
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 12 May 2022
Sec. Food Chemistry
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.857351
This article is part of the Research Topic Natural Food Additives: Sustainable Development of Bio-Based Molecules View all 4 articles
Filamentous microalga Klebsormidium sp. has huge potential to become a natural and healthy additive in aquatic feed since it contains various bioactive nutrients, such as linoleic acid (LA), carotenoids, and chlorophylls. Therefore, an eight-week feeding experiment was performed to evaluate the effects of dietary Klebsormidium sp. on the growth performance, antioxidant and anti-inflammatory status, metabolism, and mid-intestine morphology of Litopenaeus vannamei. Two isonitrogenous and isolipid diets supplemented with and without 5% Klebsormidium sp. were prepared. Results showed that L. vannamei fed with Klebsormidium sp. had better growth performance and feed utilization by optimizing mid-intestine morphology and improving the carbohydrate metabolism. In addition, Klebsormidium sp. also enhanced the antioxidant capacity of L. vannamei by downregulating antioxidant parameters (hepatopancreas T-SOD, hepatopancreas GSH-PX, hemolymph T-SOD, hemolymph MDA) and RNA expression levels of antioxidant genes (gsh-px and cat). Furthermore, the supplementations of dietary Klebsormidium sp. significantly improved hepatopancreas health by downregulating RNA expression levels of pro-inflammatory related genes (relish and rho). Therefore, a dose of 5% Klebsormidium sp. is recommended for the daily diet of L. vannamei to improve the growth performance, antioxidant and anti-inflammatory status, metabolism, and mid-intestine morphology of shrimp.
High-density farming and environmental pollution are two main reasons that limit the development of the shrimp industry since they lead to the growth of pathogenic microorganisms in water, making shrimp vulnerable to bacterial diseases (1, 2). To address this issue, antibiotics are widely used in aquaculture to prevent and treat bacterial diseases (3). However, the problem of long-term antibiotic exposure in aquatic animals is the antibiotic residue in aquatic products. This causes adverse effects on humans when seafood with numerous antibiotic residues is consumed. For example, it reduces the effectiveness of antibiotics in human if they face infections (4), and changes the diversity of gut microbiota (5). In this situation, there is a need to identify healthy and economic additives for substituting the use of antibiotics in the aquaculture field.
Crustaceans are generally considered to have a low ability to biosynthesize de novo polyunsaturated fatty acids (PUFAs). They lack Δ12 and Δ15 desaturase enzymes, which makes it hard for them to convert the oleic acid (OA) into linoleic acid (LA) and linolenic acid (LNA) (6). Therefore, shrimps require essential fatty acids (EFAs) in their daily diet. Besides, the supply of PUFAs at optimal levels and ratios in their diet is also beneficial for their growth performance (7), anti-inflammatory status (8, 9), and resistance to diseases (10–13). Therefore, substances rich in PUFAs might be an excellent choice as aquaculture feeding additives.
Klebsormidium sp., a filamentous microalga that is rich in LA with a rapid growth rate (14), can exist in typical extreme environments such as drought (15, 16) and deep freeze climate (17, 18). Except for n-6 PUFAs, Klebsormidium sp. is also a rich source of various bioactive pigments (carotenoids and chlorophylls) (19, 20). Therefore, this microalga fits the criterion of healthy and natural additive in aquatic feed. However, until recently, knowledge about the effect of dietary Klebsormidium sp. on aquatic animals is unclear.
Litopenaeus vannamei, belonging to the genus Penaeus, is mainly found in the tropical waters along the Pacific coast of the United States (21). For nearly two decades, L. vannamei has gained more attention and is widely cultured in China, India, and some Southeast Asian countries due to its delicious meat and high nutritional value (22). However, with the development of green aquaculture, the use of antibiotics is strictly limited during farming worldwide. Therefore, it is necessary to identify a healthy and cost-effective aquatic additive for substituting antibiotics, which are used for improving the health of shrimp. In the present study, an eight-week feeding experiment was performed to investigate the growth performance, antioxidant and anti-inflammatory status, metabolism, and mid-intestine morphology of L. vannamei fed with and without dietary Klebsormidium sp. The study results might provide a reference for formulating the diet of L. vannamei.
Strains of Klebsormidium sp. were obtained from the Culture Collection of Algae at the University of Göttingen (SAG) and scaled up in our laboratory in the following manner: Briefly, a 160-L vertical flat-plate glass photobioreactor and a BG-11 medium (23) with initial 9 mmol L−1 nitrogen (nitrogen source: sodium nitrate) and 1% bubbled CO2 (v/v) were used to culture Klebsormidium sp. Continuous illumination (24 h) with 300 μmol photons m−2 s−1 was provided for cultivating the Klebsormidium sp. for 15 days.
Klebsormidium sp. contained 17.97% crude protein, 31.95% crude lipid (including 9.23% LA), and 29.31% carbohydrate (dry matter) (Table 1).
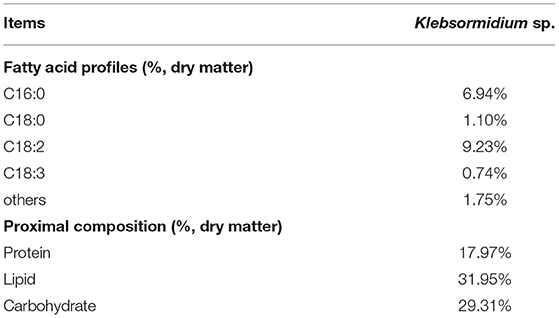
Table 1. Main fatty acid profiles (%, dry matter) and proximal compositions (%, dry matter) of Klebsormidium sp.
As shown in Table 2, two artificial diets (containing approximately 40% crude protein and 7% crude lipid) with 0% (D1) and 5% (D2) Klebsormidium sp., respectively, were prepared using the method detailed in our earlier study (24). Briefly, dry ingredients were weighed and mixed thoroughly in a Hobart-type mixer (A-200T, Canada). Then, pre-weighed fish oil, soybean lecithin, soybean oil, and distilled water (35%, v/w) were added to the mixture until a homogenous mixture was obtained. Then, the wet dough was passed through a mono screw extruder (South China University of Technology, China) with a 1.2 mm diameter die. Diets were then dried and stored at −20°C until use.
Experimental L. vannamei were obtained from the Chinese Academy of Fishery Science (Lingshui, China). Shrimp were fed the D1 group diet to acclimatize them to the experimental environment for 1 month before the feeding trial. Then, after a 24-h starvation treatment, 320 shrimp (initial body weight 0.64 ± 0.02 g) were distributed randomly into the recirculating water system with eight cylindrical fiber tanks (300 L). Each diet was randomly assigned to quadruplicate tanks. The feeding frequency was three times per day at 06:00, 12:00, and 18:00 with 8% of total shrimp weight for 56 days. During the experimental period, environmental conditions were maintained as follows: water temperature: 26.8–28.1°C; pH: 7.5–7.7; salinity: 29–32‰; dissolved oxygen: > 7.0 mg/L; total ammonia nitrogen: < 0.1 mg/L; and sulfide: < 0.05 mg/L.
At the end of the feeding trial and after a 24-h starvation treatment, all experimental L. vannamei from each tank were weighed and counted. Then, eight shrimp, collected randomly from each tank, were anesthetized (MS-222, Sigma, USA) for obtaining the blood sample. Their hepatopancreas was separated for studying antioxidant parameters and mRNA expression analysis. At the same time, a similar section of the L. vannamei mid-intestine was obtained and fixed in 4% paraformaldehyde for intestinal histological examination. Blood samples were stored at 4°C for 12 h and then centrifuged (7,100 g, 10 min, 4°C) to obtain hemolymph. All hepatopancreas and hemolymph samples were separated rapidly and then maintained in liquid nitrogen until examination.
Moisture, crude lipid, and crude protein of diets and Klebsormidium sp. were determined using the standard method of AOAC (25). Briefly, crude protein content (N × 6.25) was determined by the Kjeldahl method (1030- Autoanalyzer; Tecator, Höganäs, Sweden); crude lipid content inspection was performed according to the Soxhlet extractor method (Soxtec System HT6, Tecator, Sweden); moisture content was examined by drying in the ventilated oven at 105°C for 24 h. The carbohydrate content of Klebsormidium sp. was analyzed using the modified phenol-sulfuric acid method (5). Analysis of the fatty acid profiles of Klebsormidium sp. was done using Agilent Gas Chromatograph (Agilent 6890 N GC, Agilent Technologies, USA) following Zhang et al. (26).
We followed the method detailed in our previous study for homogenizing hepatopancreas (27). Briefly, hepatopancreas was homogenized (1:9) in phosphate buffer and then the homogenate was centrifuged (10 min, 4°C, 1,200 g). Afterward, the supernatant was collected for further analysis.
Activities of total superoxide dismutase (T-SOD) (A001-1), total antioxidant capacity (T-AOC) (A015–2), glutathione peroxidase (GSH-PX) (A005-1), and the content of malondialdehyde (MDA) (A003-1) were measured following kits' instructions (Nanjing Jiancheng Bioengineering Institute, China) (Kits' instructions can be seen in additional appended files).
The section of the mid-intestine was stained according to the study of Zhao et al. (28). Specifically, the mid-intestine section was stained using H&E, and the histological was observed under the microscope (Olympus CKX41 microscope, Japan).
Hepatopancreas RNA isolation and expression quantification were performed according to the method detailed in our previous study (29). Briefly, total RNA was isolated using Trizol® reagent (Invitrogen, USA) following the manufacturer's instructions. To ascertain RNA quality and quantity, we used 1% agarose gel electrophoresis and a spectrophotometer, respectively (NanoDrop 2000, Thermo Fisher, United States). Afterward, cDNA was synthesized using the PrimeScript TM RT reagent Kit (Takara, Japan), following the manufacturer's instructions. Real-time PCR for target genes was performed using an SYBR® Premix Ex TaqTM II (Takara, Japan) and quantified on the LightCycler 480 (Roche Applied Science, Basel, Switzerland). We used ef1a as the housekeeping gene for gene expression in the present study (30). Relative mRNA expression levels of target genes were determined using the 2−ΔΔCT method (31). Primers related to the present study are shown in Table 3.
All data in our present study are shown as means ± standard error (SE). Data analysis was performed in SPSS 22.0 (SPSS, Chicago, IL, USA), followed by an independent sample t-test where p < 0.05 was regarded as the significant difference between groups.
As shown in Table 4, the diet supplemented with Klebsormidium sp. significantly improved the growth performance and feed utilization of L. vannamei. Substantially higher growth performance parameters [weight gain rate (WGR) and specific growth rate (SGR)] of L. vannamei were obtained in the D2 group than in the D1 group (p < 0.05). The feed conversion ratio (FCR) of L. vannamei fed with Klebsormidium sp. was significantly lower compared to the control group (p < 0.05). After the 8-week diet treatment, the survival rate (SR) of L. vannamei ranged from 96.25 to 99.38% in the present study (p > 0.05).
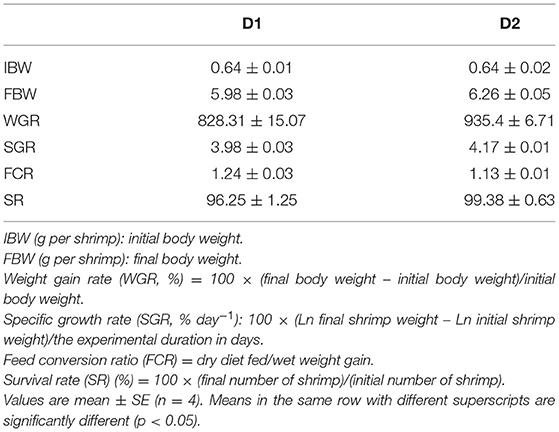
Table 4. Growth performance and feed utilization of Litopenaeus vannamei fed diets supplemented with/without Klebsormidium sp. for 56 days.
The antioxidant parameters of L. vannamei fed with and without Klebsormidium sp. are shown in Table 5. L. vannamei fed with Klebsormidium sp. showed significantly lower antioxidant enzyme activities (hepatopancreas T-SOD, hepatopancreas GSH-PX, and hemolymph T-SOD) compared to the control group (p< 0.05). In addition, significantly lower hemolymph MDA content was found in the dietary Klebsormidium sp. treatment group than in the control group (p< 0.05). No statistical differences in hepatopancreas T-AOC, hepatopancreas MDA content, hemolymph T-AOC, and hemolymph GSH-PX were found between the two experimental groups (p > 0.05).
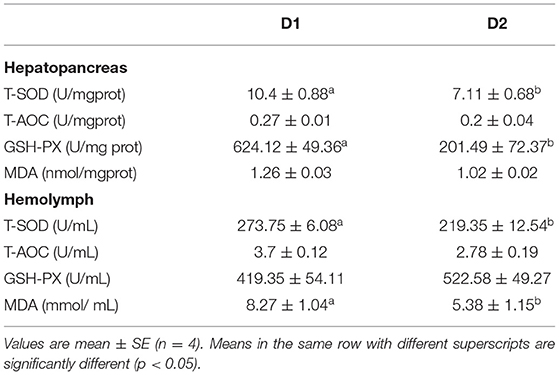
Table 5. Hepatopancreas and hemolymph antioxidant parameters of L. vannamei fed diets supplemented with/without Klebsormidium sp. for 56 days.
The RNA expression levels of antioxidant genes in the hepatopancreas are shown in Figure 1. The RNA expression levels of catalase (cat) and gsh-px in the D2 group were significantly lower than that in the D1 group (p< 0.05). However, dietary Klebsormidium sp. could not alter the RNA expression level of sod in the hepatopancreas (p > 0.05).
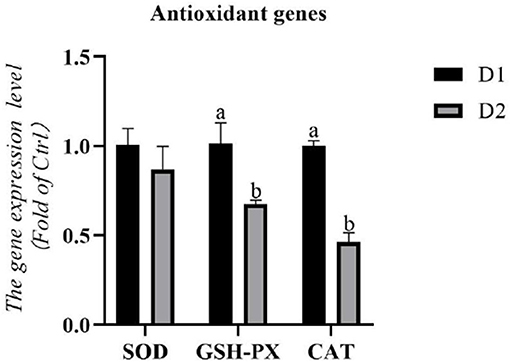
Figure 1. Hepatopancreas RNA expression levels of antioxidant genes of Litopenaeus vannamei fed diets supplemented with/without Klebsormidium sp. for 56 days. a, bThe small letters indicated significant differences at p < 0.05.
The RNA expression levels of pro-inflammatory related genes in the hepatopancreas are shown in Figure 2. Remarkably lower RNA expression levels of pro-inflammatory related genes (relish and rho) were obtained in the D2 group than that in the D1 group (p< 0.05). There was no significant difference in the RNA expression level of hsp70 between the experimental groups (p > 0.05).
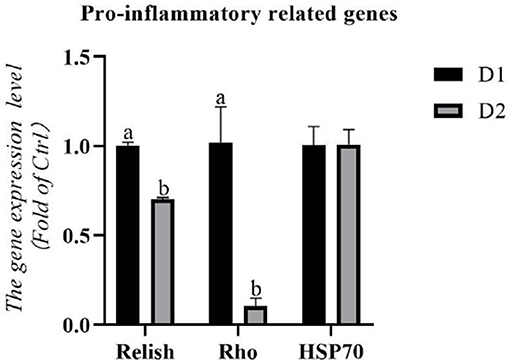
Figure 2. Hepatopancreas RNA expression levels of pro-inflammatory related genes of L. vannamei fed diets supplemented with/without Klebsormidium sp. for 56 days. a, bThe small letters indicated significant differences at p < 0.05.
As shown in Figure 3, the diet supplemented with Klebsormidium sp. could not alter the RNA expression levels of digestive enzyme genes (chymotrypsin and trypsin) in the hepatopancreas of L. vannamei (p > 0.05).
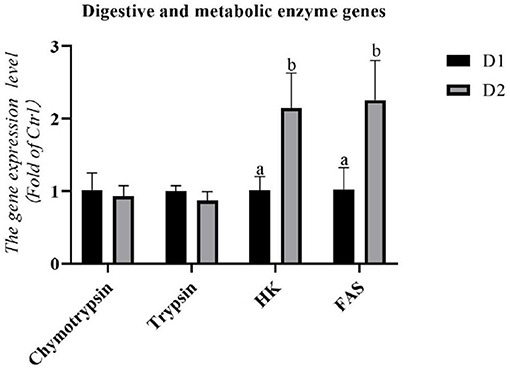
Figure 3. Hepatopancreas RNA expression levels of digestive and metabolic enzyme genes of L. vannamei fed diets supplemented with/without Klebsormidium sp. for 56 days. a, bThe small letters indicated significant differences at p < 0.05.
The RNA expression levels of metabolic enzyme genes in the hepatopancreas are shown in Figure 3. Significantly higher mRNA expression of hexokinase (hk) and fatty acid synthase (fas) were found in the D2 group compared to the D1 group (p< 0.05).
The mid-intestine morphology of L. vannamei fed with and without Klebsormidium sp. is shown in Figure 4. Mid-intestine morphology parameters (the intestinal mucosal layer thickness and the intestinal villi height) in the D2 group were significantly higher than that of the D1 group (p< 0.05).
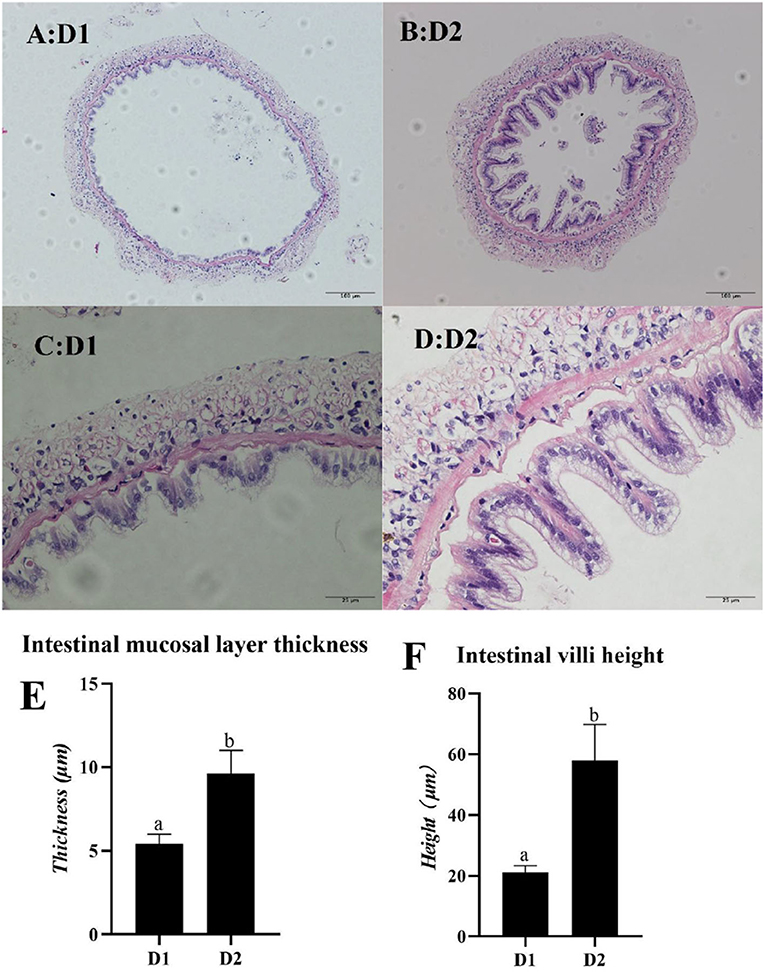
Figure 4. Light microscopy of mid-intestine morphology of L. vannamei fed diets supplemented with/without Klebsormidium sp. for 56 days. Scale bars (A,B) = 100 μm; Scale bars (C,D) = 25 μm. (E,F) represent the mid-intestinal mucosal layer thickness and intestinal villi height of L. vannamei. a, bThe small letters indicated significant differences at p < 0.05.
As a green additive in aquatic feed, microalgae have gained more attention in recent years due to their high nutritional value and convenience of scaling up the culture (37). Different microalgae might contain various nutrients, such as polyunsaturated fatty acid (PUFAs) (38, 39), pigment (40, 41), vitamins (42), minerals (43), and algae polysaccharides (44), which are beneficial to the health of aquatic animals. Therefore, microalgae have huge potential to substitute synthetic additives and reduce the budget of the aquatic feed in aquaculture (45).
In the present study, L. vannamei fed with Klebsormidium sp. obtained better growth performance parameters (WGR and SGR) and feed utilization (FCR) than the control group. These results are similar to the studies of L. vannamei fed with Haematococcus pluvialis (46), Trachinotus ovatus fed with Tribonema sp. (28), and Oplegnathus fasciatus fed with Spirulina (47). However, no significant difference in growth performance of Carassius auratus gibelio fed with/without Tribonema sp. was reported in the study of Chen et al. (48). Different results in previous studies might be attributed to differences in animal species, animal size, microalgae species, the dose of dietary microalgae, and the experimental condition. The degree of development of the intestinal morphology was one of the essential indices to affect the growth performance and feed utilization of animals because the gut contacted and absorbed the nutrients directly (49). In particular, higher intestinal villi height indicated a larger contact area between the gut and nutrients and combined with a thicker intestinal mucosal layer, it implied better absorption ability of animals (50, 51). The present study demonstrated that dietary Klebsormidium sp. supplements were significantly beneficial to mid-intestine morphology parameters (the intestinal villi height and the intestinal mucosal layer thickness) of L. vannamei. A previous study showed that a diet supplemented with fish oil (EPA, DHA-rich) or perilla oil (ALA-rich) had protective effects on the intestine morphology of rat samples since n-3 PUFA could mitigate the TNBS-induced colitis, thus reducing the death of intestinal epithelial cells (52, 53). You et al. (54) and other researchers (28, 55, 56) also pointed out that dietary n-3 PUFAs are beneficial to the gut morphology development of aquatic animals. In the present study, better mid-intestine morphology of L. vannamei was obtained in the D2 group, which might be because of rich LA in Klebsormidium sp. that could be converted to EPA and DHA (57), resulting in effective growth performance and feed utilization of L. vannamei.
The growth performance of L. vannamei is also influenced by its metabolic capacity because better metabolic capacity indicates a better nutrient utilization ability in shrimp (58). In addition, hexokinase could convert D-hexose into D-hexose-6-phosphate, which is one of the most important rate-limiting steps in the glycolysis reaction (59). In the present study, the mRNA expression level of hk was significantly upregulated in L. vannamei fed with Klebsormidium sp. Layam and Reddy (60) had shown that dietary Spirulina increased the hexokinase activity of streptozotocin-diabetic rats. However, few studies have been published about the regulating mechanism of microalgae on glycolysis. A previous study demonstrated that conjugated linoleic acid could activate the AMPK pathway in chick embryos (61), an essential pathway associated with regulating cellular energy homeostasis. Therefore, in the present study, the upregulation of the mRNA expression level of hk might be attributed to the LA in Klebsormidium sp., activating the AMPK pathway and then regulating the glycolysis of L. vannamei. Without a doubt, further study of this subject is required. In the present study, the improvement of carbohydrate metabolism capacity may contribute to improving the growth performance and feed utilization of L. vannamei. In addition, other micronutrients like carotenoids (62) in Klebsormidium sp. might be attributed to promoting the growth.
Respiratory bursts will occur if aquatic animals are subjected to environmental stressors, which could produce reactive oxygen species (ROS) for reducing oxidative stress (63). This is one of the effective self-defense mechanisms in cells. However, overproduction of ROS might also attack normal physiological cells and thus cause oxidative damage to cells (64). In this situation, cells would activate the antioxidant system and enhance the activity of antioxidant enzymes (like SOD, GSH-PX, CAT) to scavenge the overproduction of ROS for protecting the cells from oxidative stressors (65, 66). Therefore, the activity of antioxidant enzymes could be regarded as an important index to evaluate the antioxidant capacity of L. vannamei. Except for antioxidant enzymes, the MDA content, which reflects the damage degree of cell structure, can also be used to assess the oxidative state of shrimp (64, 67). In the present study, significantly lower antioxidant enzyme activities (hepatopancreas T-SOD, hepatopancreas GSH-PX, and hemolymph T-SOD), RNA expression levels of hepatopancreas antioxidant genes (gsh-px and cat), and hemolymph MDA content were obtained in the dietary Klebsormidium sp. treating group compared to the control group. Previous studies have shown that PUFAs-rich microalgae (such as Nannochloropsis, Tetraselmis, and Thalassiosira) could bring various health benefits (68, 69). Besides, the inhibition effect of free radical-induced DNA break by LA has been demonstrated in in vitro studies, indicating its potential medicinal value (70). Therefore, in the present study, a diet supplemented with Klebsormidium sp. could improve the antioxidant capacity of L. vannamei, which was correlated to the LA.
Apart from the respiratory burst, an inflammatory response is another important self-defense mechanism of cells for eliminating pathogens (71). However, an excessive inflammatory response might attack healthy tissues and cells and cause various pathological diseases (72). Among them, the NF-κB pathway was one of the crucial inflammatory responses of L. vannamei (32). Relish and rho are two well-known transcription factors involved in the NF-κB pathway (32, 73). In the present study, L. vannamei fed with Klebsormidium sp. obtained remarkably lower RNA expression levels of relish and rho than the control group, indicating that Klebsormidium sp. could mitigate the pro-inflammatory response. This result might be due to the rich LA in Klebsormidium sp. Previous studies showed that n-3 PUFA (EPA, DHA) could inhibit the inflammatory response of rodent samples by reducing the inflammatory mediators (like prostaglandin E2 (PGE2), leukotriene B4 (LTB4), TNF-α, and IL-6) (74–76). Therefore, Klebsormidium sp. could improve the anti-inflammatory capacity of L. vannamei by suppressing the NF-κB pathway.
Overall, diet supplementations of 5% Klebsormidium sp. were beneficial to L. vannamei since they could improve the growth performance, antioxidant and anti-inflammatory status, carbohydrate metabolism, and mid-intestine morphology of shrimp. Therefore, the dose of 5% Klebsormidium sp. is recommended for the daily diet of L. vannamei.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the Experimental Animal Ethics Committee of Sun Yat-sen University.
HF, JN, and WZ designed the study. LH cultured the Klebsormidium sp. HF and ZZ analyzed data. HF carried out the investigation and wrote this paper. WZ modified the language. All authors contributed to the article and approved the submitted version.
This work was supported by Project of Science and Technology of Guangdong Province (2021B0202050002), Project of China Agriculture Research System of MOF and MARA 48 (CARS 48), Project of Science and Technology of Guangdong Province (2019B110209005), and Youth Science, and Technology Innovation Talent of Guangdong TeZhi Plan Talent (2019TQ05N129).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank our supervisors and colleagues for their assistance with the experiment.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.857351/full#supplementary-material
1. Anh PT, Kroeze C, Bush SR, Mol APJ. Water pollution by intensive brackish shrimp farming in south-east vietnam: causes and options for control. Agric Water Manag. (2010) 97:872–82. doi: 10.1016/j.agwat.2010.01.018
2. Prangnell DI, Castro LF, Ali AS, Browdy CL, Zimba PV, Laramore SE, et al. Some limiting factors in superintensive production of juvenile Pacific white shrimp, Litopenaeus vannamei, in no-water-exchange, biofloc-dominated systems. J World Aquac Soc. (2016) 47:396–413. doi: 10.1111/jwas.12275
3. Xiong W, Sun Y, Zhang T, Ding X, Li Y, Wang M, et al. Antibiotics, antibiotic resistance genes, and bacterial community composition in fresh water aquaculture environment in China. Microb Ecol. (2015) 70:425–32. doi: 10.1007/s00248-015-0583-x
4. Liu X, Steele JC, Meng X-Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: a review. Environ Pollut. (2017) 223:161–9. doi: 10.1016/j.envpol.2017.01.003
5. Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. (1956) 28:350–6. doi: 10.1021/ac60111a017
6. Tan X, Luo Z, Xie P, Liu X. Effect of dietary linolenic acid/linoleic acid ratio on growth performance, hepatic fatty acid profiles and intermediary metabolism of juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture. (2009) 296:96–101. doi: 10.1016/j.aquaculture.2009.08.001
7. Codabaccus MB, Bridle AR, Nichols PD, Carter CG. Effect of feeding Atlantic salmon (Salmo salar L) a diet enriched with stearidonic acid from parr to smolt on growth and n-3 long-chain PUFA biosynthesis. Br J Nutr. (2011) 105:1772–82. doi: 10.1017/S0007114510005714
8. Zúñiga J, Cancino M, Medina F, Varela P, Vargas R, Tapia G, et al. N-3 PUFA supplementation triggers PPAR-α activation and PPAR-α/NF-κB interaction: anti-inflammatory implications in liver ischemia-reperfusion injury. PLoS ONE. (2011) 6:e28502. doi: 10.1371/journal.pone.0028502
9. Reifen R, Karlinsky A, Stark AH, Berkovich Z, Nyska A. α-Linolenic acid (ALA) is an anti-inflammatory agent in inflammatory bowel disease. J Nutr Biochem. (2015) 26:1632–40. doi: 10.1016/j.jnutbio.2015.08.006
10. Molendi-Coste O, Legry V, Leclercq IA. Why and how meet n-3 PUFA dietary recommendations? Gastroenterol Res Pract. (2010) 2011 364040. doi: 10.1155/2011/364040
11. Hulbert AJ, Turner N, Storlien LH, Else PL. Dietary fats and membrane function: implications for metabolism and disease. Biol Rev. (2005) 80:155–69. doi: 10.1017/S1464793104006578
12. De Lau LML, Bornebroek M, Witteman JCM, Hofman A, Koudstaal PJ, Breteler MMB. Dietary fatty acids and the risk of Parkinson disease: the Rotterdam study. Neurology. (2005) 64:2040–5. doi: 10.1212/01.WNL.0000166038.67153.9F
13. Salter AM. Dietary fatty acids and cardiovascular disease. Animal. (2013) 7:163–71. doi: 10.1017/S1751731111002023
14. Xu Z, He Q, Gong Y, Wang Y, Chi Q, Liu G, et al. Assessment of a novel oleaginous filamentous microalga Klebsormidium sp. Lgx80 (Streptophyta, Klebsormidiales) for Biomass and Lipid Production. J Phycol. (2021) 57:1151–66. doi: 10.1111/jpy.13137
15. Morison MO, Sheath RG. Responses to desiccation stress by Klebsormidium rivulare (Ulotrichales, Chlorophyta) from a Rhode Island stream. Phycologia. (1985) 24:129–45. doi: 10.2216/i0031-8884-24-2-129.1
16. Karsten U, Holzinger A. Light, temperature, and desiccation effects on photosynthetic activity, and drought-induced ultrastructural changes in the green alga Klebsormidium dissectum (Streptophyta) from a high alpine soil crust. Microb Ecol. (2012) 63:51–63. doi: 10.1007/s00248-011-9924-6
17. Elster J, Degma P, Kováčik L, Valentová L, Šramková K, Pereira AB. Freezing and desiccation injury resistance in the filamentous green alga Klebsormidium from the Antarctic, Arctic and Slovakia. Biologia. (2008) 63:843–51. doi: 10.2478/s11756-008-0111-2
18. Nagao M, Matsui K, Uemura M. Klebsormidium flaccidum, a charophycean green alga, exhibits cold acclimation that is closely associated with compatible solute accumulation and ultrastructural changes. Plant Cell Environ. (2008) 31:872–85. doi: 10.1111/j.1365-3040.2008.01804.x
19. George B, Pancha I, Desai C, Chokshi K, Paliwal C, Ghosh T, et al. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus–A potential strain for bio-fuel production. Bioresour Technol. (2014) 171:367–74. doi: 10.1016/j.biortech.2014.08.086
20. Ma C, Zhang Y-B, Ho S-H, Xing D-F, Ren N-Q, Liu B-F. Cell growth and lipid accumulation of a microalgal mutant Scenedesmus sp. Z-4 by combining light/dark cycle with temperature variation. Biotechnol Biofuels. (2017) 10:1–13. doi: 10.1186/s13068-017-0948-0
21. Vidal OM, Granja CB, Aranguren F, Brock JA, Salazar M. A profound effect of hyperthermia on survival of Litopenaeus vannamei juveniles infected with white spot syndrome virus. J world Aquac Soc. (2001) 32:364–72. doi: 10.1111/j.1749-7345.2001.tb00462.x
22. Qian Y-F, Xie J, Yang S-P, Huang S, Wu W-H, Li L. Inhibitory effect of a quercetin-based soaking formulation and modified atmospheric packaging (MAP) on muscle degradation of Pacific white shrimp (Litopenaeus vannamei). LWT-Food Sci Technol. (2015) 63:1339–46. doi: 10.1016/j.lwt.2015.03.077
23. Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. (1971) 35:171–205. doi: 10.1128/br.35.2.171-205.1971
24. Niu J, Xie S-W, Fang H-H, Xie J-J, Guo T-Y, Zhang Y-M, et al. Dietary values of macroalgae Porphyra haitanensis in Litopenaeus vannamei under normal rearing and WSSV challenge conditions: effect on growth, immune response and intestinal microbiota. Fish Shellfish Immunol. (2018) 81:135–49. doi: 10.1016/j.fsi.2018.06.010
25. Horwitz W. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs/Edited by William Horwitz. Gaithersburg (Maryland); AOAC International (2010).
26. Zhang W, Wang F, Gao B, Huang L, Zhang C. An integrated biorefinery process: Stepwise extraction of fucoxanthin, eicosapentaenoic acid and chrysolaminarin from the same Phaeodactylum tricornutum biomass. Algal Res. (2018) 32:193–200. doi: 10.1016/j.algal.2018.04.002
27. Fang H, Xie J, Zhao W, Liu Z, Liu Y, Tian L, et al. Study supplementation of astaxanthin in high-fat diet on growth performance, antioxidant ability, anti-inflammation, non-specific immunity and intestinal structure of juvenile Trachinotus ovatus. Aquac Nutr. (2021) 27:2575–86. doi: 10.1111/anu.13386
28. Zhao W, Fang H-H, Gao B-Y, Dai C-M, Liu Z-Z, Zhang C-W, et al. Dietary Tribonema sp. supplementation increased growth performance, antioxidant capacity, immunity and improved hepatic health in golden pompano (Trachinotus ovatus). Aquaculture. (2020) 529:735667. doi: 10.1016/j.aquaculture.2020.735667
29. Fang H, Xie J, Liao S, Guo T, Xie S, Liu Y, et al. Effects of dietary inclusion of shrimp paste on growth performance, digestive enzymes activities, antioxidant and immunological status and intestinal morphology of hybrid snakehead (Channa maculata ♀ × Channa argus ♂). Front Physiol. (2019) 10:1027. doi: 10.3389/fphys.2019.01027
30. Cesar JRO, Yang J. Expression patterns of ubiquitin, heat shock protein 70, α-actin and β-actin over the molt cycle in the abdominal muscle of marine shrimp Litopenaeus vannamei. Mol Reprod Dev. (2007) 74:554–9. doi: 10.1002/mrd.20605
31. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2– ΔΔCT method. Methods. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
32. Xie S, Fang W, Wei D, Liu Y, Yin P, Niu J, et al. Dietary supplementation of Haematococcus pluvialis improved the immune capacity and low salinity tolerance ability of post-larval white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. (2018) 80:452–7. doi: 10.1016/j.fsi.2018.06.039
33. Duan Y, Wang Y, Zhang J, Liu Q, Ding X. Morphologic, digestive enzymes and immunological responses of intestine from Litopenaeus vannamei after lipopolysaccharide injection. J Invertebr Pathol. (2018) 153:186–94. doi: 10.1016/j.jip.2018.03.003
34. Cardona E, Saulnier D, Lorgeoux B, Chim L, Gueguen Y. Rearing effect of biofloc on antioxidant and antimicrobial transcriptional response in Litopenaeus stylirostris shrimp facing an experimental sub-lethal hydrogen peroxide stress. Fish Shellfish Immunol. (2015) 45:933–9. doi: 10.1016/j.fsi.2015.05.041
35. Xie S, Wei D, Fang W, Yin P, Liu Y, Niu J, et al. Survival and protein synthesis of post-larval White Shrimp, Litopenaeus vannamei were affected by dietary protein level. Anim Feed Sci Technol. (2020) 263:114462. doi: 10.1016/j.anifeedsci.2020.114462
36. Yang P, He C, Qin Y, Wang W, Mai K, Qin Q, et al. Evaluation of composite mixture of protein sources in replacing fishmeal for Pacific white shrimp (Litopenaeus vannamei): Based on the changing pattern of growth performance, nutrient metabolism and health status. Aquac Reports. (2021) 21:100914. doi: 10.1016/j.aqrep.2021.100914
37. Priyadarshani I, Rath B. Commercial and industrial applications of micro algae–A review. J Algal Biomass Util. (2012) 3:89–100. Available online at: https://d1wqtxts1xzle7.cloudfront.net/44276968/paper14vol3no4-with-cover-page-v2.pdf?Expires=1650622337&Signature=f3hr04B77ePRS2FMPkFgJCIDbuW1kBPoa62B1Jkhs3Qaat7sDb9x6lJzYjaEKlZQwhni7rkkEPGXr1ZCi2ZJC57UnAdB9NIsiUTaW-BWKBUaTDW2-QrXHUtLv4Qq3TKUTd0CwtyN00qeI46WN9QlFYk0rcz1hUPD9wKLGYF2IScSZ4Z7-Va2QAa3lGmqU4x2jMsHakKplkuMC2Dx2OmUF53wvSKyQIoLqf9xB3u~~ICzcUS1MGy44ABAELYrSSUcAJ-MKz9xBPd2NuuTBqw8p1IF7NImFi6KoPbq8e0pSULbl1Z-UJKed9hTqmdR5tqcFF8XrQLFVGxGTQ2DZ8wiqg__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA
38. Khozin-Goldberg I, Iskandarov U, Cohen Z. LC-PUFA from photosynthetic microalgae: occurrence, biosynthesis, and prospects in biotechnology. Appl Microbiol Biotechnol. (2011) 91:905–15. doi: 10.1007/s00253-011-3441-x
39. Spolaore P, Joannis-Cassan C, Duran E, Isambert A. Commercial applications of microalgae. J Biosci Bioeng. (2006) 101:87–96. doi: 10.1263/jbb.101.87
40. Ahmad MT, Shariff M, Yusoff F, Goh YM, Banerjee S. Applications of microalga Chlorella vulgaris in aquaculture. Rev Aquac. (2020) 12:328–46. doi: 10.1111/raq.12320
41. Yaakob Z, Ali E, Zainal A, Mohamad M, Takriff MS. An overview: biomolecules from microalgae for animal feed and aquaculture. J Biol Res. (2014) 21:1–10. doi: 10.1186/2241-5793-21-6
42. Brown MR, Mular M, Miller I, Farmer C, Trenerry C. The vitamin content of microalgae used in aquaculture. J Appl Phycol. (1999) 11:247–55. doi: 10.1023/A:1008075903578
43. Fox, Joe M, Zimba PV. Minerals and trace elements in microalgae. In: Microalgae in Health and Disease Prevention. Academic Press (2018). p. 177–93. doi: 10.1016/B978-0-12-811405-6.00008-6
44. Raposo MF, de Morais RM, Bernardo de Morais AM. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar Drugs. (2013) 11:233–52. doi: 10.3390/md11010233
45. Shah MR, Lutzu GA, Alam A, Sarker P, Chowdhury MAK, Parsaeimehr A, et al. Microalgae in aquafeeds for a sustainable aquaculture industry. J Appl Phycol. (2018) 30:197–213. doi: 10.1007/s10811-017-1234-z
46. Ju ZY, Deng D-F, Dominy W. A defatted microalgae (Haematococcus pluvialis) meal as a protein ingredient to partially replace fishmeal in diets of Pacific white shrimp (Litopenaeus vannamei, Boone, 1931). Aquaculture. (2012) 354–55:50–5. doi: 10.1016/j.aquaculture.2012.04.028
47. Kim S-S, Rahimnejad S, Kim K-W, Lee K-J. Partial replacement of fish meal with Spirulina pacifica in diets for parrot fish (Oplegnathus fasciatus). Turkish J Fish Aquat Sci. (2013) 13:197–204. doi: 10.4194/1303-2712-v13_2_01
48. Chen W, Wang Y, Han D, Zhu X, Xie S, Han D, et al. Two filamentous microalgae as feed ingredients improved flesh quality and enhanced antioxidant capacity and immunity of the gibel carp (Carassius auratus gibelio). Aquac Nutr. (2019) 25:1145–55. doi: 10.1111/anu.12930
49. Chen X, Xie J, Liu Z, Yin P, Chen M, Liu Y, et al. Modulation of growth performance, non-specific immunity, intestinal morphology, the response to hypoxia stress and resistance to Aeromonas hydrophila of grass carp (Ctenopharyngodon idella) by dietary supplementation of a multi-strain probiotic. Comp Biochem Physiol Part C Toxicol Pharmacol. (2020) 231:108724. doi: 10.1016/j.cbpc.2020.108724
50. Zhou Q-C, Buentello JA, Gatlin DM III. Effects of dietary prebiotics on growth performance, immune response and intestinal morphology of red drum (Sciaenops ocellatus). Aquaculture. (2010) 309:253–7. doi: 10.1016/j.aquaculture.2010.09.003
51. Chen Y, Zhu X, Yang Y, Han D, Jin J, Xie S. Effect of dietary chitosan on growth performance, haematology, immune response, intestine morphology, intestine microbiota and disease resistance in gibel carp (C arassius auratus gibelio). Aquac Nutr. (2014) 20:532–46. doi: 10.1111/anu.12106
52. Shoda R, Matsueda K, Yamato S, Umeda N. Therapeutic efficacy of N-3 polyunsaturated fatty acid in experimental Crohn's disease. J Gastroenterol. (1995) 30:98–101.
53. Hassan A, Ibrahim A, Mbodji K, Coëffier M, Ziegler F, Bounoure F, et al. An α-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating NF-κB in rats with TNBS-induced colitis. J Nutr. (2010) 140:1714–21. doi: 10.3945/jn.109.119768
54. You C, Chen B, Wang M, Wang S, Zhang M, Sun Z, et al. Effects of dietary lipid sources on the intestinal microbiome and health of golden pompano (Trachinotus ovatus). Fish Shellfish Immunol. (2019) 89:187–97. doi: 10.1016/j.fsi.2019.03.060
55. Zhu W, Tan P, Lou B, Chen R, Wang L, Xu D. Supplementation of a soybean oil-based diet with tributyrin influences growth, muscle composition, intestinal morphology, and expression of immune-related genes of juvenile yellow drum (Nibea albiflora richardson, 1846). Aquac Int. (2020) 28:2027–43. doi: 10.1007/s10499-020-00572-7
56. Bou M, Berge GM, Baeverfjord G, Sigholt T, Østbye T-K, Romarheim OH, et al. Requirements of n-3 very long-chain PUFA in Atlantic salmon (Salmo salar L): effects of different dietary levels of EPA and DHA on fish performance and tissue composition and integrity. Br J Nutr. (2017) 117:30–47. doi: 10.1017/S0007114516004396
57. González-Félix ML, Lawrence AL, Gatlin III DM, Perez-Velazquez M. Nutritional evaluation of fatty acids for the open thelycum shrimp, Litopenaeus vannamei: I. Effect of dietary linoleic and linolenic acids at different concentrations and ratios on juvenile shrimp growth, survival and fatty acid composition. Aquac Nutr. (2003) 9:105–13. doi: 10.1046/j.1365-2095.2003.00231.x
58. Xie S, Tian L, Jin Y, Yang H, Liang G, Liu Y. Effect of glycine supplementation on growth performance, body composition and salinity stress of juvenile Pacific white shrimp, Litopenaeus vannamei fed low fishmeal diet. Aquaculture. (2014) 418:159–64. doi: 10.1016/j.aquaculture.2013.10.023
59. Soñanez-Organis JG, Peregrino-Uriarte AB, Sotelo-Mundo RR, Forman HJ, Yepiz-Plascencia G. Hexokinase from the white shrimp Litopenaeus vannamei: cDNA sequence, structural protein model and regulation via HIF-1 in response to hypoxia. Comp Biochem Physiol Part B Biochem Mol Biol. (2011) 158:242–9. doi: 10.1016/j.cbpb.2010.12.006
60. Layam A, Reddy CK. Antidiabetic property of spirulina. Chemistry. (2007) 35:29–33. Available online at: https://d1wqtxts1xzle7.cloudfront.net/54551889/Antidiabetic_property_of_Spirulina-with-cover-page-v2.pdf?Expires=1650622472&Signature=fh~IOldTPFmd~kaG0xVpfkWmobahwmkjInwqmulGX7zOULxz5mtvncefDGolNEVYpdjsxLJobBMBai7goPatnYu8qtkvFgKxVGUSiKyL-oHbrV0Xcasgn2VKVKFvHSEOpQfVLr3FEv0iHuYcIa-fbUGVavRrD4g25wDsZoVpcT2dlSXTWIpdRUsp~cvliBKfedFOT7xmyI8lNqQESVLHj7-PzKHjRxq-h2nT5vtfORxDKSKd-3d2ueCjfJf-Jz9CyYjizbRP70kxTG11daznj9bjKndl2SIecLogptJF~U1m3dwKfN0mxkn2o5KPBmDwXVnRra7A3ki~DgNRzUFNNw__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA
61. Fu C, Zhang Y, Yao Q, Wei X, Shi T, Yan P, et al. Maternal conjugated linoleic acid alters hepatic lipid metabolism via the AMPK signaling pathway in chick embryos. Poult Sci. (2020) 99:224–34. doi: 10.3382/ps/pez462
62. Liu F, Qu Y-K, Wang A-M, Yu Y-B, Yang W-P, Lv F, et al. Effects of carotenoids on the growth performance, biochemical parameters, immune responses and disease resistance of yellow catfish (Pelteobagrus fulvidraco) under high-temperature stress. Aquaculture. (2019) 503:293–303. doi: 10.1016/j.aquaculture.2019.01.008
63. Storey KB. Oxidative stress: animal adaptations in nature. Brazilian J Med Biol Res. (1996) 29:1715–33.
64. Sevcikova M, Modra H, Slaninova A, Svobodova Z. Metals as a cause of oxidative stress in fish: a review. Vet Med. (2011) 56:537–46. doi: 10.17221/4272-VETMED
65. Lopes PA, Pinheiro T, Santos MC, da Luz Mathias M, Collares-Pereira MJ, Viegas-Crespo AM. Response of antioxidant enzymes in freshwater fish populations (Leuciscus alburnoides complex) to inorganic pollutants exposure. Sci Total Environ. (2001) 280:153–63. doi: 10.1016/S0048-9697(01)00822-1
66. Nwani CD, Lakra WS, Nagpure NS, Kumar R, Kushwaha B, Srivastava SK. Toxicity of the herbicide atrazine: effects on lipid peroxidation and activities of antioxidant enzymes in the freshwater fish Channa punctatus (Bloch). Int J Environ Res Public Health. (2010) 7:3298–312. doi: 10.3390/ijerph7083298
67. Slaninova A, Smutna M, Modra H, Svobodova Z. Reviews oxidative stress in fish induced by pesticides. Neuroendocrinol Lett. (2009) 30:2. Available online at: https://www.researchgate.net/profile/Andrea-Slaninova/publication/40757648_A_review_Oxidative_stress_in_fish_induced_by_pesticides/links/0f317534fbbf746635000000/A-review-Oxidative-stress-in-fish-induced-by-pesticides.pdf
68. Panis G, Carreon JR. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: a microalgae process model and a techno-economic assessment all through production line. Algal Res. (2016) 18:175–90. doi: 10.1016/j.algal.2016.06.007
69. Khan MNA, Choi JS, Lee MC, Kim E, Nam TJ, Fujii H, et al. Anti-inflammatory activities of methanol extracts from various seaweed species. J Env Biol. (2008) 29:465–9. Available online at: https://www.researchgate.net/profile/Jae-Suk-Choi/publication/23981781_Anti-inflammatory_activities_of_methanol_extracts_from_various_seaweed_species/links/55e57aad08aede0b5735a0b7/Anti-inflammatory-activities-of-methanol-extracts-from-various-seaweed-species.pdf
70. Hiramoto K, Yasuhara Y, Sako K, Aoki K, Kikugawa K. Suppression of free radical-induced DNA strand breaks by linoleic acid and low density lipoprotein in vitro. Biol Pharm Bull. (2003) 26:1129–34. doi: 10.1248/bpb.26.1129
71. Quispe RL, Justino EB, Vieira FN, Jaramillo ML, Rosa RD, Perazzolo LM. Transcriptional profiling of immune-related genes in Pacific white shrimp (Litopenaeus vannamei) during ontogenesis. Fish Shellfish Immunol. (2016) 58:103–7. doi: 10.1016/j.fsi.2016.09.024
72. Yu Y, He J, Li S, Song L, Guo X, Yao W, et al. Fibroblast growth factor 21 (FGF21) inhibits macrophage-mediated inflammation by activating Nrf2 and suppressing the NF-κB signaling pathway. Int Immunopharmacol. (2016) 38:144–52. doi: 10.1016/j.intimp.2016.05.026
73. Mishra AK, Sharma V, Mutsuddi M, Mukherjee A. Signaling cross-talk during development: Context-specific networking of Notch, NF-κB and JNK signaling pathways in Drosophila. Cell Signal. (2021) 82:109937. doi: 10.1016/j.cellsig.2021.109937
74. Ibrahim A, Mbodji K, Hassan A, Aziz M, Boukhettala N, Coëffier M, et al. Anti-inflammatory and anti-angiogenic effect of long chain n-3 polyunsaturated fatty acids in intestinal microvascular endothelium. Clin Nutr. (2011) 30:678–87. doi: 10.1016/j.clnu.2011.05.002
75. Mullen A, Loscher CE, Roche HM. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J Nutr Biochem. (2010) 21:444–50. doi: 10.1016/j.jnutbio.2009.02.008
Keywords: Klebsormidium sp., Litopenaeus vannamei, growth performance, antioxidant, hepatopancreas health
Citation: Fang H, Zhuang Z, Huang L, Zhao W and Niu J (2022) Dietary Klebsormidium sp. Supplementation Improves Growth Performance, Antioxidant and Anti-Inflammatory Status, Metabolism, and Mid-Intestine Morphology of Litopenaeus Vannamei. Front. Nutr. 9:857351. doi: 10.3389/fnut.2022.857351
Received: 18 January 2022; Accepted: 07 April 2022;
Published: 12 May 2022.
Edited by:
Sandrina A. Heleno, Polytechnic Institute of Bragança (IPB), PortugalReviewed by:
Ye Yuan, Shantou University, ChinaCopyright © 2022 Fang, Zhuang, Huang, Zhao and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhao, end0am54eXNjQDE2My5jb20=; Jin Niu, bml1ajNAbWFpbC5zeXN1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.