
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 07 September 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.980749
Despite the growing evidence from meta-analyses on vitamin D’s anti-obesity properties, their results are controversial. The current umbrella review was performed to assess the available evidence and provide a conclusive explanation in this regard. The international databases PubMed, Scopus, Embase, Web of Science and Google Scholar were systematically searched till March, 2022. A random-effects model was used to run the meta-analysis. All meta-analyses that examined the effect of vitamin D supplementation on BW, BMI, WC, and fat mass were included. Findings of 14 meta-analyses revealed that vitamin D supplementation reduced body mass index (BMI) (ES: −0.11 kg/m2; 95% CI: −0.18, −0.05, p?0.001; I2 = 61.0%, p < 0.001), and waist circumference (WC) (ES = −0.79 cm; 95% CI: −1.20, −0.37; p < 0.001; I2 = 46.5%, p = 0.096) in comparison to control group. However, the effects of vitamin D on body weight (ES = −0.16 kg, 95% CI: −0.36, 0.04; p = 0.125; I2 = 57.0%, p = 0.017), and fat mass (ES: 0.02, 95% CI: −0.20, 0.24, p = 0.868; I2 = 0.0%, p = 0.531) were not considerable. Vitamin D supplementation significantly improved levels of obesity indices such as BMI, and WC.
According to the World Health Organization definition, a body mass index (BMI) of ≥ 30 kg/m2 is considered as obesity which currently is a global public health concern. Cardiovascular disease, stroke, type 2 diabetes mellitus (T2DM) and certain types of cancer as the leading causes of preventable death, are obesity-related conditions. According to data from 2018 to 2019 from the National Health and Nutrition Examination Survey (NHANES), almost 1 in 3 adults (30.7%) are overweight, more than 2 in 5 adults (42.4%) are obese, and about 1 Out of every 11 adults (9.2%) have severe obesity. By another mean, more than 2.5 billion adult people are overweight and obese worldwide (1, 2).
The Obesity studies emphasize the link between the metabolism of macronutrients and deficiency of serum vitamins levels with weight gain. Fat-soluble vitamins are required for various actions and prevention of diseases (3). Vitamin D as a fat soluble vitamin not only is involved in calcium and phosphorus homeostasis, and bone metabolism, but also plays an important role in regulating collagen type 1 production, muscle function, cell differentiation, insulin secretion, and the immune system (4). Some chronic disorders such as insulin resistance, metabolic syndrome, atherosclerosis, neurodegenerative diseases and obesity are associated with vitamin D deficiency (5–7). An inverse association between adipose tissue and obesity degree with serum levels of vitamin D has been observed in studies (8–10). Cheng et al. reported that vitamin D deficiency was three times higher in subjects with high body fat concentration (7). More fat concentration in the body causes the storage of more vitamin D in adipose tissue. Vitamin D volume dilution mechanism that has been considered by researchers today, suggested that vitamin D is distributed in muscles, fat, and liver. With obesity the amount of vitamin D are increased in these tissues, resulting in lower serum levels of vitamin D (11, 12). Another possible mechanism is increased lipogenesis in vitamin D deficiency condition. Elevated parathyroid hormone secretion in vitamin D deficiency results in more influx of calcium into the adipocyte, stimulating lipogenesis (13). Several clinical trial studies investigated effect of vitamin D supplementation on anthropometric indices in obese people. While some studies have shown a positive effect of supplementation on body fat and body mass index, some other studies have reported no effect of vitamin D supplementation on total body fat (14–16).
The results of the meta-analysis are also contradictory in this regard. While some meta-analysis studies show a positive effect of the vitamin D supplement on weight loss and waist circumference (17, 18), some have reported ineffectiveness of the supplementation on anthropometric indices (19, 20). Therefore, we conducted present umbrella meta-analysis to clarify the effect of vitamin D supplementation on anthropometric indices in different health conditions.
Google Scholar, PubMed, Scopus, and Web of Science were systematically searched from the earliest date available up to March, 2022. The study implementation of the study was finished on June 2022. The used MeSH terms and keywords include: (“vitamin d”OR “ergocalciferols”) AND (treatment) OR (supplementation) OR (vitamin d3) OR (vitamin d2) OR (intake) AND (“Body composition”) OR (“Weight Loss”) OR (“body weight”) OR (“body weight changes”) OR (“body mass index”) OR (obesity) OR (“body weight”) OR (BMI)[OR (“waist circumference”) OR (WC) OR (“fat mass”) OR (“lean mass”) AND (“systematic review”) OR (“meta-analysis”). In addition, a manual search of the references of eligible studies was done to minimize the risk of missing relevant papers. PRISMA guidelines were followed during implementation of all steps of this study.
PICO criteria for current umbrella meta-analysis were as follows: Population/Patients (P: adults, 18-year-old or above, who were treated with vitamin D); Intervention (I: vitamin D); Comparison (C: control or placebo group); Outcome (O: obesity indices including body weight, BMI, waist circumference (WC) and fat mass. Meta-analysis studies in English examining the effects of vitamin D supplementation on anthropometric indices including body weight, BMI, WC, and fat mass which have reported the effect sizes (ES) and corresponding confidence intervals (CI) were considered eligible. Original studies, editorials, letters to the editor, and studies with low-quality score were excluded.
Two reviewers (VM, FHK) conducted the process of screening and inclusion of articles independently. Additionally, the reference lists of all included studies were screened for eligible studies. Following data were extracted from the included meta-analyses: first author’s name, publication year, country, type of vitamin D, supplementation dosage, duration range of supplementation, study population, number of participants, participant demographics, and main results [effect size with 95% confidence intervals (95% CIs)]. Any disagreements were resolved by discussion with the third reviewer (MZ).
The Assessment of Multiple Systematic Reviews 2 (AMSTAR2) tool was employed to evaluate the methodological quality of systematic reviews and meta-analyses by two reviewers (VM, FHK), independently. AMSTAR2 questionnaire contains 16 items asking reviewers to respond with a “Yes” or “Partial Yes” or “No” or “No Meta-analysis” option. According to the AMSTAR2 checklist, quality was classified as “Critically low quality,” “low quality,” “moderate quality,” and “high quality” (21). We evaluated the overall certainty of the evidence of the included meta-analyses using GRADE tools. The quality of evidence was classified into four categories based on evaluation criteria, i.e., high, moderate, low, and very low (22).
ESs and CIs were used to calculate the overall effect sizes. We assessed data in terms of heterogeneity for each pooled analysis based on Cochrane Q test and I2 statistics. I2-values of 25, 50, and 75% were categorized as low, moderate, and high heterogeneity, respectively (23). I2-value > 50% or p < 0.1 for the Q-test were considered as significant heterogeneity among the studies. Random-effects model was used to conduct the meta-analysis. To find the possible sources of heterogeneity, subgroup analyses based on age, sample size, dose, duration, type of effect size, gender, and health condition were performed. To determine the overall effect size dependence on a specific study, sensitivity analysis was utilized. The small-study effect evaluated by the Begg’s and Egger’s tests. Also, publication bias was assessed by visual inspection of the funnel plots whenever the number of included studies were ≥ 10. Trim and fill analysis was conducted where the publication bias was present. Version 16 of Stata (Stata Corporation, College Station, TX, United States) was used to perform the analysis in this study. P-value < 0.05 was considered as significant level.
PRISMA flow diagram is shown in Figure 1. We found 152 articles after searching electronic databases. Total number of 42 duplicate articles were removed. Then the remaining 110 articles were screened carefully by title and abstract. Among those, full-text of 18 articles was evaluated. At the end, 14 meta-analyses were included in the current umbrella review. An overview of the characteristics of qualified articles is shown in Table 1. The included studies were published from 2013 to 2021, and the mean age of participants was between 27 and 62 years. The average amount of administered vitamin D among studies was between 1,000 and 59,000 IU/day. The duration of vitamin D supplementation ranged from 8 to 43 weeks. The location of the studies were as follows: four in China (20, 24–26), two in Iran (18, 27), two in United States (28, 29), one each in Philippines (30), Bahrain (17), India (19), Australia (31), United Kingdom (32), and Brazil (33). Table 1 describes the quality of randomized controlled trials that were included in the included meta-analyses.
The results of the AMSTAR2 questionnaire are presented in Table 2. Eleven of the papers were of high quality, two were moderate, and one was critically low quality. All quality outcomes were estimated as moderate using the GRADE system (based on indirectness) (Table 3).
Overall, seven meta-analyses with nine effect sizes comprising a total of 7,507 participants have investigated the impact of vitamin D supplementation on BW levels (Figure 2). Combining their findings using random-effects model, it was found that BW was not significantly affected after vitamin D supplementation (ES = −0.16 kg, 95% CI: −0.36, 0.04; p = 0.125, I2 = 57.0%, p = 0.017). Also, the findings of subgroup analysis implied that mean age of participants, dosage, sample size, study population, and duration of intervention were the sources of heterogeneity (Table 4). No significant difference was observed performing sensitivity analysis. No significant publication bias was observed performing Begg’s test (P = 0.251).

Figure 2. The effects of vitamin D supplementation on body weight are depicted in a forest plot with mean differences and 95 percent confidence intervals (CIs).
The effect of vitamin D supplementation on BMI was assessed in 14 meta-analyses with 15 effect sizes, including 15,256 participants. The pooled estimate indicated that in subjects who consumed vitamin D supplements, BMI significantly was decreased (ES = −0.11 kg/m2; 95% CI: −0.18, −0.05 p < 0.001) (Figure 3A). Furthermore, a high between-study heterogeneity was observed (I2 = 61.0%, p < 0.001), which was decreased with subgrouping according to the study population, mean age, type of effect size, sample size, treatment dosage, and duration (Table 4). Vitamin D supplement > 5,000 IU/day, intervention duration of ≤ 16 weeks and supplementation on subjects with NAFLD contributed to a greater reduction in BMI (Table 4). There was no significant difference with removing one single study using sensitivity analysis. Based on Begg’s, and Egger’s tests, no significant small-study effect has been detected (p = 0.718, and p = 384, respectively). Moreover, an uneven distribution of studies was observed after visual inspection of the funnel plot (Figure 3B). Therefore, trim and fill analysis was performed (no imputed studies) and the findings were still significant (ES = −0.11 kg/m2; 95% CI: −0.18, −0.05, p? 0.05).
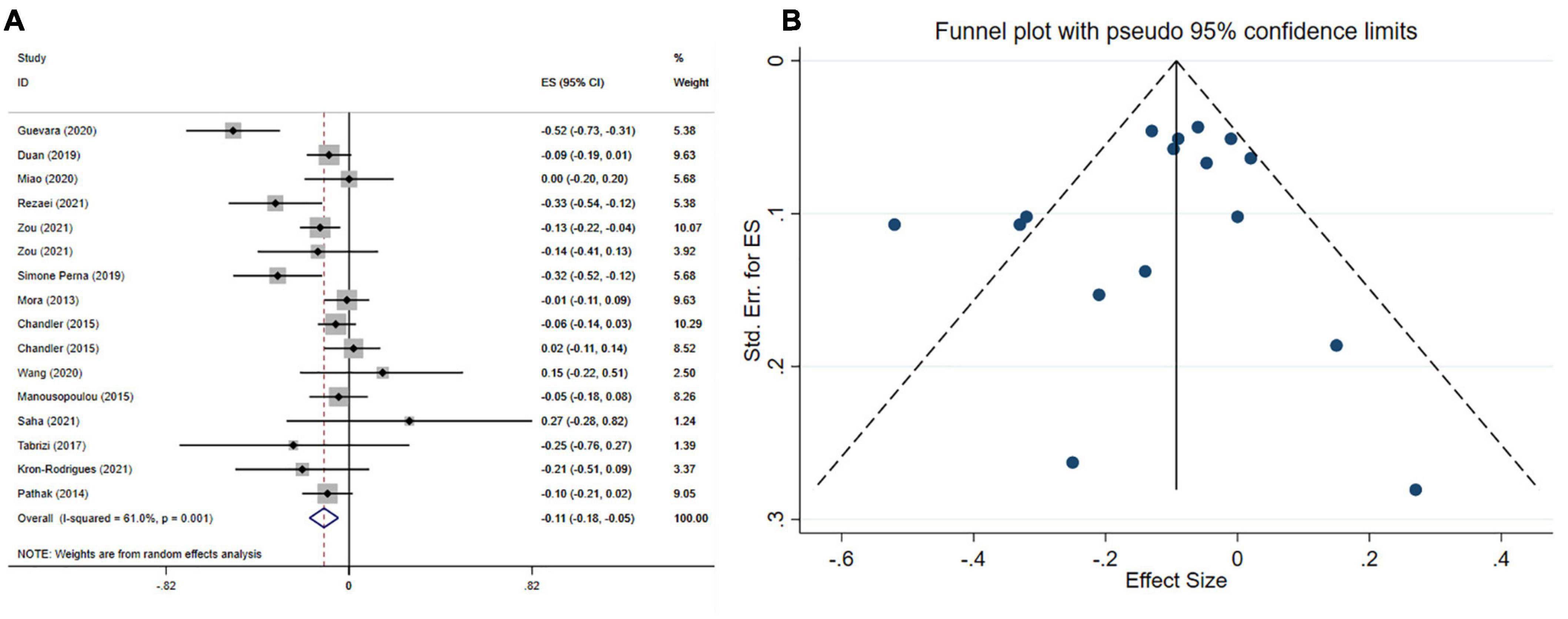
Figure 3. Forest plot (A) funnel plot with a mean difference and 95% confidence intervals (CIs) (B) publication bias in the studies reporting, the effects of vitamin D supplementation on BMI levels.
Five meta-analyses with six effect sizes, including 2,161 participants have evaluated the effect of vitamin D administration on WC levels. The obtained pooled effect size revealed that vitamin D meaningfully reduced WC (ES = −0.79 cm; 95% CI: −1.20, −0.37; p < 0.001) (Figure 4A). No significant heterogeneity was detected among studies (I2 = 46.5%, p = 0.096). The effect size was not affected by sensitivity analysis. Begg’s test has indicated no significant publication bias (p = 0.999).
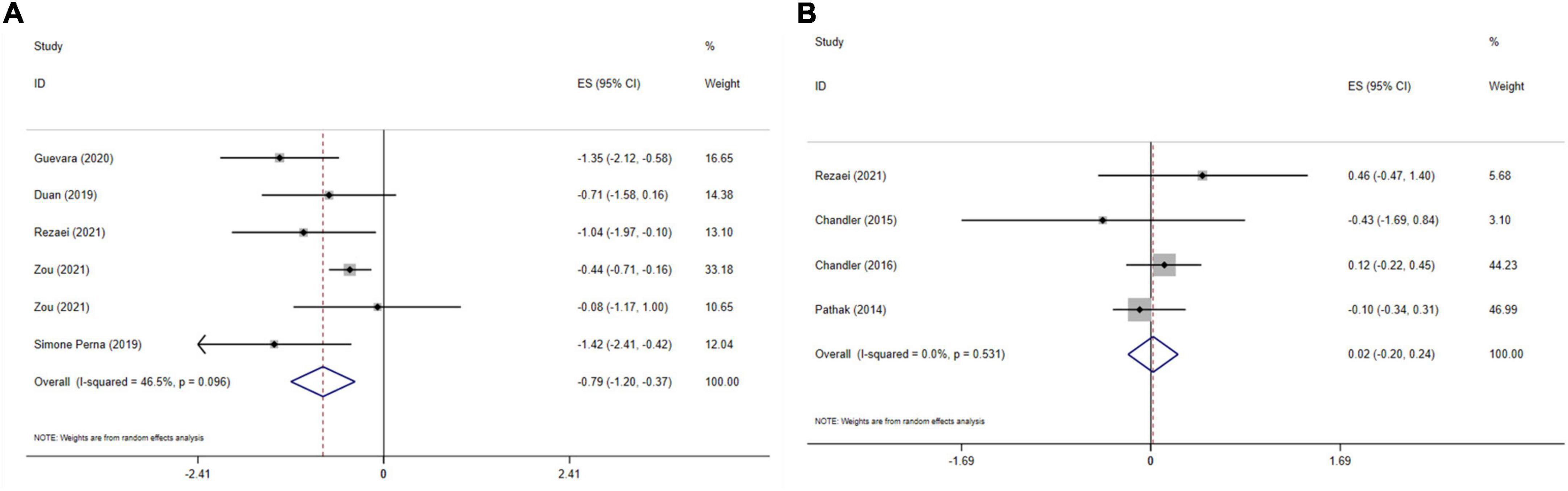
Figure 4. The effects of vitamin D supplementation on WC (A), and fat mass (B) are depicted in a forest plot with mean differences and 95 percent confidence intervals (CIs).
The effect of vitamin D on fat mass is presented in Figure 4B. Combining four effect sizes and total of 3,185 participants demonstrated that vitamin D had no significant impact on fat mass (ES: 0.02, 95% CI: −0.20, 0.24, p = 0.868). There was no significant heterogeneity among studies (I2 = 0.0%, p = 0.531). Sensitivity analysis showed that no study affected the overall effect size. Based on Begg’s test, no significant publication bias was detected (p = 0.734).
The present umbrella meta-analysis on the effect of vitamin D treatment on BW, BMI, fat mass and WC summarized the results of 14 meta-analyses including 116 trials. The findings of this study lend support to the theory that vitamin D supplementation was efficient in declining BW and BMI, whilst no significant associations were observed regarding FM and WC. Intervention duration of ≤ 16 weeks, administered dosage of > 5,000 IU, mean age of > 50 years and study population (overweight, obesity, NAFLD, diabetes) for both genders led to more improvements in BMI.
There is a bidirectional relationship between obesity and the metabolism and storage of vitamin D (29). According to observational studies, the risk of vitamin D deficiency (VDD) among obese individuals is high; however, the causality direction of this relationship is indistinct. Hence, it is not clear whether VDD is the cause or result of obesity (34). Four main mechanisms have been mentioned for the low vitamin D level in obese subjects: First, the less sun exposure compared to healthy people, since obese individuals usually avoid outdoor activity (35). Vitamin D ingested by food or synthesized in the skin undergoes two important hydroxylation stages. In the liver, vitamin D is transformed to 25(OH)D3, and in the liver, 25(OH)D3 is synthesized into 1,25 (OH) 2D. The active form of vitamin D after binding to its receptor forms a heterodimer with retinoid X receptor (RXR) and translocate into the nucleus. The so called VDR-RXR complex interacts with specific DNA regions and regulates multiple genes such as CYP27B1 expression in pancreases beta-cells (36). VDR polymorphisms display a negative role in obesity (35). Second, the high concentration of 1, 25 (OH)D in obese individuals declines the concentration of 25 (OH)D. Third, vitamin D is dispossessed inside the adipose tissue. Fourth, the volumetric dilution of fat mass, declines 25 (OH)D concentration (35). VDD and abundance of fat accumulation, together decrease the activity of alpha-hydroxylase, the main enzyme responsible for the biotransformation of calciferol in the liver. This phenomenon results in the accumulation of inactive forms of vitamin D and reduction of its bioavailability, since abdominal fat is a good storage site for vitamin D (24, 36).
VDD influences the risk of obesity by directly elevating adipogenesis or indirectly modulating inflammation, oxidative stress, metabolism, differentiation of preadipocytes into adipocytes, and gene regulation (24). VDD enhances parathyroid hormone (PTH) level and increases the invasion of calcium into adipocyte tissue and furthermore enhances lipogenesis, stimulating catecholamine to induce lipolysis and accumulate fat in adipocytes (17, 24). PTH mediates this action via a sympathetic nervous system thermogenesis and lipolysis mechanism (37). Evidence suggests that supplementing the active form of vitamin D, may alleviate substrate oxidation, improve insulin sensitivity, suppress PTH, promote adiponectin secretion and eventually stimulate weight loss (28). Vitamin D effects insulin sensitivity by enhancing the expression of insulin receptor in peripheral cells via a Ca2+-dependent mechanism, since vitamin D is responsible for the regulation and passage of intracellular Ca2+ (36). Vitamin D also influences adipocyte apoptosis, adipogenesis regulation, lipid metabolism, calcium absorption, and is associated with β cell function and/or insulin resistance (27, 29, 33).
Today obesity has been recognized a state of chronic, low-grade systemic inflammation. Within this situation, adipocytes secrete pro and anti-inflammatory cytokines, hormones, and acute phase reactants. Other inflammatory molecules such as preadipocytes, mast cells, and macrophages are also responsible for enhancing inflammation in obese subjects. Vitamin D acts as an acute phase reactant in the inflammatory situation caused by obesity and suppress 25(OH)D concentration. Vitamin D has a positive correlation with the adiponectin hormone in obese subjects. Thus, enhancing 25(OH)D concentration, helps increase the process of weight loss by declining inflammation (35). Additionally, 1,25(OH)2D regulates the expression of adipokines in visceral adipose tissue by up-regulating the expression of genes responsible in the secretion of leptin, adiponectin, tumor necrosis factor (TNF-alpha), plasminogen activator inhibitor type I, transforming growth factor (TGF) type I, and resistin (36). Vitamin D 1,25 (OH) 2D inhibits proinflammatory cytokines such as IL-1b, IL-6, IL-8, IL-12, Vascular endothelial growth factor (VEGF), C-reactive protein (CRP), and Nuclear factor kappa B (NF-kB) and mitogen activated protein kinase signaling pathways and reduce the expression of toll-like receptors. Also, the active form of vitamin D increases IL-4, IL-13, IL-10, and macrophage transformation (36). Hence, vitamin D is efficient in declining weight via controlling inflammation caused obesity. Figure 5 displays the mechanism of action of vitamin D in obesity.
Although the effect of vitamin D on WC and FM were assessed in few studies, most of the studies did not observe beneficial results. The diverse results claimed for body fat may be due to the various fat mass measures used (truncal fat, whole-body fat, and body fat percentage) instead of standard methods such as dual-energy X-ray absorptiometry (DXA) or the age of study participants since fat mass alleviates as individuals become older (29, 31). Moreover, the differences in doses of vitamin D administered and differences in serum level of vitamin D are mentioned as reason for these findings (27). However, the limited number of studies assessing the effect of vitamin D on WC and FM could be mentioned as an important reason for the insignificant results observed.
Based on subgroup analyses, vitamin D supplementation was more efficient in declining BMI when administered for individuals older than 50 years. However, since BMI is influenced by age, BMI decline may be due to other reasons rather than vitamin D supplementation. In older individuals, fat mass is increased and lean body mass and skeletal body mass are declined. Thus, these factors are also explanations for the reduction of BMI in older subjects (29). In regards to gender differences, according to Table 4, the number of studies assessing the effect of vitamin D supplementation on BMI in females were low and the majority of studies had matched basic characteristics. Thus, although gender does impact one’s ability to lose weight (28) along with hormonal changes in the body such as menopause (32), subgroup analyses indicated beneficial effects for vitamin D supplementation in both genders. This could be due to the high prevalence of vitamin D deficiency in both genders and the effectiveness of vitamin D in compensating deficiencies in VDD subjects. Subgroup analyses for duration of intervention indicated significant results for both cut-offs (Table 4). This may be because the majority of studies had not claimed how long each study had maintained their achieved level of 25(OH)D. Also, while vitamin D administration for short term can normalize serum levels, cessation of supplementation may affect the retention of beneficial effects (28). Additionally, vitamin D supplementation should be applied for a certain timing in order to observe significant changes in BMI (24). Similar findings were also observed regarding vitamin D dosage. The differences in serum levels of vitamin D is an important factor affecting the appropriate dose of vitamin D. Moreover, adequate response to vitamin D is inversely associated with baseline BMI, thus body size should be deliberated when determining suitable dose of supplementation (27). In overweight and obese subjects, as well as patients diagnosed with NAFLD and T2DM, the percentage of central fat is high, subjects have a sensitive response to supplementation and easily display the beneficial effects of vitamin D. Hence, vitamin D administration is efficient in declining BMI in this group of patients (24). However, the insignificant results for PCOS patients may be due to the limited number of studies, poor methodological biases reported, small sample size, short duration of intervention, and variations in doses and units of vitamin D (20, 25).
Subgroup analyses based on the effect of vitamin D supplementation on body weight indicated significant findings regarding NAFLD patients; however, it was only applied in one study which makes judgment partially difficult. In Rezaei et al.’s study, the majority of studies were accomplished in Iran with similar basic characteristics; therefore, the results of this meta-analysis could not be generalized to the whole population (27).
The most notable strength of this umbrella meta-analysis was performing sub-group analyses, controlling publication bias and conducting comprehensive search of the literature. The majority of the included studies reported that more than half of the performed RCTs were methodologically qualified according to Cochrane, Jadad, and SIGN tools. Also, the current review was registered in PROSPERO or Cochrane library. There were few limitations that must be noted as well. First, the significant between-study heterogeneity reported among studies which was reduced in certain subgroups. Second, the various range of study populations with different characteristics. Third, the included studies were accomplished in certain geographic regions (mostly Asian regions) which may have enhanced the possibility of selection bias. Forth, omitting the effect of environmental factors such as sunlight or diet on serum 25(OH)D status.
The present umbrella meta-analysis confirms the potential benefits of vitamin D supplementation in reducing anthropometric indices such as BMI and BW, but not WC and fat mass. Moreover, vitamin D supplementation with a dosage of > 5,000 on overweight and obese subjects, NAFLD and diabetic patients, subjects older than 50 years and with intervention duration ≤ 16 weeks contribute to a more pronounced influence in lowering BMI. In this regards, vitamin D could be administered as a complementary treatment in the management of overweight and/or obesity.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
VM, FG, and MZ designed the research and wrote the manuscript. VM and FG conducted the systematic search. VM and FK screened the articles and extracted the data. VM and MZ analyzed and interpreted the data. FG and FK drew the tables. ZG and MZ had primary responsibility for the final content. All authors read and approved the final manuscript.
This research protocol was approved and supported by the Student Research Committee, Tabriz University of Medical Sciences (Registration code: 70065).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.980749/full#supplementary-material
3. Thomas-Valdés S, Tostes MDGV, Anunciação PC, da Silva BP, Sant’Ana HMP. Association between vitamin deficiency and metabolic disorders related to obesity. Crit Rev Food Sci Nutr. (2017) 57:3332–43.
4. Vraniæ L, Mikolaševiæ I, Miliæ S. Vitamin D deficiency: consequence or cause of obesity? Medicina (Kaunas). (2019) 55:541.
5. Aarts E, van Groningen L, Horst R, Telting D, van Sorge A, Janssen I, et al. Vitamin D absorption: consequences of gastric bypass surgery. Eur J Endocrinol. (2011) 164:827.
6. Autier P, Boniol M, Pizot C, Mullie PJ. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. (2014) 2:76–89. doi: 10.1016/S2213-8587(13)70165-7
7. Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. (2010) 59:242–8. doi: 10.2337/db09-1011
8. Antolín SH, Martínez MDCG, De Frutos VÁJ. Concentraciones deficientes de vitamina D en pacientes con obesidad mórbida. Estudio Caso Control. (2010) 57:256–61.
9. Beydoun MA, Boueiz A, Shroff M, Beydoun H, Wang Y, Zonderman A, et al. Associations among 25-hydroxyvitamin D, diet quality, and metabolic disturbance differ by adiposity in adults in the United States. J Clin Endocrinol Metab. (2010) 95:3814–27. doi: 10.1210/jc.2010-0410
10. Lu L, Yu Z, Pan A, Hu FB, Franco OH, Li H, et al. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. (2009) 32:1278–83.
11. Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, et al. Vitamin D3 in fat tissue. Endocrine. (2008) 33:90–4.
12. Walsh JS, Bowles S, Evans AL. Vitamin D in obesity. Curr Opin Endocrinol Diabetes Obes. (2017) 24:389–94.
13. McCarty M, Thomas CA. PTH excess may promote weight gain by impeding catecholamine-induced lipolysis-implications for the impact of calcium, vitamin D, and alcohol on body weight. Med Hypotheses. (2003) 61:535–42. doi: 10.1016/s0306-9877(03)00227-5
14. Gallagher JC, Yalamanchili V, Smith LM. The effect of vitamin D supplementation on serum 25OHD in thin and obese women. J Steroid Biochem Mol Biol. (2013) 136:195–200. doi: 10.1016/j.jsbmb.2012.12.003
15. Salehpour A, Hosseinpanah F, Shidfar F, Vafa M, Razaghi M, Dehghani S, et al. A 12-week double-blind randomized clinical trial of vitamin D3supplementation on body fat mass in healthy overweight and obese women. Nutr J. (2012) 11:78. doi: 10.1186/1475-2891-11-78
16. Saliba W, Barnett-Griness O, Rennert G. The relationship between obesity and the increase in serum 25 (OH) D levels in response to vitamin D supplementation. Osteoporos Int. (2013) 24:1447–54. doi: 10.1007/s00198-012-2129-0
17. Perna S. Is vitamin D supplementation useful for weight loss programs? A systematic review and meta-analysis of randomized controlled trials. Medicina. (2019) 55:368.
18. Rezaei S, Tabrizi R, Nowrouzi-Sohrabi P, Jalali M, Shabani-Borujeni M, Modaresi S, et al. The effects of vitamin D supplementation on anthropometric and biochemical indices in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Pharmacol. (2021) 12:732496. doi: 10.3389/fphar.2021.732496
19. Saha S, Saha S. Changes in anthropometric and blood 25-hydroxyvitamin D measurements in antenatal vitamin supplemented gestational diabetes mellitus patients: a systematic review and meta-analysis of randomized controlled trials. J Turk Ger Gynecol Assoc. (2021) 22:217. doi: 10.4274/jtgga.galenos.2021.2020.0197
20. Wang L, Wen X, Lv S, Tian S, Jiang Y, Yang X. Effects of vitamin D supplementation on metabolic parameters of women with polycystic ovary syndrome: a meta-analysis of randomized controlled trials. Gynecol Endocrinol. (2021) 37:446–55.
21. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008.
22. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6.
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60.
24. Duan L, Han L, Liu Q, Zhao Y, Wang L, Wang Y. Effects of vitamin D supplementation on general and central obesity: results from 20 randomized controlled trials involving apparently healthy populations. Ann Nutr Metab. (2020) 76:153–64.
25. Miao CY, Fang XJ, Chen Y, Zhang Q. Effect of vitamin D supplementation on polycystic ovary syndrome: a meta-analysis. Exp Therap Med. (2020) 19:2641–9.
26. Zou Y, Guo B, Yu S, Wang D, Qiu L, Jiang Y. Effect of vitamin D supplementation on glycose homeostasis and islet function in vitamin D deficient or insufficient diabetes and prediabetes: a systematic review and meta-analysis. J Clin Biochem Nutr. (2021) 69:229–37.
27. Tabrizi R, Moosazadeh M, Lankarani KB, Akbari M, Heydari ST, Kolahdooz F, et al. The effects of vitamin D supplementation on metabolic profiles and liver function in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr. (2017) 11:S975–82.
28. Mora N, Rieke K, Plitcha J, Segura A, Leehey D, DeShong K, et al. 25-Hydroxyvitamin D supplementation and BMI change: a meta-analysis of randomized controlled trials. J Obes Weight Loss Ther. (2013) 3:181.
29. Chandler PD, Wang L, Zhang X, Sesso HD, Moorthy MV, Obi O, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. (2015) 73:577–93.
30. Guevara J, Velilia A, Tiu S, Ti A, Tinio K, Torres A, et al. Efficacy of vitamin D alone or in combination with weight-reduction programs in weight loss among adults with above-normal BMI: a systematic review and meta-analysis. Eur Heart J. (2021) 42(Suppl. 1):ehab724.2603.
31. Pathak K, Soares M, Calton E, Zhao Y, Hallett J. Vitamin D supplementation and body weight status: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. (2014) 15:528–37.
32. Manousopoulou A, Al-Daghri NM, Garbis SD, Chrousos GP. Vitamin D and cardiovascular risk among adults with obesity: a systematic review and meta-analysis. Eur J Clin Investig. (2015) 45:1113–26. doi: 10.1111/eci.12510
33. Kron-Rodrigues MR, Rudge MVC, Lima SAM. Supplementation of vitamin D in the postdelivery period of women with previous gestational diabetes mellitus: systematic review and meta-analysis of randomized trials. Rev Brasil Ginecol Obstetr. (2021) 43:699–709.
34. Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. (2012) 336:1040–4.
35. Pourshahidi LK. Vitamin D and obesity: current perspectives and future directions. Proc Nutr Soc. (2015) 74:115–24.
36. Zakharova I, Klimov L, Kuryaninova V, Nikitina I, Malyavskaya S, Dolbnya S, et al. Vitamin D insufficiency in overweight and obese children and adolescents. Front Endocrinol. (2019) 10:103. doi: 10.3389/fendo.2019.00103
37. Zhu W, Cai D, Wang Y, Lin N, Hu Q, Qi Y, et al. Calcium plus vitamin D 3 supplementation facilitated fat loss in overweight and obese college students with very-low calcium consumption: a randomized controlled trial. Nutr J. (2013) 12:1–8.
38. Viguiliouk E, Kendall CW, Kahleová H, Rahelic D, Salas-Salvadó J, Choo VL, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr. (2019) 38:1133–45. doi: 10.1016/j.clnu.2018.05.032
Keywords: vitamin D, anthropometric indices, obesity, body mass index, umbrella meta-analysis
Citation: Musazadeh V, Zarezadeh M, Ghalichi F, Kalajahi FH and Ghoreishi Z (2022) Vitamin D supplementation positively affects anthropometric indices: Evidence obtained from an umbrella meta-analysis. Front. Nutr. 9:980749. doi: 10.3389/fnut.2022.980749
Received: 28 June 2022; Accepted: 16 August 2022;
Published: 07 September 2022.
Edited by:
Lilia Castillo-Martinez, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), MexicoReviewed by:
Saba Khaliq, University of Health Sciences, PakistanCopyright © 2022 Musazadeh, Zarezadeh, Ghalichi, Kalajahi and Ghoreishi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meysam Zarezadeh, emFyZXphZGVobUB0YnptZWQuYWMuaXI=; Zohreh Ghoreishi, em9ocmVoLmdob3JlaXNoeUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.