
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 29 September 2020
Sec. Nutrition and Sustainable Diets
Volume 4 - 2020 | https://doi.org/10.3389/fsufs.2020.569625
This article is part of the Research Topic Everything Edamame: Biology, Production, Nutrition, Sensory and Economics View all 13 articles
Edamame is a food-grade soybean that is harvested at the green-immature stage (R6) and sold fresh or frozen for consumption after steaming or boiling. Limited studies have been conducted on high-temperature sterilization of edamame in cans and on acid preservation of edamame. The objectives of this study were to evaluate the color retention and texture of edamame when pasteurized in acidic brine as compared to boiling, to assess the effect of sucrose and turmeric addition on acid-treated edamame, and to characterize varietal differences when edamame lines were pasteurized in an acidic brine at either R6 or R8 stage. All studies were conducted using industry-standard processing conditions for acid-preservation of food in glass containers. The results of this research indicated that acid processing caused losses in intensity of green color and hue, but much smaller than those reported when heat-processing edamame in cans. We also observed a small and borderline significant increase in texture (p = 0.0790) in acid-preserved samples. In addition, we found that green color is positively affected by the addition of turmeric to the brine, but not of sucrose. Finally, the varieties R07-589 (red-brown seed coat) and R09-345 (black seed coat) acid-processed at R8 had color and texture similar to canned in-kind substitute products. In conclusion, acid-preservation, and addition of turmeric to immature edamame, helped maintain an acceptable quality of the processed products.
Edamame is a food-grade soybean (Glycine max (L.) Merrill) that is high in protein and phytochemicals and low in saturated fats, making it a good food product for addition to soups and salads, or as healthy snack alternative to chips and candy (Masuda, 1991; Rayaprolu et al., 2015). Edamame is normally harvested by picking pods at the R6 physiological stage when the seeds are still green and fill 80–90% of the pods (Fehr et al., 1971; Konovsky et al., 1994; Shanmugasundaram and Yan, 2004). However, edamame can also be harvested when the seed is mature and dry at the R8 reproductive stage. While the seed color at R6 is green, it will either stay green or turn yellow, black, red-brown, or brown at the R7 stage, depending on the genetics of seed-coat and cotyledon color for the particular variety (Kiuchi et al., 1987). While all commodity-soybean have yellow seed coat, some of the edamame lines are selected to have green cotyledons to facilitate harvest, for these lines do not turn yellow if harvest is slightly delayed. Very few food-grade soybean lines are available with colored coats for commercialization. However, the pigments responsible for the various seed coat colors at the R8 reproductive stage can have health benefits, such as inflammation-preventing anthocyanins and procyanidins in the black, red-brown and brown seed coats (Takahata et al., 2001; Kim et al., 2006; Nizamutdinova et al., 2009).
Edamame is preserved in several ways. Currently, the market primarily consists of either fresh, frozen, roasted (Mentreddy et al., 2002), or freeze-dried products (Rayaprolu et al., 2015). While roasting and freeze-drying products have become commercial options, improved pasteurization techniques to create a shelf-stable product with high moisture content are necessary. Mozzoni et al. (2009a) and Czaikoski et al. (2013, 2018) attempted to conduct high-temperature sterilization using retorts; however, quality attributes of the end product, including texture and color are lower in thermal-processed edamame than in fresh or frozen products (Mozzoni et al., 2009a; Czaikoski et al., 2018). The standard for an edamame product is to have a firm texture with high green/low yellow intensity (Mozzoni et al., 2009a; Czaikoski et al., 2013; Rayaprolu et al., 2015). Czaikoski et al. (2013) estimated that adding 34.3 g mL−1 of sucrose can increase hue of pasteurized edamame. In addition, turmeric has been used in food preservation as a healthy alternative to synthetic food coloring of chicken breast filets (Abdeldaiem, 2014). Mozzoni et al. (2009a) found that adding CaCl2 to the brine will increase firmness of the canned product, and that increasing pH of the brine will decrease intensity of green color (IGC), the later an undesirable attribute. The negative correlation between pH and IGC (or hue) is due to the acidity of the brine and heat process that subtracts a Mg2+ ion converting the chlorophyll pigment into pheophytin (von Elbe and Schwartz, 1996). Reducing the thermal processing time can limit this reaction, resulting in a higher hue value and intensity of green color (Czaikoski et al., 2013); however, this must be balanced with bromatological safety requirements. In contrast, if the pH of the brine is <4.5, the edamame can be pasteurized instead of sterilized, requiring less thermal processing (Abbatemarco and Ramaswamy, 1994). Reduced thermal processing will result in a firmer texture (Czaikoski et al., 2013) and less break down of the chlorophyll (von Elbe and Schwartz, 1996). The objective of this research was, therefore, to study the color preservation and texture of edamame harvested at the R6 or R8 reproductive growth stages when processed using industry-standard acid-preservation methodologies.
Three varieties (fixed factor), “R07-10397,” “R09-345,” and “R07-589,” were grown in 2016 at the University of Arkansas Vegetable Research Station in Kibler, AR on a very fine sandy loam, coarse-silty, mixed, superactive, thermic, non-acid, typic udifluvents soil (Roxana series) (Soil Survey Staff, 2017) under standard agronomic practices as reported by Mozzoni et al. (2009b). Harvesting occurred once the plots reached the R6 or R8 physiological stage. In addition, a frozen sample of commercial edamame variety “8080,” harvested at the R6 stage, was obtained from American Vegetable Soybean and Edamame, Inc. (Mulberry, AR).
Once the plots were at R6 stage, entire plants were harvested and the pods were stripped using an edamame motive–power threshing machine (KE-6) (Doubletreasure Enterprise Inc., Plano, TX). The edamame was shelled from the pods using a “Little Sheller” (Taylor mfg. Co, Inc., Moultrie, GA), and samples were placed in a refrigerator at 4°C and subjected to blanching within 24 h. Blanching was conducted using a 100°C water bath for 90 s to reduce 99% initial lipoxygenase activity as reported by Mozzoni et al. (2009b) and confirmed by Xu et al. (2012). Since acid preservation was conducted once after all samples (R6 and R8) were harvested, the R6 samples were flash frozen with liquid nitrogen immediately after blanching to maintain cell structure during freezer storage (Luyet, 1968). Prior to the acid preservation, the frozen edamame samples were thawed by placing in an 82.2°C water bath consisting of 0.47 L of 5% distilled white all-purpose vinegar for 30 s and immediately cooled to ambient temperature by placing in cool water for 1 min.
The same edamame varieties were also harvested at the mature (R8) reproductive stage. The varieties were chosen to represent different mature seed coat and cotyledon colors, namely “R07-10397” had green, “R07-589” red-brown, and “R09-345” black seed color. After harvesting, the samples were stored in a cool, dry place in cloth bags until processing.
Previous research from this group (Mozzoni et al., 2009a) and others (Czaikoski et al., 2013) involved the execution of Central Composite Rotatable Designs to identify optimum brine composition for pasteurization of edamame. Based on these findings, our base brine consisted of a solution containing 0.56 L water, 0.4 L of 5% distilled white all-purpose vinegar, 60.0 g of NaCl, and 2.6 g of CaCl2. The purpose of the vinegar was to bring the pH below 4.5 (Czaikoski et al., 2013) and the CaCl2 was used to maintain a firm texture of the edamame after the thermal processing (Mozzoni et al., 2009a). Glass jars (236.6 ml) were filled with 148.8 g of shelled and blanched edamame and 88.7 ml of brine. The closed jars were placed three-quarters of the way into boiling water for 6 min for thermal processing. To ensure a commercially-sterile pasteurized product, the jars were tested for temperature and pH. Two test jars were opened immediately after the thermal processing to check for a minimum temperature of 85°C in the cold spot (McGlynn, 2000), located between 1/3 and 1/2 of the jar's height, following standard industry processing conditions for the selected glass jars, brine volume, and fresh weight of product utilized (Fellows, 2000; Mozzoni et al., 2009a). Brine pH was measured using a Symphony SP79P pH Meter (VWR, Radnor, PA) 2 weeks after processing. After thermal processing, the jars were immediately cooled to ambient temperature using tap water for 10 min. Thermal processing was done at the pilot plant of Bryant Preserving Co. in Alma, AR on August 31, 2016. Each treatment was subjected to three replications (random factor).
To test the effect of acidic preservation on edamame color and texture, a sample of edamame variety “8080” was subjected to acidic processing as described in section Base Brine and Standard Acidic Processing Conditions, and compared to a non-processed sample of the same variety that was cooked in boiling water for 6 min (the water remained boiling after the addition of the sample) on a stove top at the University of Arkansas' test kitchen.
The effect of sucrose and turmeric on color and texture was investigated using a commercial variety “8080” with a two-factor factorial experimental design with three levels for each factor. The factors and levels were sucrose (0, 29.5, and 59 g L−1), and oleoresin turmeric (0, 0.26, 0.53 ml L−1) added to the brine. Brine's pH was readjusted prior to the addition of the edamame sample, and acid processing was conducted as described in section Base Brine and Standard Acidic Processing Conditions. As control, a 60-g sample of frozen edamame “8080” was cooked in boiling water for 6 min.
Three edamame varieties, “R07-10397,” “R09-345,” and “R07-589” were harvested and handled as described in section Immature Edamame Harvest and Blanching, and subjected to acid processing in jars as described in section Base Brine and Standard Acidic Processing Conditions. These acid-preserved samples were compared for differences in texture and color to a 60-g sample of edamame variety “8080” cooked in boiling water for 6 min.
Prior to processing, the dry samples were soaked in distilled water for 24 h to promote uniform texture and expansion during the thermal process (Nordstrom and Sistrunk, 1977). Acidic preservation was conducted as described in section Base Brine and Standard Acidic Processing Conditions. The acid-processed product of the three colored varieties were compared to a non-processed control, where the variety was soaked in water for 24 h but not acid-treated, and to an in-kind commercial product. For the latter, R07-10397 (green seed) variety was compared to thawed and cooked samples of the commercial “8080” edamame variety, R07-589 (red-brown seed coat) was compared to canned samples of commercially-available Pinto and Kidney beans, and R09-345 (black seed coat) was compared to canned samples of commercially-available black beans. After processing, the cold spot temperature and the pH of the brine was examined to ensure successful preservation. The cold spot temperature was confirmed above 85°C, and the average pH of the jars were 4.28 with a standard deviation of 0.05.
A single-bite test on a TMS 2000 texture analyzer (Food Technology Corp., Sterling VA, USA) with an Allo Kramer shear cell (10 blades) was used to measure texture. The settings of the instrument were max force at 50 kg, return distance at 40 mm, return speed at 3 mm sec−1, and contact force at 500 g. The texture was reported as the peak force in Newtons (N) the blades required to penetrate the sample (Mozzoni et al., 2009a). Twenty grams of edamame were used for the texture analysis of R6 materials, and 10 gram-samples were used in the R8 stage materials due to an increase in firmness of the mature edamame. Texture analysis was not conducted on water-soaked treatment from 2.4 because the material was too hard for the load cell of our equipment.
The color of the samples was measured with a HunterLab Color Flex (Hunter Associates Laboratory Inc., Reston, VA, U.S.A.). Three values were recorded, L*, a*, and b* which represent the brightness/darkness, redness/greenness, and the blueness/yellowness of the sample, respectively. An increasing L* value indicates a brighter sample, a smaller a* value indicates a greener sample, and larger b* value indicates a more yellow sample (Hunter Associates Laboratory Inc., Reston, VA, U.S.A). The instrument was calibrated with a black glass tile first, then with a white standard tile. The white tile had L*, a*, and b* values of 93.76, −0.93, and 1.02, respectively. Prior to sampling, the calibration was validated with a green standard tile with values L* = 52.96, a* = −25.30, and b* = 13.71. The intensity of green color (IGC) was calculated as (–a*/b*) (Mozzoni et al., 2009a), and Hue was calculated as (degrees(ATAN2)(a*,b*)) (Rayaprolu et al., 2015), respectively. The intensity of green color indicates the ratio of green to yellow, and the hue, measured in degrees, indicates how close the color is to pure red (0°), yellow (90°), green (180°), or blue (270°) (Lawless and Heymann, 1998).
Experimental factors were analyzed with SAS v.9.4 (SAS Institute, 2014) using the PROC MIXED procedure. Least square means (LSM) of the main effects and their interactions were estimated with the Type 3 method and the means were separated by interpreting the p-values generated by the DIFF option.
When comparing the effect of processing on acidified brine to the samples cooked and non-further processed of variety “8080,” we observed that the acid treatment caused a borderline non-significant effect in reducing L*-value (p = 0.1302), significant effects on a*-value (p = 0.0001), b*-value (p = 0.0050), intensity of green (p = 0.0001), and hue (p = 0.0001), and borderline non-significant effect on texture (p = 0.0790) (Table 1). The processing in acid caused an undesirable decrease in hue and intensity of green color, but also caused a desirable increase of the texture of the processed samples.

Table 1. Color and texture of edamame cultivar “8080” when blanched and processed in acidic brine (Acid-treated) vs. blanched without further processing (Control).
Adding sucrose to the brine did not have a significant effect on color or texture of edamame “8080.” Brine with 0.0, 29.5 or 59.0 g L−1 of sucrose resulted in Hue values of 88.68, 88.41, and 88.34, respectively, which are not significant at α = 0.05. Similarly, the aforementioned sucrose treatments resulted in Force readings of 470.40, 454.40, and 465.70 N, respectively, also not-statistically different at α = 0.05.
In contrast, addition of various levels of turmeric caused significant differences (p < 0.05) on a*, b*, hue, and intensity of green color, but texture remained unaffected (Table 2). Adding 0.53 or 0.26 ml L−1 of turmeric to the brine resulted in the lowest a* value, and the largest intensity of green color and hue value. The b* value was significantly smaller when adding turmeric at the level of 0.26 vs. 0.53 ml L−1. When no turmeric was added, the edamame had a significantly lower intensity of green color and hue value (p < 0.05) (Table 2). The brine-processed edamame had L* value similar to the frozen and boiled check control, irrespective of turmeric levels; however, the frozen and boiled check control had a hue value that was closer to a pure green color than the brine-processed samples regardless of turmeric levels.
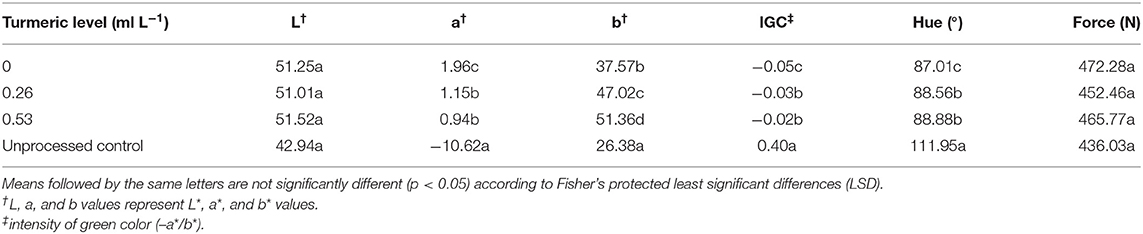
Table 2. Average color and texture for edamame variety “8080” after pasteurization in an acidic brine with different levels of the turmeric effect, and a 6-min boiled control not subject to acidic brine treatment that simulates the standard blanched-frozen-cooked product.
Three varieties (R07-10397, R07-589, and R09-345) were harvested at the R6 reproductive stage while the pods were still green when processed on an acidified brine, and compared against edamame check 8080 cooked in boiling water. We observed that R07-589 had the lowest brightness (L*) value and greatest a* value, significantly different than the check variety 8080 and the other two edamame lines. Concomitantly, Hue and IGC values for R07-589 were lower than any of the other treatments. It is worth noting that the frozen and boiled edamame check 8080 had the greatest IGC and hue closer to true green (111.07° for 8080 vs. 120.00° for true green) than any of the acid-preserved edamame varieties (Table 3).
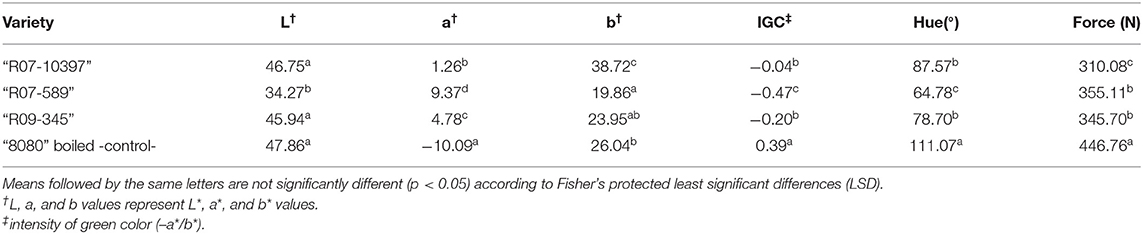
Table 3. Color values and texture of three edamame varieties harvested at the R6 stage and acid-processed after pasteurization, and an unprocessed sample of a commercial variety blanched used as check.
The hue value (91.69°) and IGC (0.029) for the processed product of R07-10397 at R8 was lower than the frozen sample of 8080 and an unprocessed sample of R07-10397, indicating less green color (Table 4). However, the hue value of R07-10397 after processing was similar to the processed edamame sample reported by Czaikoski et al. (2013) (93.50°), and was higher than 8080 (88.56°) after processing with sucrose and turmeric (Table 4). Furthermore, the texture of processed R07-10397 was not significantly different than “8080” (408.94 vs. 442.12 N, respectively).
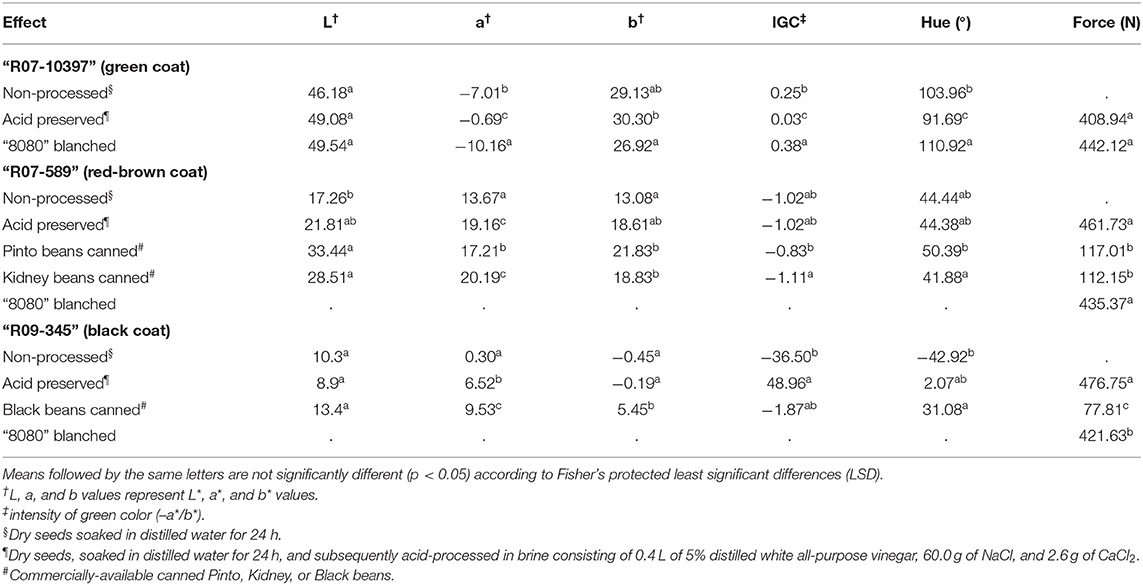
Table 4. Color and texture (force) values of three edamame varieties with colored seed coats harvested at the mature (R8) growth stage.
As the varieties R07-589 approach the R8 stage, the seed cotyledons and embryo gradually change from green to yellow, and the seed coats turn red-brown. The L* value of R07-589 (17.26) prior to processing was significantly lower (p < 0.05) than the canned product of pinto (33.44) and kidney beans (28.51); however, after processing, the L* value of R07-589 (21.81) and the pinto and kidney beans (33.44 and 28.51, respectively) were not significantly different. The hue value of the processed sample of R07-589 (44.38°) was not significantly different than the unprocessed sample and the canned samples of pinto and kidney beans (Table 4), but we observed the texture of acid-preserved R07-589 to be not-statistically different than “8080” check and significantly harder than that of canned Pinto and Kidney beans.
As for the case of R07-589, as the mature embryo and cotyledons of R09-345 change color as the seed mature, and seed coats are fully black at R8 stage. We observed no differences in L* value for R09-345 between water-soaked or acid-preserved samples, nor the L* value was different than that of canned black beans (Table 4). However, texture of R09-345 preserved in acidified brine was significantly harder than that of “8080” edamame check, and about 6-fold greater than that of canned black beans (Table 4).
Mozzoni et al. (2009a) established a protocol to blanch edamame before sterilization to deactivate lipoxygenase activity; and developed a base brine consisting of NaCl and CaCl2. Czaikoski et al. (2013) adapted the protocol set by Mozzoni et al. (2009a) by pasteurizing in an acidic brine and evaluating levels of sucrose to retain green color. Czaikoski et al. (2013) concluded that beans processed with sucrose were significantly greener than without; however, the processed product was significantly less green than the beans in natura. Although McGlynn et al. (1993) reported a brine below pH 4.5 can result in a firmer texture after thermal processing, Czaikoski et al. (2013) observed a product that was less firm than beans in natura.
The overall objective of this research was to improve the methodologies established by Mozzoni et al. (2009a) and Czaikoski et al. (2013), resulting in a product that would be commercially acceptable. Similar to Czaikoski et al. (2013, 2018), we observed a loss in green color of acid-processed edamame as compared to boiled samples; however, the color retention of samples is much larger than that of the color of heat-processed canned edamame reported by Mozzoni et al. (2009a). In Mozzoni's trial, intensity of green color dropped from 0.45 to −5.33 for blanching vs. canned product, or 5.78 absolute points; whereas in our current experiment the intensity of color is reduced from 0.39 to −0.04, or 0.43 absolute points. The samples processed under our acid conditions had greater color retention as compared to those processed in a retort by Mozzoni et al. (2009a).
Czaikoski et al. (2013) estimated adding 34.3 g L−1 of sucrose can increase the hue value by 1.17°. Although our research did not find sucrose to have a significant effect on color, the hue value dropped (less green) by 0.34° after adding 59 g L−1 of sucrose. The discrepancy between the two results may be due to the fact the suggestion of 34.3 g L−1 reported by Czaikoski et al. (2013) was a projection as it was outside of the central composite design to evaluate the effects of added sucrose. Abdeldaiem (2014) suggested adding turmeric can be a healthy alternative to artificial dyes, as it can have health benefits such as antioxidant and antimicrobial activities. Furthermore, Cleary and McFeeters (2006) inferred using turmeric in a pasteurization process can minimize off-flavors due to oxidation. In our research, we found the addition of 0.26 ml L−1 of turmeric would result in a hue closer to green than that without turmeric addition.
The variety R07-10397 (green seed coat and cotyledon), harvested mature at the R8 growth stage had the highest hue (most green); therefore, it may give the best chance to preserve an edamame product with acceptable color. This variety matures with a green cotyledon in addition to a green seed coat, which may increase the seed's ability to retain a higher green color after processing. The brightness (L*) value of R07-589 harvested mature and dry (R8 growth stage) improved after processing, in agreement with the report of Rayaprolu et al. (2015) that indicate an increase in brightness after processing. Furthermore, the hue value of R07-589 after processing was similar to that of the soaked sample, canned pinto beans, and canned kidney beans. These results indicate thermal processing in an acidic brine did not significantly alter the red-brown color of R07-589. Similarly, the L* value of the soaked and of the processed sample of R09-345 was not statistically different than the canned black beans, indicating that the thermal process in an acidic brine did not alter the black color of the beans. The retention of red-brown and black color for R07-589 and R09-345, respectively, after processing indicates the pigments causing the colors did not break down due to the thermal process or the acidic brine, leaving a product that would be aesthetically acceptable with healthy attributes. Lau et al. (2000) reported vegetables will soften during thermal processing. In this study, however, the texture of the processed edamame in the sucrose by turmeric test (R6 growth stage) and the three mature (R8) varieties were similar to the commercial check (“8080”). Maintaining the texture can be attributed to the addition of CaCl2 (Mozzoni et al., 2009a) and decrease in duration of thermal processing (Czaikoski et al., 2013).
In conclusion, acid-preservation of edamame and addition of turmeric to the brine maintained the quality characteristics of color and texture of the processed product; traits that were otherwise further degraded when edamame was canned in retorts. Therefore, acid-preservation of edamame either at immature or mature stages could be a commercially viable alternative, provided bromatological studies are conducted to confirm sterility of end product.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
DM: conceptualization, methodology, investigation, formal analysis, writing—original draft, writing—review, and editing. LM: formal analysis, supervision, writing—review, and editing. MO: investigation and methodology. LF-P: writing—review and editing. PC: conceptualization, methodology, formal analysis, project administration, and supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Bryant Preserving Co in Alma, AR for their collaboration in this research, and the Arkansas Soybean Promotion Board for their sponsorship of this project.
Abbatemarco, C., and Ramaswamy, H. S. (1994). End-over-end thermal processing of canned vegetables: effect on texture and color. Food Res. Int. 27, 327–334. doi: 10.1016/0963-9969(94)90188-0
Abdeldaiem, M. H. (2014). Use of yellow pigment extracted from turmeric (Curcuma Longa) rhizomes powder as natural food preservative. Am. J. Food Sci. Technol. 2, 36–47. doi: 10.12691/ajfst-2-1-6
Cleary, K., and McFeeters, R. F. (2006). Effects of oxygen and turmeric on the formation of oxidative aldehydes in fresh-pack dill pickles. J. Agric. Food Chem. 54, 3421–3427. doi: 10.1021/jf052868k
Czaikoski, K., Leite, R. S., Mandarino, J. M. G., Carrao-Panizzi, M. C., da Silva, J. B., and Ida, E. I. (2013). Canning of vegetable-type soybean in acidified brine: effect of the addition of sucrose and pasteurization time on color and other characteristics. Ind. Crops Prod. 45, 472–476. doi: 10.1016/j.indcrop.2012.09.009
Czaikoski, K., Leite, R. S., Mandarino, J. M. G., Carrao-Panizzi, M. C., da Silva, J. B., and Ida, E. I. (2018). Physicochemical characteristics of canned vegetable-type soybean processed with zinc at different pasteurization times. Pesqui. Agropecu. Bras. 53, 840–848. doi: 10.1590/s0100-204x2018000700008
Fehr, W. R., Caviness, C. E., Burmood, D. T., and Pennington, J. S. (1971). Stage of development descriptions for soybeans, Glycine Max (L.) Merrill. Crop Sci. 11, 929–931. doi: 10.2135/cropsci1971.0011183X001100060051x
Fellows, P. (2000). “Heat Sterilization,” in Food Processing Technology: Principles and Practice, 2nd Edn, ed P. Fellows (Boca Raton, FL: CRC Press LLC), 250–277. doi: 10.1201/NOE0849308871
Kim, E. H., Kim, S. H., Chung, J. I., Chi, H. Y., Kim, J. A., and Chung, I. M. (2006). Analysis of phenolic compounds and isoflavones in soybean seeds (Glycine max (L.) Merrill) and sprouts grown under different conditions. Eur. Food Res. Technol. 222, 201–208. doi: 10.1007/s00217-005-0153-4
Kiuchi, Y., Ishikawa, H., Nitta, N., and Sasaki, T. (1987). Collection of crop genetic resources and their characteristics in Iwate Prefecture In: characteristics of local soybean varieties. Tohoku Nogyo Kenkyu 40, 139–140.
Konovsky, J., Lumpkin, T. A., and McClary, D. (1994). “Edamame: the vegetable soybean,” in Understanding the Japanese Food and Agrimarket: a Multifaceted Opportunity, ed A. D. O'Rourke (Binghamton, NY: Haworth Press), 173–181. doi: 10.1201/9781003075172-15
Lau, M. H., Tang, J., and Swanson, B. G. (2000). Kinetics of textural and color changes in green asparagus during thermal treatments. J. Food Eng. 45, 231–236. doi: 10.1016/S0260-8774(00)00069-8
Lawless, H. T., and Heymann, H. (1998). Sensory Evaluation of Food. New York, NY: Chapman and Hall. doi: 10.1007/978-1-4615-7843-7
Luyet, B. J. (1968). Investigations on Freezing and Freeze-Drying of Selected Fruits and Vegetables. Natick, MA: Technical report 69-8-FL. U.S. Army Natick Laboratories.
Masuda, R. (1991). “Quality requirement and improvement of vegetable-type soybean,” in Vegetable-Type Soybean: Research Needs for Production and Quality Improvement, ed S. Shanmugasundaram (Taiwan: Asian Vegetable Research and Development Center), 92–102.
McGlynn, W. (2000). The Importance of food pH in commercial canning operations. Division of Agricultural Sciences and Natural Resources. Oklahoma State University Cooperative Extension Service, Web. Available online at: http://pods.dasnr.okstate.edu/docushare/dsweb/Get/Document-603/FAPC-118pod.pdf (acccesssed November 14, 2017).
McGlynn, W. G., Davis, D. R., and Honarmand, F. (1993). Gluconic acid influences texture and color of canned asparagus. J. Food Sci. 58, 614–615. doi: 10.1111/j.1365-2621.1993.tb04338.x
Mentreddy, S. R., Mohamed, A. I., Joshee, N., and Yadav, A. K. (2002). “Edamame: a nutritious vegetable crop,” in Trends in New Crops and New Uses, eds J. Janick and A. Whipkey (Alexandria, VA: ASHS Press), 432–438.
Mozzoni, L. A., Chen, P., Morawicki, R. O., Hettiarachchy, N. S., Brye, K. R., and Mauromoustakos, A. (2009b). Quality attributes of vegetable soybean as a function of boiling time and condition. Int. J. Food Sci. Technol. 44, 2089–2099. doi: 10.1111/j.1365-2621.2009.02038.x
Mozzoni, L. A., Morawicki, R. O., and Chen, P. (2009a). Canning of vegetable soybean: procedures and quality evaluations. Int. J. Food Sci. Technol. 44, 1125–1130. doi: 10.1111/j.1365-2621.2009.01929.x
Nizamutdinova, I. T., Kim, Y. M., Chung, J. I., Shin, S. C., Jeong, Y. K., Seo, H. G., et al. (2009). Anthocyanins from black soybean seed coats stimulate wound healing in fibroblasts and keratinocytes and prevent inflammation in endothelial cells. Food Chem. Toxicol. 47, 2806–2812. doi: 10.1016/j.fct.2009.08.016
Nordstrom, C. L., and Sistrunk, W. A. (1977). Effect of type of bean, soak time, canning media and storage time on quality attributes and nutritional value of canned dry beans. J. Food Sci. 42, 795–798. doi: 10.1111/j.1365-2621.1977.tb12605.x
Rayaprolu, S., Hettiarachchy, N., Aldoury, M., Cho, S., Moseley, D., and Chen, P. (2015). Physical and textural attributes of freeze-dried genetically modified and non-genetically modified soy beans. J. Food Nutr. Sci. 3, 119–125. doi: 10.11648/j.jfns.20150303.17
Shanmugasundaram, S., and Yan, M. R. (2004). “Global expansion of high value vegetable soybean,” in VII World Soybean Conference/IV International Soybean Processing and Utilization Conference/III Brazilian Soybean Congress (Foz do Iguassu), 915–920.
Soil Survey Staff (2017). Natural Resources Conservation Service, United States Department of Agriculture. Web soil survey. Available online at: https://websoilsurvey.sc.egov.usda.gov/ (accessed December 11, 2017).
Takahata, Y., Ohnishi-Kameyama, M., Furuta, S., Takahashi, M., and Suda, A. (2001). Highly polymerized procyanidins in brown soybean seed coat with a high radical-scavenging activity. J. Agric. Food Chem. 49, 5843–5847. doi: 10.1021/jf010307x
von Elbe, J. H., and Schwartz, S. J. (1996). “Colorants,” in: Food Chemistry, 3rd Edn, ed O. Fennema (New York, NY: Marcel Dekker), 651–722.
Keywords: edamame, acidic-pasteurization, turmeric, color, texture
Citation: Moseley D, Mozzoni L, Orazaly M, Florez-Palacios L and Chen P (2020) Quality of Acid-Preserved Edamame Soybean at Immature and Mature Stages. Front. Sustain. Food Syst. 4:569625. doi: 10.3389/fsufs.2020.569625
Received: 04 June 2020; Accepted: 26 August 2020;
Published: 29 September 2020.
Edited by:
Martin Williams, United States Department of Agriculture, United StatesReviewed by:
Jacek Słupski, University of Agriculture in Krakow, PolandCopyright © 2020 Moseley, Mozzoni, Orazaly, Florez-Palacios and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leandro Mozzoni, bG1venpvbkB1YXJrLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.