
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 31 March 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.846591
This article is part of the Research Topic Diet-Microbe-Host Interactions in Metabolic Syndrome View all 12 articles
 Mengjun Wang1†
Mengjun Wang1† Junliang Liu2†
Junliang Liu2† Zhao Zhang2*
Zhao Zhang2* Haixiong Zhang2
Haixiong Zhang2 Ning Wang1
Ning Wang1 Xi Chen3
Xi Chen3 Xuemei Han2
Xuemei Han2 Qian Lu2
Qian Lu2 Shanshan Chi2
Shanshan Chi2Background: Dietary interventions may modulate inflammatory indicators, but the correlations between dietary intervention and inflammatory markers in metabolic syndrome (MetS) settings remain opaque.
Objective: To evaluate the effects of dietary intervention on interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) in patients with MetS by systematic review and meta-analysis.
Methods: Databases, including PubMed, Embase, Cochrane Library, Scopus, and Google scholar, were searched from June 2011 to June 2021 for relevant available articles. Standardized mean difference (SMD) was generated as effect size by meta-analysis for continuous variants, including IL-1β, IL-6, TNF-α, and CRP levels. Then, according to study characteristics by dietary patterns of the intervention, subgroup analyses were performed.
Results: Finally, 13 studies comprising a total of 1,101 participants were included for the meta-analysis. IL-6 levels in dietary patients were significantly lower than controls (SMD = −0.30, 95% CI = −0.55, 0.04, p = 0.02, I2 = 64%). However, IL-1β, TNF-α, and CRP levels did not change significantly compared with the control group. Sensitivity analyses further yielded similar results.
Conclusions: Dietary intervention may help decrease IL-6 rather than IL-1β, TNF-α, or CRP levels in patients with MetS.
Metabolic syndrome (MetS) has become a global epidemic disease due to population aging and lifestyle changes, including diets (1). The various definition and criteria for identifying MetS (2) includes interrelated factors, such as abdominal obesity, insulin resistance, hyperglycemia, hypertension, and dyslipidemia (low high-density lipoprotein and increased triglyceride) (3). Sub-clinical TH1–lymphocyte-mediated innate and chronic low-grade inflammation might partially account for its occurrence (4). The interleukin-1 (IL-1) family, particularly IL-1β, is a group of cytokines that play a central role in the regulation of responses associated with immune and obesity-associated inflammation (5). IL-6 is a major pro-inflammatory cytokine in chronic inflammation that is closely related to insulin resistance, neurodegeneration, cardiovascular disease (CVD), and malignancy (6). A few pro-inflammatory cytokines [IL-6 and tumor necrosis factor-α (TNF-α)] can promote the upgrade of plasma C-reactive protein (CRP) level (7).
Bad eating habits are the controllable factors accelerating the development of inflammation and related diseases, including MetS (8). According to literature, food can modify inflammatory responses. It is also strongly linked to the pathogenesis of MetS (9). A healthy diet could contribute most to managing obesity and MetS (10). Currently, nutritional epidemiology tends to illustrate the relationship between dietary intervention and inflammatory diseases, but do not demonstrate the exact food species (11).
Although some trials have proved that dietary intervention can reduce the serum level of inflammatory factors, it is still controversial if there is an association between dietary intervention and the serum level of inflammatory factors in patients with MetS.
Hence, in this study, we aimed to perform a systematic review and meta-analysis to investigate the effects of dietary intervention on IL-1β, IL-6, TNF-α, and CRP levels in MetS.
The current systematic review and meta-analysis was implemented in accordance with the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (12).
Database, including PubMed, Embase, Cochrane Library, Scopus, and Google scholar, were searched from June 15, 2011 to June 15, 2021. We used the following mesh terms: “inflammatory markers,” “diet” in combination with “metabolic syndrome.”
Inclusion criteria were as follows:
(1) Randomized controlled trial (RCT) studies;
(2) Conducted dietary intervention/s of more than 4 weeks;
(3) Meets the diagnostic criteria of MetS;
(4) Studies that provided numbers, means, and standard difference (SDs) of IL-1β, IL-6, TNF-α, and CRP.
Exclusion criteria were as follows:
(1) literature reviews, in vitro study, animal study, or case report;
(2) Included patients who had co-morbidities other than MetS;
(3) (Patients were administrated drugs which might change the levels of cytokines of interest;
(4) No full text.
The following information were collected: (1) publication data (first author's name, publication year, and country), (2) study design, (3) total number of participants, their age, sex, BMI, and study duration, (4) glucose, insulin, blood lipid levels, and blood pressure status, and (5) mean and standard difference for IL-1β, IL-6, TNF-α, and CRP levels.
Qualities of enrolled studies were assessed according to the Cochrane Risk of Bias Tool (13). Two investigators independently assessed the quality and extracted data of all included studies. Any discrepancy was adjudicated by a senior investigator.
Review Manager 5.3 was implemented in our analyses. p < .05 was considered to be statistically significant. Standardized mean difference (SMD) was generated as effect size by meta-analysis for continuous variants, including IL-1β, IL-6, TNF-α, and CRP levels. If I2 <50% and p > 0.01, a fixed-effect model would be used. Otherwise, a random effect model would be implemented. If I2 > 75%, further analysis encompassing sensitive analysis, subgroup analysis, or meta-regression was carried out to explore the source of heterogeneity. Publication bias was evaluated by funnel plot and Egger's tests.
Eventually, 13 articles (14–26) reporting 1,101 patients were enrolled in this study (Figure 1). The baseline characteristics are summarized in Supplementary Table 1. Intervention duration varied from 8 weeks to 6 months. Studies followed different MetS diagnostic criteria, namely, six studies used the Adult Treatment Panel III criteria (17, 18, 20, 21, 24, 26); three studies used National Cholesterol Education Programme/Adult Treatment Panel III (NCEP-ATP III) criteria (14, 15, 19); two studies used the Joint Interim Statement (JIS) (16, 23); and two studies used International Diabetes Federation criteria (IDF) (22, 24). The dietary patterns of the intervention received by the intervention groups were as follows: low-calorie diet, fatty acids, berry, and whole wheat. We selected the following inflammatory markers: IL-1β, IL-6, TNF-α, and CRP for analysis. They were measured by enzyme-linked immunosorbent assay (ELISA) in all the studies.
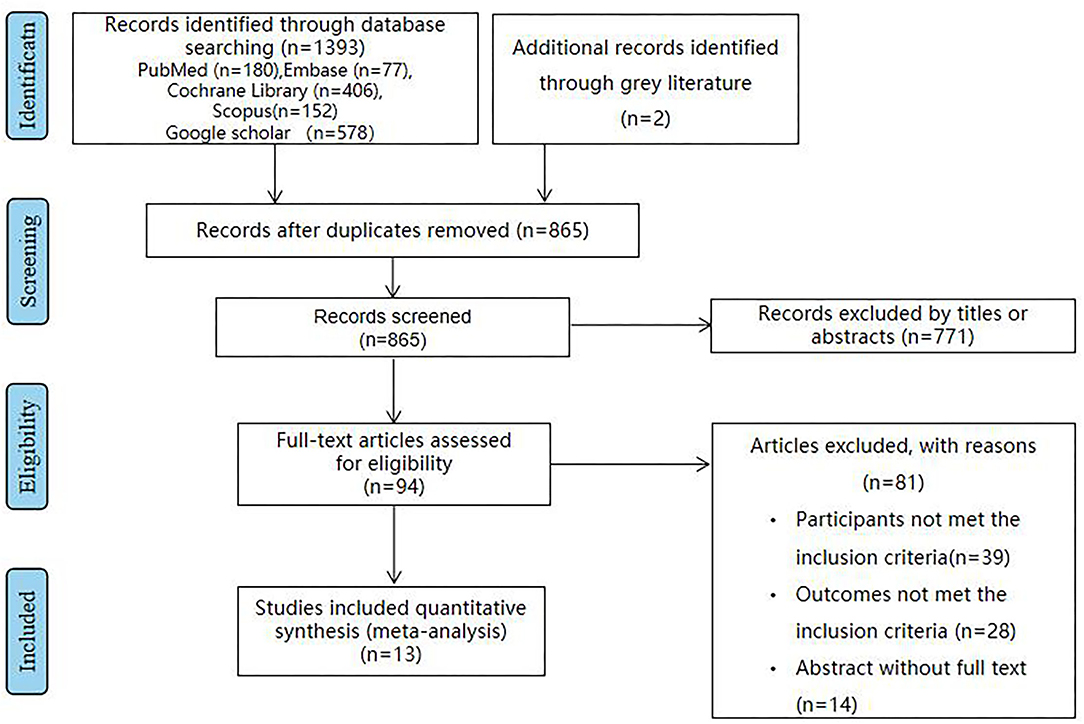
Figure 1. The flowchart shows the article selection process we performed. It shows the process by which relevant studies were retrieved from the databases, assessed, and selected, or excluded. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram for study search was used.
In the included 13 trails, the following six types of dietary intervention patterns were investigated: berry (16, 20, 26), fatty acids (14, 15, 19), low-calorie diet (22, 23), whole wheat (18, 21, 24), whole egg (17), and pistachio nuts (25). The berry intervention patterns included cranberry and black raspberry. Subjects received the same dose of berry diet or placebo. The fatty acids diet intervention patterns referred to diet alone or diet plus omega-3 polyunsaturated fatty acids (PUFAs) supplementation. The low-calorie diet included 50–60% carbohydrate, <30% total fat, and <10% saturated fat. The whole wheat diet included wholegrain or refined cereal products. The whole egg intervention pattern referred to three whole eggs containing 0 g carbohydrate, 16 g protein, and 12 g fat. The pistachio nuts diets referred to participants advised to take pistachios for 20% of total energy.
A risk of bias summary is depicted in Figure 2, and the risk of bias estimation within each of the studies selected is shown in Figure 3. Random sequence generation was adequate in the 5 trials. Three of them have received the maximal score, and none was considered low quality.
Based on data from 2 trials (101 participants) (15, 21), we found no effect of diet intervention on IL-1β change compared with control (SMD = −0.08, 95% CI: −0.30, 0.14; Figure 4).
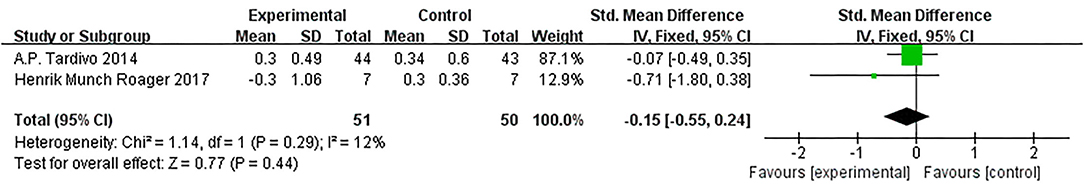
Figure 4. Results of a meta-analysis for the effects of interleukin−1β (IL-1β). Study effect sizes of IL-1β levels differences between metabolic syndrome (MetS) and controls. Each data marker represents a study, and the size of the data marker is proportional to the total number of individuals in that study. The summary effect size for the IL-1β levels of each study is denoted by a diamond. Effect estimates are reported as standardized mean difference (SMD) and 95% confidence intervals. I2 represents the magnitude of heterogeneity. p ≤ 0.05 is considered as significant.
Of the 11 included studies (14–16, 18–25), when all the data were pooled in the meta-analysis, overall IL-6 levels in dietary patients were significantly lower than controls (SMD = −0.30, 95% CI = −0.55, 0.04, p = 0.02, I2 = 64%). Figure 5 demonstrates the subgroup analyses for the IL-6 levels of between dietary intervention and the controls. The IL-6 of participants who underwent low-calorie diet, berry, whole wheat, and fatty acids dietary intervention decreased compared with the control group (SMD = −0.17, 95% CI = −0.64, 0.30; SMD = −0.34, 95% CI = −0.76, 0.08; SMD = −0.04, 95% CI = −0.60, 0.52; SMD = −0.75, 95% CI = −1.12, −0.38). Figure 6 demonstrates the subgroup analyses by MetS assessment method and shows how the NCEP ATP III and JIS assessment decreased compared with the control group (SMD = −0.75, 95% CI = −1.12, −0.38; SMD = −0.55, 95% CI = −1.01, −0.09).
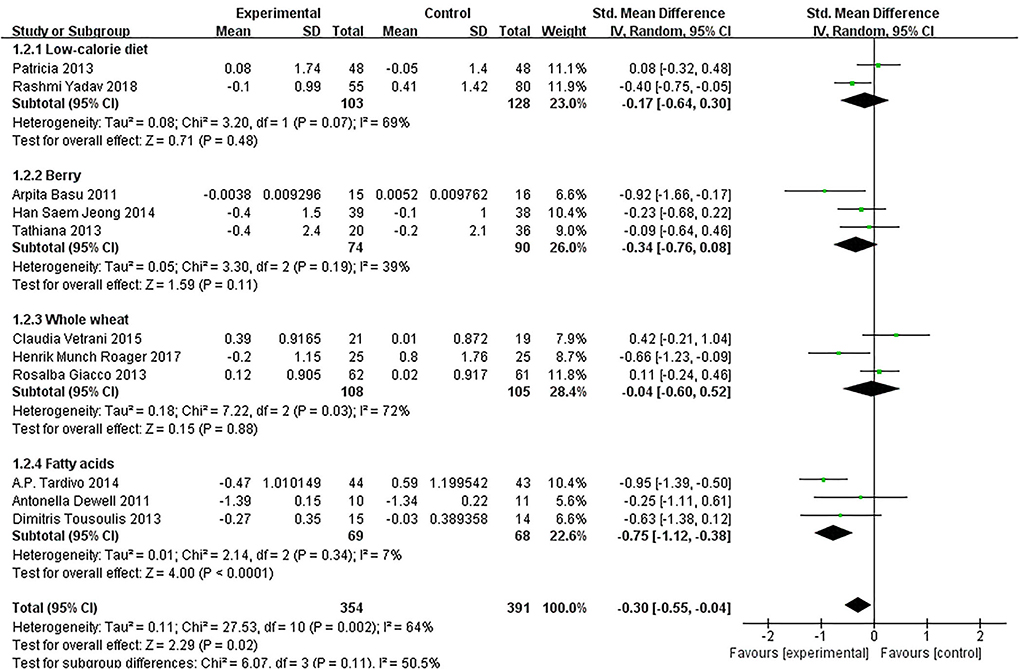
Figure 5. The subgroup analyses for the interleukin-6 (IL-6) levels by dietary intervention. Study effect sizes of the subgroup analyses for the IL-6 levels differences by dietary intervention between MetS and controls.
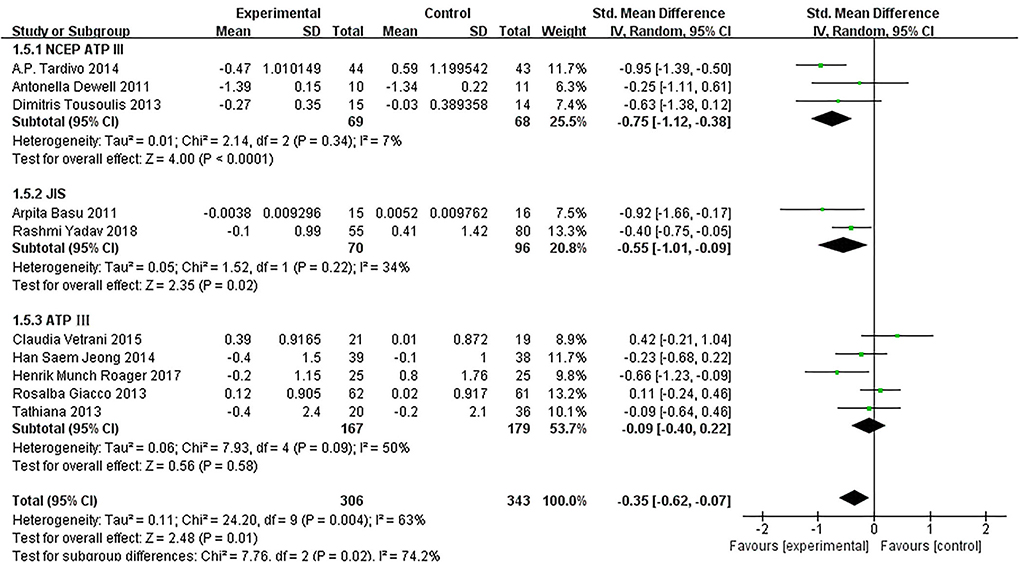
Figure 6. The subgroup analyses for the IL-6 levels by MetS assessment method. Study effect sizes of the subgroup analyses for the IL-6 level differences by MetS assessment method between MetS and controls.
As presented in Figure 7, our overall pooled analysis did not reveal the association between dietary intervention and the TNF-α levels (15, 17, 18, 20–24, 26) (SMD = −0.11, 95% CI: −0.28, 0.06). According to the assessment method of MetS, the effect estimate did not change considerably (Figure 8).
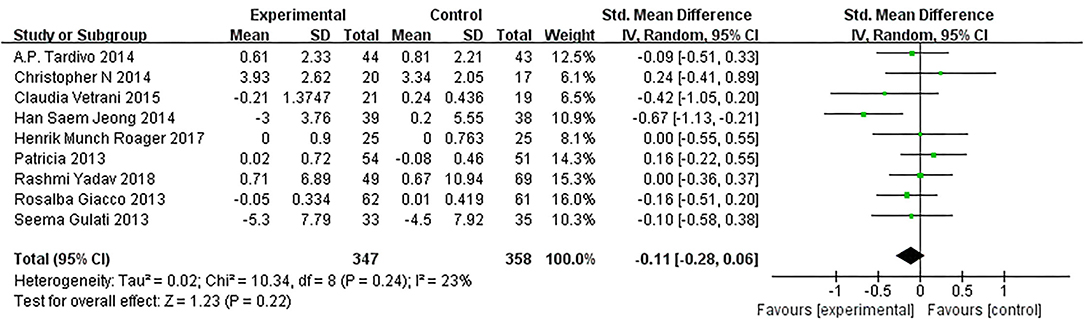
Figure 7. Results of a meta-analysis for the effects of tumor necrosis factor α (TNF-α). Study effect sizes of the subgroup analyses for the TNF-α levels differences between MetS and controls.
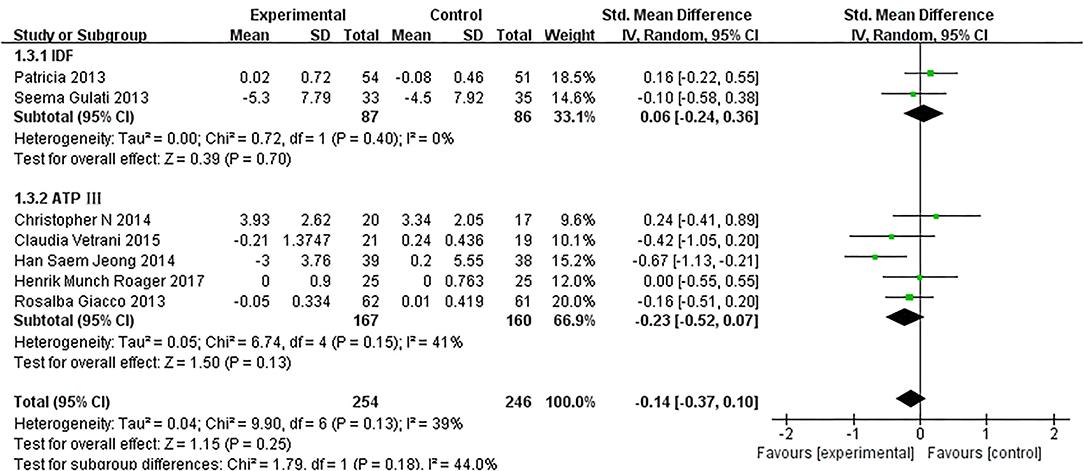
Figure 8. The subgroup analyses for the TNF-α levels by MetS assessment method. Study effect sizes of the subgroup analyses for the TNF-α levels differences by MetS assessment method between MetS and controls.
Based on data from 8 trials (458 participants) (15, 16, 18, 20–22, 26), changes on CRP due to dietary intervention were not found compared with control (SMD: 0.03, 95% CI: −0.20, 0.25; Figure 9). Similarly, the subgroup of MetS definitions did not change (Figure 10).
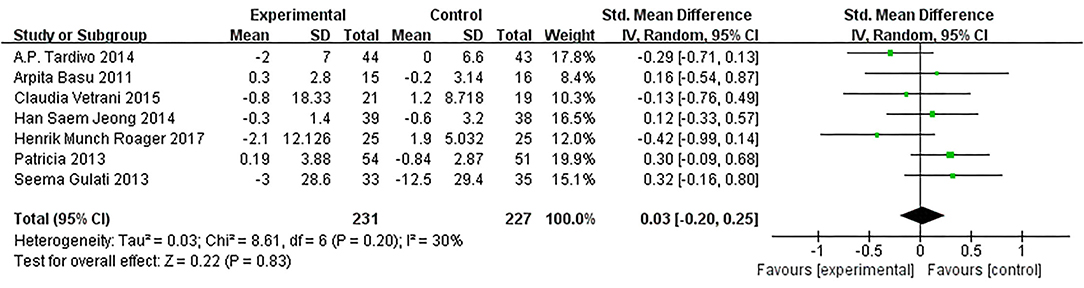
Figure 9. Results of a meta-analysis for the effects of c-reactive protein (CRP). Study effect sizes of the subgroup analyses for the CRP levels differences between MetS and controls.
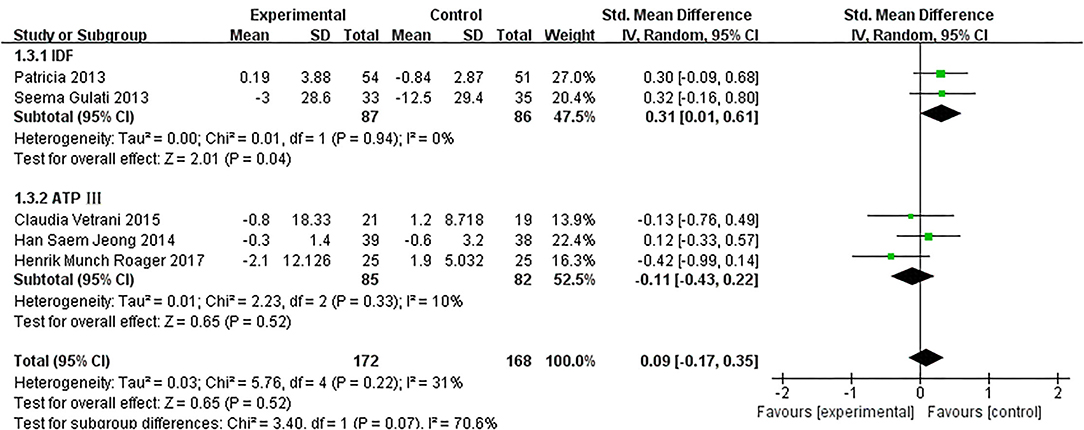
Figure 10. The subgroup analyses for the CRP levels by MetS assessment method. Study effect sizes of the subgroup analyses for the CRP levels differences by MetS assessment method between MetS and controls.
The scatter funnel plot in IL-6 levels appeared symmetrical, indicating the absence of publication bias. Additionally, Egger's test detected no publication bias (p = 0.320). The number of studies that analyzed IL-1β, TNF-α, and CRP levels was <10. Hence, it was inadequate to perform a publication bias test.
To our knowledge, this is the first meta-analysis to provide evidence that dietary intervention could improve immunological properties, particularly IL-6, in MetS. Further analysis based on subgroups indicated that these results were affected by diet patterns of MetS. Despite this, it did not reveal the association between dietary intervention and the IL-1β, CRP, and TNF-α levels.
Chronic systemic inflammation had long been considered as a major factor in the development and progression of several non-communicable diseases (NCDs), including MetS, diabetes mellitus, obesity, and cancer (27). This was a low-grade variation in the immune homeostasis that adversely regulated metabolic processes over time (28). The occurrence and development of MetS was related to pro-inflammatory cytokines.
Interleukin-1β could increase insulin resistance and promote apoptosis of β cells in animals (29). Although we did not find that dietary intervention could significantly reduce the level of the pro-inflammatory cytokine IL-1β, the anti-IL-1β agents improve insulin secretion and β cell function and reduce inflammation in humans (30).
Interleukin-6 was shown to be a vital mediator of acute phase response with a pleiotropic effect on inflammation during immune response. We found that IL-6 significantly decreased after dietary intervention, especially with fatty acids diet. It may be that dietary intervention can produce immediate changes in MetS, leading to significant changes in IL-6. The JIS and the IDF definitions substantially identify more individuals with MetS than subjects with MetS diagnosed by the NCEP ATP III/ATP III definitions (31). As for the results of subgroup analysis, the NCEP ATP III and JIS assessment show they were statistically significant in identifying patients with MetS, but there was no sufficient evidence.
Tumor necrosis factor-α induced phosphorylation of insulin receptor substrate 1 (IRS-1), preventing insulin from binding to the receptor, which consequently led to insulin resistance (32). In addition, TNF-α and IL-6 mainly came from adipose tissue, which were significantly increased in adults with MetS as they were positively correlated with the degree of obesity (33). Adipose tissue could induce a wide range of acute-phase proteins, such as CRP, fibrinogen, and thrombopoietin, and induce systemic acute phase response (34). These findings supported the role of IL-6, TNF-α, and IL-1β, which are all related to the occurrence of insulin resistance. On the other hand, TNF-α and IL-1β inhibit β Cells and promote their apoptosis (32). Hence, only dietary intervention significantly reduced IL-6 levels, while the rest of the pro-inflammatory cytokines showed a similar trend. We found that these indicators did not reach statistical differences through dietary intervention. This may be due to the patients being in a state of low-grade inflammation. This raises the possibility that data might change significantly if the inclusion standards were to be raised.
Low-calorie diets had been shown to improve the level of inflammatory factors, particularly with advancing age and improving obesity and MetS parameters. However, this was achieved through a significant reduction of total energy intake and supplementing micronutrients (35). The research indicated that after 1 year of low-calorie diet intake, severely obese patients did not show more weight loss, significant changes in MetS indicators, or levels of inflammation markers than those on conventional weight-loss diet (36).
In addition, one of the most commonly used dietary modifications consists of increasing the protein content of the diet. The role of this modification in inflammation was controversial (37). The DiOGenes project reported (apparently for the first time) that dietary protein content influences inflammation, specifically CRP concentrations. This pan-European-controlled dietary intervention study compared a high-protein diet with a low-protein diet in overweight and obese adults and found that the lower protein content appeared to be associated with a further decrease of CRP as compared with the high-protein diet. Excess protein supplementation enhanced phosphorus overload which can lead to acidosis and exacerbated insulin resistance (38). In addition, the meta-analysis by Namazi et al. (39) found no significant association between the pro-inflammatory diet and MetS.
Targeting anti-inflammatory therapies had been applied to patients with MetS. In doing so, the IL1-β inhibitor, Canakinumab, increased insulin secretion (40). In addition, IL-6 inhibitors, tocilizumab, and anti-TNF drugs, were shown to possibly primarily affect insulin-sensitive tissues (41). In the future, the combination of diet and anti-inflammatory drugs may become one of the new approaches to treat MetS.
Firstly, most eligible studies did not adjust potential confounding factors. Secondly, due to the influence of gender, race, ethnicity, and social factors, our findings should be interpreted in different geographic contexts. Thirdly, the reason for the analysis might be due to the limited time of intervention for the inclusion of RCT, leading to no significant changes in inflammatory marker levels. In addition, individual differences, different methods of intervention, and sample size might also directly affect the analysis results. Lastly, according to different diagnostic criteria, long-term trends may have confounded the results and limited generalizability.
Our study suggests that dietary intervention might decrease IL-6, IL-1β, CRP, and TNF-α in MetS. However, different diets might have different protective mechanisms for MetS. More time and more appropriate dietary patterns are needed to improve the inflammatory state of MetS. In addition, more research is needed to clarify the mechanisms underlying the effect of dietary intervention on this population's inflammatory markers. Anti-inflammatory agents combined with dietary intervention may be therapeutically useful in treating and preventing MetS.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
ZZ contributed to the study design, wrote the manuscript, reviewed and edited the manuscript. MW, JL, ZZ, HZ, XC, and NW conducted the literature search and performed data extraction and data analysis. MW, JL, and XC did the statistical analyses. MW, JL, XC, XH, QL, and SC contributed to the writing of the manuscript. All authors approved the submitted manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.846591/full#supplementary-material
MetS, metabolic syndrome; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; CRP, C-reactive protein; SMD, Standardized mean difference; CI, Confidence interval; CVD, cardiovascular disease; RCT, Randomized controlled trial; SDs, standard difference; IDF, International Diabetes Federation criteria; JIS, Joint Interim Statement; ATP III, Adult Treatment Panel III criteria; NCEP ATP III, National Cholesterol Education Program, Adult Treatment Panel III; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FPG, Fasting plasma glucose; NR, not reported; ELISA, enzyme-linked immunosorbent assay; PUFAs, polyunsaturated fatty acids; NCDs, non-communicable diseases; IRS-1, insulin receptor substrate 1; DiOGenes, Diet, Obesity, and Genes.
1. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens. Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
2. Alberti KG, Zimmet P, Shaw J. IDF epidemiology task force consensus group. the metabolic syndrome—a new worldwide definition. Lancet. (2005) 366:1059–62. doi: 10.1016/S0140-6736(05)67402-8
3. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; World heart mfederation; International. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
4. Alicka M, Marycz K. The effect of chronic inflammation and oxidative and endoplasmic reticulum stress in the course of metabolic syndrome and its therapy. Stem Cells Int. (2018) 2018:4274361. doi: 10.1155/2018/4274361
5. Besedovsky HO, Rey AD. Physiologic versus diabetogenic effects of interleukin-1: a question of weight. Curr Pharm Des. (2014) 20:4733–40. doi: 10.2174/1381612820666140130204401
6. Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. (2000) 148:209–14. doi: 10.1016/S0021-9150(99)00463-3
7. Marques-Vidal P, Schmid R, Bochud M, Bastardot F, Von Känel R, Paccaud F, et al. Adipocytokines, hepatic and inflammatory biomarkers and incidence of type 2 diabetes. The CoLaus study. PLoS ONE. (2012) 7:e51768. doi: 10.1371/journal.pone.0051768
8. O'Neil A, Shivappa N, Jacka FN, Kotowicz MA, Kibbey K, Hebert JR. Pro-inflammatory dietary intake as a risk factor for CVD in men: a 5-year longitudinal study. Br J Nutr. (2015) 114:2074–82. doi: 10.1017/S0007114515003815
9. Julibert A, Bibiloni MDM, Mateos D, Angullo E, Tur JA. Dietary fat intake and metabolic syndrome in older adults. Nutrients. (2019) 11:1901. doi: 10.3390/nu11081901
10. Martins AD, Majzoub A, Agawal A. Metabolic syndrome and male fertility. World J Mens Health. (2019) 37:113–27. doi: 10.5534/wjmh.180055
11. Michels KB, Schulze MB. Can dietary patterns help us detect diet-disease associations? Nutr Res Rev. (2005) 18:241–8. doi: 10.1079/NRR2005107
12. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
13. Higgins J, Altman D, Sterne J. Chapter 8: assessing risk of bias in included studies. In: Higgins J, Churchill R, Chandler J, Cumpston M, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (Updated June 2017). Chichester: Cochrane (2017).
14. Dewell A, Marvasti FF, Harris WS, Tsao P, Gardner CD. Low- and high-dose plant and marine (n-3) fatty acids do not affect plasma inflammatory markers in adults with metabolic syndrome. J Nutr. (2011) 141:2166–71. doi: 10.3945/jn.111.142240
15. Tardivo AP, Nahas-Neto J, Orsatti CL, Dias FB, Poloni PF, Schmitt EB, et al. Effects of omega-3 on metabolic markers in postmenopausal women with metabolic syndrome. Climacteric. (2015) 18:290–8. doi: 10.3109/13697137.2014.981521
16. Basu A, Betts NM, Ortiz J, Simmons B, Wu M, Lyons TJ. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr Res. (2011) 31:190–6. doi: 10.1016/j.nutres.2011.02.003
17. Blesso CN, Andersen CJ, Barona J, Volk B, Volek JS, Fernandez ML. Effects of carbohydrate restriction and dietary cholesterol provided by eggs on clinical risk factors in metabolic syndrome. J Clin Lipidol. (2013) 7:463–71. doi: 10.1016/j.jacl.2013.03.008
18. Vetrani C, Costabile G, Luongo D, Naviglio D, Rivellese AA, Riccardi G, et al. Effects of whole-grain cereal foods on plasma short chain fatty acid concentrations in individuals with the metabolic syndrome. Nutrition. (2016) 32:217–21. doi: 10.1016/j.nut.2015.08.006
19. Tousoulis D, Plastiras A, Siasos G, Oikonomou E, Verveniotis A, Kokkou E, et al. Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis. (2014) 232:10–6. doi: 10.1016/j.atherosclerosis.2013.10.014
20. Jeong HS, Hong SJ, Lee TB, Kwon JW, Jeong JT, Joo HJ, et al. Effects of black raspberry on lipid profiles and vascular endothelial function in patients with metabolic syndrome. Phytother Res. (2014) 28:1492–8. doi: 10.1002/ptr.5154
21. Roager HM, Vogt JK, Kristensen M, Hansen LBS, Ibrügger S, Mærkedahl RB, et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut. (2019) 68:83–93. doi: 10.1136/gutjnl-2017-314786
22. Lopez-Legarrea P, de la Iglesia R, Abete I, Navas-Carretero S, Martinez JA, Zulet MA. The protein type within a hypocaloric diet affects obesity-related inflammation: the RESMENA project. Nutrition. (2014) 30:424–9. doi: 10.1016/j.nut.2013.09.009
23. Yadav R, Yadav RK, Khadgawat R, Pandey RM. Comparative efficacy of a 12 week yoga-based lifestyle intervtention and dietary intervention on adipokines, inflammation, and oxidative stress in adults wih metabolic syndrome: a randomized controlled trial. Transl Behav Med. (2019) 9:594–604. doi: 10.1093/tbm/iby060
24. Giacco R, Lappi J, Costabile G, Kolehmainen M, Schwab U, Landberg R, et al. Effects of rye and whole wheat versus refined cereal foods on metabolic risk factors: a randomised controlled two-centre intervention study. Clin Nutr. (2013) 32:941–9. doi: 10.1016/j.clnu.2013.01.016
25. Simão TN, Lozovoy MA, Simão AN, Oliveira SR, Venturini D, Morimoto HK, et al. Reduced-energy cranberry juice increases folic acid and adiponectin and reduces homocysteine and oxidative stress in patients with the metabolic syndrome. Br J Nutr. (2013) 110:1885–94. doi: 10.1017/S0007114513001207
26. Gulati S, Misra A, Pandey RM, Bhatt SP, Saluja S. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: a 24-wk, randomized control trial. Nutrition. (2014) 30:192–7. doi: 10.1016/j.nut.2013.08.005
27. Vissers LE, Waller MA, van der Schouw YT, Hebert JR, Shivappa N, Schoenaker DA, et al. The relationship between the dietary inflammatory index and risk of total cardiovascular disease, ischemic heart disease and cerebrovascular disease: findings from an Australian population-based prospective cohort study of women. Atherosclerosis. (2016) 253:164–70. doi: 10.1016/j.atherosclerosis.2016.07.929
28. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. (2011) 121:2111–7. doi: 10.1172/JCI57132
29. Ibarra Urizar A, Friberg J, Christensen DP, Lund Christensen G, Billestrup N. Inflammatory cytokines stimulate bone morphogenetic protein-2 expression and release from pancreatic beta cells. J Interferon Cytokine Res. (2016) 36:20–9. doi: 10.1089/jir.2014.0199
30. Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. (2007) 356:1517–26. doi: 10.1056/NEJMoa065213
31. Athyros VG, Ganotakis ES, Tziomalos K, Papageorgiou AA, Anagnostis P, Griva T, et al. Comparison of four definitions of the metabolic syndrome in a Greek (Mediterranean) population. Curr Med Res Opin. (2010) 26:713–9. doi: 10.1185/03007991003590597
32. Smitka K, Marešová D. Adipose tissue as an endocrine organ: an update on pro-inflammatory and anti-inflammatory microenvironment. Prague Med Rep. (2015) 116:87–111. doi: 10.14712/23362936.2015.49
33. Popko K, Gorska E, Stelmaszczyk-Emmel A, Plywaczewski R, Stoklosa A, Gorecka D, et al. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur J Med Res. (2010) 15 (Suppl. 2):120–2. doi: 10.1186/2047-783X-15-S2-120
34. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. (1990) 265:621–36. doi: 10.1042/bj2650621
35. Sitzmann BD, Brown DI, Garyfallou VT, Kohama SG, Mattison JA, Ingram DK, et al. Impact of moderate calorie restriction on testicular morphology and endocrine function in adult rhesus macaques (Macaca mulatta). Age. (2014) 36:183–97. doi: 10.1007/s11357-013-9563-6
36. Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. (2004) 140:778–85. doi: 10.7326/0003-4819-140-10-200405180-00007
37. Santesso N, Akl EA, Bianchi M, Mente A, Mustafa R, Heels-Ansdell D, et al. Effects of higher- versus lower-protein diets on health outcomes: a systematic review and meta-analysis. Eur J Clin Nutr. (2012) 66:780–8. doi: 10.1038/ejcn.2012.37
38. Mitch WE. Beneficial responses to modified diets in treating patients with chronic kidney disease. Kidney Int Suppl. (2005) S133–5. doi: 10.1111/j.1523-1755.2005.09430.x
39. Namazi N, Larijani B, Azadbakht L. Dietary inflammatory index and its association with the risk of cardiovascular diseases, metabolic syndrome, and mortality: a systematic review and meta-analysis. Horm Metab Res. (2018) 50:345–58. doi: 10.1055/a-0596-8204
40. Zhao G, Dharmadhikari G, Maedler K, Meyer-Hermann M. Possible role of interleukin-1β in type 2 diabetes onset and implications for anti-inflammatory therapy strategies. PLoS Comput Biol. (2014) 10:e1003798. doi: 10.1371/journal.pcbi.1003798
Keywords: diets, inflammatory markers, IL-6, metabolic syndrome, meta-analysis
Citation: Wang M, Liu J, Zhang Z, Zhang H, Wang N, Chen X, Han X, Lu Q and Chi S (2022) Effects of Dietary Intervention on Inflammatory Markers in Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front. Nutr. 9:846591. doi: 10.3389/fnut.2022.846591
Received: 31 December 2021; Accepted: 01 March 2022;
Published: 31 March 2022.
Edited by:
Gratiela Gradisteanu Pircalabioru, University of Bucharest, RomaniaReviewed by:
Evelyn Frias-Toral, Catholic University of Santiago de Guayaquil, EcuadorCopyright © 2022 Wang, Liu, Zhang, Zhang, Wang, Chen, Han, Lu and Chi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhao Zhang, d21qMTk4NzExMTI1MjFAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.