- Whitelaw Group, The Roslin Institute and Royal (Dick) School of Veterinary Studies, University of Edinburgh, Edinburgh, United Kingdom
The global demand for animal-based food products is anticipated to increase by 70% by 2050. Meeting this demand in a way that has minimal impact on the environment will require the implementation of advanced technologies. Genome editing of livestock is a tool that will allow breeders to improve animal welfare, performance and efficiency, paving the way to a more sustainable future for livestock agriculture. Currently, genome editing of livestock is limited to specialized laboratories due to the complexity of techniques available for the delivery of genome editing reagents into zygotes and reproductive cells. The emergence of three cutting-edge reproductive technologies—(i) zygote electroporation, (ii) zygote transduction of recombinant adeno-associated virus (rAAV), and (iii) surrogate sire technology—will provide livestock breeders with a new toolkit of delivery strategies for genome editing. The simplicity of these technologies will enable widespread on-farm application in major livestock species by seamlessly integrating into current breeding systems. We believe it is timely to highlight these three cutting-edge reproductive technologies for genome editing and have outlined pipelines for their implementation in on-farm settings. With a nuanced regulatory framework these technologies could fast-track livestock genetic gain and help secure a sustainable future for livestock.
Introduction
Preparing to feed a balanced and nutritious diet to the projected 9.7 billion people on the globe by 2050 will be one of the greatest challenges humanity has ever faced. The FAO estimates demand for animal-based food products will increase by 70% in this time (Alexandratos and Bruinsma, 2012). Increasing reliance on plant-based diets and artificial meat production will contribute to improving food security and the sustainability of commercial agriculture, however outright omission of animal protein from human diets risks nutritional deficiencies and malnutrition, particularly in developing regions. Meeting the anticipated increase in demand for animal food products in a way that has minimal impact on the environment and ensures high animal welfare standards will likely require the implementation of advanced technologies, including genome editing and cutting-edge reproductive technologies. Considering the huge potential of these technologies, it would be negligent not to examine their inherent possibilities further.
Traditional livestock breeding is restricted by genetic linkage and the available genetic variation within a breed. Genome editing allows animal breeders to overcome these biological impediments and introduce polymorphisms that are not present in the gene pool of elite brood stock, or even create novel changes predicted to result in improved gain (Laible et al., 2014). This powerful technology allows animal breeders to specifically and efficiently alter an animal's DNA. Precise genetic alterations to remove deleterious mutations, such as recessive lethal genetic variants, or introduce desirable traits, such as hornlessness, heat tolerance and disease resistance; without affecting other genetic characteristics of their herd (Davis et al., 2017; Mueller et al., 2019; Proudfoot et al., 2019).
The genome editor toolbox currently contains variants of ZFNs, TALENs and CRISPR-Cas, and these have been successfully used in livestock species such as pigs, cattle, sheep, goats, and chickens (Tait-Burkard et al., 2019). These genome editors all work on the principle of introducing double strand DNA breaks (DSBs) at a user-defined target site in the genome, stimulating endogenous cellular DNA repair pathways to make modifications. DSBs will most frequently be repaired by non-homologous end joining (NHEJ), which is error prone and can result in the introduction of nucleotide insertions or deletions. However, if a homologous DNA repair template (HDRT) is provided, then the repair can then occur via the homology-directed repair (HDR) pathway. In this manner, DNA sequences can be precisely modified or introduced into the genome (Fernandez et al., 2017).
Of the genome editing tools currently available, CRISPR-Cas has quickly become the “go to” technology due to its ease-of-use, high efficiency and low cost. Since it's repurposing into a genome editor in 2012, there has been a flurry of research activity around the technology and an ever expanding toolbox of CRISPR-Cas reagents, including base-editors and nickase systems, which can drive editing without the need for introducing DSBs (Pickar-Oliver and Gersbach, 2019). Due to the simplicity and range of CRISPR-Cas tools now available, it is foreseeable that a significant number of genome edited livestock will be produced over the next decade. Within this article, we describe livestock genome editing strategies based on CRISPR-Cas, although ZFNs and TALENs could also achieve the same results.
A precondition for applying CRISPR-Cas in livestock are reproductive technologies that enable efficient delivery of CRISPR-Cas reagents into zygotes or reproductive cells. Although genome editors have rapidly developed, the reproductive technologies for delivering CRISPR-Cas remain complex and inefficient at a large scale. Somatic cell nuclear transfer (SCNT) and zygote microinjection are the conventional techniques. Both are technically challenging, costly, labor-intensive, and require expert skills with bulky micromanipulation equipment; restricting their use to a small number of specialized laboratories (Sheets et al., 2016).
New reproductive technologies that simplify the delivery of CRISPR-Cas reagents into livestock reproductive cells are needed to disseminate the benefits of genome editing beyond research institutes and corporate biotechnology enterprises. The emergence of three cutting-edge reproductive technologies—(i) zygote electroporation, (ii) zygote transduction with recombinant adeno-associated virus (rAAV), and (iii) surrogate sire technology (SST)—will provide animal breeders with a new toolkit for delivering CRISPR-Cas to reproductive cells. The simplicity of these approaches will allow livestock genome editing to occur on-farm.
We believe it is timely to highlight these three cutting-edge reproductive technologies and have outlined pipelines for their implementation in on-farm settings. It is hoped these cutting-edge reproductive technologies will disperse the capacity to genome edit farm animals, fast-tracking genetic gains and helping to secure a sustainable future for livestock agriculture.
Zygote Electroporation
Zygote electroporation is a recently developed method that overcomes many of the shortcomings of conventional SCNT and microinjection delivery approaches. This technique allows direct introduction of genome editing tools into zygotes by application of voltage to zygotes suspended in a medium containing CRISPR-Cas reagents. The pulses of electricity cause pores to form in the zygote membrane, allowing the genome editing tools in the suspension to pass through the pores in the zygote membrane and into the nucleus where CRISPR-Cas can begin genome editing activity (Miao et al., 2019).
Electroporation is a well-established method for introducing reagents into mammalian cells but has only recently been refined for application to zygotes. Initial efforts required the enzymatic removal of the zona pellucida, a protective membrane surrounding the zygote. However, the removal of the zona pellucida makes zygotes sticky and difficult to work with, which restricted adoption of zygote electroporation. Optimization of the technique now makes it possible to introduce CRISPR-Cas into zona-intact zygotes, making it significantly more attractive to users.
Several livestock genome editing facilities have now employed zona-intact zygote electroporation to successfully edit the genomes of pigs and cattle (Laible, 2018; Miao et al., 2019; Namula et al., 2019). The simplicity of this approach allows for zygote electroporation to be incorporated into on-farm embryo transfer (ET) programs, which are increasingly common in commercial livestock farming.
In on-farm settings, we foresee a pipeline where donor females are super-ovulated and oocytes collected for in vitro fertilization - as per a conventional ET program. After fertilization, the zygotes undergo electroporation to introduce CRISPR-Cas reagents. The genome edited embryos are then matured in vitro and the editing of each zygote is confirmed by portable biopsy sequencing. Validated embryos are then transferred into recipient females to give birth to genetically superior animals (Figure 1).
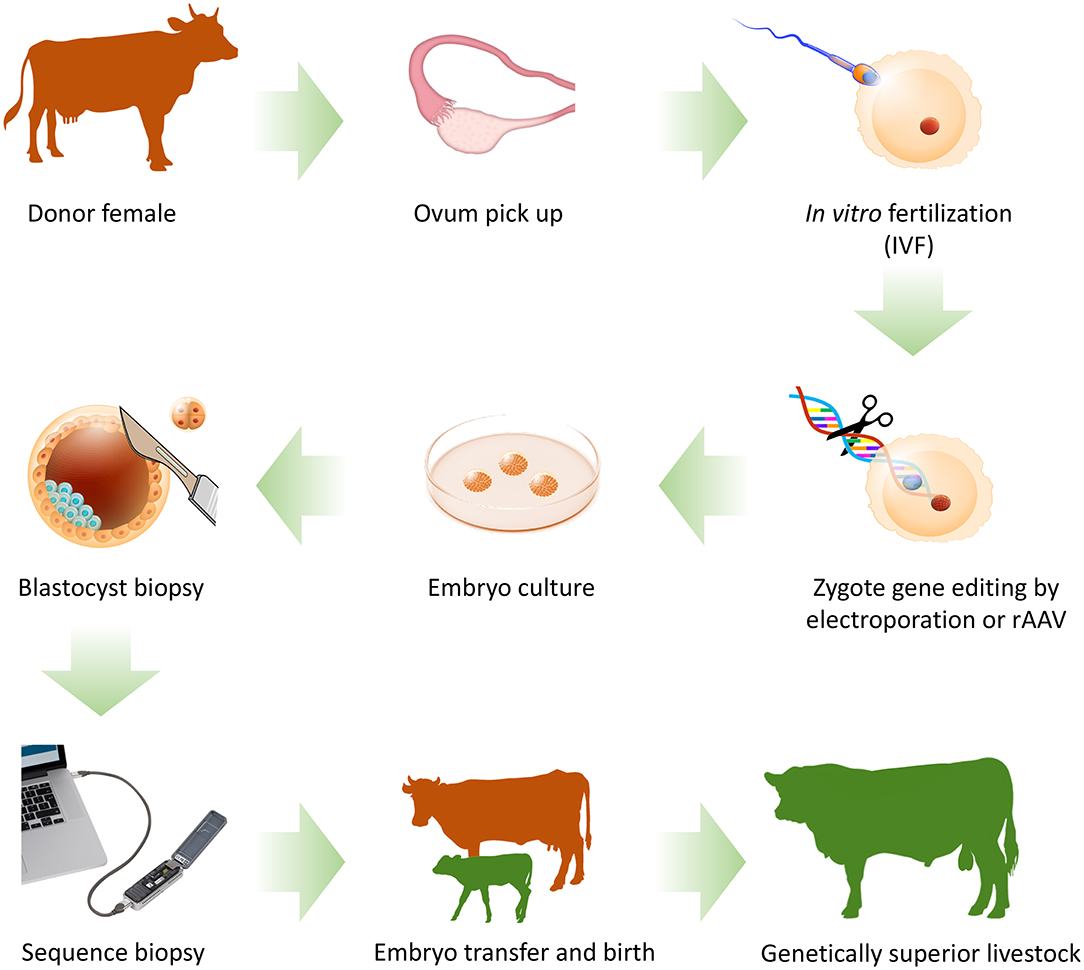
Figure 1. On-farm genome editing by zygote electroporation or zygote transduction of recombinant adeno-associated viruses (rAAV). Oocytes are collected from donor females using ovum pick up. Collected oocytes are matured and fertilized in vitro. Validated genome editing reagents are introduced into the zygote using electroporation or transduction. Embryos are cultured in vitro to blastocyst stage. A biopsy is taken from each blastocyst, DNA is extracted and sequenced on-farm using a portable DNA sequencer. Embryos with the desired edits are transferred into recipient females, who give birth to genome edited offspring. Animals with confirmed genotypes are added into the breeding program to disseminate their superior genetics.
Equipment, expertise, throughput and efficiency inhibits SCNT or zygote microinjection from being routinely applied on-farm. One of the key benefits of electroporation is that CRISPR-Cas reagents and HDRTs can be designed on a basic computer, ordered online and supplied ready-to-use in a short time frame. Compared to conventional microinjection-based approaches, electroporation is compatible with high throughput, potentially editing hundreds of zygotes simultaneously with reduced physical damage and improved embryo survival rates. The portable equipment and minimum training requirements would allow zygote electroporation to be integrated into established ET programs with little disruption.
Although electroporation has many benefits over SCNT and zygote microinjection, there are still several limitations (Laible, 2018). Primarily, only short HDRTs have been successfully used in zygote electroporation. This limits the genetic alterations to <1 kb in length. Secondly, like zygote microinjection, electroporation can be associated with mosaicism in the genome edited offspring. This occurs when the genome editing does not occur prior to the first zygotic cleavage but when the embryo has progressed to the 2 cell stage or beyond. Optimization of the timing of genome editor delivery following fertilization will likely result in a reduction in the frequency of mosaic offspring. Although mosaicism is undesirable, it is not a major concern as it can be bred out in a single generation.
Zygote Transduction with Recombinant Adeno-Associated Virus (rAAV)
Due to its non-pathogenic nature, rAAVs can be used as vehicles to deliver CRISPR-Cas and HDRTs. This strategy has proven effective in editing the genome of mice zygotes but like electroporation it has been used on other mammalian cell types for many years (Kaulich and Dowdy, 2015). To generate genome edited livestock using rAAV, oocytes are collected from donor mothers and fertilized. The fertilized zygotes are then bathed in a solution containing rAAVs that enter the zygote and drive genome editing by expressing CRISPR-Cas and providing HDRT. Once validated by biopsy sequencing, edited embryos are then transferred to recipient females to develop into genetically superior livestock (Figure 1).
Many of the benefits of rAAV transduction are similar to zygote electroporation when compared to SCNT and microinjection. Transduction with rAAV does not require physical damage to the zygotes which significantly improves embryo survival rates. The technique increases throughput and does not require any additional equipment or skills within on-farm ET programs. Furthermore, as rAAV is non-pathogenic, it can be handled safely at biosafety level 1, making it suitable for most on-farm settings.
Although this approach is yet to be applied to livestock, in mice, rAAV has been applied to zona-intact zygotes to successfully generate genome edited pups. It has very high embryo survival rates with editing in up to 100% of offspring (Yoon et al., 2018). One rAAV vector can comfortably accommodate a 3.25 kb HDRT, and even larger DNA sequences could be integrated if multiple rAAV vectors are designed to sequentially integrate HDRTs (Bak and Porteus, 2017). No other technique exhibits such simplicity for precise integration of large DNA sequences, and this is likely where rAAV transduction will be of most value in livestock genome editing.
The single stranded DNA genome of rAAV integrates into non-homologous host genomic sites at very low frequencies (~0.1%). rAAV is also quickly diluted as the cells undergoes multiple rounds of division, making it ideally suited for manipulating the genome of zygotes (Yoon et al., 2018). However, like most genome editing delivery approaches, mosaicism has been observed in rAAV genome edited mice. We expect optimization of embryo collection and the timing of rAAV transduction will reduce the frequency of mosaicism in rAAV edited offspring.
Customized rAAV vectors can be ordered from commercial suppliers as ready-to-use reagents (Sandoval et al., 2019). The design does require knowledge of viral genetics and is time-consuming, however online tools are available to assist and standardized rAAV kits could be developed for specific breeds and traits. Genome editing zygotes by rAAV transduction would not require the purchase of any additional equipment over a standard ET program and adding rAAV to zygotes in culture is a relatively straightforward procedure. The low cost and skill-level required by operators could see this technology widely adopted in on-farm settings in the near future.
Surrogate Sire Technology
Surrogate sire technology (SST) describes the creation of male animals lacking endemic germline stem cells, and therefore their ability to produce mature sperm. Spermatogonial stem cells (SSCs) from a donor male can then be transplanted into recipient testes of a surrogate sire, providing a source of self-propagating stem cells which can produce mature sperm containing the genetic information of the donor (Giasetti et al., 2019). The recipient male can then disseminate the genetics of the donor by natural breeding, operating as an ambulatory artificial insemination system.
Commercial application of SST relies on a supply of males lacking their own germline stem cells. To achieve this, researchers have generated male mice and pigs lacking a functional copy of NANOS2, a highly-conserved mammalian gene that plays an essential role in the maintenance of SSCs (Park et al., 2017). The resulting animals are physiologically healthy, displaying no abnormal phenotypes other than a complete lack of native SSCs. The transplantation of donor SSCs into the otherwise physiologically normal testicular environment of these animals leads to the establishment of the donor cell SSC population and eventually to the production of donor-derived sperm. Researchers are now working to establish NANOS2 knockout cattle, sheep and goats.
To integrate genome editing into established SST procedures, CRISPR-Cas and HDRTs can be delivered whilst donor SSCs are expanded during ex vivo cell culturing. CRISPR-Cas has been used to successfully edit the genome of mouse SSCs during ex vivo cell culture. The edited mouse SSCs were then transplanted into the testes of recipient males and lead to the generation of offspring harboring the edited donor genetics (Wang et al., 2017). These findings suggest the same will be achievable for mammalian livestock species, enabling the dissemination of genome-edited livestock genetics through surrogate sires.
Genome editing with SST would be particularly valuable for disseminating beneficial traits through expansive ranch-style operations or in developing regions where other genome editing approaches are limited by equipment and expertise availability or the scale of the operation. For on-farm settings, a vet could collect a needle testicular biopsy from a donor animal and ship the sample to a laboratory. SSCs would be expanded from the biopsy, with the desired genome edits introduced during the cell culture process. The cells would then be transplanted into juvenile sire recipients, and delivered to the farmer for integration into their breeding operation (Figure 2). With this genome editing pipeline, SST keeps on-farm equipment and expertise requirements to a minimum and empowers the farmer to maintain their own herd blood lines whilst benefiting from the technology.
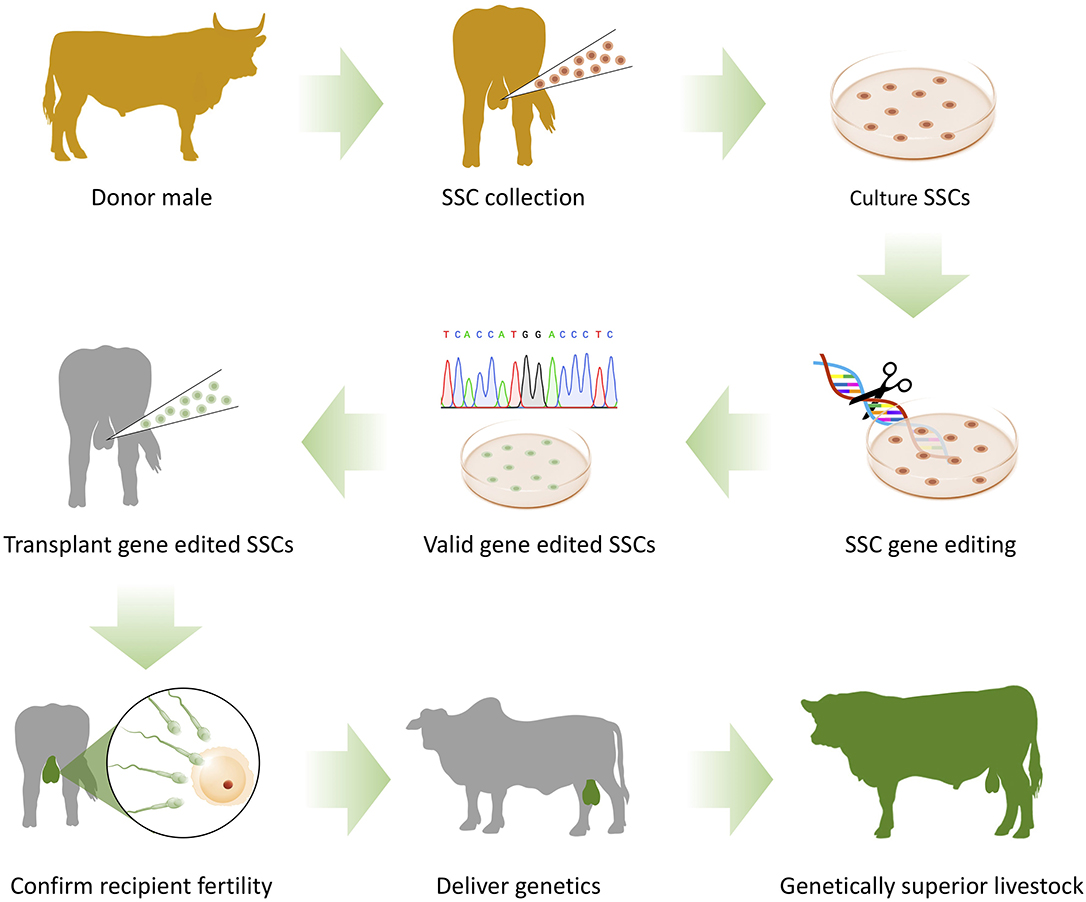
Figure 2. Genome editing with surrogate sire technology (SST). A spermatogonial stem cell (SSC) sample is collected on-farm by needle testicular biopsy from a donor male with suitable genetic merit. SSC sample is shipped to a laboratory that cultures and expands the cells in vitro. Genome editing tools are added into SSCs in culture to introduce trait/s of interest. Gene edited SSCs are validated and transplanted into the testis of germline ablated recipient male. SSC colonization of the testis and fertility of recipient male is confirmed before delivering surrogate sires to the farmer. Surrogate sires are then introduced into the breeding program to disseminate the superior germline genetics.
In regions such as Africa and South America, robust bulls of indigenous breeds could be used as surrogate sires to carry elite genome edited semen. Within the pig industry, SST boars could rapidly disseminate advantageous genome edited traits. This technology allows livestock breeders to achieve breeding objectives in less time. Using genomic prediction tools, elite SSCs from sexually immature males could be obtained, genome edited and transplanted into maturing germline ablated recipients within months. Instead of waiting until breeding maturity for each animal, generation intervals could be skipped, accelerating the rate of genetic gain.
Risks Associated With Unintended or Off-Target Editing
Until recently, genome editing approaches in livestock were commonly performed using an editor to introduce a DSB at the target site, with a double-stranded DNA (dsDNA) molecule, typically a plasmid, supplied as a repair template. Although this approach has seen success, it is not uncommon for the plasmid repair template to insert elsewhere in the genome. This issue was recently highlighted when the US Food and Drug Administration (FDA) found additional plasmid sequence proximal to the target site in genome edited hornless bulls (Norris et al., 2019). There are straightforward methods available to screen for unintended repair template integration and once identified the unwanted integration can be bred out using standard breeding strategies. This was the approach taken with the genome edited hornless bulls (Young et al., 2019). Most livestock genome editing efforts have now transitioned to using single stranded DNA (ssDNA) repair templates, which have a significantly reduced frequency of unintended genomic integration. Despite the reduced risks with ssDNA repair templates, comprehensive screening of founder livestock is important to maintain public and political trust in the technology.
Off-target editing occurs when a genome editor cuts at an unplanned site in the genome. It was a significant concern with early genome editing experiments as the impact of off-target effects remained contentious. However, the latest genome editing technologies have improved and quantified specificity, reducing off-target effects, and concerns. The goal of improving CRISPR-Cas reagents and increasing genome editing specificity has been primarily driven through biomedical research looking to apply genome editing to treat or cure human disease. The work done in the biomedical arena has significantly expanded the toolkit of CRISPR-Cas reagents available, which now includes base editors that can edit single nucleotides in the genome without the need to induce DSBs (Eid et al., 2018). Although current CRISPR-Cas reagents are adequate for on-farm application, further improvements in the specificity and efficiency of CRISPR-Cas will deliver reagents that carry a risk of off-target effect substantially lower than the frequency of spontaneous mutations naturally occurring in animal genomes.
Regulations
The science is clear: genome editing could improve animal welfare and performance while reducing the environmental footprint of livestock production. Furthermore, dietary DNA is generally regarded as safe to consume, as naturally occurring DNA variations are a routine ingredient in the food products we consume. What remains unclear is the regulatory pathway to bring genome edited animal-derived foods to market (Zhou et al., 2019). Currently, regulations vary substantially between geo-political regions. Harmonizing the regulations associated with genome editing in food species is imperative to allow livestock farmers access to genome editing tools that could increase global food security in a sustainable manner.
Genome editing provides an opportunity to align the interests of producers and consumers. Despite the foreseeable benefits, the EU applies an inhibitory regulatory framework on genome edited foods and the US currently mandates premarket new animal drug regulatory evaluation for all genome edited food animals (Van Eenennaam et al., 2019). Although the EU and the US have an oversupply of food, the prohibitive regulatory frameworks they currently implement may have detrimental knock-on effects in developing countries who stand to benefit the most from genome editing technology.
As the tide of public acknowledgment to the benefits and safety of genome editing appears to be turning, the technology will continue to gain media attention and public debate. In the US and EU there are movements from within government and the scientific community to modernize regulatory frameworks. In the US, President Trump recently signed an “Executive Order on Modernizing the Regulatory Framework for Agricultural Biotechnology Products,” while in the EU a report by the European Academies' Science Advisory Council on “Genome Editing: Scientific opportunities, public interests, and policy options in the EU” was delivered to the European Parliament and European Commission to prompt a rethink of EU's stance on genome edited foods.
Outside of the EU and US, countries such as Japan, Argentina, Brazil, Canada, and Australia have to varying extents deregulated genome editing of livestock. These proactive countries are likely to gain a competitive advantage and leave other non-subscribing nations scrambling to prevent genome edited foods entering their supply chain. Unlike previous transgenic technologies, the genetic alteration in genome edited foods are often “scarless,” in that they contain no foreign DNA. Without any plausible methodology for discriminating “naturally occurring” from intentionally edited DNA variations, regulators will have difficulty enforcing importation restrictions on genome edited foods.
Concluding Remarks
The amalgamation of genome editing and cutting-edge reproductive technologies offers a powerful tool for improving the livestock breeding landscape. Success in creating precise and heritable germline edits in diverse livestock species for a plethora of traits has demonstrated the potential benefits of this technology. However, to spread the beneficial impacts across economies, geographic regions and societies, strategies of translating established genome editing protocols into livestock breeding systems are necessary. Zygote electroporation and rAAV transduction of genome-editing reagents evades the associated, costs, labor, and facilities required by traditional methods. SST converged with genome editing could offer a commercially valuable tool to farmers with natural breeding programs. This array of cutting-edge reproductive technologies makes it technically plausible to apply genome editing in on-farm settings to rapidly improve productivity, fertility, sustainability, and animal welfare with minimal infrastructure and moderate fiscal inputs. The key to unlocking these benefits now lays in the hands of regulators.
Author Contributions
GM and HS envisaged the article and contributed to writing. AS contributed to writing the manuscript. SL assisted with editing and conceptual development. GM generated the figures.
Funding
GM was supported by the UK Commonwealth Scholarship Commission. HS was supported by Edinburgh Global Scholarship from Edinburgh University. AS was supported by Erasmus+. This work was part funded by the BBSRC through ISP BB/P013759/1.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alexandratos, N., and Bruinsma, J. (2012). World Agriculture Towards 2030/2050: The 2012 Revision. ESA Working paper No. 12-03. Rome: FAO.
Bak, R. O., and Porteus, M. H. (2017). CRISPR-mediated integration of large gene cassettes using AAV donor vectors. Cell Rep. 20, 750–756. doi: 10.1016/j.celrep.2017.06.064
Davis, S. R., Spelman, R. J., and Littlejohn, M. D. (2017). BREEDING AND GENETICS SYMPOSIUM:Breeding heat tolerant dairy cattle: the case for introgression of the “slick” prolactin receptor variant into Bos taurus dairy breeds. J. Anim. Sci. 4, 1788–1800. doi: 10.2527/jas.2016.0956
Eid, A., Alsharaf, S., and Mahfouz, M. M. (2018). CRISPR base editors: genome editing without double-stranded breaks. Biochem. J. 475, 1955–1964. doi: 10.1042/BCJ20170793
Fernandez, A., Josa, S., and Montoliu, L. (2017). A history of genome editing in mammals. Mamm. Genome. 28:6299. doi: 10.1007/s00335-017-9699-2
Giasetti, M. I., Ciccarelli, M., and Oatley, J. M. (2019). Spermatogonial stem cell transplantation: insights and outlook for domestic animals. Annu. Rev. Anim. Biosci. 7, 385–401. doi: 10.1146/annurev-animal-020518-115239
Kaulich, M., and Dowdy, S. F. (2015). Combining CRISPR/Cas9 and rAAV templates for efficient gene editing. Nucleic. Acid. Ther. 25, 287–296. doi: 10.1089/nat.2015.0545
Laible, G. (2018). “Production of transgenic livestock: overview of transgenic technologies,” in Animal Biotechnology 2, eds H. Niemann and C. Wrenzycki (Berlin: Springer), 95–121. doi: 10.1007/978-3-319-92348-2_6
Laible, G., Wei, J., and Wagner, S. (2014). Improving livestock for agriculture – technological progress from random transgenesis to precision genome editing heralds a new era. Biotech. J. 10, 109–120. doi: 10.1002/biot.201400193
Miao, D., Giasetti, M. I., Ciccarelli, M., Lopez-Biladeau, B., and Oatley, J. M. (2019). Simplified pipelines for genetic engineering of mammalian embryos by CRISPR-Cas9 electroporation. Biol. Reprod. 101, 177–187. doi: 10.1093/biolre/ioz075
Mueller, M. L., Cole, J. B., Sonstegard, T. S., and Van Eenennnaam, A. L. (2019). Comparison of gene editing versus conventional breeding to introgress the POLLED allele into the US dairy cattle population. J. Dairy Sci. 5, 4215–4226. doi: 10.3168/jds.2018-15892
Namula, Z., Wittayarat, M., Hirata, M., Hirano, T., Nguyen, N. T., Le, Q. A., et al. (2019). Genome mutation after the introduction of the gene editing by electroporation of Cas9 protein (GEEP) system into bovine putative zygotes. In Vitro Cell. Dev. Biol. Anim. 55, 237–242. doi: 10.1007/s11626-019-00385-w
Norris, A. L., Lee, S. S., Greenlees, K. J., Tadesse, D. A., Miller, M. F., and Lombardi, H. (2019). Template plasmid integration in germline genome-edited cattle. bioRxiv [Preprint]. doi: 10.1101/715482
Park, K.-E., Kaucher, A. V., Powell, A., Waqas, M. S., Sandmaier, S. E. S., Oatley, M. J., et al. (2017). Generation of germline ablated male pigs by CRISPR/Cas9 editing of the NANOS2 gene. Sci. Rep. 7:40176 doi: 10.1038/srep40176
Pickar-Oliver, A., and Gersbach, C. A. (2019). The next generation of CRISPR–Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 20, 490–507. doi: 10.1038/s41580-019-0131-5
Proudfoot, C., Lillico, S., and Tait-Burkard, C. (2019). Genome editing for disease resistance in pigs and chickens. Anim. Front. 9, 6–12. doi: 10.1093/af/vfz013
Sandoval, I. M., Collier, T. J., and Manfredsson, F. P. (2019). “Design and assembly of CRISPR/Cas9 lentiviral and rAAV vectors for targeted genome editing,” in Viral Vectors for Gene Therapy, eds F. Manfredsson and M. Benskey (New York, NY: Humana Press), 29–45. doi: 10.1007/978-1-4939-9065-8_2
Sheets, T. P., Park, C.-H., Park, K.-E., Powell, A., Donovan, D. M., and Telugu, B. P. (2016). Somatic cell nuclear transfer followed by CRIPSR/Cas9 Microinjection results in highly efficient genome editing in cloned pigs. Int. J. Mol. Sci. 17:2031. doi: 10.3390/ijms17122031
Tait-Burkard, C., Doeschl-Wilson, A., McGrew, M. J., Archibald, A. L., Sang, H. M., Houston, R. D., et al. (2019). Livestock 2.0 – genome editing for fitter, healthier, and more productive farmed animals. Genome Biol. 19:204. doi: 10.1186/s13059-018-1583-1
Van Eenennaam, A. L., Wells, K. D., and Murray, J. D. (2019). Proposed U.S. regulation of gene-edited food animals is not fit for purpose. Npj Sci. Food 3:3. doi: 10.1038/s41538-019-0035-y
Wang, Y., Ding, Y., and Li, J. (2017). “CRISPR-Cas9-mediated gene editing in mouse spermatogonial stem cells,” in RNAi and Small Regulatory RNAs in Stem Cells, ed B. Zhang (New York, NY: Humana Press), 293–305. doi: 10.1007/978-1-4939-7108-4_20
Yoon, Y., Wang, D., Tai, P. W. L., Riley, J., Gao, G., and Rivera-Perez, J. A. (2018). Streamlined ex vivo and in vivo genome editing in mouse embryos using recombinant adeno-associated viruses. Nat. Comm. 9:412. doi: 10.1038/s41467-017-02706-7
Young, A. E., Mansour, T. A., McNabb, B. R., Owen, J. R., Trott, J. F., Brown, C. T., et al. (2019). Genomic and phenotypic analyses of six offspring of a genome-edited hornless bull. Nat. Biotechnol. doi: 10.1038/s41587-019-0266-0. [Epub ahead of print].
Keywords: genome editing, CRISPR, genetic engineering, pigs, cattle, livestock, sustainability
Citation: McFarlane GR, Salvesen HA, Sternberg A and Lillico SG (2019) On-Farm Livestock Genome Editing Using Cutting Edge Reproductive Technologies. Front. Sustain. Food Syst. 3:106. doi: 10.3389/fsufs.2019.00106
Received: 12 August 2019; Accepted: 30 October 2019;
Published: 15 November 2019.
Edited by:
Yiping Qi, University of Maryland, College Park, United StatesReviewed by:
Mark Paul Running, University of Louisville, United StatesAgnieszka Barbara Najda, University of Life Sciences of Lublin, Poland
Copyright © 2019 McFarlane, Salvesen, Sternberg and Lillico. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gus R. McFarlane, Z3VzLm1jZmFybGFuZUBlZC5hYy51aw==
 Gus R. McFarlane
Gus R. McFarlane Hamish A. Salvesen
Hamish A. Salvesen Anna Sternberg
Anna Sternberg Simon G. Lillico
Simon G. Lillico