- Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires – IEGEBA (CONICET – UBA), Buenos Aires, Argentina
Urban environments have been characterized by their temporal stability of resources, which could promote stability in bird composition. Several studies have found that bird communities in urban environments persist over the years, showing a similar species composition in the short term. However, studies analyzing continuous changes in urban communities over the long term are scarce. This study aimed to analyze the stability or directional changes (instability) in bird communities along an urban gradient. Bird counts were conducted in urban, suburban, and periurban areas over 8-10 years in 2002-2019. Changes in species composition were analyzed over periods ranging from one year to the next, to changes from one year to the seventeenth. Urban bird communities were more similar between years than suburban and periurban communities. Compositional changes were greater as time lags increased, indicating directional compositional shifts. The magnitude of these changes was similar across the urban gradient. The Chimango Caracara (Milvago chimango), the Picazuro Pigeon (Patagioenas picazuro), the Rufous Hornero (Furnarius rufus), and the Red-bellied Thrush (Turdus rufiventris) significantly increased their abundances during the period, while the House Sparrow (Passer domesticus) significantly decreased its abundance. Regional changes in species abundance, urban vegetation succession, and biotic interactions could explain the changes in bird communities.
Introduction
Analyzing interannual variation in biotic communities is fundamental to understanding community assembly. Temporal dynamics of communities may be related to changes in environmental local conditions or biotic interactions and regional environmental changes that promote species density fluctuations or range distributional changes (Väisänen et al., 1986; Wiens, 1989; Dornelas et al., 2014). In the actual global change scenario, long-term analysis of communities allows us to identify the factors responsible for population changes (Storch et al., 2023).
Urbanization is one of the most drastic anthropogenic land use changes, causing fragmentation, habitat loss, alteration of hydrologic and biogeochemical cycles, and biodiversity loss (Grimm et al., 2008). Several authors have proposed that urbanization promote a temporal stabilization of environmental conditions, ultimately dampening temporal dynamics of biotic communities (Shochat et al., 2006; Leveau, 2018). Urban areas have shown lower interannual fluctuations of primary productivity than natural or rural areas (Shochat et al., 2004; Leong and Roderick, 2015). This dampening of resources in urban areas has been associated with the higher interannual persistence of bird species in urban areas (Suhonen et al., 2009; Leveau and Leveau, 2012). For example, Leveau et al. (2015) have found that urban bird assemblages had a lower interannual dissimilarity than assemblages of rural areas.
Although several long-term studies of avian communities have been carried out in urban areas (Campbell et al., 2022; Echeverry-Galvis et al., 2023; Skjelvik and Dale, 2024; and references in Fidino and Magle, 2017), it is unknown if urbanization is related to the stabilization of bird communities. Collins et al. (2000) have proposed an analytical method to assess community stability by analyzing the relationship between community dissimilarity and different time intervals or time lags (Figure 1). The regression slope between both variables indicates the rate of change in community composition, whereas the regression coefficient indicates the proportion of signal versus noise (Collins et al., 2000). Thus, the increasing dissimilarity with time lag suggests an unstable directional change because species composition changes with time (Figure 1). On the other hand, a negative relationship between composition dissimilarity and time lag would indicate another type of instability, because community composition is converging to the initial community of the time series. The lack of correlation between species dissimilarity and time lag suggests stability.
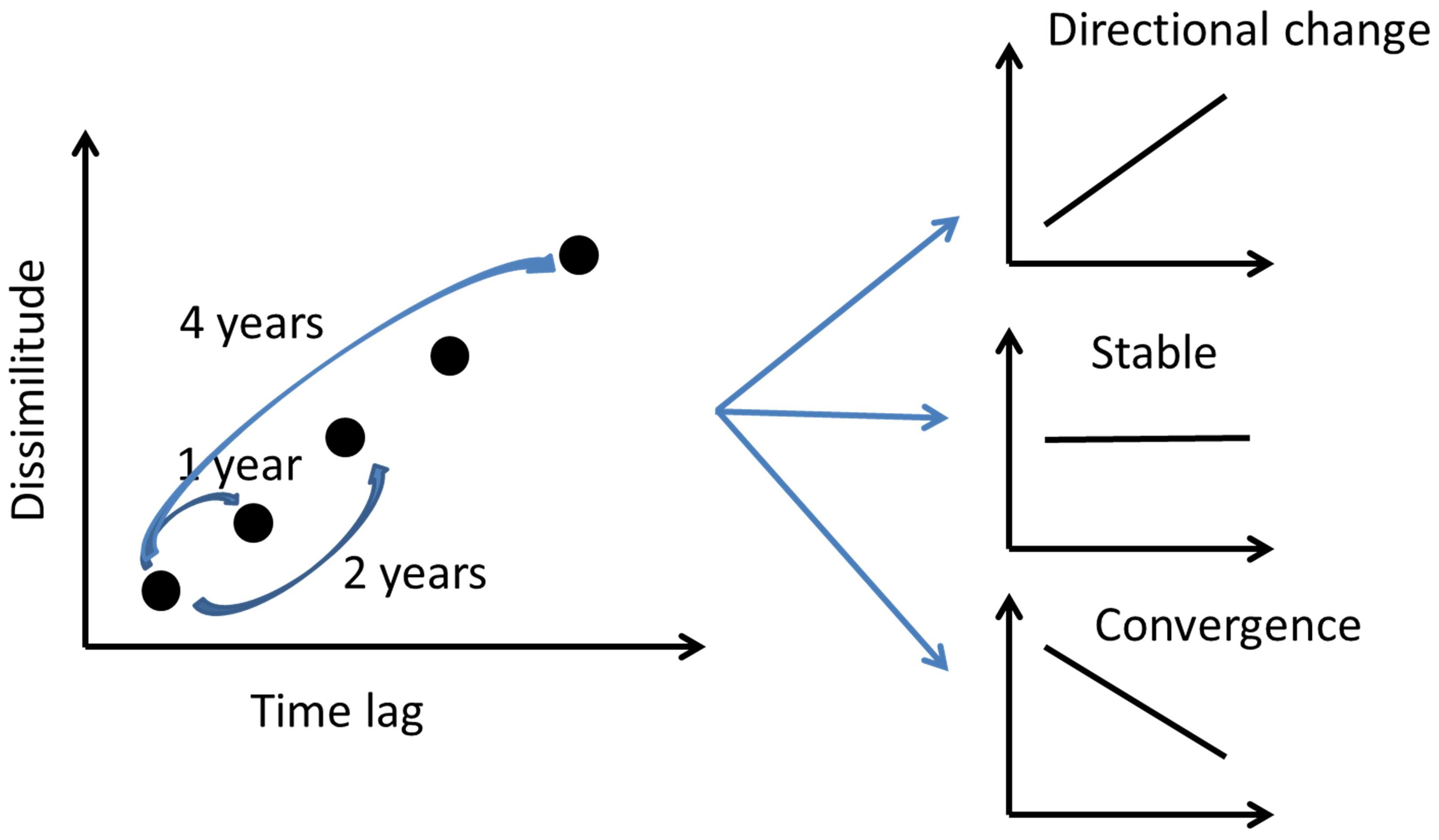
Figure 1. Schematic representation of the community stability analysis based on the relation between species composition dissimilarity and different time intervals or time lags. Source: Original figure based on the idea of Collins et al. (2000).
This study aims to analyze bird community composition change along the urban gradient of Mar del Plata City over 18 years. Specifically, the study aimed to analyze along the urban gradient: 1) the long-term abundance change of bird species; 2) the mean interannual change of bird composition; and 3) the rate of community composition changes with the passing of years. The habitat structure and level of urbanization of the study area were stable during the period, e.g. no change in land use was noticeable. However, the region had an increasing crop cover, climate warming, and increasing precipitations (Baeza and Paruelo, 2020; Ferrelli et al., 2021a, Ferrelli et al., 2021b), presumably causing population increases and distributional range expansions in several bird species (Gavier-Pizarro et al., 2012; Grande et al., 2015; Leveau, 2021; Vazquez et al., 2024). These species, such as doves, raptors, and swallows, can inhabit urban environments (La Sorte et al., 2018). Recently, Skjelvik and Dale (2024) have shown that local bird species population trends in urban parks were positively related to regional population trends. We expect bird assemblages of the most urbanized areas would be more persistent between years, e.g. their mean interannual species composition will be more similar than in less urbanized areas. However, species composition directional changes would occur with increasing time lags along the urban gradient due to the regional population increases of bird species that can invade urban areas.
Materials and methods
Study area
The study was carried out in Mar del Plata City (38°00′S 57°33′W, 15-38 m.a.s.l.), a coastal tourist city in central-east Argentina (Figure 2). The city has a population of 682605 (National Census 2022), and since 2001 has increased by 21% (National Institute of Statistics and Censuses, https://www.indec.gob.ar/). Mar del Plata has a temperate oceanic climate, with cold winters (July mean temperature = 7.5°C; period 1991-2020) and relatively warm summers (mean January temperature = 20.3°C) (Servicio Meteorológico Nacional, 2023). The mean annual precipitation is 946.1 mm (Servicio Meteorológico Nacional, 2023). The dominant native vegetation of the region is a pseudo-steppe of mesophytes with hill scrub (Oyarzabal et al., 2018). However, the human transformation of the landscape has led to a dominance of crops, grazing lands and exotic tree plantations.
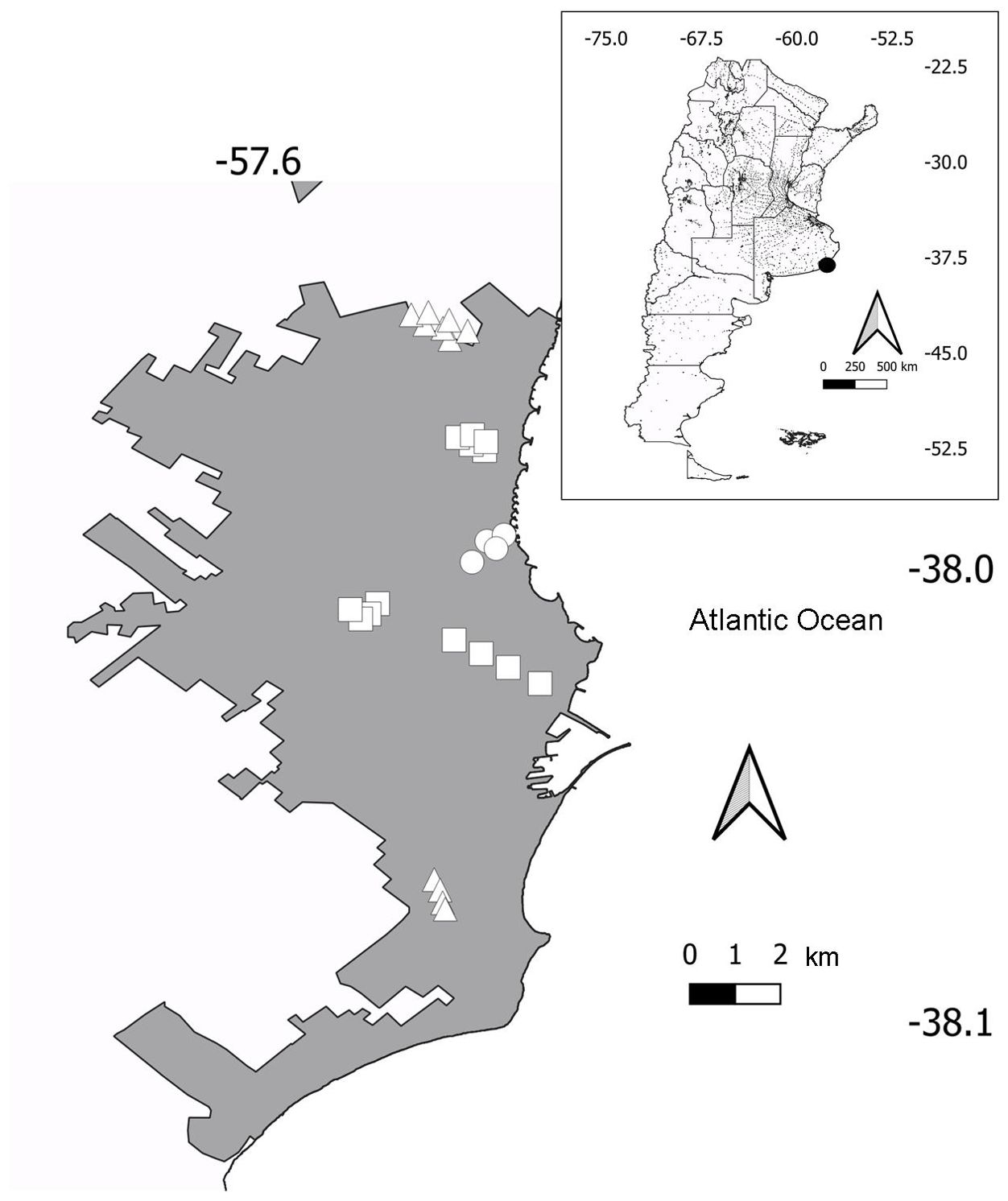
Figure 2. Location of Mar del Plata City in Argentina (inset) and urban (circles), suburban (squares), and periurban (triangles) transects in Mar del Plata.
Bird surveys
According to definitions in Marzluff et al. (2001) and MacGregor-Fors (2011), three habitat types were surveyed (see Leveau and Leveau, 2004, Leveau and Leveau, 2012): 1) urban habitat or highly developed was the commercial and administrative center of the city, dominated by buildings and paved streets (mean impervious cover = 95.19%); 2) suburban habitat or moderately developed, composed of detached houses with yards and paved streets (mean impervious cover = 54.45%); and 3) periurban habitat or sparsely developed, composed of detached houses with yards with more vegetation than the suburban habitat and located on the city fringe (mean impervious cover = 17.49%). Bird surveys were carried out during the first four hours after dawn once in December by 100 x 50 m transects, separated at least by 200 m (see more details in Leveau and Leveau, 2012). The period of the year coincided with the bird breeding season (de la Peña, 2013). Bird counts were mainly carried out by LML with the help of Carlos M. Leveau during 2002-2005, while only LML did surveys during the rest of the periods.
The number of transects varied between habitats (Figure 2). The urban habitat had four transects, the suburban habitat 13 transects, and the periurban habitat 11 transects. Transects of the urban habitat were visited during 2002-2005, 2009-2012, and 2018-2019, totaling ten years of surveys. Four suburban and periurban transects were surveyed during the same period. However, nine suburban transects did not have surveys during 2009, whereas seven periurban transects did not have surveys during 2009 and 2010.
Statistical analysis
Species abundance (individuals/transect) was correlated with the years along the urban gradient using Spearman rank (rs) correlation with the cor function in R (R Core Team, 2019) (Table 1). Only species with three or more records were analyzed. To group the different trends of species abundances through the years, the Spearman rank correlation of each species was grouped using cluster analysis with the function hclust in R (R Core Team, 2019). Dendrograms were obtained using the Euclidean distance between species correlations with single, complete, and average linkage methods. The best linkage method was tested through cophenetic correlations between the Euclidian distance matrix and the cophenetic distance representing the distance of species in the dendrogram (Borcard et al., 2011). Then, the average method had the highest cophenetic correlation and was used to construct the dendrogram.
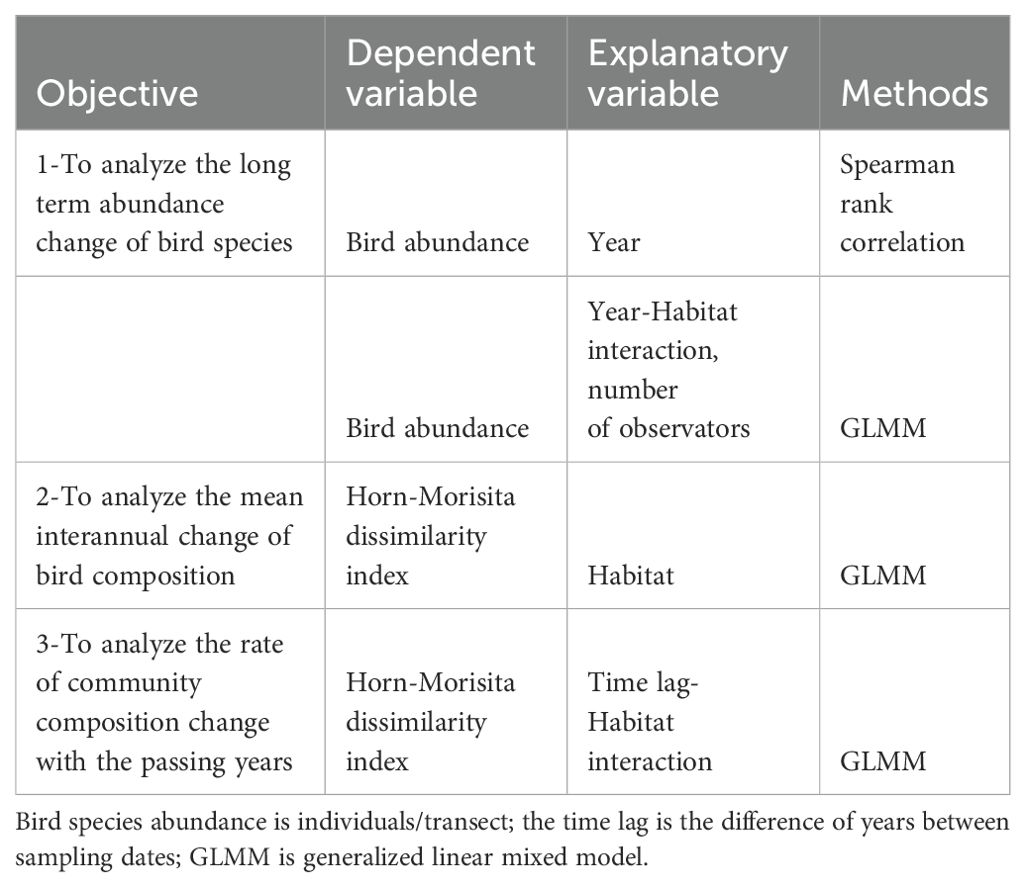
Table 1. Summary of statistical approaches used to each study objective along the urban gradient in Mar del Plata City during 2002-2019, Argentina.
To test for different interannual species abundance trends along the urban gradient, the species abundance in each transect was related to the interaction between habitat and year, and the number of observers during each year (Table 1). Species trends between habitat types were assessed through generalized linear mixed models (GLMM) using the glmmTMB function of the glmmTMB package (Brooks et al., 2023). A negative binomial (nbinom2) distribution of errors was used. The transect was deemed as a random factor. Models were constructed by backward elimination of non-significant variables (P > 0.05) from the full model using the anova function. Final models were contrasted against null models using a likelihood ratio test (LRT test) (P < 0.05).
To test for differences of interannual dissimilarity between habitats and directional changes of bird assemblages in the three habitat types (Table 1), the Horn-Morisita dissimilarity index was calculated between years for each transect with the vegdist function in vegan (Oksanen et al., 2022). The Horn-Morisita index is based on differences in the species proportion of two samples and is not biased by differences in sample size (Wolda, 1981). The dissimilarity between years was related to the time lag through a GLMM with the lmer function of the lme4 package (Bates et al., 2015). Time lag was measured at different time intervals (Figure 1, Collins et al., 2000). Time lags were transformed to the square root to avoid the probability that the smaller number of points at larger time lags will bias the analysis (Collins et al., 2000). The mixed model included the interaction between time lag and habitat type, and each transect was deemed as a random factor. Models were constructed by backward elimination of non-significant variables (P > 0.05) from the full model using the anova function. Final models were contrasted against null models using a likelihood ratio test (LRT test) (P < 0.05). The signal of the community composition change with time was assessed for each habitat type by running a GLMM and calculating the regression coefficient with the function rsquared of the piecewiseSEM package (Lefcheck, 2016). This function allows us to calculate the marginal r2 due to time lag and the conditional r2 due to both time lag and the random effects (the transects) (Lefcheck, 2016).
All diagnostic analyses were carried out with the DHARMa package (Hartig, 2022). Final models were plotted using the visreg function of the visreg package (Breheny and Burchett, 2017).
Results
A total of 47 bird species and 4408 bird detections were made (Table 2). The total richness was lower in urban than in suburban and periurban habitats. Columba livia and Passer domesticus were the most common species in the urban habitat, Passer domesticus and Zenaida auriculata were the most common in the suburban habitat, whereas Patagioenas picazuro and Passer domesticus were the most common species in the periurban habitat (Table 2).
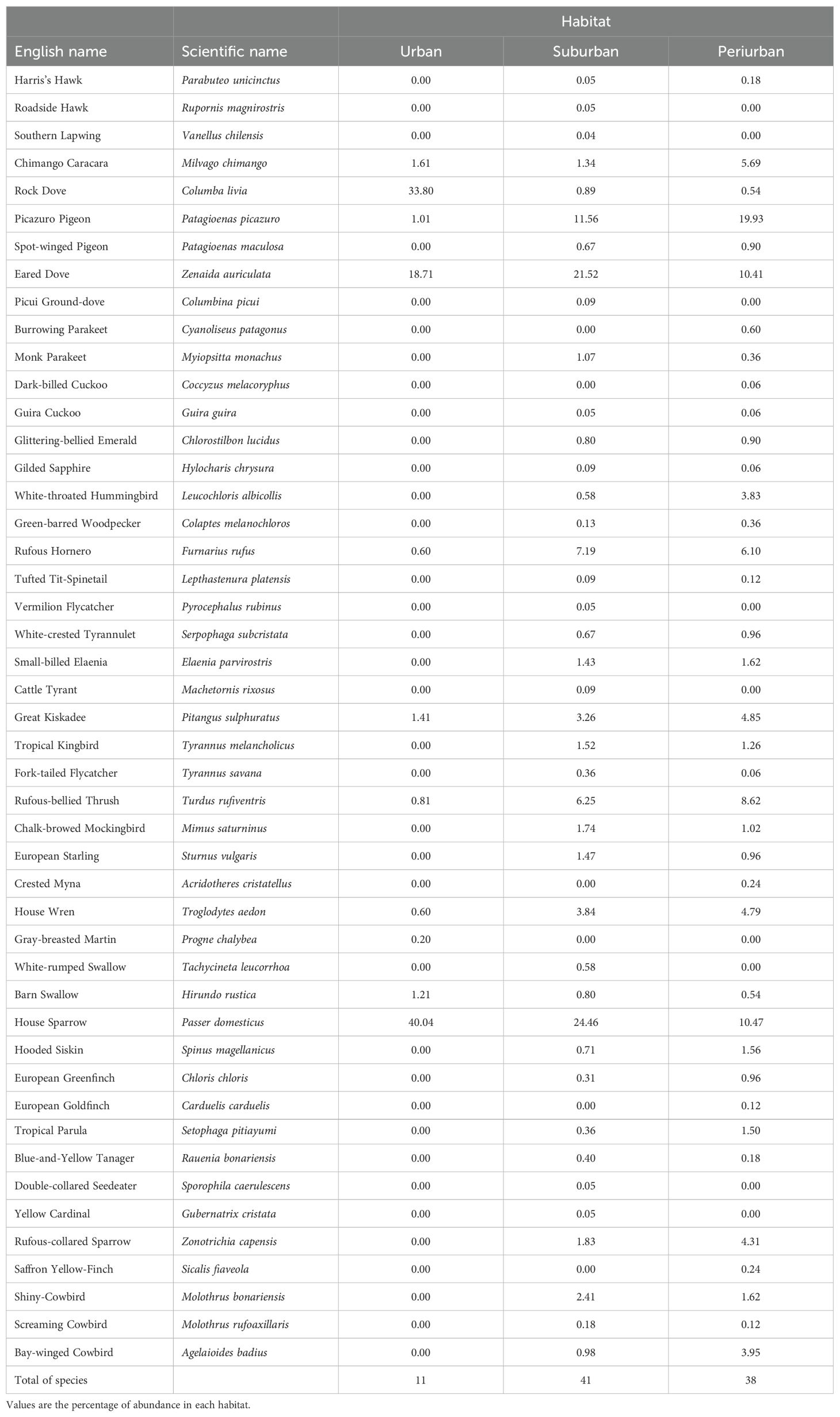
Table 2. List of species recorded along the urban gradient of Mar del Plata City, Argentina, during 2002-2019.
According to the correlations between species abundances and years along all sites, four groups were obtained (Figure 3). A group of species such as Tyrannus savana, Agelaioides badius and Mimus saturninus had no general correlation between abundances and years. A second group of species such as Molothrus bonariensis, Zonotrichia capensis, and Passer domesticus showed average negative correlations between abundances and years (Figure 3). The other two groups of species showed increases in abundance through the years. Species such as Leucochloris albicollis, Zenaida auriculata, and Setophaga pitiayumi showed moderate increases (mean rs = 0.14), whereas Sturnus vulgaris and Hirundo rustica showed strongest interannual increases (mean rs = 0.36).
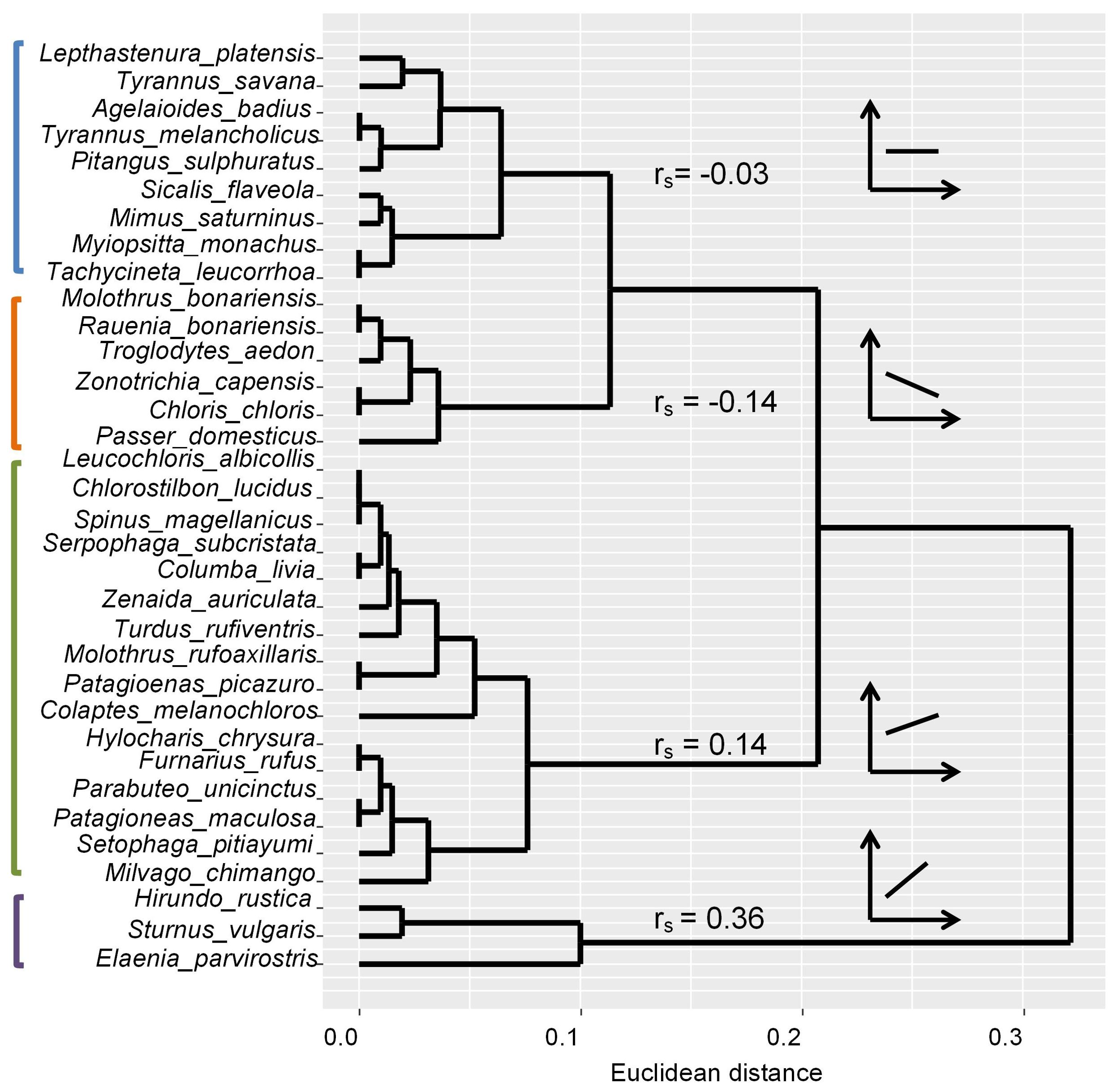
Figure 3. Dendrogram showing four groups (cut off 0.10 Euclidian distance) based on species distances of Spearman rank (rs) correlations between species abundances and years during 2002-2019. Mean correlation values for species in each group are provided.
Some species showed different interannual changes of abundances between the three habitats. For example, Patagioenas picazuro and Turdus rufiventris showed abundances increases in urban and suburban habitats, whereas Pitangus sulphuratus only showed increases in the urban habitat (Table 3, Figure 4). Zenaida auriculata showed decreasing abundances in suburban habitats, and increasing abundances in urban and periurban habitats (Table 3, Figure 4). Other species, such as Milvago chimango, Spinus magellanica, and Passer domesticus had similar trends along the three habitats (Table 3).
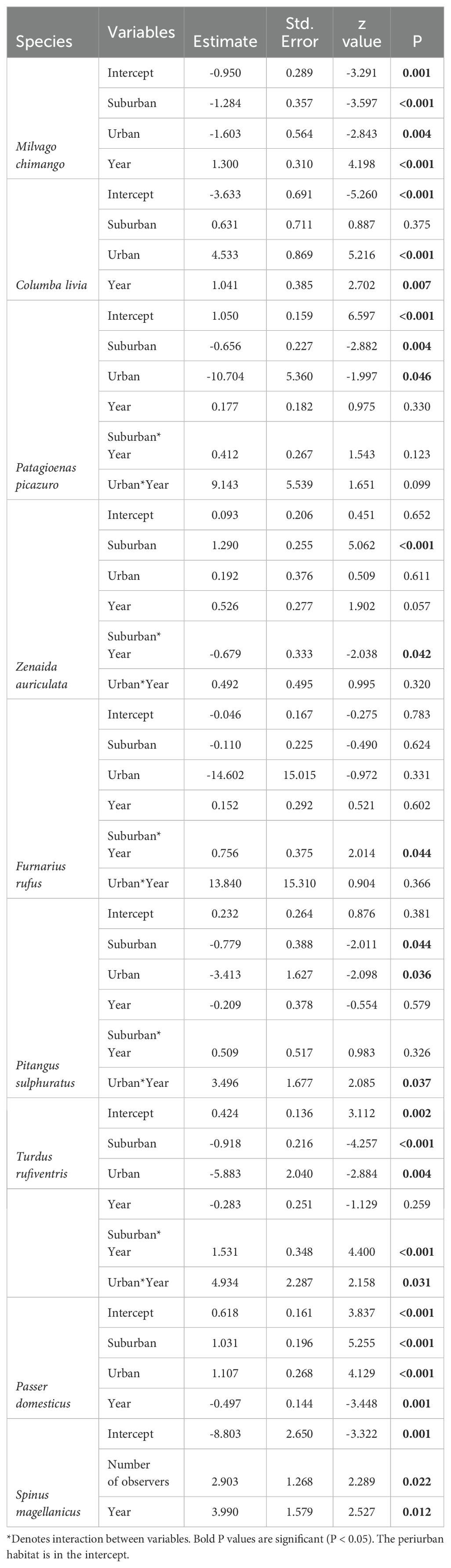
Table 3. Final generalized linear mixed models between species abundances, sampling effort (number of observers), habitat, and year along the urban gradient of Mar del Plata City, Argentina.
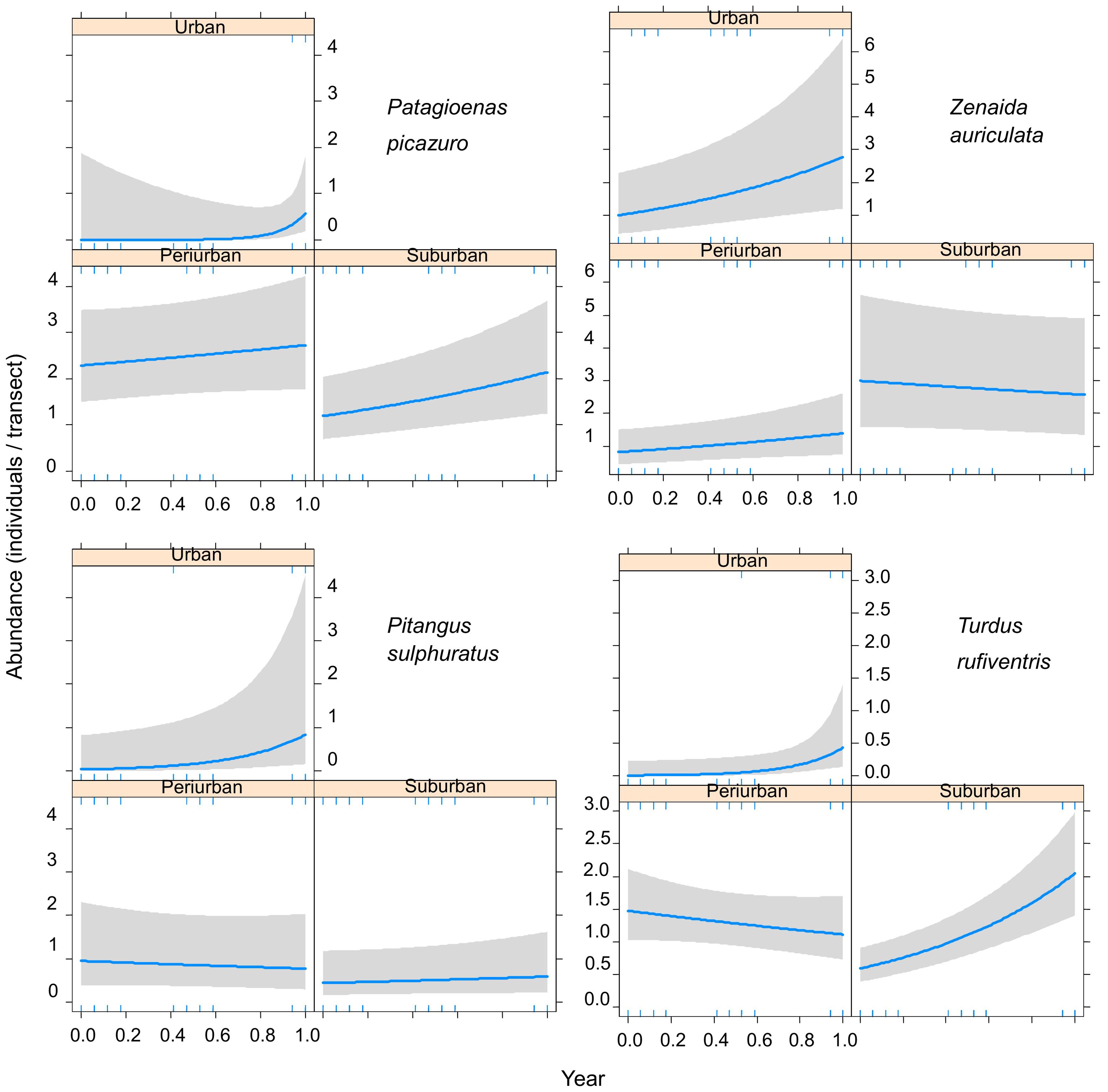
Figure 4. Final generalized linear mixed models showing the relationship between bird abundance and years during 2002-2019 in three urban habitats of Mar del Plata City, Argentina. Blue lines indicate the fitted model and grey bands indicate 95% confidence intervals. The year was standardized.
The dissimilarity of bird assemblages was related to the passing of years and habitats (LRT = 51.58, df = 3, P < 0.001, Table 4). The interannual dissimilarity of bird assemblages was lower in urban habitats than in suburban and periurban habitats (Figure 5). Therefore, urban habitats had more similar species relative abundances between years compared to suburban and periurban habitats. The dissimilarity of assemblages increased with the passing of years and the rate of increase was similar between habitats (Figure 5). Thus, the relative abundance of species changed continuously through the passing of the years along the three habitats, showing a pattern of instability of assemblages. However, the signal of these trends was low for the three habitats, and most of the variability was explained by the joint effect of time lag and random effects (Table 5). Therefore, this pattern suggests that there was a significant variation of dissimilarity trends among transects (Figure 6).
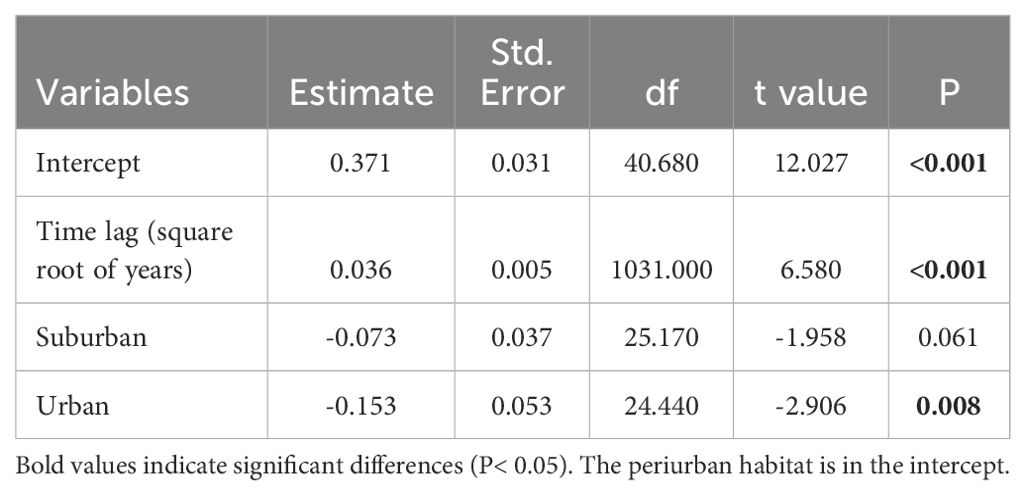
Table 4. Final generalized linear mixed model showing the relationship between the site interannual Horn-Morisita dissimilarity of bird species assemblages, time lag, and habitats along the urban gradient of Mar del Plata City, Argentina.
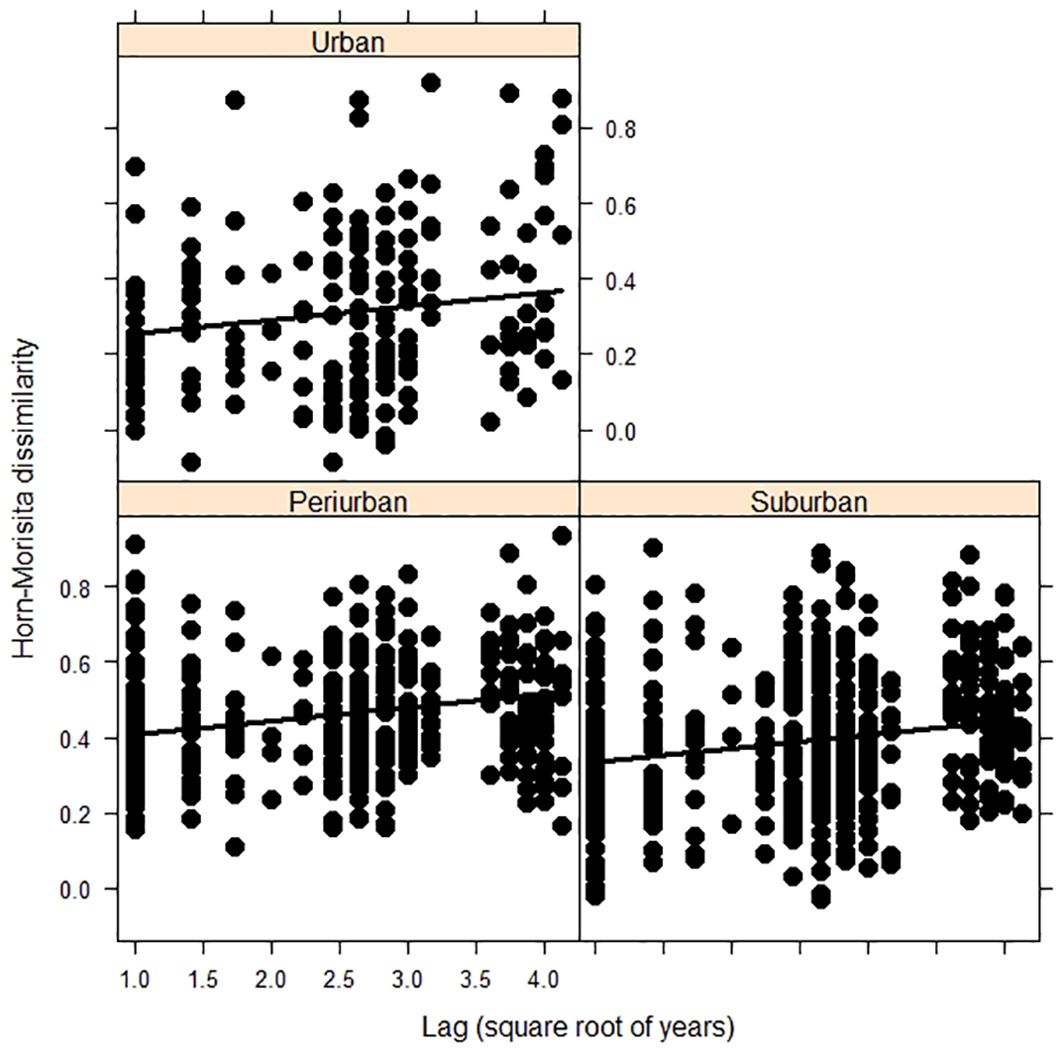
Figure 5. Final generalized linear mixed models showing the relationship between bird composition dissimilarity and time lags years during 2002-2019 in three urban habitats of Mar del Plata City, Argentina. Black lines indicate the fitted model.

Table 5. R-squared values of generalized linear mixed models between bird composition dissimilarity and time lag in urban, suburban, and periurban habitats of Mar del Plata City, Argentina.
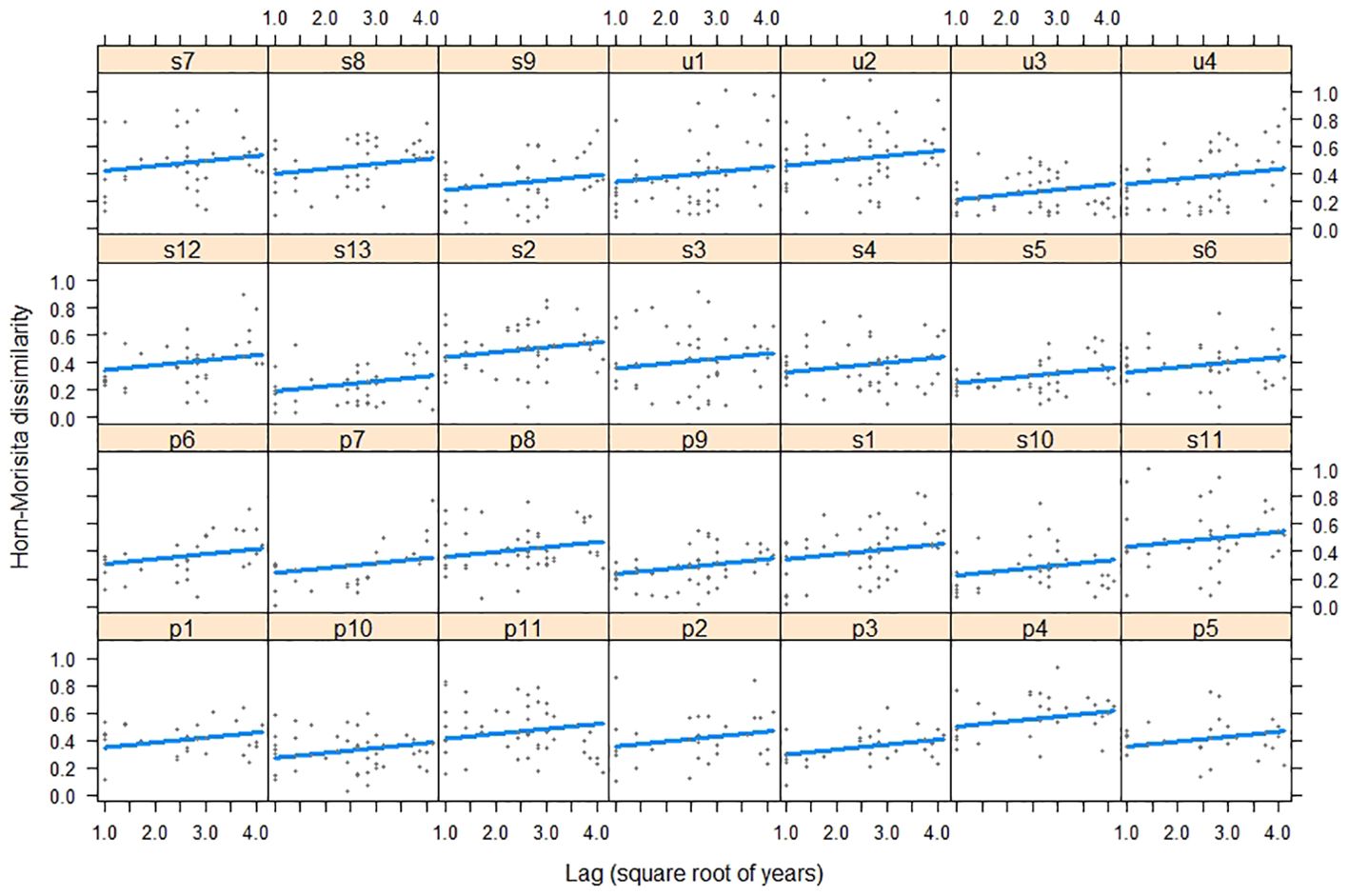
Figure 6. Relationship between bird composition dissimilarity and time lags years during 2002-2019 in transects along the urban gradient of Mar del Plata City, Argentina. u: urban habitat (N = 4), s: suburban habitat (N = 13), and p: periurban habitat (N = 11).
Discussion
Our results showed that some species had stable or decreasing interannual abundance trends, but most species showed linear increases. More detailed analyses revealed that some species, such as Patagioenas picazuro, Zenaida auriculata, and Pitangus sulphuratus, had different temporal trends between urban, suburban, and periurban habitats. These changes led to directional changes of avian assemblages which, contrary to what was expected, were of similar rate between the different levels of urbanization.
Bird communities along the urban gradient
The species richness of communities declined in the urban habitat compared to suburban and periurban habitats. The high cover of impervious surfaces in the urban habitat allowed the numerical dominance of Passer domesticus, Columba livia and Zenaida auriculata, which nest on buildings and street trees and can take advantage of discarded food by humans (Leveau and Leveau, 2004; La Sorte et al., 2018). On the other hand, the higher vegetation cover and habitat diversity in suburban and periurban habitats allowed a greater species richness (MacArthur and MacArthur, 1961; Leveau and Leveau, 2004).
Species interannual trends
Some species, such as Passer domesticus and Zonotrichia capensis showed yearly decreasing trends in their abundances. In the case of Passer domesticus, several studies in Argentina and worldwide have found similar results (Shaw et al., 2008; Dandapat et al., 2010; Berigan et al., 2020; Palacio et al., 2018; Campbell et al., 2022). Possible causes of Passer domesticus declines are: an increase in pollution; an increase in human socio-economic status; the increase in predation pressure; the decrease in herbaceous vegetation cover; and exposure to diseases (reviewed in Shaw et al., 2008 and Bernat-Ponce et al., 2020). In our study, we found interannual increases in ecological similar species to Passer domesticus, such as Zenaida auriculata and Sturnus vulgaris. These species are omnivorous ground foragers that could compete with Passer domesticus. We have seen aggressive exclusion from feeders of individuals of Passer domesticus from individuals of Zenaida auriculata. On the other hand, potential nest and adult predators of Passer domesticus, such as Milvago chimango and Parabuteo unicinctus, have also increased their abundance. Thus, we suggest that biotic interactions could be related to the Passer domesticus declines in Mar del Plata City.
Zonotrichia capensis is a ground nester species that could be negatively impacted by nest predation (Jokimäki and Huhta, 2000). Therefore, the increase of nest predators such as Milvago chimango could be related to the decline of the Zonotrichia capensis in Mar del Plata City.
Several species that have been expanding geographically their breeding range in the region during the last three decades, such as Parabuteo unicinctus, Hylocharis chrysura, Hirundo rustica, and Sturnus vulgaris (Pinto, 2005; Grande et al., 2015; Winkler et al., 2017; Ojeda et al., 2022; Leveau, 2021), showed strong or moderate interannual increases in their abundances in Mar del Plata City. Regional changes associated with increased crop cover, the construction of paved roads with concrete bridges, and climate warming probably enhanced the availability of food and nesting resources for these species (Pinto, 2005; Winkler et al., 2017; Leveau, 2021). Our results suggest that these species have found suitable habitats in urban areas during their expansion process. For example, Parabuteo unicinctus has suitable nesting substrates in wooded residential areas and urban parks, and increased food availability due to the abundance increase of pigeons and doves (Leveau, 2021). In the case of Hirundo rustica and Sturnus vulgaris, urban areas provide suitable nesting sites in buildings (Leveau, 2021), whereas Sturnus vulgaris can benefit from food availability in lawn areas (Ibañez et al., 2023).
Increased crop cover and climate warming in the region probably favored other resident bird species abundance regional increment, thus favoring their population increases in Mar del Plata City. For example, Milvago chimango, Patagioenas picazuro, Patagioenas maculosa, Zenaida auriculata, and Myiopsitta monachus have been shown to boost their abundances during the period 2002-2012 in the northern part of the pampean region (Gavier-Pizarro et al., 2012; Goijman et al., 2015). Assuming that these population trends remained in the rest of the pampean region, they could have influenced the bird population dynamics in Mar del Plata City. This hypothesis support recent findings of Skjelvik and Dale (2024), who found that local long-term bird trend abundances in urban green spaces of Oslo were correlated with regional bird trends. Therefore, regional and local environmental changes would mold bird population long-term dynamics in urban areas (Skjelvik and Dale, 2024).
Local environmental factors such as vegetation succession may be related to the population trends of bird species (Clements, 1916; Mönkkönen and Helle, 1989). For example, Zenaida auriculata, Turdus rufiventris, and Pitangus sulphuratus increased their abundance in the urban habitat. These species use wooded streets for foraging and nesting (Curzel et al., 2021). Street tree height and crown size are expected to increase with time (Rosenzweig, 1995; Zhao et al., 2016), thus increasing bird resource availability. On the other hand, Elaenia parvirostris has been recorded only in suburban and periurban habitats and had a strong abundance increase over time. This species inhabits wooded and residential areas with high habitat diversity (Cueto and Lopez de Casenave, 2000; Leveau, 2013) and was probably favored by increased vegetation complexity promoted by succession.
Interannual changes of species assemblages
The mean dissimilarity among years was lower in the urban habitat than in the suburban and periurban habitats. Therefore, the interannual presence of species was more persistent in the most urbanized areas. This pattern agrees with previous results obtained in the study area over three years (Leveau and Leveau, 2012; Leveau et al., 2015) and elsewhere (Suhonen et al., 2009). In our study area, the urban habitat was composed of a few dominant species, such as Columba livia, Zenaida auriculata, and Passer domestics. Their high abundances could lower the probability of local extinction due to environmental stochastic events or individual stochastic variation of birth, death, or offspring production (Vellend, 2010).
Although the change signal was low, the three habitats showed similar rates of compositional changes with time lags. The low signal attributed to the sole effect of time lag, and the greater effect attributed to both time lags and transects suggested that the signal of change varied among transects. This pattern agrees with the hierarchical patch dynamics (Wu and Loucks, 1995), where patches differ in their rates of change over time (see Collins and Xia, 2015). In our case, transects may represent patches with different environmental characteristics that allow species invasions and instability or species persistence over time and stability. For example, the establishment of new populations of Parabuteo unicinctus in Mar del Plata city may require sites well connected to other green areas (Leveau, 2021).
Contrary to what was expected, bird assemblages in the three habitat types were similarly unstable. This instability along the gradient could indicate that large-scale disturbances, such as increasing crop cover or climate warming, are directing the local changes in bird assemblages. The local interannual increases of several species that also showed regional increases and distributional expansions support this hypothesis. In turn, the increases in these species are supposed to cause the local population declines of several species by increasing competition and predation. Due to the successional vegetation change correlated with the regional changes in land cover and climate, it is difficult to disentangle their relative roles in the directional changes of bird assemblages.
The increased human population in Mar del Plata City during the study period could have negatively impacted bird communities by increased pedestrian traffic and noise (Fernández-Juricic, 2000; Carral-Murrieta et al., 2020). In addition, increased noise could have decreased bird species detectability (Rodríguez Arancibia et al., 2022). However, most species in our study showed increased abundance or were stable over time, suggesting that human population increase had a negligible effect on the analyzed bird communities.
Conclusions
The results obtained showed that bird assemblages along the urban gradient of Mar del Plata City had increasing dissimilarity with the passing of years. Therefore, bird assemblages were unstable with continuous changes in species abundances. Due to the rate of changes being similar between the urban habitats, the interannual changes in species abundances were probably influenced by regional changes in land uses and climate which promoted population increases of some bird species, such as pigeons and doves. However, species declines along the urban gradient, such as those of House Sparrows, are thought to be related to increased competition with doves. The results highlight the importance of long-term studies in urban areas to get more insights into assemblage dynamics.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://www.researchgate.net/publication/383603275_Data_from_Leveau_2024_Frontiers_in_Ecology_and_Evolution.
Author contributions
LL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Partially funded by the Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación, PICT 2015–0978 and PICT-2018-03871.
Acknowledgments
A thank Carlos M. Leveau for his help during field work.
Conflict of interest
The author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Baeza S., Paruelo J. M. (2020). Land use/land cover chang [amp]]ndash;2014) in the Rio de la Plata grasslands: an analysis based on MODIS NDVI time series. Remote Sens. 12, 381. doi: 10.3390/rs12030381
Bates D., Mächler M., Bolker B. M., Walker S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Software 67, 1–48. doi: 10.18637/jss.v067.i01
Berigan L. A., Greig E. I., Bonter D. N. (2020). Urban house sparrow (Passer domesticus) populations decline in North America. Wilson J. Ornithology 132, 248–258. doi: 10.1676/1559-4491-132.2.248
Bernat-Ponce E., Gil-Delgado J. A., López-Iborra G. M. (2020). Replacement of semi-natural cover with artificial substrates in urban parks causes a decline of house sparrows Passer domesticus in Mediterranean towns. Urban Ecosyst. 23, 471–481. doi: 10.1007/s11252-020-00940-4
Breheny P., Burchett W. (2017). Visualization of regression models using visreg. R J. 9, 56–71. doi: 10.32614/RJ-2017-046
Brooks M., Bolker B., Kristensen K., Maechler M., Magnusson A., McGillycuddy M. (2023). Package ‘glmmtmb’. R Packag Vers 1, 7.
Campbell C. E., Jones D. N., Awasthy M., Castley J. G., Chauvenet A. L. (2022). Big changes in backyard birds: An analysis of long-term changes in bird communities in Australia’s most populous urban regions. Biol. Conserv. 272, 109671. doi: 10.1016/j.biocon.2022.109671
Carral-Murrieta C. O., García-Arroyo M., Marín-Gómez O. H., Sosa-López J. R., MacGregor-Fors I. (2020). Noisy environments: untangling the role of anthropogenic noise on bird species richness in a Neotropical city. Avian Res. 11, 1–7. doi: 10.1186/s40657-020-00218-5
Clements F. E. (1916). Plant succession: an analysis of the development of vegetation (No. 242). Carnegie institution of Washington.
Collins S. L., Micheli F., Hartt L. (2000). A method to determine rates and patterns of variability in ecological communities. Oikos 91, 285–293. doi: 10.1034/j.1600-0706.2000.910209.x
Collins S. L., Xia Y. (2015). Long-term dynamics and hotspots of change in a desert grassland plant community. Am. Nat. 185, E30–E43. doi: 10.1086/679315
Cueto V. R., Lopez de Casenave J. N. (2000). Bird assemblages of protected and exploited coastal woodlands in east-central Argentina. Wilson Bull. 112, 395–402. doi: 10.1676/0043-5643(2000)112[0395:BAOPAE]2.0.CO;2
Curzel F. E., Bellocq M. I., Leveau L. M. (2021). Local and landscape features of wooded streets influenced bird taxonomic and functional diversity. Urban Forestry Urban Greening 66, 127369. doi: 10.1016/j.ufug.2021.127369
Dandapat A., Banerjee D., Chakraborty D. (2010). The case of the Disappearing House Sparrow (Passer domesticus indicus). Veterinary World 3, 97.
de la Peña M. R. (2013). Nidos y Reproducción de las Aves Argentinas; Ediciones Biológica (Santa Fe, Argentina: Ediciones Biologica).
Dornelas M., Gotelli N. J., McGill B., Shimadzu H., Moyes F., Sievers C., et al. (2014). Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299. doi: 10.1126/science.1248484
Echeverry-Galvis M. A., Lozano Ramírez P., Amaya-Espinel J. D. (2023). Long-term Christmas Bird Counts describe neotropical urban bird diversity. PloS One 18, e0272754. doi: 10.1371/journal.pone.0272754
Fernández-Juricic E. (2000). Local and regional effects of pedestrians on forest birds in a fragmented landscape. Condor 102, 247–255. doi: 10.1093/condor/102.2.247
Ferrelli F., Brendel A. S., Perillo G. M. E., Piccolo M. C. (2021b). Warming signals emerging from the analysis of daily changes in extreme temperature events over Pampas (Argentina). Environ. Earth Sci. 80, 422. doi: 10.1007/s12665-021-09721-4
Ferrelli F., Brendel A. S., Piccolo M. C., Perillo G. M. E. (2021a). Evaluación de la tendencia de la precipitación en la región pampeana (Argentina) durante el período 1960-2018. Ra’e Ga 51, 41–57. doi: 10.5380/raega.v51i0
Fidino M., Magle S. B. (2017). “Trends in long-term urban bird research,” in Ecology and conservation of birds in urban environments. Eds. Murgui E., Hedblom M. (Springer, Cham, Switzerland), 161–184. doi: 10.1007/978-3-319-43314-1
Gavier-Pizarro G. I., Calamari N. C., Thompson J. J., Canavelli S. B., Solari L. M., Decarre J., et al. (2012). Expansion and intensification of row crop agriculture in the Pampas and Espinal of Argentina can reduce ecosystem service provision by changing avian density. Agriculture Ecosyst. Environ. 154, 44–55. doi: 10.1016/j.agee.2011.08.013
Goijman A. P., Conroy M. J., Bernardos J. N., Zaccagnini M. E. (2015). Multi-season regional analysis of multi-species occupancy: implications for bird conservation in agricultural lands in east-central Argentina. PloS One 10, e0130874. doi: 10.1371/journal.pone.0130874
Grande J. M., Santillán M. A., Orozco P. M., Liébana M. S., Reyes M. M., Galmes M. A., et al. (2015). Barn Swallows keep expanding their breeding range in South America. Emu 115, 256–260. doi: 10.1071/MU14097
Grimm N. B., Faeth S. H., Golubiewski N. E., Redman C. L., Wu J., Bai X., et al. (2008). Global change and the ecology of cities. Science, 319 (5864), 756-760.
Hartig F. (2022).DHARMa: residual diagnostics for hierarchical (Multi-level/mixed) regression models. In: R package version 0.4.6. Available online at: https://CRAN.Rproject.org/package=DHARMa (Accessed 22 December 2022).
Ibañez L., Palacio F. X., Maragliano R. E., Montalti D. (2023). The presence of an invasive bird, the Common Starling, in an urban landscape: habitat use and relationships with other bird species. J. Ornithology 164, 537–546. doi: 10.1007/s10336-023-02047-x
Jokimäki J., Huhta E. (2000). Artificial nest predation and abundance of birds along an urban gradient. Condor 102, 838–847. doi: 10.1093/condor/102.4.838
La Sorte F. A., Lepczyk C. A., Aronson M. F., Goddard M. A., Hedblom M., Katti M., et al. (2018). The phylogenetic and functional diversity of regional breeding bird assemblages is reduced and constricted through urbanization. Diversity Distributions 24, 928–938. doi: 10.1111/ddi.12738
Lefcheck J. S. (2016). piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods in Ecology and Evolution 7 (5), 573-579.
Leong M., Roderick G. K. (2015). Remote sensing captures varying temporal patterns of vegetation between human-altered and natural landscapes. PeerJ 3, e1141. doi: 10.7717/peerj.1141
Leveau L. M. (2013). Relaciones aves–habitat en el sector suburbano de mar del plata, Argentina. Ornitología Neotropical 24, 201–212.
Leveau L. M. (2018). Urbanization, environmental stabilization and temporal persistence of bird species: a view from Latin America. PeerJ 6, e6056. doi: 10.7717/peerj.6056
Leveau L. M. (2021). The Harris Hawk (Parabuteo unicinctus) in urban areas of Argentina: arrival in Mar Del Plata City and green area use in Buenos Aires City. Animals 11, 1023. doi: 10.3390/ani11041023
Leveau L. M., Isla F. I., Bellocq M. I. (2015). Urbanization and the temporal homogenization of bird communities: a case study in central Argentina. Urban Ecosyst. 18, 1461–1476. doi: 10.1007/s11252-015-0469-1
Leveau L. M., Leveau C. M. (2004). Comunidades de aves en un gradiente urbano de la ciudad de Mar del Plata, Argentina. El Hornero 19, 13–21. doi: 10.56178/eh.v19i1
Leveau L. M., Leveau C. M. (2012). The role of urbanization and seasonality on the temporal variability of bird communities. Landscape Urban Plann. 106, 271–276. doi: 10.1016/j.landurbplan.2012.03.008
MacArthur R. H., MacArthur J. W. (1961). On bird species diversity. Ecology 42, 594–598. doi: 10.2307/1932254
MacGregor-Fors I. (2011). Misconceptions or misunderstandings? On standardization basic terms definitions urban ecology. Landscape Urban Plann. 100, 347–349. doi: 10.1016/j.landurbplan.2011.01.013
Marzluff J. M., Bowman R., Donnelly R. (2001). “A historical perspective on urban bird research: trends, terms, and approaches,” in Avian ecology and conservation in an urbanizing world, 1–17. Springer Science+Business Media, New York.
Mönkkönen M., Helle P. (1989). Migratory habits of birds breeding in different stages of forest succession: a comparison between the Palaearctic and the Nearctic. Annales Zoologici Fennici 26, 323–330.
Ojeda V., Chazarreta M. L., Masello J. F., Buglione-Rodríguez F., Failla M. (2022). European starlings expand into Patagonia. Time action. Global Ecol. Conserv. 39, e02295. doi: 10.1016/j.gecco.2022.e02295
Oksanen J., Simpson G., Blanchet F., Kindt R., Legendre P., Minchin P., et al. (2022).Vegan: community ecology package. In: R package version 2.6-2. Available online at: https://CRAN.R-project.org/package=vegan (Accessed 22 December 2022).
Oyarzabal M., Clavijo J., Oakley L., Biganzoli F., Tognetti P., Barberis I., et al. (2018). Unidades de vegetación de la Argentina. Ecología Austral 28, 40–63. doi: 10.25260/EA.18.28.1.0.399
Palacio F. X., Ibañez L. M., Maragliano R. E., Montalti D. (2018). Urbanization as a driver of taxonomic, functional, and phylogenetic diversity losses in bird communities. Can. J. Zoology 96, 1114–1121. doi: 10.1139/cjz-2018-0008
Pinto E. D. E. (2005). Range expansion of the European Starling Sturnus vulgaris in Argentina. Ardeola 52, 359–364.
R Core Team (2019). “R: a language and environment for statistical computing,” in R foundation for statistical computing, version 3.6.1. Vienna.
Rodríguez Arancibia J., Escobar M. A., Villaseñor N. R. (2022). Efecto del ruido, cobertura arbórea y hora del día sobre la detectabilidad de aves en ecosistemas urbanos. El Hornero 37, 12–12. doi: 10.56178/eh.v37i2.406
Rosenzweig M. L. (1995). Species diversity in space and time (Cambridge: Cambridge University Press).
Servicio Meteorológico Nacional (2023). Estadísticas climatológicas normales: república Argentina - período 1991-2020 (SMN), 847.
Shaw L. M., Chamberlain D., Evans M. (2008). The House Sparrow Passer domesticus in urban areas: reviewing a possible link between post-decline distribution and human socioeconomic status. J. Ornithology 149, 293–299. doi: 10.1007/s10336-008-0285-y
Shochat E., Stefanov W. L., Whitehouse M. E. A., Faeth S. H. (2004). Urbanization and spider diversity: Influences of human modification of habitat structure and productivity. Ecol. Appl. 14, 268–280. doi: 10.1890/02-5341
Shochat E., Warren P. S., Faeth S. H., McIntyre N. E., Hope D. (2006). From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 21, 186–191. doi: 10.1016/j.tree.2005.11.019
Skjelvik C. E., Dale S. (2024). Bird population changes in urban green spaces explained by regional population trends. Urban Ecosyst., 1339–1347. doi: 10.1007/s11252-024-01527-z
Storch D., Koleček J., Keil P., Vermouzek Z., Voříšek P., Reif J. (2023). Decomposing trends in bird populations: Climate, life histories and habitat affect different aspects of population change. Diversity Distributions 29, 572–585. doi: 10.1111/ddi.13682
Suhonen J., Jokimäki J., Kaisanlahti-Jokimäki M. L., Hakkarainen H., Huhta E., Inki K., et al. (2009). Urbanization and stability of a bird community in winter. Ecoscience 16, 502–507. doi: 10.2980/16-4-3280
Väisänen R. A., Järvinen O., Rauhala P. (1986). How are extensive, human-caused habitat alterations expressed on the scale of local bird populations in boreal forests? Ornis Scandinavica 17, 282–292. doi: 10.2307/3676839
Vazquez M. S., Scorolli A. L., Zalba S. M. (2024). Range expansion of native thrushes in South America. Ornithology Res., 1–11. doi: 10.1007/s43388-024-00195-z
Vellend M. (2010). Conceptual synthesis in community ecology. Q. Rev. Biol. 85, 183–206. doi: 10.1086/652373
Wiens J. A. (1989). The ecology of bird communities (Vol. 2) (Cambridge: Cambridge University Press).
Winkler D. W., Gandoy F. A., Areta J. I., Iliff M. J., Rakhimberdiev E., Kardynal K. J., et al. (2017). Long-distance range expansion and rapid adjustment of migration in a newly established population of barn swallows breeding in Argentina. Curr. Biol. 27, 1080–1084. doi: 10.1016/j.cub.2017.03.006
Wolda H. (1981). Similarity indices, sample size and diversity. Oecologia 50, 296–302. doi: 10.1007/BF00344966
Wu J., Loucks O. L. (1995). From balance of nature to hierarchical patch dynamics: a paradigm shift in ecology. Q. Rev. Biol. 70, 439–466. doi: 10.1086/419172
Keywords: avian, disturbance, composition, persistence, stability, urbanization
Citation: Leveau LM (2024) Long-term directional changes in urban bird communities of Mar del Plata City, Argentina. Front. Ecol. Evol. 12:1457476. doi: 10.3389/fevo.2024.1457476
Received: 30 June 2024; Accepted: 21 August 2024;
Published: 09 September 2024.
Edited by:
Salvador García-Ayllón Veintimilla, Polytechnic University of Cartagena, SpainReviewed by:
Charles Nilon, University of Missouri, United StatesChan Ryul Park, National Institute of Forest Sicence (NIFoS), Republic of Korea
Copyright © 2024 Leveau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucas M. Leveau, lucasleveau@yahoo.com.ar
 Lucas M. Leveau
Lucas M. Leveau