
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Hortic. , 07 February 2025
Sec. Breeding and Genetics
Volume 4 - 2025 | https://doi.org/10.3389/fhort.2025.1520119
This article is part of the Research Topic Trends in Mutation Breeding, Seed Priming and Tissue Culture-Based Genetic Improvement of Leguminous and Non-Leguminous Crops for Stress Tolerance View all articles
Cowpea (Vigna unguiculata L.) is an underutilised vegetable legume indigenous to and predominantly cultivated and consumed in Africa. However, its reach in agricultural production and consumption has expanded globally. This resilient crop is known for its ability to withstand various environmental stressors, making it suitable for marginal crop production systems commonly used by small-scale farmers. Although cowpea exhibits tolerance to drought, it is notably sensitive to salinity stress and biotic agents. The degree of tolerance to drought varies among different cultivars, which requires further research to develop more resilient varieties.The changing climate patterns and associated uncertainties highlight the urgent need to breed more resilient and productive cowpea cultivars. Conventional plant breeding techniques have produced new varieties of cowpeas, yet the limited genetic diversity within cultivated cowpeas poses challenges for future conventional breeding efforts. New breeding techniques (NBTs), including gene editing tools, single base pair alterations, and DNA methylation methods, offer promising alternatives to accelerate cowpea improvement. However, such approaches are also faced with challenges associated with the success of organogenesis (OG) and somatic embryogenesis (SE) in tissue culture. This review examines challenges and advances in the use of tissue culture to enhance cowpea productivity and resilience against abiotic and biotic stresses.
Cowpea is an indigenous African legume that is widely cultivated, distributed, and traded worldwide (Boukar et al., 2020; Horn et al., 2022). As a place of origin, the African continent continues to dominate global cowpea production, with West Africa contributing a staggering 95% of total global production (Phillip et al., 2019; Boukar et al., 2020). The African and global production of cowpeas faces challenges that limit its optimal growth and reproduction and ultimately its productivity. Various biotic and abiotic factors, including cropping systems, soil type and nutrition, pests, and diseases affect cowpea productivity (Horn and Shimelis, 2020; Omoigui et al., 2020; Togola et al., 2020; Wabwayi et al., 2020; Omomowo and Babalola, 2021; Ravelombola et al., 2022; Song et al., 2023). Despite the limitations of these factors, cowpea remains a vital crop in Africa, where 52% of its production is consumed as food, while 16% is lost to waste (Nkomo et al., 2021). The grain is the most valuable part of the cowpea plant for human consumption, and its versatility is showcased in Nigeria’s diverse array of cowpea-based dishes and popular street foods. Cowpeas are an important alternative source of nutrition to staple crops that are often high in calories. They contain protein (25%), potassium (112 mg/100 g), dietary fibre (11%), and a high lysine content (Bhowmik et al., 2021; Affrifah et al., 2022).
Beyond food for human consumption, cowpea allocation includes 13% for animal feed, 10% for seed production, and 9% for miscellaneous uses (Nkomo et al., 2021). Moreover, cowpea offers significant agronomic benefits, enriching the soil with 30-125 kg N/ha through nitrogen fixation and providing a valuable residue for subsequent crops (Ennin-Kwabiah et al., 1993). Its shade tolerance and compatibility with various cereals and root crops make it an ideal intercrop for marginal lands and drought-prone tropical regions, where it can thrive in challenging conditions (Tarawali et al., 1997).
Vigna unguiculata L., commonly known as cowpea, is a self-pollinating annual diploid legume (2n = 2x = 22) belonging to the Fabaceae family (Leguminosae). Cowpea consists of distinct species and subspecies: Vigna unguiculata subsp. unguiculata, Vigna unguiculata subsp. dekindtiana, Vigna unguiculata subsp. stenophylla, Vigna unguiculata subsp. tenuis, Vigna nervosa, Vigna vexillata, Vigna oblongifolia, Vigna frutescens, Vigna reticulata, Vigna luteola, Vigna pygmaea, Vigna gazensis, Vigna nuda, Vigna kirkii, Vigna platyloba, and Vigna wittei (Padulosi and Ng, 1990; Boukar et al., 2020). Among the listed species and subspecies of cowpea, Vigna unguiculata subsp. Unguiculata and Vigna vexillata have been successfully domesticated (Panzeri et al., 2022), with Vigna unguiculata subsp. Unguiculata commonly cultivated by farmers across the world. The centre of diversity for domesticated cowpea is reported to be North and East Africa, while Southern Africa is the centre of diversity for wild relatives (Boukar et al., 2020). Typically, in some parts of the continent, wild species/subspecies of cowpea are predominantly found near cultivated cowpea fields, often thriving in the peripheral areas and even within the fields themselves, where they can co-exist with their domesticated counterpart. This shows that Africa is a golden mine for the diversity of cowpea species (domesticated and wild relatives) translating to a diverse gene pool. However, cowpea still exhibits limited genetic variability, a characteristic that limits its potential for improvement through traditional breeding approaches (Horn and Shimelis, 2020). The strong cross-incompatibility barriers that exist between the domesticated and wild relatives hinder the introgression of desirable traits from the wild into the cultivated gene pool. These factors have collectively contributed to the slow progress in developing elite cowpea varieties with superior characteristics through conventional breeding programs, highlighting the need for innovative approaches to enhance the genetic improvement of this vital legume crop.
Introducing new breeding techniques (NBTs) in crop improvement has seen accelerated improvements in soybean production, the leading cultivated legume globally (Cai et al., 2023; Vollmann, 2016). The wide acceptance and success of NBTs in soybean production provide a pathway for the application and acceptance of NBTs for other legume crops such as cowpeas. It is worth noting that the success of NBTs depends on the success of plant regeneration in tissue culture. Various attempts have been made to improve cowpeas using NBTs such as clustered regularly interspaced palindromic repeats (CRISPR) associated protein 9 (Cas9) targeted genome editing (Bhowmik et al., 2021). However, the recalcitrancy associated with the transformation of cowpea results in low transformation efficiencies in tissue culture. To this end, organogenesis (OG) and somatic embryogenesis (SE) have been the two pathways of choice in cowpea regeneration in tissue culture. However, it is almost two decades since the optimisation of SE in cowpea with researchers using the OG pathway irrespective of SE advantage of direct gene transfer to the plant cell (Anand et al., 2000; Ramakrishnan et al., 2005; Popelka et al., 2006). The OG pathway relies on the usage of seed and vegetative plant organs as a starting material to generate a plantlet (small whole-growing plant in vitro). The OG pathway can be achieved through both direct (without intervening callus stage) and indirect (with intervening callus stage) strategies. Direct strategy is time and cost saving, with organs directly transformed and plantlets directly subjected to selection and growth with the target gene or gene manipulation. The SE pathway relies on the use of somatic cells to generate embryo-like structured cells that develop into plantlets (Bidabadi and Mohan, 2020). Similarly to OG, two strategies are taken, direct (a rare event in tissue culture where embryos are directly formed from the cell) and indirect (where the callus is first produced before embryo formation). Different factors such as the age of tissue/organ used, genotype, tissue culture medium, type, and concentrations of phytohormones influence the success of OG and SE. This ultimately influences the transformation efficiencies observed in vitro. Both OG and SE have been used in cowpea improvement and regeneration with various regeneration rates reported (Anand et al., 2000; Ramakrishnan et al., 2005; Raveendar et al., 2009; Bett et al., 2019; Bala, 2022). The findings of previous research show that differences in cowpea regeneration rates in vitro are influenced by the composition of the MS media. In this review, we summarise the challenges and opportunities in cowpea production, conventional breeding, and NBTs contribution to cowpea improvement, and explore different in vitro avenues through which researchers improve the efficiencies of tissue in cowpea improvement.
Legumes are notably a crucial component of sustainable and nutritious diets, offering a rich source of essential nutrients and serving as an alternative to calorie-dense meat- based diets. The protein-rich nutrition of legumes makes them ideal plant-based targets for sustainable agriculture during challenging climate changes. As a leguminous crop, cowpea (Vigna unguiculata L.) is an alternate crop with origins in Africa, mainly produced by small-scale farmers. Cowpea is a versatile and valuable crop that plays a crucial role in mixed farming systems. Its ability to fix nitrogen through specialised mechanisms enriches the soil, making it an excellent companion crop that boosts the productivity of other crops. Furthermore, cowpea provides a rich source of protein for animal feed and human consumption, further solidifying its status as a multipurpose crop. As diverse as it is, cowpea faces major constraints that limit its production. Such constraints include drought stress, salinity stress, extreme temperatures, pest and disease infestations, and parasitic weeds (Horn and Shimelis, 2020; Togola et al., 2020; Wabwayi et al., 2020; Omomowo and Babalola, 2021; Ravelombola et al., 2022; Song et al., 2023). These directly affect the crop growth rate by reducing the nodulation and photosynthetic efficiency of the cowpea crop. This occurs due to leaf necrosis, stem rot, and wilting caused by sensitivity to biotic agents, as well as reduced stomatal conductance and chlorophyll content as a result of an exposure to abiotic stress factors (Omomowo and Babalola, 2021). Damage due to oxidative stress as a consequence of biotic and abiotic stress factors accelerates damage to cells and tissues of sensitive cowpea crops, leading to crop wilting and death in severe cases (Song et al., 2023). Cowpea tolerance levels to biotic and abiotic stresses vary significantly, revealing certain levels of genetic diversity among cultivated cowpea varieties. This inherent genetic variation suggests that some cowpea lines may possess natural resilience to certain constraints. Taking advantage of this potential, we can further enhance the resilience of cowpea germplasm, paving the way for more efficient and sustainable production systems.
Cowpea yields in Africa are woefully underperforming, with average yields of 0.1 to 0.6 tons per hectare, compared to the estimated potential of 1.5 to 3.0 tons per hectare. This disparity underscores the need for targeted interventions to unlock the full potential of the crop (Horn and Shimelis, 2020; Omomowo and Babalola, 2021; Atakora et al., 2023). This equates to an economic loss for small-scale farmers in Africa, who tend to benefit from the economic returns from high grain yields. The market forecast places cowpea at USD 7.6 billion in 2024 and USD 9.43 billion in 2029 (Market Data Forecast, 2024). Despite the projected size of the cowpea market, its recognition as an important crop remains limited due to production constraints. Reducing cowpea production constraints through research and development investments would help improve cowpea grain yields. The African adoption of the policies put in place by the Indian government to promote pigeon pea production and utilisation (Research and Markets, 2022) would also benefit the producers as local demand would also increase. This, in turn, would positively influence the market share of cowpeas, as Africa is the largest producer.
Similar to other crops, breeding objectives for cowpeas emanate from each challenge that a respective breeder seeks to address. Researchers have focused on breeding early/medium maturing cultivars to help cowpea plants avoid drought and reach a reproductive stage on time (Asiwe, 2022; Owusu et al., 2022). Given that small-scale farmers engage in cowpea cultivation only relying on rainfall, this objective holds significant importance. Due to changing weather patterns, farmers experience erratic rainfall, with rain not covering the entire growing season. This exposes crops to drought stress at crucial periods of growth and development, such as the filling stage of the pods. Drought stress triggers a cascade of physiological responses, including chlorophyll breakdown, stomatal closure, reduced photosynthetic efficiency (measured by Fv/Fm) and disruption of photosynthetic processes. Collectively, these changes impair the ability of the plant to undergo photosynthesis, ultimately impacting its growth and productivity. Researchers have observed these physiological changes at various stages of cowpea growth, including the seedling stage (Cui et al., 2020; Ravelombola et al., 2020; Tengey et al., 2023) and the reproductive stage (Nunes et al., 2022). When exposed to drought stress, sensitive and moderately tolerant cowpea varieties experience a diminishing leaf water status, which, if it occurs in the late vegetative and reproductive stages, affects grain yield and quality. Therefore, the breeding of early or medium-maturing and drought-tolerant cowpea varieties is important in efforts to improve stagnant cowpea yields.
In addition to breeding for early or medium maturity, cowpea breeding has focused on improving grain yield, quality, and insect pest resistance, among other things (Asiwe, 2022; Singh et al., 2023; Togola et al., 2023). Pre-harvest and post-harvest insect pest infestations are most devastating for cowpea producers. Pre-harvest infestation reduces grain yield and quality, and, in severe cases, results in a total loss of yield, while post- harvest infestation results in the loss of seed lots (Mofokeng and Gerrano, 2021; Togola et al., 2023). For example, aphids (Aphis craccivora) are among the economically important pre-harvest insect pests in cowpea production. They are prominent biotrophic agents that infect cowpea seedlings with increased infestation, causing them to spread to flower buds (Togola et al., 2020; Mofokeng and Gerrano, 2021). While feeding on plants, aphids release toxic secretions into the plant, creating a sickened plant with delayed growth and development (MacWilliams et al., 2023). To counteract this, farmers rely on the application of pesticides to control aphids, a practice that is not economically sustainable and environmentally friendly. Host resistance, which has been identified in cultivated and wild cowpea species in Africa, is a sustainable alternative (Togola et al., 2020; MacWilliams et al., 2023). Host resistance is a phenomenon that refers to a plant’s ability to produce bioactive compounds that affect insect biology and growth (antibiosis resistance), affect insect behaviour without endangering it (antixenosis resistance), and the ability of the plant to withstand insect damage (plant tolerance) (MacWilliams et al., 2023). A screening of 375 cowpea varieties for host resistance to aphids revealed that three varieties exhibited increased production of bioactive compounds, specifically kaempferol and quercetin, which conferred resistance to aphid infestation (Togola et al., 2020). The low percentage of cultivated cowpea varieties with host resistance is indicative of a bottleneck in achieving host resistance through traditional breeding programs. Further developments in host resistance identification led to the discovery of two marker genes, QAC-vu7.1 and QAC-vu1.1, hypothesised to be key targets for cowpea host resistance (MacWilliams et al., 2023). Biotechnological approaches could utilise these to increase the number of cultivated cowpea varieties with host resistance.
Soil salinity is another important environmental stress factor in cowpea production and breeding. Researchers have widely used selective breeding methods to identify cultivars tolerant of salinity stress (Murillo-Amador et al., 2006; Taffouo et al., 2009; Ravelombola et al., 2022). Among the cultivars recommended for salinity-prone environments are Bambey 21 from the Senegalese Institute of Agronomic Research (ISRA) and IT97K-556- 4 and IT04K-332-1 from the International Institute of Tropical Agriculture (IITA). Selective breeding forms an important part of cultivar development as it identifies potential parental lines for breeding purposes. However, developing a new cultivar using physical and chemical mutagens (Odeigah et al., 1998; Nanhapo et al., 2024), and classical cowpea breeding methods such as pedigree, bulk, backcross, and recurrent selection (Horn and Shimelis, 2020; Asiwe, 2022; Singh et al., 2023) is time-consuming and laborious (Figures 1A, B). As a result, from 2000 to 2024, there were a total of 37 cowpea cultivars (from the UPOV code VIGNA_UNG and VIGNA_UNG_UNG) with active plant breeders’ rights registered on the International Union for the Protection of New Variety of Plants (UPOV) system. However, these numbers could be higher since South Africa’s six newly registered cowpea cultivars (De Bruyn and Sekele, 2023) do not appear in the UPOV search engine for 2000– October 2024. Although that is the case, the insufficient global list of active plant breeders’ rights amid spiralling cowpea production challenges, such as biotic and abiotic constraints aggravated by climate-change consequences, suggests a challenging future ahead.
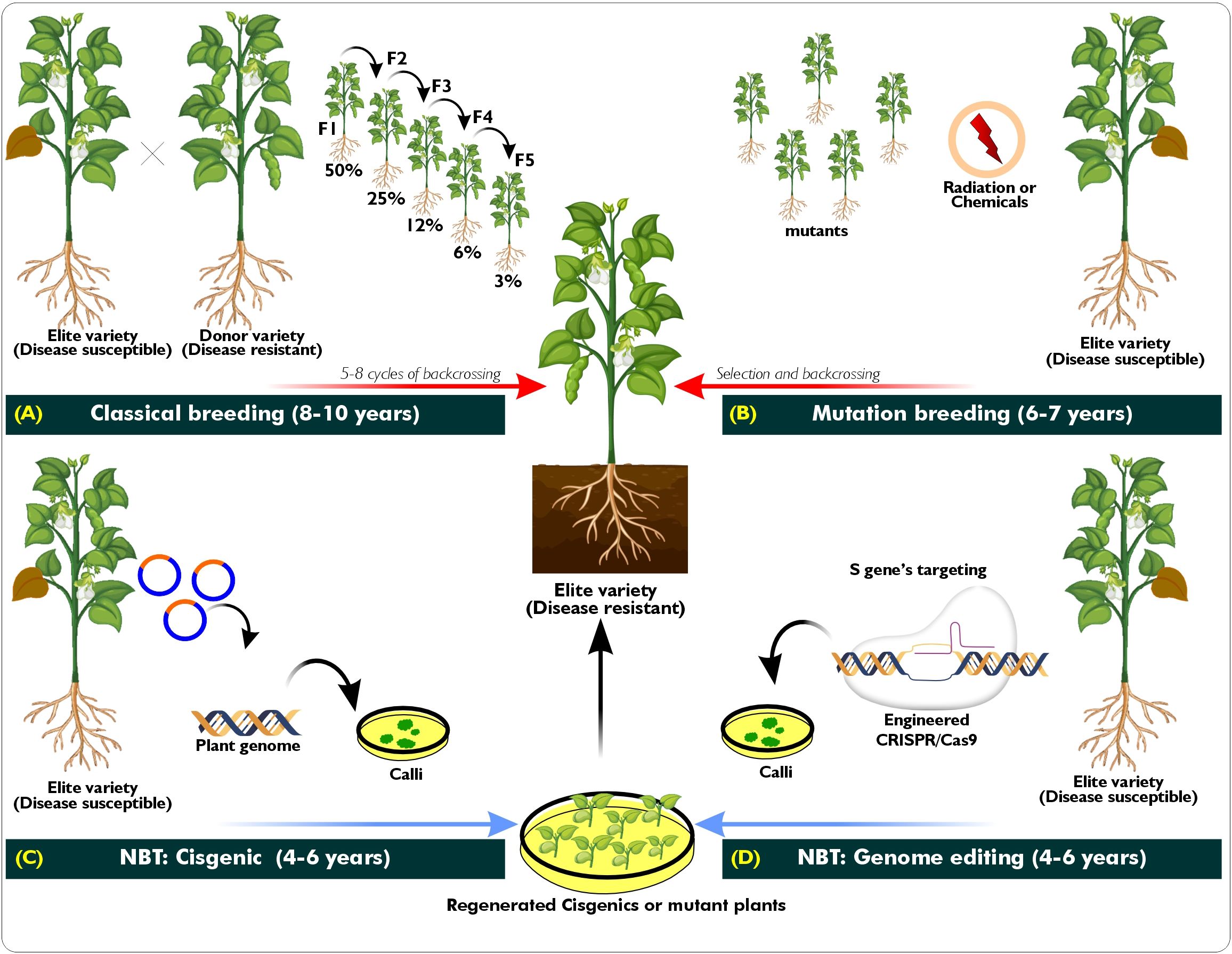
Figure 1. A schematic illustration of conventional breeding and NBT methods for the improvement of disease traits in cowpea. (A) The breeding pathway for disease-resistant elite varieties involves crossing a susceptible elite variety (recurrent parent) with a resistant variety (donor parent). Multiple backcrossings are required to introgress a homozygous mutation into a farmer- preferred genotype. Eight to ten years is required to generate an improved variety. (B) The seeds of the susceptible variety are mutagenised and then backcrossed for a period ranging from 6 to 7 years. (C) Cisgenesis is an NBT strategy that involves changing cowpea with Agrobacterium. This makes it easier to transfer gene(s) from the same species or species that can reproduce with each other. (D) Genome editing with CRISPR/Cas9 is another NBT method. This can be done with agrobacterium- mediated transformation, also known as protoplast fusion, which causes precise mutations in certain parts of the cowpea genome. This process generates multiple mutant lines from which the best- performing phenotype emerges.
Another limiting factor in improving cowpeas using classical breeding methods is the autogamous nature of the crop, which narrows its genetic base compared to many crops (Boukar et al., 2020). Recent studies of genetic diversity in cowpea germplasm from various geographical locations, using SSRs, SNPs, and SilicoDArT markers, reveal a moderate level of genetic diversity (Nkhoma et al., 2020; Gumede et al., 2022; Guimarães et al., 2023). Most markers representing this moderate diversity have only identified diversity in segments of the genome linked to yield. Consequently, the available germplasm may not be adequate for breeding cowpeas with other desirable traits such as nutritional quality and resistance to biotic and abiotic stresses. Boukar et al. (2020) proposed introgression breeding in cowpeas to overcome the limitations posed by classical breeding’s narrow genetic base. This involved utilising cowpea’s wild relatives to access their valuable gene pool, currently underutilised. However, reports of success using wild cowpea relatives to improve elite varieties are scarce. According to Rawal et al. (1976), this may be due to a lack of understanding of their biology, the presence of numerous undesirable traits, and cross-incompatibility. Cowpea faces not only a narrow genetic base, but also an eroding genetic base, further challenging traditional breeding efforts (Horn and Shimelis, 2020). Such obstacles may lead to reliance on secondary and tertiary gene pools, further limiting classical breeding due to incompatibility and infertility that arise with deviation from the primary gene pool. NBTs can be used for gene transfer and editing, increasing the success of cowpea improvement amid a narrow genetic base bottleneck.
To achieve higher grain yields in cowpea, innovative plant breeding strategies for crop improvement must be prioritised. Such strategies include the use of NBTs which are already being utilised and adopted in soybean improvement programs. The publicly available cowpea genome encourages the adoption of NBTs for cowpea improvement. However, the integration of such strategies must involve the farming community as they have direct access to diverse germplasm mostly comprising landraces. Improving farmer preferred varieties with farmers’ knowledge and participation could increase the chances of acceptance of the newly developed resilient varieties as farmers will be aware of the technology used for improvement. The incorporation of both conventional plant breeding and NBTs with cowpea farming communities’ knowledge and practices could maximise the opportunity to combat environmental stressors facing cowpea production.
Traditional approaches to crop improvement often overlook the complex relationships between traits, environmental factors, and farmer adoption. However, a more holistic approach is gaining prominence (Mir et al., 2012). Integrated breeding, which acknowledges these complexities, is now taking centre stage. By combining conventional and cutting-edge techniques, integrated breeding aims to create high-yielding, stress- resilient crop varieties. This approach involves collaboration among researchers, farmers, and policymakers to align breeding objectives with local farming community needs. The complexity of addressing climate resilience in cowpea production underscores the need for integrated breeding approaches. Participatory breeding, for instance, engages farmers in the selection process, ensuring developed varieties align with local agricultural practices and preferences (Lawali et al., 2024; Hamidou et al., 2023). This method not only enhances variety relevance but also considers specific agrosystems, socio-economic conditions, gender considerations, and other constraints faced by farmers. By leveraging indigenous knowledge, participatory breeding facilitates the identification of local varieties (landraces) with desirable traits, allowing breeders to focus on enhancing those local adaptations. Research has shown that while drought tolerance is highly valued among all genders, women exhibit distinct preferences for traits related to processing and marketing (Jinbaani et al., 2023). Engaging farmers in the evaluation and selection process democratises breeding programs and leverages local knowledge about conditions, pest management, and nutritional preferences. This is particularly important for cowpeas, which are sensitive to environmental changes and benefit from farmers’ first-hand experiences (Singh et al., 2013). By incorporating these insights, breeding programs can develop varieties that are not only high-yielding but also suitable for the end uses that matter most to farmers, enhancing adoption rates. Furthermore, advances in physiology, genomics, and molecular biology have greatly enhanced the understanding of crop genetics. By combining this deep knowledge, breeders can improve selection efficiency by targeting specific traits with greater precision. This targeted approach has proven particularly effective in developing crops resistant to abiotic stress (Mir et al., 2012).
The past decade has witnessed significant biotechnological advancements, leading to the emergence of New Breeding Techniques (NBTs) Girma, D. (2022). These innovations have given rise to various biotechnological techniques, which can be broadly categorised as NBTs (Figures 1C, D). Notably, NBTs can be distinguished into two main groups: those that involve the transfer of foreign DNA into the target crop and those that do not. These technological advances offer enormous potential to meet the global challenges associated with food and nutritional security, posed by population growth, consumer demand, and climate change. For crop improvement, technological innovations related to NBTs make use of available genome sequences. NBTs allow for the development of crops with new desirable traits, thus enhancing crop germplasm.
The availability of the cowpea genome sequence has facilitated the adoption of NBTs in cowpea development (Lonardi et al., 2019). The application of NBTs in cowpea involves various genomic alterations, including expression of foreign genes, genome-editing, RNA interference (RNAi), and DNA methylation. These techniques have been successfully employed in cowpea research (Cruz and Aragão, 2014; Kumar et al., 2022; Bridgeland et al., 2023; Singh et al., 2024). Notably, most NBTs involve the introduction of foreign DNA into the genome of the targeted crop, resulting in genetically modified organisms (GMOs). A prominent example of a genetically modified cowpea is the Pod borer-resistant (PBR) Cowpea. Developed through agrobacterium-mediated transfer of the Bacillus thuringiensis (Bt) gene, this cowpea produces a protein that disrupts the digestive system of legume pod borer larvae, conferring resistance. Successfully commercialised in several African countries, including Nigeria, Ghana, and Kenya, the PBR cowpea demonstrates the potential of NBTs to enhance crop resilience and improve food security.
Other NBTs techniques are such as (i) editing the genome using various methods, such as oligonucleotide-directed mutagenesis (ODM) and sequence- specific nuclease (SSN); (ii) different types of traditional transformation methods, such as cisgenesis, intragenesis, grafting, and agro-infiltration; and (iii) creating negative segregants by changing the epigenetic landscape (through RNA-dependent DNA methylation, RdDM). These enable precise, targeted, and reliable changes in the genome and are therefore different from GMOs, which involve the integration of foreign DNA (transgenesis). Unlike chemical- or radiation-induced mutagenesis, often traditionally used as a basis for crop improvement, NBTs do not create multiple, unknown, unintended mutations throughout the genome. For several of the techniques, the resultant plant product is free of genes foreign to the species and would not be distinguishable from the product generated by conventional breeding techniques. Adopting epigenetic approaches, which alter gene expression, prevents DNA sequence changes. Therefore, in certain cases, we may not be able to identify the production method of the new crop variety.
In recent years, genome editing methods, particularly CRISPR/Cas9, have emerged as powerful tools for crop improvement, offering promising solutions for various crops (Adegbaju et al., 2024). Since its inception, CRISPR/Cas9 mediated gene editing has been demonstrated to offer great potential for resilience towards environmental stress factors in legumes (Singh et al., 2024). The popularity of CRISPR/Cas9 can be attributed not only to its ease of use but also to its ability to generate improved germplasm without introducing foreign DNA. Despite cowpea’s recalcitrance to transformation, researchers have made progress using CRISPR/Cas-mediated gene editing, albeit with varying outcomes. Studies employing Agrobacterium-mediated gene delivery have reported success rates ranging from 4% to 67% in cowpea transformation, using embryogenic axis and hairy root culture as explants (Ji et al., 2019; Che et al., 2021). However, alternative approaches, such as genome editing using CRISPR/Cas9 technology with protoplasts for transient transformation, have also been explored. Initially, these efforts met with limited success, with one study reporting zero transformation success (Juranić et al., 2020). Nevertheless, subsequent optimization of cowpea protoplast transformation with CRISPR/Cas9 technology has yielded positive results, highlighting the importance of factors such as protoplast isolation method, constitutive promoter, temperature, plasmid concentration, and buffer percentage (Bridgeland et al., 2023). While protoplast transformation may offer a viable alternative to Agrobacterium-mediated transformation, its application is still constrained by limitations in regeneration efficiency, optimal culture conditions, environmental stress factors, and genetic constraints (Omomowo and Babalola, 2021).
Cowpea is highly susceptible to biotic and abiotic stress factors that limit yield potential (Omomowo and Babalola, 2021; Wijerathna-Yapa and Hiti-Bandaralage, 2023; Basavaraj et al., 2024). Conventional breeding through repetitive crossing is not always possible due to the self-pollinated nature of this species, which possesses very limited genetic variability (Boukar et al., 2016, 2020; Nair et al., 2023). The little success achieved through conventional cowpea breeding for insect pest and disease resistance is due to the narrow genetic base and barriers to crossing with wild-type genotypes (Fang et al., 2007; Marubodee et al., 2015). Wild-type relatives of cowpea such as Vigna vexillate L. possess genes that confer resistance to pests, however, are cross-incompatible with domesticated cowpea varieties (Barone et al., 1992; Boukar et al., 2020). This prevents the crossing of domesticated cultivars with wild-type to transfer resistance genes to cultivated varieties (Boukar et al., 2020; Panzeri et al., 2022). Biotechnological methods of genetic transformation can serve to transfer such genes under in vitro conditions (Bett et al., 2017). Exploring tissue culture propagation methods could help regenerate in vitro transformed cowpea explants produced from NBT approaches (Bakshi et al., 2012; Tang et al., 2012). Most cowpea breeding programs intend to develop a system that will effectively allow the efficient introduction of agronomically important traits into domesticated cowpea varieties (Bett et al., 2017). The goal is to produce a genetically engineered cultivar that would withstand adverse stresses and thereby prevent losses in crop yield.
Plant regeneration is achieved by employing two in vitro tissue culture pathways, namely organogenesis (OG) and somatic embryogenesis (SE) which facilitate the rapid multiplication of plants under sterile conditions (Sani et al., 2018; Pratap et al., 2018). The implementation of NBTs relies on the ability of OG and SE pathways to regenerate plants from the explants (Figure 2). Plant regeneration through tissue culture is based on the totipotent nature of meristematic plant cells, which is a phenomenon that allows fully functional plants to be regenerated from competent cells under proper culture conditions (Feher, 2019). The meristematic cells of the explants undergo in vitro proliferation and become committed to a morphogenic pathway such as OG or SE. Cowpea, similar to other legumes with large seeds, exhibits recalcitrance to regeneration and transformation, which limits the use of tissue culture techniques for its improvement (Sahoo and Jaiwal, 2008; Chaudhury et al., 2007). Numerous factors determine the morphogenic response of the cultured explant. Integrating clonal propagation tools (SE and OG) with NBTs improves the speed of genetic improvement of crop species, particularly the recalcitrant cowpea. These biotechnological tools complement existing cowpea conventional breeding programs that intend to develop germplasm with desirable traits (Popelka et al., 2006; Bett et al., 2017).
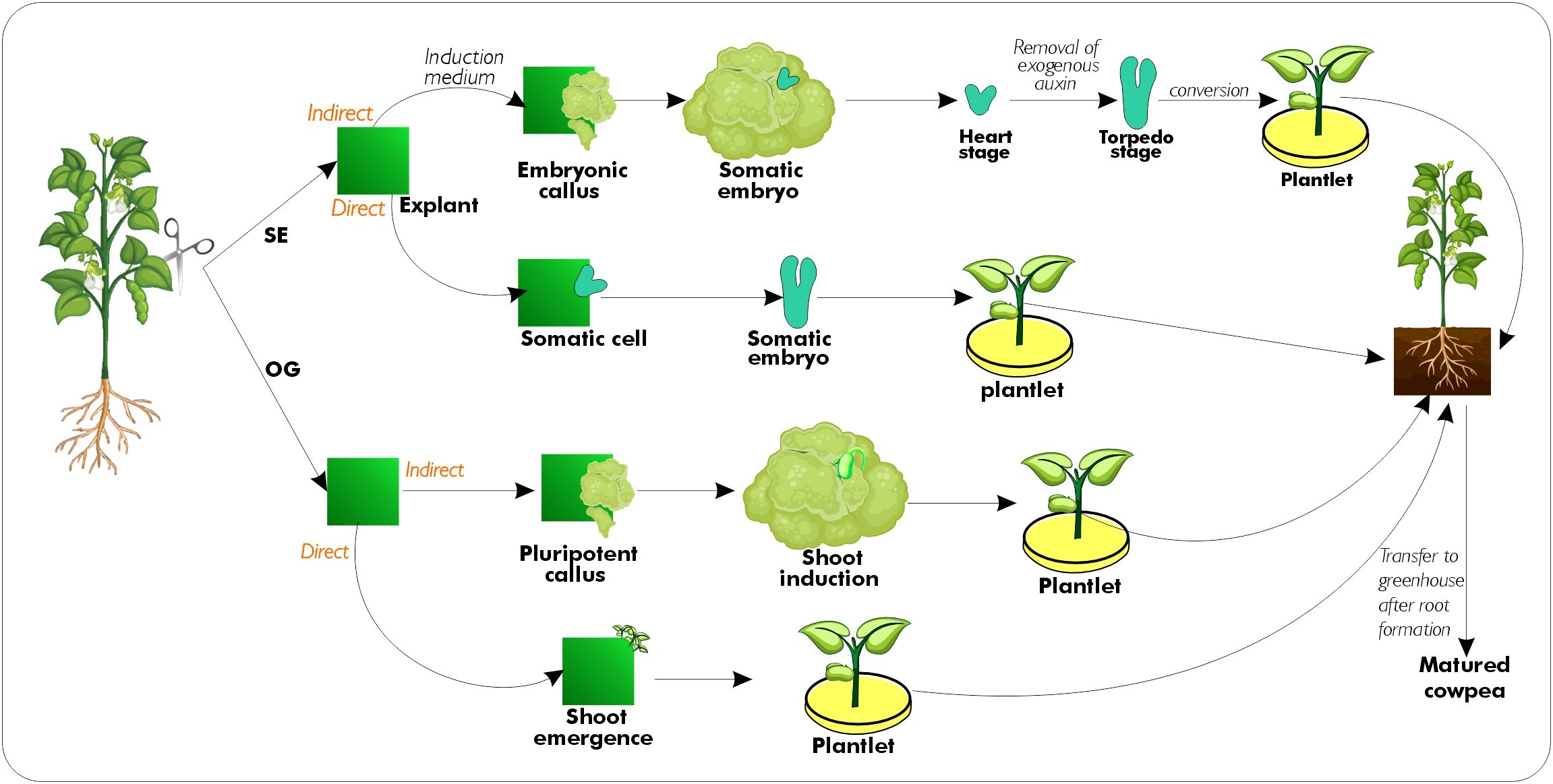
Figure 2. A schematic diagram that describes stages in somatic embryogenesis and organogenesis. Plant regeneration can occur directly on the explant or indirectly through the formation of a pluripotent callus, which differentiates into the desired tissue or organ. Conversion refers to the ex- vitro survival of somatic seedlings and their continued growth.
Somatic embryogenesis is an artificially induced morphogenic plant regeneration process in which plant somatic tissues made up of meristematic cells undergo dedifferentiation to produce competent embryos without gametes fusion (Sugimoto et al., 2010; Bull and Michelmore, 2022). The resultant embryogenic tissues are highly prolific and originate from single cells, ensuring the production of highly uniform qualitative regenerants in a relatively short period (Manman et al., 2013; Chokheli et al., 2020). Since its inception and use in carrots, the single-cell origin of SE from the induced callus has been considered the most valuable tool in plant tissue culture due to its higher proliferation rate (Zimmerman, 1993; Popelka et al., 2006; Bidabadi and Mohan, 2020). SE reduces the occurrence of chimeric and somaclonal variations that affect the production of true-to- type plants, leading to undesirable regenerated plants and affecting germplasm preservation (Krishna et al., 2016; Bidabadi and Mohan, 2020).
In tissue culture, SE can be induced with the addition of exogenous plant growth regulators or by subjecting cells to stressful conditions. Auxinic herbicides, such as 2,4- dichlorophenoxyacetic acid (2,4-D), are frequently used to induce SE. In this process, they function as auxins, stressor, and/or trigger endogenous synthesis and accumulation of auxins. After auxin accumulation, explants are immediately transferred to a medium without auxin. This leads to the formation of organiser cells- a specific type of cell that plays a vital role in the development and arrangement of tissues and organs during embryonic development. Subsequently, these organiser cells divide at the outer edge to form globular structures along with the surrounding epidermis (Smetana et al., 2019). Somatic embryogenesis comprises four distinct stages: (1) induction of somatic embryos; (2) embryo proliferation; (3) embryo maturation; and (4) embryo germination (Hartmann et al., 1997). The somatic embryo can develop through two pathways: direct SE, where SEs develop directly from the wounded site of explant, and indirect SE, where SEs develop from the embryogenic callus (Figure 2). In the culture of somatic embryos, somatic embryogenesis can also be induced directly or through the callus. This is different from primary SE, which is induced from explant cells, and is known as secondary SE (Gaj, 2004). Although secondary SE is a valuable tool for plant biotechnology, it can also cause genetic instability, somaclonal variation, and epigenetic changes. Many crop species have exhibited somatic embryogenesis; however, the germination ability of these somatic embryos into plants has been notably inadequate. In some instances, a conversion rate of as low as 3%-5% has been observed (Parth et al., 2022). This is due to incomplete development of the somatic embryos that may appear normal. To achieve a complete development of somatic embryos, all essential growth stages must be properly completed. Embryo maturation and germination have been identified as the main limiting factors for somatic embryogenesis in several crops including cowpea (Ramakrishnan et al., 2005), olive (Rugini et al., 2016), and Persian walnut (Vahdati et al., 2008). Somatic embryo maturation determines the germination potential of embryos as they accumulate dietary resources and proteins that improve their ability to withstand desiccation. In several plant species, the maturation of the somatic embryo is facilitated by abscisic acid (ABA). ABA has been reported to promote embryo maturation in Persian walnut (Vahdati et al., 2008), rapeseed (Finkelstein et al.,1985), cork oak (García-Martín et al., 2005), and it suppresses secondary somatic embryogenesis (Ammirato, 1983). In cowpea, the addition of ABA (5 µM) and L- proline (20 mg/L) to the maturation medium has improved embryo maturation to 40% (Ramakrishnan et al., 2005), compared to 26% reported by Anand et al. (2000). ABA is known to trigger the expression of genes that are normally expressed during the drying phase of seeds. The products of these genes impart tolerance to desiccation in embryos (Florin et al., 1993). ABA has been reported to increase desiccation tolerance in somatic embryos of carrots (Tetteroo, et al., 1996) and celery (Kim and Janick, 1989). Sometimes, the residual ABA, used during maturation, can interfere with further development. The culture regime during maturation may also induce dormancy. Therefore, dormancy-breaking treatments such as cold or gibberellic acid application can improve somatic embryo germination and plantlet growth. To this end, respective usage of cytokinin and N-phenyl-1,2,3- thidiazol-5yl-urea (TDZ) have been reported for cowpea plantlet growth, leading to 21- 33% regeneration efficiency (Table 1). Once the embryogenic shoot or axis is developed, a spontaneous rooting occurs while in some instances rooting is facilitated by the addition of hormones, and thus the entire plantlet is regenerated via the somatic embryogenesis process.
Organogenesis is the process by which shoots and roots develop from cultured explants without the appearance of callus, often called direct organogenesis, however, if the callus- forming stage precedes organogenesis, this is called indirect organogenesis (Abbas et al., 2021; Belanger et al., 2024). Even though it can occur without intervention in some instances, callus formation is activated by exogenous synthetic or organic auxin and cytokinin media supplementation in cowpea and other plant species explants (Markin et al., 2023; Mosoh et al., 2024). The balanced addition of phytohormones ensures the successful transition of callus into whole plants to complete the regeneration process. The process of OG is a multi-stage regeneration process which entails initiation of shoots, proliferation of adventitious shoots, elongation of shoots, and rooting of individual shoots. Plants regenerate from differentiating buds of the meristem, forming a complete functional plant (Raveendar et al., 2009; Markin et al., 2023).
Cultured explants initiate adventitious shoots by subculturing them onto the same shoot initiation medium for a period of 2 to 4 weeks (Das Bhowmik et al., 2019). Different tissue types are being used as explants in cowpea regeneration through OG. Explants from the cotyledonary node (Bakshi et al., 2011) and shoot apices (Bala, 2022) excised from germinated seedlings were used while Bett et al. (2019) established that the optimal explant was an attached embryogenic axis of the imbibed mature seeds. During in vitro culture, the cotyledonary node cells of the detached embryogenic axis of the shoot apical meristem undergo rapid cell division and dedifferentiation to acquire organic competence for shoot regeneration (Che et al., 2021). The process of OG relies on the optimal auxin-to-cytokinin ratio to successfully induce organogenesis. The formation of multiple shoots is significantly affected by the concentration and type of phytohormones as well as MS medium composition (Bala, 2022). The mean number of shoots per explant usually varies significantly with varying Benzylaminopurine (BAP) concentrations, such as an average of 2.89 shoots produced by an MS medium supplemented with a combination of 1.5 mg/L BAP and 0.1 mg/L naphthalene (NAA), while 1.5 mg/L BAP alone produced an average of 2.51 shoots (Bala, 2022).
Regenerated multiple adventitious shoots are individually elongated in an elongation medium supplemented with gibberellic acid or cytokinin (Che et al., 2021). The elongation of shoots normally takes between 4 to 6 weeks (Adesoye et al., 2010). The speed of shoot regeneration and elongation is determined by the type and concentration of phytohormone used. The highest mean shoot length of 2.60 and 2.61 were achieved with 1.5 mg/L BAP and 0.1 mg/L NAA, respectively (Bala, 2022). As the concentration of BAP decreases the shoot regeneration and elongation decrease while NAA alone offers limited success in shoot regeneration and elongation. The elongated shoots are usually subjected to a rooting treatment to achieve a well-developed root system. Some shoots can readily root in a medium devoid of a rooting hormone due to the presence of NAA in the preceding elongation phase.
The acquisition of regenerative competence in plants is generally regulated by several critical external and internal factors (Mendez-Hernandez et al., 2019). SE induction is primarily regulated by the interaction of factors that act as external stimuli triggering an embryogenic pathway in plants through the dedifferentiation of cells into a competent embryogenic state and gene reprogramming (Guan et al., 2016). Dedifferentiation is the process through which differentiated cells regain the ability to become distinct meristematic tissue capable of dividing mitotically. The following factors drive the acquisition of embryogenic competence in plant somatic cells.
Various genotypes often show variability in terms of their responses to culture (Table 1). Some genotypes easily undergo the morphogenic process of either SE or OG, while some show recalcitrance to tissue culture amenability (Bull and Michelmore, 2022). Successful induction of SE and OG depends on the judicious selection of optimal explants at the appropriate developmental and physiological stage (Mendez-Hernandez et al., 2019). The success of establishing tissue culture is influenced by the age of the explant (Bhatia et al., 2004). Fresh soft tissues are generally readily amenable to in vitro manipulation compared to old tissues. Explants must contain competent cells capable of undergoing SE induction when exposed to proper inductive conditions (Feher, 2019). Cowpea SE has been achieved using primary leaf explants (Anand et al., 2000; Ramakrishnan et al., 2005), while cowpea OG is achieved using cotyledonary nodes explants (Bakshi et al., 2011), cotyledonary nodes with attached embryonic axis (Bett et al., 2019), embryonic axis (Popelka et al., 2006; Adesoye et al., 2010; Kaur et al., 2016; Che et al., 2021), and shoot apices (Bala, 2022; Markin et al., 2023). Mature seeds are used as starting plant material even though immature seeds result in a higher regeneration rate (Popelka et al., 2006). The easy accessibility to mature seeds without having to raise plants for production of immature seeds prior commencement of in vitro cultivation for explant preparation is the benefit that comes with the use of mature seeds. The type of explant prepared determines the number of shoots produced, and the choice of explant may vary with the genotype (Bhatia et al., 2004).
The selection of tissue type and age are among the important factors to consider for a successful regeneration system of choice. For OG, the young cotyledonary node is the most responsive explant for the induction of multiple shoots under optimal conditions (Bakshi et al., 2012; Tang et al., 2012, 2022). It has been shown that seedling explant preconditioning promotes various shoot responses in shoot multiplication medium according to genotype (Kumari et al., 2021). Preconditioning of mature seeds by imbibition was optimal for shoot regeneration from attached embryogenic axis (Bett et al., 2019). The optimal length of exposure period of genotypes to preconditioning treatment results in an increase of the mean number and the length of multiple shoots. Preconditioning enhances the ability to obtain high competence of donor cultures (Sehaole and Mangena, 2024). For SE, the primary leaf explant gives viable cells but is largely influenced by the concentration of 2,4-Dichlorophenoxyacetic acid (2,4-D). As such, the reduction of 2,4-D has been reported to improve regeneration efficiency of the cells obtained from primary leaf explant from 21.8 to 32% (Table 1). As previously reported by Popelka et al. (2006), the conditions to which the explant is exposed during SE determines the success or failure of the regeneration system.
Phytohormones are plant growth regulators (PGR) that play a significant role in plant tissue culture processes (Feher, 2019; Mendez-Hernandez et al., 2019). Cowpea regeneration is driven by the type and concentration of PGRs. The classification of PGRs is based on their physiological mode of action. Both auxin and cytokinin regulate cell division in plants (Mendez-Harnandez et al., 2019). The balance between endogenous and exogenous phytohormone levels are critical to determining the developmental fate of meristematic cells of an explant (Bull and Michelmore, 2022).
The SE pathway is driven by both auxins and cytokinins, and the molar concentration balance between them determines the morphogenic response (Markin et al., 2023). The discovery of synthetic auxins such as 2,4-D, and Indole-3-acetic acid (IAA) has enabled the induction of embryogenic competence in plant cells. They can act alone or in combination with cytokinin to induce callus formation in plants (Gao et al., 2023a; 2023b). However, it is argued that an explant at an optimal developmental stage can potentially undergo SE induction without supplementing the media with PGRs (Lelu et al., 1999). In cowpea, 2,4-D and IAA have been popularly used to achieve callus tissue dedifferentiation during the SE induction process. A high concentration of 2,4-D can decrease the regeneration frequency and therefore inhibit the transition of callus to somatic embryos (Alsafran et al., 2022). Most importantly, SE induction depends on the application of external stress achieved by supplementing the medium with PGRs (Nzama et al., 2024). The withdrawal of auxin and the addition of cytokinin in an inductive medium facilitate the dedifferentiation of the callus from somatic embryos (Bernula et al., 2020). Examples of cytokinin used in plant tissue culture are benzyl adenine (BA), BAP, and TDZ, a potent non- purine growth regulator, among others (Jahan et al., 2011).
Numerous types of cytokinin are used to induce OG in optimal explants of cowpea genotypes. According to Tang et al. (2012), cytokinin BAP alone or in combination with a lower concentration of auxin is the most effective way to induce multiple shoots. It was also found that the inclusion of auxin IBA had a worthless effect on shoot multiplication from axillary buds (Aasim et al., 2009). An average of 4 shoots per explant were produced at BAP concentrations between 1.25 and 1.5 mg/L. Axillary bud cultures in a medium supplemented with 2 mg/L of BAP produced abnormal shoots with reduced proliferation and suppressed elongation. The number of multiple shoots decreased with increasing BAP concentration (Diallo et al., 2008). However, Raveendar et al. (2009) achieved an average of 13.5 multiple shoots in a medium supplemented with 0.5 µM BAP. Preconditioning of seedlings with a high dose of cytokinin before plant regeneration has been shown to increase subsequent shoot regeneration efficiency in plants (Jahan et al., 2011; Bakshi et al., 2012). Prolonged exposure to higher doses of cytokinin such as TDZ and BA resulted in an increased seedling length and the formation of secondary roots. The comparison of TDZ and BA equimolar doses in the same medium showed that TDZ is superior to BA as it produced a 1.5 to -2-fold increase in the mean number of new shoots (Bakshi et al., 2012). A study by Markin et al. (2023), successfully established that plant-derived extracts can support in vitro cowpea propagation in the absence of synthetic plant growth regulators. This organogenic response induced by these explants could be explained by the amounts of vitamins, minerals, and phytohormones present in the extracts coupled with explant growth in culture (Markin et al., 2023).
The inductive and regenerative capacity of both embryogenic and organogenic explants is reliant on the chemical composition of the medium used which is mostly based on the molar ratios of nitrogenous compounds constituting a particular basal medium. The induction of embryogenic tissue and the formation of proembryos are controlled by the chemical composition of the induction medium (Nzama et al., 2024). Numerous media formulations are available in the published literature and their optimisation has been undertaken to enable the propagation of various highly recalcitrant species such as cowpea (Reeves et al., 2018). The nitrogen source is the most critical component of the tissue culture medium that is usually adjusted or optimised during the propagation of species (Li et al., 2022; Nzama et al., 2024). Organic and inorganic nitrogen sources are the two types of nitrogen source used in tissue culture medium. Organic nitrogen sources are popularly bovine powdered milk referred to as enzymatic casein hydrolysate (CH) and an amino acid such as L-glutamine (L-gln) (Ageel and Elmeer, 2011; Daniel et al., 2018). There are numerous types of amino acids used in plant tissue culture, examples are asparagine, arginine, etc. The effectiveness of amino acids in plant tissue culture depends primarily on their type and concentration to enhance the morphogenesis of the explant to maintain or produce advanced new in vitro cell fates (Duarte-Ake et al., 2022). The mola ratios of the inorganic nitrogen components of the medium are also the most critical to induce morphogenic responses (Nzama et al., 2024). Most induction media comprise ammonium nitrate and potassium nitrate as inorganic nitrogen sources, and their molar ratios are responsible for the induction of cellular morphogenetic responses such as the induction of somatic embryogenesis and the proliferation of the resulting proembryos (Carlsson et al., 2019). Ammonium- to- nitrate ion molar ratios of 1:1, 1:2, and 1:4 are often used for different stages of an SE process (Nzama et al., 2024). The 1:1 and 1:2 molar ratios are used in the early stages of the SE process, i.e., induction, proliferation, and maturation of embryogenic somatic embryos, while 1:4 is used effectively for induction of roots and elongation of hypocotyls-epicotyls to produce a physiologically normal plant ready for acclimation. The improper balance between the two inorganic sources would result in numerous anomalies that are tissue culture-induced.
Organogenesis has been the most successful mode of regeneration for cowpea using B5, BM and popularly MS basal medium and its modifications (Manman et al., 2013). The modifications of MS basal medium such as shoot induction, elongation induction, root induction media are often used (Che et al., 2021). Sometimes, the explants are maintained in MS medium with no modifications except transferring to fresh medium until shoot regeneration. The MS medium used can contain either synthetic phytohormone or organic extracts and result in up to 90% regeneration efficiency (Bala, 2022; Markin et al., 2023). Just recently, Markin et al. (2023) demonstrated the beneficial use of plant fruit explants in promoting in vitro propagation of cowpea through OG. This study showed the amenability of three cowpea varieties (AGRAC 216, Tintinwa A and Asontem) to in vitro propagation using MS basal medium with plant-derived explants as sources of PGRs in the absence of synthetic PGRs (Table 1). These extracts were banana, coconut water, orange juice, and tomato juice.
The phenotype and genotype of intact plants are used for the evaluation of abnormalities. The appearance of regenerated plants is used to differentiate between normal and aberrant phenotypes. Aberrant phenotypes show tissue bleaching and tissue swelling due to disturbed morphogenesis during development. The discoloration of albino regenerants is readily visible in aberrant phenotypes after regeneration by tissue culture (Alikina et al., 2016; Gajecka et al., 2021; Hung et al., 2021). Plantlets with stunted growth and waterlogged organs are also readily recognisable in regenerants (Polivanova and Bedarev, 2022). Waterlogging or hyperhydricity can be avoided by reducing the water potential in the culture medium by using elevated concentrations of gelling agents during tissue culture phases, such as maturation and germination (Ivanova, 2009; Jan et al., 2021). Adsorption of metabolites produced during the in vitro development of regenerants by supplementing germination media with activated charcoal can also be used (Thomas, 2008). Activated charcoal adsorbs the remnants of PGRs used during the induction and maturation phases of the SE process. Careful consideration of the stage at which activated charcoal is used is important as it may interfere with medium hormonal balance thereby affecting the overall regeneration process. The culture environment is also responsible for inducing anomalies experienced during in vitro growth (Abdalla et al., 2022). The photoperiod during development impacts the normal development of regenerants (Ahmad et al., 2014). The early stages of development during the SE process take place in the dark, whereas germination takes place in the subdued light for a few weeks and then open light at a specific photosynthetic photon flux density (PPFD).
Molecular approaches are also used to determine the induced changes in DNA sequence due to in vitro induced stresses. Dedifferentiation during the explant transition from the original explants to the callus stage is responsible for the induction of changes in DNA sequences (Kumar and Mohapatra, 2021). New genotypes are made during the transient in vitro propagation pressures that explants undergo due to the use of PGRs. At higher concentrations of PGRs, the genotypic instability is observed where several polymorphisms are experienced and detected using DNA-based molecular approaches such as randomly amplified polymorphic DNA (RAPD), single-site repeats (SSRs), single nucleotide polymorphisms (SNPs), among others (Sivakumar et al., 2011). Karyotype- based approaches can also be used to count the number of chromosomes and their appearance could be used to infer the different appearance and physiology of regenerants (Zhao et al., 2005). Flow cytometry is an example of an approach used for cytological analyses of the chromosomal structure of regenerants (Nunes et al., 2017). Evaluation of anomalies in regenerants is based on molecular, physiology, and physical characteristics (Chen et al., 1998; AI-Zahim et al., 1999; Zhao et al., 2005). A thorough evaluation of abnormal plants could be elucidated based on proteomic, metabolomic, transcriptomic, and genomic levels. Existing technologies enable the use of these techniques to reach a conclusive decision about the genotype and phenotype of the regenerant.
Tissue culture-induced variations are termed somaclonal variations (SV) (Bairu et al., 2011; Sun et al., 2013). They could be both desirable and undesirable, depending on the breeding objective. When disease-susceptible plants suddenly start to show tolerance after undergoing a change in DNA sequence compared to their original explant genotype. This would be an example of a beneficial occurrence of SV resulting in disease tolerance that would prevent undesirable crop yield losses. An undesirable SV occurrence would be when a disease-free explant produces disease-prone or susceptible regenerants, which would eventually result in devastating crop yield losses.
Prolonged culture of induced embryogenic tissue is known to produce SV due to extended exposure to a mutagen, 2,4-D, which is normally used for SE induction (Rival et al., 2013). Prolonged culture is used to maintain highly prolific cultures of resultant cell lines, however, shortening the subculture period reduces the rate of in vitro-induced variation during plant regeneration (Farahani et al., 2011; Sun et al., 2013). A tissue cryopreservation process is used to prevent SV induction that would later result in aberrant genotypes with abnormal physical appearances (Nunes et al., 2017). Cryopreservation is the process of preservation of juvenile tissues at ultralow temperatures such as -196°C or below for infinite periods (Salaj et al., 2011). All metabolic processes are halted at such low temperatures, and losses of cell lines due to SV are minimised. However, it is also possible that cryopreservation could introduce SV, since the use of the cryoprotectant dimethylsulfoxide (DMSO) could introduce changes in the DNA makeup of genotypes of preserved cell lines (Nunes et al., 2017). This also highlights the need to screen all genotypes for SV before commercial deployment. DNA methylation is also a major cause of epigenetic changes in regenerants (Kumar and Mohapatra, 2021). Such changes could be either heritable or non-heritable. If a desirable change is passed down several generations, the desirable traits get incorporated into such germplasm and future regenerants could consistently express it. On the contrary, an undesirable change could interfere with breeding objectives and eventually affect the end product of the breeding programs and commercialisation of the product.
Propagation of species by OG from cotyledonary nodes is preferable compared to SE as the risks associated with the production of chimeric plantlets due to the de novo regeneration pathway are often very low (Tzfira et al., 1997; Belanger et al., 2024). Formation of chimeric plants is due to a single plant tissue containing a mixture of transformed and non-transformed sections (Das Bhowmik et al., 2019). Transformation and regeneration of cowpea by Che et al. (2021) produced no signs of chimerism and this could reflect the incomplete transformation events during the process resulting in both transformed and non-transformed sections of the same tissue. Higher percentage of chimera formation in cowpea and low transgenic plant recovery are major problems encountered during plant regeneration (Das Bhowmik et al., 2019). Some tissue-cultured propagules after regeneration fail to induce root formation, and this is highly genotype- dependent (Che et al., 2021). Leaf tissue necrosis and profuse callusing of the regenerating shoots are often encountered and result in the death of plantlets after growth cessation (Manman et al., 2013).
To reach the optimal yield potential of 3 tons per hectare and realise the highest potential economic returns in cowpea production, it is without a doubt that the incorporation of NBTs should be at the centre of cowpea improvement. The biotic and abiotic stress factors that occur together with the existing narrow genetic base pose challenges in improving cowpea yields. The genetic background of wild relatives of cowpea offers alternatives for cowpea improvement toward biotic and abiotic stress factors. However, the level of cross-incompatibility between cultivated and wild relatives that exhibit resistance or tolerance to biotic or abiotic stress factors hinders progress in cowpea improvement through conventional breeding strategies (Boukar et al., 2020). To circumvent this, a transgenic approach has been applied that led to the development of Bt-cowpea (Popelka et al., 2006). The recent commercialisation of Maruca vitrata-resistant GM cowpea in Nigeria is also a significant milestone in cowpea improvement using technological approaches (Boukar et al., 2020). With scepticism of the products developed from foreign DNA (transgenic crops), these two interventions might not be fully utilised for cowpea, further necessitating a look into the use of other NBTs such as CRISPR/Cas9 to develop cowpea resistance. Similar to GMO technology, CRISPR/Cas9-mediated gene editing faces a bottleneck of cowpea in vitro recalcitrancy, which is an area of research interest for many scientists. To this end, there is no available information on the release and commercialisation of cowpea products of CRISPR/Cas9 and other non-transgenic NBTs. Therefore, continuous research is needed to address the challenges encountered with the in vitro propagation of cowpeas.
Significant progress has been made towards cowpea genetic transformation and regeneration despite the low regeneration and transformation rates using in vitro propagation technologies such as SE and OG (Belanger et al., 2024). The most successful mode of regeneration for cowpea cultivars has been OG using cotyledonary node explants probably due to their easy availability and amenability to transformation compared to other explants such as those of embryonic origin that may be sensitive to handling and processing (Manman et al., 2013). The possibility of producing multitudes of somatic embryos through SE regeneration is very promising for cowpea regeneration, as the production of potentially high numbers of cowpea plantlets is possible given that the optimal culture conditions that favour regeneration are established (Chokheli et al., 2020). Another advantage of SE is the exposure of the plant cell for easier permeability of gene targets to the host compared to OG. However, researchers have been largely focusing on the use of OG and improving the gene delivery to the host (Bett et al., 2019). This is due to the direct usage of prepared explants for transformation in the OG pathway compared to the development of cells from the explants under SE. The low transformation efficiencies remain an obstacle to be overcome in OG. The generation of cells in SE also encounters some challenges. Induction of SV due to prolonged passages of explants and the use of high concentrations of phytohormones is generally one of the main stumbling blocks in the SE pathway (Rival et al., 2013; Sun et al., 2013). A solution would be to establish optimal phytohormone concentrations and avoid prolonged time in culture for proliferating tissue masses. The phase of transition of the somatic embryo into cotyledonary plantlets is preceded by the use of phytohormones that drive dedifferentiation during proliferation. For successful plant regeneration, such phytohormones should be withdrawn post-proliferation phase to avoid their carryover, which popularly hinders further plant regeneration and subsequently results in aberrant plant genotypes often characterised abnormal physical appearances such as stunted growth and albinism (Bernula et al., 2020; Alsafran et al., 2022).
Determining an optimal medium chemical formulation, such as the balance of ionic molar ratios of inorganic and organic nitrogen compounds, types and concentrations of phytohormones, could improve the morphogenesis processes in cowpea (Li et al., 2022; Nzama et al., 2024). Despite all the progress made in cowpea genetic transformation and regeneration, there is still a long way to go before optimal conditions are established and applied in a large-scale plant regeneration and genetic transformation program. Future studies should focus on optimising regeneration and genetic transformation protocols to produce genetically engineered cowpea cultivars capable of withstanding biotic and abiotic stresses while ensuring maximal yield production. For SE, it has been evident that optimisation must focus on the concentration of phytohormone and media composition. The explant type seems to be providing the required cells in SE, but media composition and phytohormone concentration determine regeneration efficiency (Table 1), hence we propose their continued optimisation. For OG, there is generally high regeneration efficiency, however, the very low transformation efficiency limits the large-scale incorporation of NBTs in cowpeas. Thus far the high regeneration efficiency in OG is not benefitting the cowpea improvement but rather micropropagation for multiplication and conservation. Further optimisation of OG should focus on establishing a high proliferating (high cell density) tissue type that could allow high transformation efficiency of cowpea. That could drive much-needed success in the development of resilient cowpea varieties using NBTs instead of lengthy conventional approaches.
MV: Conceptualization, Writing – original draft, Writing – review & editing. PN: Writing – original draft, Writing – review & editing. MA: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aasim M., Khawar K. M., Ozcan S. (2009). In vitro micropropagation from plumular apices of Turkish cowpea (Vigna unguiculata L.) cultivar Akkiz. Scientia Horticulturae. 122468–471. doi: 10.1016/j.scienta.2009.05.023
Abbas A., Rehman A. U., Javed M. M. (2021). Exploring the potential of in vitro tissue culture in breeding programs of legume and pulse crops: utilization and present condition. Bull. Biol. Allied Sci. Res. 2021 (1), 36–36. doi: 10.54112/bbasr.v2021i1.36
Abdalla N., El-Ramady H., Seliem M. K., El-Mahrouk M. E., Taha N., Bayoumi Y., et al. (2022). An academic and technical overview on plant micropropagation challenges. Horticulturae 8, 677. doi: 10.3390/horticulturae8080677
Adegbaju M. S., Ajose T., Adegbaju I. E., Omosebi T., Ajenifujah-Solebo S. O., Falana O. Y., et al. (2024). Genetic engineering and genome editing technologies as catalyst for africa’s food security: the case of plant biotechnology in nigeria. Front. Genome Editing. 6, 1398813. doi: 10.3389/fgeed.2024.1398813
Adesoye A. I., Togun A. O., Machuka J. (2010). Transformation of cowpea (Vigna unguiculata L. Walp.) by Agrobacterium infiltration. J. Appl. Biosci. 30, 1845–1860.
Affrifah N. S., Phillips R. D., Saalia F. K. (2022). “Cowpeas: Nutritional profile, processing methods and products: A review,” in Legume Science, vol. 4. (United States: John Wiley and Sons Inc). doi: 10.1002/leg3.131
Ageel S., Elmeer K. (2011). Effects of casein hydrolysates and glutamine on callus and somatic embryogenesis of the date palm (Phoenix dactylifera L.). New York Sci. J. 4, 121–125.
Ahmad N. A., Abbasi B. H., Hina Fazal H. F., Khan M. A., Afridi M. S. (2014). Effect of reverse photoperiod on in vitro regeneration and piperine production in Piper nigrum L. Comptes Rendus Biologies. 337 (1), 19–28. doi: 10.1016/j.crvi.2013.10.011
AI-Zahim M., Ford-Liod B., Newbury H. (1999). Detection of somaclonal variation in garlic (Allium Sativum L.) using RAPD and cytological analysis. Plant Cell Rep. 18, 473–477. doi: 10.1007/s002990050606
Alikina О., Chernobrovkina M., Dolgov S., Miroshnichenko D. (2016). Tissue culture efficiency of wheat species with different genomic formulas. Crop Breed. Appl. Biotechnol. 16, 307–314. doi: 10.1590/1984-70332016v16n4a46
Alsafran M., Wickramanayake K., Usman K., Ahmed T. (2022). Efficient shoot regeneration of medicinal plant Haplophyllum tuberculatum by direct and indirect organogenesis and genetic fidelity assessment using inter simple sequence repeats markers. Front. Plant Sci. 13, 1–12. doi: 10.3389/fpls.2022.995825
Ammirato P. V. (1983). Handbook of plant cell culture Vol. 1. Eds. Evans D. A., Sharp W. R., Ammirato P. V., Yamada Y. (New York: Macmillan Publishing Co), 82:123.
Anand R. P., Ganapathi A., Anbazhagan V. R., Vengadesan G., Selvaraj N. (2000). High frequency plant regeneration via somatic embryogenesis in cell suspension cultures of cowpea, Vigna unguiculata (L.) Walp. Soc. In Vitro Biol. 36, 475–480. Available at: https://www.jstor.org/stable/4293392 (Accessed June 10, 2024).
Asiwe N. A. J. (2022). “Advanced breeding approaches for developing cowpea varieties in dryland areas of Limpopo Province, South Africa,” in Legumes Research – Volume 1 (Croatia: IntechOpen). doi: 10.5772/intechopen.101028
Atakora K., Essilfie M. E., Agyarko K., Dapaah H. K., Santo K. G. (2023). Evaluation of yield and yield components of some cowpea (Vigna unguiculata(L.) Walp) genotypes in forest and transitional zones of Ghana. Agric. Sci. 14, 878–897. doi: 10.4236/as.2023.147059
Bairu M. W., Aremu A. O., Staden J. V. (2011). Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 63, 147–173. doi: 10.1007/s10725-010-9554-x
Bakshi S., Roy N. K., Sahoo L. (2012). Seedling preconditioning in thidiazuron enhances axillary shoot proliferation and recovery of transgenic cowpea plants. Plant Cell Tissue Organ Culture 110, 77–91. doi: 10.1007/s11240-012-0132-y
Bakshi S., Sadhukhan A., Mishra S., Sahoo L. (2011). Improved Agrobacterium- mediated transformation of cowpea via sonication and vacuum infiltration. Plant Cell Rep. 30, 2281–2292. doi: 10.1007/s00299-011-1133-8
Bala I. (2022). Somatic embryogenesis in local cowpea (Vigna unguiculata L. (Walp.) varieties. Fudma J. Sci. 6, 247–252. doi: 10.33003/fjs-2022-0601-895
Basavaraj P. S., Jangid K. K., Babar R., Gangana Gowdra V. M., Gangurde A., Shinde S., et al. (2024). Adventitious root formation confers waterlogging tolerance in cowpea (Vigna unguiculata (L.) Walp.). Front. Sustain. Food Syst. 8, 1–12. doi: 10.3389/fsufs.2024.1373183
Barone A., Del Giudice A., Ng N. Q. (1992). Barriers to interspecific hybridization between vigna unguiculata and Vigna vexillata. Sexual Plant Reprod. 5, 195–200. doi: 10.1007/BF00189811
Belanger J. G., Copley T. R., Hoyos-Villegas V., Charron J. B., O’Donoughue L. (2024). A comprehensive review of inplanta stable transformation strategies. Plant Methods 20, 1–24. doi: 10.1186/s13007-024-01200-8
Bernula D., Benkő P., Kaszler N., Domonkos I., Valkai I., Szőllősi R., et al. (2020). Timely removal of exogenous cytokinin and the prevention of auxin transport from the shoot to the root affect the regeneration potential of Arabidopsis roots. Plant Cell Tissue Organ Culture 140, 327–339. doi: 10.1007/s11240-019-01730-3
Bett B., Gollasch S., Moore A., Harding R., Higgins T. J. V. (2019). An improved transformation system for cowpea (Vigna unguiculata l. Walp) via sonication and a kanamycin-geneticin selection regime. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00219
Bett B., Gollasch S., Moore A., James W., Armstrong J., Walsh T., et al. (2017). Transgenic cowpeas (Vigna unguiculata L. Walp) expressing Bacillus thuringiensis Vip3Ba protein are protected against the Maruca pod borer (Maruca vitrata). Plant Cell Tissue Organ Culture 131, 335–345. doi: 10.1007/s11240-017-1287-3
Bhatia P., Ashwath N., Senaratna T., Midmore D. (2004). Tissue culture studies of tomato (Lycopersicon esculentum). Plant Cell Tissue Organ Culture 78, 1–21. doi: 10.1023/B:TICU.0000020430.08558.6e
Bhowmik P., Konkin D., Polowick P., Hodgins C. L., Subedi M., Xiang D., et al. (2021). “CRISPR/Cas9 gene editing in legume crops: Opportunities and challenges,” in Legume Science, vol. 3. (United States: John Wiley and Sons Inc). doi: 10.1002/leg3.96
Bidabadi S. S., Mohan J. S. (2020). Cellular, molecular, and physiological aspects of in vitro plant regeneration. Plants 9. doi: 10.3390/plants9060702
Boukar O., Abberton M., Oyatomi O., Togola A., Tripathi L., Fatokun C. (2020). Introgression breeding in cowpea [Vigna unguiculata (L.) Walp. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.567425
Boukar O., Fatokun C. A., Huynh B. L., Roberts P. A., Close T. J. (2016). Genomic tools in cowpea breeding programs: status and perspectives. Front. Plant Sci. 7, 1–13. doi: 10.3389/fpls.2016.00757
Bridgeland A., Biswas S., Tsakirpaloglou N., Thomson M. J., Septiningsih E. M. (2023). Optimization of gene editing in cowpea through protoplast transformation and agroinfiltration by targeting the phytoene desaturase gene. PloS One 18. doi: 10.1371/journal.pone.0283837
Bull T., Michelmore R. (2022). Molecular determinants of in vitro plant regeneration: Prospects for enhanced manipulation of lettuce (Lactuca sativa L.). Front. Plant Sci. 13, 1–17. doi: 10.3389/fpls.2022.888425
Cai Y., Chen L., Hou W. (2023). “Genome Editing Technologies Accelerate Innovation in Soybean Breeding,” in Agronomy, vol. 13. (Switzerland: Multidisciplinary Digital Publishing Institute (MDPI). doi: 10.3390/agronomy13082045
Carlsson J., Egertsdotter U., Svennerstam H. (2019). Nitrogen utilization during germination of somatic embryos of Norway spruce: revealing the importance of supplied glutamine for nitrogen metabolism. Tree 33, 383–394. doi: 10.1007/s00468-018-1784-y
Chaudhury D., Madanpotra S., Jaiwal R., Saini R., Kumar P. A., Jaiwal P. K. (2007). Agrobacterium tumefaciens-mediated high frequency genetic transformation of an Indian cowpea (Vigna unguiculata L. Walp) cultivar and transmission of transgenes into progeny. Plant Sci. 172, 692–700. doi: 10.1016/j.plantsci.2006.11.009
Che P., Chang S., Simon M. K., Zhang Z., Shaharyar A., Ourada J., et al. (2021). Developing a rapid and highly efficient cowpea regeneration, transformation and genome editing system using embryonic axis explants. Plant J. 106, 817–830. doi: 10.1111/tpj.15202
Chen W., Chen T., Fu Y., Hsieh R., Chen W. (1998). Studies on somaclonal variation in Phalaenopsis. Plant Cell Rep. 18, 7–13. doi: 10.1007/s002990050523
Chokheli V. A., Dmitriev P. A., Rajput V. D., Bakulin S. D., Azarov A. S., Varduni T. V., et al. (2020). Recent development in micropropagation techniques for rare plant species. Plants 9, 1733. doi: 10.3390/plants912-1733
Cruz A. R. R., Aragão F. J. L. (2014). RNAi-based enhanced resistance to Cowpea severe mosaic virus and Cowpea aphid-borne mosaic virus in transgenic cowpea. Plant Pathol. 63, 831–837. doi: 10.1111/ppa.12178
Cui Q., Xiong H., Yufeng Y., Eaton S., Imamura S., Santamaria J., et al. (2020). Evaluation of drought tolerance in arkansas cowpea lines at seedling stage. HortScience 55 (7), 1132–1143. doi: 10.21273/HORTSCI15036-20
Daniel M. A., David R. H., Caesar S. A., Ramakrishnan M., Duraipandiyan V., Ignacimuthu S., et al. (2018). Effect of l-glutamine and casein hydrolysate in the development of somatic embryos from cotyledonary leaf explants in okra (Abelmoschus esculentus L. monech). South Afr. J. Bot. 114, 223–231. doi: 10.1016/j.sajb.2017.11.014
Das Bhowmik S. S., Cheng A. Y., Long H., Tan G. Z. H., Hoang T. M. L., Karbaschi M. R., et al. (2019). Robust genetic transformation system to obtain non-chimeric transgenic chickpea. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00524
De Bruyn E., Sekele T. (2023). The South African Plant Variety Journal. South Africa: South African Government.
Diallo M. S., Ndiaye A., Sagna M., Gassama-Dia Y. K. (2008). Plants regeneration from African cowpea (Vigna unguiculata L.) variety. Afr. J. Biotechnol. 16, 2828–2833.
Duarte-Ake F. D., Marquez-Lopez R. E., Monroy-Gonzalez Z., Borbolla-Perez V., Loyola Vargas V. M. (2022). The source, level, and balance of nitrogen during the somatic embryogenesis process drive cellular differentiation. Planta 256, 1–26. doi: 10.1007/s00425-022-04009-8
Ennin-Kwabiah S., Asafu-Agyei J., Asafo-Adjei B. (1993). Cassava as an intercrop with soybean in Ghana. Proc. second Natl. workshop root tuber Crops plantain Kumasi Ghana., 16–23.
Finkelstein R. R., Tenbarge K. M., Shumway J. E., Crouch M. L. (1985). Role of ABA in maturation of rapeseed embryos. Plant Physiol 78 (3), 630–636. doi: 10.1104/pp.78.3.630
Fang J., Chao C. T., Roberts P. A., Ehlers J. D. (2007). Genetic diversity of cowpea [Vigna unguiculata (L.) Walp.] in four West African and USA breeding programs as determined by AFLP analysis. Genet. Resour. Crop Evol. 54, 1197–1209. doi: 10.1007/s10722-006-9101-9
Farahani F., Yari R., Masoud S. (2011). Somaclonal variation in Dezful cultivar of olive (Olea europaea subsp. europaea). Gene Conserve 10, 216–221.
Feher A. (2019). Callus, dedifferentiation, totipotency, somatic embryogenesis: what these terms mean in the era of molecular plant biology? Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00536
Florin B., Tessereau H., Lecouteux C., Didier C., Petiard V. (1993). “Long-term preservation of somatic embryos,” in Synseeds. applications of synthetic seeds to crop improvement. Ed. Redenbaugh K. (London: CRC Press), 133–161.
Gaj M. D. (2004). Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) heynh. Plant Growth Regul. 43, 27–47. doi: 10.1023/B:GROW.0000038275.29262.fb
Gajecka M., Marzec M., Chmielewska B., Jelonek J., Zbieszczyk J., Szarejko I. (2021). Changes in plastid biogenesis leading to the formation of albino regenerants in barley microspore culture. BMC Plant Biol. 21, 1–24. doi: 10.1186/s12870-020-02755-z
Gao F., Cao X., Qin C., Chen S., Cai J., Sun C., et al. (2023a). Effects of plant regulators and sucrose on proliferation and quality of embryogenic tissue in Picea pungens. Sci. Rep. 13, 1–11. doi: 10.1038/541598-023-39389-8
Gao F., Shi F., Wang R., Tretyakova L. N., Nosov A. M., Shen H., et al. (2023b). Optimization of key technologies for induction of embryogenic callus and maturation of somatic embryos in Korean pine (Pinus karaiensis). Forests 14, 1–14. doi: 10.3390/f14040850
García-Martín G., Manzanera J., González-Benito M. (2005). Effect of exogenous ABA on embryo maturation and quantification of endogenous levels of ABA and IAA in quercus suber somatic embryos. Plant Cell Tissue Organ Culture 80, 171–177. doi: 10.1007/s11240-004-1056-y
Girma D. (2022). The emergence of products of new breeding techniques and challenges to ethiopia’s biosafety regulatory regime. Ethiopian J. Agric. Sci. 32 (2), 57–69.
Guan Y., Li S.-G., Fan X.-F., Su Z.-H. (2016). Application of somatic embryogenesis in woody plants. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00938
Guimarães J. B., Nunes C., Pereira G., Gomes A., Nhantumbo N., Cabrita P., et al. (2023). Genetic diversity and population structure of cowpea (Vigna unguiculata (L.) Walp.) landraces from portugal and mozambique. Plants 12 (4), 846. doi: 10.3390/plants12040846
Gumede M. T., Gerrano A. S., Amelework A. B., Modi A. T. (2022). Analysis of genetic diversity and structure of cowpea (Vigna unguiculata (L.) Walp) genotypes using single nucleotide polymorphism markers. Plants. 11 (24), 3480. doi: 10.3390/plants11243480
Hamidou H. H., Saïdou A., Joly H. I., Tchoffo R. M., Issa M. S. S., Souley M. N. S., et al. (2023). Inferring multilocal typologies of agrosystems and farmers’ practices: A methodological basis for the setting of participatory breeding designs. Heliyon 9, e13992. doi: 10.1016/j.heliyon.2023.e13992
Hartmann H. T., Kester D. E., Davies F. T., Geneve R. L. (1997). Plant propagation: principles and practices. 6th edition (Prentice Hall International, INC).
Horn L. N., Nghituwamhata S. N., Isabella U. (2022). Cowpea production challenges and contribution to livelihood in sub-saharan region. Agric. Sci. 13, 25–32. doi: 10.4236/as.2022.131003
Horn L. N., Shimelis H. (2020). “Production constraints and breeding approaches for cowpea improvement for drought prone agro-ecologies in Sub-Saharan Africa,” in Annals of Agricultural Sciences, vol. 65. (Egypt: Faculty of Agriculture, Ain-Shams University), 83–91). doi: 10.1016/j.aoas.2020.03.002
Hung C. Y., Zhang J., Bhattacharya C., Li H., Kittur F. S., Oldham C. E., et al. (2021). Transformation of long-lived albino Epipremnum aureum ‘Golden Pothos’ and restoring chloroplast development. Front. Plant Sci. 12, 647507. doi: 10.3389/fpls.2021.647507
Ivanova M. V. (2009). Regulation of hyperhydricity in Aloe polyphylla propagated in vitro (Doctoral dissertation) (South Africa: University of KwaZulu-Natal), 1–121.
Jahan A. A., Anis M., Aref I. M. (2011). Preconditioning of axillary buds in thidiazuron- supplemented liquid media improves in vitro shoot multiplication in Nyctanthes arbor- tristis L. Appl. Biochem. Biotechnol. 163, 851–859. doi: 10.1007/s12010-010-9089-7
Jan T., Gul S., Khan A., Pervez S., Noor A., Amin H., et al. (2021). Range of factors in the reduction of hyperhydricity associated with in vitro shoots of salvia santolinifolia bioss. Braz. J. Biol. 83, e246904. doi: 10.1590/1519-6984.246904
Ji J., Zhang C., Sun Z., Wang L., Duanmu D., Fan Q. (2019). Genome editing in cowpea Vigna unguiculata using CRISPR-cas9. Int. J. Mol. Sci. 20. doi: 10.3390/ijms20102471
Jinbaani A. N., Owusu E. Y., Mohammed A.-R., Tengey T. K., Mawunya M., Kusi F., et al. (2023). Gender trait preferences among smallholder cowpea farmers in northern Ghana: lessons from a case study. Front. Sociol 8. doi: 10.3389/fsoc.2023.1260407
Juranić M., Nagahatenna D. S. K., Salinas-Gamboa R., Hand M. L., Sánchez-León N., Leong W. H., et al. (2020). A detached leaf assay for testing transient gene expressionand gene editing in cowpea (Vigna unguiculata [L.] Walp.). Plant Methods 16. doi: 10.1186/s13007-020-00630-4
Kaur A., Sharma M., Sharma C., Kaur H., Kaur N., Sharma S., et al. (2016). Pod borer resistant transgenic pigeon pea (Cajanus cajan L.) expressing cry1Ac transgene generated through simplified Agrobacterium transformation of pricked embryo axes. Plant Cell Tissue Organ Culture 127, 717–727. doi: 10.1007/s11240-016-1055-9
Kim Y.-H., Janick J. (1989). ABA and polyox-encapsulation or high humidity increases survival of desiccated somatic embryos of celery. HortScience 24, 674–676.
Krishna H., Alizadeh M., Singh D., Singh U., Chauhan N., Eftekhari M., et al. (2016). Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech. 6, 1–18. doi: 10.1007/s13205-016-0389-7
Kumar S., Mohapatra T. (2021). Dynamics of DNA methylation and its functions in plant growth and development. Front. Plant Sci. 12, 1–17. doi: 10.3389/fpls.2021.596236
Kumar S., Mukherjee S. K., Sahoo L. (2022). A Method for developing RNAi-derived resistance in cowpea against geminiviruses. Methods Mol. Biol. 2408, 191–210. doi: 10.1007/978-1-0716-1875-2_13\
Kumari P., Singh S., Yadav S., Tran L. P. (2021). Influence of different types of explants in chickpea regeneration using thidiazuron seed-priming. J. Plant Res. 134, 1149–1154. doi: 10.1007/s10265-021-01312-5
Lawali S., Boureima S., Idi S. (2024). A gender-responsive breeding approach to the intensification of sesame (Sesamum indicum L.) production in the Maradi region of Niger. Front. Sociol. 9. doi: 10.3389/fsoc.2024.1254094
Lelu M. A., Bastien C., Drugeault A., Gouez M. L., Klimaszewska K. (1999). Somatic embryogenesis and plantlet development in Pinus sylvestris and Pinus pinaster on medium with and without growth regulators. Physiol. Plant 105, 719–728. doi: 10.1034/j.1399-3054.1999.105417.x
Li F., Yao J., Hu L., Chen J., Shi J. (2022). Multiple methods synergistically promote the synchronization of somatic embryogenesis through suspension culture in the new hybrid between Pinus elliottii and Pinus caribaea. Front. Plant Sci. 13, 857972. doi: 10.3389/fpls.2022.857972
Lonardi S., Muñoz-Amatriaín M., Liang Q., Shu S., Wanamaker S. I., Lo S., et al. (2019). The genome of cowpea (Vigna unguiculata [L.] Walp.). Plant J. 98, 767–782. doi: 10.1111/TPJ.14349
MacWilliams J. R., Nabity P. D., Mauck K. E., Kaloshian I. (2023). Transcriptome analysis of aphid-resistant and susceptible near isogenic lines reveals candidate resistance genes in cowpea (Vigna unguiculata). BMC Plant Biol. 23 (1), 22. doi: 10.1186/s12870-022-04021-w
Manman T., Qian L., Huaqiang T., Yongpeng Z., Jia L., Huanxin L. (2013). A review of regeneration and genetic transformation in cowpea (Vigna unguiculata L. Walp). African. J. Agric. Res. 8, 1115–1122. doi: 10.5897/AJAR12.2059
Market Data Forecast (2024). Cowpeas Market (India: Market Data Forecast). Available at: https://www.marketdataforecast.com/market-reports/global-cowpeas-market (Accessed August 09, 2024).
Markin G., Eleblu J. S., Amissah J. N., Reynolds S., Soraru C., Craze M. S., et al. (2023). Plant fruit extracts enhance the in vitro propagation of cowpea (Vigna unguiculata) on Murashige and Skoog media. Plant Cell Tissue Organ Culture 155, 81–90. doi: 10.1007/s11240-023-02554-y
Marubodee R., Ogiso-Tanaka E., Isemura T., Chankaew S., Kaga A., Naito K., et al. (2015). Construction of an SSR and RAD-marker based molecular linkage map of vigna vexillata (L.) A. Rich. PloS One 10, e0138942. doi: 10.1371/journal.pone.0138942
Mendez-Hernandez H. A., Ledezma-Rodriguez M., Avilez-Montalvo R. N., Juarez- Gomez Y. L., Skeete A., Avilez-Montalvo J., et al. (2019). Signaling overview of plant somatic embryogenesis. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00077
Mir R. R., Zaman-Allah M., Sreenivasulu N., Trethowan R., Varshney R. K. (2012). Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops. Theor. Appl. Genet. 125, 625–645. doi: 10.1007/s00122-012-1904-9
Mofokeng M. A., Gerrano A. S. (2021). Efforts in breeding cowpea for aphid resistance: A review. Acta Agriculturae Scandinavica Section B—Soil Plant Sci. 71 (6), 489–497. doi: 10.1080/09064710.2021.1923797
Mosoh D. A., Khandel A. K., Verma S. K., Vendrame W. A. (2024). Optimizing callus induction and indirect organogenesis in non-dormant corm explants of Gloriosa superba (L.) via media priming. Front. Horticulture 3, 1378098. doi: 10.3389/fhort.2024.1378098
Murillo-Amador B., Troyo-Diéguez E., García-Hernández J. L., López-Aguilar R., Ávila- Serrano N. Y., Zamora-Salgado S., et al. (2006). Effect of NaCl salinity in the genotypic variation of cowpea (Vigna unguiculata) during early vegetative growth. Scientia Hortic. 108, 423–431. doi: 10.1016/j.scienta.2006.02.010
Nair R. M., Pujar M., Cockel C., Scheldeman X., Vandelook F., van Zonneveld M., et al. (2023). Global strategy for the conservation and use of Vigna (Bonn, Germany: Global Crop Diversity Trust). doi: 10.5281/zenodo.7565174
Nanhapo P. I., Valombola J. S., Wanga M. A., Elungi K., Awala S. K. (2024). Optimum gamma radiation doses to enhance genetic diversity in selected cowpea (Vigna unguiculata) genotypes. Reprod. Breed. 4 (2), 83–87. doi: 10.1016/j.repbre.2024.01.001
Nkomo G. V., Sedibe M. M., Mofokeng M. A. (2021). Production constraints and improvement strategies of cowpea (Vigna unguiculata L. Walp.) genotypes for drought Tolerance. Int. J. Agron. 5536417, 1–9. doi: 10.1155/2021/5536417
Nkhoma N., Shimelis H., Laing M. D., Shayanowako A., Mathew I. (2020). Assessing the genetic diversity of cowpea [Vigna unguiculata (L.) Walp.] germplasm collections using phenotypic traits and SNP markers. BMC Genet. 21, 1–16. doi: 10.1186/s12863-020-00914-7
Nunes C., Moreira R., Pais I., Semedo J., Simões F., Veloso M. M., et al. (2022). Cowpea physiological responses to terminal drought–comparison between four landraces and a commercial variety. Plants 11 (5), 593. doi: 10.3390/plants11050593
Nunes S., Marum L., Farinha N., Pereira V. T., Almeida T., Dias M. C., et al. (2017). Plant regeneration from ploidy-stable cryopreserved embryogenic lines of the hybrid Pinus elliottii x P. caribaea. Ind. Crops Products 105, 215–224. doi: 10.1016/j.indcrop.2017.05.015
Nzama P. S., Myburg A. A., Hills P. N. (2024). Synergistic effects of L-glutamine and inorganic nitrogen molar ratios enhance the induction of somatic embryogenesis of Pinus maximinoi H.E. Moore. Plant Cell Tissue Organ Culture 157, 1–15. doi: 10.1007/s11240-024-02748-y
Odeigah P. G. C., Osanyinpeju A. O., Myers G. O. (1998). Induced mutations in cowpea, Vigna unguiculata (Leguminosae). Revista de biología tropical 46 (3), 579–586.
Omoigui L., Kamara A., Kamai N., Ekeleme F., Aliyu T. (2020). Guide To Cowpea Production in Nigeria: Feed the Future Nigeria Integrated Agriculture Activity Transforming African Agriculture. Ibadan, Nigeria: International Institute for Tropical Agriculture. Available at: www.iita.org (Accessed September 20, 2024).
Omomowo O. I., Babalola O. O. (2021). Constraints and prospects of improving cowpea productivity to ensure food, nutritional security and environmental sustainability. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.751731
Owusu E. Y., Kusi F., Kena A. W., Akromah R., Attamah P., Awuku F. J., et al. (2022). Genetic control of earliness in cowpea (Vigna unguiculata (L) Walp). Heliyon 8. doi: 10.1016/j.heliyon.2022.e09852
Padulosi S., Ng N. Q. (1990). Wild Vigna species in Africa. Their collection and potential utilization, in Cowpea Genetic Resources. Eds. Ng N. Q., Monti L. M. (Ibadan, Nigeria: International Institute of Tropical Agriculture), 58–77.
Panzeri D., Nissim W. G., Labra M., Grassi F. (2022). Revisiting the domestication process of african vigna species (Fabaceae): background, perspectives and challenges. Plants 11. doi: 10.3390/plants11040532
Parth D., Shikha D., Rutul R., Ghanshyam P. (2022). “Chapter 5 - Plant tissue culture: Somatic embryogenesis and organogenesis,” in Advances in Plant Tissue Culture. Eds. Rai A. C., Kumar A., Modi A., Singh L. (Oxford: Academic Press), 109–130.
Phillip D., Nin-Pratt A., Zambrano P., Wood-Sichra U., Kato E., Komen J., et al. (2019). Insect-resistant Cowpea in Nigeria: an ex ante economic assessment of a crop improvement initiative (Discussion paper. 1896) (Washington DC: International Food Policy Research Institute). doi: 10.2499/p15738coll2.133541
Polivanova O. B., Bedarev V. A. (2022). Hyperhydricity in plant tissue culture. Plants 11, 1–12. doi: 10.3390/plants11233313
Popelka J. C., Gollasch S., Moore A., Molvig L., Higgins T. J. V. (2006). Genetic transformation of cowpea (Vigna unguiculata L.) and stable transmission of the transgenes to progeny. Plant Cell Rep. 25, 304–312. doi: 10.1007/s00299-005-0053-x
Pratap A., Prajapati U., Singh C. M., Gupta S., Rathore M., Malviya N., et al. (2018). Potential, constraints and applications of in vitro methods in improving grain legumes. Plant Breed. 137, 235–249. doi: 10.1111/pbr.2018.137.issue-3
Ramakrishnan K., Gnanam R., Sivakumar P., Manickam A. (2005). In vitro somatic embryogenesis from cell suspension cultures of cowpea [Vigna unguiculata (L.) Walp. Plant Cell Rep. 24, 449–461. doi: 10.1007/s00299-005-0965-5
Raveendar S., Premkumar A., Sasikumar S., Ignacimuthu S., Agastian P. (2009). Development of a rapid, highly efficient system of organogenesis in cowpea Vigna unguiculata (L.) Walp. South Afr. J. Bot. 75, 17–21. doi: 10.1016/j.sajb.2008.05.009
Ravelombola W., Shi A., Chen S., Xiong H., Yang Y., Cui Q., et al. (2020). Evaluation of cowpea for drought tolerance at seedling stage. Euphytica 216, 1–19. doi: 10.1007/s10681-020-02660-4
Ravelombola W., Shi A., Huynh B. L., Qin J., Xiong H., Manley A., et al. (2022). Genetic architecture of salt tolerance in a Multi-Parent Advanced Generation Inter-Cross (MAGIC) cowpea population. BMC Genomics 23. doi: 10.1186/s12864-022-08332-y
Rawal K. M., Rachie K. O., Franckowiak J. D. (1976). Reduction in seed size in crosses between wild and cultivated cowpea. J. Hered. 67, 253–254. doi: 10.1093/oxfordjournals.jhered.a108725
Reeves C., Hargreaves C., Trontin J. F., Lelu-Walter M. A. (2018). Simple and efficient protocols for the initiation and proliferation of embryogenic tissue of Douglas-fir. Trees 32, 175–190. doi: 10.1007/s00468-017-1622-7
Research and Markets (2022). Pigeon Pea Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2022-2027 Research and Markets. Available online at: https://www.researchandmarkets.com/reports/5578021/pigeon-pea-market-global-industry-trends (Accessed April 30, 2024).
Rival A., Ilbert P., Labeyrie A., Torres E., Doulbeau S., Personne A., et al. (2013). Variations in genomic DNA methylation during the long-term in vitro proliferation of oil palm embryogenic suspension cultures. Plant Cell Rep. 32, 359–368. doi: 10.1007/s00299-012-1369-y
Rugini E., Cristofori V., Silvestri C. (2016). Genetic improvement of olive (Olea europaea l.) by conventional and in vitro biotechnology methods. Biotechnol. Adv. 34 (5), 687–696. doi: 10.1016/j.biotechadv.2016.03.004
Sahoo L., Jaiwal P. K. (2008). “Biotechnology of Vigna,” in A compendium of transgenic crop plants. Eds. Kole C., Hall T. C. (Oxford: Blackwell), p. 115–132.
Salaj T., Matusikova I., Fraterova L., Pirselova B., Salaj J. (2011). Regrowth of embryogenic tissues of Pinus nigra following cryopreservation. Plant Cell Tissue Organ Culture 106, 55–61. doi: 10.1007/s11240-010-9893-3
Sani L. A., Usman I. S., Bugaje S. M. (2018). Optimization of protocol for multiple shoot regeneration from apical meristem of embryogenic axes in cowpea (Vigna unguiculataL. Walp). Bayero J. Pure Appl. Sci. 10, 428–433. doi: 10.4314/bajopas.v10i1.84S
Sehaole E. M., Mangena P. (2024). N6-benzyladenine (BAP)-Based seed preconditioning enhances the shoot regeneration of seedling-derived explants for subsequent indirect gene transfer in soybeans (Glycine max [L.] merrill.). Int. J. Plant Biol. 15, 254–266. doi: 10.3390/ijpb15020022
Singh R., van Heusden A. W., Kumar R., Visser R. G., Yadav R. C. (2013). Genetic diversity of mungbean (Vigna radiata L.) in iron and zinc content as impacted by farmers' varietal selection in Northern India. Ecol. Food Nutr. 52, 148–162. doi: 10.1080/03670244.2012.706006
Singh A., Mamo T., Singh A., Mahama A. A. (2023). “Chapter 9: Cowpea Breeding,” in Crop Improvement (United States of America: Iowa State University Digital Press). Available at: https://iastate.pressbooks.pub/cropimprovement/chapter/cowpea-breeding/ (Accessed June 1, 2024).
Singh S., Mishra V., Riyazuddin R., Chugh V., Upadhyay S. K. (2024). CRISPR/Cas9- mediated genome editing for trait improvement and stress tolerance in Leguminosae (legume family). Plant Breed., 1–19. doi: 10.1111/pbr.13234
Sivakumar P., Rajesh S., Gnanam R., Manickam A. (2011). Effect of in vitro culture conditions on somaclonal variation in cowpea (Vigna unguiculata Walp.) using RAPD markers. Acta Biologica Hungarica 62, 34–44. doi: 10.1556/ABiol.61.2011.1.3
Smetana O., Mäkilä R., Lyu M., Amiryousefi A., Sánchez Rodríguez F., Wu M. F., et al. (2019). High levels of auxin signalling define the stem-cell organizer of the vascular cambium. Nature 565 (7740), 485–489. doi: 10.1038/s41586-018-0837-0
Song X., Xu Z., Zhang J., Liang L., Xiao J., Liang Z., et al. (2023). NO and GSH alleviate the inhibition of low-temperature stress on cowpea seedlings. Plants 12. doi: 10.3390/plants12061317
Sugimoto K., Jiao Y., Meyerowitz E. M. (2010). Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev. Cell 18, 463–471. doi: 10.1016/j.devcel.2010.02.004
Sun S., Zhong J., Li S., Wang X. (2013). Tissue culture-induced somaclonal variation of decreased pollen viability in torenia (Torenia fournieri Lindi.). Botanical Stud. 54, 36. doi: 10.1186/1999-3110-54-35
Taffouo V. D., Kouamou J. K., Ngalangue L. M. T., Ndjeudji B. A. N., Akoa A. (2009). Effects of salinity stress on growth, ions partitioning, and yield of some cowpea (Vigna Unguiculata L. Walp) cultivars. Int. J. Bot. 5, 135–143. Available at: https://scialert.net/fulltext/?doi=ijb.2009.135.143:~:text=The%20results%20obtained%20during%20vegetative,in%20environments%20with%20varying%20salinity (Accessed May 5, 2024).
Tang Y., Chen L., Li X. M., Li J., Luo Q., Lai J., et al. (2012). Effect of culture conditions on the plant regeneration via organogenesis from cotyledonary node of cowpea (Vigna unguiculata L. Walp). Afr. J. Biotechnol. 11, 3270–3275. doi: 10.5897/AJB11.3214
Tang Q., Guo X., Zhang Y., Li Q., Chen G., Sun H., et al. (2022). An optimized protocol for indirect organogenesis from root explants of Agapanthus praecox subsp. orientalis ‘Big Blue’. Horticulturae 8, 715. doi: 10.3390/horticulturae8080715
Tarawali S. A., Singh B. B., Peters M., Blade. S. F. (1997). Cowpea haulms as fodder. Pages 313-325 in Advances in cowpea research. Eds. Singh B. B., Raj D. R. M., Dashiell K. E., Jackai L. E. N. (Ibadan, Nigeria: Co-publication of International Institute of Tropical Agriculture (IITA) and Japan International Research Center for Agricultural Sciences (JIRCAS).
Tengey T. K., Gyamfi R. A., Sallah E. K., Issahaku M., Ndela D. N., Seidu M., et al. (2023). Seedling stage drought screening of candidate cowpea (Vigna unguiculata (L) Walp.) genotypes. Cogent Food Agric. 9. doi: 10.1080/23311932.2023.2212463
Tetteroo F. A., de Bruijn A. Y., Henselmans R. N., Wolkers W. F., van Aelst A. C., et al. (1996). Characterization of membrane properties in desiccation-tolerant and-intolerant carrot somatic embryos. Plant Physiol. 111 (2), 403–412. doi: 10.1104/pp.111.2.403
Thomas T. D. (2008). The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 26, 618–631. doi: 10.1016/j.biotechadv.2008.08.003
Togola A., Boukar O., Servent A., Chamarthi S., Tamò M., Fatokun C. (2020). Identification of sources of resistance in cowpea mini core accessions to Aphis craccivora Koch (Homoptera: Aphididae) and their biochemical characterization. Euphytica 216. doi: 10.1007/s10681-020-02619-5
Togola A., Datinon B., Laouali A., Traoré F., Agboton C., Ongom P. O., et al. (2023). Recent advances in cowpea IPM in West Africa. Front. Agron. 5. doi: 10.3389/fagro.2023.1220387
Tzfira T., Jensen C. S., Wang W., Zuker A., Vinocur B., Altman A., et al. (1997). Transgenic Populus tremula: a step-by-step protocol for its Agrobacterium- mediated transformation. Plant Mol. Biol. Rep. 15, 219–235. doi: 10.1023/A:1007484917759
Vahdati K., Bayat S., Ebrahimzadeh H., Jariteh M., Mirmasoumi M. (2008). Effect of exogenous ABA on somatic embryo maturation and germination in persian walnut (Juglans regia l). Plant Cell Tissue Organ Culture 93, 163–171. doi: 10.1007/s11240-008-9355-3
Vollmann J. (2016). Soybean versus other food grain legumes: A critical appraisal of the United Nations International Year of Pulses 2016. Bodenkultur: J. Land Management Food Environ. 67, 17–24. doi: 10.1515/boku-2016-0002
Wabwayi N., Odhiambo J. A., Basweti E. A. (2020). Evaluation of cowpea rust disease incidence and severity on selected cowpea genotypes in Western Kenya. Afr. J. Agric. Res. 16, 1015–1024. doi: 10.5897/ajar2020.14877
Wijerathna-Yapa A., Hiti-Bandaralage J. (2023). Tissue culture—A sustainable approach to explore plant stresses. Life 13, 780. doi: 10.3390/life13030780
Zhao Y., Grout B., Crisp P. (2005). Variations in morphology and disease susceptibility of micropropagated rhubarb (Rheum rhaponticum) PC49, compared to conventional plants. Plant Cell Tissue Organ Culture 82, 357–361. doi: 10.1007/s11240-005-1836-z
Zimmerman J. L. (1993). Somatic embryogenesis: A model for early development in higher plants. Plant Cell 5, 1411–1423. Available at: https://academic.oup.com/plcell/article/5/10/1411/5984653 (Accessed July 1, 2024).
Keywords: organogenesis, somatic embryogenesis, new breeding techniques (NBTs), tissue culture, cowpea
Citation: Vacu MV, Nzama PS and Adegbaju MS (2025) Insights on the utilisation of tissue culture to aid new breeding techniques for cowpea (Vigna unguiculata L.) improvement. Front. Hortic. 4:1520119. doi: 10.3389/fhort.2025.1520119
Received: 30 October 2024; Accepted: 03 January 2025;
Published: 07 February 2025.
Edited by:
Phetole Mangena, University of Limpopo, South AfricaReviewed by:
Cristian Silvestri, University of Tuscia, ItalyCopyright © 2025 Vacu, Nzama and Adegbaju. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Malizukiswe Vincent Vacu, VmluY2VudC52YWN1QHVsLmFjLnph
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.