- 1Center of Clinical Medical Research, The Affiliated Suqian First People's Hospital of Xuzhou Medical University, Suqian, China
- 2Institute of Molecular Biology and Translational Medicine, The Affiliated People's Hospital, Jiangsu University, Zhenjiang, China
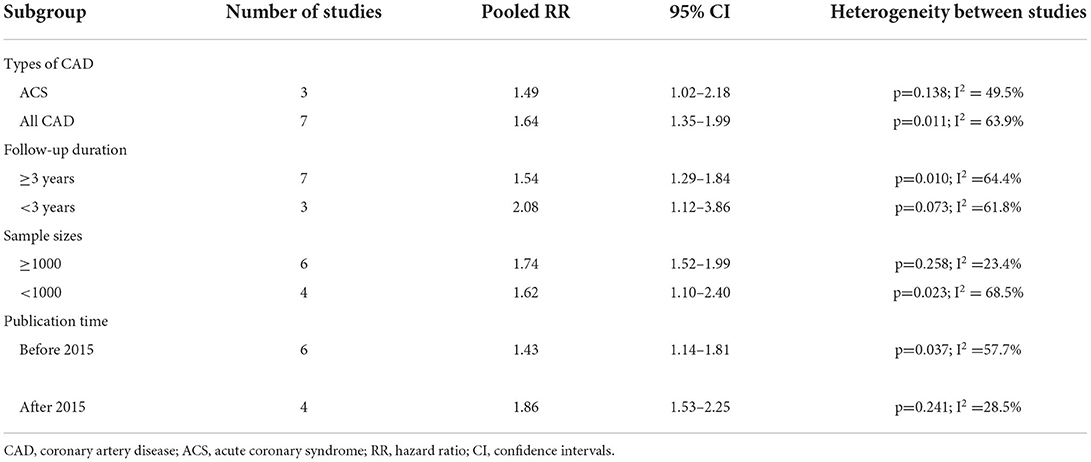
Background: A consensus has not been made about the predictive value of blood vitamin D level in patients with coronary artery disease (CAD). This meta-analysis aimed to assess the association between blood 25-hydroxyvitamin D level and adverse outcomes in patients with CAD.
Methods: Two independent authors searched the articles indexed in PubMed and Embase databases until June 28, 2022. Cohort studies or post-hoc analysis randomized trials evaluating the value of 25-hydroxyvitamin D level in predicting cardiovascular or all-cause mortality, and major adverse cardiovascular events ([MACEs] including death, non-fatal myocardial infarction, heart failure, revascularization, stroke, etc.) were included.
Results: The literature search identified 13 eligible studies for our analysis, including 17,892 patients with CAD. Meta-analysis showed that the pooled adjusted risk ratio (RR) was 1.60 (95% confidence intervals [CI] 1.35–1.89) for all-cause mortality, 1.48 (95% CI 1.28–1.71) for cardiovascular mortality, and 1.33 (95% CI 1.18–1.49) for MACEs. Leave-out one study sensitivity analysis suggested that the predictive values of blood 25-hydroxyvitamin D level were reliable.
Conclusions: Low blood 25-hydroxyvitamin D level is possibly an independent predictor of cardiovascular or all-cause mortality and MACEs in patients with CAD. Baseline 25-hydroxyvitamin D level may provide useful information in CAD patients.
Introduction
Coronary artery disease (CAD) is the most common type of heart disease worldwide, which can be manifested as stable ischemic heart disease or acute coronary syndrome (ACS). Despite the improvement in medical therapy and surgical revascularization, CAD remains a major determinant of morbidity and premature death (1). A more aggressive secondary prevention can be implemented by employing early risk stratification for cardiovascular events and death in patients with CAD.
Biomarkers play an important role in risk stratification and management of CAD (2, 3). Vitamin D is a hormone precursor that maintains calcium homeostasis. The blood level of 25-hydroxyvitamin D is identified as the best estimation of vitamin D state (4). Increasing attention has been focused on the effect of vitamin D on the management of cardiovascular disease (5). Vitamin D deficiency or insufficiency is prevalent in patients with CAD (6–8). Low blood 25-hydroxyvitamin D level is emerging as a predictive biomarker for patients with CAD (9–13). However, inconsistent findings (14–17) have been recorded on the predictive value of Vitamin D deficiency in these patients.
No previous meta-analysis has systematically assessed the predictive association of Vitamin D deficiency with adverse outcomes in patients with CAD. Therefore, the current meta-analysis aimed to evaluate the predictive value of blood 25-hydroxyvitamin D level of patients with CAD in terms of cardiovascular death, all-cause mortality, and cardiovascular events.
Methods
Search strategy
The current meta-analysis was carried out under the Preferred Reporting Items for Systematic Reviews and Meta-analysis guideline (18). Two independent authors identified the eligible studies indexed in PubMed and Embase databases using the following combination of items: (“vitamin D” OR “25-hydroxyvitamin D”) AND (“coronary artery disease” OR “coronary heart disease” OR “ischemic heart disease” OR “ischaemic heart disease” OR “acute coronary syndrome” OR “myocardial infarction” OR “angina”) AND (“death” OR “mortality” OR “cardiovascular event”) AND (“follow-up” OR “follow up” (Supplementary Text S1 in Supplementary material). The final updated search was conducted on June 28, 2022. References of pertinent articles were manually scanned to identify potentially eligible studies. To minimize publication bias, we also reviewed the ClinicalTrials.gov and full-text database of Chinese Excellent Doctoral and Master's Dissertations to identify any gray and unpublished literature.
Study selection
The inclusion criteria are as follows: (1) population: patients were with CAD; (2) exposure: blood 25-hydroxyvitamin D level at baseline; (3) comparison: patients with the bottom vs. reference top 25-hydroxyvitamin D level; (4) outcome measures: cardiovascular or all-cause mortality, and major adverse cardiovascular events ([MACEs] including death, non-fatal myocardial infarction, heart failure, revascularization, stroke, etc.); (5) reported multivariable adjusted risk estimate for the above mentioned outcomes; and (6) study design: retrospective or prospective cohort studies or post-hoc analysis randomized trials. When multiple articles were obtained from the same population, we selected the publication with the longest follow-up. The exclusion criteria are as follows: (1) studies reported the in-hospital outcomes; (2) studies provided risk summary by continuous 25-hydroxyvitamin D level; and (3) cross-sectional study or meeting abstract.
Data extraction and quality assessment
The following data was abstracted by two authors independently: last name of the first author, publication year, origin of study, study design, subtype of CAD, number of patients, gender distribution, age of patients at enrollment, length of follow-up, cutoff value of vitamin D deficiency, definition of MACEs, endpoints, fully adjusted risk summary, and confounders included in the fully adjusted models. Two authors independently assessed the study quality according to the Newcastle-Ottawa Scale (NOS) for cohort studies (maximum score of 9 points) (19). Studies with NOS point ≥ 7 indicated high methodological quality. Any discrepancies were settled by discussing with a third author (Y Fan) to reach consensus.
Statistical analysis
All data were analyzed using STATA 12.0 (STATA Corp LP, College Station, TX, USA). To evaluate the association between blood 25-hydroxyvitamin D level and adverse outcomes, we poled the most fully adjusted risk ratios (RR) and 95% confidence intervals (CI) with the bottom vs. the reference top category of 25-hydroxyvitamin D level. Heterogeneity between studies was determined using the Cochran's Q statistic (p <0.10 was considered significant) and the I2 statistic (I2≥ 50% was considered significant). A random effect model was used for data analysis in the presence of statistically significant heterogeneity. A fixed-effect model was used in the absence of significant heterogeneity. To test the credibility of the pooling results, we conducted a leave-out one study sensitivity analysis to recalculate the risk estimates. Subgroup analyses were conducted to investigate the effect of the types of CAD, sample sizes, publication time, and length of follow-up. Begg's test, Egger's test, and funnel plot were used to investigate the publication bias. The certainty of evidence was summarized via the GRADE analysis.
Results
Search results and study characteristics
A total of 1,114 records were identified from initial electronic database search. After excluding duplicates, 632 records were left. After reading the titles and abstracts, 597 obviously unrelated records were excluded. Thirty-five articles were retrieved for full-text assessment, and 13 studies (9–17, 20–23) satisfied the inclusion criteria (Figure 1).
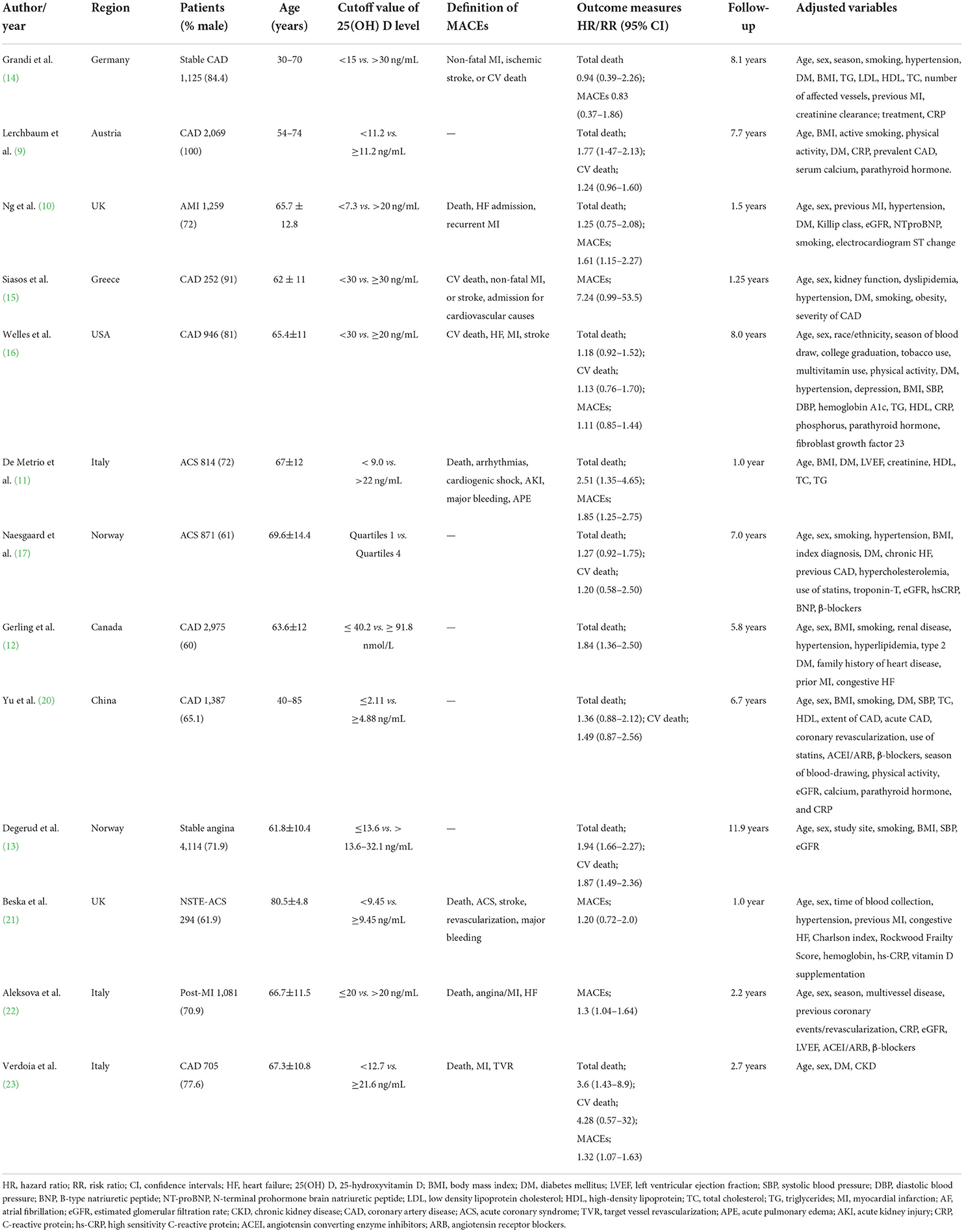
The main features of the included studies are presented in Table 1. These included studies were published between 2010 and 2021. All articles adopted the prospective designs. Four studies (10, 11, 17, 21) included patients with ACS, one study (22) enrolled patients with post-acute myocardial infarction (AMI), one study (13) included stable angina patients, and others did not report the specific type of CAD. Sample sizes ranged from 252 to 4,114, with a total of 17,892 patients with CAD. The length of follow-up varied from 12 months to 11.9 years. According to the NOS criteria, all studies were deemed as high methodological quality (Supplementary Table S1).
All-cause mortality
Ten studies (9–14, 16, 17, 20, 23) investigated the value of 25-hydroxyvitamin D level in predicting all-cause mortality. Figure 2 provides a pooling risk summary of the association between 25-hydroxyvitamin D level and all-cause mortality. Under a random effect model meta-analysis, the pooled adjusted RR of all-cause mortality was 1.60 (95% CI 1.35–1.89) for the bottom vs. the reference top category of 25-hydroxyvitamin D level, having significant heterogeneity (I2 =60.1%; p = 0.007). Leave-out one study sensitivity analysis did not alter the statistical significance of the original risk estimate. In addition, the value of blood 25-hydroxyvitamin D level in predicting all-cause mortality was consistently found in each named subgroup (Table 2). Publication bias was not detected in this outcome according to the Begg's test (p = 1.000), Egger's test (p = 0.567), and symmetrical funnel plot (Supplementary Figure S1).
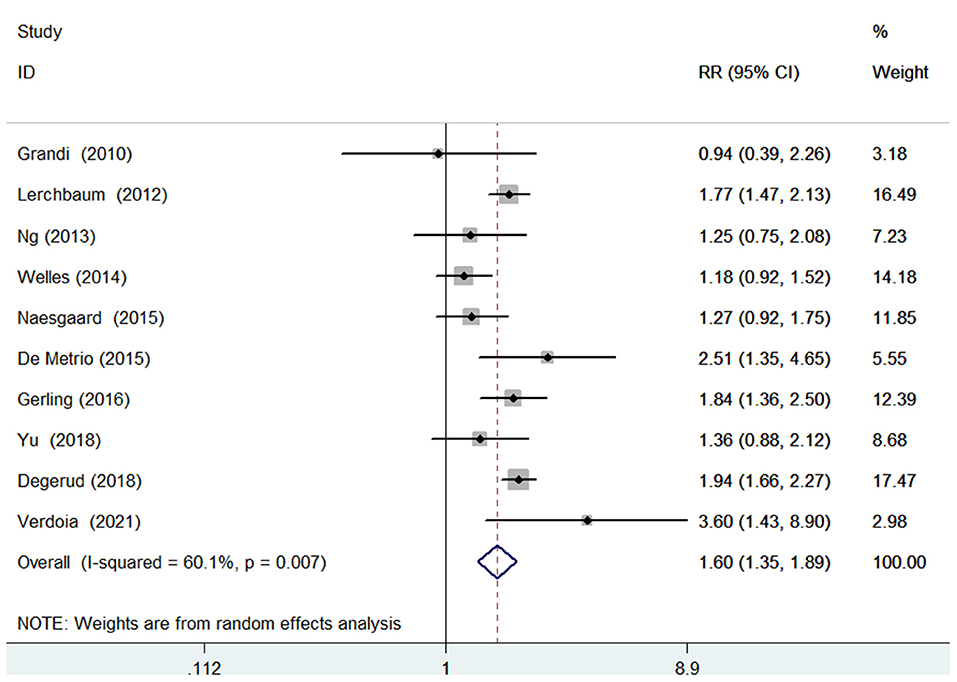
Figure 2. Forest plots showing pooled RR with 95% CI of all-cause mortality for the bottom vs. the reference top category of 25-hydroxyvitamin D level.
Cardiovascular mortality
Six studies (9, 13, 16, 17, 20, 23) evaluated the value of 25-hydroxyvitamin D level in predicting cardiovascular mortality. Figure 3 shows a pooling risk estimate of the association between 25-hydroxyvitamin D level and cardiovascular mortality. Based on fixed-effect model meta-analysis, the pooled adjusted RR of cardiovascular mortality was 1.48 (95% CI 1.28–1.71) for the bottom vs. the reference top category of 25-hydroxyvitamin D level, and no significant heterogeneity was observed between studies (I2 = 44.0%; p = 0.112). Sensitivity analysis confirmed the robustness of the originally pooling risk estimate. The Begg's test (p = 1.000), Egger's test (p = 0.567), and symmetrical funnel plot (Supplemental Figure S2) suggested a low likelihood of publication bias.
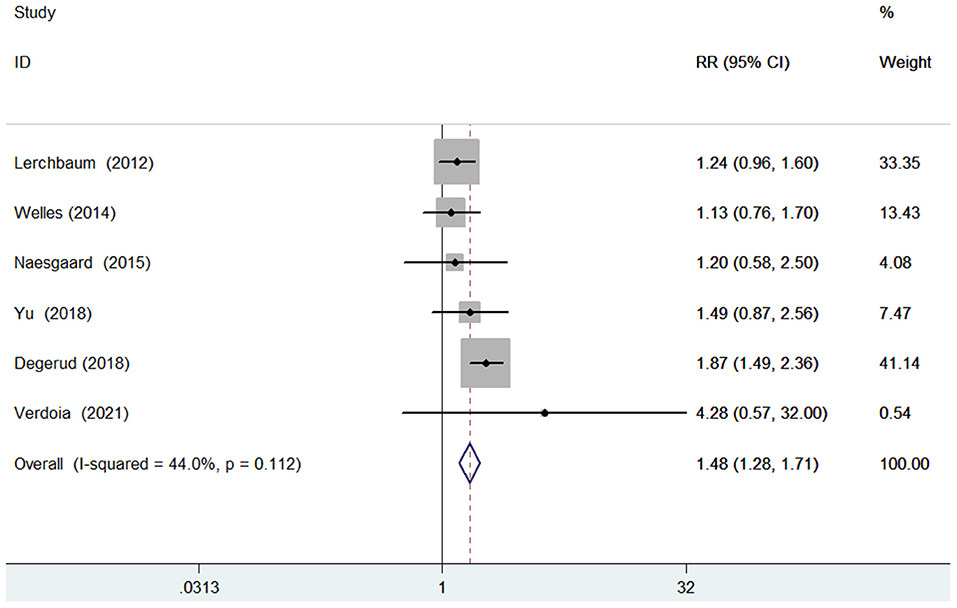
Figure 3. Forest plots showing pooled RR with 95% CI of cardiovascular mortality for the bottom vs. the reference top category of 25-hydroxyvitamin D level.
Major adverse cardiovascular events
Seven studies (10, 11, 14–16, 21–23) evaluated the value of 25-hydroxyvitamin D level in predicting MACEs. Figure 4 provides a pooling risk estimate of the association between d-dimer level and MACEs. A fixed-effect model meta-analysis suggested that the pooled adjusted RR of MACEs was 1.33 (95% CI 1.18–1.49) for the bottom vs. the reference top category of 25-hydroxyvitamin D level, and no significant heterogeneity (I2 = 29.9%; p = 0.189) was observed between studies. Leave-out one study sensitivity analysis did not change the originally statistical significance of the pooling risk estimate. No evidence of publication bias was found according to the results of the Begg's test (p = 0.902), Egger's test (p = 0.428), and symmetrical funnel plot (Supplementary Figure S3).
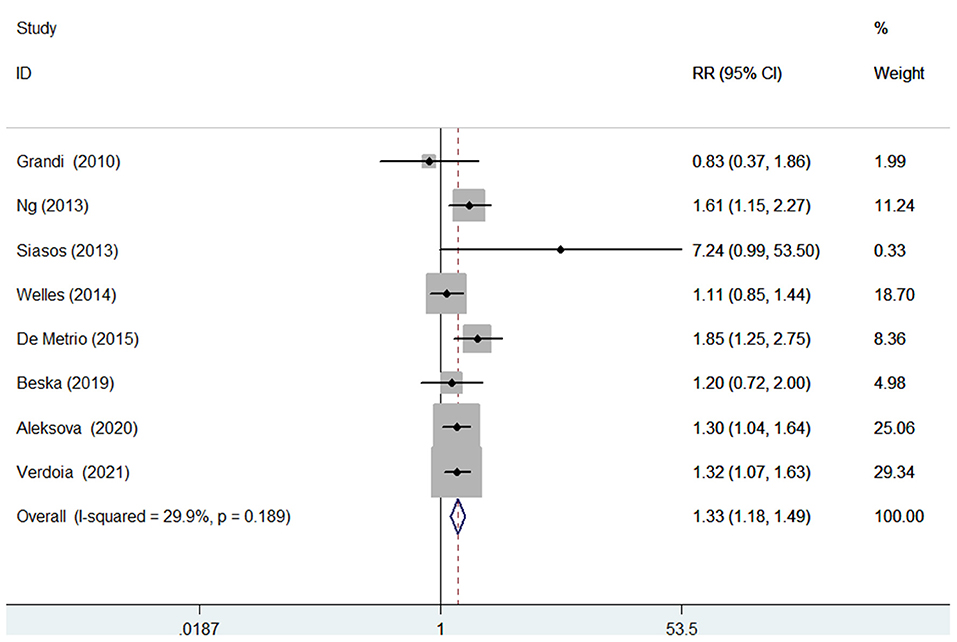
Figure 4. Forest plots showing pooled RR with 95% CI of major adverse cardiovascular events for the bottom vs. the reference top category of 25-hydroxyvitamin D level.
GRADE certainty of evidence
All-cause mortality was grouped as low quality, cardiovascular mortality was classified high quality, and MACEs was classified moderate quality (Supplementary Table S2).
Discussion
The current meta-analysis focused on the predictive value of baseline 25-hydroxyvitamin D level in patients with CAD. This meta-analysis mainly found that 25-hydroxyvitamin D level at baseline was a significant predictor of MACEs, cardiovascular, and all-cause mortality in patients with CAD, even after adjusted multiple important confounders. Based on the comparison between the bottom and reference top 25-hydroxyvitamin D level, patients with the bottom 25-hydroxyvitamin D level conferred a 60 and 48%, higher risk of all-cause mortality and cardiovascular mortality, respectively. For the cardiovascular events, patients with the bottom category of 25-hydroxyvitamin D level had an approximately 33% higher risk of MACEs.
Several studies not meeting our criteria for inclusion also assessed the predictive value of 25-hydroxyvitamin D level in patients with CAD. The predictive role of 25-hydroxyvitamin D level was further supported via continuous variable analysis. In patients with stable angina pectoris, per 10 nmol/L decrease in 25-hydroxyvitamin D conferred a 9 and 10% higher risk of all-cause mortality and cardiovascular mortality, respectively (13). Based on Ludwigshafen Risk and Cardiovascular Health study, each standard deviation (SD) decrease in 25-hydroxyvitamin D level is associated with a 25% higher risk of all-cause mortality during 9.8 years follow-up in stable patients with CAD (24). Apart from the long-term outcomes, low 25-hydroxyvitamin D level is an independent predictor of cardiovascular mortality in patients with ACS (25). In patients with ST segment elevation myocardial infarction, low 25-hydroxyvitamin D level on admission is associated with high risk of no-reflow phenomenon (26).
The different types of CAD may affect the predictive value of 25-hydroxyvitamin D level. Based on subgroup analysis, the value of 25-hydroxyvitamin D level in the prediction of all-cause mortality was lower in patients with ACS (pooled RR 1.49) than in all CAD patients (pooled RR 1.64). Considering the lack of sufficient data, whether the predictive role of 25-hydroxyvitamin D level was affected by ACS subtypes was not determined. In addition, the predictive role of 25-hydroxyvitamin D level was weakened with the lengthening of follow-up in the subgroup analysis.
Several potential mechanisms may be implicated into the association of vitamin D deficiency with adverse outcomes in patients with CAD. First, low vitamin D can activate the activity of the renin-angiotensin-aldosterone system (27); Second, vitamin D deficiency may harm CAD patients by enhancing inflammation (28, 29). Finally, low 25-hydroxyvitamin D level was closely related to the occurrence of no-reflow phenomenon after percutaneous coronary intervention (26, 30) and the severity of CAD (31).
A recent meta-analysis of four randomized clinical trials suggested that vitamin D supplementation is associated with improvements in diastolic blood pressure and parathyroid hormone in patients with CAD having vitamin D deficiency (32). However, survival and cardiovascular events were not assessed in this meta-analysis. Based on our meta-analysis, CAD patients with low blood 25-hydroxyvitamin D level should be identified as high-risk group and be closely monitored. Future randomized controlled trials are required to demonstrate whether vitamin D supplementation could improve the prognosis of patients with CAD.
Several potential limitations should be addressed in this meta-analysis. Firstly, blood 25-hydroxyvitamin D level was only detected once rather than dynamic measurement, possibly causing classification bias. Secondly, the cut-off values of lower 25-hydroxyvitamin D level, which were used for predicting adverse outcomes, varied across studies, thus making it hard for clinicians to identify patients that need supplementation of vitamin D. Thirdly, significant heterogeneity was found for all-cause mortality. The different cut-off values of low 25-hydroxyvitamin D level, types of the CAD, or length of follow-up may contribute to the existing heterogeneity. Fourthly, this meta-analysis did not analyze the predictive role of 25-hydroxyvitamin D level by continuous data analysis because of the lack of sufficient data. Fifth, when a U-shaped association of 25-hydroxyvitamin D level with worse outcomes is observed (13, 33), the selection of the bottom 25-hydroxyvitamin D level as the reference may have led to underestimation of the actual risk summary. Finally, blood level of 25-hydroxyvitamin D is strongly correlated with time spent outdoors. The lack of adjusting season or time spent outdoors may have affected the pooling risk estimate.
Conclusion
Low 25-hydroxyvitamin D level may be an independent predictor of MACEs, cardiovascular and all-cause mortality in patients with CAD. Baseline 25-hydroxyvitamin D level may provide important prognostic information in CAD patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
Study conception/design and interpretation of data: YF and XW. Literature search, data extraction, and quality assessment: HZ and PW. Statistical analysis: YJ and YS. Writing the manuscript: HZ. All the authors approved the version of the manuscript.
Funding
This work is supported by (1) Suqian Science and Technology Support Project Fund (K201907), (2) Jiangsu 333 Talent Fund (BRA2020016), and (3) Zhenjiang Key Research and Development Fund (SH2021038).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.984487/full#supplementary-material
References
1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 Update: a report from the american heart association. Circulation. (2017) 135:e146–603. doi: 10.1161/CIR.0000000000000485
2. Chacko S, Haseeb S, Glover BM, Wallbridge D, Harper A. The role of biomarkers in the diagnosis and risk stratification of acute coronary syndrome. Future Sci OA. (2018) 4:FSO251. doi: 10.4155/fsoa-2017-0036
3. McCarthy CP, McEvoy JW, Januzzi JL. Biomarkers in stable coronary artery disease. Am Heart J. (2018) 196:82–96. doi: 10.1016/j.ahj.2017.10.016
4. Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. (2004) 80:1706S−9S. doi: 10.1093/ajcn/80.6.1706S
5. Cosentino N, Campodonico J, Milazzo V, De Metrio M, Brambilla M, Camera M, et al. Vitamin D and cardiovascular disease: current evidence and future perspectives. Nutrients. (2021) 13:3603. doi: 10.3390/nu13103603
6. Dziedzic EA, Gasior JS, Saniewski T, Dabrowski M. Vitamin D deficiency among Polish patients with angiographically confirmed coronary heart disease. Pol Merkur Lekarski. (2021) 49:278–82.
7. Akhtar T, Aggarwal R, Jain SK. Serum vitamin D level in patients with coronary artery disease and association with sun exposure: experience from a tertiary care, teaching hospital in India. Adv Med. (2019) 2019:6823417. doi: 10.1155/2019/6823417
8. Knezevic Pravecek M, Vukovic-Arar Z, Miskic B, Hadzibegovic I. Vitamin D deficiency in acute coronary syndrome - clinically relevant or incidental finding? Cent Eur J Public Health. (2017) 25:185–90. doi: 10.21101/cejph.a4577
9. Lerchbaum E, Pilz S, Boehm BO, Grammer TB, Obermayer-Pietsch B, Marz W. Combination of low free testosterone and low vitamin D predicts mortality in older men referred for coronary angiography. Clin Endocrinol (Oxf). (2012) 77:475–83. doi: 10.1111/j.1365-2265.2012.04371.x
10. Ng LL, Sandhu JK, Squire IB, Davies JE, Jones DJ. Vitamin D and prognosis in acute myocardial infarction. Int J Cardiol. (2013) 168:2341–6. doi: 10.1016/j.ijcard.2013.01.030
11. De Metrio M, Milazzo V, Rubino M, Cabiati A, Moltrasio M, Marana I, et al. Vitamin D plasma levels and in-hospital and 1-year outcomes in acute coronary syndromes: a prospective study. Medicine (Baltimore). (2015) 94:e857. doi: 10.1097/MD.0000000000000857
12. Gerling ME, James MT, Wilton SB, Naugler C, Southern DA, Galbraith PD, et al. Serum total 25-OH vitamin D adds little prognostic value in patients undergoing coronary catheterization. J Am Heart Assoc. (2016) 5(10). doi: 10.1161/JAHA.116.004289
13. Degerud E, Nygard O, de Vogel S, Hoff R, Svingen GFT, Pedersen ER, et al. Plasma 25-hydroxyvitamin d and mortality in patients with suspected stable angina pectoris. J Clin Endocrinol Metab. (2018) 103:1161–70. doi: 10.1210/jc.2017-02328
14. Grandi NC, Breitling LP, Vossen CY, Hahmann H, Wusten B, Marz W, et al. Serum vitamin D and risk of secondary cardiovascular disease events in patients with stable coronary heart disease. Am Heart J. (2010) 159:1044–51. doi: 10.1016/j.ahj.2010.03.031
15. Siasos G, Tousoulis D, Oikonomou E, Maniatis K, Kioufis S, Kokkou E, et al. Vitamin D serum levels are associated with cardiovascular outcome in coronary artery disease. Int J Cardiol. (2013) 168:4445–7. doi: 10.1016/j.ijcard.2013.06.151
16. Welles CC, Whooley MA, Karumanchi SA, Hod T, Thadhani R, Berg AH, et al. Vitamin D deficiency and cardiovascular events in patients with coronary heart disease: data from the Heart and Soul Study. Am J Epidemiol. (2014) 179:1279–87. doi: 10.1093/aje/kwu059
17. Naesgaard PA, Ponitz V, Aarsetoey H, Brugger-Andersen T, Grundt H, Harris WS, et al. Prognostic utility of vitamin D in acute coronary syndrome patients in coastal Norway. Dis Markers. (2015) 2015:283178. doi: 10.1155/2015/283178
18. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
19. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (assessed June 18, 2022).
20. Yu C, Xue H, Wang L, Chen Q, Chen X, Zhang Y, et al. Serum bioavailable and free 25-hydroxyvitamin d levels, but not its total level, are associated with the risk of mortality in patients with coronary artery disease. Circ Res. (2018) 123:996–1007. doi: 10.1161/CIRCRESAHA.118.313558
21. Beska B, Chan D, Gu S, Qiu W, Mossop H, Neely D, et al. The association between vitamin D status and clinical events in high-risk older patients with non-ST elevation acute coronary syndrome undergoing invasive management. PLoS ONE. (2019) 14:e0217476. doi: 10.1371/journal.pone.0217476
22. Aleksova A, Ferro F, Gagno G, Padoan L, Saro R, Santon D, et al. Diabetes mellitus and vitamin D deficiency:comparable effect on survival and a deadlyassociation after a myocardial infarction. J Clin Med. (2020) 9:2127. doi: 10.3390/jcm9072127
23. Verdoia M, Nardin M, Rolla R, Negro F, Gioscia R, Afifeh AMS, et al. Prognostic impact of Vitamin D deficiency in patients with coronary artery disease undergoing percutaneous coronary intervention. Eur J Intern Med. (2021) 83:62–7. doi: 10.1016/j.ejim.2020.08.016
24. Kleber ME, Goliasch G, Grammer TB, Pilz S, Tomaschitz A, Silbernagel G, et al. Evolving biomarkers improve prediction of long-term mortality in patients with stable coronary artery disease: the BIO-VILCAD score. J Intern Med. (2014) 276:184–94. doi: 10.1111/joim.12189
25. Correia LC, Sodre F, Garcia G, Sabino M, Brito M, Kalil F, et al. Relation of severe deficiency of vitamin D to cardiovascular mortality during acute coronary syndromes. Am J Cardiol. (2013) 111:324–7. doi: 10.1016/j.amjcard.2012.10.006
26. Sen O, Sen SB, Topuz AN, Topuz M. Vitamin D level predicts angiographic no-reflow phenomenon after percutaneous coronary intervention in patients with ST segment elevation myocardial infarction. Biomark Med. (2021) 15:1357–66. doi: 10.2217/bmm-2020-0689
27. Kota SK, Kota SK, Jammula S, Meher LK, Panda S, Tripathy PR, et al. Renin-angiotensin system activity in vitamin D deficient, obese individuals with hypertension: An urban Indian study. Indian J Endocrinol Metab. (2011) 15:S395–401. doi: 10.4103/2230-8210.86985
28. Murr C, Pilz S, Grammer TB, Kleber ME, Meinitzer A, Boehm BO, et al. Vitamin D deficiency parallels inflammation and immune activation, the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chem Lab Med. (2012) 50:2205–12. doi: 10.1515/cclm-2012-0157
29. Liu Y, Peng W, Li Y, Wang B, Yu J, Xu Z. Vitamin D deficiency harms patients with coronary heart disease by enhancing inflammation. Med Sci Monit. (2018) 24:9376–84. doi: 10.12659/MSM.911615
30. Verdoia M, Viglione F, Boggio A, Stefani D, Panarotto N, Malabaila A, et al. Vitamin D deficiency is associated with impaired reperfusion in STEMI patients undergoing primary percutaneous coronary intervention. Vascul Pharmacol. (2021) 140:106897. doi: 10.1016/j.vph.2021.106897
31. Chen WR, Qian YA, Chen YD, Shi Y, Yin DW, Wang H, et al. The effects of low vitamin D on coronary artery disease. Heart Lung Circ. (2014) 23:314–9. doi: 10.1016/j.hlc.2013.08.012
32. Bahrami LS, Ranjbar G, Norouzy A, Arabi SM. Vitamin D supplementation effects on the clinical outcomes of patients with coronary artery disease: a systematic review and meta-analysis. Sci Rep. (2020) 10:12923. doi: 10.1038/s41598-020-69762-w
Keywords: 25-hydroxyvitamin D, coronary artery disease, major adverse cardiovascular events, mortality, meta-analysis
Citation: Zhang H, Wang P, Jie Y, Sun Y, Wang X and Fan Y (2022) Predictive value of 25-hydroxyvitamin D level in patients with coronary artery disease: A meta-analysis. Front. Nutr. 9:984487. doi: 10.3389/fnut.2022.984487
Received: 02 July 2022; Accepted: 22 July 2022;
Published: 10 August 2022.
Edited by:
Yulong Li, University of Nebraska Medical Center, United StatesReviewed by:
Huiyin Tu, Zhengzhou University, ChinaWenfeng Hu, University of Nebraska Medical Center, United States
Copyright © 2022 Zhang, Wang, Jie, Sun, Wang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Fan, anN6amZhbnl1JiN4MDAwNDA7MTYzLmNvbQ==; Xiaoyan Wang, dGRzenl5JiN4MDAwNDA7MTI2LmNvbQ==
†These authors have contributed equally to this work
 Hailing Zhang1†
Hailing Zhang1† Yimeng Sun
Yimeng Sun Xiaoyan Wang
Xiaoyan Wang