- 1Department of Allergy, Allergy and Immunology Center, Aichi Children’s Health and Medical Center, Obu, Japan
- 2Department of Pediatrics, NTT East Sapporo Hospital, Sapporo, Japan
- 3Department of Pediatrics, Hamamatsu Medical Center, Hamamatsu, Japan
- 4Department of Pediatrics, Bantane Hospital, Fujita Health University, Aichi, Japan
Some food allergic patients who have undergone oral immunotherapy develop exercise-induced allergic reactions on desensitization (EIARDs). This study investigated basophil activation status during the exercise provocation test (EPT) performed to diagnose EIARD. EPT was performed on 20 participants, and in vivo basophil activation status was analyzed using activation markers CD203c and CD63. The results showed that there was no significant difference between EPT-positive and negative subjects for basophil activation status throughout EPT. Consequently, in vivo basophil activation after ingestion of the causative food may not be associated with EIARDs. New tests are desired for predicting EIARDs.
Introduction
Although oral immunotherapy (OIT) is an effective treatment for inducing desensitization in patients with persistent food allergies (1), they sometimes develop allergic reactions induced by exercise after food ingestion. This occurs even after desensitization, and is referred to as exercise-induced allergic reactions on desensitization (EIARDs) (2). The pathophysiology of EIARDs remains unclear. Dua et al. reported that exercise reduced the symptom-provoked threshold by 45% in patients allergic to peanuts (3). Therefore, even patients desensitized to a certain amount of the causative antigen after OIT could develop allergic symptoms through lowering of the symptom-provoked threshold with exercise. Because of the augmented small intestinal permeability with exercise, increasing the amount of the absorbed causative antigen might trigger symptoms (4).
Another typical exercise-induced allergic symptom is food-dependent exercise-induced anaphylaxis (FDEIA). Exercise may enhance the clinical reactions by both lowering the threshold and increasing the severity of the allergic reaction (5, 6). These findings indicate that the pathophysiology of EIARDs is at least partially similar to that of FDEIA.
The exercise provocation test (EPT) for diagnosis of EIARDs is important for determining if patients can ingest causative food safely in daily life, given that exercise after meals is generally inevitable. The test itself is burdensome and may cause severe allergic reactions. Therefore, alternative tests are needed.
Basophils play an important role in IgE-mediated allergic reactions. Recent reports showed that basophil activation is observed after the ingestion of causative foods, even before the occurrence of allergic reactions (7, 8). Based on this finding, we hypothesized that basophils are activated by ingestion of the causative food before the onset of symptoms of EIARDs and that exercise induced further basophil activation leading to EIARDs. If the hypothesis is proven correct, evaluation of the activation status of basophils might help the diagnosis of EIARDs even without EPT. Therefore, we analyzed the basophil-activation status during EPTs for diagnosing EIARDs.
Method
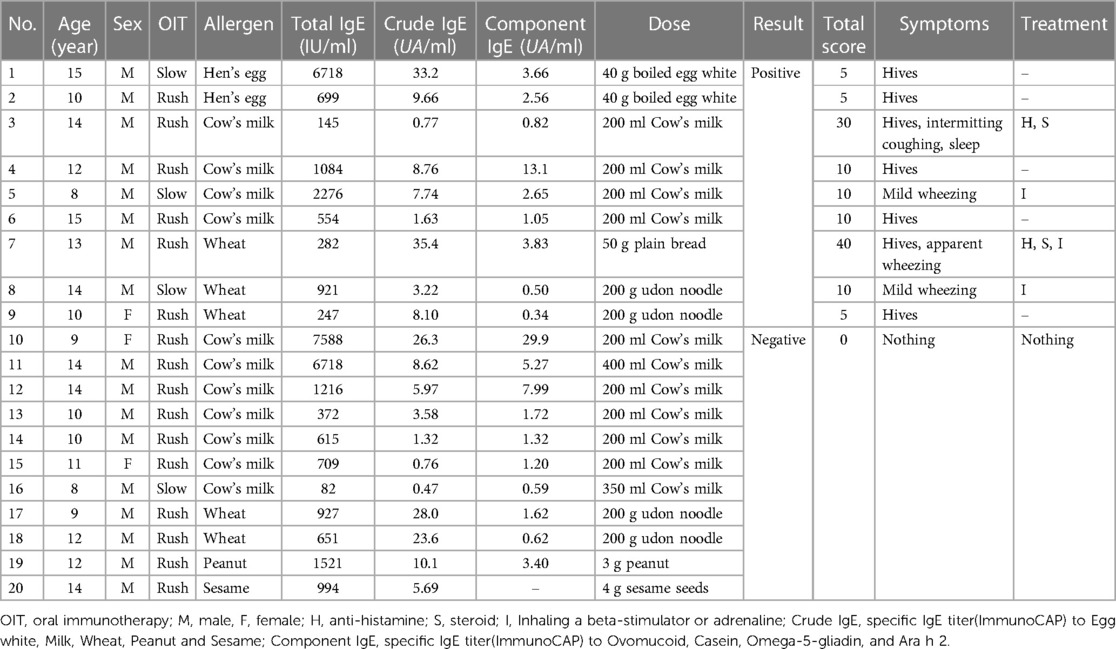
Twenty patients underwent EPTs on their desensitization status between April 2018 and January 2019 after rush (2) or slow (9) oral immunotherapy for each causative food. We judged desensitization to the causative foods by daily ingesting full dose (10) of causative antigen or more without symptoms at least 2 months before EPT. Two patients were allergic to hen's egg, 11 to cow's milk, 5 to wheat, 1 to peanuts, and 1 to sesame seeds. We measured total and specific IgE titers for each causative antigen within 3 months before the EPT. The patient's characteristics are shown in Table 1.
EPTs were performed in our hospital, as previously reported (2). Initially, patients consumed full doses (10) of the causative foods, and after 30 min engaged in free running for approximately 10 min to achieve a target heart rate of >180 beats/min. The provoked symptoms were scored using the total score (TS) of “Anaphylaxis Scoring Aichi” (2, 9). Blood samples were collected during the EPTs: before eating (sample 1), 30 min later (sample 2), immediately after exercise (sample 3), and at the peak of the provoked symptoms (sample 4) (Figure 1). To assess the basophil-activation status after causative food ingestion, peripheral blood samples were incubated with phosphate-buffered saline without further ex vivo stimulation. The in vivo basophil-activation status was evaluated using the Allergenicity kit (Beckman Coulter, Brea, CA) with the anti-CD63 antibody (Anti-Hu CD63-APC; EXBIO, Praha, Vestec, Czech Republic), following the protocol defined by the kit (Supplementary Figure S1). We compared the changes in the activation status by both mean fluorescence intensity (MFI) and CD203c high or CD63 high basophils, which were gated over the top 5% of the expression in comparison to sample 1. In addition, we measured plasma histamine, serum tryptase levels (LSI Medicine Corporation, Tokyo, Japan), and basophil count, which were evaluated using an automated analyzer.
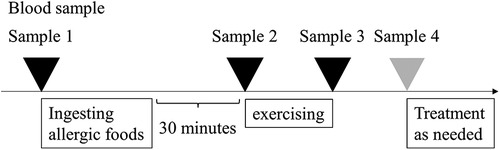
Figure 1. Schematic illustration of the exercise provocation test. The exercise provocation test was performed 30 min after consuming allergic foods. Sample 1; before eating, Sample 2; 30 min later, Sample 3; immediately after exercise, Sample 4; at the peak of the provoked symptoms.
The primary outcome was the change in the expressions of CD203c, CD63, and their MFI on no-stimulation basophils from sample 1 to sample 2 in the patients with a positive/negative result of the EPTs. Secondary outcomes included the change in the expressions of CD203c, CD63, and their MFI of all samples, plasma histamine, tryptase, and peripheral blood basophil counts. To evaluate the validity of the measurement system, we assessed the in vitro basophil-activation status in sample 1 under stimulation by anti-IgE antibodies or each causative antigen. We used the EZR software program (11) (Saitama Medical Center, Jichi Medical University, Saitama, Japan) to analyze data and determined statistical significance (P < 0.05) using the Mann–Whitney U or Wilcoxon rank sum tests. This study was approved by the Institutional Review Board of Aichi Children's Health and Medical Center (20170791). Written informed consent was obtained from all patients before enrollment.
Results
Nine (45%) of the 20 patients experienced provoked symptoms; the median TS was 10 points (5–40). None of the patients developed anaphylactic shock or needed an adrenaline injection. Basophil activation with in vitro stimulation in all patients under anti-IgE antibody and in 19 patients under the causative antigens was observed (data not shown). The expression of CD203c or CD63 and the MFI did not increase in samples 2, 3 or 4 compared to sample 1 (Figure 2). There was no significant difference between EPT-positive and negative subjects for all experimental outcomes (Figure 3 and Supplementary Figure S2). Of the nine EPT-positive patients, two patients—with the highest and second-highest TS—had levels over the standard in-plasma histamine levels: 27.0 and 8.8 ng/ml in samples 4 and 3, respectively. (The reference range is <1.24 ng/ml). However, these subjects did not demonstrate abnormally elevated tryptase levels (normal range <11.4 µg/L, Supplementary Figure 2).
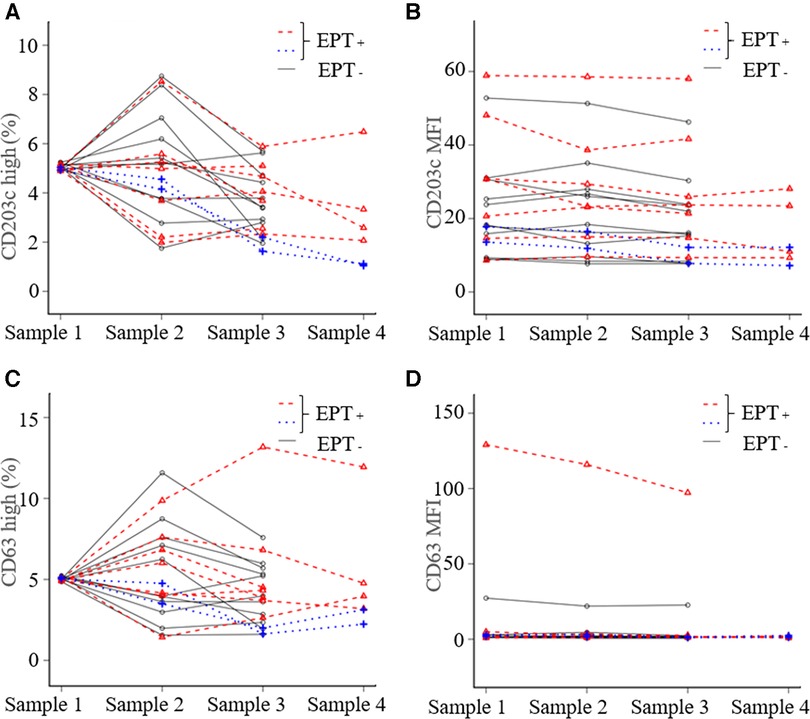
Figure 2. In vivo basophil activation status during the exercise provocation test. The basophil activation status was evaluated based on the expression of activation markers (CD203c and CD63). We compared the changes in the activation status by both mean fluorescence intensity (MFI) and CD203c-high or CD63-high basophils, which were gated over the highest 5% of the expression in comparison to sample 1. (A) CD203c high, (B) CD203c MFI, (C) CD63 high, and (D) CD63 MFI. The blue dotted lines represent the patients who showed the highest and the second-highest TS. Sample 1; before eating, Sample 2; 30 min later, Sample 3; immediately after exercise, Sample 4; at the peak of the provoked symptoms. EPT, exercise provocation test.
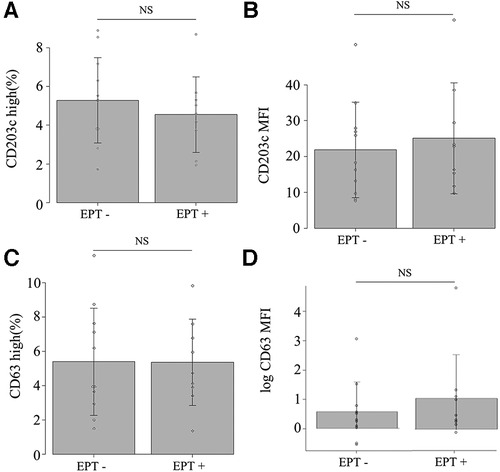
Figure 3. In vivo basophil activation at sample 2 (30 min after eating). (A) CD203c high, (B) CD203c MFI, (C) CD63 high, and (D) CD63 MFI. NS: not significant on the Mann–Whitney U test. EPT, exercise provocation test.
Discussion
Recent studies have reported that anaphylaxis is associated with reduced basophils in peripheral blood because activated basophils may migrate out of the peripheral blood (12). Other studies reported that basophil activation and degranulation might not occur in the peripheral blood, but in migrated tissues during anaphylaxis (8). These results indicate that most basophils migrate to the tissue that develops an allergic reaction. In our study, the patient with the highest TS had a gradually decreasing basophil count and did not have an increased expression of CD203c and CD63; this supports these previous described research findings (Figures 1, 4).
The association between the degranulation of basophils and the upregulation of CD203c and CD63 remains unclear. Increased expression of CD63 after ingestion of a red meat was shown not only in 9 out of 12 patients with α-gal allergy but also in 5 out of 13 healthy patients (7). Moreover, CD203c and CD63 expression of peripheral basophils was not upregulated, despite the histamine release (8, 13). Another study also demonstrated that patients with anaphylaxis showed no basophil activation in the peripheral blood, despite decreased intracellular histamine levels (12). In our study, the upregulation of CD203c and CD63 on basophils was not observed in EIARD-positive patients; these suggest that in vivo basophil activation after ingestion of the causative food may not be associated with EIARDs.
Consequently, detecting the activated basophils by CD203c or CD63 expression may not be a suitable method for predicting EIARDs and the exact cause of EIARDs remains unknown. Rather, we should consider determining basophil counts and factors involved in basophil migration, such as CCL2 (8) or factors causing severe allergic symptoms, such as PAF (13). Although no-stimulation basophil activation for patients with food allergies has already been reported, no consensus has been reached as to how this approach should be used. Keet et al. showed that MFI of the CD203c and CD63 on basophils under a no-stimulation condition significantly decreased with sublingual and oral immunotherapy for milk allergy (14). This suggests that evaluating no-stimulation basophil activation may be valid and effective in predicting whether or not the patients would be desensitized.
This study was exploratory and preliminary in nature; consequently, the sample size was small. Only a few patients developed severe allergic reactions, and experienced increases in total tryptase and plasma histamine, however, we could evaluate activation status of peripheral basophils during severe reaction of EIARDs in the patients.
However, to the best of our knowledge, this is the first study to evaluate basophil activation during EPT for patients with EIARDs. We failed to prove that peripheral basophils activate before—and even during—symptom provocation. We also did not evaluate the utility of basophil-activation status for diagnosing EIARDs; however, our findings may contribute to our understanding of the role of basophils in EIARDs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Aichi Children's Health and Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
JK: Writing – original draft, Data curation, Formal analysis, Investigation, Visualization. SS: Conseptualization, Funding acquisition, Methodology, Project administration, Formal analysis, Writing – review & editing. AS: Investigation, Writing – review & editing. MT: Investigation, Resources, Writing – review & editing. AM: Writing – review & editing. YT: Writing – review & editing. TM: Investigation, Formal analysis, Writing – review & editing. YK: Resources, Writing – review & editing. KI: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was partially funded by a grant from Nipponham Foundation for the Future of Food.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing. We gratefully appreciate the work of performing OIT and EPT from the past and present staff of Aichi Children's Health and Medical Center.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2023.1298137/full#supplementary-material
References
1. Pajno GB, Fernandez-Rivas M, Arasi S, Roberts G, Akdis CA, Alvaro-Lozano M, et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. (2018) 73(4):799–815. doi: 10.1111/all.13319
2. Furuta T, Tanaka K, Tagami K, Matsui T, Sugiura S, Kando N, et al. Exercise-induced allergic reactions on desensitization to wheat after rush oral immunotherapy. Allergy. (2020) 75:1414–22. doi: 10.1111/all.14182
3. Dua S, Ruiz-Garcia M, Bond S, Durham SR, Kimber I, Mills C, et al. Effect of sleep deprivation and exercise on reaction threshold in adults with peanut allergy: a randomized controlled study. J Allergy Clin Immunol. (2019) 144:1584–94.e2. doi: 10.1016/j.jaci.2019.06.038
4. Pals KL, Chang RT, Ryan AJ, Gisolfi CV. Effect of running intensity on intestinal permeability. J Appl Physiol. (1997) 82:571–6. doi: 10.1152/jappl.1997.82.2.571
5. Christensen M J, Eller E, Mortz C G, Brockow K, Bindslev-Jensen C. Exercise lowers threshold and increases severity, but wheat-dependent, exercise-induced anaphylaxis can be elicited at rest. J Allergy Clin Immunol Pract. (2018) 6:514–20. doi: 10.1016/j.jaip.2017.12.023
6. Christensen M J, Eller E, Mortz C G, Brockow K, Bindslev-Jensen C. Wheat-dependent cofactor-augmented anaphylaxis: a prospective study of exercise, aspirin, and alcohol efficacy as cofactors. J Allergy Clin Immunol Pract. (2019) 7:114–21. doi: 10.1016/j.jaip.2018.06.018
7. Commins SP, James HR, Stevens W, Pochan SL, Land MH, King C, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J Allergy Clin Immunol. (2014) 134:108–15. doi: 10.1016/j.jaci.2014.01.024
8. Korošec P, Gibbs BF, Rijavec M, Custovic A, Turner PJ. Important and specific role for basophils in acute allergic reactions. Clin Exp Allergy. (2018) 48:502–12. doi: 10.1111/cea.13117
9. Sugiura S, Kitamura K, Makino A, Matsui T, Furuta T, Takasato Y, et al. Slow low-dose oral immunotherapy: threshold and immunological change. Allergol Int. (2020) 69:601–9. doi: 10.1016/j.alit.2020.03.008
10. Ebisawa M, Ito K, Fujisawa T, Aihara Y, Ito S, Imai T, et al. Japanese guidelines for food allergy 2020. Allergol Int. (2020) 69:370–86. doi: 10.1016/j.alit.2020.03.004
11. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. (2013) 48:452–8. doi: 10.1038/bmt.2012.244
12. Yamaga S, Yanase Y, Ishii K, Ohshimo S, Shime N, Hide M. Decreased intracellular histamine concentration and basophil activation in anaphylaxis. Allergol Int. (2020) 69:78–83. doi: 10.1016/j.alit.2019.05.009
13. Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. (2008) 358:28–35. doi: 10.1056/NEJMoa070030
Keywords: oral immunotherapy, exercise-induced allergic reactions on desensitization, exercise provocation test, basophil activation, CD203c, CD63
Citation: Kunizaki J, Sugiura S, Sakai A, Teshigawara M, Makino A, Takasato Y, Matsui T, Kondo Y and Ito K (2023) Evaluation of peripheral basophil activation during exercise provocation test for desensitized patients. Front. Allergy 4:1298137. doi: 10.3389/falgy.2023.1298137
Received: 21 September 2023; Accepted: 29 November 2023;
Published: 22 December 2023.
Edited by:
Lucie Mondoulet, Independent Researcher, Kremlin Bicêtre, FranceReviewed by:
Matthew P. Giannetti, Harvard Medical School, United StatesJeffrey M Wilson, University of Virginia, United States
© 2023 Kunizaki, Sugiura, Sakai, Teshigawara, Makino, Takasato, Matsui, Kondo and Ito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Komei Ito a291bWVpX2l0b2hAbXguYWNobWMucHJlZi5haWNoaS5qcA==
 Jun Kunizaki
Jun Kunizaki Shiro Sugiura
Shiro Sugiura Akira Sakai1,3
Akira Sakai1,3 Miyuki Teshigawara
Miyuki Teshigawara Atsushi Makino
Atsushi Makino Teruaki Matsui
Teruaki Matsui Yasuto Kondo
Yasuto Kondo Komei Ito
Komei Ito