of the patients was collected in the morning on an empty stomach for laboratory testing. Laboratory indicators, including TG, TC, HDL, LDL, ALT, AST, GGT, and FBG, were determined by automatic biochemical analyzer. The patient’s HbA1c level was measured by the glycosylated hemoglobin analyzer.
2.3. Detection of Helicobacter pylori
Helicobacter pylori was examined for physical examination population through 13C or 14C urease test. The process of 13C breath test is performed as follows: (a) collect a breath sample after 3 h of fasting; (b) take 13C urea capsule with warm water; (c) wait for 30 min; (d) blow to the special breath card to take the breath sample after taking the medicine; (e) analyze two samples on the instrument. The process of 14C breath test is as follows: (a) take 14C urea capsule and add water to take it; (b) Wait for 20 min; (c) exhale to the gas gathering card for 1–3 min; (d) insert the gas gathering card into the detector for detection.
2.4. Definition of NAFLD
Nonalcoholic fatty liver disease was defined as hepatic steatosis diagnosed by imaging or histology after excluding viral hepatitis, excessive drinking, autoimmune liver disease and hereditary liver disease (26). Ultrasonography was performed by at least two experienced ultrasound doctors who were unaware of the purpose of the study. The patient was positioned supine with the abdomen fully exposed, and the patient’s abdominal organs were examined by the ultrasound probe. The diagnostic criteria of hepatic steatosis under ultrasound were: diffuse enhancement of near-field echoes in the liver (“bright liver”) with stronger echoes than in the kidneys; indistinct intrahepatic ductal structures; and gradual attenuation of far-field echoes in the liver (13). Those who have 2 of the above 3 abdominal ultrasound findings can be diagnosed as fatty liver.
2.5. Definition of hypertension and dyslipidemia
Hypertension was defined as a systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg. TC [≥ 240 mg/dl (6.20 mmol/l)], TG [>200 mg/dl (2.25 mmol/l)], HDL [<40 mg/dl (1.03 mmol/l)], LDL [>160 mg/dl (4.13 mmol/l)], meeting one of the above were defined as dyslipidemia (27).
2.6. Statistical analysis
In this study, continuous variables were expressed as mean ± standard deviation (SD) using t-test. Categorical variables were expressed as counts and percentages using chi-square test. Multivariate logistic regression was used to analyze the relationship between Helicobacter pylori infection and NAFLD after adjusting for confounding factors, and the odds ratio (OR) and 95% confidence interval (CI) were calculated. The generalized additive model (GAM) was used to investigate the potential nonlinear relationship between HbA1c and H. pylori infection. All statistical analyses were conducted using R (4.1.3) software, and p < 0.05 were considered statistically significant.
3. Result
3.1. Baseline characteristics
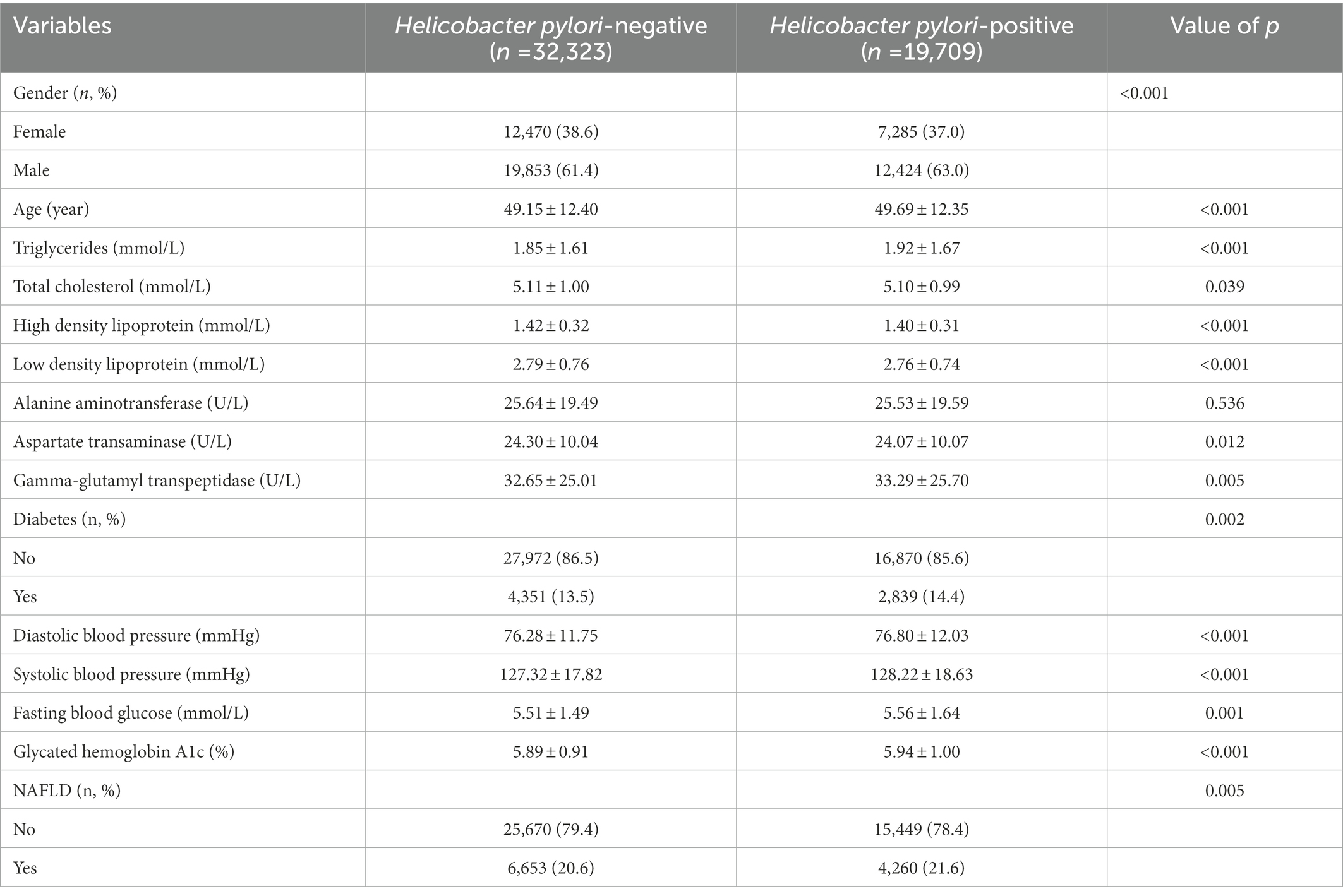
Baseline characteristics of all physical examination populations were shown in Table 1. Among the 52,032 participants, 19,755 (38.0%) were female and 32,277 (62.0%) were male. Men had a higher rate of H. pylori infection, 63.0% vs. 61.4%. People with diabetes had a higher rate of H. pylori infection, 14.4% vs. 13.5%, as well as higher H. pylori infection among people with NAFLD, 21.6% vs. 20.6%.
3.2. Risk factors for NAFLD In diabetic and non-diabetic populations
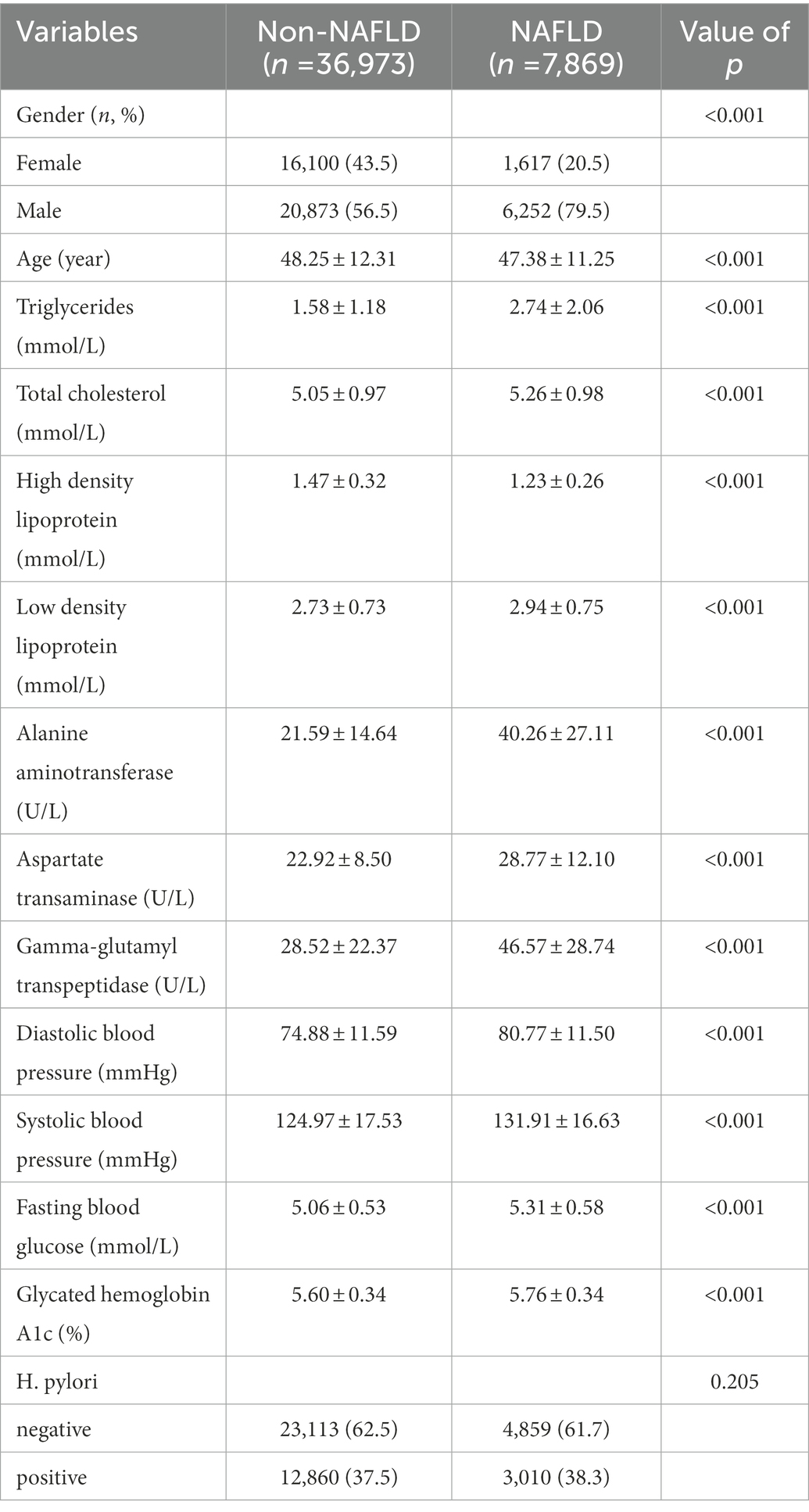
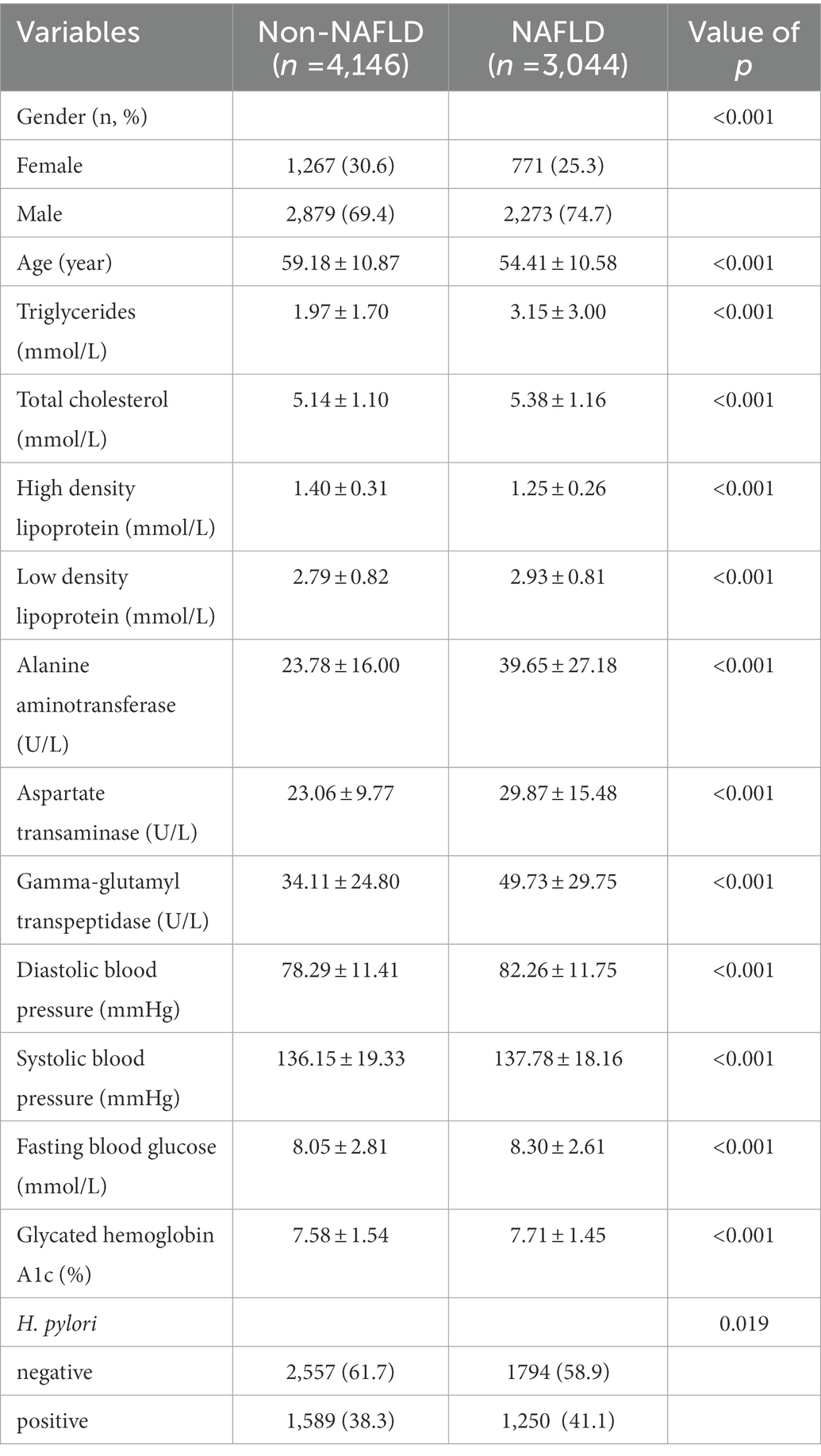
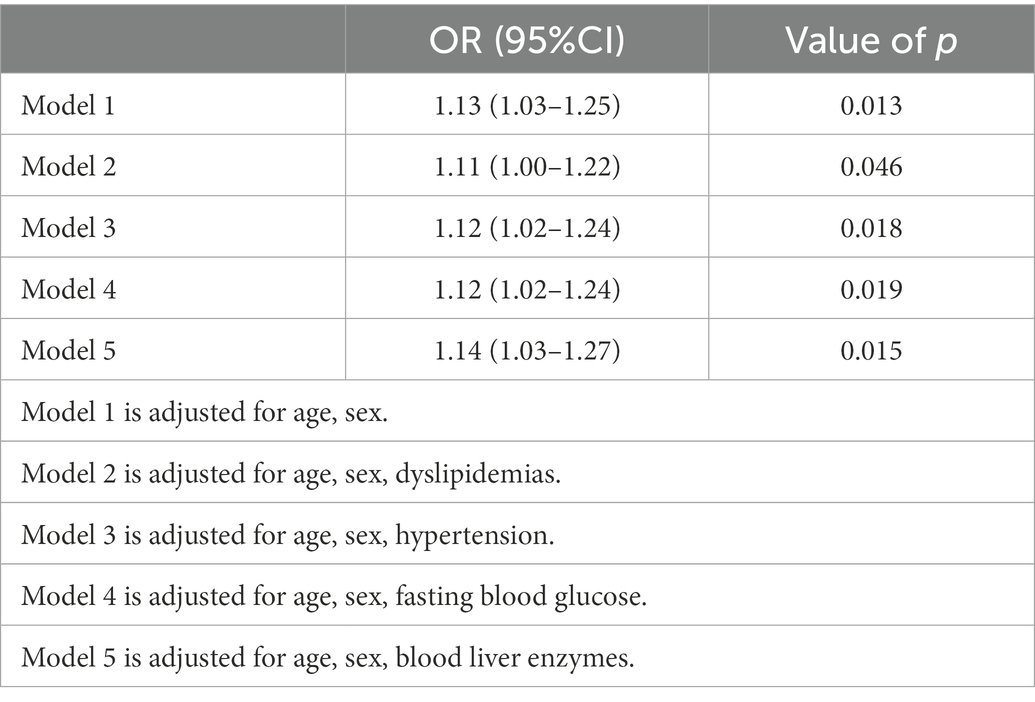
To further investigate the relationship of H. pylori infection on the development of NAFLD in different populations, a subgroup analysis of risk factors for NAFLD was carried out in diabetic and non-diabetic populations using univariate analysis. The baseline characteristics of NAFLD population in the non-diabetic group were shown in Table 2. In the NAFLD population, the proportion of male patients increased significantly, 79.5% vs. 56.5%. However, no significant correlation was seen between H. pylori infection and NAFLD (p = 0.205). Table 3 showed the baseline characteristics of NAFLD in the diabetic population. Among patients with NAFLD, there were statistical differences between gender, age, lipids, blood liver enzymes, blood glucose, blood pressure, and H. pylori infection versus non-NAFLD patients. The differences in H. pylori infection among different populations in people with NAFLD were shown in Figure 1.
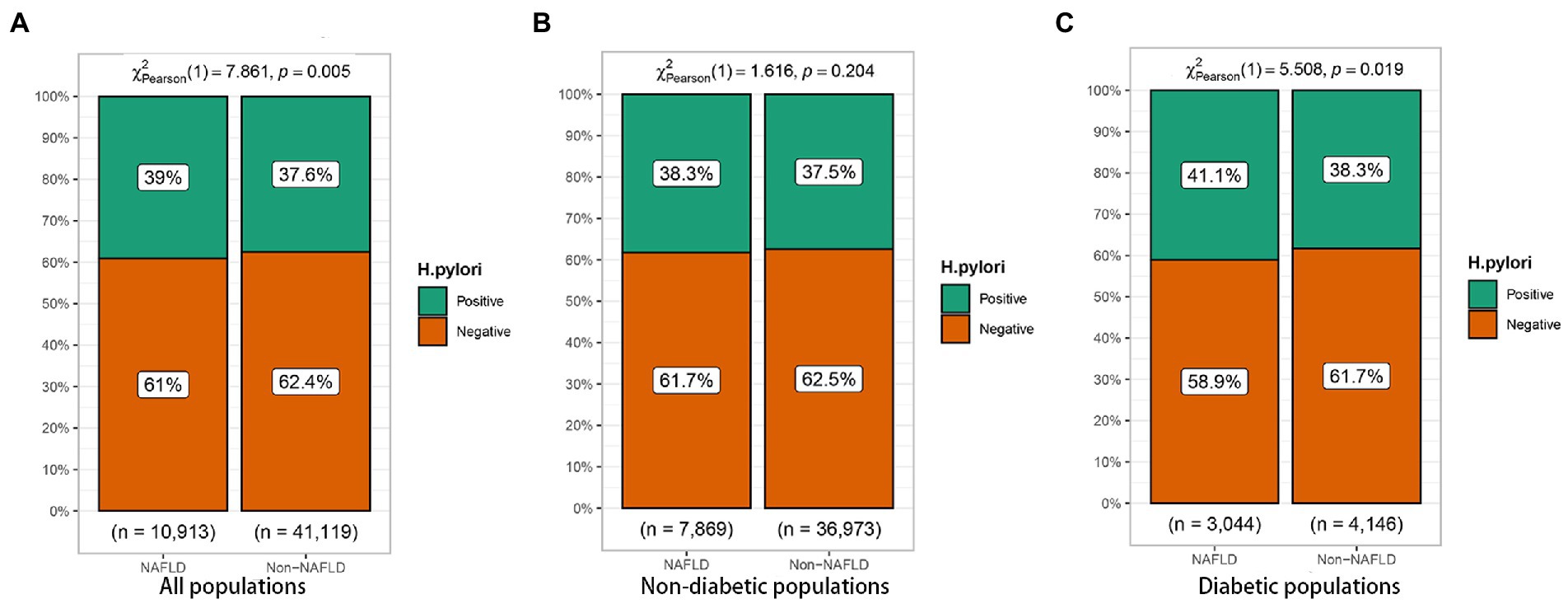
Figure 1. Relationship between Helicobacter pylori infection and nonalcoholic fatty liver disease (NAFLD) in different populations. (A) All populations. (B) Non-diabetic populations. (C) Diabetic populations.
3.3. Effect of Helicobacter pylori infection on NAFLD
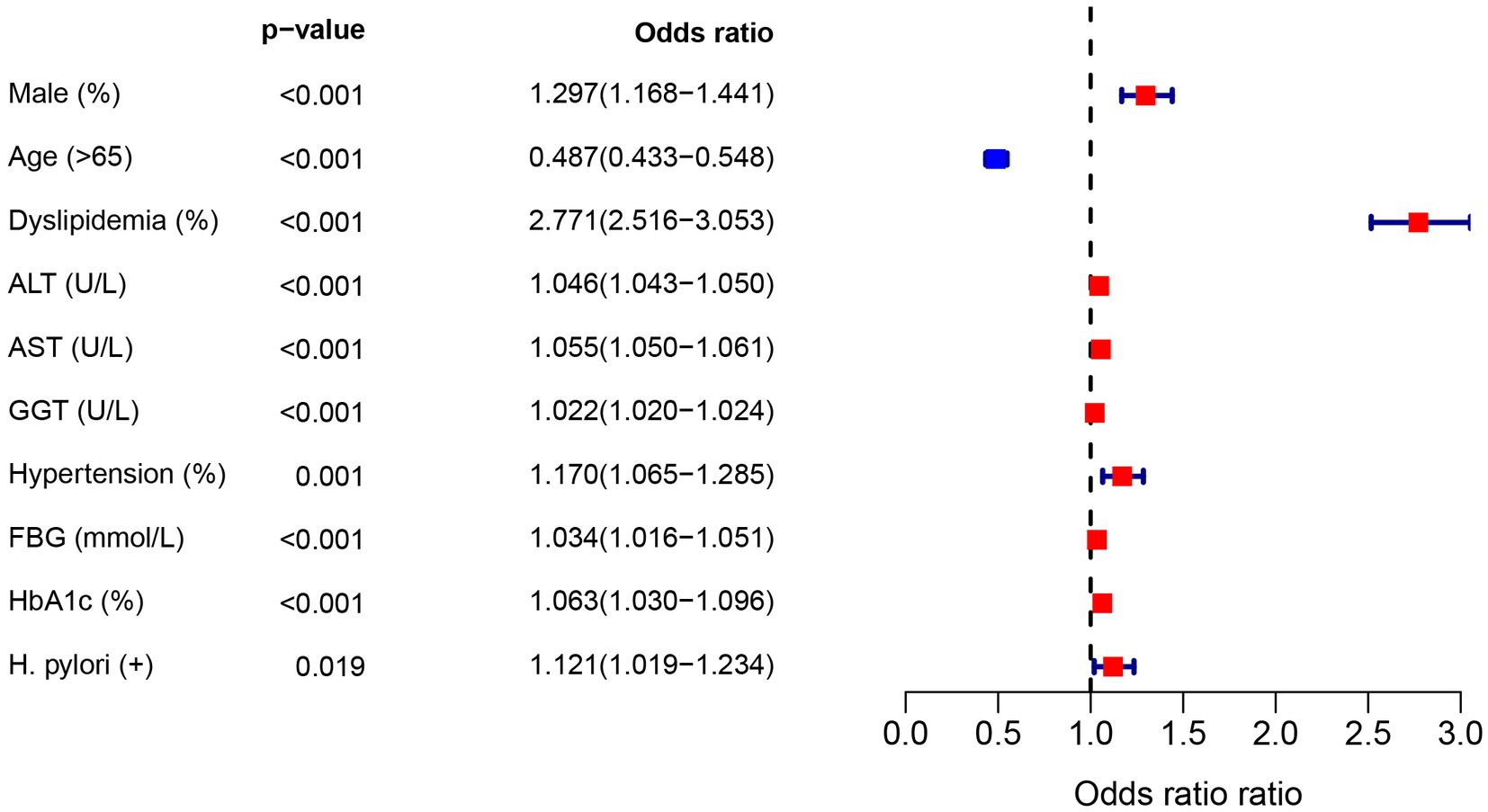
In the diabetic population, univariate logistic regression analysis showed an increased risk of NAFLD due to H. pylori (OR = 1.12, 95%CI: 1.02–1.23), as shown in Figure 2. In addition, gender, age, dyslipidemia, blood liver enzymes, hypertension, and FBG were all risk factors for NAFLD (p < 0.05). In order to control the influence of confounding factors, in addition to gender and age, dyslipidemia, hypertension, FBG and blood liver enzymes were adjusted, respectively. Helicobacter pylori infection was still a risk factor for NAFLD, Table 4.
3.4. Effect of HbA1c on Helicobacter pylori infection
We further explored the effect of HbA1c on H. pylori infection in diabetic patients. In curve fitting, we found a nonlinear relationship between HbA1c and H. pylori infection, Figure 3. After adjusting the sex and age, the impact of HbA1c on H. pylori infection was analyzed by using two-stage linear regression, Table 5. When HbA1c did not reach the threshold of 10.8%, the risk of H. pylori infection increased with elevated HbA1c (OR = 1.13, p < 0.001), but after reaching the threshold, the HP infection rate decreased with the increase of HbA1c (OR = 0.83, p = 0.013).
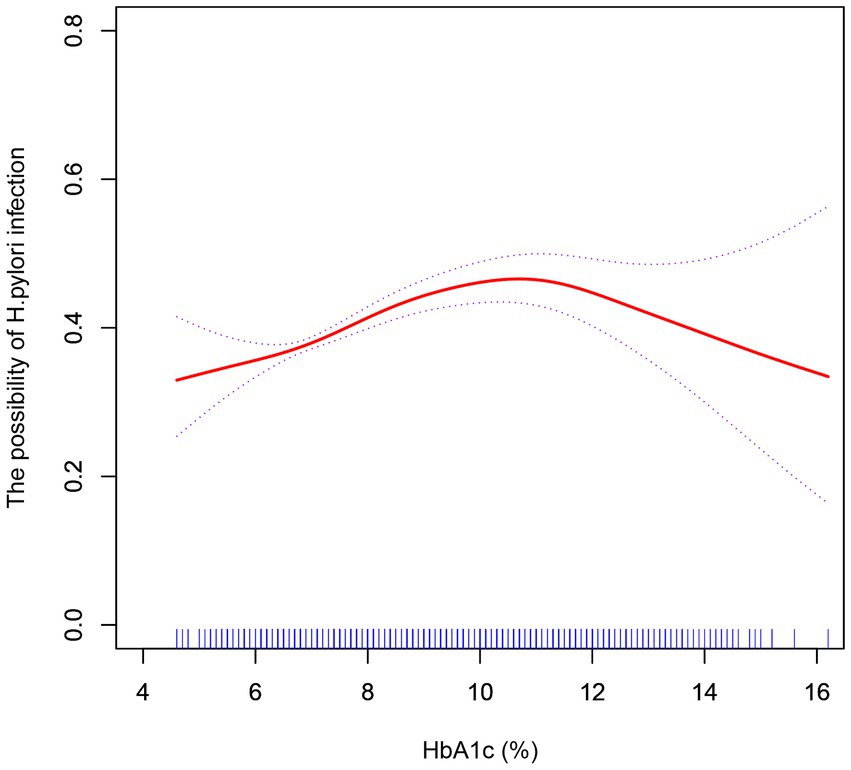
Figure 3. After adjustment for confounding factors (age, sex), a smoothed curve fit was used to show a non-linear correlation between HbA1c and H. pylori infection.
3.5. Association between Helicobacter pylori infection status and NAFLD
Of the 778 diabetic patients, 460 (59.1%) patients did not have NAFLD on first physical examination. The incidence of NAFLD was 13.2% in the persistent negative group, 23.0% in the persistent positive group, 17.2% in the eradicated infection group, and 17.0% in the new infection group. The incidence of NAFLD was significantly lower in the persistent negative group compared to the persistent infection group (p = 0.031); the incidence of NAFLD in the new infection and eradicated infection groups was significantly lower compared with the persistent positive group, but non-statistically significant difference was found (p > 0.05), as shown in Figure 4.
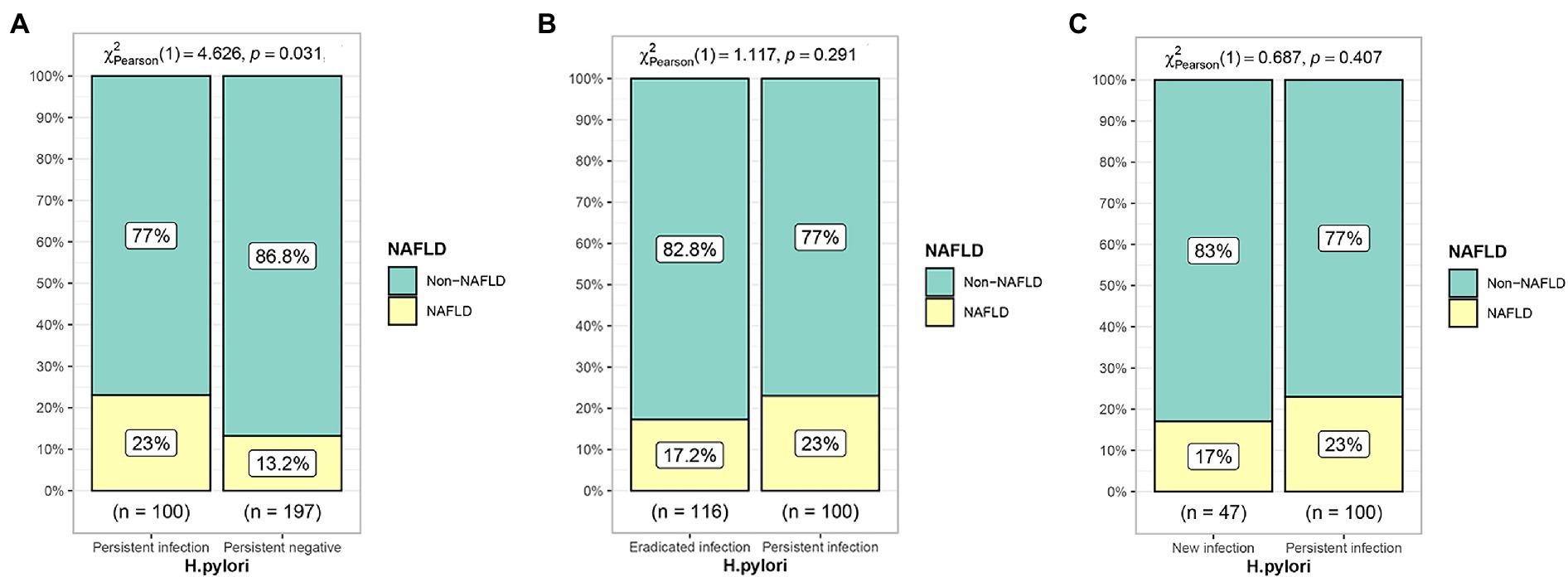
Figure 4. Effect of different H. pylori infection status on the development of NAFLD. (A) Persistent infection group compared with persistent negative group. (B) Eradicated infection group compared with persistent infection group. (C) New infection group compared with persistent infection group.
4. Discussion
Nonalcoholic fatty liver disease is a metabolic disease that can manifest as simple fatty liver in its early stages, but as the disease progresses, it can manifest as steatohepatitis, cirrhosis, and even liver cancer (28). The pathogenesis of NAFLD may be related to genetic and environmental, as well as metabolic factors, and some studies have suggested an association between NAFLD and H. pylori infection in recent years (29, 30). Several studies have found that H. pylori upregulates the expression of various inflammatory factors, including C-reactive protein, tumor necrosis factor, various interleukins, and promotes insulin resistance levels (10, 31). In addition, H. pylori infection may increase intestinal permeability, cause intestinal microbial disorders, and promote the entry of bacterial toxins into the liver, thus leading to chronic inflammation of liver (12, 32, 33).
However, the relationship between NAFLD and H. pylori infection remains controversial. In addition, there are no studies that classify populations according to the presence or absence of diabetes to exclude effect of diabetes on NAFLD in H. pylori infection status. Our study, in a large sample population, confirmed the relationship between NAFLD and H. pylori infection. However, due to the fact that conditions such as insulin resistance and genetic susceptibility in the diabetic population differ from those in the non-diabetic population, there may be differences in the role of H. pylori infection on NAFLD, so we studied diabetic patients separately. In the non-diabetic population, H. pylori infection did not significantly contribute to NAFLD. However, we found a statistically significant association between H. pylori infection and NAFLD in the diabetic population. After adjusting for multiple confounders in multifactorial logistic regression, H. pylori infection remained an independent risk factor for the development of NAFLD. Moreover, we found a significant relationship between H. pylori infection and HbA1c in diabetic patients, with or without adjustment for confounding factors, which was consistent with previous studies (34, 35). However, there was a threshold effect between HbA1c and H. pylori infection, which may not be mentioned in other studies. At HbA1c < 10.8%, the risk of H. pylori infection increased with increasing HbA1c. However, when HbA1c > 10.8%, the rate of H. pylori infection decreased instead. This could mean that prolonged poor glycemic control leads to changes in the gastrointestinal flora, as well as the intestinal mucosal barrier, and that other intestinal microorganisms are more likely to cause infection than H. pylori (36, 37). Therefore, the control of blood glucose in diabetic patients is still of positive significance for reducing H. pylori infection. However, this still needs to be validated in depth by prospective cohort studies. Furthermore, as confirmed by our cohort study, persistent H. pylori infection significantly increases the incidence of NAFLD. Eradicating H. pylori infection in diabetic population may have positive implications for reducing the incidence of NAFLD.
Previously, lifestyle changes and diet control were the main treatment options for NAFLD. For the past few years, a variety of drugs such as anti-diabetic drugs, anti-obesity drugs, and antioxidants have been investigated for the treatment of NAFLD as well as NASH (38, 39). These drugs focus on regulating metabolic dysregulation; however, the complex pathogenesis of NAFLD and the important role of immune-mediated inflammation in NAFLD development may lead to the failure of many drug candidates to achieve the expected efficacy at the late stage of the trial (38, 40). Therefore, testing for H. pylori infection and treating it before treating NAFLD may have some positive implications for subsequent drug therapy.
Our study confirms that H. pylori infection plays an independent role in the development of NAFLD in diabetic population. This may be the first time that the effect of H. pylori infection on NAFLD has been studied in a large diabetic population. In addition, we explore the nonlinear relationship of HbA1c to H. pylori infection in a diabetic population. The survey, however, still has several limitations. First of all, this is a single-center study with its own limitations. Multicenter, longitudinal studies may be needed to provide more robust evidence. Second, the diagnosis of NAFLD is mainly based on ultrasonography and lacks confirmation by liver biopsy data (41). However, due to the high sensitivity and specificity of ultrasonography for the diagnosis of steatohepatitis, this test is widely used in clinical practice (42). In addition, the invasive nature of liver puncture makes it inappropriate for such a large sample of people undergoing physical examination. Finally, although some methods are being used to adjust for confounding factors, there may still be a potential influence of other factors.
5. Conclusion
Helicobacter pylori infection is an independent risk factor for NAFLD in diabetic population. In addition, long-term H. pylori infection may increase the incidence of NAFLD. Controlling glycemia and eradicating H. pylori infection in diabetic population may benefit to reduce the incidence of NAFLD.
6. Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Taizhou Hospital (K20220790). The patients/participants provided their written informed consent to participate in this study.
Author contributions
JZ made great contributions to the concept, design and data acquisition of the article. YC and NY was mainly involved in data analysis. CS and JW were involved in article writing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Taizhou Science and Technology Program (No. 1901ky18).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.Wotherspoon, AC, Ortiz-Hidalgo, C, Falzon, MR, and Isaacson, PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. (1991) 338:1175–6. doi: 10.1016/0140-6736(91)92035-z
2.Zamani, M, Ebrahimtabar, F, Zamani, V, Miller, WH, Alizadeh-Navaei, R, Shokri-Shirvani, J, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. (2018) 47:868–76. doi: 10.1111/apt.14561
3.Vakil, N, Malfertheiner, P, and Chey, WD. Helicobacter pylori infection. N Engl J Med. (2010) 363:595–6. doi: 10.1056/NEJMc1006158
4.de Korwin, JD, Ianiro, G, Gibiino, G, and Gasbarrini, A. Helicobacter pylori infection and extragastric diseases in 2017. Helicobacter. (2017) 22:1. doi: 10.1111/hel.12411
5.Pellicano, R, Ianiro, G, Fagoonee, S, Settanni, CR, and Gasbarrini, A. Review: extragastric diseases and Helicobacter pylori. Helicobacter. (2020) 25:e12741. doi: 10.1111/hel.12741
6.Lonardo, A, Bellentani, S, Argo, CK, Ballestri, S, Byrne, CD, Caldwell, SH, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis. (2015) 47:997–1006. doi: 10.1016/j.dld.2015.08.004
7.Younossi, Z, Anstee, QM, Marietti, M, Hardy, T, Henry, L, Eslam, M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
8.Fazel, Y, Koenig, AB, Sayiner, M, Goodman, ZD, and Younossi, ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. (2016) 65:1017–25. doi: 10.1016/j.metabol.2016.01.012
9.Fabbrini, E, and Magkos, F. Hepatic Steatosis as a marker of metabolic dysfunction. Nutrients. (2015) 7:4995–5019. doi: 10.3390/nu7064995
10.Cheng, DD, He, C, Ai, HH, Huang, Y, and Lu, NH. The possible role of helicobacter pylori infection in non-alcoholic fatty liver disease. Front Microbiol. (2017) 8:743. doi: 10.3389/fmicb.2017.00743
11.Polyzos, SA, Kountouras, J, Papatheodorou, A, Patsiaoura, K, Katsiki, E, Zafeiriadou, E, et al. Helicobacter pylori infection in patients with nonalcoholic fatty liver disease. Metabolism. (2013) 62:121–6. doi: 10.1016/j.metabol.2012.06.007
12.Sumida, Y, Kanemasa, K, Imai, S, Mori, K, Tanaka, S, Shimokobe, H, et al. Helicobacter pylori infection might have a potential role in hepatocyte ballooning in nonalcoholic fatty liver disease. J Gastroenterol. (2015) 50:996–1004. doi: 10.1007/s00535-015-1039-2
13.Chen, CX, Mao, YS, Foster, P, Zhu, ZW, Du, J, and Guo, CY. Possible association between Helicobacter pylori infection and nonalcoholic fatty liver disease. Appl Physiol Nutr Metab. (2017) 42:295–301. doi: 10.1139/apnm-2016-0499
14.Lu, LJ, Hao, NB, Liu, JJ, Li, X, and Wang, RL. Correlation between Helicobacter pylori infection and metabolic abnormality in general population: a cross-sectional study. Gastroenterol Res Pract. (2018) 2018:7410801. doi: 10.1155/2018/7410801
15.Abdel-Razik, A, Mousa, N, Shabana, W, Refaey, M, Elhelaly, R, Elzehery, R, et al. Helicobacter pylori and non-alcoholic fatty liver disease: a new enigma? Helicobacter. (2018) 23:e12537. doi: 10.1111/hel.12537
16.Kim, TJ, Sinn, DH, Min, YW, Son, HJ, Kim, JJ, Chang, Y, et al. A cohort study on helicobacter pylori infection associated with nonalcoholic fatty liver disease. J Gastroenterol. (2017) 52:1201–10. doi: 10.1007/s00535-017-1337-y
17.Wang, W, Fan, M, Gong, R, Zhang, Y, Zeng, J, Xu, S, et al. Helicobacter pylori infection is not an independent risk factor of non-alcoholic fatty liver disease in China. BMC Gastroenterol. (2022) 22:81. doi: 10.1186/s12876-022-02148-6
18.Fan, N, Peng, L, Xia, Z, Zhang, L, Wang, Y, and Peng, Y. Helicobacter pylori infection is not associated with non-alcoholic fatty liver disease: a cross-sectional study in China. Front Microbiol. (2018) 9:73. doi: 10.3389/fmicb.2018.00073
19.Cai, O, Huang, Z, Li, M, Zhang, C, Xi, F, and Tan, S. Association between helicobacter pylori infection and nonalcoholic fatty liver disease: a single-center clinical study. Gastroenterol Res Pract. (2018) 2018:8040262. doi: 10.1155/2018/8040262
20.Wernly, S, Wernly, B, Semmler, G, Völkerer, A, Rezar, R, Semmler, L, et al. Non-alcoholic fatty liver disease is not independently associated with Helicobacter pylori in a central European screening cohort. Minerva Med. (2022). doi: 10.23736/s0026-4806.22.07928-9
21.Azami, M, Baradaran, HR, Dehghanbanadaki, H, Kohnepoushi, P, Saed, L, Moradkhani, A, et al. Association of Helicobacter pylori infection with the risk of metabolic syndrome and insulin resistance: an updated systematic review and meta-analysis. Diabetol Metab Syndr. (2021) 13:145. doi: 10.1186/s13098-021-00765-x
22.Mantovani, A, Petracca, G, Beatrice, G, Tilg, H, Byrne, CD, and Targher, G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. (2021) 70:962–9. doi: 10.1136/gutjnl-2020-322572
23.Chang, Y, Jung, HS, Yun, KE, Cho, J, Cho, YK, and Ryu, S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol. (2013) 108:1861–8. doi: 10.1038/ajg.2013.349
24.Adams, LA, Anstee, QM, Tilg, H, and Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. (2017) 66:1138–53. doi: 10.1136/gutjnl-2017-313884
25.Mantovani, A, Byrne, CD, Bonora, E, and Targher, G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care. (2018) 41:372–82. doi: 10.2337/dc17-1902
26.Chalasani, N, Younossi, Z, Lavine, JE, Charlton, M, Cusi, K, Rinella, M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. (2018) 67:328–57. doi: 10.1002/hep.29367
27.Kopin, L, and Lowenstein, C. Dyslipidemia. Ann Intern Med. (2017) 167:Itc81–itc96. doi: 10.7326/aitc201712050
28.Ofosu, A, Ramai, D, and Reddy, M. Non-alcoholic fatty liver disease: controlling an emerging epidemic, challenges, and future directions. Ann Gastroenterol. (2018) 31:288–95. doi: 10.20524/aog.2018.0240
29.Aron-Wisnewsky, J, Vigliotti, C, Witjes, J, Le, P, Holleboom, AG, Verheij, J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. (2020) 17:279–97. doi: 10.1038/s41575-020-0269-9
30.Liu, R, Liu, Q, He, Y, Shi, W, Xu, Q, Yuan, Q, et al. Association between helicobacter pylori infection and nonalcoholic fatty liver: a meta-analysis. Medicine (Baltimore). (2019) 98:e17781. doi: 10.1097/md.0000000000017781
31.Polyzos, SA, Kountouras, J, Zavos, C, and Deretzi, G. The association between helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter. (2011) 16:79–88. doi: 10.1111/j.1523-5378.2011.00822.x
32.Fukuda, Y, Bamba, H, Okui, M, Tamura, K, Tanida, N, Satomi, M, et al. Helicobacter pylori infection increases mucosal permeability of the stomach and intestine. Digestion. (2001) 63:93–6. doi: 10.1159/000051918
33.Boursier, J, Mueller, O, Barret, M, Machado, M, Fizanne, L, Araujo-Perez, F, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. (2016) 63:764–75. doi: 10.1002/hep.28356
34.Chen, J, Xing, Y, Zhao, L, and Ma, H. The association between helicobacter pylori infection and Glycated hemoglobin a in diabetes: a meta-analysis. J Diabetes Res. (2019) 2019:3705264–10. doi: 10.1155/2019/3705264
35.Dai, YN, Yu, WL, Zhu, HT, Ding, JX, Yu, CH, and Li, YM. Is Helicobacter pylori infection associated with glycemic control in diabetics? World J Gastroenterol. (2015) 21:5407–16. doi: 10.3748/wjg.v21.i17.5407
36.van Heck, JIP, Gacesa, R, Stienstra, R, Fu, J, Zhernakova, A, Harmsen, HJM, et al. The gut microbiome composition is altered in long-standing type 1 diabetes and associates with glycemic control and disease-related complications. Diabetes Care. (2022) 45:2084–94. doi: 10.2337/dc21-2225
37.Li, J, Zhang, H, and Wang, G. Correlations between inflammatory response, oxidative stress, intestinal pathological damage and intestinal flora variation in rats with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. (2020) 24:10162–8. doi: 10.26355/eurrev_202010_23236
38.Negi, CK, Babica, P, Bajard, L, Bienertova-Vasku, J, and Tarantino, G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism. (2022) 126:154925. doi: 10.1016/j.metabol.2021.154925
39.Polyzos, SA, Kang, ES, Boutari, C, Rhee, EJ, and Mantzoros, CS. Current and emerging pharmacological options for the treatment of nonalcoholic steatohepatitis. Metabolism. (2020) 111:154203. doi: 10.1016/j.metabol.2020.154203
40.Reccia, I, Kumar, J, Akladios, C, Virdis, F, Pai, M, Habib, N, et al. Non-alcoholic fatty liver disease: a sign of systemic disease. Metabolism. (2017) 72:94–108. doi: 10.1016/j.metabol.2017.04.011
41.Papatheodoridi, M, and Cholongitas, E. Diagnosis of non-alcoholic fatty liver disease (NAFLD): current concepts. Curr Pharm Des. (2018) 24:4574–86. doi: 10.2174/1381612825666190117102111
Keywords: Helicobacter pylori, nonalcoholic fatty liver disease, diabetes, threshold effect, glycosylated hemoglobin
Citation: Chen Y, You N, Shen C, Wu J and Zhang J (2023) Helicobacter pylori infection increases the risk of nonalcoholic fatty liver disease in diabetic population. Front. Nutr. 10:1076579. doi: 10.3389/fnut.2023.1076579
Edited by:
Carmine Gazzaruso, University of Milan, Milan, ItalyReviewed by:
Giovanni Tarantino, University of Naples Federico II, ItalySalvatore Vaccaro, IRCCS Local Health Authority of Reggio Emilia, Italy
Copyright © 2023 Chen, You, Shen, Wu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinshun Zhang, ✉ dHp6anNAcXEuY29t
 Yi Chen1
Yi Chen1 Jinshun Zhang
Jinshun Zhang