- Pest and Pathogen Ecology, NIAB at East Malling, Kent, United Kingdom
European apple canker, caused by Neonectria ditissima, is a damaging disease of apple in many production regions worldwide. The pathogen infects apple trees through artificial or natural wounds. The most damaging phase of the disease is that cankers on main stems post-planting, most likely originating from infection in nurseries, can result in tree death in young orchards. Apple cultivars differ in their responses to the pathogen, which may be additionally affected by specific site factors. An experiment was conducted to study i) the susceptibility of seven cultivars to N. ditissima at three sites and ii) the effects of cold storage duration prior to planting on subsequent development of both main stem and peripheral cankers. Planting date had significant (albeit minimal effects) on the development of peripheral cankers only. Canker development differed greatly among the three sites and between the tested cultivars, with ‘Grenadier’ and ‘Golden Delicious’ being most resistant at all sites. The relative performance of cultivars in terms of canker development was generally consistent across the three sites. Nevertheless, the interaction between cultivar and site was still statistically significant for the development of main stem cankers, indicating that some site-specific factors may interact with cultivars to affect development of latent infections. Given the close proximity of the three sites (similar climatic conditions), the results indicate that further research is needed to investigate the effects of soil characteristics on canker development post-planting.
Introduction
European apple canker, caused by Neonectria ditissima, is one of the most damaging diseases of apple trees worldwide. N. ditissima has a complex lifecycle with potential for all year-round production of ascospores and conidia, which infect wounds; it also infects fruit, leading to losses in store as a post-harvest rot (Saville and Olivieri, 2019). The most damaging phase of the disease is the canker on the main trunk of a young tree in newly established orchards, most of which result from infection in nurseries but remain latent until post-planting in orchards (McCracken et al., 2003; Børve et al., 2019). Modern nurseries apply high inputs of fungicide, nutrients and water to encourage vigorous growth in the first two years, which may mask latent infection by N. ditissima. Upon abiotic and biotic stress experienced by the trees in cold-store, in transit and during the post-planting establishment stage, these latent infections may develop into canker lesions. Modern intensive fruit wall orchard systems (c. 3000 trees/ha), where trees are much smaller than in traditional orchards coupled with popular modern varieties being much more susceptible, might have partially contributed to some observed high incidences of tree death from trunk cankers during orchard establishment.
There are many types of entry sites for the pathogen, the main ones being pruning cuts, picking and leaf scars (Amponsah et al., 2015), with as little as three spores required for infection of large wounds (Walter et al., 2016). Leaf scars and pruning cuts are considered particularly important because they occur all year round (Xu et al., 1998; McCracken et al., 2003). Following infection by N. ditissima, trees may remain asymptomatic for a period of time, ranging from a few weeks to several years (McCracken et al., 2003). The length of the incubation period decreases with increasing wound size (Amponsah et al., 2015) and inoculum doses (Walter et al., 2016; Xu et al., 1998). The long incubation time leads to the phenomenon where canker symptoms originating from nursery infections only become visible post-planting (McCracken et al., 2003; Børve et al., 2019).
Current methods of control, based on protective fungicides, are only partially effective, and non-sustainable; furthermore, a number of effective fungicides against the disease are no longer permitted (Walter et al., 2015, Walter et al., 2019). In the absence of existing biological fungicides providing disease control, current recommendations rely mainly on pruning wound protection with pruning paints, and leaf scar protection using copper- and captan-based fungicides in combination with inoculum control (Walter et al., 2015, Walter et al., 2019, Weber & Børve, 2021). An integrated approach for managing N. ditissima is urgently needed (Saville and Olivieri, 2019).
Commercial varieties that satisfy current grower and consumer preferences do not have effective resistance against N. ditissima. Although absolute host resistance has not been observed for N. ditissima, quantitative differences between genotypes in their response to this pathogen have been determined (Gomez-Cortecero et al., 2016; Bus et al., 2019). Breeding cultivars with durable resistance against N. ditissima remains a long-term goal. In addition, there is evidence for inconsistencies in the relative performance of cultivars in their canker susceptibility between studies (Gomez-Cortecero et al., 2016; Scheper et al., 2018). It is not yet clear to what extent the relative performance of cultivars in terms of their susceptibility to N. ditissima is affected by site specific conditions.
One essential aspect of canker management is to minimise canker development during the post-planting establishment stage (12-24 months), particularly those cankers on main stems most likely originating from latent infection in nurseries. The experience of the UK apple growing industry suggests that specific sites are particularly prone to canker expression following planting. In addition, growers’ experience indicates that lengthening the time trees spent in cold storage between lifting and planting could worsen canker development in orchards.
The present research had three specific objectives: (1) to determine the extent of site differences in post-planting canker development, (2) to assess the extent to which cultivar differences in canker development are consistent across sites, and (3) to determine the effect of tree cold-storage duration on post-planting canker development. Seven scion cultivars grafted onto one rootstock produced in the same field of the same nursery were inoculated in the nursery, planted at three sites and assessed for canker development for 30 months after planting.
Materials and methods
Treatments and experimental design
Seven scion apple cultivars, grafted to ‘M9 T337’ rootstocks, with varying susceptibility to N. ditissima, were planted at three sites in Kent, UK. The present research aims to study the effects of cultivars and planting dates on canker development rather than site specific factors. Thus the three sites were selected because of historical severe canker development. Scion varieties were ‘Royal Gala’, ‘Braeburn’, ‘Scifresh’, ‘Nicoter’, ‘Civni’, ‘Grenadier’, and ‘Golden Delicious’. ‘Scifresh’, ‘Nicoter’ and ‘Civni’ are regarded as highly susceptible to N. ditissima whereas ‘Grenadier’ and ‘Golden Delicious’ tolerant/resistant against N. ditissima. Half of the trees were planted in December 2018, immediately after trees were lifted from the nursery (FPM [Frank P Matthews], Hereford, UK). The other half of trees were stored in a misted cold store at +2°C until April 2019 when they were planted out at the same sites.
At each site, there were 504 trees, half of which were planted in December 2018 and the other half in April 2019. There were 72 trees per scion per site. A split-plot design was used for the experiment at each site. Within each site, there were six blocks; within each block there were seven plots, each with two subplots. Within each block, each plot was randomly assigned to one of seven cultivars; within each plot, each subplot was randomly assigned to one of the two planting times. There were six trees per subplot. For some blocks, there were insufficient number of trees for specific cultivars due to tree mortality in the nursery; these gaps were filled with other scion varieties, which was fully taken into account in data analysis. The experiment was terminated in August 2021.
Plants and management
In November 2018 (at approximately 50% leaf fall) whilst still growing at the nursery, all trees were sprayed with a moderate level of N. ditissima conidial suspension (10-4 macro conidia per ml at 500 L per ha). This inoculation was used to ensure a moderate and uniform level of latent infections of leaf scars by N. ditissima, increasing the chance of useful data to be collected.
Trees were planted at three orchards in East Sutton (Site 1), Brenchley (Site 2), and Pluckley (Site 3), Kent, UK. Table 1 shows the location of three orchards as well as summary of soil analysis for the three orchards.
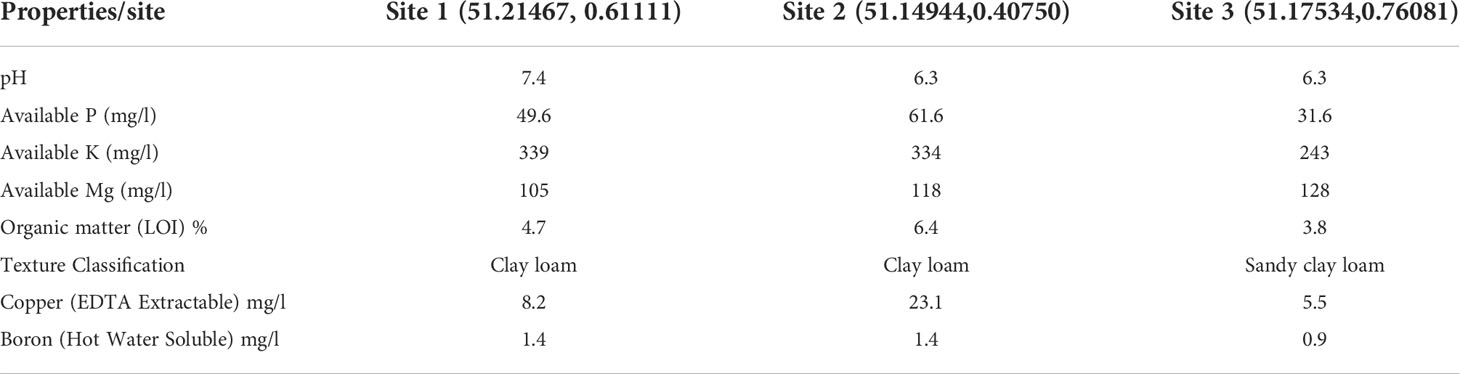
Table 1 Summary of soil analysis for each of the three experimental orchards at the end of the experiment.
The experimental plantings were managed following commercial practices except for: i) no products were specifically used to treat N. ditissima, such as fungicide treatment at leaf-fall, ii) pruning was not conducted (to prevent cankers from being removed), and iii) canker lesions were not cut out or dead trees with cankers removed from the trial sites during the experimental duration.
Canker assessment
Assessments of canker development were carried out four times: October 2019, June and November 2020, and May 2021. On each occasion, all parts of every tree were assessed for visible canker lesions. Number and location of canker lesions on each tree was classified into one of five categories: A – rootstock and graft union canker; B – canker on main trunk above graft union; C – canker on first branch from trunk; D – canker on shoot from C; E – canker on shoot from D) (McCracken et al., 2003).
Statistical analysis
All variables were summarised per subplot basis as number of canker lesions on the main stems (sum of categories A and B) and peripheral branches (sum of categories C, D and E), and number of dead trees due to cankers. On the final assessment date (May 2021), individual trees were classified into one of three categories: visibly healthy, nearly dead, and dead.
Linear mixed models were applied to the data set, in which assessment time, planting time and cultivar were treated as fixed effect factors whereas site, block within site, plot (split plot design) and subplot (experimental unit for repeated measurements) were treated as random effect factors. Number of lesions per tree was first logarithmically transformed before analysis. For tree health status on the last assessment date, data were pooled across all blocks at a given site in order to increase statistical power. Logit transformation was applied to the incidence of dead or healthy trees. Logit-transformed data were then subjected to linear mixed model analysis with the site treated as a random effect factor, and planting time and cultivar as fixed effect factors.
Linear mixed models were fitted with the lme4 (Bates et al., 2015) package in R 4.1.2. Testing for individual model terms was made through the lmerTest package (Kuznetsova et al., 2017): the Kenward-Roger test was used for testing fixed effects whereas the approximate Chi-square test of nested models was used for testing random effects.
Results
Main stem cankers
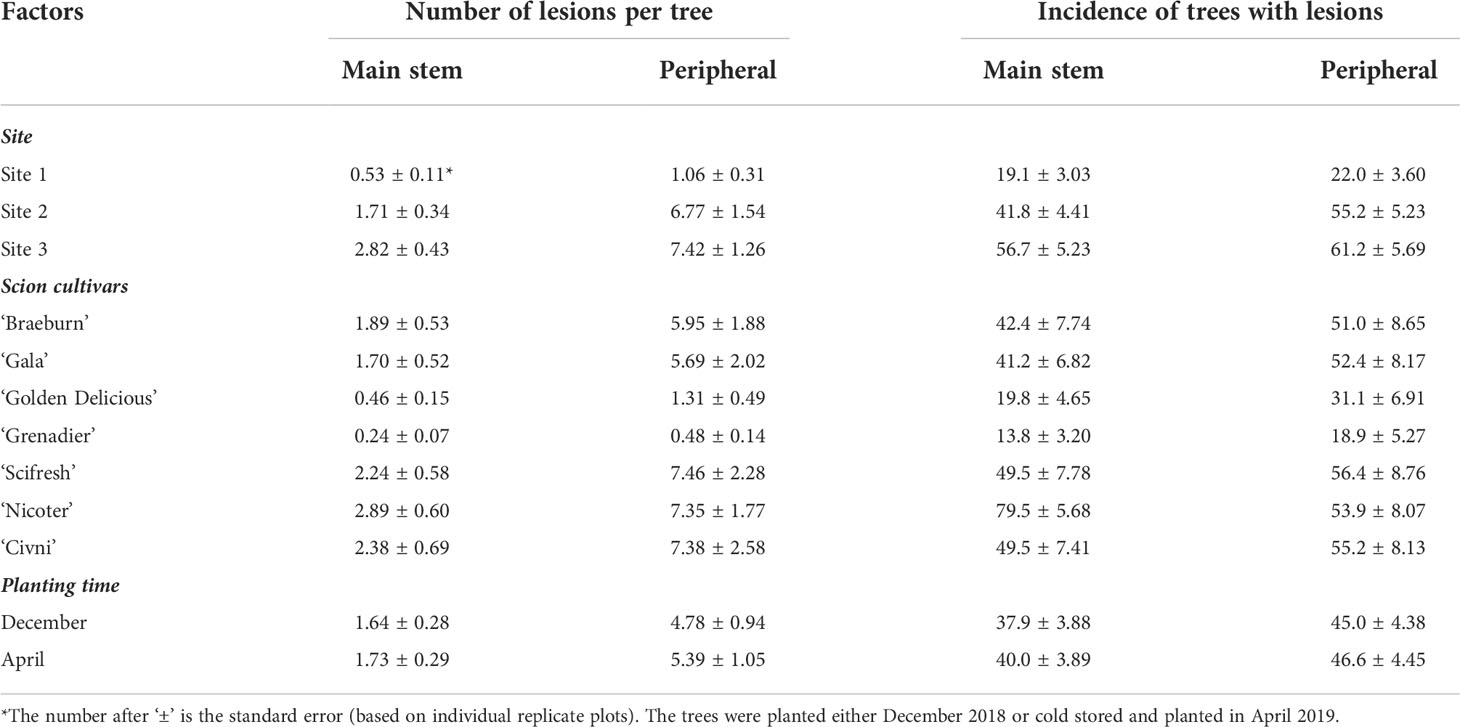
Average number of canker lesions on the main stem across all cultivars was 0.11, 0.72, 1.48 and 4.43 per tree for assessment in October 2019, June 2020, November 2020, and May 2021, respectively. The overall incidence of trees with main stem lesions (category A or B) increased from 6.7% at the first assessment to 64.0% at the last assessment. Canker development varied greatly among the three sites (Table 2). Average number of main stem lesions was 0.53, 1.71 and 2.82 per tree for Site 1, 2 and 3 orchards, respectively. The overall incidence of trees with main trunk lesions was 19.8%, 41.8% and 56.7% for Site 1, 2 and 3 orchards, respectively. The overall differences in the main stem canker development between the two planting times (i.e. storage duration) were small (Table 2).
Seven scion genotypes differed in the canker development (Table 2, Figure 1). Overall, ‘Golden Delicious’ and ‘Grenadier’ had a much lower canker incidence than the other five cultivars. ‘Nicoter’ had a much higher incidence of canker lesions on main trunks (ca. 79.5%) than ‘Braeburn’, ‘Scifresh’, ‘Gala’ and ‘Civni’ (between 40 and 49%), even though the incidence of peripheral cankers was similar for these five scion cultivars (Table 2).
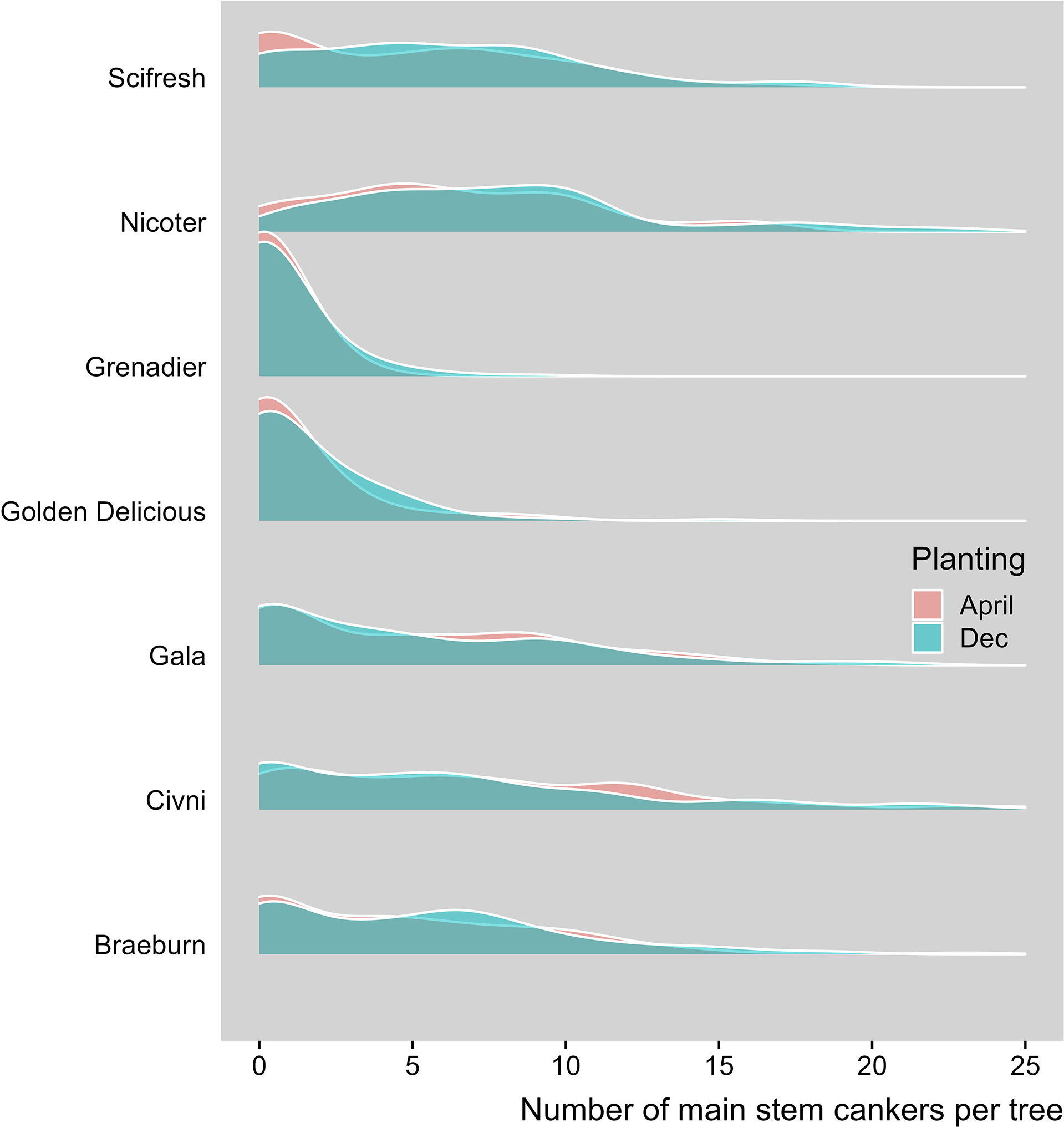
Figure 1 Density plot with number of main stem canker lesions per tree on horizontal aixs and proportion of trees on vertical axis (the final May 2021 assessment). Seven apple scion cultivars planted at three sites where trees were planted either in December 2018 or cold-stored and planted April 2019.
Variance estimates and their statistical significance are given in Table 3; it should be noted that statistical significance was not necessarily correlated with the magnitude of the variance estimate. Thus, although the variance estimate for the site factor was the largest, it was only close to statistical significance (Table 3). On the other hand, variance estimates for both plot and block terms were very small but statistically significant (Table 3). Variance estimates for the site and its interaction with assessment time were greater than the residual variance (Table 3). Figure 2 shows the nature of the interaction between site and assessment time. The increase in canker over time throughout the assessment period was greatest for Site 3 and least for Site 1, irrespective of cultivar and planting time. The interactions of cultivar with site and with both site and assessment time were statistically significant (Table 3). Cultivar differences were most pronounced at Site 3 and least at Site 1 (Figure 2). Variance estimates for any random-effect terms involving the planting time was both small and statistically not significant. Variance estimates for block, plot and subplot were all small although statistically significant in some cases.
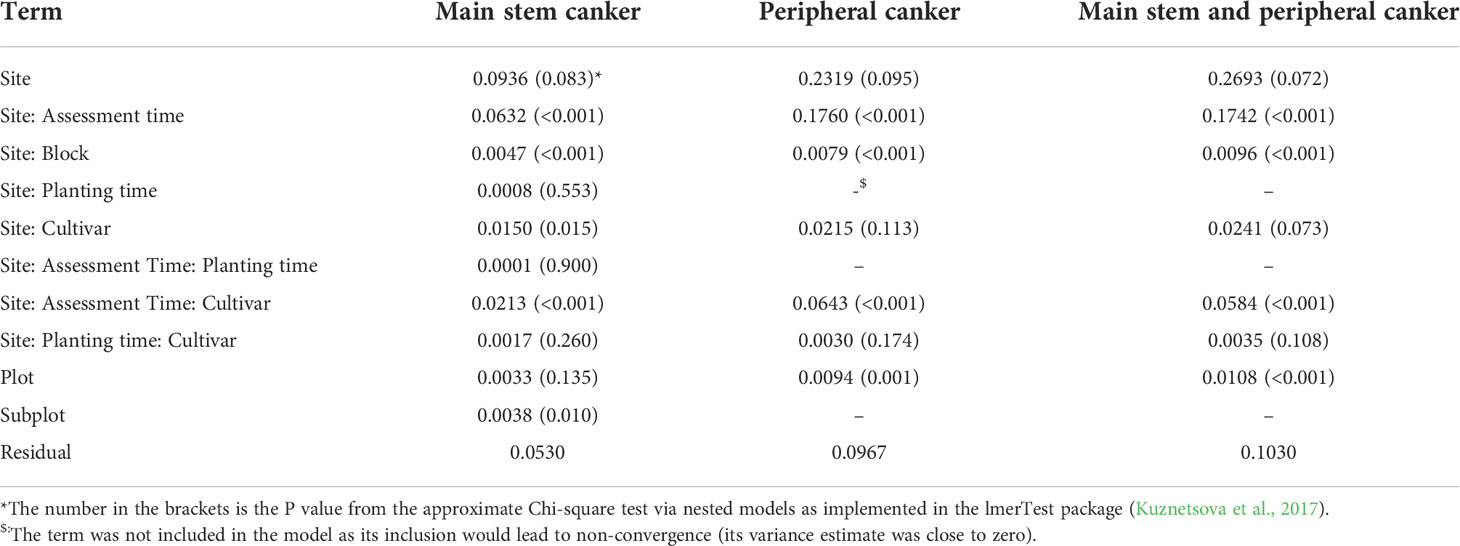
Table 3 Estimates of variances for all random effect factors in the linear mixed model analysis of canker data on individual scion cultivars summarised over individual plots across three sites where trees were planted either in December 2018 or cold-stored and planted in April 2019.
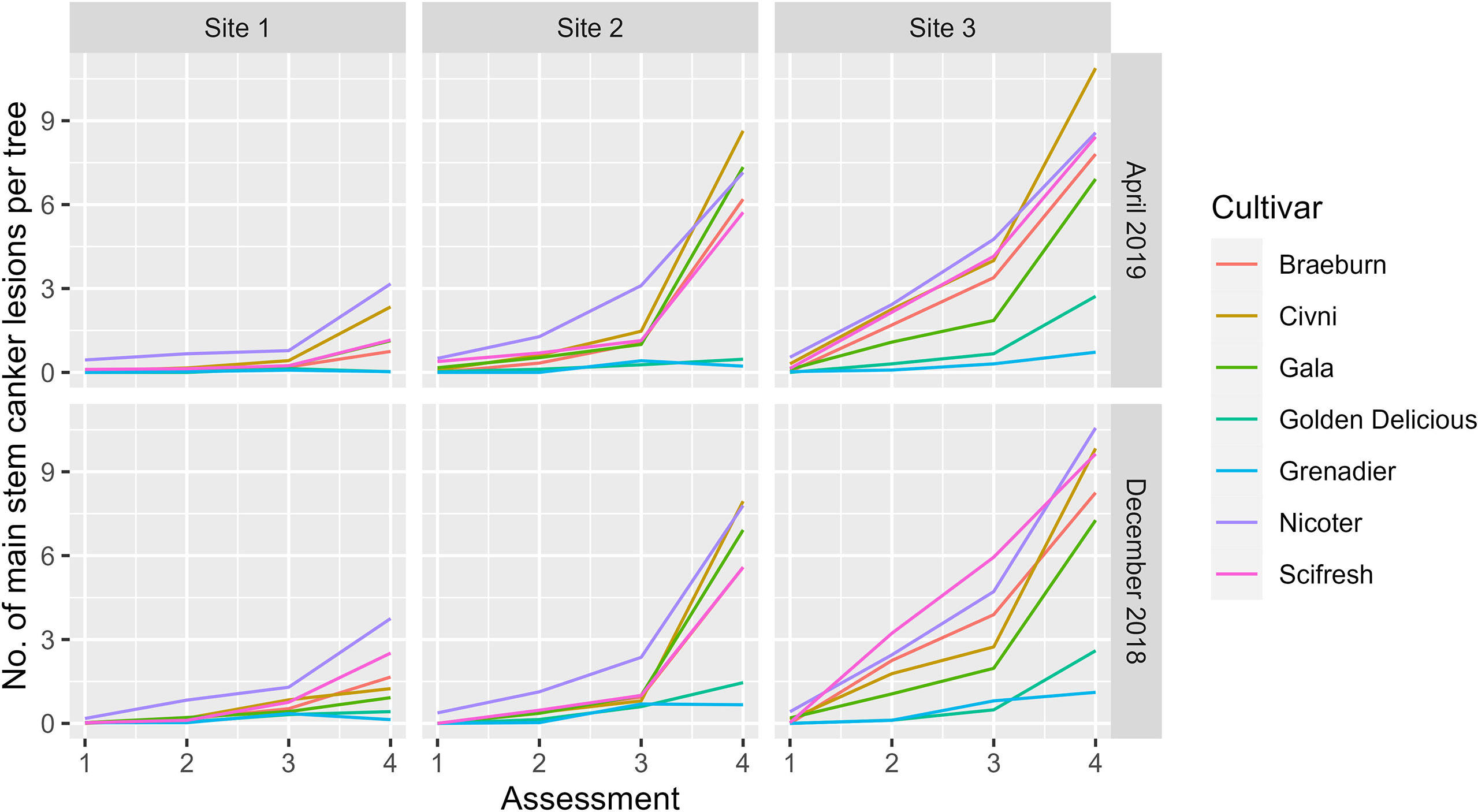
Figure 2 Mean number of main stem canker lesions on individual trees of seven apple scion cultivars across three sites and four assessments (Autumn 2019 (1), Spring 2020 (2), Autumn 2020 (3), and Spring 2021 (4)) Trees were planted either in December 2018 or cold-stored and planted in April 2019.
For the fixed effect factors, both time and cultivar, as well as their interaction, were highly significant (Table 4, Figure 3A). Cultivar differences increased with increasing time interval from planting. This is particularly noticeable for the differences of ‘Grenadier’ and ‘Golden Delicious’ with the other five cultivars on the last assessment date. The interaction of assessment time with planting time was close to statistical significance (P = 0.066); the number of main stem lesions for the December planting increased from 0.07 (1st assessment) to 4.56 (4th assessment) whereas the corresponding values for the April planting were 0.14 and 4.30.
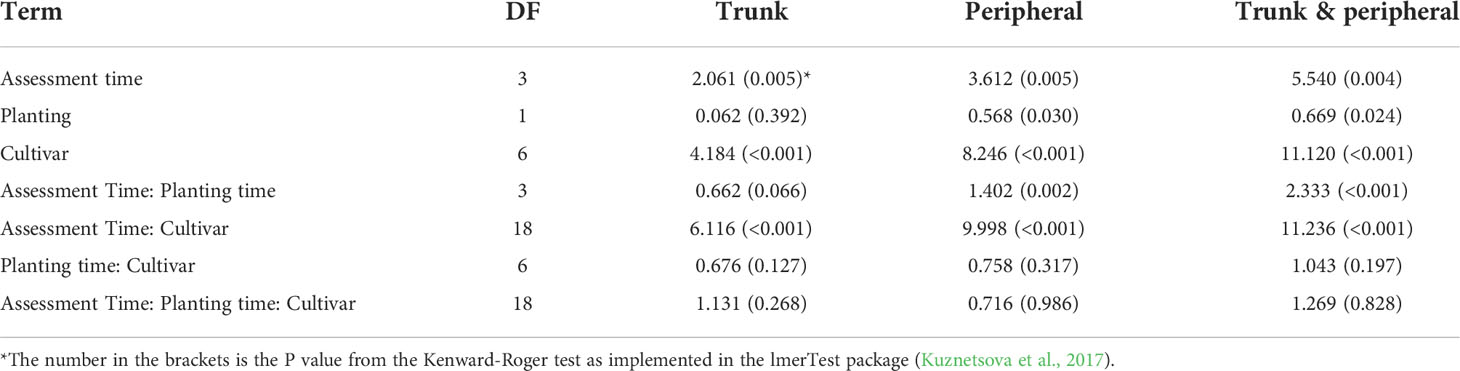
Table 4 Sum of squares of the fixed effect factors in the linear mixed model analysis of canker data on individual scion cultivars summarised over individual plots across three sites where trees were planted either in December 2018 or cold-stored and planted in April 2019.
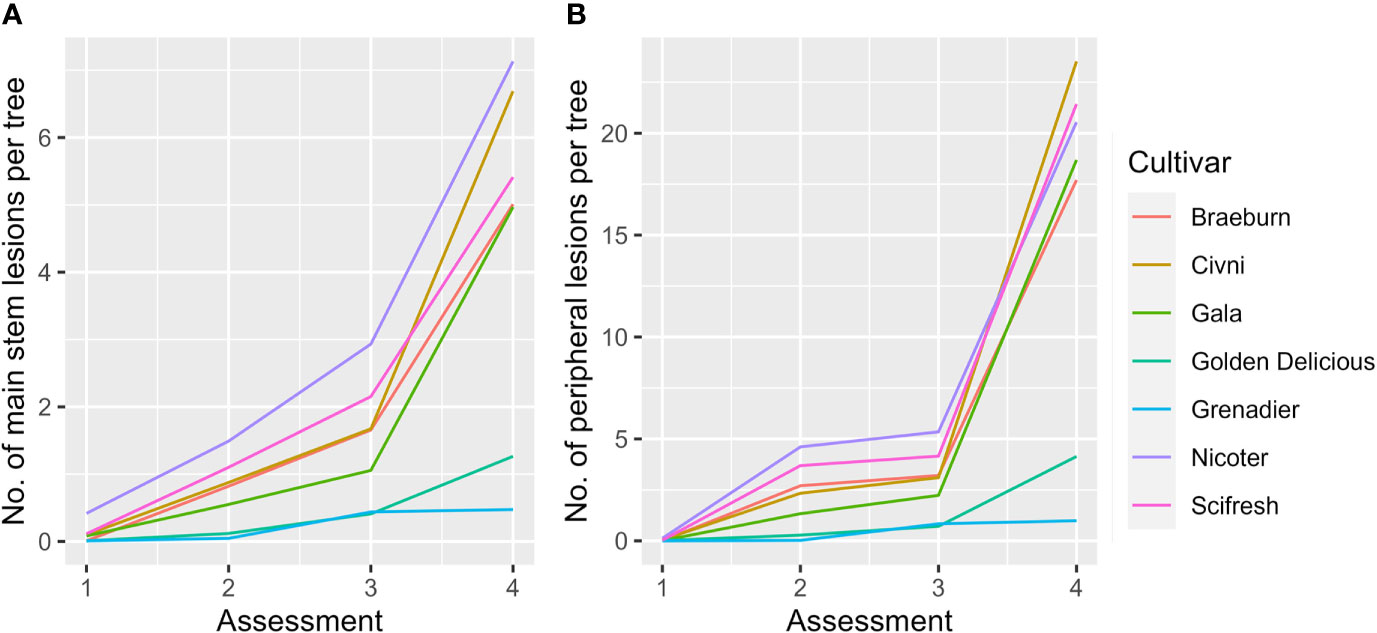
Figure 3 Mean number of main stem (A) and peripheral canker (B) lesions per tree of seven apple scion cultivars across three sites and two planting times. Four assessments were: Autumn 2019 (1), Spring 2020 (2), Autumn 2020 (3), and Spring 2021 (4).
The overall patterns of the incidence of trees with main stem lesions in relation to site, cultivar, planting date and assessment time were similar to those observed for the number of main stem lesions except that incidence values were higher and more variable.
Peripheral cankers
Peripheral canker severity increased with time. Average number of peripheral canker lesions was 0.03, 2.14, 2.81 and 15.37 per tree for assessment 1, 2, 3 and 4, respectively. The overall incidence of trees with any number or peripheral lesions increased from 2.2% on the first assessment to 76.0% on the last assessment. Canker development varied greatly among the three sites (Table 2). Canker development varied greatly among the three sites. Average number of peripheral canker lesions was 1.06, 6.77 and 7.42 per tree for Site 1, 2 and 3, respectively. The overall incidence of trees with peripheral lesions on the last assessment was 22.0%, 55.2% and 61.2% for Site 1, 2 and 3, respectively. The overall differences in the canker development between the two planting times were small (Table 2). Seven scion genotypes differed in the peripheral canker development (Table 2, Figure 4). Overall, ‘Grenadier’ (18.9%) and ‘Golden Delicious’ (31.1%) had lower incidences of peripheral cankers than the other five cultivars with the incidence ranging from 51% to 57% (Table 2).
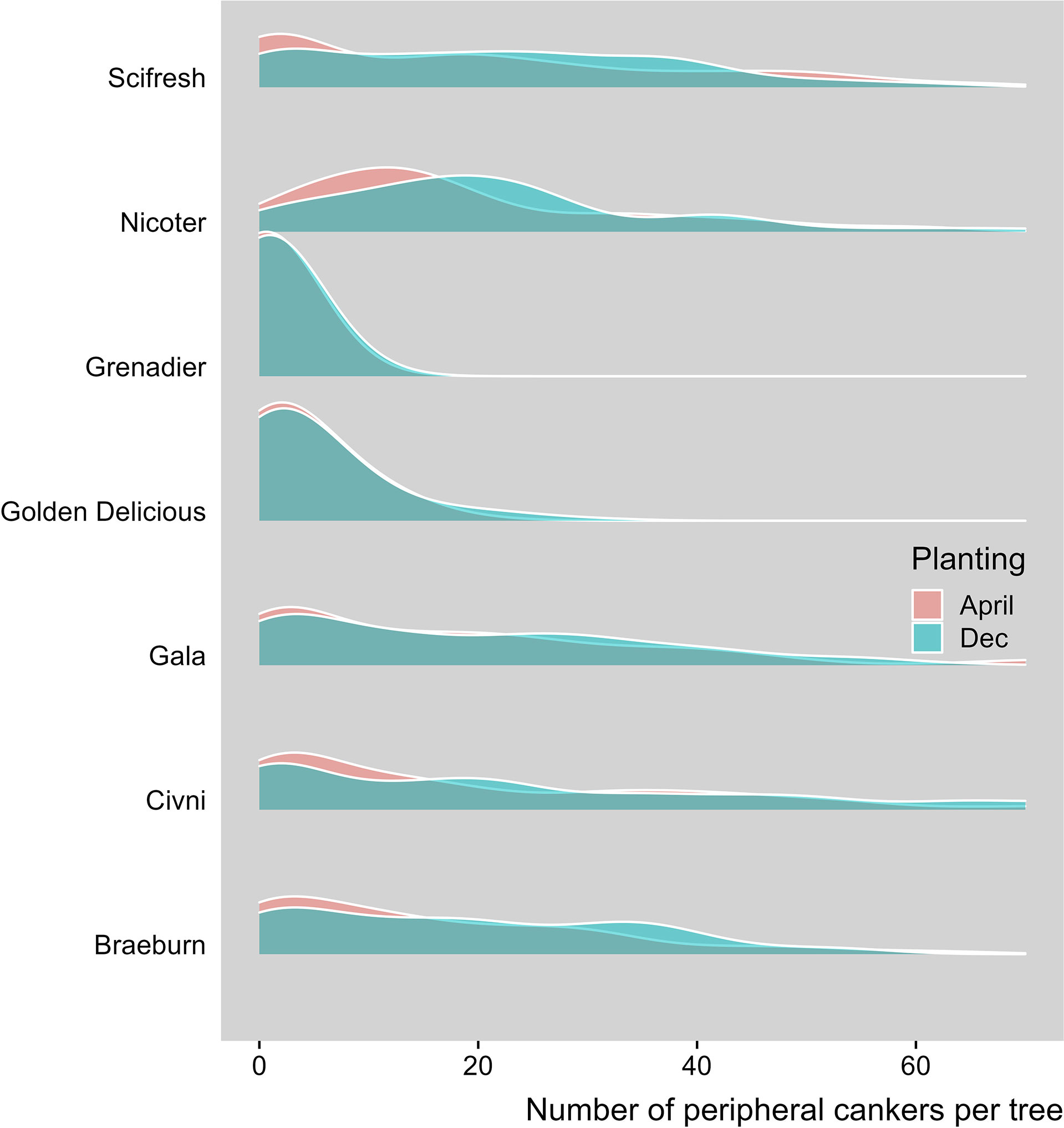
Figure 4 Density plot of number of peripheral canker lesions, when assessed in Spring 2021, of individual trees of seven apple scion cultivars planted at three sites where trees were planted either in December 2018 or cold-stored and planted in April 2019.
Variance estimates and their statistical significance are given in Table 2. As for the main stem canker, although the variance estimate for the site factor was the largest, it was only close to statistical significance (Table 3). On the other hand, variance estimates for both plot and block terms were very small but statistically significant (Table 3). Variance estimates for the site and its interaction with assessment time were greater than the residual variance (Table 3). Figure 5 shows the nature of the interaction between site and time. The increase in canker over time was much greater for Site 2 and 3, irrespective of cultivar and planting time. In contrast to the main stem canker, the interactions of site with cultivar were not significant (Table 3). However, the three-factor interaction (site, cultivar and assessment time) was significant; cultivar differences over time were greater for Site 2 and 3 (Figure 5). Variance estimates for any random-effect terms involving the planting time were both small and not significant.
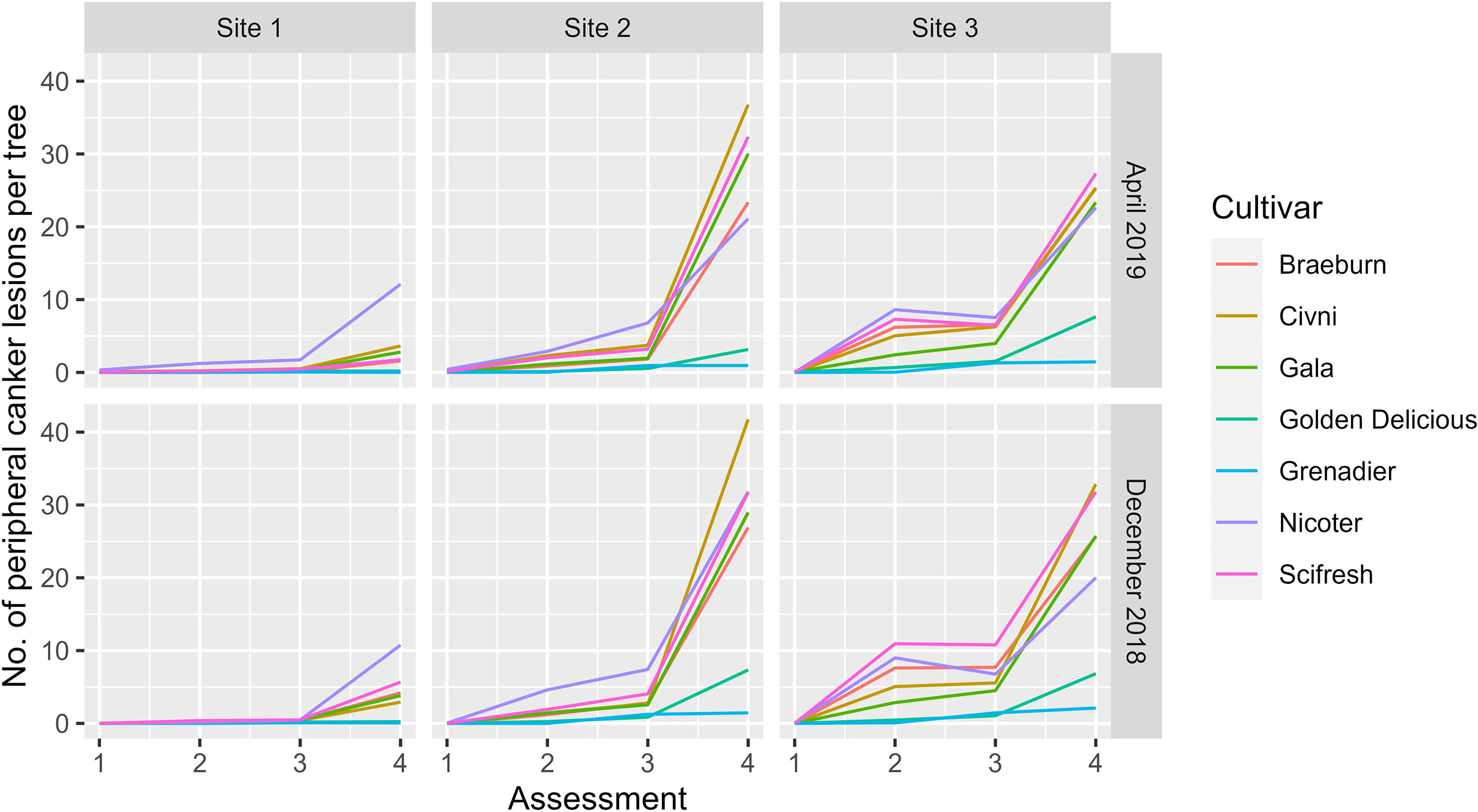
Figure 5 Mean number of peripheral canker lesions per tree of seven apple scion cultivars at three sites and four assessments [Autumn 2019 (1), Spring 2020 (2), Autumn 2020 (3), and Spring 2021 (4)] the trees were planted either in December 2018 or cold-stored and planted in April 2019.
For the fixed effect factors, both assessment time and cultivar, as well as their interaction, were highly significant (Table 4, Figure 3B). Cultivar differences increased with increasing time interval from planting. This is particularly noticeable for the differences in ‘Grenadier’ and ‘Golden Delicious’ with the other five cultivars on the last assessment date. Planting date also had significant effects, albeit small: planting in December led to slightly fewer peripheral canker lesions. The interaction of assessment time with planting time was also highly significant (P = 0.002); the number of peripheral lesions per tree for the December planting increased from 0.005 (1st assessment) to 16.3 (4th assessment) whereas the corresponding values for the April planting were 0.06 and 14.4.
When total number of canker lesions per tree (including both main stem and peripheral cankers) was analysed jointly, the results followed closely those for peripheral canker (Tables 3, 4).
Tree mortality
At the final assessment across all three sites, 254 trees died of cankers, 308 trees were dying, and 883 trees were visually healthy. In the linear mixed model analysis of healthy trees on the last assessment date, the random-effect terms of site and its interaction with cultivar were statistically significant and their variance estimates were 3.58 and 0.94, much greater than the residual variance (0.29). The nature of the interaction is shown in Figure 6. Both ‘Golden Delicious’ and ‘Grenadier’ had a very high incidence of healthy trees at all sites but the other five cultivars varied greatly among the three sites in the incidence of healthy trees. The interaction between site and planting date (variance estimate = 0.058) was not significant. Of the three fixed effect terms (planting, cultivar, and their interaction), only cultivar effect was significant (P = 0.001, Figure 7). Similar results were obtained when the incidence of dead trees was analysed.

Figure 6 Incidence of healthy, nearly dead, and dead trees for each combination of cultivar and site (tree heath was assessed in Spring 2021). Seven apple scion cultivars were planted either in December 2018 or cold-stored and planted in April 2019.
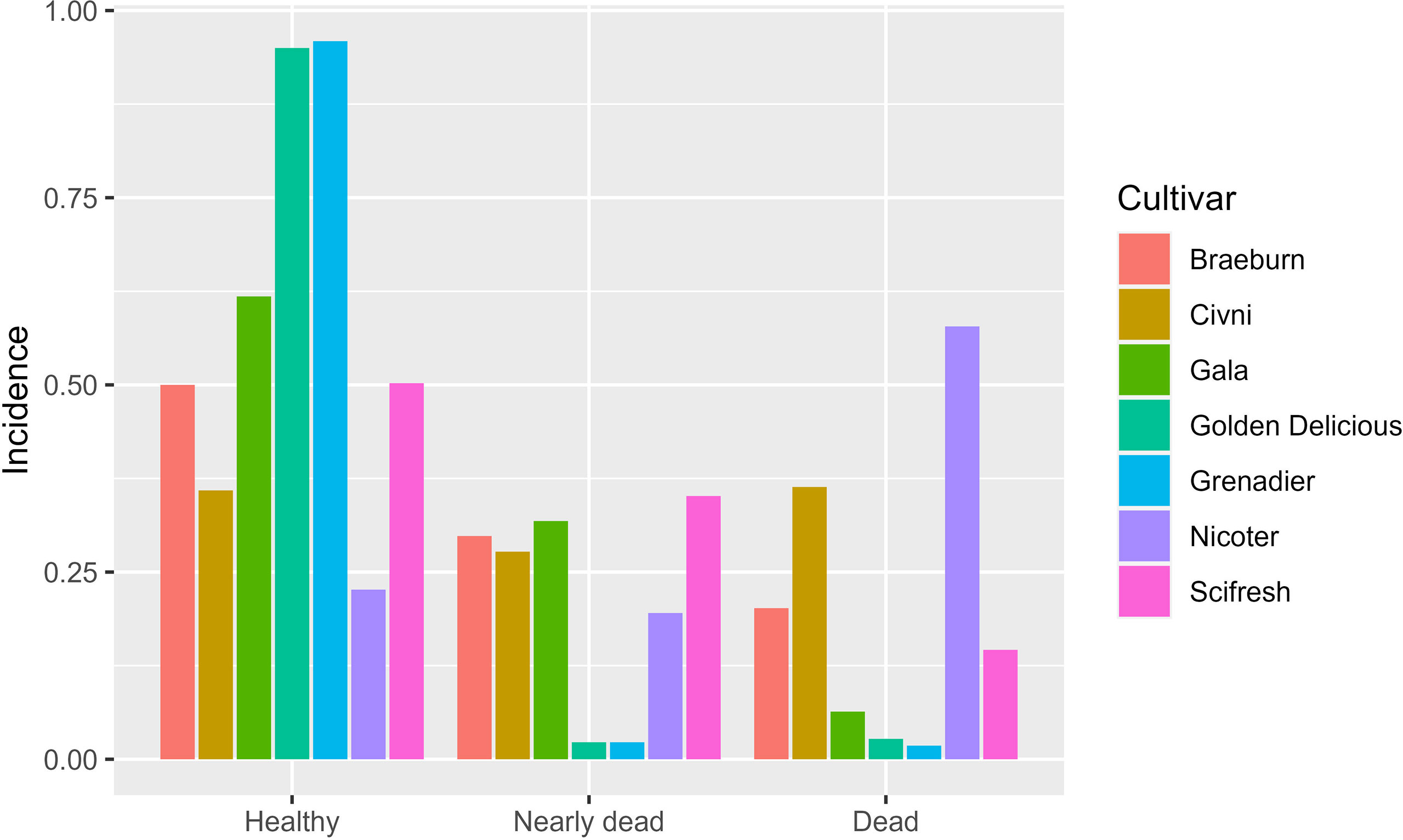
Figure 7 Overall incidence of healthy, nearly dead, and dead trees across all scion cultivars and sites (tree health was assessed in Spring 2021). Seven apple scion cultivars were planted either in December 2018 or cold-stored and planted in April 2019.
Discussion
Canker development differed greatly among the three sites for both main stem and peripheral cankers. When the site was treated as a fixed-effect factor, its effect was highly significant. Its effect as a random-effect factor was, however, not statistically significant, probably due to too few sites being used as a random-effect factor. Variance component associated with site was far greater than variance components of other factors, including the residual variance. However, in analysing the data, we treated the site as a random-effect factor in order to broaden the inference scope of cultivar performances/differences. Site differences may result from differences in general climatic conditions and possibly soil conditions. In addition, inoculum density from neighbouring orchards may differ among the sites, which could contribute to differences in peripheral cankers. Significant within-site positional effects (blocks within a site), albeit much less than among sites, suggest that differences in soil conditions may also affect canker expression indirectly through influencing tree health and development. The differences among sites and probably, to a lesser extent, between blocks within site may result partially from differences in soil fertility. A study in Germany showed that vigorously growing trees post-planting tend to have more severe canker development, which leads to the recommendation that fertilisation should be restricted during the first few years following planting, especially for highly canker-susceptible cultivars in fertile soils (Weber and Børve, 2021). However, Site 1 (the site with lowest canker incidence) had overall the smallest trees at the end of the experiment, whereas trees at the other two sites were comparatively similar in size. Unfortunately, we did not assess individual tree development in the present study. There were also significant interactions between site and time in canker development, primarily due to the faster canker development at Site 3 between the first and third assessments. Given the direct effects of climatic conditions on canker infection is well understood (Saville and Olivieri, 2019), we need to focus future research studies on detection of trees with latent canker infection and the effects of soil properties and amendments on the expression of latent infection and on predisposing trees to canker infection, especially through the orchard establishment phase. These could include soil pH, nutrient supply, water matrix potential, soil type and microbiome.
It is reassuring that the relative cultivar differences are generally consistent, in terms of number of canker lesions and trees with lesions, for both main stem and peripheral cankers, across three sites and over two planting dates, as indicated by the very limited contribution of genotype by site interaction to the observed variability in canker development. The present study eliminated potential confounding effects of specific sites or cultivars with differences in nursery infections (Weber, 2014; Weber and Børve, 2021) since all trees were from the same batch in the same nursery. In addition, differences in assessment methodologies (Ghasemkhani et al., 2015; Gomez-Cortecero et al., 2016; Scheper et al., 2018) can lead to differential ranking in cultivar responses to N. ditissima. In the present study, all trees were from the same field in the same nursery, inoculated at the same time, using the same inoculum level mimicking natural infections that resulted in peripheral cankers post-planting. Thus, the present results suggest that genetic factors responsible for reduced canker development in ‘Golden Delicious’ and ‘Grenadier’ are stable and effective across varying environments. Thus, exploiting these resistance factors in breeding could be rewarding.
The interaction between cultivar and site was nevertheless statistically significant for the main stem canker. Given the main stem cankers are more likely to result from latent infections in nurseries, the present result suggests that some site-specific factors may interact with cultivars to affect differential expression of latent infections post-planting that could differentially influence symptom development from latent infection. On the other hand, it appears that, for the new infections that occurred post-planting, cultivar response was generally consistent across the three sites. Cultivar and site interaction appears to be mostly due to the relatively more main stem cankers on ‘Nicoter’ at Site 1 and 2 in the early assessments. It is interesting to note that the soil in the former orchards is of the clay loam type, whist the orchard at Site 3 is of the sandy clay loam type. Further research is necessary to understand potential effects of soil texture on tree establishment and symptom expression of latent canker infections.
Planting date had significant but very small effects on the peripheral canker incidence. This is surprising as we would expect that symptom development of latent infection may be more likely to be affected by cold storage. Under high humidity/temperature, storage may accelerate symptom development of latent infection by N. ditissima (Wenneker et al., 2017). However, given the effect of storage time at low temperatures is so small relative to other effects, management efforts should be prioritised in other areas.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
All authors contributed to designing the experiments. TMP, HM and MPR conducted the field work. XX and RS developed the research. XX analyzed and interpreted the data, and wrote the manuscript. LRB, MPR, RS and TMP refined the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Biotechnology and Biological Science Research Council (BBSRC) [grant number: BB/P007899/1] and several industry organisations (Agriculture and Horticulture Development Board [AHDB], Adrian Scripps Limited, Avalon Fresh Limited, ENZA [T&G global subsidiary], Frank P Matthews Limited, and Worldwide Fruit Limited).
Acknowledgments
We thank Nick Dunn at Frank P Matthews, Tenbury Wells, Worcestershire, UK for carrying out grafting and storing the trees until planting. We also thank Jack Skinner at William Skinner & Son, Maidstone, UK; Mark Holden at Adrian Scripps Ltd, Tonbridge, UK and Peter and Clive Chandler at Chandler & Dunn, Canterbury, UK for providing the planting sites and carrying out orchard management operations throughout the experiment. We are also grateful to Nigel Jenner at Avalon Fresh Ltd. and Tony Harding at Worldwide Fruit Ltd. for partnering in the project and coordinating grower sites.
Conflict of interest
All authors were employed by NIAB at East Malling.
This study received funding from the Biotechnology and Biological Science Research Council (BBSRC), Agriculture and Horticulture Development Board [AHDB], Adrian Scripps Limited, Avalon Fresh Limited, ENZA [T&G global subsidiary], Frank P Matthews Limited, and Worldwide Fruit Limited). The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. All authors declare no other competing interests.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amponsah N. T., Walter M., Beresford R. M., Scheper R. W. A. (2015). Seasonal wound presence and susceptibility to Neonectria ditissima infection in New Zealand apple trees. N. Z. Plant Prot. 68, 250–256. doi: 10.30843/nzpp.2015.68.5799
Bates D., Mächler M., Bolker B. M., Walker S. C. (2015). Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/JSS.V067.I01
Børve J., Dalen M., Stensvand A. (2019). Development of Neonectria ditissima infections initiated at grafting of apple trees. Eur. J. Plant Pathol. 155, 1225–1239. doi: 10.1007/s10658-019-01851-7
Bus V. G. M., Scheper R. W. A., Walter M., Campbell R. E., Kitson B., Turner L., et al. (2019). Genetic mapping of the European canker (Neonectria ditissima) resistance locus Rnd1 from Malus ‘Robusta 5.’. Tree Genet. Genomes 15, 1–13. doi: 10.1007/s11295-019-1332-y
Ghasemkhani M., Sehic J., Ahmadi-Afzadi M., Nybom H., Garkava-Gustavsson L. (2015). Screening for partial resistance to fruit tree canker in apple cultivars. Acta Hortic. 1099, 687–690. doi: 10.17660/ActaHortic.2015.1099.84.
Gomez-Cortecero A., Saville R. J., Scheper R. W. A., Bowen J. K., de Medeiros H. A., Kingsnorth J., et al. (2016). Variation in host and pathogen in the Neonectria/Malus interactions; towards an understanding of the genetic basis of resistance to European canker. Front. Plant Sci. 7, 1365. doi: 10.3389/FPLS.2016.01365
Kuznetsova A., Brockhoff P. B., Christensen R. H. B. (2017). lmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software 82, 1–26. doi: 10.18637/JSS.V082.I13
McCracken A. R., Berrie A., Barbara D. J., Locke T., Cooke L. R., Phelps K., et al. (2003). Relative significance of nursery infections and orchard inoculum in the development and spread of apple canker (Nectria galligena) in young orchards. Plant Pathol. 52, 553–566. doi: 10.1046/j.1365-3059.2003.00924.x
Saville R. J., Olivieri L. (2019). “Fungal diseases of fruit: apple cankers in Europe,”. in Integrated Manage. Dis. Insect pests Tree fruit. Eds. Xu X., Fountain M. (Cambridge: Burleigh Dodds Science Publishing), 59–83.
Scheper R. W. A., Fisher B. M., Taylor T., Hedderley D. I. (2018). Detached shoot treatments cannot replace whole-tree assays when phenotyping for apple resistance to Neonectria ditissima. N. Z. Plant Prot. 71, 151–157. doi: 10.30843/NZPP.2018.71.137
Walter M., Manktelow D. W. L., Le Berre F., Campbell R. E., Turner L., Vorster L., et al. (2019). How much captan is required for wound protection of Neonectria ditissima conidial infection in apple? N. Z. Plant Prot. 72, 95–102. doi: 10.30843/nzpp.2019.72.273
Walter M., Roy S., Fisher B. M., Mackle L., Amponsah N. T., Curnow T., et al. (2016). How many conidia are required for wound infection of apple plants by Neonectria ditissima? N. Z. Plant Prot. 69, 238–245. doi: 10.30843/nzpp.2016.69.5886
Walter M., Stevenson O. D., Amponsah N. T., Scheper R. W. A., Rainham D. G., Hornblow C. G., et al. (2015). Control of Neonectria ditissima with copper based products in New Zealand. N. Z. Plant Prot. 68, 241–249. doi: 10.30843/nzpp.2015.68.5798
Weber R. W. S. (2014). Biologie und Kontrolle des Obstbaumkrebs-Erregers Neonectria ditissima (Syn. N. galligena) aus der Perspektive Nordwesteuropas. Erwerbs-Obstbau 56, 95–107. doi: 10.1007/S10341-014-0210-X
Weber R. W. S., Børve J. (2021). Infection biology as the basis of integrated control of apple canker (Neonectria ditissima) in Northern Europe. CABI Agric. Biosci. 2, 5. doi: 10.1186/s43170-021-00024-z
Wenneker M., de Jong P. F., Joosten N. N., Goedhart P. W., Thomma B. P. H. J. (2017). Development of a method for detection of latent European fruit tree canker (Neonectria ditissima) infections in apple and pear nurseries. Eur. J. Plant Pathol. 148, 631–635. doi: 10.1007/S10658-016-1115-3
Keywords: neonectria ditissima, trunk canker, peripheral canker, planting time, soil condition
Citation: Xu X, Passey T, Robinson-Boyer L, Mclean H, Saville R and Papp-Rupar M (2022) Development of European apple canker on different cultivars in relation to planting time at three sites in the UK. Front. Hortic. 1:995776. doi: 10.3389/fhort.2022.995776
Received: 16 July 2022; Accepted: 22 August 2022;
Published: 23 September 2022.
Edited by:
Marcel Wenneker, Wageningen University and Research, NetherlandsReviewed by:
José Manuel Mirás-Avalos, Aragon Agrifood Research and Technology Center (CITA), SpainAdrian Asanica, University of Agronomic Sciences and Veterinary Medicine of Bucharest, Romania
Copyright © 2022 Xu, Passey, Robinson-Boyer, Mclean, Saville and Papp-Rupar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangming Xu, xiangming.xu@niab.com
 Xiangming Xu
Xiangming Xu