
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 22 July 2022
Sec. Nutritional Epidemiology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.876909
This article is part of the Research Topic Dietary Intake, Eating Behavior and Health Outcomes View all 36 articles
Background: The benefits of fish fatty acid intake for non-alcoholic fatty liver disease (NAFLD) are rarely reported, although a previous study assessed the relationship between oily fish consumption and the prevalence of NAFLD.
Aims: We investigated whether oily fish and fish-based monounsaturated fatty acids, polyunsaturated fatty acids, and omega-3 fatty acids affect the development of NAFLD in South Korean adults.
Methods: In this large-scale cohort study, 44,139 participants of the Health Examinees study were selected for analysis after 5 years of follow-up. NAFLD is diagnosed with a non-invasive index, the fatty liver index. Using multivariable Cox proportional hazards models, adjusted for age, body mass index, total energy intake, education, physical activity, smoking status, and drinking (alcohol) status, we calculated the hazard ratios and 95% confidence intervals.
Results: For men, NAFLD had no statistically significant associations with quartiles of total oily fish or its fatty acid intake. However, among women, an inverse association was observed (all p for trend <0.05). Regarding the standard deviation (SD) increment of total oily fish or its fatty acid intake by one, all fatty acids from oily fish showed inverse associations for NAFLD in both men and women. After stratified analyses, we found that drinking status and menopause status were independent risk factors for NAFLD. Oily fish or its fatty acid intake has the same benefit pattern on metabolic dysfunction-associated fatty liver disease as NAFLD.
Conclusion: Oily fish and its fatty acid intake showed a preventative benefit for NAFLD and metabolic dysfunction-associated fatty liver disease, especially in South Korean women.
Non-alcoholic fatty liver disease (NAFLD) is a condition characterized by predominant macro-vesicular steatosis of the liver, progressing from insulin resistance, and it can lead to steatohepatitis, fibrosis, or cirrhosis (1–3). NAFLD has become a major worldwide public health concern due to the increased risk of chronic diseases, such as type 2 diabetes mellitus and cardiovascular disease (4, 5). In the Western countries, nearly 25% of adults suffer from NAFLD, and this condition will become the most frequent indication of the need for liver transplantation by 2030 (4, 6). The overall prevalence of NAFLD is approximately 30% among South Korean adults and is twice as high in men as in women (7).
As per the existing knowledge, there are no specific drugs or therapeutic methods against NAFLD, although lifestyle (physical activity) and diet or nutrition management appear to be mainly responsible for preventing and treating NAFLD (8–10). Findings from the previous studies confirmed that a high fructose diet or a high-fat diet could accelerate NAFLD development (8, 11, 12). Fructose consumption increases insulin resistance and visceral fat and affects the lipoprotein lipase activity, leading to increased lipid uptake into the hypertrophied adipocytes (12, 13). Similarly, a high-fat diet, especially one rich in trans-fatty acid, leads to NAFLD by inducing obesity and insulin resistance (11, 14). However, not all fat in the diet is harmful to the liver; some dietary fatty acids, such as monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), are considered to be beneficial to the liver (15). MUFAs and PUFAs can reduce lipid accumulation in the liver by affecting the activity of antioxidative enzymes (16). Oily fishes are a good source of MUFAs and PUFAs, especially omega-3 PUFAs. The most frequently consumed oily fishes in South Korea are mackerel, Pacific saury, and Spanish mackerel (17, 18).
This study aimed to investigate whether the intake of oily fish or its fatty acids affected NAFLD development among general South Korean adults, focusing on fish-based MUFAs, PUFAs, and omega-3 fatty acids. Additionally, we examined differences based on sex, age, body mass index (BMI), smoking status, drinking (alcohol) status, and menopausal status.
The Health Examinees study is a large-scale prospective cohort study investigating epidemiologic characteristics, genomic features, and gene–environment interactions of major chronic diseases, such as cancer, in South Korea (19). The study protocol has been described in detail elsewhere (19). The baseline survey was conducted among adults aged 40–69 years between 2004 and 2013, and the first follow-up survey was initiated between 2012 and 2016 (N = 65,642). Among these participants, those with liver-related diseases (fatty liver disease, acute liver disease, chronic hepatitis, cirrhosis, cholelithiasis, cholecystitis, and thyroid disease) at baseline (n = 10,268), with missing outcome measures (blood biomarkers) (n = 5517), without sensible dietary information (energy intake <800/≥4,000 kcal/day for men and <500/≥3,500 kcal/day for women) (n = 859), with alcohol abuse (alcohol intake >210 g/week for men and >140 g/week for women) (n = 4824), with missing dietary information (n = 484), or with implausible BMI value (n = 35) were excluded in the current analysis, resulting in a final sample of 43,655 adults (women, 73.26%) (20–22). Detailed information on participant selection is shown in Supplementary Figure 1.
All participants voluntarily signed an informed written consent form before enrollment. This study was performed in accordance with the guidelines specified in the Declaration of Helsinki, and the study protocol was approved by the local Institutional Review Board (IRB) of the Ethics Committee of the Korean Genome and Epidemiology Study of the Korea National Institute of Health (IRB No. E-1503-103-657).
Dietary data were collected using a 106-item semi-quantitative food frequency questionnaire (FFQ) developed for estimating food and nutrient consumption in South Korea. The reliability and validity of this questionnaire for South Koreans were established in a previous study assessed by four 3-day dietary records over four seasons (23). The dietary missing values were processed by imputation methods. Participants were asked about the average quantity and frequency of oily fish (mackerel/Pacific saury/Spanish mackerel) consumption during the past year at the survey time. Nine responses for frequency were ranged from “never or less than once per month” to “three times per day.” Moreover, the average portion sizes were estimated by photographs.
Data on each food item’s fatty acid content were obtained from the Korean Food Composition database 9.3 (24). First, food items of the FFQ containing more than one food component were separated according to their consumption ratios in each FFQ item, and consumption of each food item was converted into grams by multiplying each FFQ item’s daily consumption. Subsequently, daily fatty acid intake was calculated.
Non-alcoholic fatty liver disease was identified using the fatty liver index (FLI), a well-established non-invasive method to rule out patients with NAFLD (21, 25, 26). The FLI was calculated according to the following formula: FLI = 1/[1 + exp (−x)] × 100, x = 0.953 × ln (triglycerides) (mg/dl) + 0.139 × BMI (kg/m2) + 0.718 × ln (γ-glutamyl transferase) (U/L) + 0.053 × waist circumference (cm) − 15.745. The cut-off value for non-FLI-NAFLD was set to 30 (25–27).
For each participant, fasting venous blood was collected and processed by professionals (19). Weight (kg) and height (m) were also measured at the survey time. BMI was calculated as weight divided by the square of height.
All analyses were stratified by sex and performed using SAS 9.4 (SAS Institute, Cary, NC, United States). Participants were divided into quartiles based on their intake of total oily fish and its fatty acid. Q1 represented the lowest consumption group, and Q4 represented the highest consumption group. General characteristics are presented as means and standard deviations (SDs) for continuous variables and frequencies (n, %) for categorical variables across quartiles of oily fish or its fatty acid intake. Differences between categories were tested by the general linear model for continuous variables and the Chi-square test for categorical variables.
Linear trends across the quartiles were tested by assigning each participant the median of the category and modeling this value as a continuous variable in models. Multivariable Cox proportional hazards models were performed to assess the relationship between FLI-NAFLD and oily fish or fatty acid ratio consumption. Results from the models were presented as hazard ratios (HRs) and 95% confidence intervals (CIs). The proportional hazard assumption was tested by including time-dependent covariates in the Cox model (P = 0.5116).
Sociodemographic and lifestyle characteristics, such as age, sex, level of education, physical activity, smoking, and alcohol drinking habits, were collected using standardized questionnaires. Educational level was categorized as low (under middle school), medium (high school), and high (college and above). Physical activity was determined based on participants’ participation in any sports, to the point of sweating for over 30 min, at least twice a week (21). Individuals were categorized according to their smoking status as a non-smoker, past-smoker, and current smoker. Similarly, they were categorized based on drinking status as non-drinker and current-drinker after excluding alcohol abusers.
Stratified analyses were also performed to test whether the associations relied on the confounder of interest. In the stratified analysis, multivariate Cox models (adjusted for continuous and categorical confounders) were applied to assess the association between the highest consumption quartile and FLI-NAFLD, separately for age categories (age < or ≥ median of age, 56-years for men and 52-years for women), BMI categories (BMI < 25 kg/m2 or BMI ≥ 25 kg/m2), smoking status (non-smoker, past-smoker, and current-smoker), drinking status (non-drinker and current-drinker), and menopause status (yes or no). For male participants, the analyses were stratified by age, BMI, smoking status, and drinking status, respectively, while in the case of female participants, menopausal status was added other than the aforementioned interests. Substitution analysis used the leave-one-out to calculate the associations with NAFLD by substituting certain fatty acid for another type (28).
Model 1 was adjusted for categorical confounders (education, physical activity, smoking status, and drinking status) and continuous variables (age, BMI, and total energy intake). Model 2 was adjusted for the same covariates as model 1 except for altering BMI to waist circumference. Model 3 was adjusted for the same covariates as model 1 plus energy percent from daily carbohydrate, protein, and fat intake. The statistical significance was set at P ≤ 0.05.
Baseline general characteristics and NAFLD incidence of the 43,655 participants included in the analysis across the quartiles of oily fish are given in Table 1 and Supplementary Table 1. Compared with the lowest consumption group (Q1), the highest consumption group (Q4) had lower age and higher waist circumference, higher BMI level, high educational level, higher physical activity, and were current-drinkers with higher alcohol consumption. Moreover, in terms of blood biomarkers, Q4 showed a lower level of serum triglycerides (all P-values < 0.05).
The 1-SD increment analysis of the association between FLI-NAFLD and oily fish and its fatty acid intake revealed an inverse association among female participants. The HRs of oily fish intake, ratio of total fatty acid from oily fish and total daily diet, ratio of MUFA from oily fish and total daily diet, ratio of PUFA, and ratio of omega-3 fatty acid were 0.935 (95% CI: 0.910–0.961), 0.927 (95% CI: 0.903–0.951), 0.934 (95% CI: 0.910–0.958), 0.935 (95% CI: 0.911–0.959), and 0.935 (95% CI: 0.912–0.959), respectively (Table 2). However, the association between oily fish consumption and FLI-NAFLD was absent for male participants (Table 2).
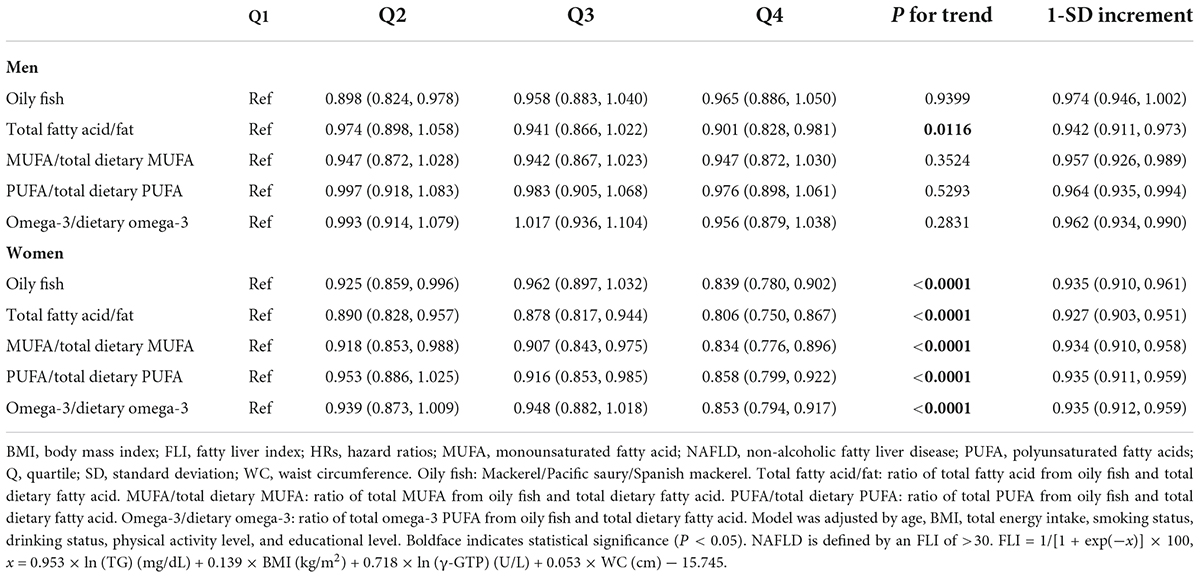
Table 2. Hazard ratios (HRs) for fatty liver index – non-alcoholic fatty liver disease (FLI-NAFLD) according to the quartiles of oily fish and its fatty acid intake.
The quartile analysis revealed that female participants consuming higher quantities of oily fish were less likely to have FLI-NAFLD (highest vs. lowest quartile, HR: 0.839; 95% CI: 0.780–0.902; P for trend <0.05; Table 2). The multivariate-adjusted analysis also revealed that the highest quartile of fatty acid ratio was significantly associated with FLI-NAFLD (Table 2). However, these significances were only present in female participants, except that the association with the total fatty acid ratio was significant in both sexes (highest vs. lowest quartile, HR: 0.901; 95% CI: 0.828–0.981; HR: 0.806; 95% CI: 0.750–0.867 in male and female participants, respectively, both P for trend <0.05; Table 2). After further adjusted analyses (models 2 and 3, Supplementary Table 2), the results stayed consistent.
The stratified analysis of participants revealed almost no differences in the effect of various groups toward FLI-NAFLD (Figures 1, 2). Among female participants, the association between the various dietary exposure groups and FLI-NAFLD was maintained regardless of age, BMI, and drinking status, whereas smoking status and menopause status were weakly associated with FLI-NAFLD (Figure 2). Among non-smokers and post-menopausal participants, oily fish or its fatty acid intake resulted in a significantly lower risk of FLI-NAFLD development (Figure 2). Age and drinking status were weakly associated with FLI-NAFLD among male participants (Figure 1).
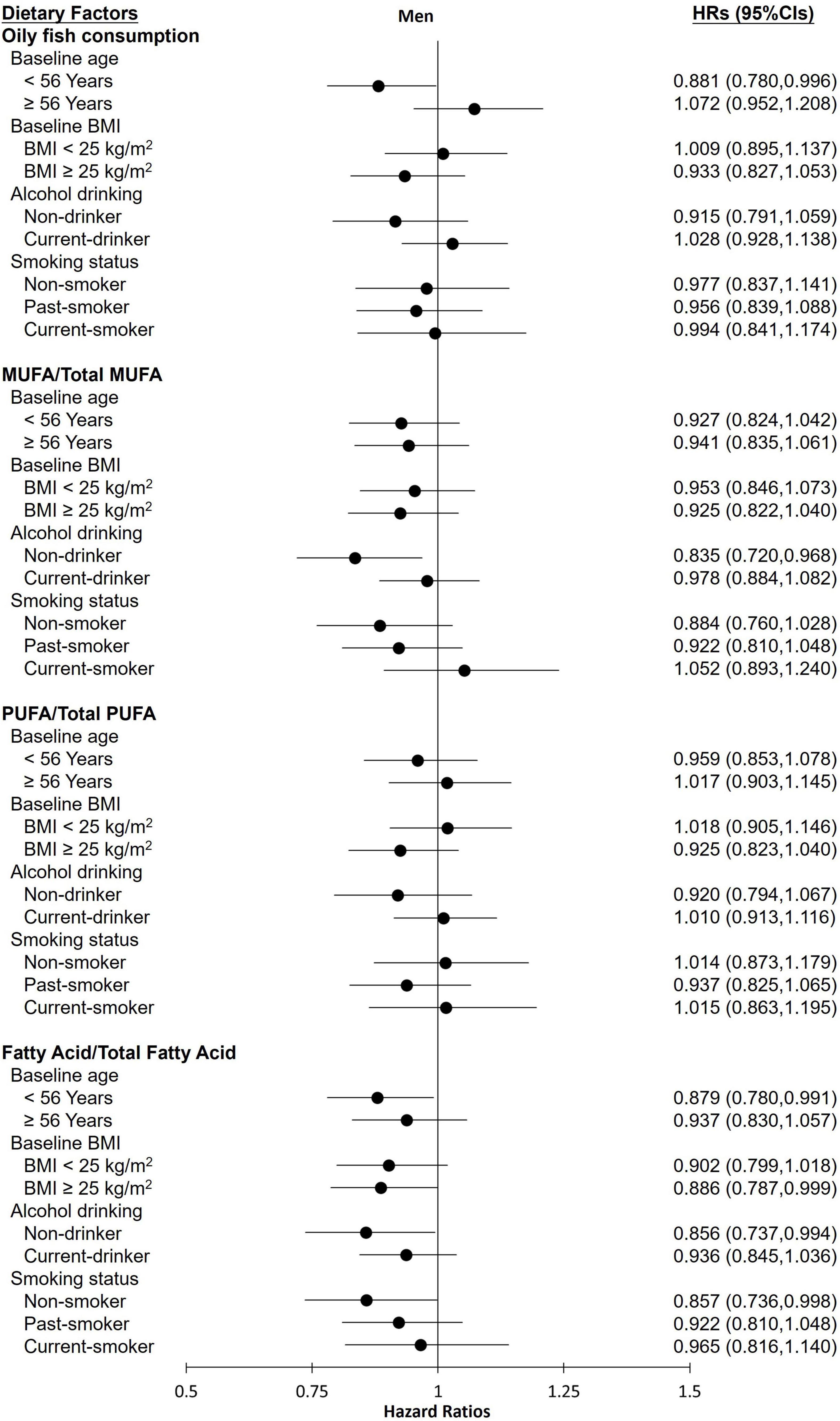
Figure 1. Hazard ratios (HRs) of fatty liver index (FLI)-non-alcoholic fatty liver disease for the highest categories compared with the lowest categories of oily fish and its fatty acid intake among male participants in the current cohort study. Analyses were stratified by body mass index (BMI), age, smoking status, and drinking status. HRs, hazard ratios; MUFAs, monounsaturated fatty acids; PUFAs, polyunsaturated fatty acids.
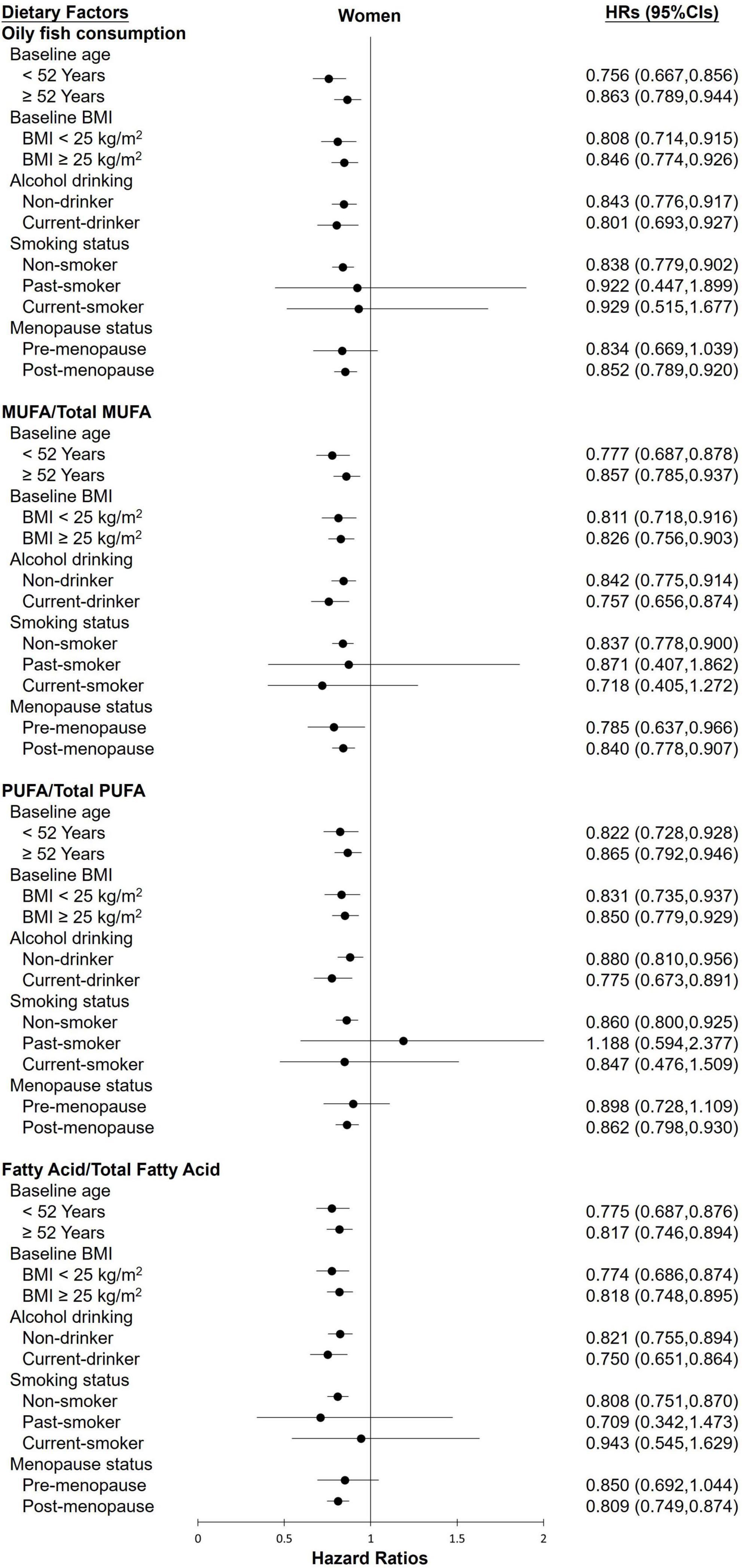
Figure 2. The HRs of FLI-non-alcoholic fatty liver disease for the highest categories compared with the lowest categories of oily fish and its fatty acid intake among female participants in the current cohort study. Analyses were stratified by body mass index (BMI), age, smoking status, drinking status, and menopause status. HRs, hazard ratios; MUFAs, monounsaturated fatty acids; PUFAs, polyunsaturated fatty acids.
The results from a leave-one-out substitution analysis are shown in Supplementary Table 3. Among female participants, after replacing fatty acid intake (from other food sources) with a fatty acid intake predominantly from oily fish, a one-unit increment of fatty acid was associated with a lower risk of FLI-NAFLD. The converse was also true. All adjusted models showed the same association pattern. However, there was no significant association for male participants.
Here, we evaluated the association between oily fish intake and its fatty acid consumption and FLI-defined NAFLD in a large-scale cohort of general adults recruited from 38 sites of South Korea.
A significant inverse association between high oily fish intake and FLI-NAFLD among female participants was found. The analysis also revealed that oily fish-sourced fatty acids, such as MUFAs, PUFAs, and omega-3 PUFAs, have preventative benefits for NAFLD. Moreover, the association continued to exist after being stratified by age, BMI, smoking status, drinking status, and menopausal status. Although covariates, such as smoking status and menopausal status, impact the effects of oily fish and its fatty acid intake on FLI-NAFLD after stratified analysis, in most ways, total intake of oily fish and its fatty acids resulted in preventative effects independently, regardless of age, BMI, and drinking alcohol status.
Non-alcoholic fatty liver disease is a common chronic disease wherein triglycerides accumulate excessively in the liver without alcohol abuse (29). Moreover, NAFLD is closely associated with diabetes and metabolic syndrome, which are both related to the pathophysiology of inflammation and insulin resistance (30, 31). Dietary MUFAs have been reported to improve lipid profile through their anti-inflammatory characteristics (30, 32). Oily fish is protective against NAFLD owing to its omega-3 PUFA contents, which impact the lipid profile (33–35). Mackerel and Pacific saury are the types of oily fishes that have been reported to be enriched in various fatty acids, especially omega-3 PUFA (36–40).
Omega-3 PUFA has been previously associated with reducing the risk of NAFLD development in various epidemiological studies (41–46). A dietary intervention study suggested that patients with NAFLD showed a lower level of circulating liver enzymes and triglycerides, with a significant improvement of adiponectin after long-term (1 year) consumption of omega-3 PUFA (42). Furthermore, a cross-sectional study revealed that fish and omega-3 PUFA intake was associated with decreasing portal and lobular inflammation and a lower risk for hepatic inflammation among children (43). In Japan, a cross-sectional study conducted in adults showed that omega-3 PUFA was not an independent risk factor for NAFLD. However, dietary eicosapentaenoic acid and eicosapentaenoic acid + docosahexaenoic acid were preventive nutrients for NAFLD and improved inflammatory change in adipose tissue in men (47, 48). These findings are partially in line with our results that oily fish and its omega-3 PUFA content are associated with a lower incidence of FLI-NAFLD, while the effect of omega-6 PUFA content has not yet been fully elucidated.
Our findings also suggest that menopausal status is an independent risk factor for FLI-NAFLD among female participants. This result is partially in line with that of a previous study, which demonstrated that menopausal status change was correlated with NAFLD through altering sex hormones, and dietary factors could exacerbate the relationship (29). The previous study convinced that smoking and drinking were associated with higher prevalence of NAFLD (49) and in current study, men and women also showed gender differences in smoking and drinking habits shown in Table 1. Less current smokers and alcohol drinkers in women than men participants maybe another explanation to significant results only found in women. Further, it is important to consider sex difference, which may be another risk factor leading to different results of this study. A previous review research reported that adipose tissue distribution, gut microbiota, and innate immune response showed some sex differences (50). Adipose tissue and innate immune response play an important role in regulating insulin resistance and inflammatory reaction (51, 52). The gut microbiota could regulate lipid/glucose metabolism by activating the farnesoid X receptor (53).
The strength of the current study is that we conducted a large-scale cohort study in South Korea, and the result is partly adapted to the general population. Moreover, in PUFA and MUFA, we focused on oily fish-sourced fatty acids instead of its supplements or other dietary sources. However, this study has certain limitations. First, the diagnosis of NAFLD was not based on a liver biopsy. However, the FLI used in the current study has been evaluated and verified in a previous study and is considered an appropriate tool for large nutritional epidemiological studies (21, 25, 26, 54). Second, rather than the exact time that FLI-NAFLD occurred, the endpoint was set on the time conducting follow-up survey. Considering soft endpoints more common in observational study, and it has little impacts on large-scale cohort study, this limitation could be negligible (55). Third, the fish-sourced fatty acid contents were not measured directly, but through linking the Korean Food Composition Database 9.3 to FFQ data, and different cooking or storage methods may lead to possible bias. Future studies should consider these factors. Finally, the lifestyle of participants was assessed by a self-reported questionnaire such as smoking, drinking, and physical activity, which may be overreported or under reported. So we grouped them as categorical variables when adjusting model to minimize the reporting bias.
In conclusion, we have demonstrated that the intake of oily fish and its fatty acid contents, such as MUFAs, PUFAs, and omega-3 PUFAs, is associated with a lower incidence of NAFLD. As a result, although we did not study their precise molecular mechanisms, oily fish may be considered effective preventative strategies for NAFLD development among South Koreans, especially for women. These findings may provide a basis for revising middle-aged and older adults’ dietary guidelines in South Korea.
The datasets presented in this article are not readily available because the datasets analyzed for this study can be available from National Genome Research Institute, Korea Centers for Disease Control and Prevention. Restrictions apply to the availability of these data, which were used under license for this study. Data described in the manuscript, codebook, and analytic code are available from the authors with the permission of National Genome Research Institute, Korea Centers for Disease Control and Prevention. Requests to access the datasets should be directed to National Genome Research Institute, Korea Centers for Disease Control and Prevention; https://kdca.go.kr/contents.es?mid=a40504060100.
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of the Ethics Committee of the Korean Genome and Epidemiology Study of the Korea National Institute of Health (IRB No. E-1503-103-657). The patients/participants provided their written informed consent to participate in this study.
SS designed and conducted the research and reviewed and revised the manuscript critically. L-JT analyzed the data, performed the statistical analysis, and wrote the first draft of the manuscript. SS and L-JT had primary responsibility for the final content. Both authors approved the final version of the article.
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (No. 2022R1F1A1074279). MSIT, Ministry of Science and ICT. This study used data from the Health Examinees study, supported by the National Genome Research Institute, Korea Centers for Disease Control and Prevention. The study sponsor/funder was not involved in the study’s design, collection, analysis, and interpretation of data, writing the report, and did not impose any restrictions regarding the publication of the report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.876909/full#supplementary-material
ALT, alanine aminotransferase; BMI, body mass index; CIs, confidence intervals; FFQ, food frequency questionnaire; FLI, fatty liver index; HRs, hazard ratios; MUFAs, monounsaturated fatty acids; NAFLD, non-alcoholic fatty liver disease; PUFAs, polyunsaturated fatty acids; SDs, standard deviations.
2. Allard JP, Aghdassi E, Mohammed S, Raman M, Avand G, Arendt BM, et al. Nutritional assessment and hepatic fatty acid composition in non-alcoholic fatty liver disease (NAFLD): a cross-sectional study. J Hepatol. (2008) 48:300–7.
3. Cortez-Pinto H, de Moura MC, Day CP. Non-alcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. (2006) 44:197–208.
5. Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. (2011) 43:617–49. doi: 10.3109/07853890.2010.518623
6. Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. (2021) 110:921–37.
7. Im HJ, Ahn YC, Wang JH, Lee MM, Son CG. Systematic review on the prevalence of nonalcoholic fatty liver disease in South Korea. Clin Res Hepatol Gastroenterol. (2021) 45:101526.
8. Zhang S, Gu Y, Bian S, Lu Z, Zhang Q, Liu L, et al. Soft drink consumption and risk of nonalcoholic fatty liver disease: results from the Tianjin chronic low-grade systemic inflammation and health (TCLSIH) cohort study. Am J Clin Nutr. (2021) 113:1265–74. doi: 10.1093/ajcn/nqaa380
9. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American association for the study of liver diseases. Hepatology. (2018) 67:328–57.
10. Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. (2017) 67:829–46.
11. Dhibi M, Brahmi F, Mnari A, Houas Z, Chargui I, Bchir L, et al. The intake of high fat diet with different trans fatty acid levels differentially induces oxidative stress and non alcoholic fatty liver disease (NAFLD) in rats. Nutr Metab (Lond). (2011) 8:65. doi: 10.1186/1743-7075-8-65
12. Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. (2013) 57:2525–31.
13. Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes. (2012) 19:81–7.
14. Nakamura A, Terauchi Y. Lessons from mouse models of high-fat diet-induced NAFLD. Int J Mol Sci. (2013) 14:21240–57. doi: 10.3390/ijms141121240
15. Berná G, Romero-Gomez M. The role of nutrition in non-alcoholic fatty liver disease: pathophysiology and management. Liver Int. (2020) 40(Suppl. 1):102–8.
16. Ristic-Medic D, Kovacic M, Takic M, Arsic A, Petrovic S, Paunovic M, et al. Calorie-restricted mediterranean and low-fat diets affect fatty acid status in individuals with nonalcoholic fatty liver disease. Nutrients. (2020) 13:15. doi: 10.3390/nu13010015
17. Teisen MN, Vuholm S, Niclasen J, Aristizabal-Henao JJ, Stark KD, Geertsen SS, et al. Effects of oily fish intake on cognitive and socioemotional function in healthy 8-9-year-old children: the FiSK Junior randomized trial. Am J Clin Nutr. (2020) 112:74–83. doi: 10.1093/ajcn/nqaa050
18. Kim SA, Lee JK, Kang D, Shin S. Oily fish consumption and the risk of dyslipidemia in Korean adults: a prospective cohort study based on the health examinees gem (HEXA-G) study. Nutrients. (2019) 11:2506. doi: 10.3390/nu11102506
19. Health Examinees Study Group. The health examinees (HEXA) study: rationale, study design and baseline characteristics. Asian Pac J Cancer Prev. (2015) 16:1591–7. doi: 10.7314/apjcp.2015.16.4.1591
20. Lee KW, Bang KB, Rhee EJ, Kwon HJ, Lee MY, Cho YK. Impact of hypothyroidism on the development of non-alcoholic fatty liver disease: a 4-year retrospective cohort study. Clin Mol Hepatol. (2015) 21:372–8. doi: 10.3350/cmh.2015.21.4.372
21. Tan LJ, Jung H, Kim SA, Shin S. The association between coffee consumption and nonalcoholic fatty liver disease in the south Korean general population. Mol Nutr Food Res. (2021) 65:e2100356. doi: 10.1002/mnfr.202100356
22. Chon HY, Lee JS, Lee HW, Chun HS, Kim BK, Tak WY, et al. Predictive performance of CAGE-B and SAGE-B models in Asian treatment-naive patients who started entecavir for chronic hepatitis B. Clin Gastroenterol Hepatol. (2021) 20:e794–807. doi: 10.1016/j.cgh.2021.06.001
23. Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, et al. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. (2007) 61:1435–41. doi: 10.1038/sj.ejcn.1602657
24. National Institute of Agricultural Sciences. Korean Food Composition Database. (2019). Available online at: http://koreanfood.rda.go.kr/kfi/fct/fctIntro/list?menuId=PS03562 (accessed on November 3, 2021).
25. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33.
26. Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL, et al. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. (2013) 11:1201–4. doi: 10.1016/j.cgh.2012.12.031
27. Jung CH, Lee WJ, Hwang JY, Yu JH, Shin MS, Lee MJ, et al. Assessment of the fatty liver index as an indicator of hepatic steatosis for predicting incident diabetes independently of insulin resistance in a Korean population. Diabet Med. (2013) 30:428–35. doi: 10.1111/dme.12104
28. Song M, Giovannucci E. Substitution analysis in nutritional epidemiology: proceed with caution. Eur J Epidemiol. (2018) 33:137–40. doi: 10.1007/s10654-018-0371-2
29. DiStefano JK. NAFLD and NASH in postmenopausal women: implications for diagnosis and treatment. Endocrinology. (2020) 161:bqaa134.
30. Cheah MCC, McCullough AJ, Goh GBB Chapter 5 - Dietary manipulations fornonalcoholic fatty liver disease (NAFLD). 2nd ed. In: RR Watson Preedy VReditors. Bioactive Food as Dietary Interventions for Diabetes. Cambridge,MA: Academic Press (2019). p. 69–88. doi: 10.1016/B978-0-12-813822-9.00005-9
31. Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res. (2020) 126:1549–64.
32. Amirkalali B, Khoonsari M, Sohrabi MR, Ajdarkosh H, Motamed N, Maadi M, et al. Relationship between dietary macronutrient composition and non-alcoholic fatty liver disease in lean and non-lean populations: a cross-sectional study. Public Health Nutr. (2021) 24:6178–90. doi: 10.1017/S1368980021001762
33. Gupta V, Mah XJ, Garcia MC, Antonypillai C, van der Poorten D. Oily fish, coffee and walnuts: dietary treatment for nonalcoholic fatty liver disease. World J Gastroenterol. (2015) 21:10621–35. doi: 10.3748/wjg.v21.i37.10621
34. Nobili V, Alisi A, Musso G, Scorletti E, Calder PC, Byrne CD. Omega-3 fatty acids: mechanisms of benefit and therapeutic effects in pediatric and adult NAFLD. Crit Rev Clin Lab Sci. (2016) 53:106–20. doi: 10.3109/10408363.2015.1092106
35. Duarte SMB, Stefano JT, Vanni DS, Carrilho FJ, Oliveira CPMS. Impact of current diet at the risk of non-alcoholic fatty liver disease (NAFLD). Arq Gastroenterol. (2019) 56:431–9.
37. Yoon MS, Heu MS, Kim JS. Fatty acid composition, total amino acid and mineral contents of commercial kwamegi. Korean J Fish Aquat Sci. (2010) 43:100–8.
38. Sardenne F, Puccinelli E, Vagner M, Pecquerie L, Bideau A, Le Grand F, et al. Post-mortem storage conditions and cooking methods affect long-chain omega-3 fatty acid content in Atlantic mackerel (Scomber scombrus). Food Chem. (2021) 359:129828. doi: 10.1016/j.foodchem.2021.129828
39. Anishchenko OV, Sushchik NN, Makhutova ON, Kalachova GS, Gribovskaya IV, Morgun VN, et al. Benefit-risk ratio of canned pacific saury (Cololabis saira) intake: essential fatty acids vs. heavy metals. Food Chem Toxicol. (2017) 101:8–14. doi: 10.1016/j.fct.2016.12.035
40. Nazemroaya S, Sahari MA, Rezaei M. Identification of fatty acid in mackerel (Scomberomorus commersoni) and shark (Carcharhinus dussumieri) fillets and their changes during six month of frozen storage at-18 degrees C. J Agric Sci Technol. (2011) 13:553–66.
41. Scorletti E, Byrne CD. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr. (2013) 33:231–48.
42. Sofi F, Giangrandi I, Cesari F, Corsani I, Abbate R, Gensini GF, et al. Effects of a 1-year dietary intervention with n-3 polyunsaturated fatty acid-enriched olive oil on non-alcoholic fatty liver disease patients: a preliminary study. Int J Food Sci Nutr. (2010) 61:792–802. doi: 10.3109/09637486.2010.487480
43. St-Jules DE, Watters CA, Brunt EM, Wilkens LR, Novotny R, Belt P, et al. Estimation of fish and omega-3 fatty acid intake in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. (2013) 57:627–33. doi: 10.1097/MPG.0b013e3182a1df77
44. Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, et al. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol. (2007) 47:711–7.
45. Yan JH, Guan BJ, Gao HY, Peng XE. Omega-3 polyunsaturated fatty acid supplementation and non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Medicine (Baltimore). (2018) 97: e12271.
46. Shapiro H, Tehilla M, Attal-Singer J, Bruck R, Luzzatti R, Singer P. The therapeutic potential of long-chain omega-3 fatty acids in nonalcoholic fatty liver disease. Clin Nutr. (2011) 30:6–19.
47. Oya J, Nakagami T, Sasaki S, Jimba S, Murakami K, Kasahara T, et al. Intake of n-3 polyunsaturated fatty acids and non-alcoholic fatty liver disease: a cross-sectional study in Japanese men and women. Eur J Clin Nutr. (2010) 64:1179–85. doi: 10.1038/ejcn.2010.139
48. Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol. (2007) 27:1918–25. doi: 10.1161/ATVBAHA.106.136853
49. Liu P, Xu Y, Tang Y, Du M, Yu X, Sun J, et al. Independent and joint effects of moderate alcohol consumption and smoking on the risks of non-alcoholic fatty liver disease in elderly Chinese men. PLoS One. (2017) 12:e0181497. doi: 10.1371/journal.pone.0181497
50. Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. (2019) 70:1457–69. doi: 10.1002/hep.30626
51. Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. (2015) 109:9–15.
52. Park YM, Pereira RI, Erickson CB, Swibas TA, Cox-York KA, Van Pelt RE. Estradiol-mediated improvements in adipose tissue insulin sensitivity are related to the balance of adipose tissue estrogen receptor alpha and beta in postmenopausal women. PLoS One. (2017) 12:e0176446. doi: 10.1371/journal.pone.0176446
53. Sharpton SR, Ajmera V, Loomba R. Emerging role of the gut microbiome in nonalcoholic fatty liver disease: from composition to function. Clin Gastroenterol Hepatol. (2019) 17:296–306.
54. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. (2020) 73:202–9.
Keywords: oily fish consumption, omega-3 fatty acid, non-alcoholic fatty liver disease – NAFLD, cohort study (or longitudinal study), South Korean adults
Citation: Tan L-J and Shin S (2022) Effects of oily fish and its fatty acid intake on non-alcoholic fatty liver disease development among South Korean adults. Front. Nutr. 9:876909. doi: 10.3389/fnut.2022.876909
Received: 16 February 2022; Accepted: 28 June 2022;
Published: 22 July 2022.
Edited by:
Rafaela Rosário, University of Minho, PortugalReviewed by:
Masahide Hamaguchi, Kyoto Prefectural University of Medicine, JapanCopyright © 2022 Tan and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sangah Shin, aXZvcnk4MzIwQGNhdS5hYy5rcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.