
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Lang. Sci., 14 August 2023
Sec. Neurobiology of Language
Volume 2 - 2023 | https://doi.org/10.3389/flang.2023.1199107
This article is part of the Research TopicSyntax, the brain, and linguistic theory: a critical reassessmentView all 11 articles
Introduction: Many studies on syntax in dementia suggest that, despite syntactic simplification, speakers with Alzheimer's disease (AD) retain their basic grammatical abilities, being mainly affected in their comprehension and production of complex syntax. Moreover, there is no single position on the origin of syntactic decline in AD, which, according to some authors, can be linked to a lexical-semantic deficit or, according to others, to either cognitive or autonomous dysfunction.
Methods: In this study, we apply the model of syntactic maturity to the analysis of oral speech production elicited by the Cookie-Theft description task. We assess a sample of 60 older adults (21 HC, 19 MCI, and 20 AD) through three indexes of syntactic maturity, measuring the proportion of sentences and clauses in discourse, their mean length, and the rate of their complexity.
Results: Our results show two important tendencies in AD: the preservation of general syntactic ability, as measured by the basic syntactic organization of speech, and the disturbance of the indexes of syntactic complexity, as measured by the overall length of utterances and their indexes of complexity.
Discussion: Although speakers with AD maintain the ability to construct grammatically acceptable sentences and produce a similar number of utterances to healthy aging speakers and speakers with MCI, the syntactic complexity of their discourse significantly changes. Importantly, such significant changes are already present at the MCI stage and are not conditioned by the lexical-semantic deficit itself. Our results may be particularly relevant to improving the detection of cognitive impairment and to theoretically discussing the relationships between language levels in aging speakers.
General preservation of syntactic ability is considered one of the hallmarks of language profile in dementia. One of the pioneering papers on changes in syntax in Alzheimer's disease (AD) found that, despite simplification of key features of syntactic complexity, speakers with dementia still produced coherent and grammatical sentences (Kemper et al., 1993). As the most frequent type of spontaneous dementia, AD is generally assumed to lead to significant disruptions in lexical semantics (Forbes-McKay and Venneri, 2005; Taler and Phillips, 2008; Verma and Howard, 2012; Lofgren and Hinzen, 2022) and phonetics (De Looze et al., 2018; Vincze et al., 2021; Ivanova et al., 2022). However, equally pronounced changes in syntax are not usually reported. Available results mainly suggest simplification, rather than significant impairment of syntactic ability in AD. This is a major challenge since, as opposed to language structures and phenomena allowing for qualitative differentiation of AD in a more targeted way (Alzheimer, 1907; in Alzheimer et al., 1991), syntactic change implies defining the level of impairment on a continuum.
Indeed, many studies show that while progression to dementia decreases the ability to produce complex utterances, such utterances remain grammatically acceptable and correct even in the moderate stage of AD. Syntactic simplification in AD would usually affect the length and the internal structure of utterances. Speakers with AD produce shorter utterances and clauses and use fewer propositions, verbal forms, and conjunctions (Kemper et al., 1993). The internal structure of their utterances is based on shorter mean dependency distances (Liu et al., 2021) and includes fewer embedded clauses (Bose et al., 2021). AD speakers use fewer coordinated and reduced structures (abbreviated subordinate clauses) (De Lira et al., 2011), fewer subordinate sentences (Croisile et al., 1996), and more sentential fragments than full sentences (Lyons et al., 1994). In some cases, AD speakers are reported to exhibit difficulties for passives (Bates et al., 1995).
At the same time, AD speakers match healthy aging speakers in formal grammatical correction and well-formation (Lyons et al., 1994). AD speakers can use recursive sentence embedding (Bánréti et al., 2016) or even sophisticated utterances (Mueller et al., 2016) like healthy elderly. Furthermore, their utterances are defined by a similar type-token ratio (Chapin et al., 2022). Importantly, despite all changes, utterances produced by AD speakers are generally informative enough, though the number of information units (that is, units of reference they might speak about) is usually reduced (Kemper et al., 1993; Croisile et al., 1996).
One intriguing question is how we can explain both the preservation (although partial) and changes in syntax in AD, and, particularly, how we can identify which of such changes are differentiating and relevant linguistic features of cognitive and language patterns of dementia. Speakers with AD rely on different, compensatory mechanisms in their cognitive and language performance. Yet, some of their syntactic features are similar to those observed in healthy aging. Indeed, some works suggest that syntax in AD bears a resemblance to some patterns of syntactic change in healthy aging. In their seminal study, Kemper et al. (2001) observed that speakers with dementia showed a similar pattern of decline in grammatical complexity (although not in the pattern of decline in propositional content) that healthy aging speakers. The authors concluded that even speakers with advanced dementia could still produce grammatical sentences. At the same time, some syntactic features change significantly in AD and can be considered a critical behavior marker of dementia progression. In fact, the processing of passives has been described as such critical behavior marker of Mild Cognitive Impairment (Sung et al., 2020). Although the manifestation of syntactic changes is more pronounced in the advanced stages of dementia (Ahmed et al., 2013), changes are significant in both the parts of speech and the syntactic structures themselves (Liu et al., 2021).
Such results on syntax in AD can be partially explained by our evidence on syntax during the lifespan and, specifically, in healthy aging. Syntactic competence is generally robust across the lifespan after its scalar development during childhood. Older adults usually preserve syntax in spite of aging-related neurocognitive changes. Despite aging-driven atrophy, gray-matter reduction, and decreased between-network connectivity in the brain, the syntactic ability is supported in older adults by neurofunctional reorganization and general functional preservation of the frontotemporal syntax system (cf. Tyler et al., 2010; Campbell et al., 2016). Furthermore, the preservation of syntax in aging is supported by the high level of automatization of the processes of integration of syntactic and semantic properties in sentential representations (Campbell et al., 2016). Neurobiological insights into the aging brain suggest that aging as a process conveys a general decline in the integrity of the left frontotemporal syntactic network, but there is no evidence for dedifferentiation of the syntactic system and, thus, for the reduction in its functional specialization (Shafro and Tyler, 2014). It is, therefore, not surprising that aged speakers recur to syntactic structures similarly to how young speakers do it, although they find it more difficult to adapt to contextual changes in syntactic patterns (e.g., when there is a shift from passive to active structures) (Heyselaar et al., 2021). Evidence from AD suggests that speakers with dementia also preserve their general syntactic competence, specifically if it is compared with other language domains, like lexical semantics or phonetics. Only fine-grained analyses, and only analyses conducted for advanced stages of AD, would suggest difficulties and/or impairments in processing syntactically constrained or ambiguous sentences (cf. Bickel et al., 2000).
Our contention is that an adequate approach to considering syntactic changes in AD must consider another, albeit related, point of ambiguity for syntax in dementia: its etiological background. The two possible positions are the lexical-semantic origin and the cognitive origin.
On the one hand, syntactic changes in healthy aging can be related to the difficulty in lexical access. Despite the effect of aging-related cognitive difficulties, these are language difficulties (for example, constrained word retrieval) that seem to be more directly involved in causing difficulties in sentence production in healthy aging (Kemper et al., 2001; Davidson et al., 2003). In AD, vocabulary and semantics are not only significantly disrupted, but are considered as a primary language symptom. Difficulties in verbal fluency and naming are prominent language characteristics of early, and even preclinical AD (Verma and Howard, 2012). Such difficulties are frequently linked to anomia, a key property of language disruption in AD. Anomia usually shows up as a general difficulty to access and recall words, resulting in a decline in both quantitative (number) and qualitative (type) presentation of lexical units (Banovic et al., 2018). Considering that changes in syntax do not appear until moderate dementia, with most errors relying on semantic deficits (Taler and Phillips, 2008), the lexical-semantic origin of such syntactic decline seems plausible. Word-finding difficulties in AD indeed lead to fragmentations and reduced coherence in language production. Similar parallels already exist at other linguistic levels, for example, in pragmatics, where disintegrated semantic knowledge predicts pragmatic disruptions, like difficulties in turn-taking or shifts in topic, or in the maintenance of conversations (cf. Van Boxtel and Lawyer, 2021). In their seminal study, Bates et al. (1995) suggested that syntactic deficits in AD are, at the abstract level, comparable to lexical deficits, in that they follow the pattern of inclusion of highly frequent or empty forms.
However, difficulties with syntactic processing and production in aging can also be due to the progressive disruption of cognitive functions properly. Healthy older adults show more errors in syntactic production due to difficulties in planning and production (Hardy et al., 2020). Crucially, tasks involving higher cognitive load (e.g., on working memory or episodic memory) unchain more acute syntactic difficulties and impairment in speakers with AD. The syntactic decline in dementia, thus, could be specific (or isolated), or otherwise result from a combination of language-cognition interplay (cf. Nasiri et al., 2022). The first option can be supported by evidence on pauses in AD discourse. Although pauses could reflect lexical retrieval difficulties, in aging they can also be the result of other types of decline, for example, global cognitive slowing down, or decline in discourse control and planning (Gayraud et al., 2011). Interestingly enough, speakers with AD present with increasing pausing in utterance-initial and clause-initial positions, suggesting difficulties in content planning and structural assembly of event representations (Lofgren and Hinzen, 2022). The second option is supported by evidence from fine-grained analyses of AD discourse. According to this, syntactic deviations in AD are not only due to deficits in formal syntactic competence but also to growing constraints for specificity in discourse referencing (e.g., in anaphoricity) (Chapin et al., 2022). In their recent fine-grained analysis, Chapin et al. (2022) related a set of syntactic changes in AD to the growing difficulty of speakers with dementia to relate events, create referential connections and, thus, establish and introduce new referents as measured by indefinite noun phrases.
Considering the above, in this paper we aim to address the etiological background of syntactic change in AD by applying the model of syntactic maturity, originally developed by Hunt (1965, 1970). In this model, the indexes of syntactic maturity do not measure the correction of the utterances, nor their internal organization (e.g., whether they are active or passive). Otherwise, they reflect how speakers cognitively support different syntactic structures by primarily considering overall embedded complexity. Consequently, the indexes of syntactic maturity directly reflect the global complexity of syntactic constituents, with no specific focus on their intrinsic internal properties. Importantly, this model allows weighting the proportion of complex over simple clauses with no effect from lexical indexes (like semantic adequacy or coherence), enabling a separation between lexical-semantic and syntactic levels.
To apply this model, our experimental design proposes the observation of possible changes in Hunt indexes in three groups of older adults: healthy speakers, speakers with AD, and speakers with MCI. MCI is commonly included in studies on cognitive and language changes in AD since in a number of cases [roughly, between 10 to 15% per annum (Shigemizu et al., 2020)] MCI can progress to dementia (Angelucci et al., 2010). Furthermore, MCI can allow the tracing of early markers of language performance in AD (Taler and Phillips, 2008). Importantly, the language performance of HC, MCI, and AD is suggested to represent a continuum of progressive, hierarchical decline in many language aspects (Liampas et al., 2022). Thus, we hypothesize that if syntactic complexity is affected in AD, speakers with MCI will also show a decline, albeit less pronounced.
Considering this, we applied the proposed model to our analysis of the Cookie-Theft description task (Goodglass et al., 2000) performed by healthy older speakers, speakers with MCI, and speakers with AD. The description of the Cookie-Theft picture minimizes the overload on memory (De Lira et al., 2014). Furthermore, our prediction was as follows: if speakers with AD present with syntactic decline on a task excluding significant overload on memory, then, such decline is syntactic in nature. This assumption considered findings from previous studies, which demonstrated more significant impairment in AD on more memory-demanding tasks (e.g., syntactic priming) than on less memory-demanding tasks (e.g., sentence completion), specifically following canonical ordering (Nasiri et al., 2022).
As stated above, the main aim of our study was to inquire into the background of syntactic changes in AD. Specifically, we wanted to test whether syntactic changes in dementia derive from the lexical-semantic deficit or, otherwise, are more autonomous in nature. In pursuit of this objective, we chose to apply the model of syntactic maturity, originally proposed by Hunt (1965, 1970) in their pioneering work in applied linguistics.
The notion of “syntactic maturity” is closely linked to that of syntactic complexity. Since the degree of syntactic complexity correlates with the speaker's capacity to express complex relations between ideas, mental states, and non-propositional actions (Beers and Nagy, 2009), its measurement is crucial for the evaluation of the speaker's cognitive state too. Hunt's model allows to assess how the full text or discourse produced by a speaker is organized in terms of the shortest grammatically allowable units (Adamson, 2019) and, at the same time, how complex these units are.
According to Hunt's model, syntactic maturity can be measured through primary indexes and secondary indexes. Primary indexes of syntactic maturity are measured as two types of units: t-units (also known as terminal units) and clauses. A t-unit is a main clause “plus all the subordinate clauses attached to or embedded in it” (Hunt, 1965, p. 141). Thus, for empirical analysis, any simple sentence, any sentence integrating a subordinate clause, and any proposition forming a sentence composed by coordination or juxtaposition, is considered as a t-unit (Delicia, 2011).
The second primary index of syntactic maturity is clause, which is defined as a subject (or a set of subjects) coordinated with a finite verb or a finite set of coordinated verbs, including impersonal verb forms (infinitive, gerund, or participle) when they do not form periphrases or semi-periphrases and act as a nucleus of a complex structure (Delicia, 2011). For empirical analysis, any clause embedded into a t-unit is considered a clause, allowing to measure how many clauses form each t-unit; this includes all simple, subordinate, and subordinating sentences. The ratio of clauses for t-units is taken as a measure of subordination (Beers and Nagy, 2009).
The interpretation of the indexes assumes that (a) the longer the t-units, (b) the higher the number of words per clause, and (c) the higher the proportion of clauses per t-unit (taken over 1), the higher the syntactic complexity (Delicia, 2011). To estimate the values of syntactic maturity, three indexes are calculated:
(a.) Index 1: mean length of t-units (the total number of words divided over the total number of t-units);
(b.) Index 2: mean length of clauses (the total number of words divided over the total number of clauses);
(c.) Index 3: the number of clauses divided over the number of t-units.
Table 1 shows examples of t-units and clauses from our sample. Specifically, it allows to see how clauses can be identified within t-units.
Hunt's model of syntactic maturity has been mainly applied to assessing normotypically developing child syntax. Yet, there have been some interesting contributions on syntactic change in language disorders from Hunt's model. Ketelaars et al. (2015) used Hunt's model to identify narrative deficits (mainly, narrative productivity) in children with pragmatic language impairment. Mozeiko et al. (2011) assessed t-units in speakers who suffered from traumatic brain injury (TBI) to find a significantly poorer performance in their grammar abilities. Pallickal and Hema (2019) also observed a significant reduction in t-units, but neither in the number of clauses nor in the number of words per clause in speakers with Wernicke's aphasia.
To the best of our knowledge, our study is the first one to apply Hunt's model of syntactic maturity in its original version for measuring syntactic competence in AD. Importantly, some studies already applied Hunt's model, although from a different perspective, to aging speakers. For example, Wainwright and Cannito (2015) analyzed t-units in older speakers to find them more prone to use referential ambiguities. Sajjadi et al. (2012) used the so-called “modified T-units” in order to analyze non-clausal utterances and message conveying in AD and semantic dementia, concluding their impairment in discourse construction. Against this background, in the present paper, we will apply the Hunt's model in its basic proposal to contribute to our understanding of the nature of syntactic changes in AD.
A total of 60 older speakers participated in the study. Of these, 19 were diagnosed with MCI following the criteria of the International Working Group on Mild Cognitive Impairment (Winblad et al., 2004), and 20 were diagnosed by the National Health System with dementia of Alzheimer's type (AD) following the NIA-AA criteria (Jack et al., 2018). The remainder were 21 healthy older adults who formed the control group (HC). AD participants were recruited from the State Reference Center for the Care of People with Alzheimer's Disease and Other Dementias, Salamanca, Spain. HC and MCI participants were recruited from among attendees of the Psychological Attention Service for the Prevention of Cognitive Problems in the Elderly, City Psychosocial Support Unit, Council of Salamanca/University of Salamanca, Spain. The Service controlled for the classification of the cognitive state of the participants.
To participate in the study, HC had to meet the following inclusion criteria: be a native speaker of Spanish; be over 60 years old; have no history of drug or alcohol abuse; have no history of psychiatric illness; have no severe sensory deficits that would preclude the administration of cognitive tests; have a minimal level of schooling years to have acquired literacy; have no diagnosis of MCI or AD.
Speakers with MCI and AD had to meet the following inclusion criteria: be a native speaker of Spanish; be over 60 years old; have no history of drug or alcohol abuse; have no history of psychiatric illness; have no severe sensory deficits that would preclude the administration of cognitive tests; have a minimal level of schooling years to have acquired literacy. Furthermore, to be classified as MCI group, speakers had to be diagnosed according to the criteria from the International Working Group on Mild Cognitive Impairment (Winblad et al., 2004). To be classified as AD group, speakers had to be diagnosed by the National Health System with dementia of Alzheimer's type (AD) following the NIA-AA criteria (Jack et al., 2018).
All participants signed the informed consent form. The study was run in accordance with the Declaration of Helsinki and its subsequent amendments, as well as the European Union regulations for medical research. The study received the approval of the Ethics Committee of the State Reference Center for the Care of People with Alzheimer's Disease and Other Dementias, Salamanca, Spain.
The sample included a balanced number of participants per diagnostic group (variance = 0.695). The mean age of the sample was 77.65 years (SD = 8.79). The mean age of participants was higher in MCI and AD than in HC, and this difference was statistically significant [F(2,57) = 5.67, p = 0.006, η2 = 0.166], with the effect size indicating a large effect. Post-hoc analysis showed that the difference was only significant between HC and AD (p = 0.005).
Participants were predominantly women (n = 46; 76.7%), but there was no statistical significance in the distribution of participants according to sex across groups [F(2,57) = 0.75, p = 0.474, η2 = 0.026]. The mean duration of schooling (in years) was 9.40 years (SD = 3.38), ranging between 4 and 17 years. There was no significant difference for mean years of schooling across groups [F(2,57) = 1.375, p = 0.261, η2 = 0.046].
All participants were assessed through the Dem-Detect toolkit (Peña-Casanova et al., 2009) for neuropsychological scoring. The cognitive assessment of each participant was conducted during three individual sessions of 1 h each.
Within a battery of neuropsychological tests, participants described the Cookie-Theft picture from the Boston Naming Test. All participants were given the same instruction to describe everything they can see in the picture. Speakers were recorded while performing the task with an iPad and a head-mounted condenser microphone, MiC plus from Apogee. Recordings were independently transcribed and annotated by two researchers following the established criteria.
Neuropsychological and language tests were used for describing and controlling for the adequacy of the sample. Furthermore, these data will not be used beyond the characterization of its neuropsychological description.
Expectedly, groups varied on their scoring for MMSE test from Folstein et al. (1975) [F(2,57) = 25.120, p < 0.001, η2 = 0.468]. HC performed at an average higher than MCI (diff = 3.7, p = 0.006) and AD (diff = 7.93, p < 0.001), and MCI performed at an average higher than AD (diff = 4.23, p = 0.002).
Groups significantly varied on the semantic verbal fluency scale (SVF) as measured by Isaac's Set Test [F(2,57) = 13.593, p < 0.001, η2 = 0.323], in line with data provided by previous studies (Fisher et al., 2004; Amieva et al., 2005; Alegret et al., 2018; Liampas et al., 2022). HC showed the highest scores for SVF with the lowest SD (M = 39.14, SD = 1.590) and minimal scoring (35). MCI performed worse than HC (M = 35.21, SD = 4.614) and better than AD (M = 29.05, SD = 9.682), with minimal scoring achieving 24 and 14, respectively. Significant differences were observed between HC and AD (p < 0.001) and MCI and AD (p = 0.009), but not between HC and MCI (p = 0.153).
Groups also varied on the phonological verbal fluency scale (PVF) [F(2,55) = 21.016, p < 0.001, η2 = 0.433], a parameter for which significant between-group variation is not as systematic in evidence (cf. Teng et al., 2013). HC showed the highest scores for PVF (M = 13.00, SD = 4.290) and minimal scoring (6). MCI performed worse than HC (M = 7.47, SD = 2.503) and better than AD (M = 5.67, SD = 4.044), with minimal scoring achieving 4 and 1, respectively. Differences were significant between HC and MCI (p < 0.001) and HC and AD (p < 0.001), but not between MCI and AD (p = 0.434).
Table 2 summarizes the main neuropsychological and language data for the sample.
All speech samples were transcribed as follows. Each recording was transliterated with no link to a specific diagnosis. Illegible sequences or words were transliterated like “XXX” for inclusion into the general word count. Non-language elements (e.g., noise, sustained sounds, etc.) and filled pauses (e.g., “mmmm”) were not included. Repetitions (e.g., “su/su mama”) and incomplete word forms (e.g., “cubiert-,” for “cubiertos”) were included. Fifteen of the 60 transcriptions (25%) were then double-checked for consistency, bordering the score of 1.
Each transcription was furthermore annotated. Identification and annotation of all categories were carried out according to a specifically designed label system for the measurement of syntactic maturity within the CORDEM corpus annotation system. Specifically, the following two labels were used: <ut></ut>, for T-Units, and <cl></cl> for clauses. To adjust the model of syntactic maturity to oral speech production, which can include non-verbal or syntagma-based utterances, as well as grammatically peripheric structures with discourse roles (e.g., discourse markers), we assumed that utterances where a verb could be possibly reconstructed [e.g., What do you see on this picture?—AD speaker: two kids = (There are/I see) two kids] would be considered as t-units.
For each transcription, the total number of produced words (n1), the total number of unique produced words (n2), and the global uttering time (s) were calculated. Data for each transcription was then merged into the global matrix of the sample to adjust to neuropsychological scores.
The following categories were collected and assessed within this model in our study:
• Total produced words (n);
• Total unique produced words (n);
• Global uttering time (excluding interviewer's utterances) (s);
• Number of t-units (<ut></ut>);
• Number of clauses (<cl></cl>);
• Mean length of t-units (Index 1);
• Mean length of clauses (Index 2);
• Mean of clauses/t-units (Index 3).
Statistical analysis was conducted using IBM SPSS Statistics for Windows (26.0). We used one-way ANOVA with Group (HC, MCI, and AD) as between-subject factor and ran it on the following dependent variables: age, sex, education level, Mini-Mental State Examination (MMSE) scoring, Semantic Verbal Fluency (SVF) scoring, Phonological Verbal Fluency (PVF) scoring, mean duration of speech production, overall produced words, overall full words, number of t-units, number of clauses, Index 1, Index 2 and Index 3. Post hoc analyses were conducted using Bonferroni post-hoc test. Correlational analysis (Pearson's correlation) was used to measure the association between SVF and indexes of syntactic maturity.
The mean duration of discourse production (measured in seconds) did not significantly differ across groups [F(2,57) = 1.131, p = 0.330, η2 = 0.038]. The mean duration for all groups was 42.85 s (SD = 20.102), with the following means for each group: HC = 48.142 (SD = 16.038), MCI = 39.631 (SD = 21.192), and AD = 40.35 (SD = 22.597).
The main effect of the group was significant in the number of overall words [F(2,57) = 7.047, p = 0.002, η2 = 0.198]. Yet, only HC and AD significantly differed in the number of overall words (p = 0.001), with HC producing 47.452 words more than AD. There were no significant differences in the number of overall words between HC and MCI (p = 0.133) and between MCI and AD (p = 0.334).
The main effect of the group was significant in the number of full words [F(2,57) = 6.041, p = 0.004, η2 = 0.175]. Again, only HC and AD significantly differed in the number of full words (p = 0.003), with HC producing 43.247 more full words than AD. There were no significant differences in the number of full words between HC and MCI (p = 0.253) and between MCI and AD (p = 0.312) (Table 3 and Figure 1).
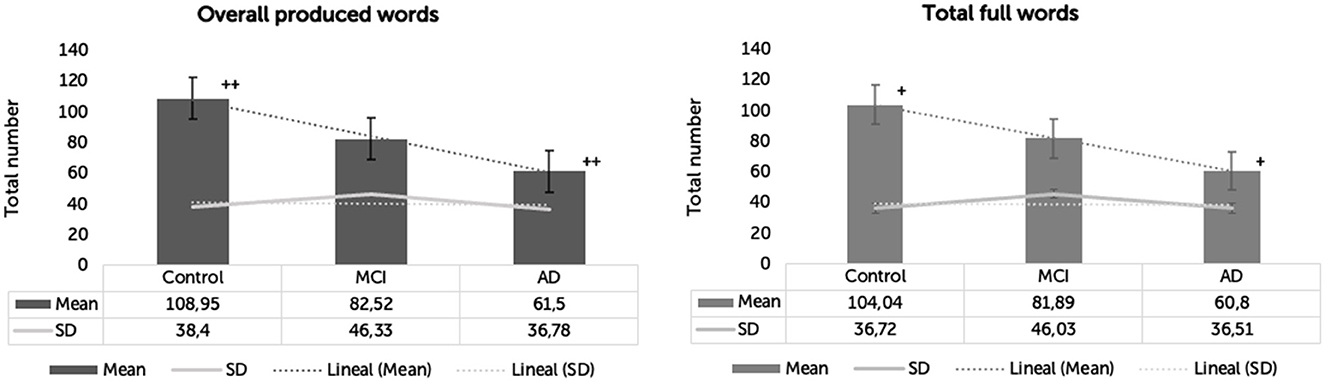
Figure 1. Overall words and total full words produced by clinical groups. *p < 0.05. +p < 0.05. **p < 0.001. ++p < 0.001.
Groups did not significantly vary in the number of t-units [F(2,57) = 0.912, p = 0.407, η2 = 0.031]. The means and SD for each group were: HC = 11.333 (SD = 5.072), MCI = 10.684 (SD = 5.508), and AD = 9.1 (SD = 5.683).
Groups significantly varied in the number of clauses [F(2,57) = 3.630, p = 0.033, η2 = 0.113]. The means and SD for each group were: HC = 18.523 (SD = 7.138), MCI = 14.89 (SD = 7.59), and AD = 12.25 (SD = 7.751). Only HC and AD significantly differed in the number of produced clauses (p = 0.029). There were no significant differences in the number of clauses between HC and MCI (p = 0.395) and between MCI and AD (p = 0.825) (Figure 2).
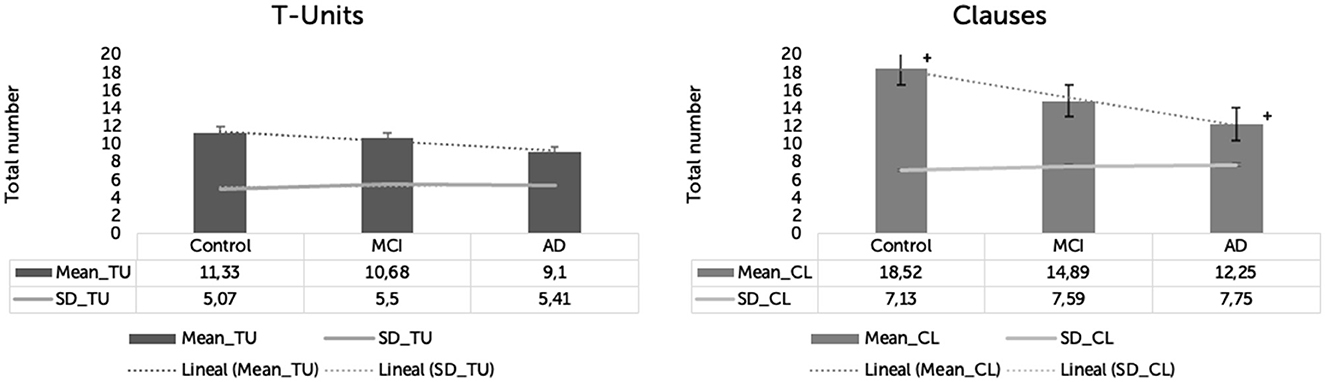
Figure 2. T-units and clauses produced by three groups in Cookie-Theft picture description task. *p < 0.05. +p < 0.05. **p < 0.001. ++p < 0.001.
Significant differences were observed in all three indexes between groups.
Differences in Index 1 were statistically significant between groups [F(2,57) = 12.945, p < 0.001, η2= 0.312]. The means and SD for each group were: HC = 10.37 (SD = 3.24), MCI = 7.53 (SD = 2.06), and AD = 6.72 (SD = 1.55). Index 1 was significantly different in HC compared to MCI (p = 0.001) and AD (p < 0.001). Differences in Index 1 were not significant between MCI and AD (p = 0.912).
Differences in Index 2 were statistically significant between groups [F(2,57) = 5.094, p = 0.009, η2 = 0.152]. The means and SD for each group were: HC = 5.95 (SD = 0.62), MCI = 5.38 (SD = 1.43), and AD = 4.78 (SD = 1.32). Index 2 was significantly different in HC compared to AD (p = 0.007). There were no significant differences in Index 2 between HC and MCI (p = 0.390) and MCI and AD (p = 0.349).
Differences in Index 3 were statistically significant between groups [F(2,57) = 7.639, p = 0.001, η2 = 0.211]. The means and SD for each group were: HC = 1.72 (SD = 0.43), MCI = 1.39 (SD = 0.18), and AD = 1.32 (SD = 0.36). Index 3 was significantly different in HC compared to MCI (p = 0.014) and AD (p = 0.002). There were no significant differences in Index 3 between MCI and AD (p = 1.000) (Figure 3).
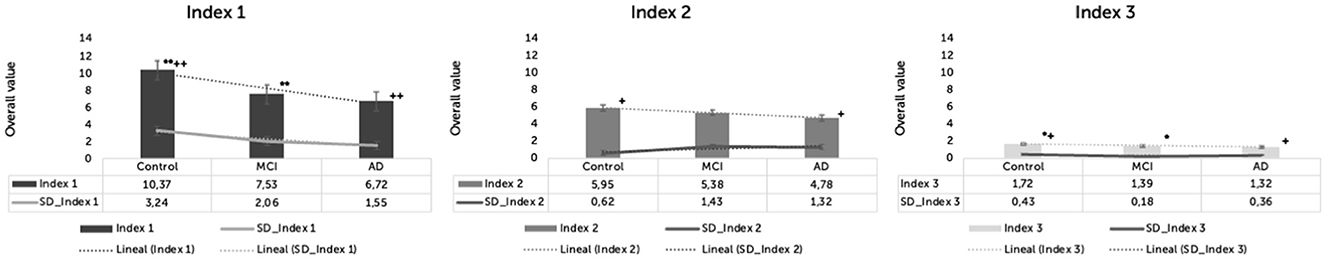
Figure 3. Indexes 1, 2, and 3 for three groups in the Cookie-Theft picture description task. *p < 0.05. +p < 0.05. **p < 0.001. ++p < 0.001.
Overall, HC and AD significantly differed in all indexes of syntactic maturity, with all indexes being lower in AD. HC and MCI significantly differed in Index 1 and Index 3, and, crucially, MCI and AD did not differ in any of the indexes of syntactic maturity.
Our results suggest that, although HC, MCI, and AD do not differ in the number of t-units, speakers with dementia produce shorter t-units, shorter clauses, and t-units with fewer clauses than HC. Thus, all indexes of syntactic maturity are significantly different between healthy older adults and speakers with dementia. At the same time, our results do not show significant differences between HC and MCI, and MCI and AD in most measures. HC and MCI do not differ in the number of t-units and clauses, but they do differ in the mean length of t-units and their index of syntactic complexity as measured by the number of clauses per t-unit. Crucially, none of the indexes of syntactic maturity shows a significant difference between MCI and AD, suggesting that these two groups do not differ in their syntactic productions measured through Hunt's model.
Table 4 summarizes the results for all measures of syntactic maturity.
To control for the possible correlation between lexical deficits and syntactic production in MCI and AD, we conducted correlation analyses between SVF scores and the three syntactic indexes of syntactic maturity.
We found positive correlations between SVF and Index 1 (r = 0.327, p = 0.011) and SVF and Index 2 (r = 0.286, p = 0.026), whereas the correlation between SVF and Index 3 was not significant (r = 0.120, p = 0.360).
In the present study, we primarily aimed to address two questions. On the one hand, we pursued the to confirm whether syntactic ability significantly changes in AD, and whether such change can be also traced in MCI. On the other hand, we wanted to address the etiological background of such change in dementia, by focusing on its probable isolating (or autonomous) nature from other declines. To meet these objectives, we opted for the application of Hunt's model of syntactic maturity to the analysis of the descriptions of the Cookie-Theft picture. To the best of our knowledge, our study is the first to apply the original version of this model to speakers with MCI and AD. Based on the results, this study shows that changes in syntactic ability are already present at the MCI stage, though it is at the full AD stage that syntactic complexity is significantly different (that is, lower in all indexes) than in HC.
In line with many studies on syntax in AD (Croisile et al., 1996; De Lira et al., 2011; Bose et al., 2021; Liu et al., 2021), our research confirms that speakers with dementia present with changes in their syntactic ability. Importantly, such syntactic changes are not surface, that is, they do not affect the basic syntactic organization of speech in dementia, which is consistent with previous findings on the general preservation of syntax in pathological aging. Truly indeed, despite producing significantly fewer words than healthy older adults (almost half as much), speakers with AD organize them sequentially in a similar proportion of sentences. Importantly, the degree of in-group (speakers with AD) and between-group (HC vs. MCI vs. AD) homogeneity for this general ability is high.
According to our results, significant changes appear in qualitative aspects of syntax and, specifically, in complex syntax. The difference in the number of t-units is not statistically significant and this result is consistent with the previously reported general preservation of syntactic ability in dementia, and cognitive impairment stage, reported in the Introduction of this work.
Our results are, however, consistent with the view that it is syntactic complexity, as measured by embedding (clauses), which is significantly reduced in dementia. Despite producing a similar proportion of sentences, AD speakers produce shorter units (either sentences or clauses) and fewer subordinations than HC. Crucially, an important finding of this work is that MCI and AD do not differ on any index of syntactic complexity, suggesting that significant changes in syntactic maturity are already present at the MCI stage. Since HC and MCI significantly differ in the mean length of t-units and the proportion of clauses over t-units only, but not in the number of produced words, the number of t-units, the number of clauses, or the mean length of clauses, we suggest that the most important changes in syntax occur in the overall length of utterances and their index of complexity. A plausible explanation for syntactic changes in AD can be related to the decline in working memory, which is consistently reported in the literature (cf. Kirova et al., 2015), and the interaction of working memory decline and language problems themselves (Lee and Kim, 2019; Nasiri et al., 2022). The same argument could potentially explain the few changes observed between HC and MCI, relative to the decline in the length and the complexity of utterances. Yet, further studies would be needed to check the plausibility of this explanation for MCI at the language level, in line with studies suggesting general memory decline already in MCI (cf. Saunders and Summers, 2010).
Relevant to this latter observation is our result on the correlation between lexical-semantic performance and syntactic indexes of HC, MCI, and AD. Since the task we analyze in this paper minimizes the effect of cognitive load (being supported by images, speakers do not have to rely on episodic memory), we can assume that syntactic production is not constrained and, thus, can be manifested to the fullest. Furthermore, relative lexical freedom of the task (although conditioned by the picture, speakers can use synonyms or hypernyms) makes it more flexible considering possible lexical-semantic deficits. In fact, the model of syntactic complexity we use in this research allows us to disregard the possible effect of the lexical-semantic deficit on syntactic productions. Interestingly enough, correlation analysis of scores on SVF task and indexes of syntactic maturity showed that a better SVF predicts a higher length of either t-units or clauses in aging speakers, but not the index of complexity as measured by the proportion of embeddings. These results suggest that the syntactic complexity is not conditioned by the lexical-semantic deficit itself and, importantly, such observation is in line with other studies reporting cognitive, rather than linguistic predictors of syntactic performance in AD (cf. Nasiri et al., 2022).
Further, ad-hoc designed analyses are needed to confirm whether the syntactic change in AD is independent of specific cognitive dysfunctions. Both possibilities could potentially exist if we consider other language levels in AD. For example, several studies proved that lexical-semantic deficit in early dementia is not related to cognitive dysfunction. Auriacombe et al. (2006) observed that changes in category verbal fluency task are quantitatively, but not qualitatively significant in early AD. Put it differently: speakers with prodromal dementia produce fewer words, but they do not show deficits in repeating them. The authors interpreted this result as proof of primarily semantic, but not a directly involved cognitive deficit proper of dementia. Similar conclusions were formulated by Liampas et al. (2022), who related word-finding problems with predominant disruptions in semantic stores, and Andreetta and Marini (2015), who reported lexical impairment to be responsible for macrolinguistic difficulties and impairment in fluent aphasia.
These observations lead us to another important question: the relevance of the type of task (or stimuli) in collecting, analyzing, and interpreting data on syntax in dementia. As stated above, AD drives important disruptions in cognitive functions involved in language control, but also in the language function itself. Furthermore, different language tasks can unchain different degrees of the implication of cognitive functions and language components. In their seminal study based on the assessment of written texts, Kemper et al. (1993) concluded that AD speakers showed syntactic simplification but, at the same time, preserved syntactic grammaticality. By contrast, a longitudinal study from Eyigoz et al. (2020) showed that speakers with future onset of AD wrote texts based on telegraphic patterns, i.e., with reduced (or even absent) grammatical structures, lacking functional words (like determiners or auxiliaries), and frequent misspellings. The typology of the task is a probable root of such contradictory results.
Discussion about the type of stimuli, however, should not be simplistically considered from the differentiation of written and oral tasks. Oral tasks, which are more commonly applied to the assessment of language and cognitive performance of older speakers usually imply different cognitive overload. Thus, different degrees of spontaneity in oral tasks lead to different cognitive loads on speakers. It is assumed that, within such a gradation, the most spontaneous oral tasks are the most demanding, since, in addition to not allowing for planning or prior memorization, they also require a very high level of cognitive and memory control and activation (Guinn et al., 2014). Instead, picture-description tasks reduce cognitive demands by minimizing overload on memory, specifically on episodic memory (Chapin et al., 2022). As our own results show, the correct identification and selection of the task can be a determining factor in correctly accessing the complexity of syntactic phenomena in dementia.
Studying syntax in AD faces another important challenge: what should we consider a baseline for assessing grammatical decline in aging and dementia? In their other relevant study, Kemper et al. (2001) observed a considerable individual variation in the initial grammatical complexity of older adults. Further longitudinal studies (e.g., Ahmed et al., 2013) confirmed that the starting profile of AD, including its prodromal or probable stages, is heterogeneous in (baseline) language abilities. Furthermore, one of the challenges in measuring syntactic disruption is related to the definition of what is syntactic complexity, how we can correctly measure it, and what cognitive predictions can be made about it. Several indexes of syntactic complexity have been used in studies on AD considering their predictive cognitive load. For example, Pakhomov et al. (2011) developed a computerized linguistic analysis system (CLAS), which assessed AD-driven changes in syntactic complexity based on the indexes of utterance length (mean number of words), the mean number of clauses (number of S nodes in parse trees), the total Yngve depth index (number of branches below each node, from right to left), the total Frazier depth index (number of branches for each word in the path to the highest node), and the total syntactic dependency length (SDL; sum of all dependency distances in the serial position of the constituent words). Their results are promising, but we still lack data on the threshold levels of syntactic normotypicality in aging.
Another intriguing question we have tried to address in this work is to which extent possible syntactic deficit in AD can be affected or predicted by the lexical-semantic deficit. Results from some of the most relevant studies suggest that lexical and syntactic impairments (or changes) are potentially dissociated in AD. Fraser et al. (2016) observed that semantic and syntactic impairments are asymmetric, that is, are presented with very low correlation. In their referential study of Iris Murdoch's written language, Garrard et al. (2005) observed similar dissociation between lexical impairment and relative syntactic preservation, and, in both cases, authors related their findings to underlying neuropathological patterns primarily affecting the temporal lobe. In this study, we found that lexical-semantic ability (as measured by SVF) predicts performance in the length of utterances and embedded clauses, but not in the syntactic complexity.
Considering such dissociation, it is crucial to look for the reasons for syntactic simplification in AD. Many of the indexes that measure syntactic production are associated with working memory and processing ability. Yet, Pakhomov et al. (2011) also suggested that both working memory and semantic difficulties could jointly affect syntactic complexity. Since cognitive impairments in AD are related to both structural dysfunction and functional disconnections in brain networks (Montembeault et al., 2019), their pattern can be insightful for our understanding of syntax in dementia. Functional connectivity changes in the language network are specifically noticeable in the left posterior middle temporal gyrus (pMTG) of people with AD, and associations between such changes and lexical deficits (mainly naming and verbal fluency), which link pMTG with lexical-semantic retrieval, are reported (Mascali et al., 2018; Montembeault et al., 2019). So, one of the important contributions from AD to our understanding of syntax in the brain comes from the evidence of how AD-related neurodegenerative processes, and the corresponding syntactic deviations, align with the predicted neuroanatomical substrate for syntactic processing and production. Difficulties in syntactic comprehension can be linked to progressive volume loss in the left temporal lobe, comprising the Wernicke's area, which is responsible for the syntactic analysis of stimuli and, mainly, for the building up of the argument structure (Bickel et al., 2000). Yet, at the same time, the preservation of the general ability to produce syntax is coherent with the most recent findings about neuroanatomical support of syntactic abilities. Syntactic abilities in the human brain are mainly supported by the inferior frontal gyrus, particularly by Broca's area and, within this, by BA44, but complex syntax significantly relies on the interactive connection of BA44 with the superior temporal gyrus (STG) (Friederici et al., 2017). Furthermore, neuroanatomical and language interactions expectedly replicate each other.
As a final word, our results are in line with recent research from Chapin et al. (2022), who suggest the necessity to recur to fine-grained, rather than coarse analysis of syntax if we want to understand its true nature. The fine-grained analysis from Chapin et al. (2022) showed that specific syntactic elements (e.g., NP vs. VP) can show up with different changes, and be, furthermore, due to different etiologies. Our work confirms this position by underlying the need to specify what we measure in syntax, how we measure it, and what is baseline we have to consider for measuring it accordingly.
For a long time, syntax has been considerably disregarded from the study of language profiles in AD. The salience and the primacy of the lexical-semantic deficit in dementia have probably been one of the main reasons for such disregard. The preservation of general syntactic ability, reported by pioneering studies on syntax in AD, is another important factor. Truly indeed, speakers with AD can construct grammatically acceptable sentences, and, as this research shows, the number of sentences they build matches with the similar index in healthy aging and speakers with Mild Cognitive Impairment.
Yet, the application of the model of syntactic maturity allowed us to demonstrate that syntax is not fully preserved in AD and already changes at the MCI stage. Specifically, we observed that speakers with dementia produce significantly shorter sentences and clauses, and rely significantly less on subordination. Our results are in line with other recent studies (e.g., Chapin et al., 2022), which suggest the necessity of fine-grained analysis for disentangling the specificity of syntactic deficits in dementia. Considering that the task we analyze (Cookie-Theft picture description task) minimizes the effect of cognitive and lexical load, and that scoring in semantic tasks does not correlate with the index of syntactic complexity, we conclude that syntactic decline in AD parallels other language and cognitive declines.
Overall, several important questions must be addressed for a better understanding of syntax in pathological aging. First, we lack a necessary background for what normotypical syntax is and how we should measure it. This is crucial for answering the questions about the patterns of syntactic change in AD. Second, we need to specify better the type of stimuli for syntax elicitation. Different language tasks drive different loads on cognition and language, so, expectedly, syntactic outcomes can vary in due order. All in all, we believe that a better understanding of syntactic ability in AD can significantly improve our understanding of human syntactic ability, as well as its neurocognitive and theoretical relationship with other language levels.
The data that support the findings of this study are available from the corresponding author upon reasonable request. Requests to access the data should be directed to OI at b2xnYS5pdmFub3ZhQHVzYWwuZXM=.
The study received the approval of the Ethics Committee of the State Reference Centre for the Care of People with Alzheimer's Disease and Other Dementias (Salamanca, Spain), attached to the Spanish Ministry of Social Rights and 2030 Agenda. All participants signed the informed consent form. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments and the European Union regulations concerning medical research.
OI and JM contributed to conception and design of the study. OI, IM-N, and EG-P organized the database. OI and JM performed the data analyses. All authors contributed to manuscript revision, read, and approved the submitted version.
This research was supported by the University of Salamanca and by the grant PIC2-2020-19 funded by the University of Salamanca to OI.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
OI and JM were declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adamson, H. D. (2019). Linguistics and English Literature: An Introduction. Cambridge: Cambridge University Press. doi: 10.1017/9781107051379
Ahmed, S., Haigh, A. M. F., de Jager, C. A., and Garrard, P. (2013). Connected speech as a marker of disease progression in autopsy-proven Alzheimer's disease. Brain 136, 3727–3737. doi: 10.1093/brain/awt269
Alegret, M., Peretó, M., Pérez, A., Valero, S., Espinosa, A., Ortega, G., et al. (2018). The role of verb fluency in the detection of early cognitive impairment in Alzheimer's disease. J. Alzheimers Dis. 62, 611–619. doi: 10.3233/JAD-170826
Alzheimer, A. (1907). Über eine eigenartige erkrankung der hirnrinde. Allg. Zschr. Psychiat. 64, 146–148.
Alzheimer, A., Förstl, H., and Levy, R. (1991). On certain peculiar diseases of old age. Hist. Psychiatry 2, 71–101. doi: 10.1177/0957154X9100200505
Amieva, H., Jacqmin-Gadda, H., Orgogozo, J. M., Le Carret, N., Helmer, C., Letenneur, L., et al. (2005). The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain 128, 1093–1101. doi: 10.1093/brain/awh451
Andreetta, S., and Marini, A. (2015). The effect of lexical deficits on narrative disturbances in fluent aphasia. Aphasiology 29, 705–723. doi: 10.1080/02687038.2014.979394
Angelucci, F., Spalletta, G., Iulio, F., di Ciaramella, A., Salani, F., Colantoni, L., et al. (2010). Alzheimer's disease (AD) and Mild Cognitive Impairment (MCI) patients are characterized by increased BDNF serum levels. Curr. Alzheimer Res. 7, 15–20. doi: 10.2174/156720510790274473
Auriacombe, S., Lechevallier, N., Amieva, H., Harston, S., Raoux, N., Dartigues, J. F., et al. (2006). A longitudinal study of quantitative and qualitative features of category verbal fluency in incident Alzheimer's disease subjects: results from the PAQUID Study. Dement. Geriatr. Cogn. Disord. 21, 260–266. doi: 10.1159/000091407
Banovic, S., Junuzovic Zunic, L., and Sinanovic, O. (2018). Communication difficulties as a result of dementia. Mater. Sociomed. 30, 221–224. doi: 10.5455/msm.2018.30.221-224
Bánréti, Z., Hoffmann, I., and Vincze, V. (2016). Recursive subsystems in aphasia and Alzheimer's disease: case studies in syntax and theory of mind. Front. Psychol. 7, 405. doi: 10.3389/fpsyg.2016.00405
Bates, E., Harris, C., Marchman, V., Wulfeck, B., and Kritchevsky, M. (1995). Production of complex syntax in normal ageing and Alzheimer's disease. Lang. Cogn. Process. 10, 487–539. doi: 10.1080/01690969508407113
Beers, S. F., and Nagy, W. E. (2009). Syntactic complexity as a predictor of adolescent writing quality: which measures? Which genre? Read. Writ. 22, 185–200. doi: 10.1007/s11145-007-9107-5
Bickel, C., Pantel, J., Eysenbach, K., and Schröder, J. (2000). Syntactic comprehension deficits in Alzheimer's disease. Brain Lang. 71, 432–448. doi: 10.1006/brln.1999.2277
Bose, A., Dash, N. S., Ahmed, S., Dutta, M., Dutt, A., Nandi, R., et al. (2021). Connected speech characteristics of Bengali speakers with Alzheimer's disease: evidence from language-specific diagnostic markers. Front. Aging Neurosci. 13, 707628. doi: 10.3389/fnagi.2021.707628
Campbell, K. L., Samu, D., Davis, S. W., Geerligs, L., Mustafa, A., Tyles, L. K., et al. (2016). Robust resilience of the frontotemporal syntax system to aging. J. Neurosci. 36, 5214–5227. doi: 10.1523/JNEUROSCI.4561-15.2016
Chapin, K., Clarke, N., Garrard, P., and Hinzen, W. (2022). A finer-grained linguistic profile of Alzheimer's disease and mild cognitive impairment. J. Neurolinguistics 63, 101069. doi: 10.1016/j.jneuroling.2022.101069
Croisile, B., Ska, B., Brabant, M. J., Duchene, A., Lepage, Y., Aimard, G., et al. (1996). Comparative study of oral and written picture description in patients with Alzheimer's disease. Brain Lang. 53, 1–19. doi: 10.1006/brln.1996.0033
Davidson, D. J., Zacks, R. T., and Ferreira, F. (2003). Age preservation of the syntactic processor in production. J. Psycholinguist. Res. 32, 541–566. doi: 10.1023/A:1025402517111
De Lira, J., Minett, T. S. C., Bertolluci, P. H. F., and Ortiz, K. Z. (2014). Analysis of word number and content in discourse of patients with mild to moderate Alzheimer's disease. Dement. Neuropsychol. 8, 260–265. doi: 10.1590/S1980-57642014DN83000010
De Lira, J., Ortiz, K., Campanha, A., Bertolucci, P., and Minett, T. (2011). Microlinguistic aspects of the oral narrative in patients with Alzheimer's disease. Int. Psychogeriatr. 23, 404–412. doi: 10.1017/S1041610210001092
De Looze, C., Kelly, F., Crosby, L., Vourdanou, A., Coen, R. F., Walsh, C., et al. (2018). Changes in speech chunking in reading aloud is a marker of mild cognitive impairment and mild-to-moderate Alzheimer's disease. Curr. Alzheimer Res. 15, 828–847. doi: 10.2174/1567205015666180404165017
Delicia, D. D. (2011). Madurez sintáctica y modos de organización del discurso: un estudio sobre la competencia gramatical adolescente en producciones narrativas y argumentativas. Onomázein 24, 173–198. doi: 10.7764/onomazein.24.08
Eyigoz, E., Mathur, S., Santamaria, M., Cecchi, G., and Naylor, M. (2020). Linguistic markers predict onset of Alzheimer's disease. Eclinical Medicine. 28, 100583. doi: 10.1016/j.eclinm.2020.100583
Fisher, N. J., Tierney, M. C., Rourke, B. P., and Szalai, J. P. (2004). Verbal fluency patterns in two subgroups of patients with Alzheimer's disease. Clin. Neuropsychol. 18, 122–131. doi: 10.1080/13854040490507235
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). Mini-mental state. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Forbes-McKay, K.E., and Venneri, A. (2005). Detecting subtle spontaneous language decline in early Alzheimer's disease with a picture description task. Neurol. Sci. 26, 243–254. doi: 10.1007/s10072-005-0467-9
Fraser, K. C., Meltzer, J. A., and Rudzicz, F. (2016). Linguistic features identify Alzheimer's disease in narrative speech. J. Alzheimers Dis. 49, 407–422. doi: 10.3233/JAD-150520
Friederici, A., Chomsky, N., Berwick, R. C., Moro, A., and Bolhuis, J. J. (2017). Language, mind and brain. Nat. Hum. Behav. 1, 713–722. doi: 10.1038/s41562-017-0184-4
Garrard, P., Maloney, L. M., Hodges, J. R., and Patterson, K. (2005). The effects of very early Alzheimer's disease on the characteristics of writing by a renowned author. Brain 128, 250–260. doi: 10.1093/brain/awh341
Gayraud, F., Lee, H. R., and Barkat-Defradas, M. (2011). Syntactic and lexical context of pauses and hesitations in the discourse of Alzheimer patients and healthy elderly subjects. Clin. Linguist. Phon. 25, 198–209. doi: 10.3109/02699206.2010.521612
Goodglass, H., Kaplan, E., and Barresi, B. (2000). Boston Diagnostic Aphasia Examination, 3rd ed. San Antonio, TX: Pearson.
Guinn, C., Singer, B., and Habash, A. (2014). “A comparison of syntax, semantics, and pragmatics in spoken language among residents with Alzheimer's disease in managed-care facilities,” in 2014 IEEE Symposium on Computational Intelligence in Healthcare and E-Health (CICARE) (Orlando, FL: IEEE), 98–103. doi: 10.1109/CICARE.2014.7007840
Hardy, S. M., Wheeldon, L., and Segaert, K. (2020). Structural priming is determined by global syntax rather than internal phrasal structure: evidence from young and older adults. J. Exp. Psychol.: Learn. Mem. Cogn. 46, 720–740. doi: 10.1037/xlm0000754
Heyselaar, E., Wheeldon, L., and Segaert, K. (2021). Structural priming is supported by different components of nondeclarative memory: evidence from priming across the lifespan. J. Exp. Psychol.: Learn. Mem. Cogn. 47, 820–837. doi: 10.1037/xlm0000955
Hunt, K. W. (1965). Grammatical Structures Written at Three Grade Levels. Urbana: National Council of Teachers of English.
Hunt, K. W. (1970). “Recent measures in syntactic development,” in Readings in Applied Transformation Grammar, ed M. Lester (New York, NY: Holt, Rinehart and Winston), 187–200.
Ivanova, O., Meilán, J. J. G., Martínez-Sánchez, F., Martínez-Nicolás, I., Llorente, T. E., Carcavilla González, N., et al. (2022). Discriminating speech traits of Alzheimer's disease assessed through a corpus of reading task for Spanish language. Comput. Speech Lang. 73, 101341. doi: 10.1016/j.csl.2021.101341
Jack, C. R., Bennett, D. A., Blennow, K., Carrillo, M. C., Dunn, B., Haeberlein, S. B., et al. (2018). NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 14, 535–562. doi: 10.1016/j.jalz.2018.02.018
Kemper, S., Thompson, M., and Marquis, J. (2001). Longitudinal change in language production: effects of aging and dementia on grammatical complexity and propositional content. Psychol. Aging 16, 600–614. doi: 10.1037/0882-7974.16.4.600
Kemper, S. LaBarge, E., Ferraro, F.R., Cheung, H., Cheung, H., and Storand, M. (1993). On the preservation of syntax in Alzheimer's disease. evidence from written sentences. Arch. Neurol. 50, 81–86. doi: 10.1001/archneur.1993.00540010075021
Ketelaars, M. P., Jansonius, K., Cuperus, J., and Verhoeven, L. (2015). Narrative competence in children with pragmatic language impairment: a longitudinal study. Int. J. Lang. Commun. Disord. 51, 162–173. doi: 10.1111/1460-6984.12195
Kirova, A. M., Bays, R. B., and Lagalwar, S. (2015). Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer's disease. Biomed Res. Int. 2015, 748212. doi: 10.1155/2015/748212
Lee, M. S., and Kim, B. S. (2019). Effects of working memory intervention on language production by individuals with dementia. Neuropsychol. Rehabil. 31, 1557–1581. doi: 10.1080/09602011.2020.1789479
Liampas, I., Folia, V., Morfakidou, R., Siokas, V., Yannakoulia, M., Sakka, P., et al. (2022). Languages differences among individuals with normal cognition, amnesic and non-amnesic MCI, and Alzheimer's disease. Arch. Clin. Neuropsychol. 38, 525–536. doi: 10.1093/arclin/acac080
Liu, J., Zhao, J., and Bai, X. (2021). Syntactic impairments of Chinese Alzheimer's disease patients from a language dependency network perspective. J. Quant. Linguist. 28, 253–281. doi: 10.1080/09296174.2019.1703485
Lofgren, M., and Hinzen, W. (2022). Breaking the flow of thought: increase of empty pauses in the connected speech of people with mild and moderate Alzheimer's disease. J. Commun. Disord. 97, 106214. doi: 10.1016/j.jcomdis.2022.106214
Lyons, K., Kemper, S., Labarge, E., Ferraro, F. R., Balota, D., Storandt, M., et al. (1994). Oral language and alzheimer's disease: a reduction in syntactic complexity. Aging Neuropsychol. Cogn. 1, 271–281. doi: 10.1080/13825589408256581
Mascali, D., DiNuzzo, M., Serra, L., Mangia, S., Maraviglia, B., Bozzali, M., et al. (2018). Disruption of semantic network in mild Alzheimer's disease revealed by resting-state fMRI. Neuroscience 371, 38–48. doi: 10.1016/j.neuroscience.2017.11.030
Montembeault, M., Chapleau, M., Jarret, J., Boukadi, M., Laforce, R., Wilson, M. A., et al. (2019). Differential language network functional connectivity alterations in Alzheimer's disease and the semantic variant of primary progressive aphasia. Cortex 117, 284–298. doi: 10.1016/j.cortex.2019.03.018
Mozeiko, J., Le, K., Coelho, C., Krueger, F., and Grafman, J. (2011). The relationship of story grammar and executive function following TBI. Aphasiology 25, 826–835. doi: 10.1080/02687038.2010.543983
Mueller, K. D., Koscik, R. L., Turkstra, L. S., Riedeman, S. K., LaRue, A., Clark, L. R., et al. (2016). Connected language in late middle-aged adults at risk for Alzheimer's disease. J. Alzheimers Dis. 54, 1539–1550. doi: 10.3233/JAD-160252
Nasiri, M., Moayedfar, S., Purmohammad, M., and Ghasisin, L. (2022). Investigating sentence processing and working memory in patients with mild Alzheimer and elderly people. PLoS ONE 17, e0266552. doi: 10.1371/journal.pone.0266552
Pakhomov, S., Chacon, D., Wicklund, M., and Gundel, J. (2011). Computerized assessment of syntactic complexity in Alzheimer's disease: a case study of Iris Murdoch's writing. Behav. Res. Methods 43, 136–144. doi: 10.3758/s13428-010-0037-9
Pallickal, M., and Hema, N. (2019). Discourse in Wernicke's aphasia. Aphasiology 34, 1138–1163. doi: 10.1080/02687038.2020.1739616
Peña-Casanova, J., Blesa, R., Aguilar, M., Gramunt-Fombuena, N., Gómez-Ansón, B., Oliva, R., et al. (2009). Spanish multicenter normative studies (NEURONORMA Project): methods and sample characteristics. Arch. Clin. Neuropsychol. 24, 307–319. doi: 10.1093/arclin/acp027
Sajjadi, S. A., Patterson, K., Tomek, M., and Nestor, P. J. (2012). Abnormalities of connected speech in semantic dementia vs Alzheimer's disease. Aphasiology 26, 847–866. doi: 10.1080/02687038.2012.654933
Saunders, N. L. J., and Summers, M. J. (2010). Attention and working memory deficits in mild cognitive impairment. J. Clin. Exp. Neuropsychol. 32, 350–357. doi: 10.1080/13803390903042379
Shafro, M. A., and Tyler, L. K. (2014). Language in the aging brain: the network dynamics of cognitive decline and preservation. Science 346, 583–587. doi: 10.1126/science.1254404
Shigemizu, D., Akiyama, S., Higaki, S., Sugimoto, T., Sakurai, T., Boroevich, K. A., et al. (2020). Prognosis prediction model for conversion from mild cognitive impairment to Alzheimer's disease created by integrative analysis of multi-omics data. Alzheimers Res. Ther. 12, 145. doi: 10.1186/s13195-020-00716-0
Sung, J. E., Choi, S., Eom, B., Yoo, J. K., and Jeong, J. H. (2020). Syntactic complexity as a linguistic marker to differentiate Mild Cognitive Impairment from normal aging. J. Speech Lang. Hear. Res. 63, 1416–1429. doi: 10.1044/2020_JSLHR-19-00335
Taler, V., and Phillips, N. A. (2008). Language performance in Alzheimer's disease and mild cognitive impairment: a comparative review. J. Clin. Exp. Neuropsychol. 30, 501–556. doi: 10.1080/13803390701550128
Teng, E., Leone-Friedman, J., Lee, G. J., Woo, S., Apostolova, L. G., Harrell, S., et al. (2013). Similar verbal fluency patterns in amnesic Mild Cognitive Impairment and Alzheimer's disease. Arch. Clin. Neuropsychol. 28, 400–410. doi: 10.1093/arclin/act039
Tyler, L. K., Shafro, M. A., Randall, B., Wright, P., Marslen-Wilson, W. D., Stamatakis, E. A., et al. (2010). Preserving syntactic processing across the adult life span: the modulation of the frontotemporal language system in the context of age-related atrophy. Cereb. Cortex 20, 352–364. doi: 10.1093/cercor/bhp105
Van Boxtel, W., and Lawyer, L. (2021). Sentence comprehension in ageing and Azheimer's disease. Lang. Linguist. Compass 15, e12430. doi: 10.1111/lnc3.12430
Verma, M., and Howard, R. J. (2012). Semantic memory and language dysfunction in early Alzheimer's disease: a review. Int. J. Geriatr. Psychiatry 27, 1209–1217. doi: 10.1002/gps.3766
Vincze, V., Szatlóczki, G., Tóth, L., Gosztolya, G., Pákáski, M., Hoffmann, I., et al. (2021). Telltale silence: temporal speech parameters discriminate between prodromal dementia and mild Alzheimer's disease. Clin. Linguist. Phon. 35, 727–742. doi: 10.1080/02699206.2020.1827043
Wainwright, A. B., and Cannito, M. P. (2015). Referential ambiguity in the narrative productions of African American Adults. Am. J. Speech Lang. Pathol. 24, 990–1000. doi: 10.1044/2015_AJSLP-14-0146
Winblad, B., Palmer, K., Kivipelto, M., Jelic, V., Fratiglioni, L., Wahlund, L.-O., et al. (2004). Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 256, 240–246. doi: 10.1111/j.1365-2796.2004.01380.x
Keywords: syntactic ability, Alzheimer's disease, aging, lexical-semantic deficit, cognitive impairment, syntactic complexity
Citation: Ivanova O, Martínez-Nicolás I, García-Piñuela E and Meilán JJG (2023) Defying syntactic preservation in Alzheimer's disease: what type of impairment predicts syntactic change in dementia (if it does) and why? Front. Lang. Sci. 2:1199107. doi: 10.3389/flang.2023.1199107
Received: 02 April 2023; Accepted: 12 July 2023;
Published: 14 August 2023.
Edited by:
Emiliano Zaccarella, Max Planck Institute for Human Cognitive and Brain Sciences, GermanyReviewed by:
Marco Calabria, Open University of Catalonia, SpainCopyright © 2023 Ivanova, Martínez-Nicolás, García-Piñuela and Meilán. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olga Ivanova, b2xnYS5pdmFub3ZhQHVzYWwuZXM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.