
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 08 November 2021
Sec. Clinical Nutrition
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.748075
Purpose: The study aimed to explore the effects of l-carnitine, acetyl-l-carnitine, and propionyl-l-carnitine on Body Mass in type 2 diabetes mellitus (T2DM) patients.
Methods: Randomized controlled trial (RCT) studies of l-carnitine, acetyl-l-carnitine, and propionyl-l-carnitine in T2DM patients were searched. The change rates of Body Mass index (BMI) from baseline values were used as an evaluation indicator. The maximal effect (Emax) model by non-linear mixed-effect modeling (NONMEM) was used as the evaluation method.
Results: A total of 10 RCT studies, 1239 T2DM patients were included for analysis, including eight studies of l-carnitine, one study of acetyl-l-carnitine, and one study of propionyl-l-carnitine. The study found that l-carnitine could reduce the Body Mass of T2DM patients. Based on only one study each for acetyl-l-carnitine and propionyl-l-carnitine, no significant effects were found in acetyl-l-carnitine or propionyl-l-carnitine. In addition, in order to achieve a plateau of efficacy (80% Emax), 2 g/day l-carnitine was required for at least 2 weeks.
Conclusions: Two g/day l-carnitine was required for at least 2 weeks to affect Body Mass in T2DM patients, and no significant effects were found in acetyl-l-carnitine or propionyl-l-carnitine.
- The present study analyzed the effects of l-carnitine, acetyl-l-carnitine, and propionyl-l-carnitine on Body Mass in T2DM patients.
- L-carnitine could reduce the Body Mass of T2DM patients, in which 2 g/day l-carnitine was required for at least 2 weeks.
- No significant effects on Body Mass were found from acetyl-l-carnitine or propionyl-l-carnitine in T2DM patients.
Type 2 diabetes mellitus (T2DM), a chronic degenerative disease where the pancreas cannot produce enough insulin and/or the insulin produced is inefficient, causing hyperglycemia, is a major health problem and one of the top 10 causes of mortality worldwide (1). According to the International Diabetes Federation (IDF) (2019), 9.3% of adults around the world, amount to 463 million people, have T2DM (1). This number is expected to increase increase to 700 million people by 2045, which is equivalent to 10.90% of the adult population worldwide (1). In addition, T2DM is also an important risk factor for chronic kidney disease, cardiovascular disease, and mortality (2).
From a clinical point of view, T2DM patients are often accompanied by obesity, atherosclerotic disease, dyslipidemia, and hypertension (3, 4), in which more than 50% of T2DM patients have been reported to be obese (3, 5). Overweight or obesity in T2DM can increase the cardiovascular disease risk and further increase the risk of death, which are important determinants of the prognosis in T2DM patients (5, 6). Therefore, intensive therapy for T2DM patients with overweight or obesity is crucial (2).
At present, many drugs have been used to control blood glucose and Body Mass in T2DM patients, among which Wang et al. report the quantitative efficacy of l-carnitine supplementation on glycemic control in T2DM patients (7). However, the effects of l-carnitine, as well as its other forms of existence, acetyl-l-carnitine, and propionyl-l-carnitine on Body Mass in T2DM patients are still unclear. The present study is to explore the effects of l-carnitine, acetyl-l-carnitine, and propionyl-l-carnitine on Body Mass in T2DM patients.
We searched and extracted the Pubmed database (https://pubmed.ncbi.nlm.nih.gov/) with the deadline of April 2021. Only English publications were included. The terms “l-carnitine,” “acetyl-l-carnitine,” “propionyl-l-carnitine,” and “type 2 diabetes mellitus” were used in the present search strategy. Inclusion criteria included: (I) randomized controlled trial (RCT), (II) with Body Mass Index (BMI) information, (III) exact dose and duration of l-carnitine, acetyl-l-carnitine, and propionyl-l-carnitine. Source, country, grouping, sample size, age, duration of treatment et al were extracted from the above-included studies.
In order to eliminate the potential baseline effect, the efficacy of l-carnitine, acetyl-l-carnitine, and propionyl-l-carnitine were evaluated using BMI change rate from the baseline value. The Formula (1) was as follows:
Et, the value of BMI at time t; Eb, the value of BMI at baseline.
The Emax model was used to evaluate the effects of l-carnitine, acetyl-l-carnitine or propionyl-l-carnitine on Body Mass in T2DM patients. In addition, in order to acquire the actual effects on BMI from l-carnitine, acetyl-l-carnitine, and propionyl-l-carnitine, the control effects need to be subtracted from the sum effects. The Formulas (2) and (3) were as follows:
EI,i,j, the sum effects on BMI from l-carnitine, acetyl-l-carnitine or propionyl-l-carnitine, including actual effects and control effects; ED,i,j, the actual effects on BMI; EC,i,j, the control effects on BMI; i, different studies; j, the time point of every study; Emax, the maximal effects on BMI; ET50, the treatment duration to reach half of the maximal effects on BMI; εi,j, the residual error of study i with j time; Ni,j, the sample size in study i with time point j. εi,j was weighted by sample size, assumed to be normally distributed, with a mean of 0 and variance of σ2/(Ni,j/100).
The inter-study variability was described by exponential error or additive error models. The Formulas (4)–(7) were as follows:
η1,i, η2,i were the inter-study variabilities, when available, they would be added into Emax, and ET50, respectively. η1,i, η2,i were assumed to be normally distributed, with a mean of 0 and variance of ω1,i2, ω2,i2, respectively.
In addition, continuous covariates and categorical covariates were evaluated by Formulas (8)–(9) and (10):
Pp, the parameter for a patient with a covariate value of COV; PT, the typical value of the parameter; COV, covariate; COVm, the median value of covariable in the population. θc, a correction coefficient of the covariate to the model parameter.
The model development was done using non-linear mixed-effect modeling (NONMEM, edition 7, ICON Development Solutions, Ellicott City, MD, USA). When a basic model was built, potential covariates were considered for adding into Emax. The change of objective function value (OFV) was used as the covariate inclusion criteria. When the decrease of OFV was >3.84 (χ2, α = 0.05, d.f. = 1), it was considered sufficient for inclusion. When the increase of OFV was >6.63 (χ2, α = 0.01, d.f. = 1), it was considered sufficient for significance in the final model (8).
The goodness-of-fit plots of the model (individual predictions vs. observations), distribution of conditional weighted residuals (CWRES) for the model (density vs. CWRES, and quantiles of CWRES vs. quantiles of normal), and individual plots from different studies were used to estimate the final model. Prediction-corrected visual predictive check (VPC) plots were used to assess the predictive performance of the final model. In addition, the medians and 2.5th−97.5th percentiles of the results from bootstrap (Simulation, n = 1,000) were used to compare with final model parameters. The efficacy prediction of l-carnitine on BMI in T2DM patients was simulated by the Monte Carlo method.
Figure 1 was the retrieval process and a total of 10 RCT studies, comprising 1,239 T2DM patients were included for analysis, including 8 studies of l-carnitine (9–16), 1 study of acetyl-l-carnitine (17), and 1 study of propionyl-l-carnitine (18). The dosages of l-carnitine, acetyl-l-carnitine, and propionyl-l-carnitine were 2–3, 2, and 2 g/day, respectively, in the included studies, and the details were shown in Table 1, and part of the literature was retrieved from the previous study (7). The risk of bias analysis was shown in Figure 2. As both acetyl-l-carnitine, and propionyl-l-carnitine had only 1 study, model-based meta-analysis (MBMA) could not be performed at this time for them. Further analysis found that no significant effects on BMI in acetyl-l-carnitine or propionyl-l-carnitine in T2DM patients. Therefore, the following MBMA analysis was mainly aimed at l-carnitine.
The actual drug effects of l-carnitine on BMI in T2DM patients is shown in Table 2, the Emax of l-carnitine on BMI in T2DM patients was −1.51% and the ET50 of l-carnitine on BMI in T2DM patients was 0.5 weeks. In addition, no covariate (in particular dosage) was incorporated into the Emax model, showing there was no significant dose-dependence from l-carnitine efficacy on BMI in T2DM patients in the present study. The Emax model of l-carnitine on BMI in T2DM patients was shown in Formulas (11):
E, efficacy of l-carnitine on BMI; Time, l-carnitine treatment duration.
The visual inspection of routine diagnostic plots, and individual predictions vs. observations, are shown in Figure 3A. The distribution of CWRES for model (density vs. CWRES, and quantilies of CWRES vs. quantiles of normal) are shown in Figures 3B,C. Individual plots from different studies are shown in Figure 3D. As we could see, there were good linear relationships between individual predictions and observations, and individual plots were also consistent meaning the good fitting of the final models. At the same time, the distribution of the model also satisfied the normal distribution.
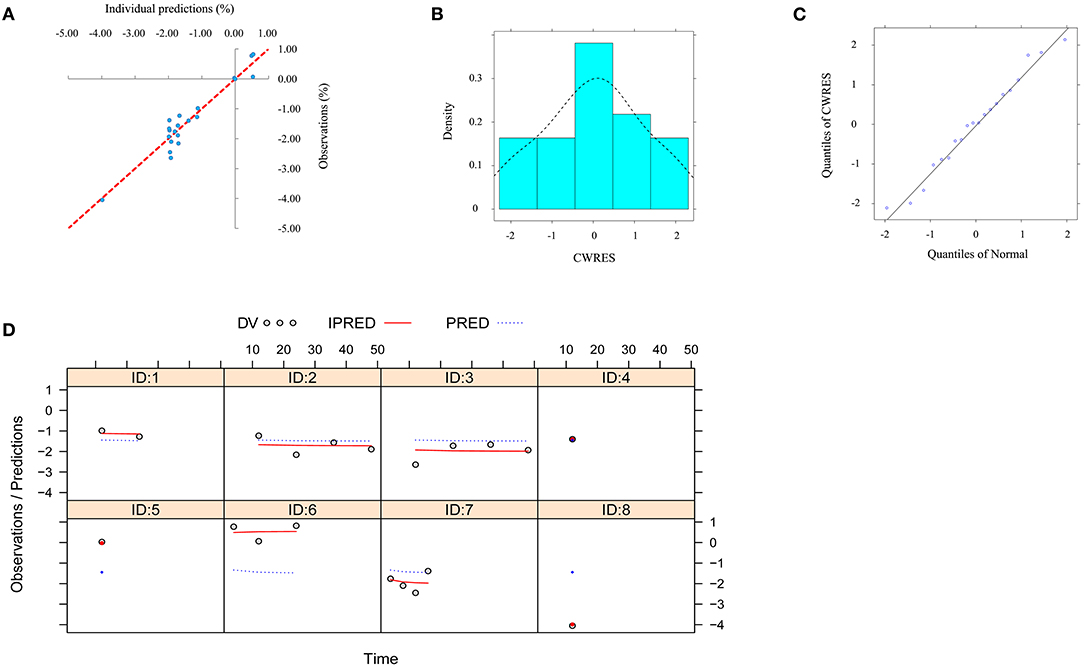
Figure 3. Model evaluation. (A) individual predictions vs. observations for the model from the effect of l-carnitine on BMI. (B) distribution of conditional weighted residuals (CWRES) for model (density vs. CWRES). (C) distribution of CWRES for model (quantiles of CWRES vs. quantiles of normal). (D) individual plots for the model from the effect of l-carnitine on BMI.
The VPC plots are shown in Figure 4, and most observed data were included in the 95% prediction intervals produced by simulation data, which shows the predictive power of the final models.
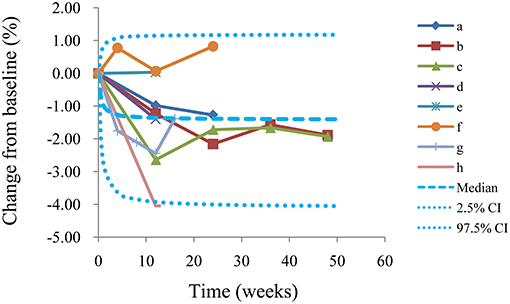
Figure 4. Visual predictive check of the model from the l-carnitine effect on BMI. Median, 2.5% CI and 97.5% CI were simulated by Monte Carlo (n = 1,000); CI, confidence interval; From a to h were studies come form El-Sheikh et al. (16), Derosa et al. (14), Derosa et al. (15), Malaguarnera et al. (13), Malaguarnera et al. (12), Galvano et al. (11), Derosa et al. (10), Liang et al. (9), respectively.
We also simulated the curve of the final model for the effect of l-carnitine on BMI via the Monte Carlo method. The trend of the efficacy of l-carnitine on BMI in T2DM patients is shown in Figure 5. As we could see from the curve, the efficacy of l-carnitine on BMI at 0.5 weeks was 50% of the Emax, at 2 weeks was 80% of the Emax (plateau stage), at 4.5 weeks was 90% of the Emax, at 9.5 weeks was 95% of the Emax. In the current study, the dose range was 2–3 g/day and there was no significant dose-dependence from l-carnitine efficacy on BMI in T2DM patients, so the lower dose of 2 g/day was selected as recommended dose. In addition, in order to achieve a plateau of efficacy (80% Emax), 2 g/day l-carnitine was required for at least 2 weeks.
Carnitine is derived from amino acids and is found in almost all cells in the body (19). Its name comes from the Latin carnus, meaning meat, because the compound is extracted from meat (19). Carnitine is a generic term, which includes l-carnitine, acetyl-l-carnitine, and propionyl-l-carnitine (20). L-carnitine plays an important role in energy metabolism (21). It transfers long-chain fatty acids to cell mitochondria for oxidation, which produces energy needed by the body (21, 22). It also transports harmful substances out of the organelle, preventing them from accumulating in the cell (21). Because of these functions, carnitine is found in high concentrations in skeletal muscle and cardiac muscle cells, which allow them to use fatty acids as an energy source (20). For most people, the body can make enough to meet its needs, but for some people, because of genetic or pharmaceutical reasons, the body cannot produce enough, it is, therefore, an essential nutrient for these individuals (23).
As is well-known, l-carnitine can adjust many events, such as metabolism of glucose and fatty acids, and has the potential to protect these cellular events in several manners including decreasing the production of reactive oxygen species at different points and maintaining mitochondrial functions (24). In addition, it has been reported that l-carnitine had many important pharmacological actions (24–31), for example, l-carnitine has a potential therapeutic effect in treating insulin resistance (32). It is also reported that l-carnitine can improve glycemia in T2DM patients (33). Wang et al.'s report provides valuable quantitative information for the efficacy of l-carnitine supplementation on glycemic control in T2DM patients (7). They find that for the efficacy of l-carnitine on fasting plasma glucose (FPG), 2 g/day l-carnitine is required for at least 36.1 weeks; For the efficacy of l-carnitine on glycated hemoglobin (HbA1c), 2 g/day l-carnitine is required for at least 106 weeks (7). However, the effects of l-carnitine, as well as its other forms of existence, acetyl-l-carnitine, and propionyl-l-carnitine on Body Mass in T2DM patients are still unclear. The purpose of this study is to explore the effects of l-carnitine, acetyl-l-carnitine, and propionyl-l-carnitine on Body Mass in T2DM patients by MBMA.
In the present study, a total of 10 RCT studies comprising 1,239 T2DM patients were included for analysis, including 8 studies of l-carnitine (9–16), 1 study of acetyl-l-carnitine (17), and 1 study of propionyl-l-carnitine (18). The dosages of l-carnitine, acetyl-l-carnitine, and propionyl-l-carnitine were 2–3, 2, and 2 g/day, respectively, in the included studies. Of course, when investigating the efficacy of a drug on Body Mass, important factors should be stable such as diet, antiglycemic drugs, and duration of T2DM. Fortunately, since our study was from RCTs, conditions in the intervention group and the control group were similar in each study. In this way, the control group effects were deducted from the intervention group, and the actual l-carnitine drug effects were obtained. In addition, we also considered the impact of various indicators in different studies on baseline values. In addition, as for both acetyl-l-carnitine, and propionyl-l-carnitine had only 1 study, MBMA analysis could not be performed at this time for them. Further analysis found no significant effects on BMI in acetyl-l-carnitine or propionyl-l-carnitine in T2DM patients.
In further analysis of the effects of l-carnitine on Body Mass in T2DM patients, we found the Emax of l-carnitine on BMI in T2DM patients was −1.51% and the ET50 of l-carnitine on BMI in T2DM patients was 0.5 weeks. In addition, no covariate (in particular dosage) was incorporated into the Emax model, showing there was no significant dose-dependence from l-carnitine efficacy on BMI in T2DM patients. In the current study, the dose range was 2–3 g/day, there was no significant dose-dependence from l-carnitine efficacy on BMI in T2DM patients, so the lower dose of 2 g/day was selected as recommended dose. In addition, in order to achieve a plateau of efficacy (80% Emax), 2 g/day l-carnitine was required for at least 2 weeks. From the current view, l-carnitine could play an important role in glucose metabolism and increase energy expenditure, meanwhile, l-carnitine had a role in lipid metabolism as well (34–36). For these two reasons, l-carnitine helps Body Mass loss by increasing energy expenditure (36). However, this study had some limitations. The number of studies currently included was limited, and additional studies were needed in the future.
Two gram per day l-carnitine was required for at least 2 weeks to affect Body Mass in T2DM patients, and no significant effects were found in acetyl-l-carnitine or propionyl-l-carnitine.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
D-DW, S-MH, and Y-MW conceived and designed the study. D-DW, T-YW, YY, and S-MH collected and analyzed data. D-DW wrote the paper. S-MH reviewed and edited the manuscript. All authors read and approved the final manuscript.
This work was supported by the Initializing Fund of Xuzhou Medical University (No. RC20552111), the Fusion Innovation Project of Xuzhou Medical University (No. XYRHCX2021011), the Xuzhou Special fund for promoting scientific and technological innovation (No. KC21257), the Suzhou Science & Technology Town Hospital pre-research fund project (No. 2019Y01), the Suzhou Science and Technology Development Plan Project (No. S YSD2019076), and the Jiangsu Pharmaceutical Society-Tianqing Hospital Pharmaceutical Fund Project (No. Q202024).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zepeda-Pena AC, Gurrola-Diaz CM, Dominguez-Rosales JA, Garcia-Lopez PM, Pizano-Andrade JC, Hernandez-Nazara ZH, et al. Effect of Lupinus rotundiflorus gamma conglutin treatment on JNK1 gene expression and protein activation in a rat model of type 2 diabetes. Pharm Biol. (2021) 59:374–80. doi: 10.1080/13880209.2021.1893757
2. Uneda K, Kawai Y, Yamada T, Kinguchi S, Azushima K, Kanaoka T, et al. Systematic review and meta-analysis for prevention of cardiovascular complications using GLP-1 receptor agonists and SGLT-2 inhibitors in obese diabetic patients. Sci Rep. (2021) 11:10166. doi: 10.1038/s41598-021-89620-7
3. Iglay K, Hannachi H, Joseph Howie P, Xu J, Li X, Engel SS, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. (2016) 32:1243–52. doi: 10.1185/03007995.2016.1168291
4. Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, Loos RJF, et al. Obesity. Nat Rev Dis Primers. (2017) 3:17034. doi: 10.1038/nrdp.2017.34
5. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. (2018) 17:83. doi: 10.1186/s12933-018-0728-6
6. American Diabetes Association. 8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes-2020. Diabetes Care. (2020) 43(Suppl 1):S89–97. doi: 10.2337/dc20-S008
7. Wang DD, Mao YZ, He SM, Yang Y, Chen X. Quantitative efficacy of L-carnitine supplementation on glycemic control in type 2 diabetes mellitus patients. Expert Rev Clin Pharmacol. (2021) 1–8. doi: 10.1080/17512433.2021.1917381
8. Wang DD, Mao YZ, He SM, Chen X. Analysis of time course and dose effect from metformin on Body Mass Index in children and adolescents. Front Pharmacol. (2021) 12:611480. doi: 10.3389/fphar.2021.611480
9. Liang Y, Li Y, Shan J, Yu B, Ho Z. The effects of oral L-carnitine treatment on blood lipid metabolism and the body fat content in the diabetic patient. Asia Pac J Clin Nutr. (1998) 7:192–5.
10. Derosa G, Cicero AF, Gaddi A, Mugellini A, Ciccarelli L, Fogari R. The effect of L-carnitine on plasma lipoprotein(a) levels in hypercholesterolemic patients with type 2 diabetes mellitus. Clin Ther. (2003) 25:1429–39. doi: 10.1016/S0149-2918(03)80130-3
11. Galvano F, Li Volti G, Malaguarnera M, Avitabile T, Antic T, Vacante M, et al. Effects of simvastatin and carnitine versus simvastatin on lipoprotein(a) and apoprotein(a) in type 2 diabetes mellitus. Expert Opin Pharmacother. (2009) 10:1875–82. doi: 10.1517/14656560903081745
12. Malaguarnera M, Vacante M, Avitabile T, Malaguarnera M, Cammalleri L, Motta M. L-Carnitine supplementation reduces oxidized LDL cholesterol in patients with diabetes. Am J Clin Nutr. (2009) 89:71–6. doi: 10.3945/ajcn.2008.26251
13. Malaguarnera M, Vacante M, Motta M, Malaguarnera M, Li Volti G, Galvano F. Effect of L-carnitine on the size of low-density lipoprotein particles in type 2 diabetes mellitus patients treated with simvastatin. Metabolism. (2009) 58:1618–23. doi: 10.1016/j.metabol.2009.05.014
14. Derosa G, Maffioli P, Ferrari I, D'Angelo A, Fogari E, Palumbo I, et al. Comparison between orlistat plus l-carnitine and orlistat alone on inflammation parameters in obese diabetic patients. Fundam Clin Pharmacol. (2011) 25:642–51. doi: 10.1111/j.1472-8206.2010.00888.x
15. Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Gravina A, Mereu R, et al. Effects of combination of sibutramine and L-carnitine compared with sibutramine monotherapy on inflammatory parameters in diabetic patients. Metabolism. (2011) 60:421–9. doi: 10.1016/j.metabol.2010.03.010
16. El-Sheikh HM, El-Haggar SM, Elbedewy TA. Comparative study to evaluate the effect of l-carnitine plus glimepiride versus glimepiride alone on insulin resistance in type 2 diabetic patients. Diabetes Metab Syndr. (2019) 13:167–73. doi: 10.1016/j.dsx.2018.08.035
17. Parvanova A, Trillini M, Podesta MA, Iliev IP, Aparicio C, Perna A, et al. blood pressure and metabolic effects of acetyl-l-carnitine in type 2 diabetes: DIABASI randomized controlled trial. J Endocr Soc. (2018) 2:420–36. doi: 10.1210/js.2017-00426
18. Santo SS, Sergio N, Luigi DP, Giuseppe M, Margherita F, Gea OC, et al. Effect of PLC on functional parameters and oxidative profile in type 2 diabetes-associated PAD. Diabetes Res Clin Pract. (2006) 72:231–7. doi: 10.1016/j.diabres.2005.10.007
20. Jiang Q, Wang C, Xue C, Xue L, Wang M, Li C, et al. Changes in the levels of l-carnitine, acetyl-l-carnitine and propionyl-l-carnitine are involved in perfluorooctanoic acid induced developmental cardiotoxicity in chicken embryo. Environ Toxicol Pharmacol. (2016) 48:116–24. doi: 10.1016/j.etap.2016.10.017
21. Bota AB, Simmons JG, DiBattista A, Wilson K. Carnitine in alcohol use disorders: a scoping review. Alcohol Clin Exp Res. (2021) 45:666–74. doi: 10.1111/acer.14568
22. Longo N, Frigeni M, Pasquali M. Carnitine transport and fatty acid oxidation. Biochim Biophys Acta. (2016) 1863:2422–35. doi: 10.1016/j.bbamcr.2016.01.023
23. Bene J, Hadzsiev K, Melegh B. Role of carnitine and its derivatives in the development and management of type 2 diabetes. Nutr Diabetes. (2018) 8:8. doi: 10.1038/s41387-018-0017-1
24. Modanloo M, Shokrzadeh M. Analyzing mitochondrial dysfunction, oxidative stress, and apoptosis: potential role of l-carnitine. Iran J Kidney Dis. (2019) 13:74–86.
25. Jiang Q, Jiang G, Shi KQ, Cai H, Wang YX, Zheng MH. Oral acetyl-L-carnitine treatment in hepatic encephalopathy: view of evidence-based medicine. Ann Hepatol. (2013) 12:803–9. doi: 10.1016/S1665-2681(19)31323-7
26. Ribas GS, Vargas CR, Wajner M. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene. (2014) 533:469–76. doi: 10.1016/j.gene.2013.10.017
27. Khalatbari-Soltani S, Tabibi H. Inflammation and L-carnitine therapy in hemodialysis patients: a review. Clin Exp Nephrol. (2015) 19:331–5. doi: 10.1007/s10157-014-1061-3
28. Song X, Qu H, Yang Z, Rong J, Cai W, Zhou H. Efficacy and safety of l-carnitine treatment for chronic heart failure: a meta-analysis of randomized controlled trials. Biomed Res Int. (2017) 2017:6274854. doi: 10.1155/2017/6274854
29. Askarpour M, Hadi A, Dehghani Kari Bozorg A, Sadeghi O, Sheikhi A, Kazemi M, et al. Effects of L-carnitine supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Hum Hypertens. (2019) 33:725–34. doi: 10.1038/s41371-019-0248-1
30. Askarpour M, Hadi A, Symonds ME, Miraghajani M, Omid S, Sheikhi A, et al. Efficacy of l-carnitine supplementation for management of blood lipids: a systematic review and dose-response meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. (2019) 29:1151–67. doi: 10.1016/j.numecd.2019.07.012
31. Askarpour M, Hadi A, Miraghajani M, Symonds ME, Sheikhi A, Ghaedi E. Beneficial effects of l-carnitine supplementation for weight management in overweight and obese adults: an updated systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol Res. (2020) 151:104554. doi: 10.1016/j.phrs.2019.104554
32. Xu Y, Jiang W, Chen G, Zhu W, Ding W, Ge Z, et al. L-carnitine treatment of insulin resistance: a systematic review and meta-analysis. Adv Clin Exp Med. (2017) 26:333–8. doi: 10.17219/acem/61609
33. Vidal-Casariego A, Burgos-Pelaez R, Martinez-Faedo C, Calvo-Gracia F, Valero-Zanuy MA, Luengo-Perez LM, et al. Metabolic effects of L-carnitine on type 2 diabetes mellitus: systematic review and meta-analysis. Exp Clin Endocrinol Diabetes. (2013) 121:234–8. doi: 10.1055/s-0033-1333688
34. Wall BT, Stephens FB, Constantin-Teodosiu D, Marimuthu K, Macdonald IA, Greenhaff PL. Chronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. J Physiol. (2011) 589(Pt 4):963–73. doi: 10.1113/jphysiol.2010.201343
35. Kim JH, Pan JH, Lee ES, Kim YJ. L-Carnitine enhances exercise endurance capacity by promoting muscle oxidative metabolism in mice. Biochem Biophys Res Commun. (2015) 464:568–73. doi: 10.1016/j.bbrc.2015.07.009
Keywords: l-carnitine, acetyl-l-carnitine, propionyl-l-carnitine, Body Mass, type 2 diabetes mellitus
Citation: Wang D-D, Wang T-Y, Yang Y, He S-M and Wang Y-M (2021) The Effects of L-Carnitine, Acetyl-L-Carnitine, and Propionyl-L-Carnitine on Body Mass in Type 2 Diabetes Mellitus Patients. Front. Nutr. 8:748075. doi: 10.3389/fnut.2021.748075
Received: 07 August 2021; Accepted: 11 October 2021;
Published: 08 November 2021.
Edited by:
Clelia Madeddu, University of Cagliari, ItalyReviewed by:
Sidney B. Peres, State University of Maringá, BrazilCopyright © 2021 Wang, Wang, Yang, He and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Dong Wang, MTM4NTIwMjk1OTFAMTYzLmNvbQ==; Su-Mei He, aGVoZTgyMDRAMTYzLmNvbQ==; You-Mei Wang, d3ltMTU4NjE3MDM4NTVAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.