
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Lang. Sci. , 09 August 2023
Sec. Neurobiology of Language
Volume 2 - 2023 | https://doi.org/10.3389/flang.2023.1217837
This article is part of the Research Topic Syntax, the brain, and linguistic theory: a critical reassessment View all 11 articles
A recently emerging generalization about language and the brain is that brain regions implicated in language that show syntax-related activations (e.g., increased activation for more complex sentence structures) also tend to show word-related activations, such as increased activation for reading real words (e.g., poet) relative to pseudowords (e.g., tevill). Fedorenko et al. (2020) generalize as follows: “...syntactic/combinatorial processing is not separable from lexico-semantic processing at the level of brain regions-or even voxel subsets-within the language network”. Based on this generalization, Fedorenko et al. have made the conclusion...” that a cognitive architecture whereby syntactic processing is not separable from the processing of individual word meanings is most likely,” arguing against “syntax-centric” views of language as promulgated by Chomsky and others. However, the notion of “lexico-semantics”, a commonly used concept in the field of neurolinguistics, obscures the fact that words are both syntactic and semantic entities. Because of this, any functional neuroimaging experiment that manipulates lexicality will almost assuredly tax both syntactic and semantic resources and is therefore inadequate for isolating conceptual-semantic processing in the brain in addition to syntax. Unlike these sorts of neuroimaging studies, robust lesion data show clear functional-anatomical dissociations within the language network. Finally, a “syntax-centric” view of language is perfectly compatible with the state of the art in neurobiology because of the multiple potential mappings between linguistic theory and neurobiology beyond the level of individual brain regions. The present work presents a critique of Fedorenko et al. (2020) as a way to explore these more general issues.
The way language works, then, is that each person's brain contains a lexicon of words and the concepts they stand for (a mental dictionary) and a set of rules that combine the words to convey relationships among concepts (a mental grammar) (Pinker, 1995).
Pinker's work had a major impact in popularizing the ideas of Chomsky and mainstream generative grammar (MGG). Like Chomsky, Pinker forcibly argued for the concept of an innate linguistic module of the human brain that allows us to learn language. However, his work included some simplifications that have become ensconced in cognitive science. A major one was “Words and Rules” (Pinker, 1999; Pinker and Ullman, 2002): the idea that language is fundamentally two diametrically opposed systems, a database of form-meaning pairs (the lexicon) and a rule-based, combinatorial system (syntax), each rooted in distinct underlying brain systems (Figure 1, left). Pinker combined the traditional Saussurean notion of human language as fundamentally a system of arbitrary form-meaning pairs with the focus of generative grammar on combinatorial syntactic operations.
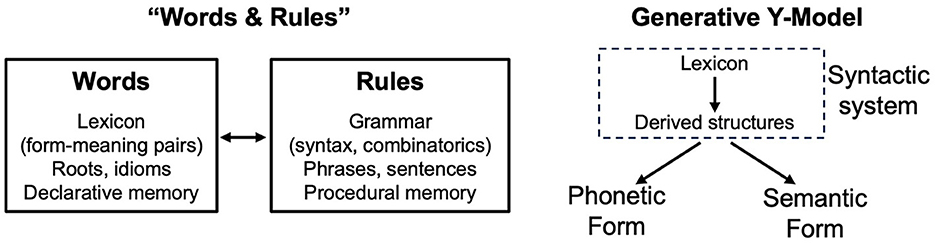
Figure 1. LEFT: the Words and Rules theory of Pinker (1999), adapted from Pinker and Ullman (2002). Separate cognitive systems are posited, each with distinct memory mechanisms, one containing a lexicon of form-meaning pairs (words) and one involved in combining lexical elements to create complex structures and semantic relations (rules). RIGHT: the Y-model posited in mainstream generative grammar (e.g., Chomsky, 1965, 1981, 1995b). A syntactic module, which combines lexical elements into complex structures, is then interpreted by two separate modules, one for phonetic form (how an expression is pronounced) and one for semantic form (the meaning of an utterance). In the Y-model, there is only an indirect and loose connection between the lexicon and meaning, whereas in the Words and Rules model, the lexicon is a repository of form-meaning pairs.
While many have taken the Words and Rules theory of Pinker to accurately summarize the MGG approach, these approaches are actually fundamentally incompatible (see also Embick and Marantz, 2005). The first models of MGG did not even contain a lexicon (Chomsky, 1955, 1957). Later models understood words as syntactic objects, inputs to syntactic computation, only later acquiring phonological and semantic expression (the inverted Y-model; Chomsky, 1965, 1981, 1995b) (Figure 1, right). Even further, Chomsky (1995a) proposed the theory of bare phrase structure, in which the labels of phrasal projections are not traditional syntactic categories like nouns and verbs but are rather derived from the lexical items themselves. For example, instead of verb phrases, there are “eat phrases”; instead of noun phrases, there are “cat phrases”; and so on. Some researchers have pointed to compelling evidence that words do not bear a direct mapping to meaning as Saussure claimed (Pietroski, 2018; Preminger, 2021), referring to phenomenon such as polysemy, in which the meaning of the same lexical item, for example, “book,” is determined by syntactic context (e.g., the book's pages were torn—a physical object, the book has challenged millions of readers to reexamine their views—an abstract collection of words).
I do not claim that this approach is incontrovertibly correct. However, almost every modern (psycho)linguistic approach acknowledges that words have syntax, a point not raised or addressed by Fedorenko et al. (2020). The most popular alternative approach to MGG, the construction grammar/usage-based approach (Jackendoff, 2002; Goldberg, 2003), while advocating for a clearly distinct approach to language, does not abolish the distinction between syntax and semantics but, rather, articulates that words and constructions are pairs of syntactic form and meaning. In addition, popular psycholinguistic models of word production involve two stages: the first stage involves going from meaning to the lemma, which includes the syntactic representation of a concept; the second stage includes going from the lemma to the phonological form (Kempen and Huijbers, 1983; Levelt, 1989; Dell and O'Seaghdha, 1992; Levelt et al., 1999).1 The idea that word retrieval involves access to syntactic information, even in the context of single-word production, is mostly uncontroversial in this literature. Thus, the idea of a coherent lexico-semantic system entirely distinct from and diametrically opposed to syntax is, in many ways, an aberration, yet it appears to have had a substantial impact on cognitive neuroscience.2
Fedorenko et al.'s claims about the neurobiological implementation of language are unusually strong and are based on the problematic notion of “lexico-semantics” reviewed earlier. First, Fedorenko et al. (2020) argue against the idea of a purely syntactic system in the brain because several previous studies “found that any language-responsive brain region or electrode that shows sensitivity to syntactic structure... is at least as sensitive, and often more sensitive, to meanings of individual words.” This is simply the observation that regions implicated in syntax activate more to real words than pseudowords;3,4 there is no evidence that these activations only reflect meaning. A syntactic system in the brain should activate more to real words relative to pseudowords, reflecting access to syntactic elements as reviewed earlier. Thus, the “lexico-semantic” activations reported in these experiments are ambiguous between lexical-syntactic and lexical-semantic processing.
Then, Fedorenko et al. (2020) performed a series of three additional functional magnetic resonance imaging experiments designed to separately target “lexico-semantic” and syntactic processing, following similar experimental designs in previous research (Dapretto and Bookheimer, 1999; Kuperberg et al., 2000, 2003; Friederici, 2003; Noppeney and Price, 2004; Menenti et al., 2011; Segaert et al., 2012). This article provides example stimuli from the critical, putatively “lexico-semantic” conditions in Fedorenko et al. (2020). In these experiments, the initial sentence or clause is followed up by a second sentence or clause that contrasts with the initial one:
Experiment 1: “Although his ears were damaged... the man could still cook” (meaning violation)
Experiment 2: “The protestor quoted the leader -> The striker cited the chief” (different words)
Experiment 3: “The scientist flattered David -> The scientist misled David” (non-synonym)
All three of these experiments conflate syntax and semantics within the “lexico-semantic” condition. In Experiment 1, the meaning violation is also a violation of the expected word, which is a syntactic, as well as a semantic, element. In addition, it is quite possible that violations of meaning are accompanied by syntactic revision processes in order to attempt to reinterpret the sentence. In Experiment 2, different words are different syntactic elements, as well as different meanings. In Experiment 3, the manipulation involves changing the final word, which is again both a syntactic and a semantic element. It is, therefore, no surprise that brain areas thought to be potentially selective to syntax (such as the inferior frontal lobe and posterior temporal lobe as postulated by many authors; Hagoort, 2005; Tyler and Marslen-Wilson, 2008; Bornkessel-Schlesewsky and Schlesewsky, 2013; Friederici, 2017; Matchin and Hickok, 2020) show activations to both the “lexico-semantic” and syntactic conditions because the “lexico-semantic” conditions always involve a “hidden” syntactic manipulation. That is, these experiments always manipulate words, which are intrinsically syntactic as well as semantic, which is a consensus position in linguistic theory as reviewed earlier.
Researchers using similar experimental conditions in brain imaging research have reported dissociations of syntactic and semantic processing (Dapretto and Bookheimer, 1999; Kuperberg et al., 2000, 2003; Friederici, 2003; Noppeney and Price, 2004; Menenti et al., 2011; Segaert et al., 2012), which seem to be discrepant with the results reported by Fedorenko et al. (2020). However, these previous authors reported whole-brain activation maps, whereas Fedorenko et al. do not. It is possible that the subtle spatial dissociations reported by previous authors would be replicated in the Fedorenko et al. experiments if whole-brain analyses had been reported.5 Regardless, it is more important that these previous authors operated under the same mistaken assumption as Fedorenko et al.: that “lexico-semantic” manipulations do not tax syntax. The fact that Fedorenko et al. do not (appear to) replicate the syntax–semantics dissociations reported by previous authors is more likely due to the fact that these original experimental designs were flawed to begin with.
Future functional neuroimaging studies investigating the syntax–semantics distinction should account for the dual semantic and syntactic nature of the lexicon by eschewing the conventional notion of “lexical-semantics” itself. Instead, researchers should develop more careful experiments that independently vary the richness of conceptual-semantic content and lexical-syntactic complexity or separately model these components during sentence comprehension (see Pylkkänen, 2019, 2020 for reviews; Hale et al., 2022).
Fedorenko et al. (2020) focus on the idea of a syntactic system in the brain that should not be activated by lexical manipulations, a prediction that does not follow from “syntax-centric” theories of MGG (e.g., Chomsky, 1965, 1981, 1995b). However, they do report a significant preference for the syntactic condition in the posterior temporal lobe for Experiment 2 when using a localizer more sensitive to syntax. It is not a coincidence that the posterior temporal lobe has been strongly implicated in syntax by recent authors (Bornkessel-Schlesewsky and Schlesewsky, 2013; Pylkkänen, 2019; Matchin and Hickok, 2020). Recent lesion-symptom mapping literature supports a strong association between syntactic comprehension deficits and damage to posterior temporal-parietal areas (Pillay et al., 2017; Rogalsky et al., 2018; Matchin et al., 2022a,b), a similar pattern that is also emerging for paragrammatic speech production deficits (Yagata et al., 2017; Matchin et al., 2020). Residual functional activation in the posterior temporal lobe after accounting for lesion effects appears to be uniquely associated with aphasia recovery (Schneck, 2022; Wilson et al., 2022). Given that lesion-symptom mapping provides a much stronger causal inference than functional neuroimaging (Rorden and Karnath, 2004), such data need to be addressed together.
Finally, while I find the evidence for a hierarchical, abstract syntactic system in the posterior temporal lobe to be highly compelling, a variety of multiple perspectives on this issue are possible. First, even if no brain region is selective for syntax, specific network configurations could be (Schnitzler and Gross, 2005; Buzsaki, 2006; Anderson, 2016; Farahani et al., 2019). Furthermore, linguistic theories do not make predictions about how much cortical surface area would be needed to process syntax and semantics or whether there must be large cortical areas dedicated to processing syntax at all (Poeppel and Embick, 2005; Embick and Poeppel, 2015). The ideas of Chomsky regarding the uniqueness and expressive power of syntax are perfectly compatible with a “slight rewiring of the brain” (Chomsky, 2005) of an evolutionarily recent hominin ancestor, augmenting a sea of brain mechanisms that resulted in the modern human language faculty (Berwick and Chomsky, 2016), regardless of whether there is clear evidence of a large swath of syntax-selective cortex.
WM conceived and wrote the entire article.
This research was supported by National Institute on Deafness and Other Communication Disorders grant P50 DC014664.
The author would like to thank the reviewers and editor for constructive feedback.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
WM declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^Some have critiqued the notion of lemmas (Krauska, 2023). However, the alternative view of speech production articulated by these authors also involves two steps with an intermediate syntactic layer.
2. ^Many researchers use the term lexico-semantic to refer to a conglomeration of lexical and conceptual processing that excludes syntax. However, there is an alternative, viable usage of lexico-semantic that refers to monadic concepts.
3. ^The term non-word should be supplanted by pseudo-word. It acknowledges the complexities of the potential higher level linguistic processing that occurs when people process them (Vitevitch and Luce, 1999).
4. ^In many of these experiments, the word lists include morphologically complex forms and morphosyntactic features such as past tense. Thus, syntax is often present in the putatively “lexico-semantic” conditions.
5. ^In many papers, Fedorenko et al. do not report whole-brain analyses. However, they are critical supplements to region of interest analyses, potentially revealing hidden patterns in the data and allowing for better comparability across studies.
Anderson, M. L. (2016). Précis of after phrenology: neural reuse and the interactive brain. Behav. Brain Sci. 39, e120. doi: 10.1017/S0140525X15000631
Berwick, R. C., and Chomsky, N. (2016). Why Only Us: Language and Evolution. Cambridge, MA: MIT Press. doi: 10.7551/mitpress/9780262034241.001.0001
Bornkessel-Schlesewsky, I., and Schlesewsky, M. (2013). Reconciling time, space and function: a new dorsal–ventral stream model of sentence comprehension. Brain Lang. 125, 60–76. doi: 10.1016/j.bandl.2013.01.010
Buzsaki, G. (2006). Rhythms of the Brain. Oxford: Oxford University Press. doi: 10.1093/acprof:oso/9780195301069.001.0001
Chomsky, N. (1965). Aspects of the Theory of Syntax. Cambridge, MA: MIT Press. doi: 10.21236/AD0616323
Chomsky, N. (1995a). Bare Phrase Structure. In H. Campos and P. Kempchinsky (Eds.), Evolution and Revolution in Linguistic Theory. Georgetown University Press.
Chomsky, N. (2005). Three factors in language design. Linguis. Inquiry 36, 1–22. doi: 10.1162/0024389052993655
Dapretto, M., and Bookheimer, S. Y. (1999). Form and content: dissociating syntax and semantics in sentence comprehension. Neuron 24, 427–432. doi: 10.1016/S0896-6273(00)80855-7
Dell, G. S., and O'Seaghdha, P. G. (1992). States of lexical access in language production. Cognition 42, 287–314. doi: 10.1016/0010-0277(92)90046-K
Embick, D., and Marantz, A. (2005). Cognitive neuroscience and the English past tense: Comments on the paper by Ullman et al.?. Brain and Lang. 93, 243–247. doi: 10.1016/j.bandl.2004.10.003
Embick, D., and Poeppel, D. (2015). Towards a computational(ist) neurobiology of language: Correlational, integrated and explanatory neurolinguistics. Lang. Cogn. Neurosci. 30, 357–366. doi: 10.1080/23273798.2014.980750
Farahani, F. V., Karwowski, W., and Lighthall, N. R. (2019). Application of graph theory for identifying connectivity patterns in human brain networks: a systematic review. Front. Neurosci. 13, 585. doi: 10.3389/fnins.2019.00585
Fedorenko, E., Blank, I. A., Siegelman, M., and Mineroff, Z. (2020). Lack of selectivity for syntax relative to word meanings throughout the language network. Cognition 203, 104348. doi: 10.1016/j.cognition.2020.104348
Friederici, A. D. (2003). The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb. Cort. 13, 170–177. doi: 10.1093/cercor/13.2.170
Friederici, A. D. (2017). Language in Our Brain: The Origins of a Uniquely Human Capacity. Cambridge, MA: MIT Press. doi: 10.7551/mitpress/9780262036924.001.0001
Goldberg, A. E. (2003). Constructions: a new theoretical approach to language. Trends Cogn. Sci. 7, 219–224. doi: 10.1016/S1364-6613(03)00080-9
Hagoort, P. (2005). On Broca, brain, and binding: a new framework. Trends Cogn. Sci. 9, 416–423. doi: 10.1016/j.tics.2005.07.004
Hale, J. T., Campanelli, L., Li, J., Bhattasali, S., Pallier, C., Brennan, J. R., et al. (2022). Neurocomputational models of language processing. Ann. Rev. Linguist. 8, 427–446. doi: 10.1146/annurev-linguistics-051421-020803
Jackendoff, R. (2002). Foundations of Language: Brain, Meaning, Grammar, Evolution. Oxford: Oxford University Press. doi: 10.1093/acprof:oso/9780198270126.001.0001
Kempen, G., and Huijbers, P. (1983). The lexicalization process in sentence production and naming: Indirect election of words. Cognition 14, 185–209. doi: 10.1016/0010-0277(83)90029-X
Krauska, A. (2023). Moving away from lexicalism in psycho- and neuro-linguistics. Front. Lang. Sci. 5, 127. doi: 10.3389/flang.2023.1125127
Kuperberg, G. R., Holcomb, P. J., Sitnikova, T., Greve, D., Dale, A. M., Caplan, D., et al. (2003). Distinct patterns of neural modulation during the processing of conceptual and syntactic anomalies. J. Cogn. Neurosci. 15, 272–293. doi: 10.1162/089892903321208204
Kuperberg, G. R., McGuire, P. K., Bullmore, E. T., Brammer, M. J., Rabe-Hesketh, S., Wright, I. C., et al. (2000). Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: an fMRI study. J. Cogn. Neurosci. 12, 321–341. doi: 10.1162/089892900562138
Levelt, W. J. M., Roelofs, A., and Meyer, A. S. (1999). A theory of lexical access in speech production. Behav. Brain Sci. 76, 1776. doi: 10.1017/S0140525X99001776
Matchin, W., Basilakos, A., Ouden, D-. B. d. e. n., Stark, B. C., Hickok, G., Fridriksson, J., et al. (2022a). Functional differentiation in the language network revealed by lesion-symptom mapping. NeuroImage 247, 118778. doi: 10.1016/j.neuroimage.2021.118778
Matchin, W., Basilakos, A., Stark, B. C., Ouden, de., Fridriksson, D. B. J., and Hickok, G. (2020). Agrammatism and paragrammatism: a cortical double dissociation revealed by lesion-symptom mapping. Neurobiol. Lang. 3, 1–47. doi: 10.1162/nol_a_00010
Matchin, W., De Ouden Hickok, D. B., Hillis, G., and Bonilha, A. E. (2022b). The Wernicke conundrum revisited: evidence from connectome-based lesion-symptom mapping in post-stroke aphasia. Brain awac 3, 219. doi: 10.1101/2021.10.25.465746
Matchin, W., and Hickok, G. (2020). The cortical organization of syntax. Cereb. Cort. 30, 1481–1498. doi: 10.1093/cercor/bhz180
Menenti, L., Gierhan, S. M. E., Segaert, K., and Hagoort, P. (2011). Shared language: overlap and segregation of the neuronal infrastructure for speaking and listening revealed by functional MRI. Psychol. Sci. 22, 1173–1182. doi: 10.1177/0956797611418347
Noppeney, U., and Price, C. J. (2004). An fMRI study of syntactic adaptation. J. Cogn. Neurosci. 16, 702–713. doi: 10.1162/089892904323057399
Pietroski, P. M. (2018). Conjoining Meanings: Semantics Without Truth Values. Oxford: Oxford University Press. doi: 10.1093/oso/9780198812722.001.0001
Pillay, S. B., Binder, J. R., Humphries, C., Gross, W. L., and Book, D. S. (2017). Lesion localization of speech comprehension deficits in chronic aphasia. Neurology 88, 970–975. doi: 10.1212/WNL.0000000000003683
Pinker, S., and Ullman, M. T. (2002). The past and future of the past tense. Trends Cogn. Sci. 6, 456–463. 3 doi: 10.1016/S1364-6613(02)01990-3
Poeppel, D., and Embick, D. (2005). “Defining the relation between linguistics and neuroscience,” in Twenty-First Century Psycholinguistics: Four Cornerstones, ed A. Cutler (Lawrence Erlbaum Associates Publishers), pp. 103–118.
Preminger, O. (2021). Natural Language Without Semiosis [Invited Talk]. SinFonIJA 14, University of Maryland. Available online at: https://www.ff.uns.ac.rs/uploads/files/Nauka/Konferencije/2021/Sinfonija%2014/materijali/Invited%20talks/Preminger%20slides.pdf (accessed May 17, 2023).
Pylkkänen, L. (2019). The neural basis of combinatory syntax and semantics. Science 366, 62–66. doi: 10.1126/science.aax0050
Pylkkänen, L. (2020). Neural basis of basic composition: what we have learned from the red–boat studies and their extensions. Philosoph. Transact. Royal Soc. B Biol. Sci. 375, 20190299. doi: 10.1098/rstb.2019.0299
Rogalsky, C., LaCroix, A. N., Chen, K.-H., Anderson, S. W., Damasio, H., Love, T., et al. (2018). The neurobiology of agrammatic sentence comprehension: a lesion study. J. Cogn. Neurosci. 30, 234–255. doi: 10.1162/jocn_a_01200
Rorden, C., and Karnath, H-. O. (2004). Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat. Rev. Neurosci. 5, 812–819. doi: 10.1038/nrn1521
Schneck, S. M. (2022). Neural correlates of language processing in post-stroke aphasia (Unpublished doctoral dissertation). Vanderbilt University, Nashville, TN, United States.
Schnitzler, A., and Gross, J. (2005). Normal and pathological oscillatory communication in the brain. Nat. Rev. Neurosci. 6, 285–296. doi: 10.1038/nrn1650
Segaert, K., Menenti, L., Weber, K., Petersson, K. M., and Hagoort, P. (2012). Shared syntax in language production and language comprehension—An fMRI study. Cerebral Cortex 22, 1662–1670. doi: 10.1093/cercor/bhr249
Tyler, L. K., and Marslen-Wilson, W. (2008). Fronto-temporal brain systems supporting spoken language comprehension. Philosoph. Transact. Royal Soc. B Biol. Sci. 363, 1037–1054. doi: 10.1098/rstb.2007.2158
Vitevitch, M. S., and Luce, P. A. (1999). Probabilistic phonotactics and neighborhood activation in spoken word recognition. J. Mem. Lang. 40, 374–408. doi: 10.1006/jmla.1998.2618
Wilson, S. M., Entrup, J. L., Schneck, S. M., Onuscheck, C. F., Levy, D. F., Rahman, M., et al. (2022). Recovery from aphasia in the first year after stroke. Brain 3, awac129. doi: 10.1093./brain/awac129
Keywords: syntax, fMRI, lexicon, lexico-semantic, generative grammar, Words and Rules
Citation: Matchin W (2023) Lexico-semantics obscures lexical syntax. Front. Lang. Sci. 2:1217837. doi: 10.3389/flang.2023.1217837
Received: 05 May 2023; Accepted: 17 July 2023;
Published: 09 August 2023.
Edited by:
Malathi Thothathiri, George Washington University, United StatesReviewed by:
Svetlana Malyutina, National Research University Higher School of Economics, RussiaCopyright © 2023 Matchin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William Matchin, matchin@mailbox.sc.edu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.