- 1Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) MultiMedica, Milan, Italy
- 2Scientific and Technological Pole, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) MultiMedica, Milan, Italy
- 3Department of Biotechnology and Life Sciences, University of Insubria, Varese, Italy
- 4Dipartimento Scienze Biomediche e Cliniche Luigi Sacco, ASST Fatebenefratelli-Sacco, Università degli Studi di Milano, Milan, Italy
A two-dose regimen of Pfizer–BioNTech COVID-19 vaccination confers 95% protection against COronaVIrus Disease 19 (COVID-19) and the safety profile is adequate. To the submission date, there were no reports in literature of acute pericarditis after BNT162b2 vaccination. However, pericarditis has been reported as a rare event associated with COVID-19 infection, which could be due to the pro-inflammatory effects of the spike protein. Recent evidence of post-vaccine myocarditis has been published. Herein we describe the case of a middle-aged healthy women who developed symptoms and signs of acute pericarditis 7–10 days after the second dose of Pfizer–BioNTech COVID-19 vaccination. Although a direct effect cannot be stated, it is important to report a potential adverse vaccine reaction effect that could be associated with the expression of SARS-CoV-2 spike protein induced from the mRNA of the vaccine.
Introduction
The Pfizer-BioNTech COronaVIrus Disease 19 (COVID-19) mRNA vaccine BNT162b2 is an mRNA-based COVID-19 vaccine, used to provide protection against infection by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Virus in people (1). It is based on the expression of spike RNA which after inoculation expresses the protein and stimulates immune cells to recognize it.
It is known that a two-dose regimen of BNT162b2 confers 95% protection against Covid-19 and the safety profile is adequate (2). The most commonly reported adverse reactions include injection site pain, fatigue, headache, myalgia, chills, arthralgia, Bell's paralysis and fever, and safety aspects are included in the EU's risk management plan (3). Currently, the only relevant side effect is severe allergic reaction (anaphylaxis). To date, there are no reports in literature of acute pericarditis after Pfizer–BioNTech COVID-19 vaccination.
A systematic review reveals a certain number of coronavirus disease 2019 (COVID-19) patients with pericarditis and summarizes the clinical features, diagnostic methods, treatment, and outcomes of the patients (4). Acute pericarditis has often a dubious underlying etiology. In the case of coronavirus disease 2019 (COVID-19) in patients with pericarditis, although there is no definitive test to prove the causal relationship, this complication could be secondary to viral biological effects of COVID-19 infection (4, 5). It is significant to reflect that pericardial disease could be a late complication of COVID-19, and to have therapeutic solutions available when facing such events (4, 5). The major protein involved with SARS-Cov2 pathogenesis is the spike protein, which causes an imbalance of the Renin-Angiotensin-Aldosterone System (RAAS) (6–9).
Herein we present the case of a middle-aged healthy woman who developed symptoms and signs of acute pericarditis 7–10 days after the second dose of Pfizer–BioNTech COVID-19 vaccination. Since the vaccine is based on endogenous spike production, attention should be paid to this possible, although never reported before, complication.
Clinical Course
A 54-year-old woman, BMI 19.6 Kg/m2, without cardiovascular risk factors and with no previous cardiac history, presented to the Emergency Department (ED) with chest pain and simultaneous left upper arm pain, with radiation to the interscapular region, enhanced by inspiration and supine position. These symptoms occurred ~7–10 days after COVID-19 vaccination. The patient had informed consent.
Upon admission, body temperature was 36.5°C, heart rate was 65 beats per minute, blood pressure was 120/80 mmHg, respiratory rate was 15 times per minute, and oxygen saturation was 96% on ambient air. Pericardial friction rubs were not present and respiratory sounds were normal.
Blood tests showed a white blood cell count of 6,650/mmc (63.3% neutrophils and 27.5% lymphocytes), hemoglobin 14.7 g/dl, C-reactive protein (CRP) at the level of 0.03 mg/dl (reference range 0.05–0.50 mg/dl), estimated glomerular filtration rate 81 ml/min/m2, and high-sensitive troponin I at the level of 1.45 ng/L (reference range 2.50–38.6 ng/L). Serology for COVID-19 showed an IgG level of 148 AU/ml (protective value >11.5 AU/ml), therefore serology for other viruses was not performed.
The electrocardiogram (ECG) showed sinus rhythm with normal atrio-ventricular and intra-ventricular conduction, and normal left ventricular repolarization (not shown). Transthoracic echocardiography (TTE) demonstrated mild posterior pericardial detachment (Figure 1A) and mild anterior pericardial effusion (Figure 1B) that was not hemodynamically significant. Cardiac chambers, biventricular systolic function and both left-sided, and right-sided heart valves were normal. Lung ultrasound excluded pleural effusion. Therefore, the patient was diagnosed with acute pericarditis. She received anti-inflammatory treatment with naproxen sodium 550 mg/die and colchicine 1 mg/die, with gradual regression of symptoms.
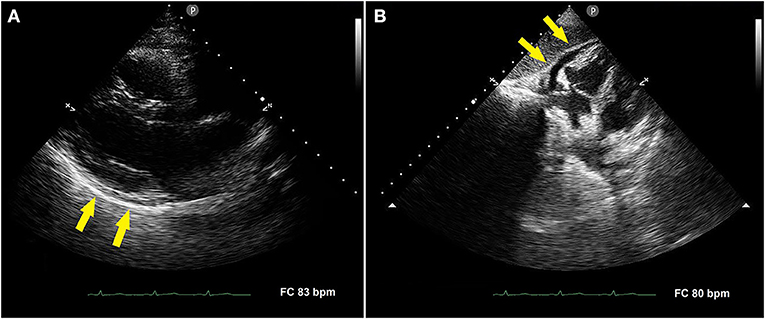
Figure 1. Transthoracic echocardiography performed in the acute phase of disease. Parasternal long-axis view showing mild posterior pericardial detachment [(A), yellow arrows]. Subcostal four-chamber view showing mild anterior pericardial effusion [(B), yellow arrows].
A subsequent TTE, performed after 4 weeks of anti-inflammatory treatment, showed the complete disappearance of both posterior pericardial detachment and anterior pericardial effusion (Figures 2A,B, respectively). Finally, a cardiac magnetic resonance imaging (MRI), performed 40 days after beginning of symptoms, confirmed the complete resolution of pericarditis.
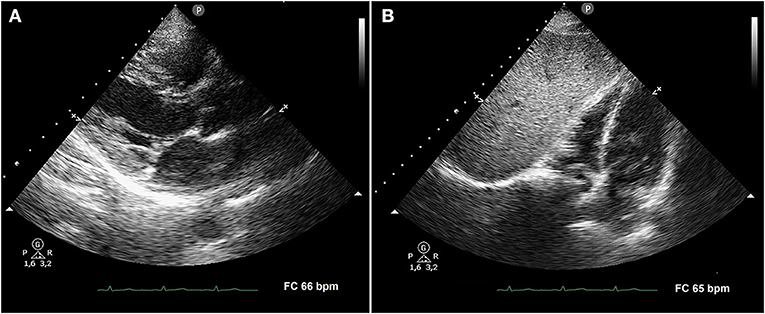
Figure 2. Transthoracic echocardiography performed after 4 weeks of anti-inflammatory treatment. Parasternal long-axis view (A) and subcostal four-chamber view (B) demonstrating the complete disappearance of both posterior pericardial detachment and anterior pericardial effusion, respectively.
Discussion
The peculiarity of the present case is that a healthy woman who received a COVID-19 vaccination (with Pfizer-BioNTech COVID-19 mRNA vaccine BNT162b2) developed symptoms and signs of acute pericarditis ~7–10 days after vaccination.
She was diagnosed with acute pericarditis, meeting 2 diagnostic criteria: typical pain and pericardial effusion (10). On the other hand, ECG, inflammatory and myocardial injury indices were normal. The CRP was tested 3 weeks after symptoms onset and this could be the reason for its value within the normal range.
As suggested by the most recent Guidelines (10, 11) anti-inflammatory therapy and colchicine were safe and effective in reducing symptoms. The patient's clinical course was favorable in the absence of any complications such as large pericardial effusion, tamponade, myopericarditis, high inflammatory indices and the response to colchicine treatment was good (12). Cardiac MRI confirmed the complete regression of pericarditis and the absence of myocardial involvement.
To the best of our knowledge, to the submission date, this is the first reported case of acute pericarditis likely related to COVID-19 vaccination in 54 year women. Rare case series of vaccine-related pericarditis have been reported when using mRNA vaccines against COVID-19, particularly in adolescents and young adults (<30 years) and mainly male; symptom onset is usually within 1 week following vaccination (13–15). Clinical course appears to be mild in most cases, these patients can usually return to their normal daily activities after their symptoms improve (13, 14). A study in forty hospitals in Washington, Oregon, Montana, and Los Angeles County (individuals 2000287 least 1 COVID-19 vaccination) showed 37 vaccinated patients who subsequently had diagnoses of acute pericarditis, mostly were male (73%), and the median age was 59 years, 13 patients were admitted to the hospital, none to intensive care and none of them died (16).
The correlation of typical chest pain and pericardial effusion with COVID-19 vaccination was “suspected” given the absence of other etiologic findings, the temporal relationship to the vaccination, and positive IgG anti-COVID-19 serology. Myocarditis and pericarditis represent serious and life-threatening inflammatory diseases involving myocardium and pericardium, potentially associated with the use of several drugs and vaccines (10, 17, 18). Rare cases of myo-pericarditis after influenza immunization have been reported (19, 20).
In the present case, COVID-19 mRNA vaccine may have triggered an inflammatory response that was responsible for pericardial injury. In particular, the mRNA vaccine might have induced a molecular mimicry mechanism between the viral spike protein and an unknown cardiac protein (21, 22). The vaccine is based on SARS-CoV-2 spike RNA to synthesize the protein antigen. Spike, besides to ACE2 (6–9), binds the toll-like receptor TLR4 (23), and spike protein binds to bacterial lipopolysaccharide (24, 25). The high anti-spike IgG antibody titer and the good response to anti-inflammatory therapy and colchicine suggested the possible immune-mediated etiology of pericarditis (26).
The patient's age (54 years old) was in alignment with that observed in the great majority of COVID-19 related pericarditis (4). Moreover, the present case revealed that the occurrence of a COVID-19 vaccine related cardiac involvement in the absence of pulmonary findings is possible.
In the present case, the normal serum level of high-sensitive troponin I, the normal cardiac chambers size and the normal biventricular systolic function assessed by TTE, allowed to exclude any myocardial injury. On the other hand, the pericardial effusion with normality of the remaining echocardiographic findings suggested exclusive pericardial involvement with no evidence of myocarditis. The diagnosis of pericarditis was formulated on the basis of two diagnostic criteria: (1) the typical chest pain and (2) the pericardial effusion.
To date, cardiac injury (troponin I elevation, ECG and echocardiography abnormalities) has been observed in ~7.2% of patients with severe and critical COVID-19 infection (27–33), while pericardial effusion was found in 4.8% of patients, which suggests that acute pericarditis could be an underdiagnosed pathology, and therefore, not properly managed and treated (5, 34, 35).
The pathogenesis of cardiac involvement associated with SARS-CoV-2 may reflect a process of replication and dissemination of the virus through the blood or the lymphatic system from the respiratory tract. Alternatively, SARS-CoV-2 could trigger an exaggerated inflammatory response that can cause myocardial injury (6, 36). Finally, a potential binding to a viral receptor of the myocyte can favor the internalization and subsequent replication of the capsid proteins and the viral genome (37, 38).
Currently, a validated test to assess COVID-19 in the pericardial fluid need to be developed. COVID-19 studies are needed to determine in pericardial fluid and how to correctly check for its occurrence (35).
Conclusion
A cardiac involvement in the absence of pulmonary findings could be suspected in symptomatic subjects who have undergone COVID-19 vaccination. Acute pericarditis is often underdiagnosed. The occurrence of an acute pericarditis related to a COVID-19 vaccination may be underpinned by an immune-mediated mechanism.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author Contributions
AS, ML, and PS: conceptualization. AS, ML, and AB: performed the clinical testing of the patient. AS, ML, AB, PS, DN, and AA: analyzed data. AS, AA, DN, ML, and PS: data curation. AS, ML, and PS: writing original draft preparation. AS, AA, and DN: writing review and editing. AA and DN: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant of the Ministero della Salute COVID-2020-12371849, to DN. This work has also been supported by the Italian Ministry of Health Ricerca Corrente—IRCCS MultiMedica.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Paola Corradino for support in literature research.
References
1. Rosenblum HG, Hadler SC, Moulia D, Shimabukuro TT, Su JR, Tepper NK, et al. Use of COVID-19 vaccines after reports of adverse events among adult recipients of janssen (johnson & johnson) and mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna): update from the advisory committee on immunization practices - United States, july 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1094–9. doi: 10.15585/mmwr.mm7032e4
2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
3. European-Medicines-Agency. COVID-19 mRNA Vaccine COMIRNATY (COVID-19 mRNA Vaccine) Risk Management Plan 2021. European Medicines Agency: European Medicines Agency (2021). Available online at: https://www.ema.europa.eu/en/documents/rmp-summary/comirnaty-epar-risk-management-plan_en.pdf (accessedJune 6, 2021).
4. Diaz-Arocutipa C, Saucedo-Chinchay J, Imazio M. Pericarditis in patients with coronavirus disease 2019: a systematic review. J Cardiovasc Med. (2021) 22:693–700. doi: 10.2459/JCM.0000000000001202
5. Sollie ZW, Vallepu SR, Tharumia Jagadeesan C, White LC, Nagalapuram V. Challenges in managing pericardial disease related to post viral syndrome after COVID-19 infection. Cureus. (2021) 13:e13461. doi: 10.7759/cureus.13461
6. Albini A, Di Guardo G, Noonan DM, Lombardo M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern Emerg Med. (2020) 15:759–66. doi: 10.1007/s11739-020-02364-6
7. Rysz S, Al-Saadi J, Sjostrom A, Farm M, Campoccia Jalde F, Platten M, et al. COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system. Nat Commun. (2021) 12:2417. doi: 10.1038/s41467-021-22713-z
8. Davidson AM, Wysocki J, Batlle D. Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor: therapeutic implications. Hypertension. (2020) 76:1339–49. doi: 10.1161/HYPERTENSIONAHA.120.15256
9. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80.e8. doi: 10.1016/j.cell.2020.02.052
10. Adler Y, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert J, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European society of cardiology (ESC) endorsed by: the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2015) 36:2921–64. doi: 10.5603/KP.2015.0228
11. Imazio M, Brucato A, Lazaros G, Andreis A, Scarsi M, Klein A, et al. Anti-inflammatory therapies for pericardial diseases in the COVID-19 pandemic: safety and potentiality. J Cardiovasc Med. (2020) 21:625–9. doi: 10.2459/JCM.0000000000001059
12. Cremer PC, Kumar A, Kontzias A, Tan CD, Rodriguez ER, Imazio M, et al. Complicated pericarditis: understanding risk factors and pathophysiology to inform imaging and treatment. J Am Coll Cardiol. (2016) 68:2311–28. doi: 10.1016/j.jacc.2016.07.785
13. Luk A, Clarke B, Dahdah N, Ducharme A, Krahn A, McCrindle B, et al. Myocarditis and pericarditis following COVID-19 mRNA vaccination: practical considerations for care providers. Can J Cardiol. (2021). doi: 10.1016/j.cjca.2021.08.001
14. Pepe S, Gregory AT, Denniss AR. Myocarditis, pericarditis and cardiomyopathy after COVID-19 vaccination. Heart Lung Circ. (2021) 30:1425–9. doi: 10.1016/j.hlc.2021.07.011
15. Vidula MK, Ambrose M, Glassberg H, Chokshi N, Chen T, Ferrari VA, et al. Myocarditis and other cardiovascular complications of the mRNA-Based COVID-19 vaccines. Cureus. (2021) 13:e15576. doi: 10.7759/cureus.15576
16. Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. (2021) e2113443. doi: 10.1001/jama.2021.13443
17. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. (2013) 34:2636–48, 2648a−8d. doi: 10.1093/eurheartj/eht210
18. Butany J, Ahn E, Luk A. Drug-related cardiac pathology. J Clin Pathol. (2009) 62:1074–84. doi: 10.1136/jcp.2008.058255
19. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the vaccine adverse event reporting system. Int J Cardiol. (2018) 273:183–6. doi: 10.1016/j.ijcard.2018.09.054
20. Mei R, Raschi E, Poluzzi E, Diemberger I, De Ponti F. Recurrence of pericarditis after influenza vaccination: a case report and review of the literature. BMC Pharmacol Toxicol. (2018) 19:20. doi: 10.1186/s40360-018-0211-8
21. Das BB, Moskowitz WB, Taylor MB, Palmer A. Myocarditis and Pericarditis Following mRNA COVID-19 vaccination: what do we know so far? Children. (2021) 8:607. doi: 10.3390/children8070607
22. Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. (2018) 15:586–94. doi: 10.1038/cmi.2017.151
23. Shirato K, Kizaki T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon. (2021) 7:e06187. doi: 10.1016/j.heliyon.2021.e06187
24. Petruk G, Puthia M, Petrlova J, Samsudin F, Stromdahl AC, Cerps S, et al. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J Mol Cell Biol. (2020) 12:916–32. doi: 10.1093/jmcb/mjaa067
25. Ouyang W, Xie T, Fang H, Gao C, Stantchev T, Clouse KA, et al. Variable induction of pro-inflammatory cytokines by commercial SARS CoV-2 spike protein reagents: potential impacts of LPS on in vitro modeling and pathogenic mechanisms in vivo. Int J Mol Sci. (2021) 22:7540. doi: 10.3390/ijms22147540
26. Maestroni S, Di Corato PR, Cumetti D, Chiara DB, Ghidoni S, Prisacaru L, et al. Recurrent pericarditis: autoimmune or autoinflammatory? Autoimmun Rev. (2012) 12:60–5. doi: 10.1016/j.autrev.2012.07.023
27. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
28. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
29. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
30. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. (2020) 395:1763–70. doi: 10.1016/S0140-6736(20)31189-2
31. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. (2020) 382:2534–43. doi: 10.1056/NEJMsa2011686
32. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. (2020) 584:430–6. doi: 10.1038/s41586-020-2521-4
33. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
34. Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, et al. The clinical and chest ct features associated with severe and critical COVID-19 pneumonia. Invest Radiol. (2020) 55:327–31. doi: 10.1097/RLI.0000000000000672
35. Fox K, Prokup JA, Butson K, Jordan K. Acute effusive pericarditis: a late complication of COVID6-19. Cureus. (2020) 12:e9074. doi: 10.7759/cureus.9074
36. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 5:819–24. doi: 10.1001/jamacardio.2020.1096
37. Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. (2001) 104:1076–82. doi: 10.1161/hc3401.095198
Keywords: COVID-19, vaccination, Pfizer-BioNTech, acute pericarditis, SARS-CoV-2
Citation: Sonaglioni A, Albini A, Noonan DM, Brucato A, Lombardo M and Santalucia P (2021) A Case of Acute Pericarditis After COVID-19 Vaccination. Front. Allergy 2:733466. doi: 10.3389/falgy.2021.733466
Received: 30 June 2021; Accepted: 06 September 2021;
Published: 01 October 2021.
Edited by:
Ingrid Terreehorst, Academic Medical Center, NetherlandsReviewed by:
Sophia Tsabouri, University of Ioannina, GreeceFrancesca Saretta, Azienda Sanitaria Universitaria Friuli Centrale (ASU FC), Italy
Kathrin Scherer Hofmeier, Aarau Cantonal Hospital, Switzerland
Copyright © 2021 Sonaglioni, Albini, Noonan, Brucato, Lombardo and Santalucia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adriana Albini, YWRyaWFuYS5hbGJpbmkmI3gwMDA0MDttdWx0aW1lZGljYS5pdA==
 Andrea Sonaglioni
Andrea Sonaglioni Adriana Albini
Adriana Albini Douglas M. Noonan
Douglas M. Noonan Antonio Brucato
Antonio Brucato Michele Lombardo
Michele Lombardo Paola Santalucia1
Paola Santalucia1