- 1Department of Medicine, School of Clinical Sciences, Monash University, Melbourne, VIC, Australia
- 2Gastroenterology and Liver Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3Cancer Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Department of Food Science and Technology, National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 5School of Agriculture and Food, Faculty of Veterinary and Agricultural Sciences, University of Melbourne, Parkville, VIC, Australia
- 6Guangdong Provincial Key Laboratory of Food, Nutrition and Health, Department of Nutrition, School of Public Health, Sun Yat-sen University, Guangzhou, China
- 7Research Center for Plants and Human Health, Institute of Urban Agriculture, National Agricultural Science and Technology Center, Chinese Academy of Agricultural Sciences, Chengdu, China
Objective: Nigella sativa (N. sativa) from the family Ranunculaceae has medicinal properties. Previous studies have reported promising findings showing that N. sativa may benefit cardiometabolic health; however, current evidence on its cardiometabolic effects on those with prediabetes and type 2 diabetes mellitus (T2DM) is still unclear. Hence, we conducted a systematic review and meta-analysis to assess the efficacy of N. sativa on cardiometabolic parameters in population with prediabetes and T2DM.
Methods: PubMed/Medline, ISI Web of Science, Scopus, and Cochrane library were systematically searched up to June 20, 2022. Meta-analyses using random-effects models were used.
Results: Eleven randomized controlled trials (RCTs) were included in the meta-analysis. N. sativa intervention resulted in significant changes in fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), c-reactive protein (CRP), and malondialdehyde (MDA), without overall changes in glucose levels after oral glucose tolerance test (OGTT), fasting insulin, homeostatic model assessment of insulin resistance (HOMA-IR), triglyceride, high-density lipoprotein cholesterol (HDL-C), and body mass index (BMI) when compared with the control group. In subgroup analyses, N. sativa supplementation enhanced serum levels of HDL-C in subjects with baseline HDL-C lower than 40 mg/dL. Furthermore, HOMA-IR and BMI values decreased in the N. sativa-supplemented group compared with the control group, when the length of follow-up was more than 8 weeks and the dose was more than 1 g/day for N. sativa supplementation, respectively.
Conclusion: Our findings indicate that N. sativa supplementation may effectively improve cardiometabolic profiles in individuals with prediabetes and T2DM.
Introduction
In accordance with the reports from the latest Global Burden of Disease (GBD 2016), 72.3% of the mortality rate is attributed to non-communicable diseases (NCDs) (1). Type 2 diabetes mellitus (T2DM) is among the most prevalent NCDs with the global estimates up to 463 million adults in 2019 which is predicted to increase to 642 million people by 2040 (2, 3). Moreover, there are 318 million individuals with prediabetes as a marker for T2DM development (4, 5). It is particularly noteworthy that its prolonged nature, accompanied by diabetes-associated complications, leading T2DM to one of the most costly disease categories (6). Insulin resistance and cardiovascular risk factors, including obesity, hyperlipidemia, and hypertension, are the hallmarks of T2DM pathogenesis (7–9). Given the epidemic of diabetes-related cardiovascular events and economic burden of T2DM, exploring cost-effective and promising therapeutic approaches should be of substantial importance (6–10).
Today, several medications are marketed to deal with the complications of diabetes. However, some standard drugs, such as biguanides, meglitinides, thiazolidinedione, etc. cause side effects such as nausea, bloating, stomach pain, dark urine, and liver problems (11). Therefore, complementary and alternative medicine (CAM) has provided the opportunity to manage diabetes along with lifestyle interventions and nutrition therapies and also make patients with T2DM to investigate a surrogate therapy to the chemical medications (12, 13). Nigella sativa (N. sativa) from the family Ranunculaceae (buttercup), commonly called black caraway, black cumin, nigella, or kalonji, is a medicinal food with a wide range of health benefits, such as a gastro-protective, hepato-protective, anti-diabetic, antihypertensive, bronchodilator, immunomodulatory, anti-inflammatory, anti-hyperlipidemic, antioxidant, and anticancer effects (14–17).
Numerous studies proposed N. sativa as an adjuvant therapy in diabetes control, since it revealed a significant reduction in glucose values following oral glucose tolerance test (OGTT), fasting plasma glucose (FPG) and hemoglobin A1c (HbA1c) levels, and insulin resistance, and also an escalation in serum fasting insulin concentrations (18–20), while recent research reported that N. sativa supplementation increased the levels of glycemic control components (21). Furthermore, it has been demonstrated that N. Sativa extract could increase the high-density lipoprotein cholesterol (HDL-C) concentration through rising the activity of plasma lecithin cholesterol acetyltransferase (LCAT), and it could also exert its antioxidant effects by enhancing the activities of antioxidant enzymes, including catalase and glutathione peroxidase, leading to its potential efficacy in amelioration of atherosclerosis (22, 23). However, there are still strong pieces of evidence suggesting null effects of N. sativa on biomarkers of inflammation (21, 24).
Considering the discrepancies across all the available evidence, the efficacy of N. sativa on cardiometabolic parameters in subjects with prediabetes and T2DM is still unclear. In order to fill this knowledge gap, we conducted an in-depth systematic review and meta-analysis based on high-quality randomized controlled trials (RCTs) to evaluate the effects of N. sativa consumption on cardiovascular risk factors in individuals with prediabetes and T2DM.
Methods
We conducted and reported the current systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) checklist (25).
Data sources and search strategy
Electronic databases (PubMed/MEDLINE, Scopus, Web of Science, and Cochrane library) were used to identify studies until June 20, 2022. A search strategy was implemented using the following keywords: (“Black cumin” OR “Nigella sativa” OR “black seed” OR “black caraway” OR “Roman coriander” OR “kalonji” OR “fennel flower” OR “pungent seeds”) AND (Intervention OR “controlled trial” OR randomized OR random OR randomly OR placebo OR “clinical trial” OR “randomized clinical trial” OR RCT OR trials OR “Cross-Over Studies” OR “Cross-Over” OR “Cross-Over Study” OR parallel OR “parallel study” OR “parallel trial”) AND (“diabetes” OR “type 2 diabetes mellitus” OR “T2DM” OR “type 2 diabetes” OR “T2D” OR “prediabetes”). Supplementary Table 1 lists search terms used across various databases. No date restrictions were applied, however, only English-language articles were eligible for inclusion. We screened the bibliographies of relevant studies and systematic reviews identified through the search strategy for additional studies. Data were requested from corresponding authors if the required data for meta-analysis were not reported.
Study selection and eligibility criteria
For further screening, endnote software was used to save records from electronic and manual searches. Researchers independently evaluated the titles and abstracts of all articles identified in the initial search (S.S. and K.N.). Discussion with a third reviewer (O.A.) resolved the disagreement regarding full-text eligibility. The eligibility of articles was determined by the Population, Intervention, Comparison, Outcomes, and Study Design (PICOS) framework (Table 1). Eligibility for the studies included: (1) Population: adult subjects (≥18 years old) and with physician's diagnosis of impaired glucose tolerance or prediabetes or T2DM; (2) Intervention: administration of Nigella sativa in different chemical forms including oil, capsule, and tablet; (3) Comparators: comparison with placebo, any pharmacological or non-pharmacological intervention(s), or usual care; (4) Outcomes: those which reported mean changes and their standard deviations (SDs) of BMI, glycemic control parameters (FPG, OGTT, HbA1c, fasting insulin, and HOMA-IR), lipid profile components (TG, TC, LDL-C, and HDL-C), c-reactive protein (CRP), and malondialdehyde (MDA) during the trial for both intervention and control groups or provided the information required to calculate those effect sizes; and (5) Study design: being an RCT in either parallel or cross-over design. Studies were excluded from this investigation if they: (1) included Nigella sativa as a part of a complex intervention; (2) lacked suitable control; (3) had no viable endpoint data in Nigella sativa or control groups; (4) were carried out on pregnant women, children, or animals, and (5) were performed <4 weeks in duration. In addition, gray literature, conference abstracts, protocols, and unpublished studies were excluded.
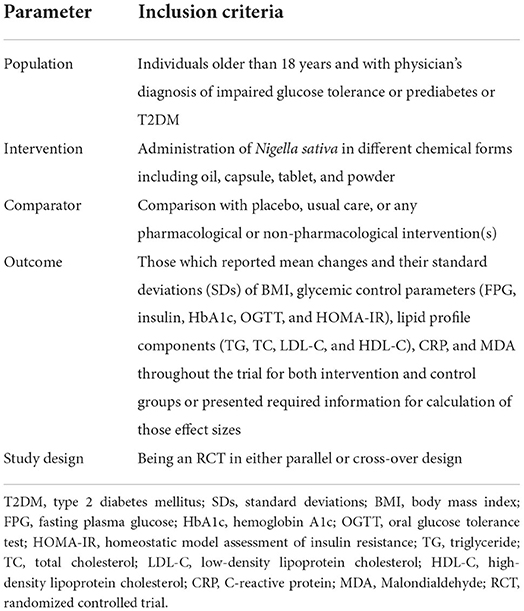
Table 1. Population, intervention, comparison, outcomes, and study design (PICOS) criteria for inclusion of studies.
Data extraction
Two independent investigators extracted the following information from each eligible clinical trial: study author; publication year; study location; study design; the number of participants; participants' ethnicity, age, comorbidities, body mass index; the type, dose, duration, and frequency of the intervention; and the study results [mean or median with SDs, standard errors (SEs), 95% CIs, or interquartile ranges (IQRs)] at study baseline, post-intervention, and/or changes between baseline and post-intervention. We converted data from each endpoint when it was reported in different units.
Quality assessment
In order to determine whether the included RCTs were likely to be biased, we used the Cochrane Risk of Bias Tool (26). The quality of each publication was evaluated by two independent authors using the following seven domains: (1) random sequence generation. (2) allocation concealment, (3) selective outcome reporting, (4) blinding of participants and personnel, (5) detection bias (blinding of data analyzers), (6) incomplete outcome data, and (7) other probable sources of biases. Each article was assigned either low-risk (L) or high-risk (H) bias label according to the Cochrane Handbook recommendations (Supplementary Table 2).
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) method was used to assess the quality of evidence for each outcome (27). Each outcome was graded by two independent reviewers (S.S. and K.N.) based on the risk of bias, inconsistency (heterogeneity), indirectness, and imprecision, according to the GRADE guidelines (27). Each outcome was rated as high, moderate, low, or very low (Table 4).
Data synthesis and meta-analysis
Mean changes and SDs for the outcomes (BMI, FPG, OGTT, HbA1c, fasting insulin, HOMA-IR, TG, TC, LDL-C, HDL-C, CRP, and MDA) in the intervention and placebo groups were used to calculate the effect sizes. For trials in which mean changes were not reported, we calculated the mean changes by considering all changes in each variable throughout the trial. We also converted 95% CIs, SEs, and IQRs to SDs applying appropriate formulas (28). Heterogeneity was determined by the I2 statistic and Cochrane's Q test. I2-value > 50% or P < 0.05 for the Q-test was characterized as significant between-study heterogeneity (29, 30). We used a random-effects model that considers the study variations to determine the overall effect sizes. To find probable sources of heterogeneity, subgroup analyses were performed according to the predefined criteria including gender (male/female), length of follow-up (8 ≥/8 < weeks), and baseline levels of outcome variables (abnormal/normal levels) and participants' baseline BMI level (normal, overweight or obese). To determine the non-linear potential effects of Nigella sativa dosage (g/day) on each indices, fractional polynomial modeling was executed. Sensitivity analysis was used to explore the extent to which inferences might depend on a particular study. The possibility of publication bias was evaluated by the formal Egger's test. The meta-analysis was carried out using Stata (Version 14.0, Stata Corp., College Station, TX). P-value <0.05 was considered as statistically significant.
Results
Study selection
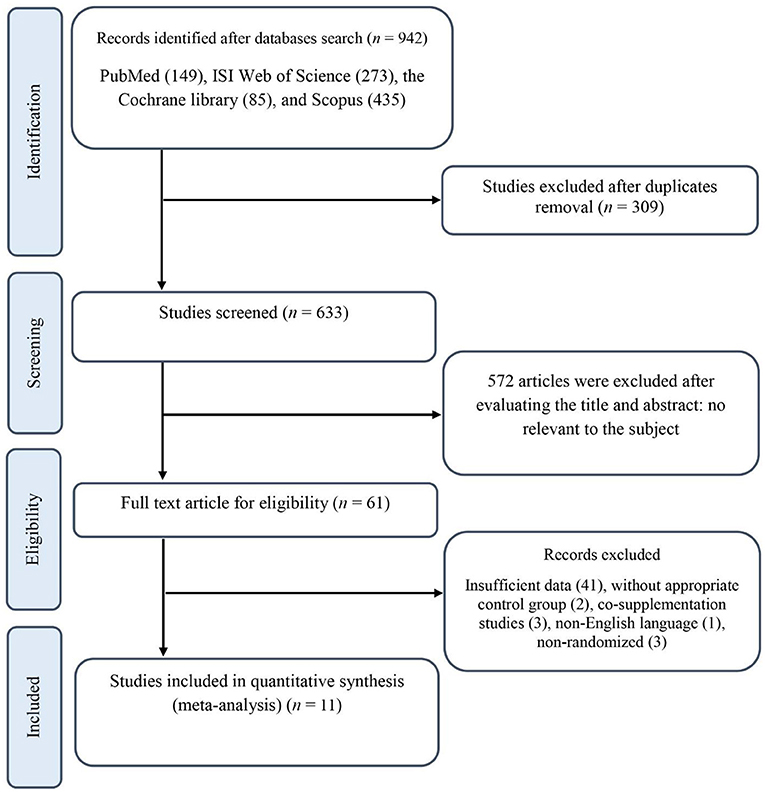
Our initial search resulted in 942 publications, 309 of which were excluded after duplicates removal. Titles and abstracts were examined for all the remaining 633 records, resulting in the exclusion of 572 publications. Next, the full texts for 61 RCTs were checked, and 50 records were excluded for the following reasons: three studies investigated the effects of N. sativa combining with other supplements where the independent effect of N. sativa could not be distinguished (31–33); three studies had non-randomized designs (34–36); one study reported its results in a non-English language (37); two studies did not have an appropriate control group (38, 39); and the 41 remaining publications provided insufficient data and/or did not meet the inclusion criteria. The final quantitative analysis included 11 trials with 666 participants. A flow diagram of the literature search process in detail is shown in Figure 1.
Study characteristics
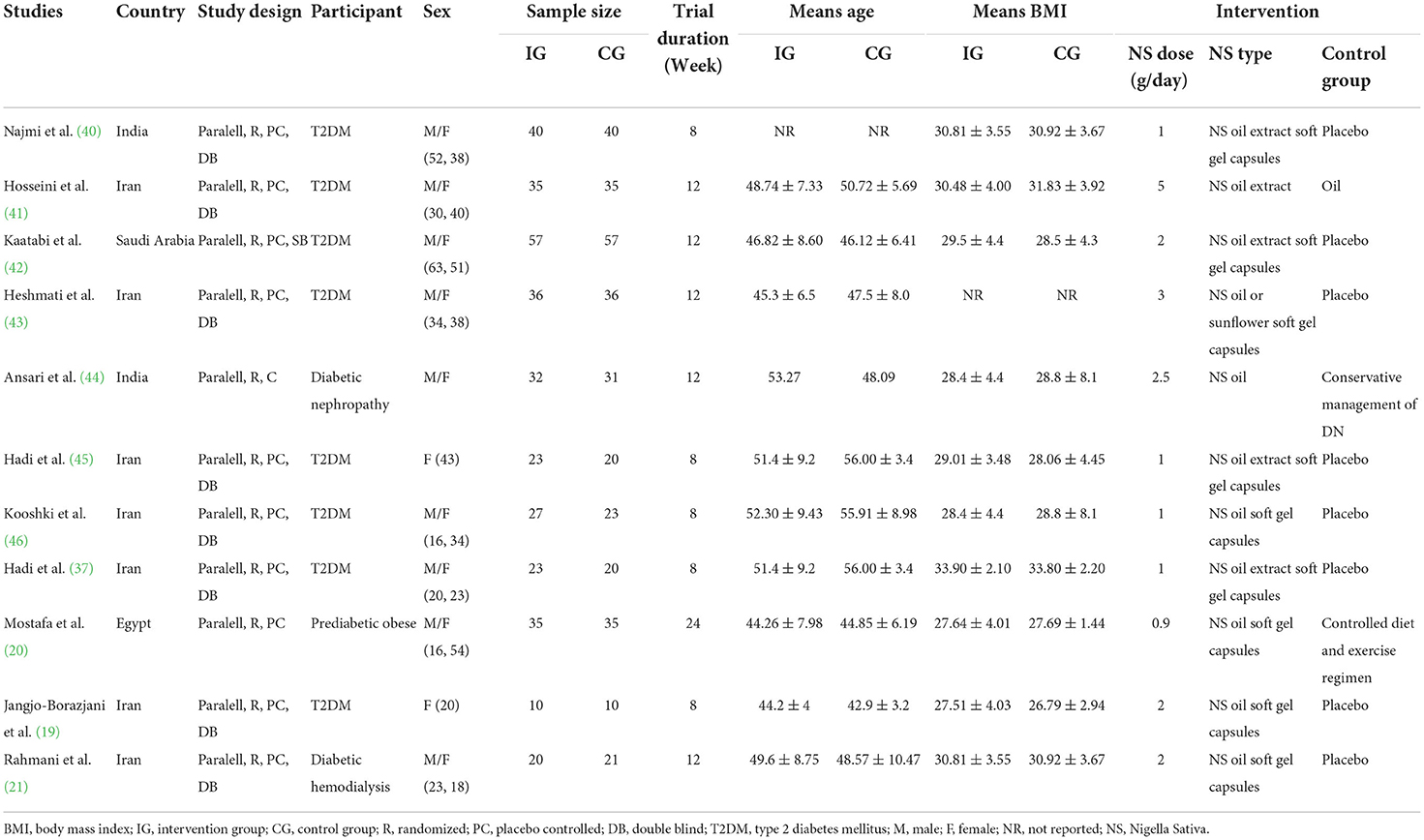
The general characteristics of the 11 included studies are outlined in Table 2. All the included trials had parallel designs and were published between 2012 and 2022. These studies were carried out in Iran (19, 21, 37, 41, 43, 45, 46), India (40, 44), Egypt (20), and Saudi Arabia (42). In total, 666 participants (338 interventions and 328 controls) were recruited with the age range between 42.9 ± 3.2 and 56 ± 3.4 years old and the BMI range between 26.79 ± 2.94 and 33.90 ± 2.10 kg/m2. Nine studies included both genders, whereas two studies were performed exclusively on females (19, 45). All the participants were individuals with T2DM except one study including those with prediabetes (20). The dose of N. sativa supplementation ranged from 0.9 to 5 g/day, with the length of follow-up ranging from 2 to 6 months throughout the studies. Of the 11 RCTs, 9 effect sizes administered oil form of the supplement (20, 21, 37, 40, 41, 43–46) and the remaining used extracts of N. sativa (19, 42) as an investigational product. All the 11 included trials had appropriate controlled designs, and the only difference between the two groups in each study was the N. sativa intervention.
Effects of N. sativa supplementation on cardiometabolic parameters
Anthropometric measurement
A total of five studies investigated the effects of N. sativa on BMI values (19, 20, 37, 40, 43). Pooled results from the random-effects model indicated that BMI values did not change significantly after N. sativa supplementation (WMD: −0.56 kg/m2; 95% CI: −1.95, 0.81; P = 0.422; Pheterogeneity < 0.001, I2 = 83.2%) (Supplementary Figure 1). Subgroup analysis demonstrated that although N. sativa had a significant lowering effect on BMI in studies with the supplemented dose of more than 1 g/day, this effect was irrespective of the health condition of the participants (Table 3).
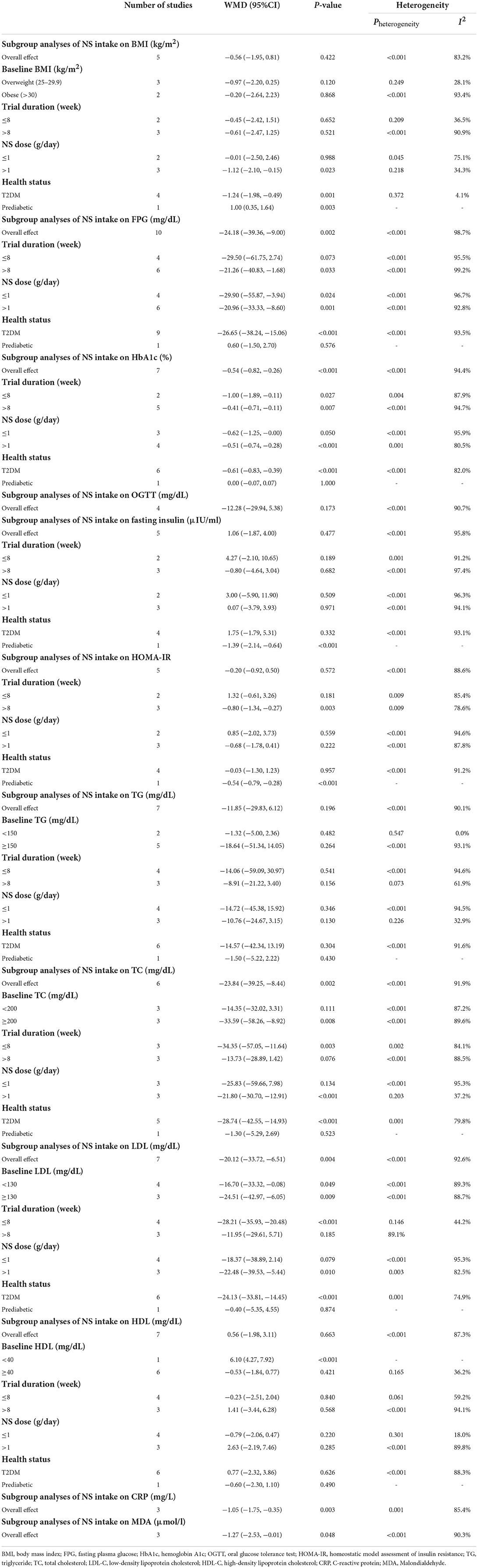
Table 3. Subgroup analyses of NS intake on body mass index, glycemic indices, lipid profile, CRP, and MDA.
Measures of glucose homeostasis
Glycemic control
Results from the random-effects model indicated that consumption of N. sativa resulted in a significant reduction in FPG (WMD: −24.18 mg/dL; 95% CI: −39.36, −9; P = 0.002; Pheterogeneity < 0.001, I2 = 98.7%) (19–21, 37, 40–44, 46), and HbA1c (WMD: −0.54%; 95% CI: −0.82, −0.26; P < 0.001; Pheterogeneity < 0.001, I2 = 94.4%) (20, 21, 37, 40–43) (Figure 2). However, there was no significant difference in OGTT (WMD: −12.28 mg/dL; 95% CI: −29.94, 5.38; P = 0.173; Pheterogeneity < 0.001, I2 = 90.7%) between groups (40–42, 44) (Supplementary Figure 1). The findings from the subgroup analyses showed that N. sativa reduced FPG in those with T2DM and individuals who were intervened with N. sativa for a longer time (more than 8 weeks) regardless of the dose of intervention. Also, a significant decrease in HbA1c was observed in subjects with T2DM irrespective of both the length of follow-up and the intervention dose of N. sativa. Subgroup analysis was not conducted on OGTT, as there were not enough studies reporting on this parameter (Table 3).
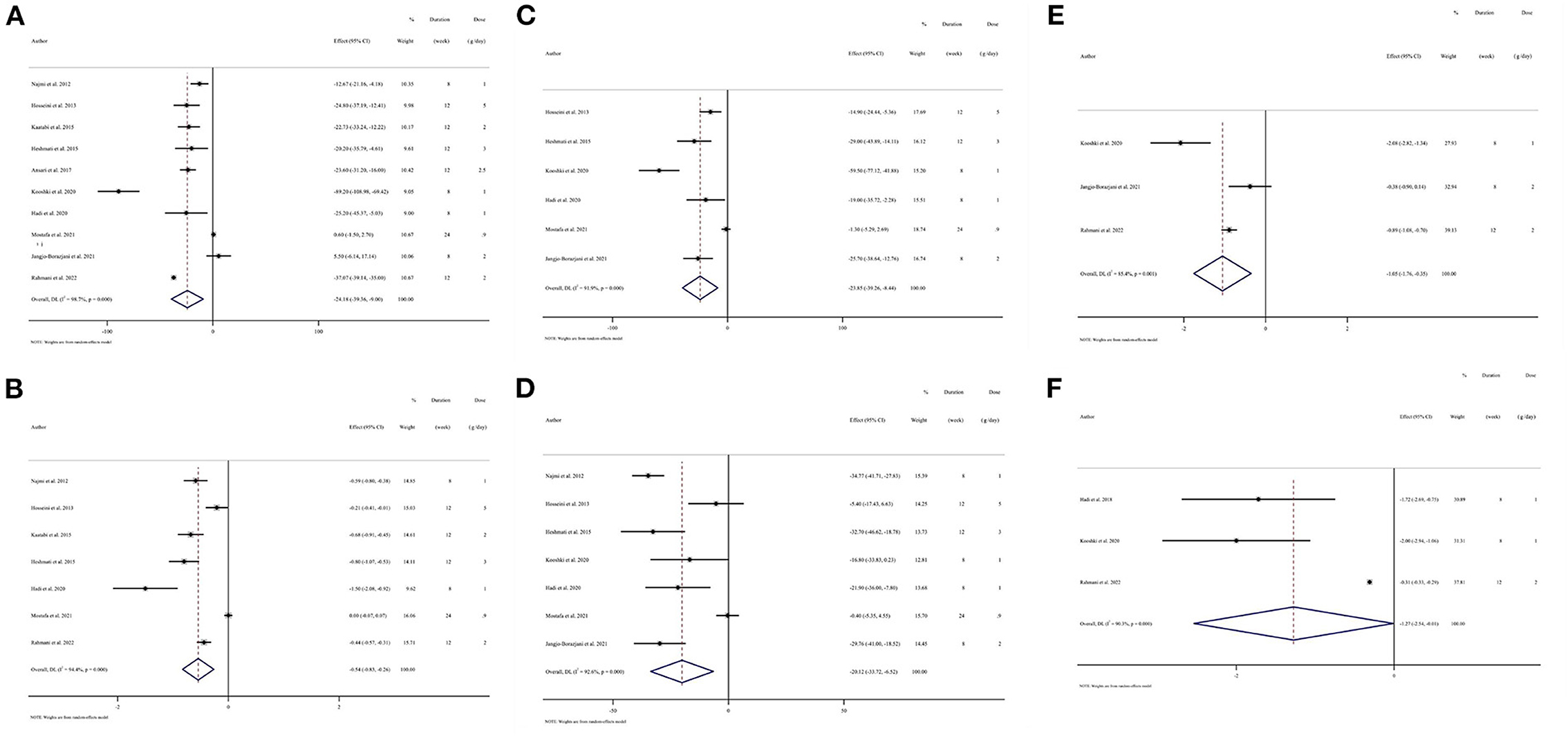
Figure 2. Forest plots of randomized controlled trials illustrating weighted mean difference (WMD) in biomarkers between the intervention and placebo groups for all eligible studies in overall analysis. (A) Fasting plasma glucose (FPG); (B) hemoglobin A1c (HbA1c); (C) total cholesterol (TC); (D) low-density lipoprotein cholesterol (LDL-C); (E) C-reactive protein (CRP); (F) Malondialdehyde (MDA).
Insulin resistance and secretion
The meta-analysis of five trials revealed no significant change in fasting insulin levels (WMD: 1.06 μIU/mL; 95% CI: −1.87, 4; P = 0.477; Pheterogeneity < 0.001, I2 = 95.8%) (19–21, 37, 43) and in HOMA-IR (WMD: −0.20; 95% CI: −0.92, 0.50; P = 0.572; Pheterogeneity < 0.001, I2 = 88.6%) (19, 20, 37, 42, 43) after N. sativa intervention (Supplementary Figure 1). On subgroup analyses, we observed that N. sativa supplementation significantly reduced fasting insulin concentration among subjects with prediabetes. Furthermore, studies with a longer intervention duration (more than 8 weeks) of N. sativa supplementation and those including individuals with prediabetes reported a significant reduction in serum HOMA-IR. Notably, no evidence of difference was shown in HOMA-IR based on the dose of intervention (Table 3).
Cardiovascular risk factors
Lipid profile
Pooled data from six studies indicated that TC levels were reduced significantly in those receiving N. sativa compared to controls (WMD: −23.84 mg/dL; 95% CI: −39.25, −8.44; P = 0.002; Pheterogeneity < 0.001, I2 = 91.9%) (19, 20, 37, 41, 43, 46) (Figure 2). Moreover, the effect of N. sativa supplementation on LDL-C was evaluated in seven clinical trials and the pooled mean difference revealed a reduction in LDL-C (WMD: −20.12 mg/dL; 95% CI: −33.72, −6.51; P = 0.004; Pheterogeneity < 0.001, I2 = 92.6%) (19, 20, 37, 40, 41, 43, 46) (Figure 2). However, combining seven effect sizes revealed that N. sativa supplementation resulted in a non-significant change in serum levels of TG (WMD: −11.85 mg/dL; 95% CI: −29.83, 6.12; P = 0.196; Pheterogeneity < 0.001, I2 = 90.1%) (19, 20, 37, 40, 41, 43, 46) (Supplementary Figure 1) and HDL-C (WMD: 0.56 mg/dL; 95% CI: −1.98, 3.11; P = 0.663; Pheterogeneity < 0.001, I2 = 87.3%) (19, 20, 37, 40, 41, 43, 46) (Supplementary Figure 1). The subgroup analyses indicated that N. sativa consumption might induce a more significant reduction of TC and LDL-C concentrations compared to the placebo group in those with T2DM, individuals with more than 1 g/day of N. sativa supplementation, and the duration of follow-up was 8 weeks or less. Additionally, reduced TC levels were significant in subjects with a baseline TC level of 200 mg/dL or more. However, LDL-C changes were not associated with the baseline levels of LDL-C. Results for TG remained non-significant across all subgroups, while after N. sativa consumption, HDL-C levels were significantly elevated in those with baseline serum levels of HDL-C lower than 40 mg/dL (Table 3).
Inflammatory and oxidative stress markers
Pooling three effect sizes, a significant reduction was seen in CRP (WMD: −1.05 mg/L; 95% CI: −1.75, −0.35; P = 0.003; Pheterogeneity = 0.001, I2 = 85.4%) and MDA (WMD: −1.27 μmol/L; 95% CI: −2.53, −0.01; P = 0.048; Pheterogeneity < 0.001, I2 = 90.3%) following N. sativa supplementation (Figure 2). However, it was impossible to perform subgroup analysis for CRP and MDA due to the limited number of included studies reporting these parameters (Table 3).
Sensitivity analysis
Sensitivity analysis for BMI, OGTT, and TG showed that the overall estimates were influenced by eliminating studies conducted by Mostafa et al. (20) (WMD: −1.24; 95% CI: −1.98, −0.49), Hadi et al. (37) (WMD: −0.57; 95% CI: −1.13, −0.01), and Najmi et al. (40) (WMD: −19.27; 95% CI: −37.44, −1.97), respectively. Moreover, the exclusion of the study conducted by Rahmani et al. (21) (WMD: −1.2; 95% CI: −2.87, 0.45) changed the overall effect size for CRP. Furthermore, the results of sensitivity analysis for MDA showed that removing the Hadi et al. (37) (WMD: −1.08; 95% CI: −2.73, 0.56) and Kooshki et al. (46) (WMD: −0.92; 95% CI: −2.29, 0.44) studies changed the overall effect sizes. Finally, sensitivity analysis for FPG, HbA1c, fasting insulin, HOMA-IR, LDL-C, and HDL-C did not indicate any sensitivity.
Publication bias
Funnel plot (Supplementary Figure 2), Egger's test (47), and Begg's test (48) (Supplementary Table 3) were applied to assess the possibility of significant publication bias. The findings from Egger's statistical test revealed that no evidence of publication bias was detected for BMI, FPG, fasting insulin, HOMA-IR, TG, LDL-C, HDL-C, CRP, or MDA. Visual inspection of funnel plot also affirmed these findings on BMI, FPG, fasting insulin, HOMA-IR, TG, LDL-C, and HDL-C. However, there were significant publication bias for the studies investigating the effectiveness of N. sativa on OGTT (P = 0.006), HbA1c (P = 0.005), and TC (P = 0.01).
GRADE assessment
The certainty of the evidence was evaluated by applying the GRADE protocol (Table 4). It was determined that the quality of evidence for FPG, LDL-C, CRP, and MDA was low due to very serious inconsistency (I2 > 75%). However, for BMI, OGTT, fasting insulin, HOMA-IR, TG, and HDL-C, the quality of evidence was downgraded to very low owing to very serious inconsistency (I2 > 75%) and imprecision (Wide CI). In addition, the quality of evidence for HbA1c and TC was also very low due to very serious inconsistency (I2 > 75%) and significant publication bias.
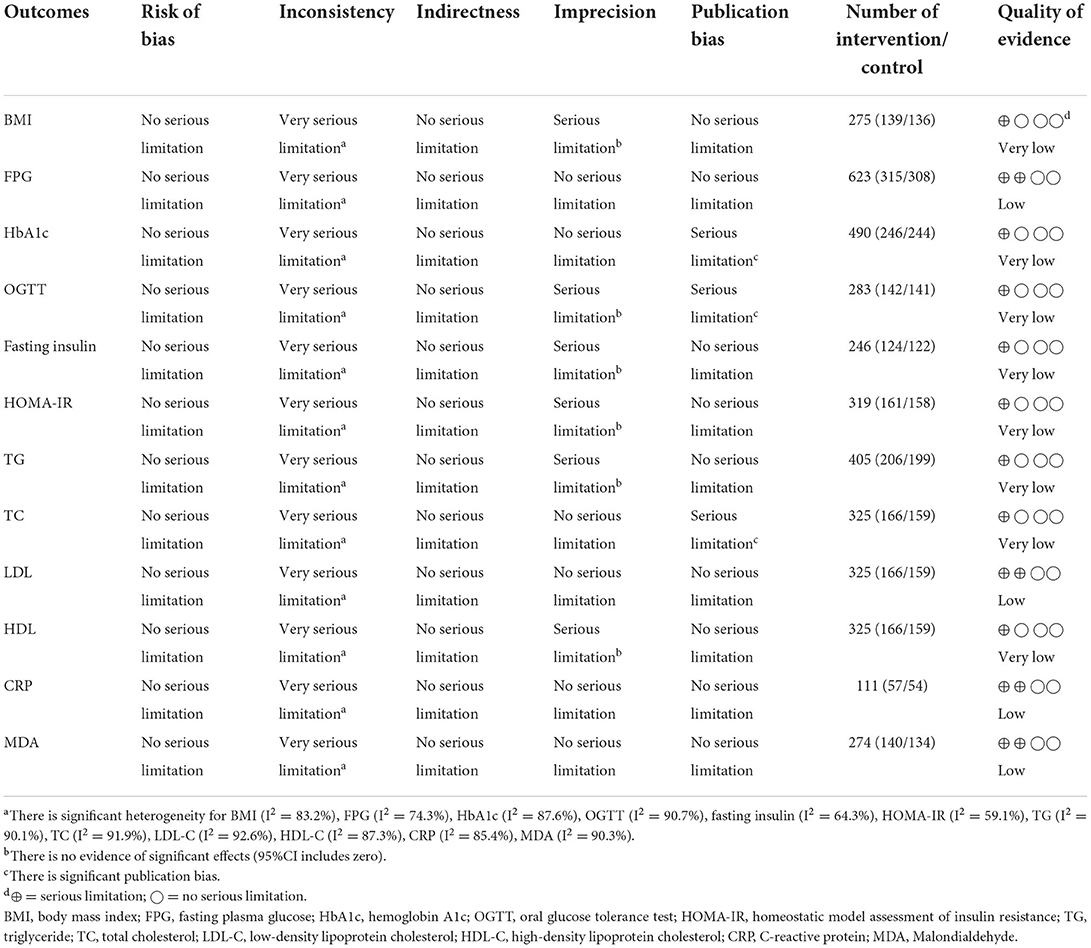
Table 4. GRADE profile of NS intake on lipid profile, glycemic indices, body mass index, CRP, and MDA.
Discussion
Many functional plants and their bioactive components have been demonstrated to possess anti-diabetic effects, such as tea (49), sweet tea (50), ginseng (29), citrus (51), pomegranate peel (52), and some medicinal plants (53). The present meta-analysis investigated the effectiveness of N. sativa consumption on cardiometabolic indicators among individuals with prediabetes and T2DM. The result indicated that N. sativa supplementation was associated with declines in glycemic control components (FPG and HbA1c), lipid profile parameters (TC and LDL-C), and biomarkers of inflammation and oxidative stress (CRP and MDA). Meanwhile, it was found that N. sativa supplementation enhanced serum levels of HDL-C in subjects with the baseline HDL-C level lower than 40 mg/dL. Furthermore, HOMA-IR and BMI values decreased in the N. sativa-supplemented group compared to the control group, when the length of follow-up was more than 8 weeks and in individuals with more than 1 g/day of N. sativa supplementation, respectively. However, there was no change in fasting serum concentration of insulin, TG, or glucose values following OGTT after N. sativa supplementation compared to the control group.
Nigella sativa contains various phytochemicals, including thymoquinone (TQ) which is the main ingredient of this plant. Moreover, thymol and dithymoquinone are other components of N. sativa (54). Current evidence proposes that N. sativa intake contributes to the modulation of cardiometabolic parameters in diabetes through the improvement of glucose homeostasis and lipid profiles, resulting in the prevention of atherosclerosis and cardiovascular events as diabetes complications (55). In 2017, a systematic review and meta-analysis of seven RCTs demonstrated that intake of N. sativa improves glycemic control parameters by reducing serum levels of FPG and HbA1c, and improving lipid profile through decreasing TC and LDL-C concentrations, without any significant alterations in TG or HDL-C (56). This is in line with the present study, which reveals that N. sativa favorably affects glycemic and lipid profiles. Moreover, our results revealed that N. sativa supplementation might modulate inflammation and oxidative stress by decreasing CRP and MDA levels.
Our findings are also supported by the previous systematic reviews and meta-analyses demonstrating the hypoglycemic efficacy of N. sativa among various non-diabetic populations (57–59). Hallajzadeh et al. (58) reported the beneficial effects of N. sativa on FPG and HbA1c without any change in serum insulin levels. Moreover, N. sativa consumption had a significant lowering effect on FPG, HbA1c, and OGTT values in a study conducted by Askari et al. (57). N. sativa could preserve pancreatic β-cell integrity, enhance the quantity of islets, and consequently make a contribution to the pancreatic β-cells proliferation and decreasing insulin resistance in diabetic experimental models and clinical studies (38, 43, 60, 61). Other probable anti-hyperglycemic mechanisms of N. sativa are as follows. Thymoquinone, a main bioactive compound in N. sativa, may reduce the gene expression of fructose-1,6-bisphosphatase and glucose-6-phosphatase, resulting in diminishing the hepatic gluconeogenesis (62, 63). N. sativa may also exert its hypoglycemic effects through activation of the AMP-activated protein kinase (AMPK) pathway, resulting in enhancement of pancreatic insulin secretion (22, 62). N. sativa may reduce glucose absorption by inhibiting the sodium glucose co-transporter (64). Similarly, it was reported that N. sativa could decrease intestinal glucose absorption and increase tissue glucose uptake by improving insulin release (42, 65). It is noteworthy that in the present study, we did not find any favorable effects of N. sativa supplementation on fasting serum levels of insulin and insulin resistance. This discrepancy might be partly due to the limited number of included studies and different forms of N. sativa supplementation. Therefore, further studies are warranted in order to verify the effectiveness of N. sativa on insulin secretion and/or insulin resistance.
Insulin resistance precedes the development of T2DM, leading to increased levels of free fatty acids (FFAs), and gives rise to TG formation, which in turn results in developing dyslipidemia among subjects with T2DM (66–68). Lipid-lowering property of N. sativa has been proposed to be attributed to its bioactive ingredients. Polyunsaturated fatty acids (PUFAs) (e.g., linoleic acid) in N. sativa were reported to inhibit the secretion of very-low density lipoprotein cholesterol (VLDL-C) and enhance fatty acid oxidation (69). Its high content of soluble fibers could contribute to reducing cholesterol absorption and elevating bile production (36). Furthermore, its TQ was reported to suppress gene expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-COAR), up-regulate insulin receptors in hepatic cells, and enhance the uptake of LDL-C (70). A recent meta-analysis among the general population revealed a significant lowering effect of N. sativa on TC and LDL-C concentrations, while no effect on TG or HDL-C levels (59). These findings are in total agreement with the present study. However, another systematic review and meta-analysis among healthy and unhealthy participants demonstrated its efficacy on serum levels of TG in addition to TC and LDL-C concentrations (58). This discrepancy might be caused by the different target populations and the number of the included studies.
Low-grade chronic inflammation and oxidative stress seem to be independent risk factors for the development of T2DM (70, 71). One of the well-established indicators of inflammation in individuals with T2DM is CRP (72). The current finding was affirmed by a recent systematic review and meta-analysis, which reported that elevated CRP concentrations were linked to the increased incidence of T2DM (73). Moreover, it is also notable that studies showed that MDA, as a toxic by-product of oxidation, increases T2DM pathogenesis (74). Emerging evidence supports the beneficial effects of N. sativa on indicators of inflammation through inhibition of the nuclear factor-kappa B (NF-κB) pathway and oxidative stress via increasing the expression of antioxidant enzymes, such as superoxide dismutase, in T2DM (75). However, previous systematic reviews and meta-analyses did not show this favorable effect (58, 76). These findings are in contrast to the present study, which demonstrated that N. sativa intervention could reduce CRP and MDA concentrations in individuals with prediabetes and T2DM. The possible explanation for this discrepancy might be due to the great data range and standard deviation among the included studies.
Obesity usually co-occurs with diabetes (77). Available experimental evidence proposed that N. sativa supplementation could exert its anti-obesity effect through appetite suppression (78, 79). A meta-analysis of 11 RCTs in the general population by Namazi et al. (80) revealed that N. sativa oil significantly decreased BMI values in adult humans. However, the present study failed to show its beneficial effects on BMI. This might be mainly due to the limited number of studies included in this meta-analysis reporting on the effectiveness of N. sativa on BMI values and the differences in the health status of the participants.
Strengths and limitations
This is the first systematic review and meta-analysis investigating the effectiveness of N. sativa supplementation on a wide range of cardiometabolic indicators in individuals with prediabetes and T2DM. The previous study included 505 participants (7 papers) (56), whereas our systematic review includes 666 participants (11 papers). In addition, subgroup analyses and grading the overall certainty of evidence across the studies according to the GRADE guidelines were conducted. Despite all the aforementioned strengths, the present study has some limitations that should be taken into consideration. First, we were unable to stratify the efficacy of N. sativa on each endpoint based on different forms of N. sativa (extract vs. oil) due to the limited number of included studies. Second, the RCTs had relatively small sample sizes, with only one study including more than 114 participants. Third, all the studies were carried out in Asia, except one of them performed in Africa, which may lead to generalizability limitations. Fourth, in our analysis, statistical heterogeneity is evident. Poor methodological quality and/or differences in treatment regimens (doses/durations) or the form of N. sativa used may contribute to these differences. Finally, we did not consider the effects of some confounding factors, such as smoking status and diabetes duration, owing to the insufficient reporting of them.
Conclusions
The results of the present study have indicated that N. sativa may improve cardiometabolic parameters by ameliorating glucose homeostasis and alleviating dyslipidemia, inflammation, and oxidative stress in individuals with prediabetes and T2DM. Overall, our results suggest that N. sativa supplementation can be a potential adjuvant therapy in the management of prediabetes and T2DM. In the future, more well-designed clinical studies are guaranteed to shed light on these findings.
Author contributions
SS and KN contributed to the literature search and data extraction. SS, KN, and OA contributed to data analysis. SS, KN, and KA drafted the manuscript and which was critically revised for important intellectual content by all authors. SS, KN, PZ, and H-BL contributed to the methodology, statistical analysis, and manuscript drafting. R-YG supervised the study, is a guarantor and had full access to all the data and takes responsibility for the integrity of the data, and the accuracy of the data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Agricultural Science and Technology Innovation Program (ASTIP-IUA-2022002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.977756/full#supplementary-material
References
1. Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1151–210. doi: 10.1016/S0140-6736(17)32152-9
2. Afroz A, Alramadan MJ, Hossain MN, Romero L, Alam K, Magliano DJ, et al. Cost-of-illness of type 2 diabetes mellitus in low and lower-middle income countries: a systematic review. BMC Health Serv Res. (2018) 18:972. doi: 10.1186/s12913-018-3772-8
3. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
4. Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. (2017) 128:40–50. doi: 10.1016/j.diabres.2017.03.024
5. Cho NH, Shaw J, Karuranga S, Huang Y, da Rocha Fernandes J, Ohlrogge A, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for (2045). Diabetes Res Clin Pract. (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
6. Ettaro L, Songer TJ, Zhang P, Engelgau MM. Cost-of-illness studies in diabetes mellitus. Pharmacoeconomics. (2004) 22:149–64. doi: 10.2165/00019053-200422030-00002
7. Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Cardiovasc. Diabetol. Clin. Metab. Inflam. Facets. (2008) 45:1–16. doi: 10.1159/000115118
8. Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. (2013) 19:5695–703. doi: 10.2174/1381612811319320005
9. Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. (2007) 30:263–9. doi: 10.2337/dc06-1612
10. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. (2015) 6:1246–58. doi: 10.4239/wjd.v6.i13.1246
11. Hossain MA, Pervin R. Current antidiabetic drugs: review of their efficacy and safety. Nutr Therap Interv Diabetes Metab Syndrome. (2018) 455–73. doi: 10.1016/B978-0-12-812019-4.00034-9
12. Coppell KJ, Kataoka M, Williams SM, Chisholm AW, Vorgers SM, Mann JI. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment–Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. (2010) 341:c3337. doi: 10.1136/bmj.c3337
13. Xie W, Zhao Y, Zhang Y. Traditional chinese medicines in treatment of patients with type 2 diabetes mellitus. Evid Based Complement Alternat Med. (2011) 2011:726723. doi: 10.1155/2011/726723
14. Al-Naqeep G, Al-Zubairi AS, Ismail M, Amom ZH, Esa NM. Antiatherogenic potential of Nigella sativa seeds and oil in diet-induced hypercholesterolemia in rabbits. Evid Based Complement Alternat Med. (2011) 2011:213628. doi: 10.1093/ecam/neq071
15. Ermumcu MSK, Sanlier N. Black cumin (Nigella sativa) and its active component of thymoquinone: effects on health. Food Health. (2017) 3:170–83. doi: 10.3153/JFHS17020
16. Tavakkoli A, Mahdian V, Razavi BM, Hosseinzadeh H. Review on clinical trials of black seed (Nigella sativa) and its active constituent, thymoquinone. J Pharmacopunct. (2017) 20:179–93. doi: 10.3831/KPI.2017.20.021
17. Darand M, Darabi Z, Yari Z, Saadati S, Hedayati M, Khoncheh A, et al. Nigella sativa and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: results from a randomized, double-blind, placebo-controlled, clinical trial. Complement Ther Med. (2019) 44:204–9. doi: 10.1016/j.ctim.2019.04.014
18. Hamdan A, Haji Idrus R, Mokhtar MH. Effects of Nigella sativa on type-2 diabetes mellitus: a systematic review. Int J Environ Res Public Health. (2019) 16:911. doi: 10.3390/ijerph16244911
19. Jangjo-Borazjani S, Dastgheib M, Kiyamarsi E, Jamshidi R, Rahmati-Ahmadabad S, Helalizadeh M, et al. Effects of resistance training and Nigella sativa on type 2 diabetes: implications for metabolic markers, low-grade inflammation and liver enzyme production. Arch Physiol Biochem. (2021) 21:1–9. doi: 10.1080/13813455.2021.1886117
20. Mostafa TM, Hegazy SK, Elnaidany SS, Shehabeldin WA, Sawan ES. Nigella sativa as a promising intervention for metabolic and inflammatory disorders in obese prediabetic subjects: a comparative study of Nigella sativa versus both lifestyle modification and metformin. J Diabetes Complications. (2021) 35:107947. doi: 10.1016/j.jdiacomp.2021.107947
21. Rahmani A, Niknafs B, Naseri M, Nouri M, Tarighat-Esfanjani A. Effect of Nigella sativa oil on oxidative stress, inflammatory, and glycemic control indices in diabetic hemodialysis patients: a randomized double-blind, controlled trial. Evid Based Complement Alternat Med. (2022) 2022:2753294. doi: 10.1155/2022/2753294
22. Abdelrazek HMA, Kilany OE, Muhammad MAA, Tag HM, Abdelazim AM. Black seed thymoquinone improved insulin secretion, hepatic glycogen storage, and oxidative stress in streptozotocin-induced diabetic male wistar rats. Oxid Med Cell Longev. (2018) 2018:8104165. doi: 10.1155/2018/8104165
23. Saxena S, Pandey PT, Kumar V, Saxena JK. Antidyslipidemic and anti-oxidant activities of Nigella sativa seeds extract in hyperlipidemic rats. Int J Pharmaceut Sci Res. (2020) 25:4321–8. doi: 10.13040/IJPSR.0975-8232.11(9).4321-28
24. Hadi V, Kheirouri S, Alizadeh M, Khabbazi A, Hosseini H. Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled clinical trial. Avicenna J Phytomed. (2016) 6:34–43.
25. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
26. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
27. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. (2004) 328:1490. doi: 10.1136/bmj.328.7454.1490
28. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
29. Naseri K, Saadati S, Sadeghi A, Asbaghi O, Ghaemi F, Zafarani F, et al. The efficacy of ginseng (panax) on human prediabetes and type 2 diabetes mellitus: a systematic review and meta-analysis. Nutrients. (2022) 14:2401. doi: 10.3390/nu14122401
30. Naseri K, Saadati S, Yari Z, Asbaghi O, Hezaveh ZS, Mafi D, et al. Beneficial effects of probiotic and synbiotic supplementation on some cardiovascular risk factors among individuals with prediabetes and type 2 diabetes mellitus: a GRADE-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials. Pharmacol Res. (2022) 182:106288. doi: 10.1016/j.phrs.2022.106288
31. Memon AR, Shah SS, Memon AR, Naqvi SHR. Effect of combination of Nigella sativa and Trigonella foenum-graecum with glibenclamide on serum triglyceride, HDL and creatinine levels in type-2 diabetes mellitus patients. Pak J Pharmacol. (2012) 29:1–6. Available online at: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1039.5365&rep=rep1&type=pdf
32. Rao AS, Hegde S, Pacioretty LM, DeBenedetto J, Babish JG. Nigella sativa and trigonella foenum-graecum supplemented chapatis safely improve HbA1c, body weight, waist circumference, blood lipids, and fatty liver in overweight and diabetic subjects: a twelve-week safety and efficacy study. J Med Food. (2020) 23:905–19. doi: 10.1089/jmf.2020.0075
33. Shari FH, Ramadhan HH, Mohammed RN, Al Bahadily DC. Hypolipidemic and antioxidant effects of fenugreek-Nigella sativa combination on diabetic patients in Iraq. Syst Rev Pharm. (2020) 11:911–15.
34. Badar A, Kaatabi H, Bamosa A, Al-Elq A, Abou-Hozaifa B, Lebda F, et al. Effect of Nigella sativa supplementation over a one-year period on lipid levels, blood pressure and heart rate in type-2 diabetic patients receiving oral hypoglycemic agents: nonrandomized clinical trial. Ann Saudi Med. (2017) 37:56–63. doi: 10.5144/0256-4947.2017.56
35. Bamosa A, Kaatabi H, Badar A, Al-Khadra A, Al Elq A, Abou-Hozaifa B, et al. Nigella sativa: a potential natural protective agent against cardiac dysfunction in patients with type 2 diabetes mellitus. J Family Community Med. (2015) 22:88–95. doi: 10.4103/2230-8229.155380
36. Bilal A, Masud T, Uppal AM, Naveed AK. Effects of Nigella sativa oil on some blood parameters in type 2 diabetes mellitus patients. Asian J Chem. (2009) 21:5373. Available online at: https://www.cabdirect.org/cabdirect/abstract/20093257730
37. Hadi S, Daryabeygi-Khotbehsara R, Mirmiran P, McVicar J, Hadi V, Soleimani D, et al. Effect of Nigella sativa oil extract on cardiometabolic risk factors in type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. (2021) 35:3747–55. doi: 10.1002/ptr.6990
38. Bamosa AO, Kaatabi H, Lebdaa FM, Elq AM, Al-Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol. (2010) 54:344–54.
39. Kaatabi H, Bamosa AO, Lebda FM, Al Elq AH, Al-Sultan AI. Favorable impact of Nigella sativa seeds on lipid profile in type 2 diabetic patients. J Family Community Med. (2012) 19:155–61. doi: 10.4103/2230-8229.102311
40. Najmi A, Nasiruddin M, Khan R, Haque SF. Therapeutic effect of Nigella sativa in patients of poor glycemic control. Asian J Pharm Clin Res. (2012) 5:224–8. Available online at: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-00908800/full
41. Hosseini M, Mirkarimi S, Amini M, Mohtashami R, Kianbakht S, Fallah HH. Effects of Nigella sativa L. Seed Oil in Type II Diabetic Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. (2013).
42. Kaatabi H, Bamosa AO, Badar A, Al-Elq A, Abou-Hozaifa B, Lebda F, et al. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trial. PLoS ONE. (2015) 10, e0113486. doi: 10.1371/journal.pone.0113486
43. Heshmati J, Namazi N, Memarzadeh M-R, Taghizadeh M, Kolahdooz F. Nigella sativa oil affects glucose metabolism and lipid concentrations in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Food Res Int. (2015) 70:87–93. doi: 10.1016/j.foodres.2015.01.030
44. Ansari ZM, Nasiruddin M, Khan RA, Haque SF. Protective role of Nigella sativa in diabetic nephropathy: a randomized clinical trial. Saudi J Kidney Dis Transpl. (2017) 28:9–14. doi: 10.4103/1319-2442.198093
45. Hadi S, Mirmiran P, Daryabeygi-Khotbesara R, Hadi V. Effect of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress among people with type 2 diabetes mellitus: a randomized, double-blind, placebo controlled trial. Prog Nutr. (2018) 20:127–33. Available online at: http://eprints.mums.ac.ir/id/eprint/26375
46. Kooshki A, Tofighiyan T, Rastgoo N, Rakhshani MH, Miri M. Effect of Nigella sativa oil supplement on risk factors for cardiovascular diseases in patients with type 2 diabetes mellitus. Phytother Res. (2020) 34:2706–11. doi: 10.1002/ptr.6707
47. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
48. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
49. Meng J-M, Cao S-Y, Wei X-L, Gan R-Y, Wang Y-F, Cai S-X, et al. Effects and mechanisms of tea for the prevention and management of diabetes mellitus and diabetic complications: an updated review. Antioxidants. (2019) 8:170. doi: 10.3390/antiox8060170
50. Shang A, Liu H-Y, Luo M, Xia Y, Yang X, Li H-Y, et al. Sweet tea (Lithocarpus polystachyus rehd) as a new natural source of bioactive dihydrochalcones with multiple health benefits. Crit Rev Food Sci Nutr. (2022) 62:917–34. doi: 10.1080/10408398.2020.1830363
51. Gandhi GR, Vasconcelos ABS, Wu D-T, Li H-B, Antony PJ, et al. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: a systematic review of in vitro and in vivo studies. Nutrients. (2020) 12:2907. doi: 10.3390/nu12102907
52. Liu Y, Kong KW, Wu D-T, Liu H-Y, Li H-B, Zhang J-R, et al. Pomegranate peel-derived punicalagin: ultrasonic-assisted extraction, purification, and its α-glucosidase inhibitory mechanism. Food Chem. (2022) 374:131635. doi: 10.1016/j.foodchem.2021.131635
53. Li B-Y, Xu X-Y, Gan R-Y, Sun Q-C, Meng J-M, Shang A, et al. Targeting gut microbiota for the prevention and management of diabetes mellitus by dietary natural products. Foods. (2019) 8:440. doi: 10.3390/foods8100440
54. Alasalvar C, Chang SK, Bolling B, Oh WY, Shahidi F. Specialty seeds: nutrients, bioactives, bioavailability, and health benefits: a comprehensive review. Compr Rev Food Sci Food Safety. (2021) 20:2382–427. doi: 10.1111/1541-4337.12730
55. Heshmati J, Namazi N. Effects of black seed (Nigella sativa) on metabolic parameters in diabetes mellitus: a systematic review. Complement Ther Med. (2015) 23:275–82. doi: 10.1016/j.ctim.2015.01.013
56. Daryabeygi-Khotbehsara R, Golzarand M, Ghaffari MP, Djafarian K. Nigella sativa improves glucose homeostasis and serum lipids in type 2 diabetes: a systematic review and meta-analysis. Complement Ther Med. (2017) 35:6–13. doi: 10.1016/j.ctim.2017.08.016
57. Askari G, Rouhani MH, Ghaedi E, Ghavami A, Nouri M, Mohammadi H. Effect of Nigella sativa (black seed) supplementation on glycemic control: a systematic review and meta-analysis of clinical trials. Phytother Res. (2019) 33:1341–52. doi: 10.1002/ptr.6337
58. Hallajzadeh J, Milajerdi A, Mobini M, Amirani E, Azizi S, Nikkhah E, et al. Effects of Nigella sativa on glycemic control, lipid profiles, and biomarkers of inflammatory and oxidative stress: a systematic review and meta-analysis of randomized controlled clinical trials. Phytother Res. (2020) 34:2586–608. doi: 10.1002/ptr.6708
59. Mohtashami A, Entezari MH. Effects of Nigella sativa supplementation on blood parameters and anthropometric indices in adults: a systematic review on clinical trials. J Res Med Sci. (2016) 21:3. doi: 10.4103/1735-1995.175154
60. Kanter M. Effects of Nigella sativa and its major constituent, thymoquinone on sciatic nerves in experimental diabetic neuropathy. Neurochem Res. (2008) 33:87–96. doi: 10.1007/s11064-007-9419-5
61. Benhaddou-Andaloussi A, Martineau LC, Spoor D, Vuong T, Leduc C, Joly E, et al. Antidiabetic activity of Nigella sativa. seed extract in cultured pancreatic βancreat skeletal muscle cells, and adipocytes. Pharm Biol. (2008) 46:96–104. doi: 10.1080/13880200701734810
62. Benhaddou-Andaloussi A, Martineau L, Vuong T, Meddah B, Madiraju P, Settaf A, et al. The in vivo antidiabetic activity of Nigella sativa is mediated through activation of the AMPK pathway and increased muscle Glut4 content. Evid Based Complement Alternat Med. (2011) 2011:538671. doi: 10.1155/2011/538671
63. Pari L, Sankaranarayanan C. Beneficial effects of thymoquinone on hepatic key enzymes in streptozotocin–nicotinamide induced diabetic rats. Life Sci. (2009) 85:830–4. doi: 10.1016/j.lfs.2009.10.021
64. Meddah B, Ducroc R, El Abbes Faouzi M, Eto B, Mahraoui L, Benhaddou-Andaloussi A, et al. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol. (2009) 121:419–24. doi: 10.1016/j.jep.2008.10.040
65. Hannan JMA, Ansari P, Haque A, Sanju A, Huzaifa A, Rahman A, et al. Nigella sativa stimulates insulin secretion from isolated rat islets and inhibits the digestion and absorption of (CH(2)O)(n) in the gut. Biosci Rep. (2019) 39:723. doi: 10.1042/BSR20190723
66. Ma C, Yu H, Xiao Y, Wang H. Momordica charantia extracts ameliorate insulin resistance by regulating the expression of SOCS-3 and JNK in type 2 diabetes mellitus rats. Pharm Biol. (2017) 55:2170–7. doi: 10.1080/13880209.2017.1396350
67. O'Brien T, Nguyen TT, Zimmerman BR. Hyperlipidemia and diabetes mellitus. Mayo Clinic Proc. (1998) 73:969–76. doi: 10.4065/73.10.969
68. Oza MJ, Kulkarni YA. Biochanin A improves insulin sensitivity and controls hyperglycemia in type 2 diabetes. Biomed Pharmacother. (2018) 107:1119–27. doi: 10.1016/j.biopha.2018.08.073
69. Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. (2012) 142:592s−9s. doi: 10.3945/jn.111.155259
70. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. (2014) 105:141–50. doi: 10.1016/j.diabres.2014.04.006
71. Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. (2015) 5:194–222. doi: 10.3390/biom5010194
72. Kengne AP, Batty GD, Hamer M, Stamatakis E, Czernichow S. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status: pooled analyses of 25,979 participants from four UK prospective cohort studies. Diabetes Care. (2012) 35:396–403. doi: 10.2337/dc11-1588
73. Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. (2013) 36:166–75. doi: 10.2337/dc12-0702
74. Salem M, Kholoussi S, Kholoussi N, Fawzy R. Malondialdehyde and trace element levels in patients with type 2 diabetes mellitus. Arch Hellenic Med. (2011) 28:83–8.
75. Hadi V, Pahlavani N, Malekahmadi M, Nattagh-Eshtivani E, Navashenaq JG, Hadi S, et al. Nigella sativa in controlling Type 2 diabetes, cardiovascular, and rheumatoid arthritis diseases: molecular aspects. J Res Med Sci. (2021) 26:20. doi: 10.4103/jrms.JRMS_236_20
76. Ardiana M, Pikir BS, Santoso A, Hermawan HO, Al-Farabi MJ. Effect of Nigella sativa supplementation on oxidative stress and antioxidant parameters: a meta-analysis of randomized controlled trials. Sci World J. (2020) 2020:2390706. doi: 10.1155/2020/2390706
77. Golay A, Ybarra J. Link between obesity and type 2 diabetes. Best Pract Res Clin Endocrinol Metab. (2005) 19:649–63. doi: 10.1016/j.beem.2005.07.010
78. Houcher Z, Boudiaf K, Benboubetra M, Houcher B. Effects of methanolic extract and commercial oil of Nigella sativa L. on blood glucose and antioxidant capacity in alloxan-induced diabetic rats. Pteridines. (2007) 18:8–18. doi: 10.1515/pteridines.2007.18.1.8
79. Tasawar Z, Siraj Z, Ahmad N, Lashari MH. The effects of Nigella sativa (Kalonji) on lipid profile in patients with stable coronary artery disease in Multan, Pakistan. Pak J Nutr. (2011) 219:173–81. doi: 10.3923/pjn.2011.162.167
Keywords: diabetes mellitus, prediabetes, meta-analysis, Nigella sativa, cardiometabolic, lipid profile, glycemic homeostasis
Citation: Saadati S, Naseri K, Asbaghi O, Abhari K, Zhang P, Li H-B and Gan R-Y (2022) Nigella sativa supplementation improves cardiometabolic indicators in population with prediabetes and type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 9:977756. doi: 10.3389/fnut.2022.977756
Received: 24 June 2022; Accepted: 25 July 2022;
Published: 11 August 2022.
Edited by:
Lijun Sun, Northwest A&F University, ChinaReviewed by:
Sui Kiat Chang, Universiti Tunku Abdul Rahman, MalaysiaGuoyi Tang, The University of Hong Kong, Hong Kong SAR, China
Naina Mohamed Pakkir Maideen, Dubai Health Authority, United Arab Emirates
Copyright © 2022 Saadati, Naseri, Asbaghi, Abhari, Zhang, Li and Gan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ren-You Gan, Z2FucmVueW91QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Saeede Saadati
Saeede Saadati Kaveh Naseri
Kaveh Naseri Omid Asbaghi
Omid Asbaghi Khadijeh Abhari4
Khadijeh Abhari4 Pangzhen Zhang
Pangzhen Zhang Hua-Bin Li
Hua-Bin Li Ren-You Gan
Ren-You Gan