- 1Key Laboratory of Neuroregeneration of Jiangsu and Ministry of Education, Co-innovation Center of Neuroregeneration, School of Life Science, Nantong Laboratory of Development and Diseases, Medical College, Clinical Medical Research Center, Affiliated Wuxi Clinical College of Nantong University, Nantong University, Nantong, China
- 2Clinical Medical Research Center, Wuxi No. 2 People's Hospital, Jiangnan University Medical Center, Wuxi, China
Peripheral nerve injury disease is a prevalent traumatic condition in current medical practice. Despite the present treatment approaches, encompassing surgical sutures, autologous nerve or allograft nerve transplantation, tissue engineering techniques, and others, an effective clinical treatment method still needs to be discovered. Exploring novel treatment methods to improve peripheral nerve regeneration requires more effort in investigating the cellular and molecular mechanisms involved. Many factors are associated with the regeneration of injured peripheral nerves, including the cross-sectional area of the injured nerve, the length of the nerve gap defect, and various cellular and molecular factors such as Schwann cells, inflammation factors, kinases, and growth factors. As crucial mediators of cellular communication, kinases exert regulatory control over numerous signaling cascades, thereby participating in various vital biological processes, including peripheral nerve regeneration after nerve injury. In this review, we examined diverse kinase classifications, distinct nerve injury types, and the intricate mechanisms involved in peripheral nerve regeneration. Then we stressed the significance of kinases in regulating autophagy, inflammatory response, apoptosis, cell cycle, oxidative processes, and other aspects in establishing conductive microenvironments for nerve tissue regeneration. Finally, we briefly discussed the functional roles of kinases in different types of cells involved in peripheral nerve regeneration.
1 Introduction
Peripheral nerves are defined as nerves that are not part of the brain or spinal cord but instead connect the central nervous system (CNS) to target organs for neural signal transduction. Peripheral nerve injury (PNI) is a prevalent disease condition resulting from either physical injury, including traumatic events and surgical procedures, or other disease conditions, such as diabetes, and autoimmune diseases like Guillain-Barre syndrome, systemic lupus erythematosus, and rheumatoid arthritis (1, 2). Iatrogenic injury, primarily caused by traction, cutting, surgery, and neuroma, is a common way to disrupt the continuity of axons and leads to sensory and motor dysfunction in the innervated region, significantly compromising patients' quality of life (3–5). In certain regions, the incidence of PNI caused by traffic accidents or mechanical injuries within the body has steadily risen alongside economic development (6).
There are different treatment therapies for disease condition-induced PNI, which depends on the causal diseases. For example, glucose control is the predominant method to prevent diabetic neuropathy, along with medicines for pain management (7). Guillain-Barre Syndrome (GBS), an autoimmune disorder involving demyelination of peripheral nerves, is the most common cause of acute flaccid paralysis worldwide. Traditional treatments for GBS encompass corticosteroids, plasma exchange, and intravenous (IV) administration of immunoglobulins (IVIG). Several novel therapies, such as complement inhibitors and cerebrospinal fluid (CSF) filtration, have been developing recently (8). Some patients with systemic lupus erythematosus can also get peripheral neuropathy, who are typically treated with corticosteroids and immunosuppression (9). For Sjogren's syndrome, another common autoimmune disease, apart from the clinical usage of gabapentin and pregabalin for pain relief, immunomodulatory and immunosuppressive therapies have been tested in trials (10).
Unlike the CNS, peripheral nerves possess an inherent capacity for self-repair and regeneration following injury. The treatment methods for physical PNI have evolved from initial microsurgery to current approaches, including autologous/allogeneic nerve tissue transplantation and tissue engineering material transplantation (11). However, the efficacy of these methods is exceptionally constrained due to the intricate nature of peripheral nerve differentiation and the limited understanding of the regeneration mechanisms involved in injured peripheral nerves. Consequently, it is imperative to identify and elucidate additional determinants and mechanisms that influence the regeneration process of the peripheral nerve system (PNS).
To date, numerous factors and molecules, such as Schwann cells (SCs), inflammation factors, kinases, and growth factors, have been identified as crucial regulators of peripheral nerve regeneration (PNR) (11–13). Kinases, a type of biochemical molecules ubiquitously present in cellular organisms, can catalyze the transfer of high-energy phosphate groups from high-energy donors (e.g., ATP) to substrates, primarily facilitating the transduction of various biological signals within cells. Due to their pivotal involvement in signal transduction, malfunctioning kinases often have severe detrimental effects and are associated with a series of diseases (14, 15). Here, we will review the recent progress of research on the mechanisms of PNR from the perspective of kinases.
2 Classification of kinases
The currently recognized kinases encompass protein kinases (including serine/threonine kinases and tyrosine kinases), lipid kinases, glucokinase (such as hexokinase and fructokinase), and others, with protein kinases comprising the majority. Over 500 protein kinases have been identified in the human body, encoded by more than 900 genes, accounting for ~5% of the human genome (16, 17).
2.1 Protein kinase
Based on the presence of phosphorylated groups on the receptors, protein kinases are categorized into various types, such as serine/threonine kinases, tyrosine kinases, histidine/lysine/arginine kinase, and aspartate/glutamate kinase, among others (18–24). Furthermore, protein kinases can also be classified based on their roles in signaling pathways, including AGC (Protein kinase PKA, PKG, PKC), calmodulin kinases (CaMK), mitogen-activated protein kinase (MAPK), MAPKK kinase (Raf), protein kinase B (AKT), Cyclin-dependent kinases (CDKs), protein tyrosine kinases (PTK), and other kinase families (25–31). Protein kinases play significant roles in diverse biological processes by activating target proteins and regulating cellular signaling transduction. Mutations and dysfunctions in protein kinases have been closely linked to human diseases, particularly cancer and inflammation. Consequently, protein kinases have emerged as promising pharmaceutical targets in medical applications (32, 33).
2.1.1 Serine/threonine kinase and tyrosine kinase
The two primary types of protein kinases are serine/threonine kinases (STK) and tyrosine kinases (TK), which were among the earliest kinases to be identified (34). STKs catalyze the phosphorylation of serine/threonine hydroxyl groups on target proteins, while TKs facilitate the phosphorylation of tyrosine residues. These kinases are ubiquitously found in nearly all eukaryotic multicellular organisms and are crucial in mediating cellular signal transduction (35, 36). Based on the localization, these kinases can be categorized into transmembrane receptor kinases and cytoplasmic kinases. Functionally, the transmembrane kinases bind with extracellular ligands to transmit signals into the cells. In contrast, the cytoplasmic kinases are indispensable for transmitting intercellular signals, which subsequently regulate a wide range of cellular processes encompassing cell growth, metabolism, differentiation, proliferation, division, and apoptosis through the modulation of the gene expressions in the nucleus (37, 38).
Various STKs have been identified, including PKA, PKC, CaMK, pyruvate dehydrogenase kinase (PDK), and DNA-dependent protein kinase (DNA-PK). PKA, a typical protein kinase, is widely recognized for its crucial involvement in regulating various biological processes through the cAMP/PKA signaling pathway (39). TKs, based on their location and function, can be broadly categorized into receptor tyrosine kinases (RTKs), which are transmembrane receptor proteins, and non-receptor tyrosine kinases (NRTKs), which are found in the cytoplasm and nucleus. RTKs can be further classified into two groups, which are tyrosine kinase receptor (TKR) and tyrosine kinase associated receptor (TKAR) (40). Non-receptor kinases include PTK, TEC, and Janus kinase (JAK) family members (35, 41). Numerous tyrosine kinases identified thus far have been found to originate from proto-oncogenes. For instance, the initial two categories of NRTKs were identified as resulting from structural alterations of typical tyrosine kinases (35). Furthermore, these kinases also play a role in regulating inflammation and immune processes, as evidenced by the rapid tyrosine phosphorylation of diverse proteins during the proliferation of both standard and malignant tumor cells, as well as the activation of T cells, B cells, and mast cells (42, 43).
2.1.2 Histidine/lysine/arginine kinase
Histidine protein kinases (HPK) are signaling enzymes that can phosphorylate conserved histidine residues. The phosphorylation of arginine and lysine residues follows processes similar to histidine due to their analogous basic groups. HPKs and their downstream target proteins form a two-component signal transduction system (44). A typical HPK is a transmembrane receptor containing an extracellular receptor region at the N-terminal end and an intracellular signal region at the C-terminal end. Despite the low similarity between HPK, STK, and TK, reports have suggested a potentially distant evolutionary relationship among them (45).
2.1.3 Cysteine-rich receptor-like kinase
A receptor-like kinase (RLK) is a widely present protein kinase in plants, playing a crucial role in regulating various biological processes, including development, stress adaptation, and plant defense (46). A cysteine-rich receptor-like kinase (CRK), DUF26 receptor kinase, is pivotal in numerous signaling pathways. Functioning as a plasma membrane receptor, it recognizes and receives external signals and mediates subsequent intracellular signal transmission via phosphorylation (47).
2.1.4 Aspartate/glutamate kinase
Aspartate kinase is the initial pivotal enzyme in the biosynthetic pathway of aspartate amino acids. It is widely distributed and exerts crucial roles in the metabolic pathways of plants and microorganisms. N-acetyl glutamate kinase, commonly known as NAGK, functions as the second rate-limiting enzyme in the biosynthesis of L-arginine, and it is also susceptible to feedback inhibition by the end product L-arginine (48).
2.2 Lipid kinase
Lipid kinases primarily encompass phosphoinositide lipid kinases (PIK), a class of proteins that facilitate the catalysis of phosphorylated variants of specific phosphatidylinositols. In mammals, the PIK family consists of three main categories: phosphatidylinositol 3 kinase (PI3K), phosphatidylinositol 4 kinase (PI4K), and phosphatidylinositol P (PIP) kinase (PIPK) (49). These PIK family members play crucial roles in signal transduction, generating second messengers that regulate cellular metabolism, promote overall wellbeing, and are indispensable for sustaining the energy necessary for cell growth and survival. Therefore, these lipid kinases are potential therapeutic targets for various diseases (50, 51).
2.3 Sugar kinase
Hexokinases and galactokinases are the most critical sugar kinases involved in sugar metabolism. Hexokinases typically phosphorylate glucose at the 6-position, while galactokinases catalyze the phosphorylation at the 1-position of galactose (52). Glucokinase (GK) is one of the isoenzymes of hexokinases. As a crucial glucose sensor in the human body, GK is primarily localized in pancreatic β cells and the liver, responsible for monitoring alterations in glucose concentration and activating the blood glucose regulation system to maintain blood glucose homeostasis (53).
3 Peripheral nerve injury
PNI is a prevalent affliction that primarily results in impaired nerve conduction in patients, ultimately leading to sensory and motor impairments and potentially lifelong disability (1). PNI can be mainly divided into mechanical injury and iatrogenic injury.
3.1 Mechanical injury
Mechanical injury pertains to nerve damage caused by external forces, such as traffic accidents, warfare, earthquakes, and industrial accidents. The injuries can be categorized into three types: crush injuries, transection injuries, and missing injuries. Crush injury is considered the least severe form of mechanical injury. In fundamental research, the sciatic nerve transection model is the most commonly utilized model for studying nerve damage (54). This model enables the investigation of various aspects, including the development, structure, and material transport within axons of peripheral nerves, such as chemical substances, signal molecules, and physical and chemical factors. Transection injury is a mechanical trauma that severs nerve tissue without inducing a defect. Transection injuries present greater challenges in terms of recovery compared to crush injuries, as they involve the breakage of nerve axons and endoneurium. Microsurgery, commonly employed as a treatment for transection, entails surgical sutures to restore the functionality of the injured nerve, typically improving functional recuperation (55). Missing injury, also known as nerve avulsion, is one of the most severe forms of mechanical injury, resulting in irreversible nerve damage. The treatment for missing injury depends on the gaps of the missing part. Autologous nerve transplantation or allogeneic nerve transplantation techniques are commonly employed in cases with small gaps. In contrast, larger gaps often require nerve conduits to facilitate regrowth and recovery (56–58).
3.2 Iatrogenic injury
Iatrogenic nerve lesions are nerve injuries that occur as a result of medical treatment or surgical procedures; for instance, the neurological dysfunction caused by abnormal nerve conduction due to ischemia-reperfusion (3–5).
3.3 The PNR process after PNI
Compared to the CNS, the peripheral nervous system possesses a degree of capacity for regeneration after injury (1). Waller degeneration usually occurs in the distal nerve after PNI. It mainly involves the degeneration and collapse of the local axon and myelin in the distal segment of the injured nerve, originating from the cell body, accompanied by various cytokines produced simultaneously (59).
The restoration of PNI typically entails three essential processes: eliminating incomplete myelin debris, generating new tissue, and recovering nerve impulse conduction function (13). The process of myelin debris removal commences with the degeneration and collapse of the impaired myelin sheath, prompting Schwann cells to phagocytose the myelin debris (60). Concurrently, these cells recruit a substantial quantity of inflammatory cells, including macrophages, neutrophils, and others, to expedite the clearance of the remnants (61, 62). Subsequently, ~28 days post-injury, T lymphocytes congregate at the distal end of the damaged nerve to elicit an immune response, thereby averting malignant inflammation at said location (63). Generating new tissue encompasses various events, such as the migration of neurons and elongation of axons, along with the proliferation and migration of Schwann cells around neurons. Additionally, it involves the regeneration and reconstruction of blood vessels and support systems surrounding nerve tissues (13, 64, 65). The restoration of nerve conduction function includes the proliferation of Schwann cells activated by cytokines and re-encloses nerve axons to form myelin sheaths. Ultimately, the ends of nerve axons establish correct connections with target organs and tissues, enabling proper functionality (66).
3.4 Peripheral nerve regeneration-associated genes
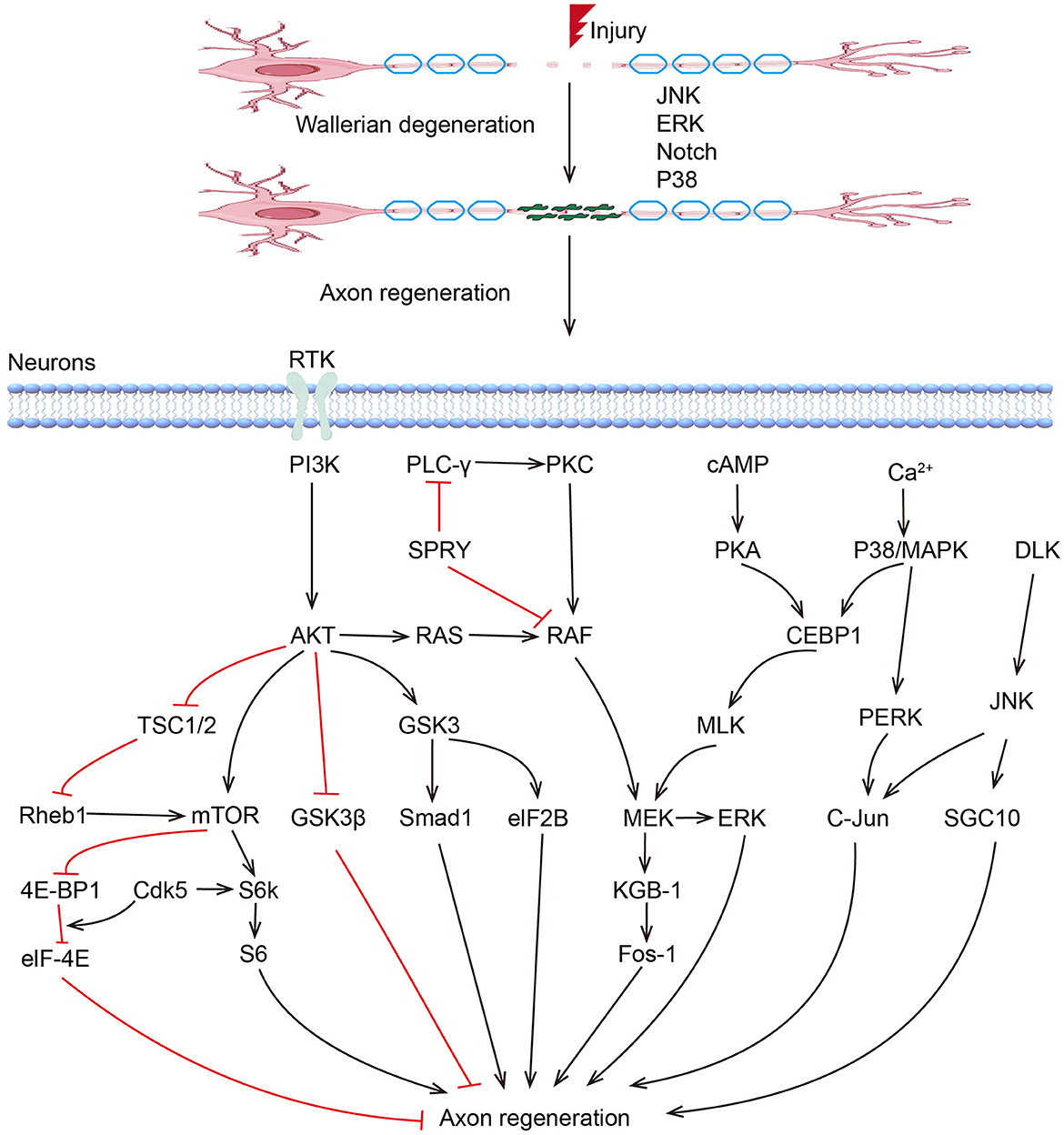
To date, an increasing number of Regeneration Associated Genes (RAGs) have been reported to play critical regulatory roles during peripheral nerve regeneration. These RAGs can be classified into several groups, including transcription factors, growth factors, miRNAs, and other non-coding RNAs (Figure 1).
3.4.1 Transcription factors
Transcription factors are the main regulatory factors for various cellular processes under physiological and pathological conditions. Many transcription factors that are differentially expressed after peripheral nerve injury play essential roles in nerve regeneration, including c-Myc, Sox11, STAT3, Atf3, c-Jun, Smad1, Sox2, Krox20, Sox10, and p53 (67). Sox11 is a protein with an HMG domain that can effectively promote peripheral nerve regeneration. It regulates multiple genes, including adhesion molecules, cytoskeletal elements, growth factors, cytokines, neuropeptides, and other molecules related to regeneration (68). Bcl11a is crucial for Schwann cell activation and peripheral nerve regeneration. It participates in regulating Schwann cell activity via regulating the expression of Nr2f2. Reduction of Bcl11a in damaged peripheral nerves leads to restricted axonal extension and myelin sheath wrapping, reduced Schwann cell proliferation and migration rates, and impaired ability of debris clearance, thereby causing recovery failure (69).
3.4.2 Growth factors
Growth factors (GFs) are neurotrophic factors known for regulating cellular proliferation, migration, and differentiation. In preclinical trials, exogenous application of GFs to the lesion site of peripheral nerves has demonstrated their high potential in repairing peripheral nerves by promoting myelin debris clearance, axonal sprouting, remyelination, neurogenesis, and neovascularization (70–72). These GFs, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin 3 (NT-3), have been shown to stimulate axonal regeneration both in vitro and in vivo (73, 74).
3.4.3 MicroRNAs
MicroRNAs (miRNAs) have been found to exhibit significant expression variation after peripheral nerve injury, reflecting their essential roles during this process. MiRNAs are prevalently involved in various aspects of peripheral nerve regeneration, encompassing inflammation, cell proliferation and migration, neurite outgrowth, and axon remyelination (75). MiRNA182 was found to be decreased in the initial stage of acute nerve injury, allowing for essential inflammatory reaction and increased SC migration by targeting fibroblast growth factor 9 (FGF9) and Neurotrimin (NTM) (76). Let-7 family can be upregulated after nerve lesion, resulting in the downregulation of Notch1 and higher levels of EGR2, consequently inducing remyelination (77, 78).
3.4.4 Other non-coding RNAs
Apart from miRNAs, other non-coding RNAs, including LncRNAs and Circ-RNAs, are also involved in PNR regulation. Silc1 is a lncRNA that is highly expressed in neuronal tissue. Previous studies have shown that Silc1 regulates nerve regeneration by cis-activating Sox 11 (79). Knockdown of lncRNA Bc088327 inhibits cell viability of SCs and induces apoptosis and cell cycle arrest in S-phase. This lncRNA may also interact with Hereglin-1β, which is involved in nerve regeneration (80). Circ-Spidr is enriched in the cytoplasm of dorsal root ganglion (DRG) neurons. It can regulate the PI3K-AKT pathway and inhibit axonal regeneration of DRG after sciatic nerve injury (81). Overexpression of Circ-Ankib1 was reported to affect SC proliferation and axonal regeneration after sciatic nerve injury by directly binding to miR-423-5 p, miR-485-5 p, and miR-666-3p and regulating Cyp26b1 expression (82).
4 Kinase and PNR
The microenvironment of PNS undergoes alterations following nerve injury, including cellular damage and subsequent release of cellular constituents, leading to significant changes in the surrounding microenvironment of the injured tissue. Then, diverse cytokines and kinases are induced and participate in the clearance and regeneration process. Kinases, which regulate numerous signaling pathways and serve as crucial signaling molecules, are intricately associated with the regeneration of injured peripheral nerves, establishing the microenvironment necessary to regenerate injured nerve tissues (83, 84). It influences the repair pace for damaged peripheral nerves by modulating various processes, including autophagy, inflammatory response, cell apoptosis, cell cycle, and oxidative stress. Due to the involvement of multiple pathways, kinases have the potential to both facilitate and impede the regeneration process of injured peripheral nerves via regulating different biological processes.
4.1 Kinase affects the PNR by regulating the autophagy process
In the context of PNR, autophagy serves as a crucial regulatory factor. Studies have pointed out that the serine/threonine kinase, known as the mammalian target of rapamycin (mTOR), exerts significant regulatory influence on the autophagy process and neuronal protection (85). Suppression of mTOR activity in mice led to a substantial reduction in the phosphorylation level of downstream p70S6K protein and, concurrently, increased expression levels of autophagy genes lc3 and beclin1, thereby facilitating autophagy and subsequently expediting the elimination of myelin debris and the regeneration process (86, 87). DLK1, a conserved MAPKKK, is an essential injury sensor regulating regeneration in motor and mechanosensory neurons by activating autophagy through a MAP kinase cascade (88).
4.2 Kinase affects PNR by regulating the inflammatory response
Inflammation is closely associated with the regeneration of injured nerve tissue and assumes a dual role, either positive or negative, during this process. It is widely acknowledged that the prompt pro-inflammatory reaction following the PNI is an indispensable prerequisite for eliminating tissue debris and facilitating effective regeneration (59). Kinases are closely associated with the inflammation response of the injured nerves. Both inflammation and nerve injury recovery can be controlled by p38 MAPK, which plays an essential physiological role in nerve regeneration. It was found that after sciatic nerve injury, the elevation in p38-MAPK phosphorylation levels inhibited the synthesis of IL-1β and TNF-α and positively influenced the regeneration of impaired tissues (89). The activation of IKK kinase facilitates the upregulation of downstream NF-κB pathway factors, attenuating the inflammatory response during PNI and fostering nerve regeneration (90, 91). Kinase DLK (Map3k12) assumes a pivotal role in the initial phase of nerve damage, exerting control over various downstream signaling molecules, including immune factors Csf1, Sarm1, and JNK/c-Jun, thereby governing the process of damage repair (92–95).
4.3 Kinase affects PNR by regulating cell apoptosis
Enhancing anti-apoptosis effects is advantageous for facilitating the restoration of impaired nerves. Rat c-Jun N-terminal kinases (JNK) and extracellular signal-regulated protein kinase (ERK) have been demonstrated to effectively inhibit the PI3K/AKT/mTOR signaling pathway, thereby reducing the expression of apoptosis-related proteins such as Bax and cleaved-Caspase3 (96, 97). Inhibited PI3K/AKT/mTOR signaling pathway was found to hinder scar tissue formation in the sciatic nerve after injury, thereby creating ample room for nerve regeneration (98). Additionally, inhibiting the activity of mTORC1 may reduce the BCL-2 expression and stimulate apoptosis, thereby negatively regulating the development of diabetic peripheral neuropathy (99).
4.4 Kinases affect PNR by regulating the cell cycle
Alteration of the cell cycle of nerve cells is also one of the most important factors influencing the regeneration process of injured nerves. In the central nervous system, AKT kinase can enhance the expression of cyclin D1 by deactivating glycogen synthase kinase-3β (GSK-3β) and reducing protein 27 kinase inhibitor 1 (p27kip1), thereby modulating the cell cycle, influencing cell proliferation rate, and impacting myelin sheath regeneration (100–102). Suppression of AKT phosphorylation can easily result in the arrest of the G1 phase, leading to cell apoptosis and hindering the subsequent recovery of the injured nerves (103). Peripheral nerve extrusion and severance resulted in a notable increase in the expression of protein kinase SKP2 while simultaneously degrading the downstream p27kip1 protein, which had an impact on the cell cycle of cells involved in the regeneration process, ultimately leading to alterations in the progression of injured nerve regeneration (104). The elimination of cyclin-dependent kinase CDK in peripheral nerves typically prevented Schwann cells from initiating the cell cycle, resulting in a significant decrease in the proliferation rate of Schwann cells. Consequently, the reduction of the proliferative Schwann cells severely impeded the regeneration of the damaged nerve myelin sheaths, which in turn affected the recovery of nerve function in the later stage (105).
4.5 Kinase affects PNR by regulating oxidative stress
PNI induces neurotoxicity, and the involvement of the oxidative defense system in nerve tissue can significantly enhance the efficacy against neurotoxicity. It was found that decreased JNK by silymarin (SLM) upregulated the expression of cyclic adenylate response element binding protein CREB, augmented the activity of superoxide dismutase and catalase, reinforced the antioxidant defense system, and suppressed apoptosis and inflammation, thereby safeguarding peripheral nerves (106). Another study has demonstrated that FGF21 might inhibit excessive ERK activation, thereby reducing cellular oxidative stress and autophagy and consequently improving remyelination and nerve regeneration after PNI (107). In addition, the alteration in PI3K kinase activity is intricately associated with excessive oxidation and apoptosis of Schwann cells (108–110).
4.6 Roles of hexokinase in PNR
High glucose has been recognized as one of the most influential factors causing diabetic peripheral neuropathy (DPN) with significant peripheral nerve damage (59). High glucose concentration can induce Schwann cells' apoptosis by affecting various cellular aspects, including oxidative stress, inflammatory reactions, endoplasmic reticulum stress, autophagy, nitrification, and signaling pathways (110). Hexokinase is the first enzyme that catalyzes the phosphorylation of glucose in glycolysis. It has been found that blockade of the enzymatic activity or disruption of the location of hexokinase significantly inhibited the neurite outgrowth of the sensory neurons (111).
5 Kinase affects PNR by regulating the function of different cells in PNS
Kinases are intricate enzymes, the mechanism of which is multilayered, complex, and cell-dependent. Here, we will briefly discuss the functional roles of kinases depending on the different cell types involved in PNR.
5.1 Kinase regulation of neurons
MAPKs play critical roles in peripheral nerve regeneration (Figure 1). Previous studies have demonstrated that P38 and JNK pathways are coordinated to regulate axon regeneration, which involves the participation of other kinases, including DLK1 and MLK1. MLK1/MEK-1/KGB-1 (JNK) MAPK Pathway is necessary for axon regeneration by facilitating the growth cone initiation and migration (112). ERK/MAPK and PI3K/AKT signal channels were found to be both activated in facial neurons after injury (113). The activation of ERK is necessary in proximal and distal nerve stumps, which promote neurite outgrowth and regeneration (114, 115). The Raf-ERK pathway was proved necessary for axon elongation in sensory neurons, while the PI3K-AKT pathway increases axon caliber and branching (116–118). Based on optogenetic systems, activated ERK and AKT successfully enhanced the axon regeneration in the peripheral nerve system in Drosophila, with an excellent ability to fine-tune and guide the axon regrowth, suggesting their application potential in treatments of peripheral nerve injuries (119). The role of GSK3 in PNR seems to be controversial (120). Although sustained GSK-3 without inhibition of PI3K/AKT was found to promote regeneration after sciatic nerve crush (121), blocking of GSK-3β enhanced axonal regeneration and remyelination in both CNS and PNS (122–124). In addition, Rho-kinase plays a negative role in neurite formation and maintenance in both CNS and PNS (125, 126). ROCK/ROCK pathway is involved in rearranging the cytoskeleton, and inhibition of the RhoA signal can counteract the inhibitory effects of other inhibitor molecules on axon growth, leading to increased axon sprouting and improved functional recovery (126, 127).
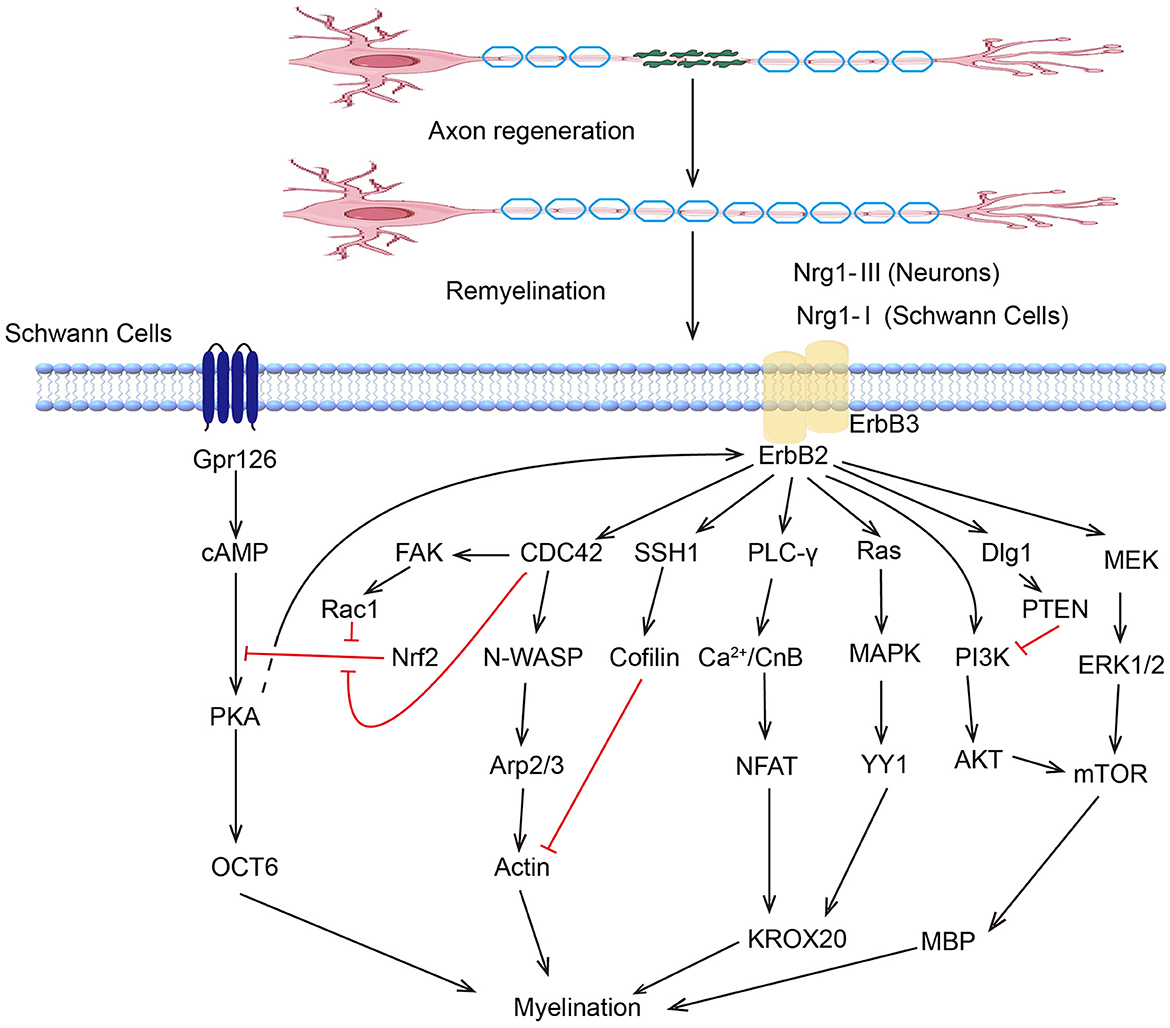
5.2 Kinase regulation of SCs
Peripheral nervous system SCs are renowned for their regenerative ability. Following the inflammatory response, the SCs proliferate rapidly and reinnervate the targeted area with new myelin sheaths, which involve various kinase signaling pathways (Figure 2). The ERK/MAPK pathway is critical in SCs, particularly following nerve injury. ERK activation leads to the upregulation of genes associated with myelin breakdown and nerve repair. This kinase is essential for the proliferation and migration of SCs and their dedifferentiation and redifferentiation processes, which are essential for PNR (128). Studies have shown that melatonin can induce dedifferentiation and proliferation of SCs through the Ras/Raf/ERK, MAPK, and GDNF/PKC pathways, suggesting its potential therapeutic value in treating PNI (129). The PI3K/AKT pathway can promote cell survival and proliferation, enhancing the regenerative potential of SCs. Stab1 was found to activate PI3K/AKT activity, inhibit SCs apoptosis, and promote PNR (130). Sam68 also participates in SC proliferation and regulates regeneration after sciatic nerve compression by activating PI3K/AKT activity (131). The AKT/mTOR pathway in neurons is crucial for myelin sheath growth by supporting protein translation and myelin protein synthesis (132). Mek/ERK1/2-MAPK and PI3K/AKT/mTOR signaling were found to affect Schwann cells' differentiation, myelination, and dysmyelination in either synergetic or independent ways (133). JNK, another member of the MAPK family, regulates the dedifferentiation and apoptosis of SCs and the expression of RAGs (134).
5.3 Kinase regulation of macrophages
In macrophages, P38 MAPK serves as a pivotal kinase in regulating inflammatory cytokine production while also facilitating the transition of macrophages from a pro-inflammatory M1 state to a pro-regenerative M2 state at the site of nerve injury through the modulation of AKT activity. This phenotypic shift is essential for mitigating inflammation and establishing a milieu conducive to nerve regeneration (135, 136).
5.4 Kinase regulation of fibroblasts
Various tissues throughout the body contain fibroblasts, which are mesenchymal cells that synthesize extracellular matrix (ECM) and basement membrane (BL) (137). The wound-healing process in injured peripheral nerves is highly regulated and involves fibroblasts. Transforming growth factor beta-activated kinase 1 (TAK1) can be activated by TGF- β, thereby affecting fibroblast activity and regulating the production of extracellular matrix and scar tissue formation (138).
5.5 Kinase regulation of endothelial cells
TEK receptor tyrosine kinase (Tie-2) controls endothelial-pericyte adhesion to maintain vascular integrity. Tie2 kinases in endothelial cells play critical roles in angiogenesis during nerve regeneration. They facilitate the response to growth factors, such as VEGF, to stimulate the formation of new blood vessels, ensuring the delivery of essential nutrients and oxygen to regenerating nerves (139, 140).
6 Conclusion
PNI diseases are frequently encountered as accidental conditions. Despite the relatively well-understood mechanisms and enhanced precision in surgical interventions, a practical clinical treatment approach still needs to be discovered, leading to suboptimal functional recovery in the long term. PNI frequently induces alterations in kinase activity and tissue inflammation, influencing the subsequent reparative processes. However, the regeneration of peripheral nerve damage is influenced by numerous factors, including the extent of nerve severance, the length and depth of the nerve defect, the level of wound inflammation, the patient's age and physical condition, as well as the activity and expression of various kinases, transcription factor expression and regulation, and nutrient factor secretion. Considering the complexity of these factors, it is evident that more than one factor alone is needed to comprehensively and effectively address the problem. Hence, to overcome the challenges associated with the disease, the examination of kinase function and effectiveness within the framework of nerve injury restoration, alongside the simultaneous analysis of diverse contributing factors, will present a potential avenue for providing innovative perspectives and strategies in managing PNI conditions. In the future, the efficacy of clinical treatment could also be enhanced by identifying drugs that target the kinase enzyme activity.
Author contributions
XZ: Funding acquisition, Writing—original draft. XD: Writing—review & editing. XL: Conceptualization, Writing—review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Project of Wuxi Health Committee (Q202229) and the General Program of Maternal and Child Health Research of Wuxi Health Commission (FYKY202304) received by XZ.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Faroni A, Mobasseri SA, Kingham PJ, Reid AJ. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev. (2015) 82–83:160–7. doi: 10.1016/j.addr.2014.11.010
2. Gwathmey KG, Satkowiak K. Peripheral nervous system manifestations of rheumatological diseases. J Neurol Sci. (2021) 424:117421. doi: 10.1016/j.jns.2021.117421
3. Wijntjes J, Borchert A, van Alfen N. Nerve ultrasound in traumatic and iatrogenic peripheral nerve injury. Diagnostics. (2020) 11:30. doi: 10.3390/diagnostics11010030
4. Desai K, Warade AC, Jha AK, Pattankar S. Injection-related iatrogenic peripheral nerve injuriesurgical experience of 354 operated cases. Neurol India. (2019) 67 (Supplement): S82–91. doi: 10.4103/0028-3886.250703
5. Hara T, Tatebe M, Kurahashi T, Hirata H. Iatrogenic peripheral nerve injuries - common causes and treatment: a retrospective single-center cohort study. J Orthop Sci. (2021) 26:1119–23. doi: 10.1016/j.jos.2020.09.009
6. Kumar A, Shukla D, Bhat DI, Devi BI. Iatrogenic peripheral nerve injuries. Neurol India. (2019) 67 (Supplement): S135–9. doi: 10.4103/0028-3886.250700
7. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. (2012) 11:521–34. doi: 10.1016/S1474-4422(12)70065-0
8. Florian IA, Lupan I, Sur L, Samasca G, Timiş TL. To be, or not to be… Guillain-Barré syndrome. Autoimmun Rev. (2021) 20:102983. doi: 10.1016/j.autrev.2021.102983
9. Florica B, Aghdassi E, Su J, Gladman DD, Urowitz MB, Fortin PR. Peripheral neuropathy in patients with systemic lupus erythematosus. Semin Arthritis Rheum. (2011) 41:203–11. doi: 10.1016/j.semarthrit.2011.04.001
10. McCoy SS, Baer AN. Neurological complications of Sjögren's syndrome: diagnosis and management. Curr Treatm Opt Rheumatol. (2017) 3:275–88. doi: 10.1007/s40674-017-0076-9
11. Gu X, Ding F, Williams DF. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials. (2014) 35:6143–56. doi: 10.1016/j.biomaterials.2014.04.064
12. Baradaran A, El-Hawary H, Efanov JI, Xu L. Peripheral nerve healing: so near and yet so far. Semin Plast Surg. (2021) 35:204–10. doi: 10.1055/s-0041-1731630
13. Scheib J, Höke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. (2013) 9:668–76. doi: 10.1038/nrneurol.2013.227
14. Jones LH. Small-molecule kinase downregulators. Cell Chem Biol. (2018) 25:30–5. doi: 10.1016/j.chembiol.2017.10.011
15. Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. (2015) 36:422–39. doi: 10.1016/j.tips.2015.04.005
16. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. (2002) 298:1912–34. doi: 10.1126/science.1075762
17. Roskoski R Jr. Properties of FDA-approved small molecule protein kinase inhibitors: a 2022 update. Pharmacol Res. (2022) 175:106037. doi: 10.1016/j.phrs.2021.106037
18. ten Dijke P, Franzén P, Yamashita H, Ichijo H, Heldin CH, Miyazono K. Serine/threonine kinase receptors. Prog Growth Factor Res. (1994) 5:55–72. doi: 10.1016/0955-2235(94)90017-5
19. Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. (2000) 69:373–98. doi: 10.1146/annurev.biochem.69.1.373
20. Attwood PV. Histidine kinases from bacteria to humans. Biochem Soc Trans. (2013) 41:1023–8. doi: 10.1042/BST20130019
21. Dumas R, Cobessi D, Robin AY, Ferrer JL, Curien G. The many faces of aspartate kinases. Arch Biochem Biophys. (2012) 519:186–93. doi: 10.1016/j.abb.2011.10.016
22. Pavlíková D, Pavlík M, Staszková L, Motyka V, Száková J, Tlustos P, et al. Glutamate kinase as a potential biomarker of heavy metal stress in plants. Ecotoxicol Environ Saf. (2008) 70:223–30. doi: 10.1016/j.ecoenv.2007.07.006
23. Matthews HR. Protein kinases and phosphatases that act on histidine, lysine, or arginine residues in eukaryotic proteins: a possible regulator of the mitogen-activated protein kinase cascade. Pharmacol Ther. (1995) 67:323–50. doi: 10.1016/0163-7258(95)00020-8
24. Pereira CA. Arginine kinase: a potential pharmacological target in trypanosomiasis. Infect Disord Drug Targets. (2014) 14:30–6. doi: 10.2174/1871526514666140713144103
25. Leroux AE, Schulze JO, Biondi RM. AGC kinases, mechanisms of regulation and innovative drug development. Semin Cancer Biol. (2018) 48:1–17. doi: 10.1016/j.semcancer.2017.05.011
26. Takemoto-Kimura S, Suzuki K, Horigane SI, Kamijo S, Inoue M, Sakamoto M, et al. Calmodulin kinases: essential regulators in health and disease. J Neurochem. (2017) 141:808–18. doi: 10.1111/jnc.14020
27. Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. (1995) 9:726–35. doi: 10.1096/fasebj.9.9.7601337
28. Chong H, Vikis HG, Guan KL. Mechanisms of regulating the Raf kinase family. Cell Signal. (2003) 15:463–9. doi: 10.1016/S0898-6568(02)00139-0
29. Harris TE, Lawrence JC Jr. TOR signaling. Sci STKE. (2003) 2003:re15. doi: 10.1126/stke.2122003re15
31. Fu XY. From PTK-STAT signaling to caspase expression and apoptosis induction. Cell Death Differ. (1999) 6:1201–8. doi: 10.1038/sj.cdd.4400613
32. Lahiry P, Torkamani A, Schork NJ, Hegele RA. Kinase mutations in human disease: interpreting genotype-phenotype relationships. Nat Rev Genet. (2010) 11:60–74. doi: 10.1038/nrg2707
33. Tsai CC, Yue Z, Shen J. How electrostatic coupling enables conformational plasticity in a tyrosine kinase. J Am Chem Soc. (2019) 141:15092–101. doi: 10.1021/jacs.9b06064
34. Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. (1988) 241:42–52. doi: 10.1126/science.3291115
35. Neet K, Hunter T. Vertebrate non-receptor protein-tyrosine kinase families. Genes Cells. (1996) 1:147–69. doi: 10.1046/j.1365-2443.1996.d01-234.x
37. Radha V, Nambirajan S, Swarup G. Association of lyn tyrosine kinase with the nuclear matrix and cell-cycle-dependent changes in matrix-associated tyrosine kinase activity. Eur J Biochem. (1996) 236:352–9. doi: 10.1111/j.1432-1033.1996.00352.x
38. Ruetten H, Thiemermann C. Effects of tyrphostins and genistein on the circulatory failure and organ dysfunction caused by endotoxin in the rat: a possible role for protein tyrosine kinase. Br J Pharmacol. (1997) 122:59–70. doi: 10.1038/sj.bjp.0701345
39. Taylor SS, Ilouz R, Zhang P, Kornev AP. Assembly of allosteric macromolecular switches: lessons from PKA. Nat Rev Mol Cell Biol. (2012) 13:646–58. doi: 10.1038/nrm3432
40. Amatu A, Sartore-Bianchi A, Bencardino K, Pizzutilo EG, Tosi F, Siena S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann Oncol. (2019) 30(Suppl_8):viii5–15. doi: 10.1093/annonc/mdz383
41. Tsygankov AY. Non-receptor protein tyrosine kinases. Front Biosci. (2003) 8:s595–635. doi: 10.2741/1106
42. Jacobsen FA, Scherer AN, Mouritsen J, Bragadóttir H, Thomas Bäckström B, Sardar S, et al. Role for the non-receptor tyrosine kinase Abl2/Arg in experimental neuroinflammation. J Neuroimmune Pharmacol. (2018) 13:265–76. doi: 10.1007/s11481-018-9783-8
43. Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, et al. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and AKT. Nat Med. (2015) 21:906–13. doi: 10.1038/nm.3908
44. Koretke KK, Lupas AN, Warren PV, Rosenberg M, Brown JR. Evolution of two-component signal transduction. Mol Biol Evol. (2000) 17:1956–70. doi: 10.1093/oxfordjournals.molbev.a026297
45. Wolanin PM, Thomason PA, Stock JB. Histidine protein kinases: key signal transducers outside the animal kingdom. Genome Biol. (2002) 3:Reviews3013. doi: 10.1186/gb-2002-3-10-reviews3013
46. Diévart A, Clark SE. LRR-containing receptors regulating plant development and defense. Development. (2004) 131:251–61. doi: 10.1242/dev.00998
47. Lee DS, Kim YC, Kwon SJ, Ryu CM, Park OK. The Arabidopsis cysteine-rich receptor-like kinase CRK36 regulates immunity through interaction with the cytoplasmic kinase BIK1. Front Plant Sci. (2017) 8:1856. doi: 10.3389/fpls.2017.01856
48. Pauwels K, Abadjieva A, Hilven P, Stankiewicz A, Crabeel M. The N-acetylglutamate synthase/N-acetylglutamate kinase metabolon of Saccharomyces cerevisiae allows coordinated feedback regulation of the first two steps in arginine biosynthesis. Eur J Biochem. (2003) 270:1014–24. doi: 10.1046/j.1432-1033.2003.03477.x
49. Brown JR, Auger KR. Phylogenomics of phosphoinositide lipid kinases: perspectives on the evolution of second messenger signaling and drug discovery. BMC Evol Biol. (2011) 11:4. doi: 10.1186/1471-2148-11-4
50. Rincón E, Gharbi SI, Santos-Mendoza T, Mérida I. Diacylglycerol kinase ζ: at the crossroads of lipid signaling and protein complex organization. Prog Lipid Res. (2012) 51:1–10. doi: 10.1016/j.plipres.2011.10.001
51. Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase–AKT pathway in human cancer. Nat Rev Cancer. (2002) 2:489–501. doi: 10.1038/nrc839
52. Conway LP, Voglmeir J. Functional analysis of anomeric sugar kinases. Carbohydr Res. (2016) 432:23–30. doi: 10.1016/j.carres.2016.06.001
53. Zelent D, Najafi H, Odili S, Buettger C, Weik-Collins H, Li C, et al. Glucokinase and glucose homeostasis: proven concepts and new ideas. Biochem Soc Trans. (2005) 33(Pt 1):306–10. doi: 10.1042/BST0330306
54. DeLeonibus A, Rezaei M, Fahradyan V, Silver J, Rampazzo A, Bassiri Gharb B, et al. meta-analysis of functional outcomes in rat sciatic nerve injury models. Microsurgery. (2021) 41:286–95. doi: 10.1002/micr.30713
55. Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci. (2016) 43:336–50. doi: 10.1111/ejn.13005
56. Liu Y, Yu S, Gu X, Cao R, Cui S. Tissue-engineered nerve grafts using a scaffold-independent and injectable drug delivery system: a novel design with translational advantages. J Neural Eng. (2019) 16:036030. doi: 10.1088/1741-2552/ab17a0
57. Liao C, Zheng R, Wei C, Yan J, Ding Y, Wang G, et al. Tissue-engineered conduit promotes sciatic nerve regeneration following radiation-induced injury as monitored by magnetic resonance imaging. Magn Reson Imaging. (2016) 34:515–23. doi: 10.1016/j.mri.2015.12.004
58. Chang W, Shah MB, Lee P, Yu X. Tissue-engineered spiral nerve guidance conduit for peripheral nerve regeneration. Acta Biomater. (2018) 73:302–11. doi: 10.1016/j.actbio.2018.04.046
59. Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. (2011) 8:110. doi: 10.1186/1742-2094-8-110
60. Sen MK, Mahns DA, Coorssen JR, Shortland PJ. The roles of microglia and astrocytes in phagocytosis and myelinationnsights from the cuprizone model of multiple sclerosis. Glia. (2022) 70:1215–50. doi: 10.1002/glia.24148
61. Mueller M, Leonhard C, Wacker K, Ringelstein EB, Okabe M, Hickey WF, et al. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab Invest. (2003) 83:175–85. doi: 10.1097/01.LAB.0000056993.28149.BF
62. Perkins NM, Tracey DJ. Hyperalgesia due to nerve injury: role of neutrophils. Neuroscience. (2000) 101:745–57. doi: 10.1016/S0306-4522(00)00396-1
63. Zigmond RE, Echevarria FD. Macrophage biology in the peripheral nervous system after injury. Prog Neurobiol. (2019) 173:102–21. doi: 10.1016/j.pneurobio.2018.12.001
64. Nocera G, Jacob C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol Life Sci. (2020) 77:3977–89. doi: 10.1007/s00018-020-03516-9
65. Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. (2016) 594:3521–31. doi: 10.1113/JP270874
66. Renthal W, Tochitsky I, Yang L, Cheng YC, Li E, Kawaguchi R, et al. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron. (2020) 108:128–44.e129. doi: 10.1016/j.neuron.2020.07.026
67. Zhang Y, Zhao Q, Chen Q, Xu L, Yi S. Transcriptional control of peripheral nerve regeneration. Mol Neurobiol. (2023) 60:329–41. doi: 10.1007/s12035-022-03090-0
68. Jankowski MP, McIlwrath SL, Jing X, Cornuet PK, Salerno KM, Koerber HR, et al. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. (2009) 1256:43–54. doi: 10.1016/j.brainres.2008.12.032
69. Zhang Y, Shen Y, Zhao L, Zhao Q, Zhao L, Yi S. Transcription factor BCL11A regulates Schwann cell behavior during peripheral nerve regeneration. Mol Neurobiol. (2023) 60:5352–65. doi: 10.1007/s12035-023-03432-6
70. Li R, Li D, Wu C, Ye L, Wu Y, Yuan Y, et al. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics. (2020) 10:1649–77. doi: 10.7150/thno.40919
71. Alsmadi NZ, Bendale GS, Kanneganti A, Shihabeddin T, Nguyen AH, Hor E, et al. Glial-derived growth factor and pleiotrophin synergistically promote axonal regeneration in critical nerve injuries. Acta Biomater. (2018) 78:165–77. doi: 10.1016/j.actbio.2018.07.048
72. Huo DS, Zhang M, Cai ZP, Dong CX, Wang H, Yang ZJ. The role of nerve growth factor in ginsenoside Rg1-induced regeneration of injured rat sciatic nerve. J Toxicol Environ Health A. (2015) 78:1328–37. doi: 10.1080/15287394.2015.1085943
73. Romero MI, Rangappa N, Garry MG, Smith GM. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J Neurosci. (2001) 21:8408–16. doi: 10.1523/JNEUROSCI.21-21-08408.2001
74. Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. (2009) 26:E3. doi: 10.3171/FOC.2009.26.2.E3
75. Borger A, Stadlmayr S, Haertinger M, Semmler L, Supper P, Millesi F, et al. How miRNAs regulate Schwann cells during peripheral nerve regeneration-a systemic review. Int J Mol Sci. (2022) 23:3440. doi: 10.3390/ijms23073440
76. Yu B, Qian T, Wang Y, Zhou S, Ding G, Ding F, et al. miR-182 inhibits Schwann cell proliferation and migration by targeting FGF9 and NTM, respectively at an early stage following sciatic nerve injury. Nucleic Acids Res. (2012) 40:10356–65. doi: 10.1093/nar/gks750
77. Gökbuget D, Pereira JA, Bachofner S, Marchais A, Ciaudo C, Stoffel M, et al. The Lin28/let-7 axis is critical for myelination in the peripheral nervous system. Nat Commun. (2015) 6:8584. doi: 10.1038/ncomms9584
78. Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. (2008) 56:1552–65. doi: 10.1002/glia.20761
79. Perry RB, Hezroni H, Goldrich MJ, Ulitsky I. Regulation of neuroregeneration by long noncoding RNAs. Mol Cell. (2018) 72:553–67.e555. doi: 10.1016/j.molcel.2018.09.021
80. Wang H, Wu J, Zhang X, Ding L, Zeng Q. Microarray analysis of the expression profile of lncRNAs reveals the key role of lncRNA BC088327 as an agonist to heregulin-1β-induced cell proliferation in peripheral nerve injury. Int J Mol Med. (2018) 41:3477–84. doi: 10.3892/ijmm.2018.3571
81. Mao S, Huang T, Chen Y, Shen L, Zhou S, Zhang S, et al. Circ-Spidr enhances axon regeneration after peripheral nerve injury. Cell Death Dis. (2019) 10:787. doi: 10.1038/s41419-019-2027-x
82. Mao S, Zhang S, Zhou S, Huang T, Feng W, Gu X, et al. Schwann cell-enriched circular RNA circ-Ankib1 regulates Schwann cell proliferation following peripheral nerve injury. FASEB J. (2019) 33:12409–24. doi: 10.1096/fj.201900965R
83. Yang P, Wen HZ, Zhang JH. Expression of a dominant-negative Rho-kinase promotes neurite outgrowth in a microenvironment mimicking injured central nervous system. Acta Pharmacol Sin. (2010) 31:531–9. doi: 10.1038/aps.2010.35
84. Kong L, Gao X, Qian Y, Sun W, You Z, Fan C. Biomechanical microenvironment in peripheral nerve regeneration: from pathophysiological understanding to tissue engineering development. Theranostics. (2022) 12:4993–5014. doi: 10.7150/thno.74571
85. Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. (2014) 26:2694–701. doi: 10.1016/j.cellsig.2014.08.019
86. Liu SY, Chen L, Li XC, Hu QK, He LJ. Lycium barbarum polysaccharide protects diabetic peripheral neuropathy by enhancing autophagy via mTOR/p70S6K inhibition in Streptozotocin-induced diabetic rats. J Chem Neuroanat. (2018) 89:37–42. doi: 10.1016/j.jchemneu.2017.12.011
87. Xiong J, Kong Q, Dai L, Ma H, Cao X, Liu L, et al. Autophagy activated by tuberin/mTOR/p70S6K suppression is a protective mechanism against local anaesthetics neurotoxicity. J Cell Mol Med. (2017) 21:579–87. doi: 10.1111/jcmm.13003
88. Ko SH, Apple EC, Liu Z, Chen L. Age-dependent autophagy induction after injury promotes axon regeneration by limiting NOTCH. Autophagy. (2020) 16:2052–68. doi: 10.1080/15548627.2020.1713645
89. Kato N, Matsumoto M, Kogawa M, Atkins GJ, Findlay DM, Fujikawa T, et al. Critical role of p38 MAPK for regeneration of the sciatic nerve following crush injury in vivo. J Neuroinflammation. (2013) 10:1. doi: 10.1186/1742-2094-10-1
90. Xu D, Jin T, Zhu H, Chen H, Ofengeim D, Zou C, et al. TBK1 suppresses RIPK1-driven apoptosis and inflammation during development and in aging. Cell. (2018) 174:1477–91.e1419. doi: 10.1016/j.cell.2018.07.041
91. Govindasamy N, Chung Chok K, Ying Ng P, Yian Koh R, Moi Chye S. Melatonin induced schwann cell proliferation and dedifferentiation through NF-KB, FAK-dependent but Src-independent pathways. Rep Biochem Mol Biol. (2022) 11:63–73. doi: 10.52547/rbmb.11.1.63
92. Huntwork-Rodriguez S, Wang B, Watkins T, Ghosh AS, Pozniak CD, Bustos D, et al. JNK-mediated phosphorylation of DLK suppresses its ubiquitination to promote neuronal apoptosis. J Cell Biol. (2013) 202:747–63. doi: 10.1083/jcb.201303066
93. Wlaschin JJ, Gluski JM, Nguyen E, Silberberg H, Thompson JH, Chesler AT, et al. Dual leucine zipper kinase is required for mechanical allodynia and microgliosis after nerve injury. eLife. (2018) 7:e33910. doi: 10.7554/eLife.33910
94. Gerdts J, Summers DW, Milbrandt J, DiAntonio A. Axon self-destruction: new links among SARM1, MAPKs, and NAD+ metabolism. Neuron. (2016) 89:449–60. doi: 10.1016/j.neuron.2015.12.023
95. Shin JE, Ha H, Kim YK, Cho Y, DiAntonio A. DLK regulates a distinctive transcriptional regeneration program after peripheral nerve injury. Neurobiol Dis. (2019) 127:178–92. doi: 10.1016/j.nbd.2019.02.001
96. Yu X, Fan H, Jiang X, Zheng W, Yang Y, Jin M, et al. Apatinib induces apoptosis and autophagy via the PI3K/AKT/mTOR and MAPK/ERK signaling pathways in neuroblastoma. Oncol Lett. (2020) 20:52. doi: 10.3892/ol.2020.11913
97. Liu H, Hussain Z, Xie Q, Yan X, Zeng C, Zhou G, et al. Targeting PI3K/AKT/mTOR pathway to enhance the anti-leukemia efficacy of venetoclax. Exp Cell Res. (2022) 417:113192. doi: 10.1016/j.yexcr.2022.113192
98. Chen CH, Sung CS, Huang SY, Feng CW, Hung HC, Yang SN, et al. The role of the PI3K/AKT/mTOR pathway in glial scar formation following spinal cord injury. Exp Neurol. (2016) 278:27–41. doi: 10.1016/j.expneurol.2016.01.023
99. Zhu L, Hao J, Cheng M, Zhang C, Huo C, Liu Y, et al. Hyperglycemia-induced Bcl-2/Bax-mediated apoptosis of Schwann cells via mTORC1/S6K1 inhibition in diabetic peripheral neuropathy. Exp Cell Res. (2018) 367:186–95. doi: 10.1016/j.yexcr.2018.03.034
100. Akram R, Anwar H, Javed MS, Rasul A, Imran A, Malik SA, et al. Axonal regeneration: underlying molecular mechanisms and potential therapeutic targets. Biomedicines. (2022) 10:3186. doi: 10.3390/biomedicines10123186
101. Gao L, Wang C, Qin B, Li T, Xu W, Lenahan C, et al. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase Suppresses neuronal apoptosis by increasing glycolysis and “cyclin-dependent kinase 1-mediated phosphorylation of p27 After traumatic spinal cord injury in rats. Cell Transpl. (2020) 29:963689720950226. doi: 10.1177/0963689720950226
102. Dill J, Wang H, Zhou F, Li S. Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci. (2008) 28:8914–28. doi: 10.1523/JNEUROSCI.1178-08.2008
103. Park KW, Kim DH, You HJ, Sir JJ, Jeon SI, Youn SW, et al. Activated forkhead transcription factor inhibits neointimal hyperplasia after angioplasty through induction of p27. Arterioscler Thromb Vasc Biol. (2005) 25:742–7. doi: 10.1161/01.ATV.0000156288.70849.26
104. Shen AG, Shi SX, Chen ML, Qin J, Gao SF, Cheng C. Dynamic changes of p27(kip1) and Skp2 expression in injured rat sciatic nerve. Cell Mol Neurobiol. (2008) 28:713–25. doi: 10.1007/s10571-007-9167-8
105. Li H, Yang H, Liu Y, Huan W, Zhang S, Wu G, et al. The cyclin-dependent kinase inhibitor p27(Kip1) is a positive regulator of Schwann cell differentiation in vitro. J Mol Neurosci. (2011) 45:277–83. doi: 10.1007/s12031-011-9518-2
106. Yardim A, Kucukler S, Özdemir S, Çomakli S, Caglayan C, Kandemir FM, et al. Silymarin alleviates docetaxel-induced central and peripheral neurotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Gene. (2021) 769:145239. doi: 10.1016/j.gene.2020.145239
107. Lu Y, Li R, Zhu J, Wu Y, Li D, Dong L, et al. Fibroblast growth factor 21 facilitates peripheral nerve regeneration through suppressing oxidative damage and autophagic cell death. J Cell Mol Med. (2019) 23:497–511. doi: 10.1111/jcmm.13952
108. Zhang X, Liang Z, Zhou Y, Wang F, Wei S, Tan B, et al. Artesunate inhibits apoptosis and promotes survival in Schwann cells via the PI3K/AKT/mTOR axis in diabetic peripheral neuropathy. Biol Pharm Bull. (2023) 46:764–72. doi: 10.1248/bpb.b22-00619
109. Li R, Wu Y, Zou S, Wang X, Li Y, Xu K, et al. NGF attenuates high glucose-induced ER stress, preventing Schwann cell apoptosis by activating the PI3K/AKT/GSK3β and ERK1/2 pathways. Neurochem Res. (2017) 42:3005–18. doi: 10.1007/s11064-017-2333-6
110. Liu YP, Shao SJ, Guo HD. Schwann cells apoptosis is induced by high glucose in diabetic peripheral neuropathy. Life Sci. (2020) 248:117459. doi: 10.1016/j.lfs.2020.117459
111. Wang Z, Gardiner NJ, Fernyhough P. Blockade of hexokinase activity and binding to mitochondria inhibits neurite outgrowth in cultured adult rat sensory neurons. Neurosci Lett. (2008) 434:6–11. doi: 10.1016/j.neulet.2008.01.057
112. Nix P, Hisamoto N, Matsumoto K, Bastiani M. Axon regeneration requires coordinate activation of p38 and JNK MAPK pathways. Proc Natl Acad Sci U S A. (2011) 108:10738–43. doi: 10.1073/pnas.1104830108
113. Huang HT, Sun ZG, Liu HW, Ma JT, Hu M. ERK/MAPK and PI3K/AKT signal channels simultaneously activated in nerve cell and axon after facial nerve injury. Saudi J Biol Sci. (2017) 24:1853–8. doi: 10.1016/j.sjbs.2017.11.027
114. Agthong S, Kaewsema A, Tanomsridejchai N, Chentanez V. Activation of MAPK ERK in peripheral nerve after injury. BMC Neurosci. (2006) 7:45. doi: 10.1186/1471-2202-7-45
115. Agthong S, Koonam J, Kaewsema A, Chentanez V. Inhibition of MAPK ERK impairs axonal regeneration without an effect on neuronal loss after nerve injury. Neurol Res. (2009) 31:1068–74. doi: 10.1179/174313209X380883
116. Markus A, Zhong J, Snider WD. Raf and AKT mediate distinct aspects of sensory axon growth. Neuron. (2002) 35:65–76. doi: 10.1016/S0896-6273(02)00752-3
117. Huang H, Liu H, Yan R, Hu M. PI3K/AKT and ERK/MAPK signaling promote different aspects of neuron survival and axonal regrowth following rat facial nerve axotomy. Neurochem Res. (2017) 42:3515–24. doi: 10.1007/s11064-017-2399-1
118. Hausott B, Klimaschewski L. Promotion of peripheral nerve regeneration by stimulation of the extracellular signal-regulated kinase (ERK) pathway. Anat Rec. (2019) 302:1261–7. doi: 10.1002/ar.24126
119. Wang Q, Fan H, Li F, Skeeters SS, Krishnamurthy VV, Song Y, et al. Optical control of ERK and AKT signaling promotes axon regeneration and functional recovery of PNS and CNS in Drosophila. Elife. (2020) 9:e57395. doi: 10.7554/eLife.57395
120. Diekmann H, Fischer D. Role of GSK3 in peripheral nerve regeneration. Neural Regen Res. (2015) 10:1602–3. doi: 10.4103/1673-5374.167753
121. Gobrecht P, Leibinger M, Andreadaki A, Fischer D. Sustained GSK3 activity markedly facilitates nerve regeneration. Nat Commun. (2014) 5:4561. doi: 10.1038/ncomms5561
122. Chen Y, Weng J, Han D, Chen B, Ma M, Yu Y, et al. GSK3β inhibition accelerates axon debris clearance and new axon remyelination. Am J Transl Res. (2016) 8:5410–20.
123. Leibinger M, Andreadaki A, Golla R, Levin E, Hilla AM, Diekmann H, et al. Boosting CNS axon regeneration by harnessing antagonistic effects of GSK3 activity. Proc Natl Acad Sci U S A. (2017) 114:E5454–e5463. doi: 10.1073/pnas.1621225114
124. Su H, Yuan Q, Qin D, Yang X, Wong WM, So KF, et al. Lithium enhances axonal regeneration in peripheral nerve by inhibiting glycogen synthase kinase 3β activation. Biomed Res Int. (2014) 2014:658753. doi: 10.1155/2014/658753
125. Madura T, Yamashita T, Kubo T, Fujitani M, Hosokawa K, Tohyama M. Activation of Rho in the injured axons following spinal cord injury. EMBO Rep. (2004) 5:412–7. doi: 10.1038/sj.embor.7400117
126. Hiraga A, Kuwabara S, Doya H, Kanai K, Fujitani M, Taniguchi J, et al. Rho-kinase inhibition enhances axonal regeneration after peripheral nerve injury. J Peripher Nerv Syst. (2006) 11:217–24. doi: 10.1111/j.1529-8027.2006.00091.x
127. Fujita Y, Yamashita T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci. (2014) 8:338. doi: 10.3389/fnins.2014.00338
128. Newbern JM, Snider WD. Bers-ERK Schwann cells coordinate nerve regeneration. Neuron. (2012) 73:623–6. doi: 10.1016/j.neuron.2012.02.002
129. Tiong YL, Ng KY, Koh RY, Ponnudurai G, Chye SM. Melatonin promotes Schwann cell dedifferentiation and proliferation through the Ras/Raf/ERK and MAPK pathways, and glial cell-derived neurotrophic factor expression. Exp Ther Med. (2020) 20:16. doi: 10.3892/etm.2020.9143
130. Liu HW, Bi WT, Huang HT, Li RX, Xi Q, Feng L, et al. Satb1 promotes Schwann cell viability and migration via activation of PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. (2018) 22:4268–77. doi: 10.26355/eurrev_201807_15423
131. Wu W, Liu Y, Wang Y. Sam68 promotes Schwann cell proliferation by enhancing the PI3K/AKT pathway and acts on regeneration after sciatic nerve crush. Biochem Biophys Res Commun. (2016) 473:1045–51. doi: 10.1016/j.bbrc.2016.04.013
132. Fedder-Semmes KN, Appel B. The AKT-mTOR pathway drives myelin sheath growth by regulating cap-dependent translation. J Neurosci. (2021) 41:8532–44. doi: 10.1523/JNEUROSCI.0783-21.2021
133. Ishii A, Furusho M, Bansal R. Mek/ERK1/2-MAPK and PI3K/AKT/mTOR signaling plays both independent and cooperative roles in Schwann cell differentiation, myelination and dysmyelination. Glia. (2021) 69:2429–46. doi: 10.1002/glia.24049
134. Shin YK, Jang SY, Park JY, Park SY, Lee HJ, Suh DJ, et al. The neuregulin-Rac-MKK7 pathway regulates antagonistic c-jun/Krox20 expression in Schwann cell dedifferentiation. Glia. (2013) 61:892–904. doi: 10.1002/glia.22482
135. Liu L, Guo H, Song A, Huang J, Zhang Y, Jin S, et al. Progranulin inhibits LPS-induced macrophage M1 polarization via NF-KB and MAPK pathways. BMC Immunol. (2020) 21:32. doi: 10.1186/s12865-020-00355-y
136. Perdiguero E, Sousa-Victor P, Ruiz-Bonilla V, Jardí M, Caelles C, Serrano AL, et al. p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol. (2011) 195:307–22. doi: 10.1083/jcb.201104053
137. Liu X, Duan X. Mechanisms and treatments of peripheral nerve injury. Ann Plast Surg. (2023) 91:313–8. doi: 10.1097/SAP.0000000000003480
138. Kuk H, Hutchenreuther J, Murphy-Marshman H, Carter D, Leask A. 5Z-7-oxozeanol inhibits the effects of TGFβ1 on human gingival fibroblasts. PLoS ONE. (2015) 10:e0123689. doi: 10.1371/journal.pone.0123689
139. Qiu L, He B, Hu J, Zhu Z, Liu X, Zhu J. Cartilage oligomeric matrix protein angiopoeitin-1 provides benefits during nerve regeneration in vivo and in vitro. Ann Biomed Eng. (2015) 43:2924–40. doi: 10.1007/s10439-015-1342-3
Keywords: peripheral nerve regeneration, peripheral nerve injury, kinase, molecular mechanism, microenvironment
Citation: Zhang X, Duan X and Liu X (2024) The role of kinases in peripheral nerve regeneration: mechanisms and implications. Front. Neurol. 15:1340845. doi: 10.3389/fneur.2024.1340845
Received: 19 November 2023; Accepted: 02 April 2024;
Published: 16 April 2024.
Edited by:
Hui Lu, Zhejiang University, ChinaReviewed by:
Lars Klimaschewski, Innsbruck Medical University, AustriaCopyright © 2024 Zhang, Duan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuchu Duan, dxd2002sk@ntu.edu.cn; Xiaoyu Liu, lxy2002sk@ntu.edu.cn
 Xu Zhang
Xu Zhang