- Laboratory of Organic and Metal-Organic Nitrogen-Oxygen Systems, N. D. Zelinsky Institute of Organic Chemistry, Moscow, Russia
Editorial on the Research Topic
Heterodienes in organic synthesis
Introduction
Vinylogous systems have always been in focus of organic chemists due to their unique reactivity, structure, and synthetic application (Curti et al., 2020). Heterodienes are among the most simple and valuable vinylogous systems in organic chemistry. The presence of heteroatoms in the conjugated diene induces specific polarization of the π-system leading to versatile reactivity patterns (Lopes et al., 2018).
Among the most widely utilized heterodienes in organic synthesis are α,β-unsaturated carbonyl compounds (enones), 1- and 2-azadienes, 1,2-diaza-1,3-butadienes (azoalkenes), nitro- and nitrosoalkenes, 2,3-diaza-1,3-butadienes, and α-dicarbonyl compounds and α-diimines (Figure 1A). Although they are chemically distinct species, their reactivity shares common features (Figure 1B). First, most of heterodienes are reactive Michael acceptors in reactions with various nucleophiles (Lopes et al., 2018; Weinreb, 2019). Second, similarly to normal dienes, heterodienes enter [4 + 2]-cycloaddition reactions. Due to their electron-deficient nature, heterodienes react only with electron-rich dienophiles via an inverse-electron demand Diels–Alder reaction (Baiazitov and Denmark, 2013; Png et al., 2017). Third, being highly polarized 1,4-synthons, heterodienes are convenient partners for stepwise [4 + 1]- [4 + 3]- and [4 + 4]- and other [4 + n]-annulation processes involving ylides, carbenoids, and related species (Selvaraj et al., 2020; Ushakov et al., 2022; Wang et al., 2024). Additionally, they are commonly involved in multi-component condensation reactions that lead to the formation of valuable heterocyclic scaffolds (Attanasi et al., 2009; Lopes et al., 2018; Heredia-Moya et al., 2022). Heterodiene reactions can be conducted using a variety of organo- and metal-based catalysts, enabling the asymmetric synthesis of valuable products, especially those found in natural sources and pharmaceuticals. Enantioselective Michael addition, hetero-Diels-Alder, and cascade reactions with stable heterodienes (mostly, conjugated enones and nitroalkenes) have been successfully developed in recent years (Jiang and Wang, 2013).
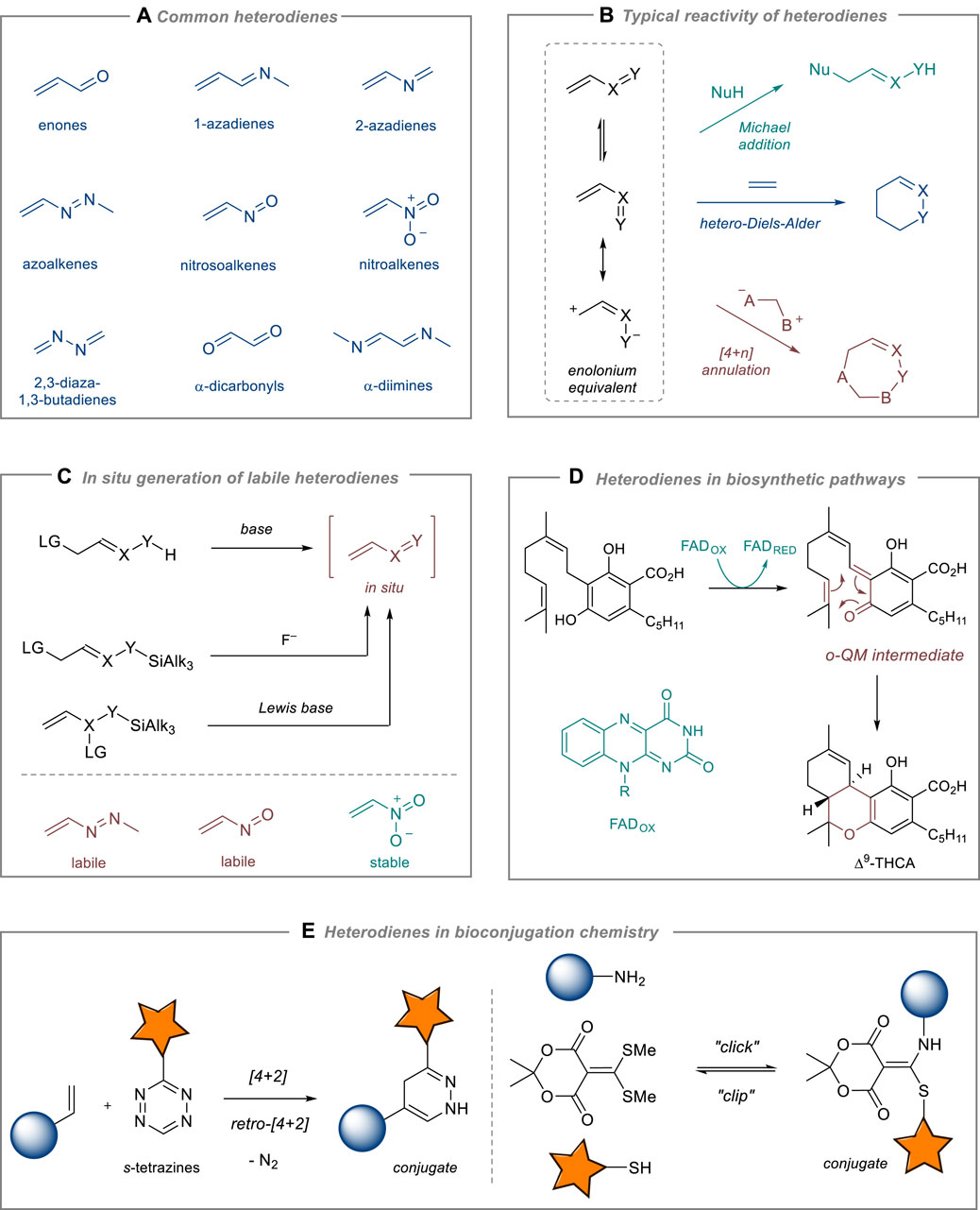
Figure 1. Chemistry and applications of heterodienes. (A) Common heterodienes. (B) Typical reactivity of heterodienes. (C) In situ generation of labile heterodienes. (D) Heterodienes in biosynthetic pathways. (E) Heterodienes in bioconjugation chemistry.
The Frontiers Research Topic “Heterodienes in Organic Synthesis” comprises a Research Topic of original research articles dealing with the chemistry and applications of heterodienes. This Research Topic consists of four articles, which reflect on modern trends in the synthetic chemistry of the azoalkenes, nitrosoalkenes, as well as α,β-unsaturated carbonyl compounds and imines.
Stability of heterodienes
Heterodienes are known to be reactive and chemically labile species. Thus, azoalkenes and nitrosoalkenes, unless stabilized with bulky or strong EWG groups, are prone to dimerization and polymerization reactions. These heterodienes are generated in situ from the corresponding stable precursors (α-halohydrazones, α-halooximes and their silyl ethers, ene-nitroso acetals, Figure 1C). In contrast, conjugated nitroalkenes are normally bench-stable, yet highly reactive heterodienes. Michael addition to nitroalkenes affords β-functionalized nitro derivatives that can be further transformed into amines (via reduction of NO2 group), carbonyls (via Nef reaction), oximes (via interrupted Nef and Meyer reactions), and other useful products (Ballini et al., 2007; Sukhorukov, 2023). Nitroalkenes are recognized for their stability and versatile chemistry, making them essential building blocks in organic synthesis along with enones (Halimehjani et al., 2014).
Heterodienes in biosynthesis
Apart from organic synthesis, heterodienes play a crucial role in the fields of biochemistry and biotechnology, with continuously expanding applications. Recent research on the biosynthesis of natural compounds has shown that Nature extensively exploits the versatile chemistry of heterodienes. The biosynthetic machinery utilizes the conjugate addition of enolate-type nucleophiles to α,β-unsaturated carbonyl compounds to synthesize structurally diverse natural products, for example, polyketides (Miyanaga, 2019). More surprisingly, the hetero-Diels-Alder reaction of unstable ortho-quinone methides (o-QMs) catalyzed by specific enzymes (in particular, hetero-Diels-Alderases) was recently discovered to be a key stage in the biosynthesis of cannabinoids (Purdy et al., 2022) and some sesquiterpenes (Chen et al., 2019) (Figure 1D). Heterodiene chemistry offers extensive possibilities for bioconjugation via fast and catalyst-free “click”-like reactions compatible with in vivo conditions, for example, [4 + 2]-cycloaddition of 1,2,4,5-tetrazines (s-tetrazines) (Oliveira et al., 2017; Zare et al., 2022). Moreover, a reversible character of the Michael addition to heterodienes has been utilized to design “clip” reactions for controllable reversible bioconjugation chemistry (Diehl et al., 2016) (Figure 1E).
Azoalkenes
Conjugated azoalkenes are highly promising intermediates in organic synthesis since they are synthetic equivalents of enolonium cation (reversely polarized synthon to enolate anion) (Attanasi et al., 2009; Uteuliyev et al., 2015). Being powerful Michael acceptors, azoalkenes react with a variety of nucleophiles leading to α-substituted hydrazones that can be further hydrolyzed to ketones. However, the use of P-nucleophiles in these reactions is very limited. The report by Alexey Sukhorukov et al. describes a convenient protocol for the Michael addition of phosphine oxides R2P(O)H to the in situ-generated azoalkenes. The developed method provides a convenient route to β-hydrazonophosphine oxides that are precursors to important organophosphorus compounds, including phosphorylated N-heterocycles, α-aminophosphonates, and vinylphosphonates.
Nitroso- and nitroalkenes
Nitroso- and nitroalkenes are extensively utilized as 4π synthons in hetero-Diels-Alder reactions with electron-rich alkenes. This methodology provides straightforward access to 1,2-oxazines and their N-oxides (cyclic nitronic esters) that serve as intermediates in the synthesis of highly functionalized natural products with multiple stereogenic centers (Denmark et al., 2008; Malykhin et al., 2024). The report by Teresa Pinho e Melo et al. deals with experimental and theoretical studies on the regioselectivity of the [4 + 2]-cycloaddition of ethyl nitrosoacrylate with pyrroles, indoles, and 1,6-dihydropyrrolo[3,2-c]carbazoles leading to fuzed 1,2-oxazine systems. Using the developed approach, a new heterocyclic system, namely, hexahydropyrido[4′,3':4,5]pyrrolo[3,2-c]carbazole, was assembled by the authors.
Other heterodienes
Multi-component one-pot reactions using heterodienes are currently undergoing significant development. In this Research Topic, Yue Zhang et al. report new photocatalytic trichloromethyl radical-triggered annulative reactions of amide-linked 1,7-diynes with polyhalomethanes. This process involves a cascade of Kharasch-type addition/nucleophilic substitution/elimination reactions leading to densely substituted polyhalogenated quinolin-2(1H)-one derivatives. In another report in this field, Fabiana Nador et al. developed a Cu-catalyzed A3-type coupling between pyridine-2-carbaldehyde, an aromatic alkyne, and a substituted tetrahydroisoquinoline to give new indolizine-dihydroisoquinoline hybrid dyes. The obtained products exhibit pH-dependent changes in the UV-Vis spectra and color which makes them attractive candidates to use as pH indicators.
Conclusion
The cutting-edge research articles published in this Frontiers Research Topic highlight that the chemistry of heterodienes continues to be an exciting and challenging research area, in which many more discoveries will be made.
Author contributions
AS: Conceptualization, Funding acquisition, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Russian Science Foundation, grant number 22-13-00230, https://rscf.ru/project/22-13-00230/.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Attanasi, O. A., De Crescentini, L., Favi, G., Filippone, P., Mantellini, F., Perrulli, F. R., et al. (2009). Cultivating the passion to build heterocycles from 1,2-Diaza-1,3-dienes: the force of imagination. Eur. J. Org. Chem. 2009, 3109–3127. doi:10.1002/ejoc.200900243
Baiazitov, R. Y., and Denmark, S. E. (2013). “Tandem [4+2]/[3+2] cycloadditions,” in Methods and applications of cycloaddition reactions in organic syntheses. Editor N. Nishiwaki (United States: John Wiley and Sons), 471.
Ballini, R., Palmieri, A., and Righi, P. (2007). Highly efficient one- or two-step sequences for the synthesis of fine chemicals from versatile nitroalkanes. Tetrahedron 63, 12099–12121. doi:10.1016/j.tet.2007.09.024
Chen, Q., Gao, J., Jamieson, C., Liu, J., Ohashi, M., Bai, J., et al. (2019). Enzymatic intermolecular hetero-diels–alder reaction in the biosynthesis of tropolonic sesquiterpenes. J. Am. Chem. Soc. 141, 14052–14056. doi:10.1021/jacs.9b06592
Curti, C., Battistini, L., Sartori, A., and Zanardi, F. (2020). New developments of the principle of vinylogy as applied to π-extended enolate-type donor systems. Chem. Rev. 120, 2448–2612. doi:10.1021/acs.chemrev.9b00481
Denmark, S. E., Nguyen, S. T., and Baiazitov, R. Y. (2008). Asymmetric synthesis of the ABCD ring system of daphnilactone B via a tandem, double intramolecular, [4+ 2]/[3+ 2] cycloaddition strategy. Heterocycles 76, 143. doi:10.3987/com-08-s(n)15
Diehl, K. L., Kolesnichenko, I. V., Robotham, S. A., Bachman, J. L., Zhong, Y., Brodbelt, J. S., et al. (2016). Click and chemically triggered declick reactions through reversible amine and thiol coupling via a conjugate acceptor. Nat. Chem. 8, 968–973. doi:10.1038/nchem.2601
Halimehjani, A. Z., Namboothiri, I. N. N., and Hooshmand, S. E. (2014). Nitroalkenes in the synthesis of carbocyclic compounds. RSC Adv. 4, 31261–31299. doi:10.1039/C4RA04069D
Heredia-Moya, J., Zurita, D. A., Cadena-Cruz, J. E., and Alcívar-León, C. D. (2022). Diaza-1,3-butadienes as useful intermediate in heterocycles synthesis. Molecules 27, 6708. doi:10.3390/molecules27196708
Jiang, X., and Wang, R. (2013). Recent developments in catalytic asymmetric inverse-electron-demand Diels-Alder reaction. Chem. Rev. 113, 5515–5546. doi:10.1021/cr300436a
Lopes, S. M. M., Cardoso, A. L., Lemos, A., and Pinho E Melo, T. M. V. D. (2018). Recent advances in the chemistry of conjugated nitrosoalkenes and azoalkenes. Chem. Rev. 118, 11324–11352. doi:10.1021/acs.chemrev.8b00375
Malykhin, R. S., Aksenova, S. A., and Sukhorukov, A. Y. (2024). An intramolecular nitroso-Meerwein–Ponndorf–Verley–Oppenauer reaction to access fused pyrrolidine scaffolds. Org. Lett. 26, 450–455. doi:10.1021/acs.orglett.3c03552
Miyanaga, A. (2019). Michael additions in polyketide biosynthesis. Nat. Product. Rep. 36, 531–547. doi:10.1039/C8NP00071A
Oliveira, B. L., Guo, Z., and Bernardes, G. J. L. (2017). Inverse electron demand Diels–Alder reactions in chemical biology. Chem. Soc. Rev. 46, 4895–4950. doi:10.1039/C7CS00184C
Png, Z. M., Zeng, H., Ye, Q., and Xu, J. (2017). Inverse-electron-demand Diels-Alder reactions: principles and applications. Chem. Asian J. 12, 2142–2159. doi:10.1002/asia.201700442
Purdy, T. N., Moore, B. S., and Lukowski, A. L. (2022). Harnessing ortho-quinone methides in natural product biosynthesis and biocatalysis. J. Nat. Prod. 85, 688–701. doi:10.1021/acs.jnatprod.1c01026
Selvaraj, K., Chauhan, S., Sandeep, K., and Swamy, K. C. K. (2020). Advances in [4+3]-Annulation/Cycloaddition reactions leading to homo- and heterocycles with seven-membered rings. Chem. Asian J. 15, 2380–2402. doi:10.1002/asia.202000545
Sukhorukov, A. Y. (2023). Interrupted Nef and Meyer reactions: a growing point for diversity-oriented synthesis based on nitro compounds. Molecules 28, 686. doi:10.3390/molecules28020686
Ushakov, P. Y., Ioffe, S. L., and Sukhorukov, A. Y. (2022). Recent advances in the application of ylide-like species in [4 + 1]-annulation reactions: an updated review. Org. Chem. Front. 9, 5358–5382. doi:10.1039/D2QO00698G
Uteuliyev, M. M., Nguyen, T. T., and Coltart, D. M. (2015). Diastereoselective addition of Grignard reagents to α-epoxy N-sulfonyl hydrazones. Nat. Chem. 7, 1024–1027. doi:10.1038/nchem.2364
Wang, Y., Jin, Z., Zhou, L., and Lv, X. (2024). Recent advances in [4 + 4] annulation of conjugated heterodienes with 1,4-dipolar species for the synthesis of eight-membered heterocycles. Org. Biomol. Chem. 22, 252–268. doi:10.1039/D3OB01626A
Weinreb, S. M. (2019). Nitrosoalkenes: underappreciated reactive intermediates for formation of carbon–carbon bonds. Synlett 30, 1855–1866. doi:10.1055/s-0037-1611899
Zare, F., Potenza, A., Greschner, A. A., and Gauthier, M. A. (2022). Consecutive alkylation, “click”, and “clip” reactions for the traceless methionine-based conjugation and release of methionine-containing peptides, and “clip” reactions for the traceless methionine-based conjugation and release of methionine-containing peptides. Biomacromolecules 23, 2891–2899. doi:10.1021/acs.biomac.2c00357
Keywords: heterodienes, vinylogous systems, Michael addition, cycloadditions, annulations, cascade reactions, N-heterocycles, bioorthogonal chemistry
Citation: Sukhorukov AY (2024) Editorial: Heterodienes in organic synthesis. Front. Chem. 12:1403024. doi: 10.3389/fchem.2024.1403024
Received: 18 March 2024; Accepted: 25 March 2024;
Published: 08 April 2024.
Edited and Reviewed by:
Iwao Ojima, Stony Brook University, United StatesCopyright © 2024 Sukhorukov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexey Yu. Sukhorukov, sukhorukov@ioc.ac.ru, a.yu.sukhorukov@gmail.com
 Alexey Yu. Sukhorukov
Alexey Yu. Sukhorukov